Abstract
Recently, plant-based food has become important in the global food market. The increasing demand for plant-based food is a consequence of the increase in both environmental awareness and care for animal welfare as well as the changes in nutritional recommendations. Therefore, food producers are concentrating on fulfilling consumer needs by creating alternatives to animal-based products with comparable nutritional and sensory qualities but from plant-based sources. One promising trend is the production of plant-based fish. Thus, this work aimed to summarize the possibilities of creating plant-based fish analogs, including a review of alternatives to fish products currently available on the market and the possible use of the various ingredients to produce plant-based fish analogs like fillets, slices, as well as sticks, or burgers. Furthermore, the plant-based ingredients were characterized for potential use in fish analogs production. Additionally, the study includes technologies used for plant-based fish analogs production, e.g., texturization, 3D and 4D printing, electrospinning, etc. Furthermore, future perspectives were given considering the challenges and limitations in this range.
1. Introduction
One dynamic developing trend in the food market is the growing popularity of vegan products [1]. Plant-based products are produced based on plant sources, which can be listed as vegetables, grains, pulses, seeds, nuts, fruits, etc. [2]. According to a report published by Allied Market Research [3], the size of the vegan food market was valued at USD 16.55 billion in 2022 and is forecast to reach USD 37.45 billion by 2030, i.e., the average annual growth rate (CAGR) in 2022–2030 will be 10.6%.
The growth of the plant-based food market has been primarily driven by people who restrict animal-based foods in their diets, including vegans [4]. The growing percentage of the population on a plant-based diet translates into an increasing market offer of vegan products. According to the Mintel report [5], every tenth product introduced to the European market in the food and drink category is labeled as vegetarian. As a result, more and more companies dealing exclusively with plant food are being established. In Europe, there were 11,655 of them in 2019, which is 93 percent more than in 2016. According to the Good Food Institute [6], plant-based fish and seafood alternatives remain a small fraction of the plant-based meat and seafood alternatives category, accounting for less than 1% of that category. However, it should be noted that in 2020 sales in this group of products in the US increased to USD 12 million, or 23%. In addition, investments in vegan seafood substitutes in the US reached USD 175 million in 2021, almost twice the amount raised in the previous year. These statistics show that, recently, work has been underway to fill the market gap with plant substitutes for fish and seafood.
There are several reasons why consumers are increasingly giving up eating fish and seafood, including ecological, ethical, or health reasons [7], which have been presented in Figure 1. Ecological aspects include overfishing, marine biodiversity reduction, and environmental destruction. Due to the growing population and increasing fish consumption, overfishing has been noted. Moreover, overfishing has led to a decline in fish populations, which has, in turn, affected marine biodiversity and ecosystem health. Additionally, fish farming practices can have a negative impact on the environment, including water pollution, habitat destruction, and the spread of disease. Unfortunately, fish are susceptible to various diseases, resulting in high mortality rates and reduced fish farm yields [8]. According to the United Nations, almost 90% of the world’s marine fish stocks are overfished. Another issue is ethical aspects and the growing popularity of animal rights protection, whose supporters increasingly raise the problem of the suffering of fish.
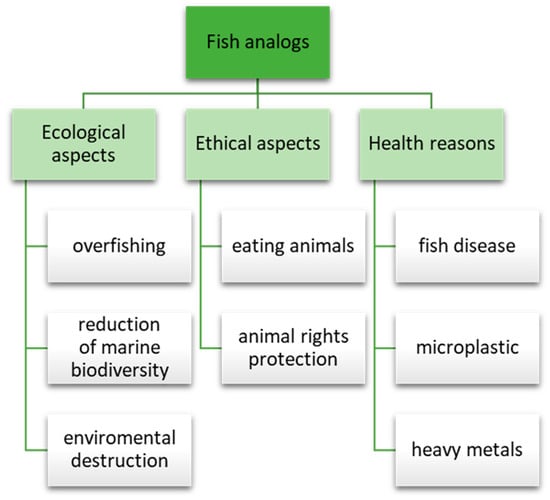
Figure 1.
Reasons for interest in fish analogs.
Furthermore, an increasing part of the population is giving up eating fish for health reasons. For example, the contamination of fish by microplastics poses a significant threat. Exposure to it, alone or in combination with other pollutants, can lead to various health issues in fish [9,10]. Furthermore, although fish contains many valuable ingredients and is an essential protein source for human growth and development, issues related to mercury, dioxins, microplastics, lead, and arsenic have recently been raised [11,12]. Heavy metals are a major source of pollution in aquatic environments and can accumulate in the tissues of fish, posing a risk to human health when consumed [13]. Considering the above reasons, in the last few years, both large companies and start-ups have tried to meet the needs of consumers—the Fact.MR report predicts a CAGR increase of the global plant-based fish market in 2021–2031 by 28%, which means that the projected market value in 2031 is 1.3 billion dollars [14].
Plant-based animal analog food products can be classified into several categories, as follows [15,16,17]:
- -
- Dairy analogs. These plant-based products mimic dairy products like milk, cheese, and yogurt. They are typically made from nuts, soy, chickpea, oats, seeds, tapioca, yeasts, etc.
- -
- Meat analogs. These are plant-based products that mimic the meat’s taste, texture, and nutritional profile. They are usually made from soy, wheat, or pea protein and can be prepared in different forms, such as burgers, sausages, and meatballs.
- -
- Seafood analogs. These plant-based products are usually made from algae, seaweed, soy protein, and gluten and can be used in a variety of dishes, such as sushi, crab cakes, and fish and chips.
- -
- Egg analogs. These are plant-based products that replicate the taste and texture of eggs. They are typically made from tofu, chickpea flour, or aquafaba.
Commonly, plant-based fish products are a sub-category of seafood analogs and are specifically designed to mimic the taste, texture, and nutritional profile of fish [16]. Such products usually have a longer shelf life and better food safety than animal-origin food [18] due to plant-derived protein, eliminating the risk of zoonotic diseases. Moreover, in order to achieve a meat-like texture, the plant-derived ingredients undergo multiple processing steps, including heat treatment (high-temperature cooking or extrusion), during which microbial contamination can be significantly reduced. However, high-temperature processing of protein products can lead to the formation of harmful substances such as heterocyclic aromatic amines, polycyclic aromatic hydrocarbons, and advanced glycation end products from the Maillard reaction. On the other hand, analogs are multi-component, so each ingredient introduced into the final product (e.g., dyes or spices) can be a source of pathogenic microflora. Additionally, meals prepared with meat analogs can be more prone to spoilage during storage due to their neutral pH. This can complicate the supply chain and requires strict control during release for consumption, compared to their meat-containing counterparts [19,20,21,22].
To prevent contamination, the packaging and storage of meat analogs should be similar to meat products. Chilled or frozen conditions in closed plastic containers or flexible bags are recommended for storing plant-based analogs [18]. Additionally, Priyadarshini et al. [23] noted that the quality of shrimp analog prepared from Pangasionodon hypophthalmus surimi was better under vacuum packaging than without vacuum (standard packaging). The study found that psychrophilic microorganisms were detected later in the vacuum-packaged samples, and the amount of total volatile base nitrogen was lower compared to standard packaging. Generally, for plant-based analogs packaging with the modified atmosphere can reduce oxidation and expand shelf life [18]. However, similarly to meat and fish products, numerous studies have explored the utilization of natural antioxidants to prolong their shelf life. Thus, it can also be used to prolong the shelf life of plant-based analogs [24].
According to the above-mentioned aspects, this article attempts to compare vegan fish analogs available on the market, ingredients used for the production of vegan meat and fish analogs, as well as methods of their preparation.
2. Fish Alternative Products Market Review
Currently, there are several plant-based fish products available on the market. In Table 1, plant-based fish products with listed ingredients as well as nutritional value, are shown. Main groups such as fillets, slices, fish sticks, or burgers can be distinguished (Figure 2). The most popular product group is fillets. The market review showed fillets are commonly found on each analyzed continent—Europe, Asia, and North and South America. Moreover, fillets occur both in breaded and non-breaded forms. In this group, the most commonly used basic raw material is soybean protein. Wheat or pea protein, as well as rice, are also often used [25]. For instance, the OmniFoods company from Hong Kong produces fish analogs (“Golden fillet”) using soybean, pea and rice protein, rapeseed oil, and potato starch. The products have also been enriched with omega-3 fatty acids [26]. Some companies use a mixture of various plant proteins. This way of designing plant-based food products is very important from the point of view of providing complementary proteins [15]. For example, the American Good Catch uses a blend of pea protein isolate, soy protein concentrate, chickpea flour, faba protein, lentil protein, soy protein isolate, and navy bean powder [27]. Vegan fish fillets are also present on the European market—Novish, a Dutch company, launched plant-based substitutes in January 2020, which made them the first vegan fish company in Europe. The products contain, e.g., rice, water, wheat flour, or rapeseed oil [28].
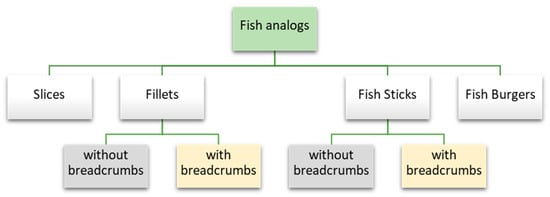
Figure 2.
Types of plant-based analogs available on the market.
It is well-known that soybean is an allergenic food product. Its proteins can cause unwanted effects like diarrhea, vomiting, urticaria, anaphylaxis, and atopy, as well as facial swelling, airway narrowing, and difficulties in breathing [29]. A lot of plant-based alternatives contain this source of protein. However, some products on the market do not contain this allergen. For example, Novish produces soy-free products, which may be good product for allergy sufferers interested in a vegan diet [28]. There are also VBites products from the United Kingdom on the European market, which are made of, e.g., rapeseed oil, yeast, konjac flour, tapioca, and wheat starch [30]. Yeast is the source of the specific taste and the protein that can be used in a wide range of foodstuffs—an inexpensive supplement to the human diet [31].
Industries try to recreate specific fish species; salmon is the most frequently chosen species. An analog of salmon produced by the Asian company May Wah is made from soy and wheat proteins, salt, soy sauce, and canola oil [32]. In addition, another often imitated species of fish is cod. For instance, cod fillet produced by the Malaysian company TKC Food uses soy protein, tapioca starch, and vegetable oils [33]. Occasionally, companies seek unique concepts which differentiate them from others. Such an approach is represented by Everbest, which developed a substitute for the Goby Fish. The product is based on soy protein, water, tapioca starch, yeast extract, and seaweed [34].
Another popular type of plant-based product is sliced smoked salmon. For example, the vegan salmon of Austrian company Revo is made of pea protein, canola, and linseed oils [35], whereas the product of Vbites is based on rapeseed oil and soya protein [30]. In contrast, the salmon alternative produced by the American enterprise Sophie’s Kitchen contains olive oil, konjac powder, pea starch, potato starch, pea protein, organic agave nectar, or seaweed powder [36]. Ran et al. [8] showed that using the water-soluble polysaccharide-konjac glucomannan could influence the texture and rheological properties to mimic the tissue of the seafood analogs. The authors stated that adding konjac glucomannan could be a good solution to simulate seafood products without the need for high-pressure extrusion.
Market research shows that fish sticks are most often produced and are common mainly in Europe. In addition, they usually occur in breaded form, and the main ingredient is wheat protein. Wheat protein has several functions, including visco-elasticity and specific texture, and due to these properties, it can be used as the main component of plant-based products [1,18]. On the market, there is Paluszki a’la rybne by the Polish company Polsoja. This product contains wheat protein, isolated soy protein, wheat starch, and wheat fiber [37]. There are several fish stick products produced using wheat protein or wheat flour. These include Sea Style Sticks [38], Vivera’s Vegan Crispy Sticks [39], Good Catch’s Fish Sticks [27], etc. Even when the product contains another source of protein, wheat proteins are added to obtain complementary protein sources [15]. Mainly tofu was used for the production of Waitrose’s Vegan Fishless Fingers. However, they also contain wheat flour, durum wheat semolina, corn barley flour, wheat gluten, and tapioca starch [40].
Furthermore, Good Catch’s company evaluated their own food additive for plant-based products, which contains different sources of proteins, such as pea protein isolate, soy protein concentrate, chickpea flour, faba protein, lentil protein, soy protein isolate, navy bean powder [27]. Furthermore, to improve the texture of the fish sticks, some companies use thickener-modified potato starch or xanthan gum, as in Paluszki a’la rybne [37] and Good Catch’s Fish Sticks [27]. In addition, different sources of oils or fats (olive oil, rapeseed oil, sunflower oil, and linseed oil) are essential to use in such products because fat is a carrier of flavor and taste in plant-based analogs [18].
The next type of plant-based fish analog is fish burgers. In comparison to previously described products, burgers are not as diverse when it comes to their composition. The main ingredients are water and pea proteins. For example, Salmon Burgers Original and Whitefish Burgers by Sophie’s Kitchen [36] are made from pea protein, sunflower oil, hemp oil, potato starch, emulsifier, yeast extract, and spices.
In addition, researchers conducted studies to obtain high-quality products using different food components. For example, shrimp analogs were obtained from sunflower oil, alginate, egg white powder, sucrose, glycine, and CaCO3. Furthermore, shrimp analogs were incorporated with ethanolic extract of coconut husk as well as cluster bean extract to extend the shelf-life of ready-to-eat vegan products from 12 days of refrigerated storage to 21 and 16 days, respectively [41].

Table 1.
The plant-based fish products available on the market.
Table 1.
The plant-based fish products available on the market.
| Type of Product | Name of the Product | Region | Producer | Ingredients | Nutritional Value | References |
|---|---|---|---|---|---|---|
| Fish fillets | Breaded Fish Fillets | Europe | Novish | Rice. Water. Flour (wheat). Rapeseed oil. Salt. Thickener (methylcellulose). Yeast. Aroma. Fiber (sugar cane, pea). Buffered vinegar powder (preservative ingredient). Modified starch (oxidized starch). Food acid (citric acid). Spices (paprika powder). | Energy: 690 kJ/165 kcal; Fat: 6.8 g. of which saturates: 0.5 g; Carbohydrates: 19.7 g. of which sugars: 1.6 g; Fibers: 6.6 g; Protein: 3.1 g; Sodium: 0.46 g; Salt: 1.2 g | [28] |
| Fish fillets | Breaded Fishless Steaks | Europe | Vbites | Water. Breadcrumb Coating (Wheat Flour. (Flour Calcium Carbonate. Niacin. Iron. Thiamine). Rapeseed Oil. Water. Salt. Yeast. Konjac Flour. Tapioca Starch. Wheat Starch. Salt. Natural Flavouring. Sugar. Wheat Fibre. Yeast Extract. Thickener: Carrageenan. Preservative: Potassium Sorbate. Color: Titanium Dioxide. | Energy: 568 kJ/136 kcal; Fat: 5.7 g. of which saturates: 0.4 g; Carbohydrate: 17.8 g. of which sugars: 1.1 g; Fibre: 2.7 g; Protein: 2 g; Salt: 1.2 g | [30] |
| Fish fillets | Classic Fillet | Asia | OmniFoods | Water. Soy Protein Concentrate. Canola Oil. Potato Starch. Corn Starch. Methylcellulose. Salt. Natural Flavor. Cane Sugar. Yeast Extract. Pea Protein. Rice Protein. Shiitake Mushroom Powder. Oats. Wheat Starch. | Energy: 670 kJ/160 kcal; Fat: 9 g. of which saturates: 0.5 g. polyunsaturates: 1.5 g. monounsaturates: 3 g; Carbohydrate: 9 g. of which sugars: 1 g; Fiber: 3 g; Protein: 11 g; Salt: 0.3 g | [26] |
| Fish fillets | Golden Fillet | Asia | OmniFoods | Fillets [Water. Soy Protein Concentrate. Canola Oil. Potato Starch. Methylcellulose. Salt. Natural Flavor. Cane Sugar. Yeast Extract. Pea Protein. Rice Protein. Shiitake Mushroom Powder. Oats]. Batter [Water. Enriched Unbleached Wheat Flour (Flour. Niacin. Reduced Iron. Thiamine Mononitrate. Riboflavin. Folic Acid). Wheat Starch. Cane Sugar. Salt. Baking Powder (Sodium Acid Pyrophosphate. Sodium Bicarbonate. Corn Starch. Monocalcium Phosphate)]. | Energy: 670 kJ/160 kcal; Fat: 10 g. of which saturates: 0.5 g. polyunsaturates: 1.5 g. monounsaturates: 3 g; Carbohydrate: 11 g. of which sugars: >1 g; Fiber: 2 g; Protein: 6 g; Salt: 0.3 g | [42] |
| Fish fillets | Plant Based Fish Fillets | North and South America | Sophie’s Kitchen | Textured vegetable protein (pea protein, pea starch). Canola oil. Rice flakes (from brown rice). Konjac powder. Seaweed powder. Potato starch. Powdered cellulose. Organic agave nectar. Turmeric. White pepper. Sea salt. Ginger. | Energy: 180 kcal; Fat: 8 g. of which saturates: 1 g; Carbohydrate: 20 g. of which sugars: 3 g; Protein: 8 g; Fiber: 4.6 g; Salt: 0.4 g | [36] |
| Fish fillets | Fish Fillets | North and South America | Gardein | Water. Soy protein concentrate. Rapeseed oil. Wheat flour. Modified corn starch. Tapioca starch. Potato starch. Wheat gluten. Methylcellulose. Isolated soy protein. Maltodextrin. Natural flavors. Corn flour. Sea salt. Salt. Spices. Onion powder. Yeast extract. Yeast. Garlic powder. Soy flour. Algae oil. Baking powder. Xanthan gum. Sugar. Seasoning. Soybean oil. Cane sugar. Citric acid. Color: Pea protein. Carrot powder. Beet fiber. | Energy:870 kJ/208 kcal. Fat: 13.5 g. of which saturates: 1 g; Carbohydrate: 12.5 g; Fiber: 2.1 g; Protein: 9.4 g; Salt: 0.4 g | [43] |
| Fish fillets | Plant -Based Breaded Fish Fillets | North and South America | Good Catch | Water. Good Catch 6-Plant Protein Blend (pea protein isolate. Soy protein concentrate. Chickpea flour. Faba protein. Lentil protein. Soy protein isolate. Navy bean powder). Wheat flour. Sunflower oil. Corn starch. Natural flavors. Methylcellulose. Yeast extracts. Sugar. Salt. Corn maltodextrin. Garlic powder. Onion powder. Corn flour. Yeast. Spices. Xanthan gum. Annatto extract. Acetic acid. | Energy: 740 kJ/177 kcal; Fat: 9.7 g. of which saturates: 0.9 g; Carbohydrate: 12.4 g. of which sugars: 0.9 g; Fiber: 0.9 g; Protein: 10.6 g | [27] |
| Fish fillets | Vegan Salmon | Europe | Vantastic Foods | Wheat fiber. Soy fiber. Water. Soy protein. Soy sauce (soy, wheat, salt, sugar). Tapioca starch. Sugar. Salt. White pepper. Color: Paprika extract; Seaweed (Laminaria japonica). | Energy: 1071 kJ/257 kcal; Fat: 17 g. of which saturates: 2.8 g; Carbohydrate: 17.8 g. of which sugars: 1.1 g; Fiber: 2.7 g; Protein: 2 g; Salt: 1.2 g | [44] |
| Fish fillets | Vegan Salmon Fillet | Asia | May Wah | Soy protein. Wheat protein. Salt. Soy sauce. Canola oil. Five spice. Pepper. Seaweed. | Energy: 268 kJ/64 kcal; Fat: 4.5 g. of which saturates: 0 g; Carbohydrate: 3 g. of which sugars: 0.5 g; Protein: 4 g; Salt: 0.3 g | [32] |
| Fish fillets | TKC Vegetarian Cod Fillet | Asia | TKC Food | Soybean Protein. Water. Modified Potato Starch. Soybean Oil. Seaweed. Sugar. Salt. Spices. Seasoning. | Energy:1381 kJ/330 kcal; Fat: 27 g. of which saturates: 5 g; Carbohydrate: 11 g. of which sugars: 0 g; Protein: 12 g; Salt: 0.7 g | [33] |
| Fish fillets | Vegan Cod Fish | Asia | Ahimsa | Isolated soy protein. Textured soy protein. Tapioca starch. Vegetable oil. Salt. Sugar. Seaweed. | Energy: 365 kJ/87 kcal; Fat: 0.4 g. of which saturates: 0.2 g; Carbohydrate: 4 g; Protein: 10.5 g; Fiber: 12.2 g; Salt: 0.5 g | [45] |
| Fish fillets | Vegetarian Goby Fish | Asia | Everbest | Isolated soy protein. Water. Soy. Modified tapioca starch. Yeast extract. Seaweed. | Energy: 812 kJ/194 kcal; Protein: 13.2 g; Fat: 11 g; Carbohydrate: 11 g | [34] |
| Slices | Smokey Salmon Slices | Europe | VBites | Water. Rapeseed Oil. Soya Protein. Thickeners: Carrageenan. Konjac Flour. Potato Starch. Salt. Natural Flavouring. Onion Powder. Sugar. Preservative: Potassium Sorbate; Color: Annatto. Iron Oxide. | Energy: 546 kJ/123 kcal; Fat: 9.5 g. of which saturates: 0.8 g; Carbohydrate: 5.1 g. of which sugars: 0.8 g; Fiber: 1.7 g; Protein: 5.6 g; Salt: 1.9 g | [46] |
| Slices | Revo Smoked Salmon | Europe | Revo | Water. Pea protein. Vegetable oils (rapeseed oil, linseed oil). Flavors. Gelling agent: calcium alginate; thickeners: carrageenan. Konjac; Sea salt. Modified starch. Beetroot concentrate. Vitamins (vitamin B2, vitamin B6, vitamin B12, vitamin D2). Smoke flavor. Color: carotene. | Energy: 401 kJ/96 kcal; Fat: 5.4 g. of which saturates: 0.8 g; Carbohydrate: 5.1 g. of which sugars: 0.5 g; Protein: 5.7 g; Salt: 3 g | [35] |
| Slices | Smoked Salmon | North and South America | Sophie’s Kitchen | Water. Olive oil. Konjac powder. Pea starch. Potato starch. Pea protein. Sea salt. Organic agave nectar. Seaweed powder. Fenugreek. Alginate (from seaweed). Paprika. Calcium hydroxide. | Energy: 669 kJ/160 kcal; Fat: 4 g. of which saturates: 0 g; Carbohydrate: 32 g; Fiber: 6 g; Protein: >2 g. Salt: 0.5 g | [47] |
| Fish Sticks | Paluszki a’la rybne | Europe | Polsoja | Water. Breadcrumbs (water, rapeseed oil, wheat flour, wheat gluten, salt, corn flour, rice flour, yeast, olive oil, paprika extract, color: paprika extract). Wheat protein. Oil rapeseed. Isolated soy protein. Salt. Wheat starch. Flavors. Wheat fiber. Thickener: Modified potato starch. Emulsifier: Methylcellulose. Dried onion juice concentrate. Dried vegetables (garlic, carrot, parsley, onion). | Energy: 891 kJ/213 kcal; Fat: 8.3 g. of which saturates: 0.7 g; Carbohydrates: 21.3 g. of which sugars: 1.8 g; Fiber: 3 g; Protein: 11.5 g; Salt: 1.6 g | [37] |
| Fish Sticks | Sea Style Sticks | Europe | Veganz | Rehydrated wheat protein. Wheat flour. Sunflower oil. Water. Spirit vinegar. Salt. Thickener: methylcellulose; natural flavorings. Wheat starch. Wheat fibers. Herbs. Spices. Linseed oil. Potato fibers. Maltodextrin. Maize Starch. Preservative: Sodium diacetate; Onion powder. Garlic powder. Iron gluconate. Cyanocobalamin. | Energy: 1029 kJ/245 kcal; Fat: 9.3 g. of which saturates: 1.1 g; Carbohydrate: 26 g. of which sugars: 1.4 g; Protein: 13 g; Salt: 1.6 g; Iron: 2.1 g; Vitamin B12: 0.38 g | [38] |
| Fish Sticks | Vegan Fishless Fingers | Europe | Waitrose | Tofu (water, soya beans). Water. Fortified wheat flour (wheat flour, calcium carbonate, iron, niacin, thiamin). Durum wheat semolina. Barley flour. Maize starch. Seaweed. Salt. Sunflower oil. Wheat gluten. Tapioca starch. Lemon zest. Dill. Garlic powder. Onion powder. Dextrose. Black pepper. Turmeric extract. Flavoring. Paprika extract. Maize maltodextrin. Lemon purée. Yeast. | Energy: 918 kJ/220 kcal; Fat: 12.7 g. of which saturates: 1.8 g; Carbohydrate: 13.1 g. of which sugars: 0.7 g; Fibre: 3.1 g; Protein: 11.8 g; Salt: 0.65 g | [40] |
| Fish Sticks | Vegan Crispy Sticks | Europe | Vivera | Reconstituted wheat protein. Breadcrumbs (wheat flour. Yeast, salt, paprika powder). Sunflower oil. Water. Wheat flour. Spirit vinegar. Thickener (methylcellulose). Natural flavors. Wheat starch. Wheat fiber. Salt. Sea salt. Linseed oil. Potato fiber. Maltodextrin. Corn starch. Herbs and spices. Preservative (sodium diacetate). Onion powder. Garlic powder. Iron. Vitamin B12. | Energy: 1029 kJ/245 kcal; Fat: 9.3 g. of which saturates: 1.1 g; Carbohydrate: 26 g. of which sugars: 1.4 g; Fiber: 3.2 g; Protein: 13 g; Salt: 1.6 g; Iron: 2.1 g; Vitamin B12: 0.38 g | [39] |
| Fish Sticks | Breaded Fish Sticks | North and South America | Good Catch | Water. Wheat flour. Good Catch 6-Plant protein blend (pea protein isolate, soy protein concentrate, chickpea flour, faba protein, lentil protein, soy protein isolate, navy bean powder). Sunflower oil. Corn starch. Natural flavors. Methylcellulose. Yeast extracts. Sugar. Corn maltodextrin. Salt. Onion powder. Yeast. Corn flour. Garlic powder. Spices. Xanthan gum. Annatto. Acetic acid. | Energy: 813 kJ/195 kcal; Fat: 9.7 g. of which saturates: 0.9 g; Carbohydrate: 15 g. of which sugars: 0.9 g; Protein: 10.6 g; Fiber: 0.9 g; Salt: 0.4 g | [48] |
| Burgers | Fish Burgers Classic Style | North and South America | Good Catch | Water. Good Catch 6-Plant Protein Blend (pea protein isolate, soy protein concentrate, chickpea flour, faba protein, lentil protein, soy protein isolate, navy bean powder). Celery. Green onions. Sunflower oil. Natural vegan flavors. Methylcellulose. Lemon juice. Corn starch. Onion powder. Salt. Yeast extract. Algal oil. Garlic powder. Spice. | Energy: 592 kJ/142 kcal; Fat: 4.4 g. of which saturates: 0.4 g; Carbohydrate: 7.9 g. of which sugars: 0.88 g; Protein: 18.6 g; Fiber: 1.8 g; Salt: 1.8 g | [49] |
| Burgers | Whitefish Burgers | North and South America | Sophie’s Kitchen | Water. Pea protein. Safflower oil. Hempseed oil. Potato starch. Dill. Contains or less methylcellulose. Pea protein isolate. Yeast extract. Salt. Organic cane sugar. Garlic powder. Parsley. Natural flavors. Lemon juice concentrate. Lemon oil. | Energy: 740 kJ/177 kcal; Fat: 8.8 g. of which saturates: 0.9 g; Carbohydrate: 7.1 g. of which sugars: 0.9 g; Protein: 16.8 g; Salt: 0.7 g | [50] |
| Burgers | Salmon Burgers Classic Style | North and South America | Good Catch | Water. Good Catch 6-Plant Protein Blend (pea protein isolate, soy protein concentrate, chickpea flour, faba protein, lentil protein, soy protein isolate, navy bean powder). Coconut oil. Natural vegan flavors. Sunflower oil. Methylcellulose. Yeast extract. Corn starch. Onion powder. Salt. Lemon juice. Lemon. Orange. Shallot. Spice. Sugar. Garlic powder. Annatto extract. Vegetable juice. | Energy: 740 kJ/177 kcal; Fat: 9.3 g. of which saturates: 6.2 g; Carbohydrate: 8.8 g. of which sugars: 0.9 g; Protein: 14.2 g; Fiber: 1.8 g; Salt: 0.5 g | [51] |
| Burgers | Salmon Burgers Original | North and South America | Sophie’s Kitchen | Water. Pea protein. Safflower oil. Hempseed oil. Potato starch. Dill. Contains or less methylcellulose. Pea protein isolate. Yeast extract. Salt. Organic cane sugar. Garlic powder. Vegetable juices (color). Natural flavors. Lemon juice concentrate. Lemon oil. | Energy: 669 kJ/160 kcal; Fat: 4 g. of which saturates:0.9 g; Carbohydrate: 7.1 g. of which sugars: 1.8 g; Protein: 15.9 g; Salt: 0.7 g | [52] |
Fish and plant-based analogs are designed to mimic the taste, texture, and appearance of real fish. However, they differ in their functional properties from animal-origin products. Fish flesh is composed of water (70–80%), proteins (15–20%), lipids (2–5%), carbohydrates (2%), minerals, and vitamins [20,25]. Compared to animal-based proteins, plant-based proteins are easier to produce, but most plant proteins are deficient in essential amino acids, making them nutritionally incomplete [53]. Therefore, combining plant proteins from various sources makes it feasible to attain a very high PDCAAS comparable to fish protein [20]. Nevertheless, the nutritional value of the plant-based products on the market is far from their animal origin [54]. Figure 3 presents the protein, fats, and carbohydrate content of salmon and cod [55] and their chosen analogs. Companies try to produce products that mimic the texture, appearance, and taste of the fish species; however, there is still a long way to go to reach nutritional levels similar to those of fish species. For example, salmon (Revo) fish analogs are characterized by low amounts of proteins and fat and high amounts of carbohydrates. In contrast, cod analogs (TKC Veg Cod Fillet) contain higher amounts of fats, carbohydrates, and lower protein than medium quantities in fish meat. Furthermore, fish analogs made from plant-based proteins have higher levels of dietary fiber. However, plant-based analogs may have lower levels of omega-3 fatty acids, which are abundant in real fish [25]. Thus, the seafood substitutes are enriched in micronutrients, such as omega-3 fatty acids and vitamins (A, B, and D), in order to ensure nutritional parity with traditional seafood products [54].
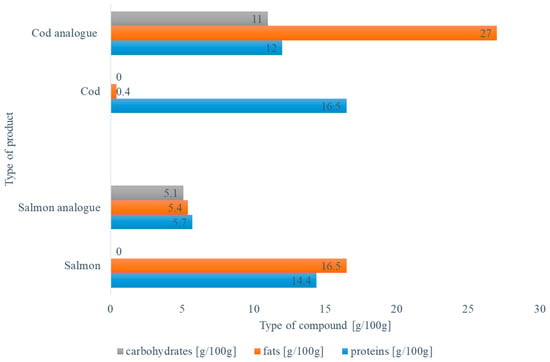
Figure 3.
Comparison of fish and fish analogs (salmon and cod nutritional value—[55], salmon analog—Revo, Cod analog—TKC Veg Cod Fillet).
Fish analogs made from plant proteins have a different texture than real fish due to the absence of muscle fibers [15]. Various techniques are being explored for obtaining good texture of the plant-based analogs; however, research is still ongoing [20]. Still, most products on the market do not replicate the structure and texture of fish or seafood but rather reproduce the sensory characteristics of processed fish products, including their appearance, texture, aroma, and flavor [25]. However, fish analogs made from plant-based proteins may have a slightly different taste compared to real fish due to the bitter taste of some plant-based proteins. Furthermore, the specific taste of the plant-based proteins can be masked and flavored to mimic the taste of different types of fish [53]. Generally, plant-based fish analogs and animal-origin products differ in their functional properties, which is currently under investigation.
3. The Possible Use of Various Ingredients to Produce Plant-Based Fish Analogs and Their Characteristics
Separate nutrients such as proteins, carbohydrates, lipids, and whole products such as legumes can be used to produce plant-based fish alternatives. Fish are a valuable source of polyunsaturated fatty acids, including omega-3 acids essential for the proper functioning of the human body. Therefore, vegetable fats rich in omega-3 fatty acids should be used in plant-based fish replacements. Fish are also characterized by high protein content, which depends on the species, the conditions of existence, and the type of muscle—it is important for proteins to be digestible and contain essential amino acids. Fish meat proteins are distinguished by their high biological value due to the presence of essential amino acids in a nutritionally favorable proportion. Conversely, vegetable proteins are considered less valuable. However, proteins from cereals and legumes can be mixed to achieve an appropriate essential amino acid profile. It is also worth mentioning that vegetable proteins are usually characterized by good emulsifying, thickening, and foaming properties [15,56].
3.1. Soya
Soya (Glycine L.) is characterized by its high-fat content, including polyunsaturated fatty acids. It is also distinguished by its high protein content, which accounts for up to 46% of grains’ total weight. The carbohydrate content, on the other hand, ranges from approximately 22.8% to 27.9%, and lipids account for a maximum of 23% of the total weight [57]. There are five principal fatty acids in soybean oil: palmitic (11%), stearic (4%), oleic (23%), linoleic (54%), and linolenic (8%) [58]. Soy protein has a significant amino acid composition as it complements cereal protein. Although soy is deficient in methionine, it contains enough lysine to overcome the lysine deficiency in cereals [59]. For example, the composition of the essential amino acids in defatted soya seed flour (g/100 g protein) is histidine 2.58; isoleucine 4.45; leucine 7.82; lysine 5.91; threonine 3.61; tryptophan 0.33; valine 4.58 [60]. Soybean flour, concentrates, and isolates are characterized by emulsification, fat absorption, viscosity, and water absorption [1]. The high amount of soy β-conglycinin (7S) is responsible for moisturizing properties, including emulsifying and foaming. There is a relationship between the proportion of soy glycinin (11S) and soy β-conglycinin (7S) which affects the gel and foaming properties [61]. Soybean is one of the leading food and oil crops, growing for over 5000 years. In 2018, the global soybean yield was 397.9 million tons [62].
3.2. Wheat
Wheat (Triticum L.) is a widely grown cereal [63]. The predominant constituents in wheat grains are carbohydrates, mainly starch, accounting for 58% of the grains’ dry matter. The protein content is 11%, while lipids account for 2%. The proteins found in wheat can be divided into four classes. Storage, metabolic, protective, and proteins have different functions [64]. Due to its rheological and viscoelastic properties, wheat protein allows for obtaining the consistency of muscle fibers. However, wheat proteins are allergenic, and one of the main allergens is the globulin-1 S allele, a glycoprotein [63,65]. Wheat grains are a valuable source of B vitamins, as well as vitamin E. They contain significant amounts of potassium, phosphorus, magnesium, and calcium. In addition, they have a nutritionally favorable ratio of omega-6 to omega-3 acids [64]. The amino acid composition of wheat lacks essential amino acids such as lysine (1.1%), threonine (1.8%), and methionine (0.7%). Newly introduced wheat varieties are characterized by increased lysine content [66,67]. In 2018, global wheat production was 762.2 million tons [68].
3.3. Legumes
There is a big group of different proteins based on legumes sources such as peas (Pisum sativum L.), beans (Phaseolus spp.), chickpeas (Cicer arietinum L.), lentils (Lens culinaris), lupin (Lupinus albus L.), etc. [69].
The most common and available on the market are pea proteins, characterized by their high nutritional value. Fresh pea seeds contain 20–50% starch, 17–22% of other carbohydrates, 6.2% to 6.5% protein, and 0.4% fat. Pea proteins have no allergenic effect and are not genetically modified. Pea flour is marked by whipping ability, water, fat binding, emulsification, gelling, texture property, and foaming [1,70]. Pea proteins are divided into globulins, albumins, prolamins, and glutelins. Globulin accounts for 55 to 65% of the total protein content. During seed germination, globulin is degraded to legumin and vicilin. Pea protein is characterized by a high lysine content (6.7 g/100 g protein). Compared to grain proteins, pea protein is characterized by a high content of leucine (7.6 g 100 g protein) and phenylalanine (5.7 g/100 g protein) but a lower content of methionine (0.9 g/100 g protein) and cysteine (1.0 g/100 g protein). The content of individual amino acids also differs between pea protein types. Pea globulin is characterized by a high content of arginine, while pea albumin contains a high amount of threonine [71,72]. In 2017, the global pea protein market was valued at USD 32.09 million. World production of dry seeds, according to 2019 FAOSTAT data was about 22 million tons and fresh peas were about 14.5 million tons [73].
The common bean (Phaseolus vulgaris L.) is included in the legume group. It is an important source of protein, as well as energy and dietary fiber. Raw (unprocessed) beans contain 1.9% lipids, 21.7% protein, and 58.8% carbohydrates. The seeds of this species are not oilseeds but have a high proportion of unsaturated fatty acids. The primary fatty acids are linoleic and linolenic (62–83% of total acids). The high proportion of PUFAs in the beans provides a favorable ratio of omega-3 to omega-6 acids [74]. Among all amino acids, beans (regardless of the method of pretreatment: autoclaved at 103 kPa, freeze-dried, and also ground) contain the highest amount of aspartic acid (10.6% in protein) and glutamic acid (13.6% in protein), as well as significant amounts of essential amino acids, especially leucine (7.2% in protein), lysine (6.2% in protein), and phenylalanine (5.4% in protein). The grains also contain small amounts of sulfur amino acids (methionine 0.7% and cysteine 0.3% in protein), characteristic of legumes. The flour of beans has the same functional properties as pea flour. Production volume in 2016 in the United States was 140 million tons, of which 114 million tons were directed to consumption [1,60,75].
Chickpea (Cicer arietinum) is one of the most widely grown legumes sold as seeds, flours, and canned foods. It is a good source of protein (17–22%) that can be extracted wet and dry. Extruded chickpea protein is rich in essential amino acids such as lysine (1.45% dry matter), leucine (1.75% dry matter), and phenylalanine (1.29% dry matter). The limiting amino acid is tryptophan (0.23% dry matter) [76,77]. Chickpeas contain 40–60% low-digestible carbohydrates and 4–8% essential fats. Chickpeas have a higher fat content than other legumes [78]. The fatty acid composition of chickpeas is 66% PUFA, 19% MUFA, and 15% SFA. They contain the most linoleic acid (51.2%) and oleic acid (32.6%). In 2018 global chickpea production was 17.19 million tons, of which 27.53% of the crop was produced in India [79].
Lentils are classed as legumes and are high in protein as well as low in fat. The seeds of lentils can be black, orange, yellow, or green. The dry matter contains 20.6–31.4% protein. Lentil proteins are categorized as having a high nutritional value and the right ratios of leucine to isoleucine and leucine to lysine. These proteins are rich in lysine (6.7 ± 0.6 g/16 g N), whereas the limiting amino acids are sulfur amino acids (methionine 0.9 ± 0.2 g/16 g N; cysteine 1.1 ± 0.3 g/16 g N). The content of carbohydrates ranges from 43.4 to 69.9 g per 100 g of dry matter. Lentils also have a high amount of fiber, totaling as much as 26.9 g per 100 g of dry matter. Lentil flour is characterized by similar properties to the flour of peas or beans. The global production of lentil grains was 63.15 billion tons in 2021 [1,80,81,82].
Lupin seed has a protein content of 44%. It contains most of the essential amino acids, with lysine (2.1%) being the most abundant. Lupin seeds are nearly devoid of starch but contain oligosaccharides, such as raffinose, stachyose, and polysaccharides [66,83]. Lupin has a relatively low oil content of 18%. The most abundant fatty acids are oleic, palmitic, linolenic, and erucic acids [70].
3.4. Rice
Rice (Oryza sativa L.) is a cereal rich in carbohydrates, mainly starch. The grain of rice is characterized by starch amounts to 75–80%. The content of water is 12%, and the content of protein is only 7%. Still, it is worth mentioning that this protein is highly digestible and has a high biological value due to its lysine content (about 4%). Rice bran has extra nutritional compounds, including cellulose, pectin, hemicellulose, lignin, polyphenolics, vitamin B9, and vitamin E isoforms [84]. Rice is characterized by a low lipid content. Oil consists mainly of bran, and rice bran oil consists of 36% oleic acid and 37% linoleic acid. In addition, the content of palmitic acid is considerable and amounts to 18% [85]. Paddy rice which is hulled, is known as brown rice, whereas white rice is obtained after milling and polishing. In 2017, rice production totaled about 693 million tons in Asia alone, and worldwide production was about 770 million tons [86].
3.5. Corn
Corn (Zea mays, maize) is one of the most important cereal crops in the world. It exhibits high tolerance to weather conditions. It can be grown in both tropical and temperate climates. Corn cob is used as a vegetable, and its grains are used as animal feed [87,88]—the fat content of corn averages 4%. Corn oil contains a high content of linoleic acid. The other primary fatty acid in corn is oleic acid, which ranges in some corn varieties, like the amount of linoleic acid [89]. Corn grains contain an average of 7.3% protein. Corn protein–zein which is one of the primary storage proteins—contains alanine (10%), proline (10%), and leucine (20%) in the highest amounts [88,89,90]. Each year, global corn production exceeds 1 billion tons, thus overtaking rice and wheat production by 25%. About 17% of the world’s corn production undergoes bioconversion to ethanol [91].
3.6. Potato Proteins
Potato (Solanum tuberosum L.) is the world’s most important food crop, next to wheat, rice, and corn [92]. The nutritional profile of raw potatoes is 83.4% water, 13.6% carbohydrate, 2.1% protein, 0.9% lipid, and 0.9% ash. The key industrial use of potatoes is for starch extraction. During this process, a diluted by-product, called potato fruit juice, is produced [93]. It is worth noting that around 8 million tons of potatoes are processed into starch in the EU annually, generating approximately 6 million m3 of potato fruit juice. Therefore, despite containing only around 1–2% of crude protein, potato fruit juice may be a source of protein preparations. Furthermore, properly isolated potato protein may enrich highly processed foods due to its nutritional, functional, and antimicrobial properties (antibacterial as well as antifungal [94,95]). The potato protein is recognized as one of the most valuable non-animal proteins comparable to egg white and milk proteins due to amino acids’ profile and digestibility [96]. It has in its composition all the essential amino acids (based on the total amino acid content): leucine 10.1%; lysine 7.7%; valine 6.7%; phenylalanine 6.3%; isoleucine 5.6%; threonine 5.3%; histidine 2.1%; methionine 2.1%; and tryptophan 1.2%, and it also contains nonessential amino acids: tyrosine 5.3%; arginine 5.2%; proline 4.9%; glycine 4.7%; serine 4.2%; cystine 1.4%; alanine; aspartic acid; and glutamic acid [93]. Furthermore, potato protein is considered a non-allergic and GRAS component with high antioxidant activity and good solubility, gel-forming, foaming, and emulsifying properties [97]. Due to these characteristics, the global potato protein market is expected to grow from USD 73.78 billion in 2021 to USD 115.44 billion by 2030, at a CAGR of 5.10% during the forecast period 2022–2030, according to the report published by The Brainy Insights [98].
3.7. Other Sources of Proteins
In addition to the previously mentioned protein sources, other sources of proteins are also used, e.g., seaweeds, sunflower proteins, rapeseed proteins, etc. Seaweeds (macroalgae) are plant-like organisms that live in the water of oceans. They are rich in minerals, vitamins, and polysaccharides; some contain high concentrations of amino acids, proteins, and fatty acids [99]. There is a relationship between the concentration of free amino acids in seaweed and the flavor of seaweed [100]. Umami is based on monosodium L-glutamate and L-aspartate but can also be the result of the synergistic effect of 5′-nucleotides, guanosine-5′-monophosphate, and inosine-5′-monophosphate [101]. In brown algae, the protein content ranges from 5.02 to 19.66%, in red algae from 0.67 to 45%, and in green algae from 3.42 to 29.8%. Seaweeds are a source of lysine [102]. The polysaccharides content ranges from 4 to 76% (about total dry weight), depending on the species [103]. In 2018 the world production of brown and red seaweeds amounted to 31.3 billion tons of fresh weight with a value of 12.4 billion USD [104].
The next source of proteins is raw sunflower seeds. The protein content of sunflower seeds varies between 45% and 50%. Some essential amino acids are present in sunflower seeds: aspartic acid, glutamic acid, serine, histidine, glycine, threonine, arginine, alanine, tyrosine, cysteine, valine, methionine, phenylalanine, isoleucine, leucine, lysine, and proline. Generally, sunflower seeds also contain around 25% oil, but the content has increased to 40% through plant breeding. The main constituents of sunflower oil are linoleic and oleic acids. The differences in the fatty acid content of sunflower oil are due to the heat treatment used in its production. It contains approximately 5% palmitic acid, 6% stearic acid, 30% oleic acid, and 59% linoleic acid. Sunflower seeds that are processed are characterized by their low carbohydrate content. Furthermore, it is worth mentioning that sunflower flour can absorb oil, make emulsions, and is characterized by foaming properties and whipping—but the last property is only as the flour is without chlorogenic acid. In 2018/19, global sunflower oil production was 51.46 billion tons [1,105].
Rapeseed is another source of protein and contains about 45% protein as well as around 42.8% fat. Compared to soya and other legumes, rapeseed protein has an excellent balance of essential amino acids. It is characterized by high levels of sulfur-containing amino acids, including cysteine and methionine [106]. In turn, the fatty acid profile is as follows: 12% oleic acid, 13% linoleic acid, 8–9% linolenic acid, 8–9% eicosenoic acid, and 50–51% erucic acid [107]. Rapeseed flour has the same properties as the flour of sunflowers [1]. World rapeseed production in 2018/19 was 70.91 billion tons [105].
4. Technologies Used for Plant-Based Fish and Meat Analogs Production
Various technological processes are employed to obtain fish and meat analogs. The most commonly used are textured vegetable proteins, produced in the extrusion process, and have a fibrous, meat-like structure. Other techniques used in the food industry are 3D and 4D printing. These technologies are much more expensive and time-consuming processes, but they allow for personalized products. The less popular techniques that may only be introduced in the food industry for the production of analogs of animal products include electrospinning, wet spinning, directional freezing, and shear cell.
4.1. Texturization of Vegetable Protein (TVP)
One method of producing meat- and fish-like products is extruding cooking plant materials for texturing fibrous substitutes for animal products. Textured vegetable proteins (TVP) are products used as substitutes for beef, poultry, or fish, which resemble these products not only in appearance but also in structure, color, and taste. They are available as powder, granules, or cubes [108]. Extrusion cooking is a popular food processing technique carried out at high temperatures for a short time. It is used to make fiber-rich products. This is how moistened, starchy, and protein-rich raw materials are plasticized and cooked in a tube by combining moisture, pressure, temperature, and mechanical shear through chemical reactions [18,109]. Textured proteins can be obtained by two methods of extrusion—wet and dry. Extrusion with a feed moisture content below 40% is called low-moisture extrusion (LME), while extrusion performed with a feed moisture content above 40% is considered high-moisture extrusion (HME). The products obtained in the LME technology are characterized by low sensory acceptability and are not used in the production of fish analogs. HME makes it possible to obtain unexpanded fiber products that mimic the texture and taste of meat products [7,110].
Textured vegetable proteins find use in the production of nutritionally balanced or fortified products, as well as dietary products and animal product substitutes [109]. They are mainly made of skimmed soy flakes or soy flour. TVP contains very little fat, cholesterol, and a minimal amount of carbohydrates while being high in protein and fiber and, therefore, rich in amino acids. Textured soy protein is the most common, although textured wheat protein is also available [108]. However, soy protein use is limited due to the negative perception of soy products by consumers related to potential health hazards [111]. Therefore, the production of TVP also uses unconventional raw materials such as mucuna bean flour, faba bean, cotton, corn, pea and lupine, lima bean and African oil bean seed, oat protein, hemp, and lentils [7,109,110].
4.2. 3D and 4D Printing
A problematic aspect when obtaining plant analogs of animal products is providing them with an appropriate texture. Therefore, there is a need to find solutions that allow obtaining analogs with similar taste or nutritional properties and an appropriate structure, e.g., fish fillets. One way to achieve the right structure is to use 3D or 4D printing. Using 3D food printing enables the preparation of personalized food, with a complex structure, obtained layer by layer, according to programmed digital models created using computer-aided design/production software (CAD/CAM) [16,112,113]. Various 3D food printing techniques have been developed for creating stable three-dimensional products, such as extrusion-based printing, inkjet printing, bioprinting, and powder bed printing [113,114]. However, the most commonly used in producing high-protein foods, i.e., meat and fish analogs, is extrusion-based printing, a fairly long-lasting food printing process, wherein fibrous meat or analog materials are extruded from a nozzle in order to form 3D structures [112,115]. In the syringe injection process, the highly viscous protein solution is extruded through the moving nozzle of a thin syringe layer by layer to form a 3D product, e.g., a muscle-like structure [25]. Although bioprinting is under development, it is also accomplished through the usual three primary methods—jetting, lasers, and extrusion [116]. Research is underway using the binder jet technique, a powder printing technology in which a liquid binder is precisely deposited to agglomerate adjacent powder particles to build a 3D-printed structure. However, it is a difficult technique due to the swelling of protein raw materials during the process [112]. Swelling of the powder particles impairs the printing process. The wet layer appears above the surface, causing misalignment and breakage of structures upon application of the next layer of powder [117]. Research conducted by Zhu et al. [112] showed that the increasing binder content in printed food products resulted in greater sample consistency and that the protein content of dry powder blends had a significant impact on the elasticity of the final product. By adjusting the amount of binder deposited, as well as the addition of calcium caseinate to the dry powder mixtures, a wide range of textures was achieved, such as textures similar to crushed dough or elastic gum.
It has been suggested that 3D printing can potentially reduce waste and energy in the food industry. For the production of meat and fish analogs with 3D printing technology, materials derived mainly from insects, plants, and cell cultures are used, which are tested for technological feasibility, environmental impact, and consumer acceptance. It is also possible to use industrial waste [113]. The 3D printing technology can be used not only to eliminate the sensory differences between meat from vegetable protein and real meat but also to adapt plant analogs to consumers’ different preferences and needs. In addition, the printed 3D model should be durable and resistant to thermal cooking processes after printing. This is due to the need for heat treatment of finished products before consumption. Examples of 3D-printed products are steak analogs, fish fillets, or protein-rich snacks [112,118,119,120].
An emerging and relatively unknown technology in the realm of food technology is 4D printing. The use of 4D printing is an additive manufacturing technique and is an extension of 3D printing. Furthermore, 4D printing is similar to 3D printing in design development and printing structures with a 3D printer [121,122]. The main differences between 4D and 3D printing are smart design and smart materials, as 4D printed structures can change shape or function [123]. Food can change color, texture, flavor, and shape when influenced by various stimulants such as temperature and pH. Therefore, it saves space in the transport process or adjusting the printed materials to the customers’ needs. Although this technology is still at the research stage, there are attempts to use vegetable proteins (e.g., soy protein isolate) to design food with a specific smell or color [122].
4.3. Other Technologies
Electrospinning is a technique that allows the production of very thin fibrils with a high aspect ratio. In the spinning process, placing a high voltage over a polymer solution produces fibrils. The protein solution is pushed through the nozzle and electrically accelerated by the electric potential gradient from the ground electrode. Consequently, the fine stream exiting the nozzle, shaped like a Tylor cone, becomes a fine fiber. At the same time, the solvent evaporates and is finally collected on the manifold connected to the electrode. For electrospinning to occur, the spun proteins must be in an unfolded conformation or internally unstructured. Vegetable proteins are usually spherical in their native state, so they should be unfolded (usually using heating) prior to electrospinning, avoiding the formation of insoluble aggregates. In order to obtain structures resembling animal proteins, an acidic solution of spirulina protein [18,25,124].
One of the new technologies that can find industrial application in producing meat and fish analogs is wet spinning [18]. In this process, the protein solution is forced into a coagulation bath containing a solvent that lowers the solubility of the protein or promotes cross-linking and fiber formation. The solvent can cause the protein to precipitate, and with the force of the nozzle, they align the proteins, forming fibers. Binders, e.g., Ca2+, are added to the solvent to allow the proteins to cross-link. It is also possible to change the acidity of the solution, which favors the formation of inter- and intra-molecular chemical bonds between the protein chains. The products obtained this way are then purified from the solvent [25,125]. For this technology to be introduced to the food industry, new solvents are constantly being sought that will be safe for both products and the environment [126]. Therefore, the production of wet spinning fibers also uses raw materials such as soy, wheat, okra protein, and zein [125].
Directional freezing is based on the use of the freezing process to obtain multilayer protein structures. The process involves producing microstructures from two-phase systems (aqueous or organic solutions, suspensions, and slurries). Variable solution concentrations and the directional freezing rate regulate the shape of the protein structures. In this method, soy proteins can be used to prepare protein products [127,128].
The shear cell technology is another technique in which a combination of shear and heat is used to form plant-based meat and fish analogs with layered fibrous structures that resemble the mouthfeel and texture of real animal products [36]. The shear device used in this technology is called a shear chamber, where intense shear can be applied. There are two types of shear cells: conical cells based on cone-plate rheometer and cylindrical shape—Couette cells, which can be used on an industrial scale. The shear cell technology is characterized by a lower variation in product quality compared to extrusion. Examples of proteins that can be subjected to this process are dairy proteins (calcium caseinate), soy protein isolate, and vital wheat gluten [85,129,130,131].
5. Conclusions and Future Perspectives
Consumer interest, ethical concerns, and environmental issues have increased the demand for plant-based products, including fish analogs. Many plant-based products currently exist on the market, and this category continues to grow at a rapid pace. Companies compete to create new products, but many plant substitutes often fail to match their animal counterparts. The problematic issues related to nutritional content must not be missed.
Various sources of protein, including soya, wheat, legumes, rice, corn, potato, seaweed, sunflower seeds, rapeseed, etc., can be used to develop fish analog plant products [131,132,133]. However, it is worth mentioning that fish analog plant products are mainly based on readily available soy and wheat proteins, but both are allergens. This means that people with allergies cannot consume many plant-based products. Furthermore, soybeans are usually associated with GMO crops, which are negatively perceived, especially in European countries. Additionally, appropriately selected technology from among texturization of vegetable protein, 3D printing, as well as less popular techniques which nowadays are rapidly developed like electrospinning, wet spinning, directional freezing, and shear cell, is one of the key aspects in obtaining an appropriate texture which resembles the texture of animal origin.
In future years it will be necessary to focus more on the nutritional value and the physical and technological properties of fish analogs. In addition, the different sources of plant proteins and other compounds, e.g., oleogels, algae, or fungus, may be used in fish analogs and affect their quality.
Author Contributions
Conceptualization, M.N., M.T., and K.P.; writing—original draft preparation, M.N., M.T., P.C., A.P., K.W., A.W., J.P., K.R. and K.P.; writing—review and editing, M.N., M.T., and K.P.; visualization, M.N.; supervision, M.N.; project administration, M.N., and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Education and Science (Poland) from the state budget within the program “Student research clubs create innovations” in the years 2022–2023 (grant number SKN/SP/534683/2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Maurya, A.; Das, S.; Prasad, J.; Yadav, A.; Singh, V.K.; Singh, B.K.; Dubey, N.K.; Dwivedy, A.K. Nanoencapsulation strategies for improving nutritional functionality, safety and delivery of plant-based foods: Recent updates and future opportunities. Plant Nano Biol. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Vegan Food Market Report, 2022: Vegan Food Market Size, Share & Trends Analysis Report by Product (Meat & Seafood, Creamer, Yogurt, Meals, Cheese, Butter), by Distribution Channel (Offline, Online), by Region, and Segment Forecasts, 2022–2030. Available online: https://www.researchandmarkets.com/reports/5665126/ (accessed on 19 March 2023).
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Mintel Report, 2017. Innovation vs. Health. Opportunities for Food Brands. Available online: https://reports.mintel.com/display/787865/ (accessed on 29 March 2023).
- Good Food Institute Report, 2021: 2021 Industry Update, Alternative Seafood. Available online: https://gfi.org/resource/alternative-seafood-state-of-the-industry-report/ (accessed on 19 March 2023).
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Lou, X.; Zheng, H.; Gu, Q.; Yang, H. Improving the texture and rheological qualities of a plant-based fishball analogue by using konjac glucomannan to enhance crosslinks with soy protein. Innov. Food Sci. Emerg. Technol. 2022, 75, 102910. [Google Scholar] [CrossRef]
- Bhuyan, S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- De Boer, J.; Schösler, H.; Aiking, H. Fish as an alternative protein—A consumer-oriented perspective on its role in a transition towards more healthy and sustainable diets. Appetite 2020, 152, 104721. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Sunderland, E.M.; Chan, H.M.; Choi, A.L.; Grandjean, P.; Mariën, K.; Oken, E.; Sakamoto, M.; Schoeny, R.; Weihe, P.; et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr. Rev. 2011, 69, 493–508. [Google Scholar] [CrossRef]
- Pandey, G.; Madhuri, S.; Shrivastav, A.B. Contamination of mercury in fish and its toxicity to both fish and humans: An overview. Int. Res. J. Pharm. 2012, 3, 44–47. [Google Scholar]
- Fact.MR, 2021: Plant-based Fish Market Segmentation by Fish (Plant-Based Tuna Products, Crab Products, Shrimp Products), by Product (Plant-Based Fish Cutlets, Fish Fillets, Fish Cutlets), by Source (Soy-Based, Canola-Based, Wheat-Based Fish Products), by Distribution Channel & Regional Forecast—2031. Available online: https://www.factmr.com/report/plant-based-fish-market (accessed on 25 October 2022).
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. npj Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Lima, M.; Costa, R.; Rodrigues, I.; Lameiras, J.; Botelho, G. A Narrative Review of Alternative Protein Sources: Highlights on Meat, Fish, Egg and Dairy Analogues. Foods 2022, 11, 2053. [Google Scholar] [CrossRef]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Chapter 6—Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 103–126. [Google Scholar] [CrossRef]
- Tóth, A.J.; Dunay, A.; Battay, M.; Illés, C.B.; Bittsánszky, A.; Süth, M. Microbial Spoilage of Plant-Based Meat Analogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, Y.; Xu, Y. Production of Fish Analogues from Plant Proteins: Potential Strategies, Challenges, and Outlook. Foods 2023, 12, 614. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F.F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Zhang, K.; Zang, M.; Wang, S.; Zhang, Z.; Li, D.; Li, X. Development of meat analogs: Focus on the current status and challenges of regulatory legislation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1006–1029. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.B.; Majumder, R.K.; Maurya, P. Effect of vacuum packaging on the shelf-life of shrimp analog prepared from Pangasionodon hypophthalmus surimi during refrigerated storage. J. Food Process. Preserv. 2022, 46, e16369. [Google Scholar] [CrossRef]
- Kołodziejczak, K.; Onopiuk, A.; Szpicer, A.; Poltorak, A. Meat analogues in the perspective of recent scientific research: A review. Foods 2021, 11, 105. [Google Scholar] [CrossRef]
- Kazir, M.; Livney, Y.D. Plant-Based Seafood Analogs. Molecules 2021, 26, 1559. [Google Scholar] [CrossRef] [PubMed]
- OMNI. Available online: https://omnifoods.co/us/product/27 (accessed on 9 September 2022).
- Vegan Essentials. Available online: https://veganessentials.com/products/good-catch-plant-based-breaded-fish-fillets (accessed on 9 September 2022).
- NOVISH. Available online: https://www.novish.eu/en/vish_product/breaded-vish-filets/ (accessed on 18 September 2022).
- Matsuo, A.; Matsushita, K.; Fukuzumi, A.; Tokumasu, N.; Yano, E.; Zaima, N.; Moriyama, T. Comparison of Various Soybean Allergen Levels in Genetically and Non-Genetically Modified Soybeans. Foods 2020, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- THE VEGAN KIND. Available online: https://thevegankind.com/p/vbites-making-waves-fish-style-steaks-200g?objectID=36217003022 (accessed on 18 September 2022).
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Lily’s Vegan Pantry. Available online: https://www.lilysveganpantry.com/product_p/r007.htm (accessed on 23 September 2022).
- Lotus V. Available online: https://www.lotusvstore.co.uk/product-page/tkc-veg-cod-fillet-250g (accessed on 18 September 2022).
- FairPrice. Available online: https://www.fairprice.com.sg/product/everbest-vegetarian-goby-fish-500-g-90095943 (accessed on 23 September 2022).
- Velivery. Available online: https://www.velivery.com/de/vegane-lebensmittel/fisch-alternativen/revo-lachs-aus-pflanzen-80g?info=relaunch (accessed on 23 September 2022).
- SophiesKITCHEN. Available online: https://www.sophieskitchen.com/fish-fillets (accessed on 23 September 2022).
- Terra Vege. Available online: https://terravege24.pl/pl/p/Paluszki-roslinne-ala-rybne-190g-Polsoja/4225 (accessed on 9 September 2022).
- Ecomarkt. Available online: https://ekomarkt.de/Veganz-Sea-Style-Sticks-210g (accessed on 18 September 2022).
- Vivera. Available online: https://vivera.com/pl/produkty/roslinne-pauluszki/ (accessed on 18 September 2022).
- WAITROSE & PARTNERS. Available online: https://www.waitrose.com/ecom/products/waitrose-ve-fishless-fingers/809255-672983-672984 (accessed on 9 September 2022).
- Priyadarshini, M.B.; Xavier, K.A.M.; Dhanabalan, V.; Nayak, B.B.; Balange, A.K. Development of ready-to-cook shrimp analogue from surimi: Effect of natural plant extracts on the chemical quality during refrigerated storage. LWT 2020, 135, 110239. [Google Scholar] [CrossRef]
- OMNI. Available online: https://omnifoods.co/us/product/28 (accessed on 9 September 2022).
- GARDEIN. Available online: https://www.gardein.com/fishless/fsh-filets (accessed on 9 September 2022).
- SPOŻYVCZAK. Available online: https://spozyvczak.com/pl/p/Weganski-losos%2C-Vantastic-Foods%2C-300g/218 (accessed on 23 September 2022).
- VegetarianWorldFoods. Available online: https://vegetarianworldfoods.com/product/vegan-cod-fish/ (accessed on 9 September 2022).
- THE VEGAN KIND. Available online: https://thevegankind.com/p/vbites-making-waves-smoked-salmon-style-slices-100g?collection=meat-seafood-seafood-salmon&queryID=56a39af50a7b35f2813f4fd781f15f2c&objectID=35525315470 (accessed on 18 September 2022).
- SophiesKITCHEN. Available online: https://www.sophieskitchen.com/smoked-salmon (accessed on 23 September 2022).
- GTFO It’s Vegan. Available online: https://gtfoitsvegan.com/product/breaded-fish-sticks-by-good-catch/ (accessed on 9 September 2022).
- Vegan Essentials. Available online: https://veganessentials.com/products/good-catch-plant-based-classic-style-fish-burgers (accessed on 9 September 2022).
- SophiesKITCHEN. Available online: https://www.sophieskitchen.com/copy-of-mediterranean-whitefish-bu (accessed on 23 September 2022).
- Vegan Essentials. Available online: https://veganessentials.com/products/good-catch-plant-based-salmon-burgers-classic-style (accessed on 9 September 2022).
- SophiesKITCHEN. Available online: https://www.sophieskitchen.com/copy-of-crab-cakes?fbclid=IwAR1q2lPdDXBtvqMe0nG7rq4INTKlBsdZFkGwGMStVbQygD-Wx3_v3Vue5Xw (accessed on 23 September 2022).
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition Through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Baune, M.C.; Gagaoua, M.; Castellari, M. Seafood alternatives: Assessing the nutritional profile of products sold in the global market. Eur. Food Res. Technol. 2022, 248, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Berk, Z. Food Process Engineering and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://app.knovel.com/hotlink/toc/id:kpFPETE002/food-process-engineering/food-process-engineering (accessed on 29 March 2023).
- Pal, J.; Shukla, B.N.; Maurya, A.K.; Verma, H.O.; Pandey, G.; Amitha, A. A review on role of fish in human nutrition with special emphasis to essential fatty acid. Int. J. Fish. Aquat. Stud. 2018, 6, 427–430. [Google Scholar]
- Agume, A.S.N.; Njintang, N.Y.; Mbofung, C.M.F. Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods 2017, 6, 12. [Google Scholar] [CrossRef]
- Gaskin, E.L.; Carrero-Colón, M.; Hudson, K.A. Combination of the elevated stearic acid trait with other fatty acid traits in soybean. J. Am. Oil Chem. Soc. 2021, 98, 221–226. [Google Scholar] [CrossRef]
- Verduci, E.; Di Profio, E.; Cerrato, L.; Nuzzi, G.; Riva, L.; Vizzari, G.; D’Auria, E.; Giannì, M.L.; Zuccotti, G.; Peroni, D.G. Use of Soy-Based Formulas and Cow’s Milk Allergy: Lights and Shadows. Front. Pediatr. 2022, 8, 591988. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Lyu, B.; Li, J.; Meng, X.; Fu, H.; Wang, W.; Ji, L.; Wang, Y.; Guo, Z.; Yu, H. The Protein Composition Changed the Quality Characteristics of Plant-Based Meat Analogues Produced by a Single-Screw Extruder: Four Main Soybean Varieties in China as Representatives. Foods 2022, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.M.; Zhang, B.; Mian, M.B.; Huang, H. Soybean Amino Acids in Health, Genetics, and Evaluation; Soybean for Human Consumption and Animal Feed: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, C.; Zhou, J.; Wang, Y.; Chen, Q.; Fu, L. Purification and identification of globulin-1 S allele as a novel allergen with N-glycans in wheat (Triticum aestivum). Food Chem. 2022, 390, 133189. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Koehler, P.; Scherf, K.A. Wheat—An Exceptional Crop Botanical Features, Chemistry, Utilization, Nutritional and Health Aspects, Chemical Composition; Woodhead Publishing: Thorston, UK, 2020; pp. 13–45. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kovis, T.J.K.; Yi, N.J. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plantbased protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in Grain, Flour, Amino Acid Composition, Protein Profiling, and Proportion of Total Flour Proteins of Different Wheat Cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef]
- Sherzod, D.D.; Sherzod, S.S.; Jaloliddin, R.A.; Azizbek, S.H.; Sarvar, O.M.J. Selection of large seed and high yielding lines of bread wheat for drought conditions. Int. Multidiscip. Res. J. 2021, 11, 4. [Google Scholar]
- Bou, R.; Navarro-Vozmediano, P.; Domínguez, R.; López-Gómez, M.; Pinent, M.; Ribas-Agustí, A.; Benedito, J.J.; Lorenzo, J.M.; Terra, X.; García-Pérez, J.V.; et al. Application of emerging technologies to obtain legume protein isolates with improved techno-functional properties and health effects. Compr. Rev. Food Sci. Food Saf. 2021, 21, 2200–2232. [Google Scholar] [CrossRef]
- Calvano, C.D.; Bianco, M.; Ventura, G.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Analysis of Phospholipids, Lysophospholipids, and Their Linked Fatty Acyl Chains in Yellow Lupin Seeds (Lupinus luteus L.) by Liquid Chromatography and Tandem Mass Spectrometry. Molecules 2020, 25, 805. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Food Sci. Nutr. 2019, 60, 2593–2605. [Google Scholar] [CrossRef]
- Sable, K. 2018. Pea Protein Market by Type (Pea Protein Isolate, Pea Protein Concentrate, and Textured Pea Protein), Form (Dry and Liquid), Application (Dietary Supplemnet, Bakery & Confectionery Good, Meat Product & Alternative, Beverage, and Others)-Global Opportunity Analysis and Industry Forecast. Allied Market Research, March 2022; pp. 1–198. Available online: https://www.marketresearch.com/Meticulous-Research-v4061/Pea-Protein-Type-Isolate-Concentrate-30992020/ (accessed on 20 February 2023).
- Los, F.G.B.; Zielinski, A.F.; Wojeicchowski, J.P.; Npgueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Suitivisedsak, N.; Evangelista, R.L.; Cheng, H.N.; Biswas, A. Composition and Functional Properties of Saline-Soluble Protein Concentrates Prepared from Four Common Dry Beans (Phaseolus vulgaris L.). J. Am. Oli Chem. Soc. 2018, 98, 1001–1012. [Google Scholar] [CrossRef]
- Bukid, F. Chickpea (Cicer arietinum L.) protein as a prospective plant-based ingredient: A review. Int. J. Food Sci. Technol. 2021, 56, 5435–5444. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; Tar’an, B.; House, J.D. Thermal processing methods differentially affect the protein quality of Chickpea (Cicer arietinum). Food Sci. Nutr. 2020, 8, 2950–2958. [Google Scholar] [CrossRef]
- Madurapperumage, A.; Tang, L.; Thavarajah, P.; Bridges, W.; Shipe, E.; Vandemark, G.; Thavarajah, D. Chickpea (Cicer arietinum L.) as a Source of Essential Fatty Acids—A Biofortification Approach. Front. Plant Sci. 2021, 12, 734980. [Google Scholar] [CrossRef]
- Kaur, R.; Prasas, K. Technological, processing and nutritional aspects od chickpea (Cicer arietinum)—A review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Choudhary, M.; Sharma, A. A review on breeding, global production and utilization of lentil. Pharma Innov. J. 2022, SP-11, 1303–1310. [Google Scholar] [CrossRef]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chem. X 2021, 9, 100112. [Google Scholar] [CrossRef]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed Protein of Lentils: Current Status, Progress, and Food Applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Neves Martins, J.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.; Tong, H.; Li, T.; Wang, L.; Chen, H. Genome-wide analysis of fatty acid desaturase genes in rice (Oryza sativa L.). Sci. Rep. 2019, 9, 19445. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Rice By-Products: Phytochemicals and Food Products Application; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ali, S.; Isaacson, J.; Kroner, J.; Saldias, S.; Kandasamy, S.; Lazarovits, G. Corn sap bacterial endophytes and their potential in plant growth-promotion. Environ. Sustain. 2018, 1, 341–355. [Google Scholar] [CrossRef]
- Tripathy, S.K. Quality protein maize (QPM): A way forward for food and nutrional security. Genom. Appl. Biol. 2019, 10, 10–19. [Google Scholar] [CrossRef]
- Barrera-Arellano, D.; Badan-Ribeiro, A.P.; Serna-Saldivar, S.O. Chapter 21—Corn Oil: Composition, Processing, and Utilization. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Eagan, MN, USA, 2019; pp. 593–613. [Google Scholar] [CrossRef]
- Acosta, J.P.; Espinosa, C.D.; Jaworski, N.W.; Stein, H.H. Corn protein has greater concentrations of digestible amino acids and energy than low-oil corn distillers dried grains with solubles when fed to pigs but does not affect the growth performance of weanling pigs. J. Anim. Sci. 2021, 99, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Serna-Saldivar, S.O. Chapter 1—Corn History and Culture. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Eagan, MN, USA, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.N.; Soladoye, O.P. Towards potato protein utilisation: Insights into separation, functionality and bioactivity of patatin. Int. J. Food Sci. Technol. 2020, 55, 2314–2322. [Google Scholar] [CrossRef]
- Galves, C.; Galli, G.; Kurozawa, L. Potato protein: Current review of structure, technological properties, and potential application on spray drying microencapsulation. Crit. Rev. Food Sci. Nutr. 2022, 2022, 2036093. [Google Scholar] [CrossRef] [PubMed]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl Microbiol Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef]
- Gebrechristos, H.Y.; Ma, X.; Xiao, F.; He, Y.; Zheng, S.; Oyungerel, G.; Chen, W. Potato peel extracts as an antimicrobial and potential antioxidant in active edible film. Food Sci Nutr. 2020, 13, 6338–6345. [Google Scholar] [CrossRef]
- Peksa, A.; Miedzianka, J. Potato Industry By-Products as a Source of Protein with Beneficial Nutritional, Functional, Health-Promoting and Antimicrobial Properties. Appl. Sci. 2021, 11, 3497. [Google Scholar] [CrossRef]
- Hussain, M.; Qayum, A.; Xiuxiu, Z.; Liu, L.; Hussain, K.; Yue, P.; Yue, S.; Koko, M.Y.F.; Hussain, A.; Li, X. Potato protein: An emerging source of high quality and allergy free protein, and its possible future based products. Food Res. Int. 2021, 148, 110583. [Google Scholar] [CrossRef] [PubMed]
- The Brainy Insights. Potato Protein Market Size by Application (Meat, Supplements, Bakery & Confectionery, Animal Feed, and Others), Type (Concentrate, Hydrolyzed and Isolate), Regions, Global Industry Analysis, Share, Growth, Trends, and Forecast. 2022. Available online: https://www.thebrainyinsights.com/report/potato-protein-market-12907 (accessed on 29 March 2023).
- Vincent, A.; Stanley, A.; Ring, J. Hidden champion of the ocean. In Seaweed as a Growth Engine for a Sustainable European Future; ASFA Monographs: London, UK, 2021. [Google Scholar]
- Moerdijk-Poortvliet, T.C.W.; de Jong, D.L.C.; Fremouw, R.; de Reu, S.; de Winter, J.M.; Timmermans, K.; Mol, G.; Reuter, N.; Derksen, G.C.H. Extraction and analysis of free amino acids and 5′-nucleotides, the key contributors to the umami taste of seaweed. Food Chem. 2022, 370, 131352. [Google Scholar] [CrossRef] [PubMed]
- Milinovic, J.; Campos, B.; Mata, P.; Diniz, M.; Noronha, J.P. Umami free amino acids in edible green, red, and brown seaweeds from the Portuguese seashore. J. Appl. Phycol. 2020, 32, 3331–3339. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Brakel, J.; Sibonga, R.C.; Dumilag, R.V.; Montalescot, V.; Campbell, I.; Cottier-Cook, E.J.; Ward, G.; Le Masson, V.; Liu, T.; Msuya, F.E.; et al. Exploring, harnessing and conserving marine genetic resources towards a sustainable seaweed aquaculture. Plants People Planet 2021, 3, 337–349. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Jia, W.; Rodriguez-Alonso, E.; Bianeis, M.; Keppler, J.K.; van der Goot, A.J. Assessing functional properties of rapeseed protein concentrate versus isolate for food applications. Technologies 2021, 68, 102636. [Google Scholar] [CrossRef]
- Cartea, E.; De Haro-Bailón, A.; Padilla, G.; Obregón-Cano, S.; del Rio-Celestino, M.; Ordás, A. Seed Oil Quality of Brassica napus and Brassica rapa Germplasm from Northwestern Spain. Foods 2019, 8, 292. [Google Scholar] [CrossRef]
- Maung, T.T.; Gu, B.Y.; Kim, M.H.; Ryu, G.H. Fermentation of texturized vegetable proteins extruded at different moisture contents: Effect on physicochemical, structural, and microbial properties. Food Sci. Biotechnol. 2020, 29, 897–907. [Google Scholar] [CrossRef]
- Omohimi, C.I.; Sobukola, O.P.; Sarafadeen, K.O.; Sanni, L.O. Effect of Thermo-extrusion Process Parameters on Selected Quality Attributes of Meat Analogue from Mucuna Bean Seed Flour. Niger. Food J. 2014, 32, 21–30. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.S.; Varela, P.; Sahlstrøma, S. Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Future Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Das, S.; Thent, Z.C. An insight into the harmful effects of soy protein: A review. Clin. Ter. 2015, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ramos, P.V.; Heckert, O.F.; Stieger, M.; van der Goot, A.J.; Schutyser, M. Creating protein-rich snack foods using binder jet 3D printing. J. Food Eng. 2022, 332, 111124. [Google Scholar] [CrossRef]
- Ramachandraiah, K. Potential Development of Sustainable 3D-Printed Meat Analogues: A Review. Sustainability 2021, 13, 938. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3D printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Dick, A.; Bhandari, B.; Prakash, S. 3D printing of meat. Meat Sci. 2019, 153, 35–44. [Google Scholar] [CrossRef]
- Min, S.; Ko, I.K.; Yoo, J.J. State-of-the-Art Strategies for the Vascularization of Three-Dimensional Engineered Organs. Vasc. Spec. Int. 2019, 35, 77–89. [Google Scholar] [CrossRef]
- Holland, S.; Foster, T.; Tuck, C. Chapter 9—Creation of Food Structures Through Binder Jetting. In Fundamentals of 3D Food Printing and Applications; Godoi, F.C., Bhandari, B.R., Prakash, S., Zhang, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 257–288. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Kumar, S.; Bhat, H.F.; Aadil, R.M.; Bekhit, A.E.-D.A. 3D printing: Development of animal products and special foods. Trends Food Sci. Technol. 2021, 118, 87–105. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Bhandari, B. 3D Printing of Steak-like Foods Based on Textured Soybean Protein. Foods 2021, 10, 2011. [Google Scholar] [CrossRef]
- Wang, T.; Kaur, L.; Furuhata, Y.; Aoyama, H.; Singh, J. 3D Printing of Textured Soft Hybrid Meat Analogues. Foods 2022, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kwon, O.-C.; Jo, W.; Lee, H.J.; Moon, M.-W. 4D Printing Technology: A Review. 3d Print. Addit. Manuf. 2015, 2, 159–167. [Google Scholar] [CrossRef]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; George, J. 4D printing: A new approach for food printing; effect of various stimuli on 4D printed food properties. A comprehensive review. Appl. Food Res. 2022, 2, 100150. [Google Scholar] [CrossRef]
- Pei, E.; Loh, G.H. Technological considerations for 4D printing: An overview. Prog. Addit. Manuf. 2018, 3, 95–107. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Brier, P.; van den Eijnden, P.; Henket, J.T.M.M.; Langelaan, M.L.P.; Stroeks, N.; van Deventer, H.C.; Martin, A.H. Food-grade electrospinning of proteins. Innov. Food Sci. Emerg. Technol. 2013, 20, 269–275. [Google Scholar] [CrossRef]
- Chen, D.; Jones, O.G.; Campanella, O.H. Plant protein-based fibers: Fabrication, characterization, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 2021, 2004991. [Google Scholar] [CrossRef]
- Cui, B.; Liang, H.; Li, J.; Zhou, B.; Chen, W.; Liu, J.; Li, B. Development and characterization of edible plant-based fibers using a wet-spinning technique. Food Hydrocoll. 2022, 133, 107965. [Google Scholar] [CrossRef]
- Guan, J.; Porter, D.; Tian, K.; Shao, Z.; Chen, X. Morphology and mechanical properties of soy protein scaffolds made by directional freezing. J. Appl. Polym. Sci. 2010, 118, 1658–1665. [Google Scholar] [CrossRef]
- Chantanuson, R.; Nagamine, S.; Kobayashi, T.; Nakagawa, K. Preparation of soy protein-based food gels and control of fibrous structure and rheological property by freezing. Food Struct. 2022, 32, 100258. [Google Scholar] [CrossRef]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Nowacka, M.; Trusinska, M.; Chraniuk, P.; Drudi, F.; Lukasiewicz, J.; Nguyen, N.P.; Przybyszewska, A.; Pobiega, K.; Tappi, S.; Tylewicz, U.; et al. Developments in Plant Proteins Production for Meat and Fish Analogues. Molecules 2023, 28, 2966. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Göbel, J.; van der Goot, A.J.; Stefanidis, G.D. Production of structured soy-based meat analogues using simple shear and heat in a Couette Cell. J. Food Eng. 2015, 160, 34–41. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential health benefits of garden pea seeds and pods: A review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Simonin, M.; Dasilva, C.; Terzi, V.; Ngonkeu, E.L.M.; Diouf, D.; Kane, A.; Béna, G.; Moulin, L. Influence of plant genotype and soil on the wheat rhizosphere microbiome: Evidences for a core microbiome across eight African and European soils. FEMS Microbiol. Ecol. 2020, 96, 6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).