Abstract
By definition, children constitute a vulnerable population, especially when they are chronically ill and/or disabled. A characteristic of chronically ill and disabled children is that they also suffer from indirect effects of their disease, such as immobilization, chronic inflammation, reduced time outdoors in the sun, osteotoxic effects of disease-targeted therapy (like glucocorticoids), and poor nutrition. All these factors may lead to bone fragility due to secondary osteoporosis, a co-morbidity that may be overlooked in the context of serious underlying diseases. The ultimate goal of osteoporosis diagnosis and monitoring in this setting is the early identification, prevention, and treatment of low-trauma long bone and vertebral fractures; indeed, vertebral fractures are a frequently under-diagnosed manifestation of overt bone fragility in this context. Efforts to prevent first-ever fractures are also meritorious, including encouragement of weight-bearing activities, optimization of nutritional status, including calcium and vitamin D supplementation, and the diagnosis and treatment of delayed growth and puberty; however, these conservative measures may be insufficient in those at high risk. Numerous natural history studies have shown that vertebral fractures are more common than non-vertebral (i.e., long bone) fractures in at-risk children. Not surprisingly, the cornerstone of secondary osteoporosis monitoring is lateral spine imaging for the early detection of vertebral collapse. Although dual-energy x-ray absorptiometry (DXA) is the gold standard to measure bone mineral density, digital X-ray radiogrammetry may be used as a surrogate measure of bone strength if dual-energy x-ray absorptiometry is not available. In the event that preventive measures fail, treatment with bisphosphonates may be appropriate. Typically, treatment with intravenous bisphosphonates is reserved for children with overt bone fragility and limited potential for spontaneous recovery. However, there is increasing attention to very high-risk children, such as boys with Duchenne muscular dystrophy, who may benefit from bisphosphonate therapy prior to first-ever fractures (given their high fracture frequency and essentially absent potential for spontaneous recovery). This article provides a contemporary overview of the definition and diagnosis of osteoporosis in children with chronic illness, along with the approach to monitoring those at risk and the evidence for currently recommended intervention strategies.
1. Introduction
The health domains of vulnerable populations can be divided into physical, psychological, and social []. The physical domain includes all infants and chronically ill and disabled children and adults. Most people in this domain have more than two chronic morbidities by the age of 65 []. Osteoporosis is a known chronic morbidity in adults by the age of 65 []. In children, osteoporosis can be categorized into primary and secondary causes. Primary osteoporosis in children is caused by a disease of the bone itself, for example, osteogenesis imperfecta. Secondary osteoporosis in children can be caused by immobilization, cytokine-release-related disorders, gastrointestinal disorders, endocrine disorders, drugs (particularly glucocorticoids), or a combination of these factors. In children with certain diseases, multiple factors can contribute to bone fragility and secondary osteoporosis; the most common of these include chronic glucocorticoid use, inflammatory disorders, and compromised mobility. Both types of osteoporosis are associated with an increased risk of fractures and morbidity and decreased quality of life [].
The diagnosis of osteoporosis in children is more challenging than in adults because bone mass varies greatly with age/pubertal stage and because peak bone mass has not yet been attained. In addition, fracture rates increase during periods of accelerated linear growth, such as during adolescence []. In 2014, The International Society of Clinical Densitometry (ISCD) put forward a definition of osteoporosis that was meant to avoid over-diagnosis in otherwise healthy children. In this specific context, the ISCD defined pediatric osteoporosis as 1. ≥2 long bone fractures before the age of 10 years or ≥3 long bone fractures before the age of 19 years in combination with a low bone mineral density (BMD) for age and gender (z-score ≤−2.0 as measured by DXA), or 2. ≥1 vertebral compression fractures (VF), independent of the BMD, in the absence of major trauma or local disease []. It is important to underscore that this conservative definition was intended for otherwise healthy children, as opposed to children at high risk for fractures, so as to avoid labeling children with osteoporosis who may have simply been unlucky on the playground or in sports activities. In children with significant risk factors for bone fragility due to chronic illness, even a single low-trauma long bone or vertebral fracture (in the absence of a “low BMD”) may be sufficient to diagnose the child with osteoporosis, as discussed in a recent paper by Ward et al. [].
This article will first discuss the diagnostic approach to bone fragility and low bone mass in childhood. We will then focus on two representative conditions associated with low bone mass: immobilization and drug-related (glucocorticoid therapy). Thereafter, we will describe methods that can be used for osteoporosis screening, prevention, and therapy. Finally, we will describe secondary osteoporosis in three groups of children with chronic diseases, to which less attention is paid in literature, more in detail: children with profound intellectual and multiple disabilities, children on the ketogenic diet, and children with congenital myopathies.
2. Diagnosis
As stated earlier, the ISCD definition of osteoporosis in children was not intended for children with significant risk factors for bone fragility due to chronic illness. Waiting for a second long bone fracture, a first vertebral fracture or a low BMD by DXA following low-trauma fractures unnecessarily delays the initiation of treatment in those with conditions known to be associated with an increased risk of bone fragility []. A vertebral fracture in children has been defined as >20% loss of vertebral height ratio according to the semi-quantitative Genant method []. The Genant method to define VF in children has been validated by showing that vertebral fractures defined in this way independently predict new (i.e., subsequent) vertebral and long bone fractures []. VFs are more common than long bone fractures in children with chronic illness treated with glucocorticoids: VFs occur in 32.5%, non-VFs in 23% of children with acute lymphoblastic leukemia [], and VFs occur in 16.3% and non-VF fractures in 10.1% of children with rheumatic disorders treated with glucocorticoids []. Thirty-nine percent of children with VF in the group of children with acute lymphoblastic leukemia were asymptomatic []. For this reason, routine monitoring by lateral spine imaging (radiography or dual-energy x-ray absorptiometry) is required for their early detection []. Untreated VFs can lead to chronic back pain and spine deformity []. Therefore, screening for VF is so important that many clinicians are now using DXA-based Vertebral Fracture Assessment, for which there are guidelines in children put forward by the ISCD [,]. This has the advantage of low radiation, lack of parallax, and showing the entire spine on one cassette (which minimizes errors in determining the affected vertebral level(s)) [].
A child with a chronic illness associated with bone fragility with a size-adjusted BMD z-score <−2.0 but without a history of fractures is not classified as having osteoporosis per se but would be considered at-risk. The definition “fractures without major trauma” has been defined as fractures occurring outside of motor vehicle accidents or falling from 10 feet (3 m) or less []. With respect to falls in the at-risk, chronic illness setting, a more appropriate definition has been used: falling from a standing height or less or at no more than walking speed [].
DXA-based BMD can be viewed as a clinical precursor to bone fragility, as it is considered a surrogate for bone strength. Because 90% of peak bone mass is acquired by the age of 18 years, chronic pediatric disease reduces BMD and thus increases fracture risk, not only in childhood and adolescence but also in adulthood [,]. There is a strong correlation between BMD and fracture risk []. However, BMD can be normal in children with fractures due to secondary osteoporosis [,]. DXA is the gold standard for the measurement of bone mass (bone mineral content, BMC) and bone mineral density (BMD) in children because of its precision, minimal radiation dose, reproducibility, availability of normative data, and correlation with prevalent and incident fractures in children [,,]. Different DXA skeletal sites for BMD measurement have been described, with the lumbar spine (L1-L4] and hip (the latter, in children over 4–5 years of age) representing key skeletal sites [,]. The main purpose thus of BMD is to provide additional evidence to justify an appropriate workup and therapeutic intervention. Follow-up of the BMD can be used as a predictor of bone fragility, with a loss of ≥ 0.5 SD considered clinically significant [,].
However, DXA has its limitations. DXA may be technically impossible to perform or interpret in children due to movement during measurement, metallic implants, contractures, and sometimes scoliosis []. Secondly, the z-scores are based on calendar age and not skeletal maturation. This may lead to inaccurate interpretation of measurements in those with delayed pubertal development [,]. Last but not least, DXA provides a measurement of areal BMD (g/cm2) rather than volumetric density (g/cm3), which can give an underestimation of BMD in children with small stature s and an overestimation of BMD in children with tall stature [].
There are other methods, like digital X-ray radiogrammetry of the hand, peripheral quantitative computed tomography (both standard (pQCT) and high resolution (HR-pQCT)), and quantitative ultrasound, that may overcome some of these limitations. However, these are currently not in routine clinical use. A recent systematic review, including a meta-analysis, reviewed the literature on the relationship of digital X-ray radiogrammetry, pQCT, and quantitative ultrasound with DXA []. According to this study, digital X-ray radiogrammetry had the strongest positive relationship with DXA (correlation coefficient of 0.71]. Digital X-ray radiogrammetry uses web-based software (for example BoneXpert), which can assess both skeletal maturation (bone age) and bone strength, expressed as the bone health index (BHI). This BHI is a measurement of cortical thickness and cortical mineralization, which results in a representation of bone quality. The BHI reference values are specific for gender and bone age. Digital X-ray radiogrammetry is less stressful for pediatric patients than DXA and is easy to obtain. Often performing an X-ray of the hand does not involve additional exposure to ionizing radiation since hand radiographs for the assessment of bone age are regularly obtained in at-risk children []. In a prospective study of 101 pediatric patients with a high probability of low bone strength, digital X-ray radiogrammetry was compared with DXA [] in children with the following profile: mean age was 11.7 years, 38 were non-ambulatory, and 52 had a neurological disorder. The mean BMD z-score was −1.3 for the group and for the non-ambulatory children −2.2. Digital X-ray radiogrammetry had a sensitivity of 67% and a specificity of 83% for BMD z-score ≤−2.0. Additionally, If the BMD z-score was <−2.0, digital X-ray radiogrammetry z-scores (being also <−2.0] demonstrated a percentage of 92.4% agreement.
(HR-) pQCT is used to measure cortical and trabecular volumetric bone mineral density separately and microarchitectural bone morphology. Movement artifacts are a real limitation []. The use of (HR-) pQCT is limited due to the lack of standardized pediatric reference data for young children, and only a few (HR-) pQTC scanners are available for patient care. The correlation coefficient of (HR-) pQCT with DXA is only 0.57 []. Quantitative ultrasound has the same correlation coefficient.
Practical information about who and how to screen for osteoporosis is given in the Section 4 further in this manuscript.
3. Risk Factors for Developing Secondary Osteoporosis
3.1. Immobilization and Secondary Osteoporosis
Immobilization is normal in children who are wheelchair-dependent. Cerebral palsy and neuromuscular disorders, such as Duchenne muscular dystrophy, are examples of diseases with immobilization. However, also severe neurodevelopmental disorders and spina bifida are frequent causes of immobilization.
The degree of immobilization in children with cerebral palsy is classified with the Gross Motor Functional Classification Scale (GMFCS) in five levels []. GMFCS level 4 means that a child can walk indoors for short distances with assistance but relies on a wheelchair outdoors. Children with GMFCS level 5 are wheelchair dependent. Immobilization in children with cerebral palsy reduces biomechanical bone loading, leading to thinner long bones and less trabecular bone formation []. In children aged 2–19 years with moderate to severe cerebral palsy, classified as GMFCS level 3–5, low BMD was found in 97% of children unable to stand and older than 9 years. This leads to reduced periosteal apposition in lower extremity bones, reducing cortical thickness. Consequently, fractures occur most commonly in the distal femur and tibia. Fractures occurred in 26% of children who were older than 10 years []. Other factors that contributed to low BMD (z score ≤−2.0] were feeding difficulty and the use of anticonvulsants. Other studies showed an incidence of fractures of 4% per year [].
The major role that immobilization plays in secondary osteoporosis and low BMD is also illustrated by boys with Duchenne muscular dystrophy. In the era preceding treatment with glucocorticoids, Larson and Henderson found that BMD was only slightly decreased (z-score lumbar spine −0.8] when the boys were ambulatory but decreased significantly after the loss of ambulation (z-score lumbar spine −1.6] []. This was also shown by Crabtree et al. in boys with Duchenne muscular dystrophy treated with glucocorticoids who became non-ambulant []. They showed that 44% of the boys sustained a fracture. Two-thirds of these fractures involved the lower extremities, and there were no vertebral fractures. Moreover, 44% of the boys who walked with support at the time of fracture never resumed walking after the fracture. In addition, Joseph et al. showed an absence of clinical vertebral fractures in glucocorticoid naïve boys []. In the authors’ experience, vertebral fractures can occur in DMD among steroid-naïve patients if routine screening is part of the bone health evaluation; however, this occurrence is rarely related to boys with DMD who are receiving glucocorticoid therapy.
Preclinical studies are useful for a more in-depth understanding of the relationship between immobilization and secondary osteoporosis. Animal models may be used to study immobilization and the cellular mechanisms of secondary osteoporosis. A recent systematic review gives an overview of known animal models [].
3.2. Drug-Induced Secondary Osteoporosis
A myriad of drugs can lead to low BMD and secondary osteoporosis. The most well-known are glucocorticoids, anticonvulsants, and methotrexate []. Glucocorticoids are often used for prolonged periods of time in (chronic) diseases in children like systemic inflammatory and autoimmune diseases, renal diseases, after organ transplantation, leukemia, and Duchenne muscular dystrophy. These diseases in themselves may also lead to fragility fractures because of reduced bone strength, for example, due to the effect of the increased cytokines (like IL1, IL6, and tumor necrosis factor-alpha) in case of systemic inflammatory and autoimmune diseases on bone metabolism []. Much is known about the adverse effects of glucocorticoids on bone strength []. Glucocorticoids cause decreased bone formation, with an additional early and transient increase in bone resorption. The final effect is increased bone turnover with early onset bone loss []. BMD rapidly decreases in the first 2 weeks after the start of glucocorticoids, leading to significant bone loss in the first 3–6 months of therapy []. This loss diminishes with time and is replaced by a chronic phase of decreased bone formation. The ultimate effect is a reduction of BMD and altered bone microarchitecture, with a predilection for the trabecular-rich spine []. This deleterious effect of glucocorticoids on bone strength can be seen in children with Duchenne muscular dystrophy. Glucocorticoids are used to delay loss of ambulation, improve or retain pulmonary function with reduced need for assisted ventilation and delay cardiomyopathy []. Before corticosteroids were used, vertebral fractures were rarely seen in children with Duchenne muscular dystrophy, and most fractures involved the lower extremities []. In boys living with Duchenne muscular dystrophy who are treated with glucocorticoids, the prevalence of vertebral fractures is >50%, with a cumulative incidence of 28% over a median follow-up of 4 years after starting with glucocorticoids [,]. In Canada, an observational cohort study was performed to increase insight into glucocorticoid-induced osteoporosis in children []. The most important observations were that vertebral fractures are the hallmark of pediatric glucocorticoid-induced secondary osteoporosis, can occur in the first year of glucocorticoid treatment, and are frequently asymptomatic. However, some children have the growth-mediated ability to restore normal vertebral body dimensions following vertebral fractures. This is important to know since this may preclude the need for intravenous osteoporosis therapy []. Children with poor growth, older children (with less residual growth), and children with ongoing bone health threats have less potential for vertebral body reshaping, which can result in permanent vertebral deformity []. Therefore, timely intervention with intravenous osteoporosis therapy is paramount in children with vertebral fractures if they have persistent risk factors for ongoing spine collapse.
Although preclinical models may help to understand the effects of glucocorticoids on bone metabolism and bone strength, up to now, there is no robust animal model to evaluate known and new interventions [].
4. Screening for Secondary Osteoporosis
The goal of screening is to identify patients with a high risk for secondary osteoporosis in order to initiate bone protection therapy in a timely fashion. This has led to screening for early rather than late signs of vertebral fractures, as well as reductions in BMD following appropriate size corrections. This aligns with a secondary prevention approach, which seeks to mitigate the progression of low BMD and bone fragility following identification in an earlier stage []. High-risk patients belong to one of the earlier-mentioned groups, especially those with immobilization with or without additional risk factors and those treated with daily or IV glucocorticoids for more than three months.
Recently, two observations have demonstrated the limitations of a “BMD-focused approach” instead of implementing a “fracture- and function-focused approach.” First, the use of a BMD z-score threshold of −2 or worse to identify a child as having osteoporosis is problematic due to variability in the z-scores arising from the reference databases [], and secondly, asymptomatic VF can occur at lumbar spine BMD z-scores >−2, thereby requiring spine imaging for vertebral fractures, especially in children using daily oral or IV glucocorticoids for more than three months where the risk of vertebral fractures is even higher []. Other functional outcomes that should also be considered in osteoporosis screening include a history of long bone fractures, growth, pain (especially indicative for vertebral fractures), degree of mobility, muscle strength, and the potential for spontaneous, medication-unassisted recovery (the latter, which is influenced by pubertal stage and residual growth potential). Screening of BMD (by DXA or digital X-ray radiogrammetry) is an adjuvant component of bone health monitoring, as it provides insight into the child’s overall bone health trajectory; to this end, BMD is most useful when implemented as a longitudinal monitoring tool, much like linear growth is tracked children as a barometer of overall well-being. The exact timing and frequency of screening BMD depends on the risk of secondary osteoporosis and, consequently, differs between patient groups and the individual patient. In general, the following guidelines can be followed:
- Patients that will be treated with daily oral or IV glucocorticoids for more than three months should be considered for a baseline spine radiograph or DXA-based vertebral fracture assessment. This is recommended since the earliest reported vertebral fracture in children treated with glucocorticoids is at 4 months after the start of glucocorticoids []. These children should also undergo a follow-up radiograph at 12 months if risk factors are persistent since this is the time point with the highest incidence of vertebral fractures [,]. Annual to biennial imaging for VF is advised afterward for patients still treated with glucocorticoids; VF imaging beyond this critical high-risk period is then customized to the magnitude of risk factors thereafter. For further information on the follow-up frequency, see Ward et al. [];
- The same principles apply to children with other risk factors for bone fragility. In children with immobilization, especially in combination with drugs that can cause secondary osteoporosis, screening should start at the latest by 6–8 years of age and then at intervals of about two years thereafter until the end of growth or earlier in case of suffering from back pain or fragility fractures [,]. Monitoring is recommended to start by this time to plan to start with treatment. Treatment should be initiated before there is not enough residual growth potential to reshape vertebral bodies following VFs. Since BMD is useful as a longitudinal measurement to assist the clinician in understanding the child’s overall bone health trajectory and in making logical decisions about the need for ongoing monitoring versus discharge from bone health care or intervention, it is recommended that a BMD measurement is carried out at least as frequently as spine radiographs according to the guidelines above, with assessments every 6 months in those children at greatest risk [,]. If the spine BMD Z-score declines by more than 0.5 on successive measurements, or there is back pain, earlier spine imaging is recommended every 1–2 years in those with persistent risk factors (as demonstrated in recent guidelines put forward in a prototypical osteoporotic condition of childhood, glucocorticoid-treated DMD) [].
5. Prevention of Secondary Osteoporosis
Numerous interventions may prevent or reduce bone fragility fractures []. This starts with recognizing the risk of bone fragility and osteoporosis, as discussed above. Children with a condition or risk factors known to be associated with low bone strength or secondary osteoporosis, such as wheelchair dependence, cytokine-release related disorders, or who are on certain drugs, have an increased risk of fragility fractures []. General osteoporosis prevention measures are effective treatment of the underlying condition and associated morbidities, optimizing nutritional state and weight-bearing activities, and the diagnosis and treatment of endocrinopathies, including delayed puberty, growth hormone deficiency, and thyroid disorders. For children with chronic illnesses, adequate treatment of the illness is a sine qua non for osteoporosis prevention and treatment []. However, sometimes the disease cannot be causally treated (i.e., severe neurodevelopmental disorder), or the treatment induces bone loss and osteoporosis, such as glucocorticoids or chemotherapy. For this reason, whenever possible, treatments should be as steroid-sparing as possible. Nutritional state is an important factor. For example, a poor nutritional state (underweight or feeding difficulties) is associated with a lower BMD in children with cerebral palsy and chronic pancreatitis [,,]. Other well-known risk factors for reduced bone strength are vitamin D deficiency and a shortage of dietary intake of calcium. Even in healthy children in Europe, the prevalence of vitamin deficiency is about one in every 4–5 children []. Children with chronic illnesses are at high risk due to a combination of limited sun exposure and often feeding problems. The recommended daily intake of vitamin D is 400–800 IU/day, depending on the 25-hydroxyvitamin D level. The optimal serum 25-hydroxyvitamin D threshold, however, remains controversial. From a practical perspective, a 25-hydroxyvitamin D level of 50 nmol/L [20 ng/mL) or more is recommended at the end of winter [,]. Incidental studies report a positive result of this intervention. Bianchi et al. [] reported a significant increase in BMD in 65% of patients with Duchenne muscular dystrophy after two years of vitamin D treatment and adjustment of dietary calcium to the recommended daily dose. Calcium is an important nutrient for adequate skeletal mineralization. The recommended dietary allowance of calcium is 700 mg/day for children between 1–3 years, 1000 mg/day between 4–8 years, and 1300 mg/day for children between 9–18 years []. Optimal calcium intake can be achieved by an adequate diet whenever possible []. The role of standard calcium supplementation in healthy children has been investigated by a meta-analysis showing only a small effect on BMD, unlikely to alter fracture risk []; the situation in chronically ill children may be different since risk factors may “stack” towards an increased risk of bone fragility. For this reason, optimizing conservation measures to enhance bone health, such as calcium and vitamin D intake, are standard approaches in the chronic illness setting.
Physical activities, including weight-bearing activities, have an anabolic effect on the growing skeleton. These physical activities increase bone mass in healthy children []. The evidence of the effect of physical or weight-bearing activities in children with chronic illnesses is still insufficient []. It is advised to encourage activities with a low risk of falling and bodily contact in ambulatory children with osteoporosis []. In non-ambulant children, modest increases in BMD have been reported following weight-bearing regimes and low amplitude, high-frequency vibration therapy [,]. Moreover, it was shown that exercise might improve bone strength under conditions of adequate calcium intake, showing the importance of implementing these general measures in tandem [,].
6. Potential for Recovery
When vulnerable children receive a diagnosis of secondary osteoporosis, there is not always the need to treat with bone-targeted therapy because of the pediatric skeleton’s ability to undergo recovery in both bone mass/density and shape. Case in point, the growing skeleton has the potency to reconstitute normal heights of vertebral bodies following a vertebral collapse, a phenomenon known as “vertebral body reshaping” []. This has been illustrated in children with acute lymphoblastic leukemia and children with inflammatory bowel disease [,]. For example, many children with acute lymphoblastic leukemia will undergo vertebral body reshaping following vertebral fractures because most are diagnosed at a young age (and have significant residual growth potential), and the disease (and its treatment) are usually transient. In a series of children with acute lymphoblastic leukemia, the cumulative VF incidence over six years was 32.5% [], and complete vertebral body reshaping occurred in 77.3% of these children. Notably, the children in which the reshaping was incomplete were older (and had less residual growth potential) and had more severe degrees of vertebral collapse.
Pediatric patients with inflammatory bowel disease, in general, are older at diagnosis, and the disease is chronic with exacerbations. Vertebral fractures have been reported at diagnosis []. The direct effects of chronic inflammation, use of glucocorticoids, delayed puberty, and poor nutrition are contributing factors causing secondary osteoporosis. Optimizing disease control can help reshape vertebral fractures and specific bones and may be the only therapeutic intervention needed for the effective reshaping of vertebral bodies [].
At the opposite end of the spectrum are children with chronic diseases and persistent risk factors for osteoporosis (i.e., due to presently incurable conditions), with the need for continuous treatment with glucocorticoids, for example, boys with Duchenne muscular dystrophy. In these children, there is no capacity for spontaneous vertebral body reshaping. For this reason, contemporary care includes monitoring for signs of osteoporosis at the time of initiation of glucocorticoid treatment and starting osteoporosis treatment at the first sign of a low-trauma long-bone fracture or VF [,,,]. In such children, strategies are currently being considered for the prevention of first-ever fractures.
These three different clinical scenarios show the importance of assessing whether the vulnerable child with a fragility fracture needs osteoporosis therapy. It is important to recognize that younger age, transient risk factors, normal growth and puberty, and less severe vertebral collapse are key factors for recovery without needing bone-targeted intervention (i.e., bisphosphonates).
7. Therapy
Currently, the most widely used agents for treating secondary osteoporosis in children are bisphosphonates []. Bisphosphonates are synthetic analogs of pyrophosphates. Classically, treatment with intravenous bisphosphonates should be considered in children with a formal diagnosis of secondary osteoporosis that manifests as at least one low-trauma vertebral or long bone fracture and limited potential for spontaneous (i.e., medication-unassisted) recovery due to older age and/or persistence of osteoporosis risk factors [,]. In previous years, the ISCD criteria for otherwise healthy children were erroneously applied to some children with risk factors for osteoporosis, which meant that bisphosphonate therapy was withheld from, for example, boys with DMD who had a single femur fracture (instead of two or more long-bone fractures by 10 years of age or three or more long bone fractures by 19 years of age plus a low BMD) [,]. However, it is now understood that these criteria were intended for otherwise healthy children so as not to over-diagnose osteoporosis in this population, and even a single low-trauma long bone fracture can represent an osteoporotic event in children with risk factors for osteoporosis []. Low-trauma long bone fractures and symptomatic vertebral fractures (or asymptomatic VF in high-risk settings such as CP, DMD, and glucocorticoid-treated disorders) are the most frequent indications for treatment with intravenous bisphosphonates. The primary function of bisphosphonates is to inactivate osteoclasts, resulting in cortical and trabecular bone thickening. This makes bones wider, denser, and stronger. There has been much discussion in the literature on the use of oral versus intravenous therapy with bisphosphonates. At this time, the scientific data support the use of the (more potent) intravenous bisphosphonates over oral bisphosphonates. This article is not the place for a thorough discussion on this theme, but we refer those wanting to know more to Ward et al. [].
There are different regimens to prescribe the most-used intravenous bisphosphonate, pamidronate. The most frequently used regimen is 1 mg/kg/day for three days every four months (in total, 9 mg/kg/year). However, a regimen of 1 mg/kg every three months (in total, 4 mg/kg/year) in children with primary osteoporosis has shown a comparable effect on BMD and reduction of fragility fractures []. Other studies also showed a comparable effect [,]. A different intravenous bisphosphonate, zoledronic acid, is also used in children. Zoledronic acid can be given every six months and is preferred by some because of its greater convenience with respect to the shorter duration of the infusion and longer duration of action compared with pamidronate. Saraff et al. showed that zoledronic acid has a comparable efficacy compared to pamidronate in children with osteogenesis imperfecta []. Additionally, Nasomyont et al. found no difference in effect on BMD Z scores between Pamidronate and zoledronic acid in patients with primary, secondary, and glucocorticoid-induced osteoporosis []. Furthermore, the costs of zoledronic acid are lower []. Conversely, Pamidronate probably has fewer adverse drug reactions, predominantly hypocalcemia [], nausea, and vomiting. It is important to note that the first-infusion reactions of intravenous bisphosphonate therapy can precipitate adrenal insufficiency in glucocorticoid-treated children. For this reason, steroid stress dosing is recommended in children at risk for adrenal suppression in this context. In a recent study of intravenous zoledronic acid versus intravenous placebo in glucocorticoid-treated children, 25% of children in the placebo group had at least one adverse event following the first zoledronic acid infusion []. This observation provided evidence that not all health events following the first intravenous bisphosphonate infusions are bisphosphonate-related but may be linked to underlying chronic illnesses.
In practice, there are still two other questions regarding the treatment with bisphosphonates in children with permanent risk factors: when should we start bisphosphonates, and how long should patients be on bisphosphonates therapy?
When should we start bisphosphonates? As said before, treatment with intravenous bisphosphonates should be considered in children fulfilling the ISCD criteria. However, in children with significant risk factors for bone fragility due to chronic illness, even a single low-trauma long bone or vertebral fracture (in the absence of a “low BMD”) may be sufficient to diagnose the child with osteoporosis, as discussed in a recent paper by Ward et al. []. recently Nasomyont et al. published a study of the treatment with bisphosphonates in 48 with primary osteoporosis, 46 with secondary osteoporosis without glucocorticoids and 29 children with glucocorticoid-induced osteoporosis []. They treated 19% of the patients with intravenous bisphosphonates without these patients fulfilling the ISCD criteria for osteoporosis. One of these indications was low, declining BMD without a history of fractures; another was low BMD in association with kidney stones in children with ongoing risk factors. In addition, Draaisma et al. treated children on a ketogenic diet with ongoing risk factors with bisphosphonates out of the ISCD criteria [].
How long should patients be on bisphosphonate therapy? Some observations in children with both primary and secondary osteoporosis have provided answers to these questions []. After discontinuing bisphosphonate therapy in children with open growth plates and persistent risk factors for osteoporosis, the newly formed bone has a low density. This may cause a point of increased bone fragility because of the “density differential” between the high-density bone (formed under treatment with bisphosphonates) and the low-density bone [], leading to fractures in the transition zone between treated and untreated bone [,]. So, if bisphosphonate therapy should be continued, for how long? A possible answer followed from a study in children with osteogenesis imperfecta []. In children with still open growth plates and discontinuing bisphosphonate therapy, the BMD z-scores declined. However, discontinuing therapy in children with closed growth plates had no effect on the BMD z-scores two years later. This has led to the recommendation of continuation of bisphosphonate therapy until the closure of the growth plates in those with persistent risk factors (such as congenital/genetic disorders and long-term acquired risk factors, such as chronic glucocorticoid therapy). Starting with a higher dose until the patient is clinically stable and tapering the dose or frequency is often implemented in order to avoid over-treatment in the context of long-term therapy considered [,].
In summary, treatment with intravenous bisphosphonates should be considered in children fulfilling the ISCD criteria (intended for otherwise healthy children with clinically significant fractures and low, appropriately size-adjusted BMD parameters). However, it should also be considered in high-risk patients outside of this conservative approach who have even a single-low-trauma long bone or vertebral fracture, a known increased risk for bone fragility, and limited potential for spontaneous recovery [,]. It is important to keep in mind that not all children with secondary osteoporosis require bisphosphonate therapy following a fragility fracture; those with transient risk factors for vertebral or long bone fractures and potential for spontaneous recovery due to younger age or less severe vertebral collapse typically do not need osteoporosis intervention, unless they have significant bone pain interfering with the quality of life.
A proposition for screening, prevention, and therapy of secondary osteoporosis is given in Figure 1.
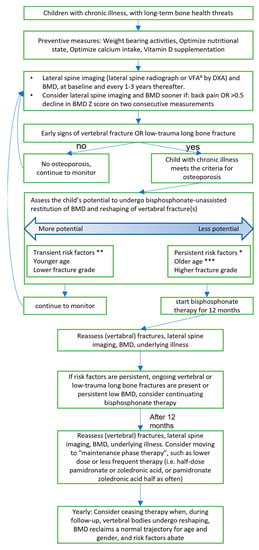
Figure 1.
Recommendation for investigation and treatment of secondary osteoporosis in children. Partly based on the previous recommendation of papers of Ward [,], Fehlings et al. [], and Simm et al. [].a VFA = Vertebral Fracture Assessment * Persistent risk factors are >3 months steroids, sub-normal mobility, poorly controlled underlying disease ** Transient risk factors are <3months steroids, short-term immobilization (<2 weeks), well-controlled underlying disease *** Older age is defined as >8 years in girls and >9 years in boys.
8. Specific Groups
While the principles with respect to at-risk children mapped out previously in this article apply to all high-risk groups, the following describes additional disease-specific nuances in the management of patients who fall into three groups: those with profound intellectual and multiple disabilities, those with neuromuscular conditions otherwise, and children receiving a therapeutic ketogenic diet for the treatment of intractable seizures.
Profound intellectual and multiple disabilities
Children with profound intellectual and multiple disabilities (PIMD) form an extremely heterogeneous group regarding cause, level of functioning, and co-morbidities []. A GMFCS of four or five in combination with a severe intellectual disability (IQ < 30 or developmental age < 2 years), regardless of the underlying disease, is often used as a criterion for PIMD. Low BMD and secondary osteoporosis are highly prevalent co-morbidities in PIMD. As low BMD often goes unnoticed, a (low-impact) fracture is a common presenting sign of low BMD or osteoporosis. Since children with PIMD are frequently limited in their communication skills, there can be a significant delay in noticing these complications. Presedo et al. reported a delay in diagnosis of a fracture in half of the patients with cerebral palsy with an average delay of 10 days [], stressing the need for a high index of suspicion in this population.
Incidence rates for fractures in children with PIMD are scarce. However, ample literature regarding fractures and low BMD in children and adults with cerebral palsy is available. As the level of (motor) functioning in severe cerebral palsy (GMFCS 4 or higher) and co-morbidities (e.g., epilepsy) in cerebral palsy in PIMD are fairly similar, these groups can be considered interchangeably regarding osteoporosis and low BMD. The incidence rates for fractures in children with severe cerebral palsy have been reported to be around 4% []. However, these numbers vary greatly. A systematic review by Mergler, including 21 studies in children with severe cerebral palsy, reported incidence rates of fractures varying from 2.7–23% []. The prevalence of low BMD was higher, with reported rates of 27–77%. Several studies have evaluated low BMD and secondary osteoporosis risk factors in children with cerebral palsy/PIMD. Although the reported significance of the risk factors varies, non-ambulatory status, feeding difficulties, and anticonvulsant use are repeatedly outlined as the foremost. Several studies have also shown tube feeding as an independent risk factor for low BMD [,], although it is unclear if this is a consequence of more excessive feeding difficulties. Other risk factors that have been reported are dietary deficits (calcium, vitamin D) and the use of other drugs, such as proton pump inhibitors [], solely progestin-containing contraceptives [], and delayed puberty. Of course, sustaining previous fractures has also been shown to be an important risk factor [], as is low BMD.
Conversely, laboratory studies (e.g., calcium, phosphorus, alkaline phosphatase) have not been shown to correlate with low BMD. As calcium and phosphatase levels in the blood are maintained at a constant level at the expense of bone composition, this is not unimaginable, but unremarkable blood tests can give the clinician a false sense of security.
Diagnosing and following up on low BMD in children with PIMD deserves special attention. Measurement by DXA, including DXA-based vertebral fracture assessment (or baseline spine radiograph), is considered the gold standard, but its availability in hospitals is limited. Furthermore, movement during measurement, scoliosis, or contractures can negatively influence the measurements. Alternatives, such as the aforementioned digital X-ray radiogrammetry, have been proven to correlate well with DXA [] in healthy children. This technique has also been investigated in children with PIMD and seems feasible, with the exception of children with (severe) anatomical deformities []. Screening for low BMD can be performed as stated before (in the section on screening).
In PIMD, it is almost without exception impossible to adequately treat the underlying disease. Following this, it is paramount to optimally treat all of the independent factors associated with low BMD. Regarding vitamin D and calcium, the recommendations mentioned in the Section 5 are valid. To adequately treat feeding difficulties, we recommend the early involvement of a multidisciplinary team, including a pediatric dietician and speech therapist.
All anticonvulsants have been, either clinically or preclinically, associated with disturbances in bone metabolism. However, enzyme-inducing anticonvulsants such as phenytoin and carbamazepine have been shown to carry a higher risk, possibly due to inducing vitamin D metabolism []. Multiple and prolonged anticonvulsant use (>2 years) is also associated with a higher fracture risk []. As in antibiotics, restraint anticonvulsant use should be considered to limit the risk of fragility fractures/low BMD. As gastro-esophageal reflux tends to diminish over time, decreasing or discontinuing proton pump inhibitors could also contribute. Lastly, systemic progestin-only contraceptives should be replaced with alternatives also containing estrogen.
Considering immobilization, loading exercises conducted by a physical therapist experienced in children with PIMD are important. Prevention of contractures should also deserve attention to maintain mobility and facilitate imaging options.
Aids, such as a standing frame implemented by a pediatric rehabilitation specialist, have also been shown to slightly improve BMD []. The Section 7 mentions osteoporosis treatment with bisphosphonate therapy.
Neuromuscular disorders
Children with neuromuscular disorders, including congenital myopathies and (congenital) muscular dystrophies, are at particular risk for reduced bone quality and, consequently, fragility fractures due to a combination of factors. Impaired mobility and altered musculoskeletal interaction reduce cortical thickness and prevent the preservation of the bone structure. Additionally, more myokines are expressed in atrophied muscles, leading to osteoclast differentiation and bone loss induction []. Further, children with a neuromuscular disease frequently experience nutritional issues due to swallowing problems and have decreased sun exposure, leading to a deficiency of calcium and vitamin D [,,,]. Children with Duchenne muscular dystrophy are particularly at risk for decreased bone strength when treated with glucocorticoids [], as mentioned before. Next, the number of falls increases, and the falling mechanism is altered in patients with neuromuscular diseases. Ambulant patients commonly report problems with walking, poor balance, frequent trips, and falls [,]. Patients with neuromuscular diseases tend to have a different falling mechanism: they fall on their proximal bones because they are not fast enough to reach out their hands in order to break their fall []. This results in more frequent long-bone fractures of the long bone that are more proximally located (i.e., humerus, femur).
It is essential to prevent long bone fractures in children with neuromuscular diseases as far as possible in order to optimize functional prognosis, quality of life, and survival [,,]. There are clues that limb immobilization in patients with neuromuscular disease may cause more loss of muscle mass than in healthy subjects []. Fractures of the lower extremities might further lead to permanent loss of ambulance [,]. Low bone strength can impair neuromuscular management and rehabilitation, including surgical treatments for scoliosis or foot deformities [,]. Pain caused by long bone fractures negatively influences rehabilitative care and, consequently, survival [,].
Routine assessment of bone quality in children with neuromuscular diseases is recommended. We propose a DXA-scan (including DXA-based vertebral fracture assessment where available) in all patients at diagnosis of the neuromuscular disease and subsequently at every one to two years, depending on the overall BMD trajectory (and more often in the patient is on glucocorticoid therapy as in boys with DMD). A digital X-ray radiogrammetry of the hand to measure the BHI is a feasible alternative when it is not possible to perform a DXA scan (movement during measurement, metallic implants, contractures, scoliosis) or age < 3 years (no normative values for DXA available for this age group). In order to detect vertebral compression fractures, a lateral thoracolumbar spine x-ray should be performed when DXA-based vertebral assessment is not possible.
Children using Ketogenic Diet
Treatment with a ketogenic diet (KDT) is a nonpharmacologic intervention for intractable childhood epilepsy. The diet consists of a high fat (mostly 80% fat), adequate protein, and a low-carbohydrate percentage that mimics a state of fasting. In intractable childhood epilepsy, treatment with a ketogenic diet can lead to a beneficial effect on seizure control, regardless of age or seizure type []. The ketogenic diet is associated with skeletal demineralization with a reduction of BMD between 0.16–0.6 z-score/year [,,]. This results in an increased incidence of bone fractures in up to 21% of the children during a follow-up period of 6 years or more [].
The ketogenic diet results in a high “acid load” via the ketone bodies, alterations in vitamin D levels, and lowering growth factors []. This also causes failure to accrue bone at a normal rate and increased bone loss due to increased bone absorption [].
Prophylactic supplementation with calcium and vitamin D was considered mandatory for all children on a ketogenic diet, but this may be insufficient in preventing ongoing declines in BMD Z-scores [,].
Moreover, during long-term follow-up of KDT, an increased incidence of kidney stones has also been found, mostly due to hypercalciuria. Even frank hypercalcemia has been described along this spectrum []. Therefore, prophylactic supplementation of calcium and vitamin D is challenging. Screening with DXA scans to evaluate for a diminished BMD is recommended in children on KDT for over 2 years by 12 of the 25 [48%) centers participating in the International Ketogenic Diet Study Group []. The main recommendation of the recent report of this study group was that screening with DXA scans should be standard. Interventions should be considered in case of abnormal results. DXA scans should be repeated a year afterward [].
These findings support the need to monitor bone strength in individuals on KDT, by following parameters, such as calcium intake, vitamin D deficiency, and activity levels. Developing kidney stones remains a major complication of KDT and has to be taken into account when assessing a patient on the diet [].
9. Conclusions
Chronically ill and/or disabled children suffer from direct and indirect effects of their disease, such as immobilization, osteotoxic drug use, chronic inflammation, reduced time outdoors, and poor nutrition. All these factors may lead to bone fragility (low-trauma fractures) due to secondary osteoporosis. Early screening and prevention of declines in bone mass and BMD, plus timely identification of vertebral and long bone fractures, are the cornerstone of osteoporosis management in this context. When conservative preventive measures fail, treatment with intravenous bisphosphonates should be considered in at-risk children with the diagnosis of secondary osteoporosis (Figure 1), where even a single long bone or vertebral fracture provides a sufficient rationale to initiate treatment in children when there are significant risk factors for bone fragility plus limited potential for spontaneous recovery. Looking ahead, prevention of first-ever fractures is on the minds of clinicians caring for the highest-risk patients, such as boys with Duchenne muscular dystrophy, either through relatively steroid-sparing therapy [] or via pre-fracture initiation of bisphosphonate therapy. Studies have facilitated the sightline to the prevention of first fractures in high-risk children, which have highlighted the children who are most likely to recover from osteoporosis, obviating the need for osteoporosis therapy versus those with the least potential for recovery, underscoring the need for anticipatory prevention.
Author Contributions
Conceptualization: A.T.M.D. and J.M.T.D.; methodology: A.T.M.D. and J.M.T.D.; formal and statistical analysis: not performed; investigation: A.T.M.D., E.J.M.J., J.G., K.B. and J.M.T.D.; data curation: not performed; writing—original draft preparation: A.T.M.D., E.J.M.J., J.G., K.B. and J.M.T.D.; writing—review and editing: A.T.M.D., E.J.M.J., J.G., K.B., L.M.W. and J.M.T.D.; supervision: A.T.M.D. and J.M.T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
An Institutional Review Board Statement was not necessary due to the type of article.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are openly available from the author.
Acknowledgments
Jos Draaisma and Joyce Geelen are members of the European Reference Network for Developmental Anomalies and Intellectual Disability (ERN-ITHACA).
Conflicts of Interest
All the authors completed the ICMJE uniform disclosure form. The authors have no conflict of interest to declare. In the last two years, Dr. Ward has been a consultant to and/or participated in clinical trials with Amgen, PTC, ReveraGen, and Santhera.
References
- Vulnerable populations: Who are they? Am. J. Manag.Care 2006, 12 (Suppl. S13), S348–S352.
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Kendler, D.L.; Khan, A.A.; Shapiro, M.C.M.; Morisset, A.; Leung, J.-P.; Reiner, M.; Colgan, S.M.; Slatkovska, L.; Packalen, M. A retrospective observational study of osteoporosis management after a fragility fracture in primary care. Arch. Osteoporos. 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Sochett, E.B.; Mäkitie, O. Osteoporosis in chronically ill children. Ann. Med. 2005, 37, 286–294. [Google Scholar] [CrossRef]
- Swain, M.; Kamper, S.J.; Maher, C.G.; Broderick, C.; McKay, D.; Henschke, N. Relationship between growth, maturation and musculoskeletal conditions in adolescents: A systematic review. Br. J. Sport. Med. 2018, 52, 1246–1252. [Google Scholar] [CrossRef]
- Bishop, N.; Arundel, P.; Clark, E.; Dimitri, P.; Farr, J.; Jones, G.; Makitie, O.; Munns, C.F.; Shaw, N. Fracture prediction and the definition of osteoporosis in children and adolescents: The ISCD 2013 Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 275–280. [Google Scholar] [CrossRef]
- Ward, L.M.; Weber, D.R.; Munns, C.F.; Högler, W.; Zemel, B.S. A Contemporary View of the Definition and Diagnosis of Osteoporosis in Children and Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, e2088–e2097. [Google Scholar] [CrossRef]
- Ward, L.M.; Ma, J.; Lang, B.; Ho, J.; Alos, N.; Matzinger, M.A.; Shenouda, N.; Lentle, B.; Jaremko, J.L.; Wilson, B. Bone Morbidity and Recovery in Children with Acute Lymphoblastic Leukemia: Results of a Six-Year Prospective Cohort Study. J. Bone Miner. Res. 2018, 33, 1435–1443. [Google Scholar] [CrossRef]
- Genant, H.K.; Wu, C.Y.; van Kuijk, C.; Nevitt, M.C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef]
- Ward, L.M.; Ma, J.; Robinson, M.-E.; Scharke, M.; Ho, J.; Houghton, K.; Huber, A.; Scuccimarri, R.; Barsalou, J.; Roth, J. Osteoporotic Fractures and Vertebral Body Reshaping in Children with Glucocorticoid-treated Rheumatic Disorders. J. Clin. Endocrinol. Metab. 2021, 106, e5195–e5207. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadiyannakis, S.; Olson, A.K. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, N.; Chapman, S.; Högler, W.; Hodgson, K.; Chapman, D.; Bebbington, N.; Shaw, N.J. Vertebral fractures assessment in children: Evaluation of DXA imaging versus conventional spine radiography. Bone 2017, 97, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Boyce, A.; Gordon, C.; Högler, W.; Kecskemethy, H.H.; Misra, M.; Swolin-Eide, D.; Tebben, P.; Ward, L.M.; Wasserman, H. The Utility of DXA Assessment at the Forearm, Proximal Femur, and Lateral Distal Femur, and Vertebral Fracture Assessment in the Pediatric Population: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 567–589. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.R.; Van Kuijk, C. Of small bones and big mistakes; bone densitometry in children revisited. Eur. J. Radiol. 2009, 71, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.C.; Berglund, L.M.; May, R.; Zemel, B.S.; I Grossberg, R.; Johnson, J.; Plotkin, H.; Stevenson, R.D.; Szalay, E.; Wong, B. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J. Bone Miner. Res. 2010, 25, 520–526. [Google Scholar] [CrossRef]
- Messina, C.; Lastella, G.; Sorce, S.; Piodi, L.P.; Rodari, G.; Giavoli, C.; Marchelli, D.; Guglielmi, G.; Ulivieri, F.M. Pediatric dual-energy X-ray absorptiometry in clinical practice: What the clinicians need to know. Eur. J. Radiol. 2018, 105, 153–161. [Google Scholar] [CrossRef]
- Schündeln, M.M.; Marschke, L.; Bauer, J.J.; Hauffa, P.K.; Schweiger, B.; Führer-Sakel, D.; Lahner, H.; Poeppel, T.D.; Kiewert, C.; Hauffa, B.P. A Piece of the Puzzle: The Bone Health Index of the BoneXpert Software Reflects Cortical Bone Mineral Density in Pediatric and Adolescent Patients. PLoS ONE 2016, 11, e0151936. [Google Scholar] [CrossRef]
- Leijten, A.D.; Hampsink, B.; Janssen, M.; Klein, W.M.; Draaisma, J.M.T. Can digital X-ray radiogrammetry be an alternative for dual-energy X-ray absorptiometry in the diagnosis of secondary low bone quality in children? Eur. J. Pediatr. 2019, 178, 1433–1441. [Google Scholar] [CrossRef]
- Mergler, S.; Rieken, R.; Tibboel, D.; Evenhuis, H.M.; van Rijn, R.R.; Penning, C. Lumbar spine and total-body dual-energy X-ray absorptiometry in children with severe neurological impairment and intellectual disability: A pilot study of artefacts and disrupting factors. Pediatr. Radiol. 2012, 42, 574–583. [Google Scholar] [CrossRef]
- Mergler, S.; de Man, S.A.; Boot, A.M.; Heus, K.G.C.B.B.-D.; Huijbers, W.A.R.; van Rijn, R.R.; Penning, C.; Evenhuis, H.M. Automated radiogrammetry is a feasible method for measuring bone quality and bone maturation in severely disabled children. Pediatr. Radiol. 2016, 46, 1017–1022. [Google Scholar] [CrossRef]
- Bachrach, L.K.; Gordon, C.M. Bone Densitometry in Children and Adolescents. Pediatrics 2016, 138, e20162398. [Google Scholar] [CrossRef] [PubMed]
- Shalof, H.; Dimitri, P.; Shuweihdi, F.; Offiah, A.C. Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis. Bone 2021, 150, 116013. [Google Scholar] [CrossRef] [PubMed]
- Digby, M.G.; Bishop, N.J.; Paggiosi, M.A.; Offiah, A.C. HR-pQCT: A non-invasive ‘biopsy’ to assess bone structure and strength. Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Saraff, V.; Högler, W. Endocrinology and Adolescence: Osteoporosis in children: Diagnosis and management. Eur. J. Endocrinol. 2015, 173, R185–R197. [Google Scholar] [CrossRef]
- Henderson, R.C.; Lark, R.K.; Gurka, M.J.; Worley, G.; Fung, E.B.; Conaway, M.; Stallings, V.A.; Stevenson, R.D. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 2002, 110, e5. [Google Scholar] [CrossRef]
- Mergler, S.; Evenhuis, H.M.; Boot, A.M.; De Man, S.A.; Heus, K.G.B.-D.; Huijbers, W.A.; Penning, C. Epidemiology of low bone mineral density and fractures in children with severe cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2009, 51, 773–778. [Google Scholar] [CrossRef]
- Larson, C.M.; Henderson, R.C. Bone Mineral Density and Fractures in Boys with Duchenne Muscular Dystrophy. J. Pediatr. Orthop. 2000, 20, 71–74. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Roper, H.; Shaw, N.J. Cessation of ambulation results in a dramatic loss of trabecular bone density in boys with Duchenne muscular dystrophy (DMD). Bone 2022, 154, 116248. [Google Scholar] [CrossRef]
- Joseph, S.; Wang, C.; Bushby, K.; Guglieri, M.; Horrocks, I.; Straub, V.; Ahmed, S.F.; Wong, S.C. Fractures and Linear Growth in a Nationwide Cohort of Boys with Duchenne Muscular Dystrophy with and Without Glucocorticoid Treatment: Results from the UK NorthStar Database. JAMA Neurol. 2019, 76, 701–709. [Google Scholar] [CrossRef]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif. Tissue Int. 2021, 108, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. Part I: Which Child with a Chronic Disease Needs Bone Health Monitoring? Curr. Osteoporos. Rep. 2021, 19, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J. Cytokines and Bone: Osteoimmunology. Handb. Exp. Pharmacol. 2020, 262, 177–230. [Google Scholar] [PubMed]
- Ward, L.M. Glucocorticoid-Induced Osteoporosis: Why Kids Are Different. Front. Endocrinol. 2020, 11, 576. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- van Staa, T.P.; Leufkens, H.G.; Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef]
- Weber, F.J.; Latshang, T.D.; Blum, M.R.; Kohler, M.; Wertli, M.M. Prognostic factors, disease course, and treatment efficacy in Duchenne muscular dystrophy: A systematic review and meta-analysis. Muscle Nerve 2022, 66, 462–470. [Google Scholar] [CrossRef]
- Singh, A.; Schaeffer, E.K.; Reilly, C.W. Vertebral Fractures in Duchenne Muscular Dystrophy Patients Managed With Deflazacort. J. Pediatr. Orthop. 2018, 38, 320–324. [Google Scholar] [CrossRef]
- Ward, L.M.; Konji, V.N.; Ma, J. The management of osteoporosis in children. Osteoporos. Int. 2016, 27, 2147–2179. [Google Scholar] [CrossRef]
- Wood, C.L.; Soucek, O.; Wong, S.C.; Zaman, F.; Farquharson, C.; Savendahl, L.; Ahmed, S.F. Animal models to explore the effects of glucocorticoids on skeletal growth and structure. J. Endocrinol. 2018, 236, R69–R91. [Google Scholar] [CrossRef]
- Ma, J.; Siminoski, K.; Alos, N.; Halton, J.; Ho, J.; Lentle, B.; Matzinger, M.A.; Shenouda, N.; Atkinson, S.; Barr, R. The choice of normative pediatric reference database changes spine bone mineral density Z-scores but not the relationship between bone mineral density and prevalent vertebral fractures. J. Clin. Endocrinol. Metab. 2015, 100, 1018–1027. [Google Scholar] [CrossRef]
- Rodd, C.; Lang, B.; Ramsay, T.; Alos, N.; Huber, A.M.; Cabral, D.A.; Scuccimarri, R.; Miettunen, P.M.; Roth, J.; Atkinson, S.A. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: A national observational study. Arthritis Care Res. 2012, 64, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.; Ma, J.; Fernandez, C.V.; Halton, J.; Alos, N.; Miettunen, P.M.; Jaremko, J.L.; Ho, J.; Shenouda, N.; Matzinger, M.A. Incident Vertebral Fractures in Children with Leukemia During the Four Years Following Diagnosis. J. Clin. Endocrinol. Metab. 2015, 100, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Berkvens, J.J.L.; Mergler, S.; Beerhorst, K.; Verschuure, P.; Tan, I.Y.; Majoie, H.J.M.; van den Bergh, J.P.W. Bone mineral density and fractures in institutionalised children with epilepsy and intellectual disability. J. Intellect. Disabil. Res. 2021, 65, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L.; Leonard, M.B.; Bechtold, S.; Högler, W.; Mughal, M.Z.; Schönau, E.; Sylvester, F.A.; Vogiatzi, M.; van den Heuvel-Eibrink, M.M.; Ward, L. Bone health in children and adolescents with chronic diseases that may affect the skeleton: The 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 281–294. [Google Scholar] [CrossRef]
- Ciancia, S.; Högler, W.; Sakkers, R.J.B.; Appelman-Dijkstra, N.M.; Boot, A.M.; Sas, T.C.J.; Renes, J.S. Osteoporosis in children and adolescents: How to treat and monitor? Eur. J. Pediatr. 2022, 182, 501–511. [Google Scholar] [CrossRef]
- Fernández, J.M.M.d.Z.; Arnal, I.R.; Segura, J.L.P.; Romero, R.G.; Martínez, G.R. Bone health impairment in patients with cerebral palsy. Arch. Osteoporos. 2020, 15, 91. [Google Scholar] [CrossRef]
- Srivastava, A.; Saini, N.; Mathias, A.; Arya, A.; Jain, S.; Yachha, S.K. Prevalence and predictive factors of undernutrition and low bone mineral density in children with chronic pancreatitis. Pancreatology 2021, 21, 74–80. [Google Scholar] [CrossRef]
- Castano, L.; Madariaga, L.; Grau, G.; García-Castaño, A. 25(OH)Vitamin D Deficiency and Calcifediol Treatment in Pediatrics. Nutrients 2022, 14, 1854. [Google Scholar] [CrossRef]
- Fehlings, D.; Switzer, L.; Agarwal, P.; Wong, C.; Sochett, E.; Stevenson, R.; Sonnenberg, L.; Smile, S.; Young, E.; Huber, J. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: A systematic review. Dev. Med. Child Neurol. 2012, 54, 106–116. [Google Scholar] [CrossRef]
- Bianchi, M.L.; Morandi, L.; Andreucci, E.; Vai, S.; Frasunkiewicz, J.; Cottafava, R. Low bone density and bone metabolism alterations in Duchenne muscular dystrophy: Response to calcium and vitamin D treatment. Osteoporos. Int. 2011, 22, 529–539. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D; Calcium. The National Academies Collection: Reports Funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Winzenberg, T.; Shaw, K.; Fryer, J.; Jones, G. Effects of calcium supplementation on bone density in healthy children: Meta-analysis of randomised controlled trials. BMJ 2006, 333, 775. [Google Scholar] [CrossRef]
- Tan, V.P.; Macdonald, H.M.; Kim, S.; Nettlefold, L.; Gabel, L.; Ashe, M.C.; McKay, H.A. Influence of physical activity on bone strength in children and adolescents: A systematic review and narrative synthesis. J. Bone Miner. Res. 2014, 29, 2161–2181. [Google Scholar] [CrossRef]
- Ozel, S.; Switzer, L.; Macintosh, A.; Fehlings, D. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: An update. Dev. Med. Child Neurol. 2016, 58, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Hough, J.P.; Boyd, R.N.; Keating, J.L. Systematic review of interventions for low bone mineral density in children with cerebral palsy. Pediatrics 2010, 125, e670–e678. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Alsop, C.; Caulton, J.; Rubin, C.; Adams, J.; Mughal, Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J. Bone Miner. Res. 2004, 19, 360–369. [Google Scholar] [CrossRef]
- Julián-Almárcegui, C.; Gómez-Cabello, A.; Huybrechts, I.; González-Agüero, A.; Kaufman, J.M.; Casajús, J.A.; Vicente-Rodríguez, G. Combined effects of interaction between physical activity and nutrition on bone health in children and adolescents: A systematic review. Nutr. Rev. 2015, 73, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Catto-Smith, A.G.A.; Zacharin, M. Pathological fractures in paediatric patients with inflammatory bowel disease. Eur. J. Pediatr. 2014, 173, 141–151. [Google Scholar] [CrossRef]
- Ward, L.M. Part 2: When Should Bisphosphonates Be Used in Children with Chronic Illness Osteoporosis? Curr. Osteoporos. Rep. 2021, 19, 289–297. [Google Scholar] [CrossRef]
- Nasomyont, N.; Hornung, L.N.; Gordon, C.M.; Wasserman, H. Outcomes following intravenous bisphosphonate infusion in pediatric patients: A 7-year retrospective chart review. Bone 2019, 121, 60–67. [Google Scholar] [CrossRef]
- Morris, H.F. Veterans Administration Cooperative Studies Project No. 147. Part VI: Laboratory costs of castings from noble and alternative ceramic metal alloys. J. Prosthet. Dent. 1988, 60, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Soto, T.; Pacaud, D.; Stephure, D.; Trussell, R.; Huang, C. Treatment of symptomatic osteoporosis in children: A comparison of two pamidronate dosage regimens. J. Pediatr. Endocrinol. Metab. 2011, 24, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Bhansali, A.; Mohanty, S.K.; Khandelwal, N.; Mathur, S.K.; Dash, R.J. Hypophosphatemic rickets and osteomalacia in polyostotic fibrous dysplasia. J. Pediatr. Endocrinol. Metab. 2003, 16, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Steelman, J.; Zeitler, P. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J. Pediatr. 2003, 142, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Saraff, V.; Sahota, J.; Crabtree, N.; Sakka, S.; Shaw, N.J.; Högler, W. Efficacy and treatment costs of zoledronate versus pamidronate in paediatric osteoporosis. Arch. Dis. Child. 2018, 103, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Choudhury, A.; Alos, N.; Cabral, D.A.; Rodd, C.; Sbrocchi, A.M.; Taback, S.; Padidela, R.; Shaw, N.J.; Hosszu, E. Zoledronic Acid vs Placebo in Pediatric Glucocorticoid-induced Osteoporosis: A Randomized, Double-blind, Phase 3 Trial. J. Clin. Endocrinol. Metab. 2021, 106, e5222–e5235. [Google Scholar] [CrossRef]
- Draaisma, J.M.; Hampsink, B.M.; Janssen, M.; van Houdt, N.B.; Linders, E.T.; Willemsen, M.A. The Ketogenic Diet and Its Effect on Bone Mineral Density: A Retrospective Observational Cohort Study. Neuropediatrics 2019, 50, 353–358. [Google Scholar] [CrossRef]
- Robinson, M.-E.; Trejo, P.; Palomo, T.; Glorieux, F.H.; Rauch, F. Osteogenesis Imperfecta: Skeletal Outcomes After Bisphosphonate Discontinuation at Final Height. J. Bone Miner. Res. 2019, 34, 2198–2204. [Google Scholar] [CrossRef]
- Rauch, F.; Munns, C.; Land, C.; Glorieux, F.H. Pamidronate in children and adolescents with osteogenesis imperfecta: Effect of treatment discontinuation. J. Clin. Endocrinol. Metab. 2006, 91, 1268–1274. [Google Scholar] [CrossRef]
- Rauch, F.; Cornibert, S.; Cheung, M.; Glorieux, F.H. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone 2007, 40, 821–827. [Google Scholar] [CrossRef]
- Harcke, H.T.; Stevenson, K.L.; Kecskemethy, H.H.; Bachrach, S.J.; Grissom, L.E. Fracture after bisphosphonate treatment in children with cerebral palsy: The role of stress risers. Pediatr. Radiol. 2012, 42, 76–81. [Google Scholar] [CrossRef]
- Simm, P.J.; Biggin, A.; Zacharin, M.R.; Rodda, C.P.; Tham, E.; Siafarikas, A.; Jefferies, C.; Hofman, P.L.; Jensen, D.E.; Woodhead, H. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J. Paediatr. Child Heal. 2018, 54, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Nakken, H.; Vlaskamp, C. A Need for a Taxonomy for Profound Intellectual and Multiple Disabilities. J. Policy Pr. Intellect. Disabil. 2007, 4, 83–87. [Google Scholar] [CrossRef]
- Presedo, A.; Dabney, K.W.; Miller, F. Fractures in patients with cerebral palsy. J. Pediatr. Orthop. 2007, 27, 147–153. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Conaway, M.; Barrington, J.W.; Cuthill, S.L.; Worley, G.; Henderson, R.C. Fracture rate in children with cerebral palsy. Pediatr. Rehabilitation 2006, 9, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Honzawa, S.; Kaga, M.; Iwasaki, Y.; Masuyama, T. Osteoporosis pathology in people with severe motor and intellectual disability. Brain Dev. 2020, 42, 256–263. [Google Scholar] [CrossRef]
- Selvin, S.T.; Thomas, S.; Bikeyeva, V.; Abdullah, A.; Radivojevic, A.; Jad, A.A.A.; Ravanavena, A.; Ravindra, C.; Igweonu-Nwakile, E.; Ali, S. Establishing the Association Between Osteoporosis and Peptic Ulcer Disease: A Systematic Review. Cureus 2022, 14, e27188. [Google Scholar]
- Arvio, M.; Kilpinen-Loisa, P.; Tiitinen, A.; Huovinen, K.; Mäkitie, O. Bone mineral density and sex hormone status in intellectually disabled women on progestin-induced amenorrhea. Acta Obstet. Gynecol. Scand. 2009, 88, 428–433. [Google Scholar] [CrossRef]
- Henderson, R.C.; Kairalla, J.; Abbas, A.; Stevenson, R.D. Predicting low bone density in children and young adults with quadriplegic cerebral palsy. Dev. Med. Child Neurol. 2004, 46, 416–419. [Google Scholar] [CrossRef]
- Thodberg, H.H.; van Rijn, R.R.; Tanaka, T.; Martin, D.D.; Kreiborg, S. A paediatric bone index derived by automated radiogrammetry. Osteoporos. Int. 2010, 21, 1391–1400. [Google Scholar] [CrossRef]
- Petty, S.J.; Paton, L.M.; O’Brien, T.; Makovey, J.; Erbas, B.; Sambrook, P.; Berkovic, S.F.; Wark, D.W. Effect of antiepileptic medication on bone mineral measures. Neurology 2005, 65, 1358–1365. [Google Scholar] [CrossRef]
- Beerhorst, K.; Schouwenaars, F.M.; Tan, I.Y.; Aldenkamp, A.P. Epilepsy: Fractures and the role of cumulative antiepileptic drug load. Acta Neurol. Scand. 2012, 125, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Caulton, J.M.; Ward, K.; Alsop, C.W.; Dunn, G.; E Adams, J.; Mughal, M.Z. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch. Dis. Child. 2004, 89, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Divieti Pajevic, P.; Bonewald, L.F.; Bauman, W.A. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [PubMed]
- Chou, E.; Lindeback, R.; D’Silva, A.M.; Sampaio, H.; Neville, K.; Farrar, M.A. Growth and nutrition in pediatric neuromuscular disorders. Clin. Nutr. 2021, 40, 4341–4348. [Google Scholar] [CrossRef]
- Kooi-van Es, M.; Erasmus, C.E.; de Swart, B.J.M.; Voet, N.B.M.; van der Wees, P.J.; de Groot, I.J.M.; van den Engel-Hoek, L. Dysphagia and Dysarthria in Children with Neuromuscular Diseases, a Prevalence Study. J. Neuromuscul Dis. 2020, 7, 287–295. [Google Scholar] [CrossRef]
- Bian, Q.; McAdam, L.; Grynpas, M.; Mitchell, J.; Harrington, J. Increased Rates of Vitamin D Insufficiency in Boys With Duchenne Muscular Dystrophy Despite Higher Vitamin D(3) Supplementation. Glob. Pediatr. Heal. 2019, 6, 2333794x19835661. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Ward, L.M.; Hadjiyannakis, S.; McMillan, H.J.; Noritz, G.; Weber, D.R. Bone Health and Osteoporosis Management of the Patient with Duchenne Muscular Dystrophy. Pediatrics 2018, 142 (Suppl. S2), S34–S42. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Carroll, K.; McGinley, J.L.; Paterson, K.L. Walking and weakness in children: A narrative review of gait and functional ambulation in paediatric neuromuscular disease. J. Foot Ankle Res. 2020, 13, 10. [Google Scholar] [CrossRef]
- Wiles, C.M.; E Busse, M.; Sampson, C.M.; Rogers, M.T.; Fenton-May, J.; van Deursen, R. Falls and stumbles in myotonic dystrophy. J. Neurol. Neurosurg. Psychiatry 2006, 77, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Horlings, C.G.; Küng, U.M.; van Engelen, B.G.; Voermans, N.C.; Hengstman, G.J.; van der Kooi, A.J.; Bloed, B.R.; Allum, J.H.J. Balance control in patients with distal versus proximal muscle weakness. Neuroscience 2009, 164, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Dowling, J.J.; North, K.; Schroth, M.K.; Sejersen, T.; Shapiro, F.; Bellini, J.; Weiss, H.; Guillet, M.; Amburgery, K. Consensus statement on standard of care for congenital myopathies. J. Child Neurol. 2012, 27, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Paoletta, M.; Liguori, S.; Curci, C.; Moretti, A. Neuromuscular Diseases and Bone. Front. Endocrinol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Glerup, H.; Steffensen, B.F.; Rejnmark, L.; Rahbek, J.; Moseklide, L. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J. Rehabil. Med. 2001, 33, 150–155. [Google Scholar] [CrossRef]
- Joya, J.E.; Kee, A.J.; Nair-Shalliker, V.; Ghoddusi, M.; Nguyen, M.-A.T.; Luther, P.; Hardeman, E.C. Muscle weakness in a mouse model of nemaline myopathy can be reversed with exercise and reveals a novel myofiber repair mechanism. Hum. Mol. Genet. 2004, 13, 2633–2645. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.D. Extremity fractures in children with neuromuscular disease. Johns Hopkins Med. J. 1979, 145, 89–93. [Google Scholar]
- Vialle, R.; Thévenin-Lemoine, C.; Mary, P. Neuromuscular scoliosis. Orthop. Traumatol. Surg. Res. 2013, 99 (Suppl. S1), S124–S139. [Google Scholar] [CrossRef]
- Strømsøe, K. Fracture fixation problems in osteoporosis. Injury 2004, 35, 107–113. [Google Scholar] [CrossRef]
- Pianucci, L.; Sonagra, M.; Greenberg, B.A.; Priestley, D.R.; Gmuca, S. Disordered eating among adolescents with chronic pain: The experience of a pediatric rheumatology subspecialty pain clinic. Pediatr. Rheumatol. Online J. 2021, 19, 16. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Bergqvist, A.G.C.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Bergqvist, A.C.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Simm, P.J.; Bicknell-Royle, J.; Lawrie, J.; Nation, J.; Draffin, K.; Stewart, K.G.; Cameron, F.J.; Scheffer, I.E.; Mackay, M.T. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 2017, 136, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Groesbeck, D.K.; Bluml, R.M.; Kossoff, E.H. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev. Med. Child Neurol. 2006, 48, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Roy, S.M.; Dekelbab, B.; Frazier, B.; Grover, M.; Haidet, J.; Listman, J.; Maden, S.; Roan, M.; Rodd, C. Hypercalcemia in Children Using the Ketogenic Diet: A Multicenter Study. J. Clin. Endocrinol. Metab. 2021, 106, e485–e495. [Google Scholar] [CrossRef] [PubMed]
- Guglieri, M.; Clemens, P.R.; Perlman, S.J.; Smith, E.C.; Horrocks, I.; Finkel, R.S.; Mah, J.K.; Deconink, N.; Goemans, N.; Haberlova, J. Efficacy and Safety of Vamorolone vs Placebo and Prednisone Among Boys with Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1005–1014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).