Abstract
This work is a bibliographic review. The search for the necessary information was carried out in the months of November 2022 and January 2023. The databases used were as follows: Pubmed, Academic Google, Scielo, Scopus, and Cochrane library. Results: In total, 101 articles were selected after a review of 486 articles from databases and after applying the inclusion and exclusion criteria. The update on the molecular mechanism of human coronavirus (HCoV) infection was reviewed, describing possible therapeutic targets in the viral response phase. There are different strategies to prevent or hinder the introduction of the viral particle, as well as the replicative mechanism ((protease inhibitors and RNA-dependent RNA polymerase (RdRp)). The second phase of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) involves the activation of hyperinflammatory cascades of the host’s immune system. It is concluded that there are potential therapeutic targets and drugs under study in different proinflammatory pathways such as hydroxychloroquine, JAK inhibitors, interleukin 1 and 6 inhibitors, and interferons.
1. Introduction
The human coronavirus (HCoV) family can cause infections in humans, being a zoonotic disease, as it is transmitted from animals (birds and mammals) to humans [1]. The symptoms shown by patients affected by HCoV vary between processes that resemble the common cold to severe conditions such as those described with severe acute respiratory syndrome coronavirus 1 [2] (SARS-CoV-1) and Middle East respiratory syndrome coronavirus virus (MERS-CoV) [3].
There are drugs with indications in other pathologies that are being tested in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection; of particular interest are those that have been shown to be effective in other coronaviruses such as SARS-CoV-1 and MERS-CoV. Two main phases have been differentiated: a viral response and a hyperinflammatory response. This article reviews different strategies to prevent or hinder the introduction of the viral particle, as well as the replicative mechanism ((protease inhibitors (PI), inhibitors of RNA-dependent RNA polymerase (RdRp) (RNA-dependent RNA polymerase), and inhibitors of intracellular transport of viral structures) [1,2,3].
2. Materials and Methods
The preparation of this work was carried out through a systematic bibliographic review of the articles found by searching the following databases: Medline/Pubmed, WOS, Scielo, Scopus, and Google Scholar. To find the best possible scientific evidence, a series of inclusion and exclusion criteria were applied.
The keywords for this review are as follows: SARS-CoV-2, COVID-19, ACE2, protease inhibitors, RdRp inhibitors, JAK inhibitors, interleukin 1 inhibitors, interleukin 6 inhibitors, and interferon. To carry out the bibliographic search, different keywords in English were used: “SARS-CoV-2”, “COVID-19”, “ACE2”, “protease inhibitors”, “RdRp inhibitors”, “JAK inhibitors”, “interleukin 1 inhibitors”, “interleukin 6 inhibitors”, and “interferon”. These have been validated by the DeCS and MeSH. Once selected, the corresponding Boolean operators, AND/OR, were used, as well as the necessary parentheses and quotation marks. The final search string is as follows: (SARS-CoV-2) OR (COVID-19) AND (ACE2) AND (protease inhibitors) AND (RdRp inhibitors) AND (JAK inhibitors) AND (interleukin 1 inhibitors) OR (interleukin 6 inhibitors) AND (interferon). The criteria that were taken into account for the selection of the relevant studies were the following. Inclusion criteria: the period between 2010 and 2023; article type: article review and article research; field: medicine; English language; sample in adult population; and studies that provide scientific evidence justified by the level of indexing of articles in journals according to the latest certainties. Exclusion criteria: articles prior to 2010; language: not English; studies in which the population was minors; and studies that do not provide scientific evidence justified by the level of indexing of articles in journals according to the latest certainties.
For the methodological evaluation of the individual studies and the detection of possible biases, the evaluation was carried out using the PEDro Evaluation Scale. This scale consists of 11 items, providing one point for each element that is fulfilled. The articles that obtained a score of 9–10 points have an excellent quality, those between 6 and 8 points have a good quality, those that obtained 4–5 points have an intermediate quality, and finally those articles that obtained less than 4 points have a poor methodological quality.
The Scottish Intercollegiate Guidelines Network classification was used in the data analysis and assessment of the levels of evidence, which focused on the quantitative analysis of systematic reviews and the reduction in systematic error. Although it took into account the quality of the methodology, it did not assess the scientific or technological reality of the recommendations.
3. Fundamental Structural Proteins
The HCoV genome encodes four fundamental structural proteins [4], which are necessary to form the viral particle:
The spike (S): responsible for the union of HCoV with host cell receptors, facilitating viral entry [5,6,7]. The union of the virus with angiotensin converting enzyme type II (ACE2) constitutes the point of entry to infect human cells, with this union being primed by the transmembrane protease, serine 2 (TMPRSS2) [8,9].
The nucleocapsid (N): binds to the ribonucleic acid (RNA) genome of HCoV, forming the nucleocapsid [10].
Membrane protein (M): the most abundant structural protein that interacts with other structural proteins for the assembly of the viral envelope [11].
The envelope (E): during replication, it is abundantly expressed in the endoplasmic reticulum, Golgi apparatus, and the endoplasmic reticulum–Golgi intermediate compartment (ERGI), although it is only expressed in small amounts in the envelope of the virion [12,13]. In the absence of protein E, reduced titers of viral particles are observed and viral maturation is prevented with incompetence for propagation. This has been observed in vitro with recombinant HCoVs lacking protein S [14,15,16,17,18].
Any of these proteins can be the basis of future vaccines against SARS-CoV-2. In the race to manufacture the anti-SARS-CoV-2 vaccine [19], there are more than 125 anti-SARS-CoV-2 vaccine candidates [20].
4. Envelope Protein
The envelope protein E is a short transmembrane protein of 76–109 amino acids (8.4–12 kDa) [21,22,23], consisting of a short amino-terminal (7–12 aa) hydrophilic end; followed by a transmembrane domain (25 aa) hydrophobic, where there is an amphipathic α-helix with properties of forming an ion conducting pore [24,25,26]; and a long carboxy-terminal end [27,28,29,30]. It can establish homotypic interactions, forming transmembrane-domain-dependent homo-oligomeric multimers [31] and generating a known ion channel protein known as viroporin [32,33].
Although many HCoVs encode two proteins that, when homooligomerized, can form viroporins, SARS-CoV1 encodes three proteins: 3a, E, and 8a. Proteins 3a and E contain a PDZ-binding motif (PBM), which can bind to more than 400 cellular proteins that contain a PDZ domain [34,35].
5. Biochemical–Molecular Mechanism of HCoV Infection
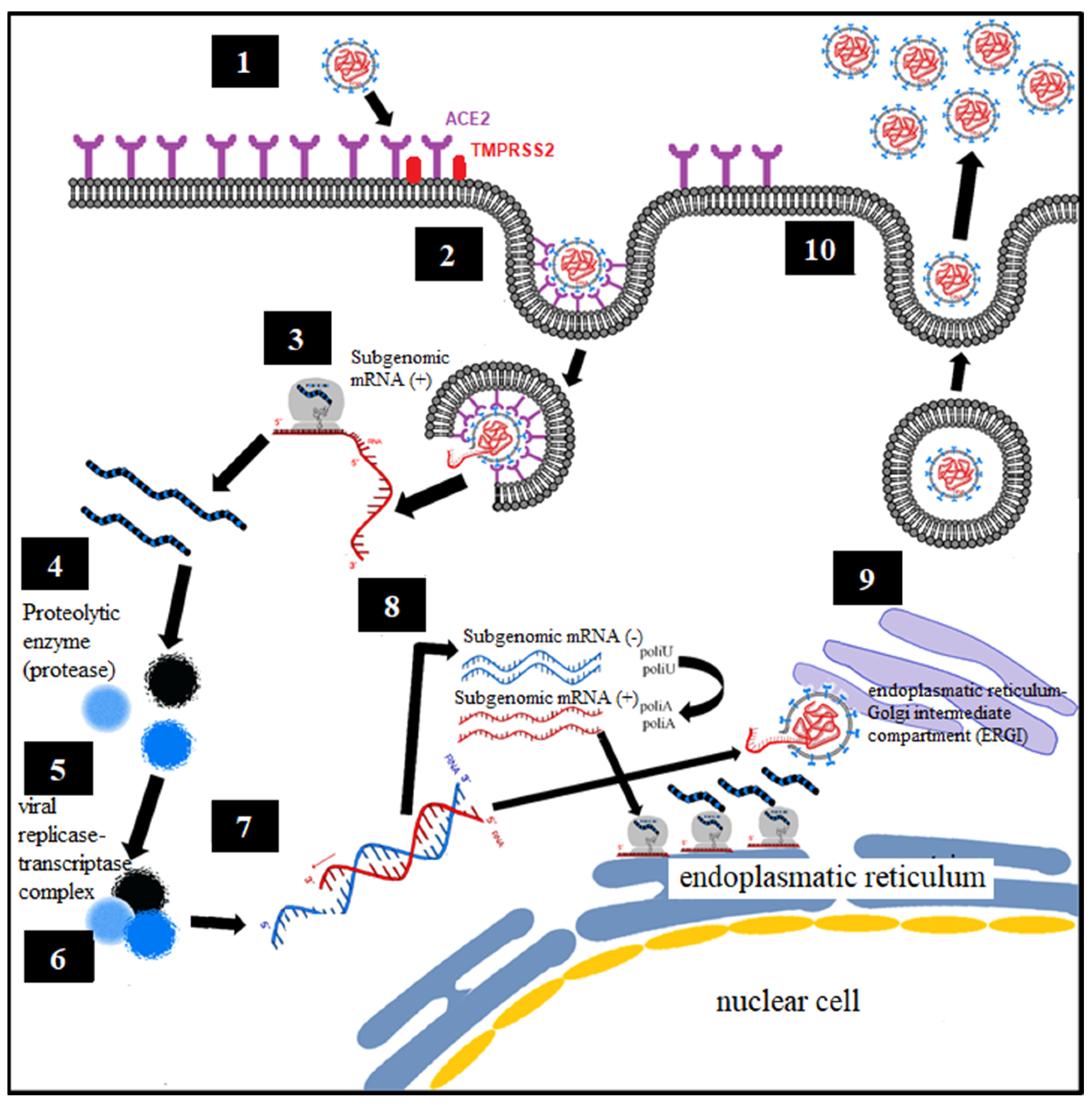
Analogous to what was known about the virology of SARS-CoV-1 and MERS, the proposed mechanism is defined [36,37] (Figure 1):
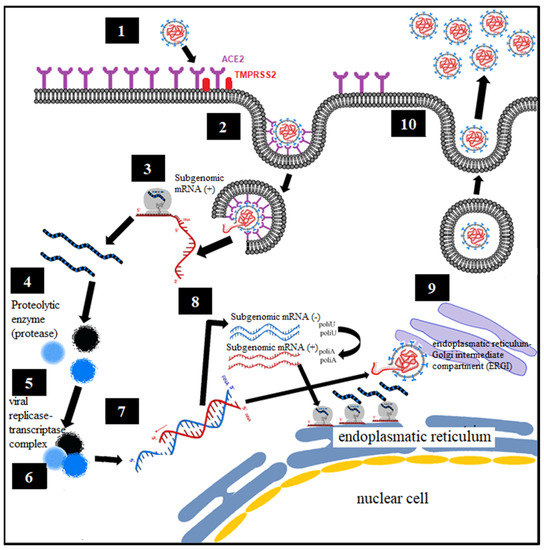
Figure 1.
Virological mechanism of SARS-CoV-1 and MERS-CoV.
- Binding of the spike protein (S) virus of SARS-CoV-2 with the angiotensin converting enzyme type II (ACE2), constituting the point of entry to infect human cells, with the said union being primed by the transmembrane protease, serine 2 (TMPRSS2) [8,9].
- Endocytosis of viral particles.
- Early translation of the positive ribonucleic acid (RNA) of SARS-CoV-2 as if it were host cell mRNA with the synthesis of early (regulatory) proteins, including polyproteins and essential viral proteases.
- Proteolysis through a protease. The polyproteins (pp1a and pp1ab) are cleaved into 16 nonstructural effector proteins by 3CLpro and PLpro.
- Formation of the replication complex together with the RNA-dependent RNA polymerase (RdRp).
- Synthesis of negative single-stranded RNA from the positive single-stranded RNA template by RNA polymerase, with formation of the replicative complex. The negative single-stranded RNA is not released, remaining associated with the replicative complex.
- The replicative complex produces synthesis of positive single-stranded RNA, mRNA, and negative single-stranded RNA.
- Late translation of positive single-stranded RNA and mRNA, with late (structural) protein synthesis on the ribosomes of the rough endoplasmic reticulum.
- Formation of viral particles with assembly in the ERGI intermediate compartment (endoplasmic reticulum–Golgi apparatus).
- Release of viral particles by exocytosis.
6. Etiopathogenic Phases of COVID-19
According to epidemiological data from the World Health Organization, the first variants of SARS-CoV-2 are as follows: Alpha (B.1.1.7) (United Kingdom, 12/2020), Beta (B.1.351) (South Africa 12/2020), Gamma (P.1) (Brazil, 01/2021), Delta (B.1.617.2) (12/2020), and Ómicron (B.1.1.529) (South Africa, 11/2021) [38].
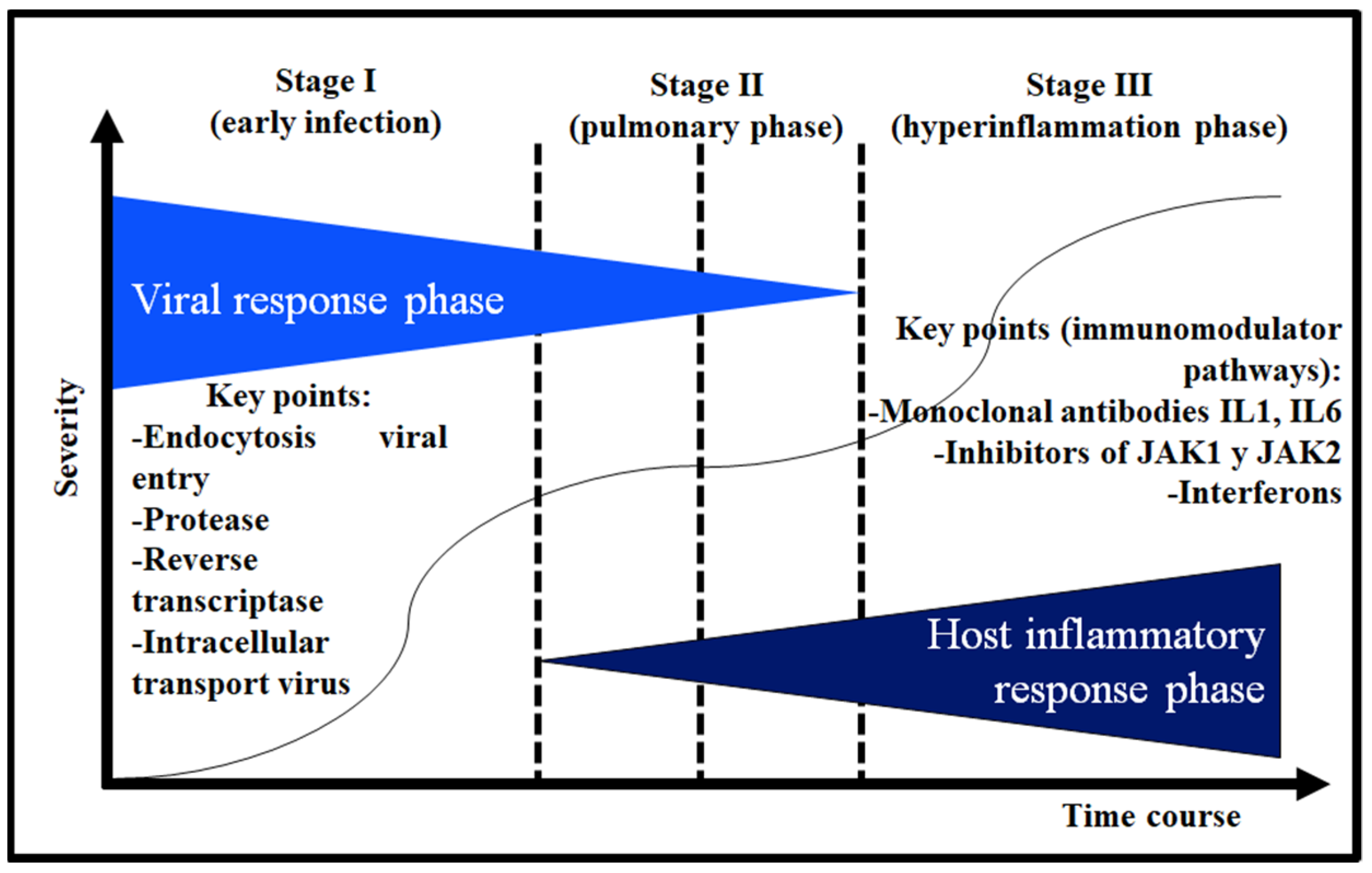
In COVID-19, an etiopathogenic model has been proposed that divides it into a response phase against SARS-CoV-2 and a host inflammatory response phase where an inflammatory cascade occurs [39]. Figure 2 shows the three stages: (I) early infection (which corresponds to the viral response phase), (II) or pulmonary phase (where the two response phases overlap), and (III) or hyperinflammatory phase. Depending on the phase in which the patient is, he will have a different therapeutic approach.
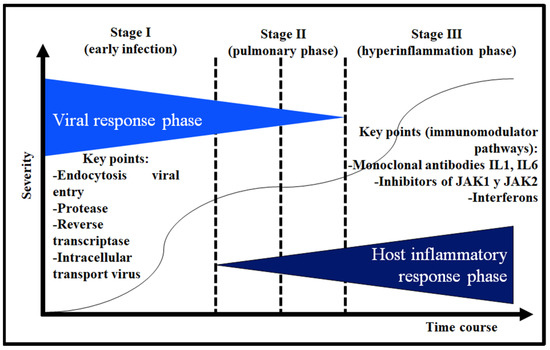
Figure 2.
Differentiated phases of viral and proinflammatory response (interleukin 1: IL 1; interleukin 6: IL-6; Janus kinase: JAK).
In a didactic way, in the viral response phase, a subdivision could be made into the following: (a) entry of the viral particle, (b) proteolysis, (c) SARS-CoV-2 RNA replication by RNA-dependent RNA polymerase, and (d) intracellular transport of viral structures (Figure 2) [38,39].
7. Physiopathology of Edema in Pulmonary Alveoli and Possible Therapeutic Targets
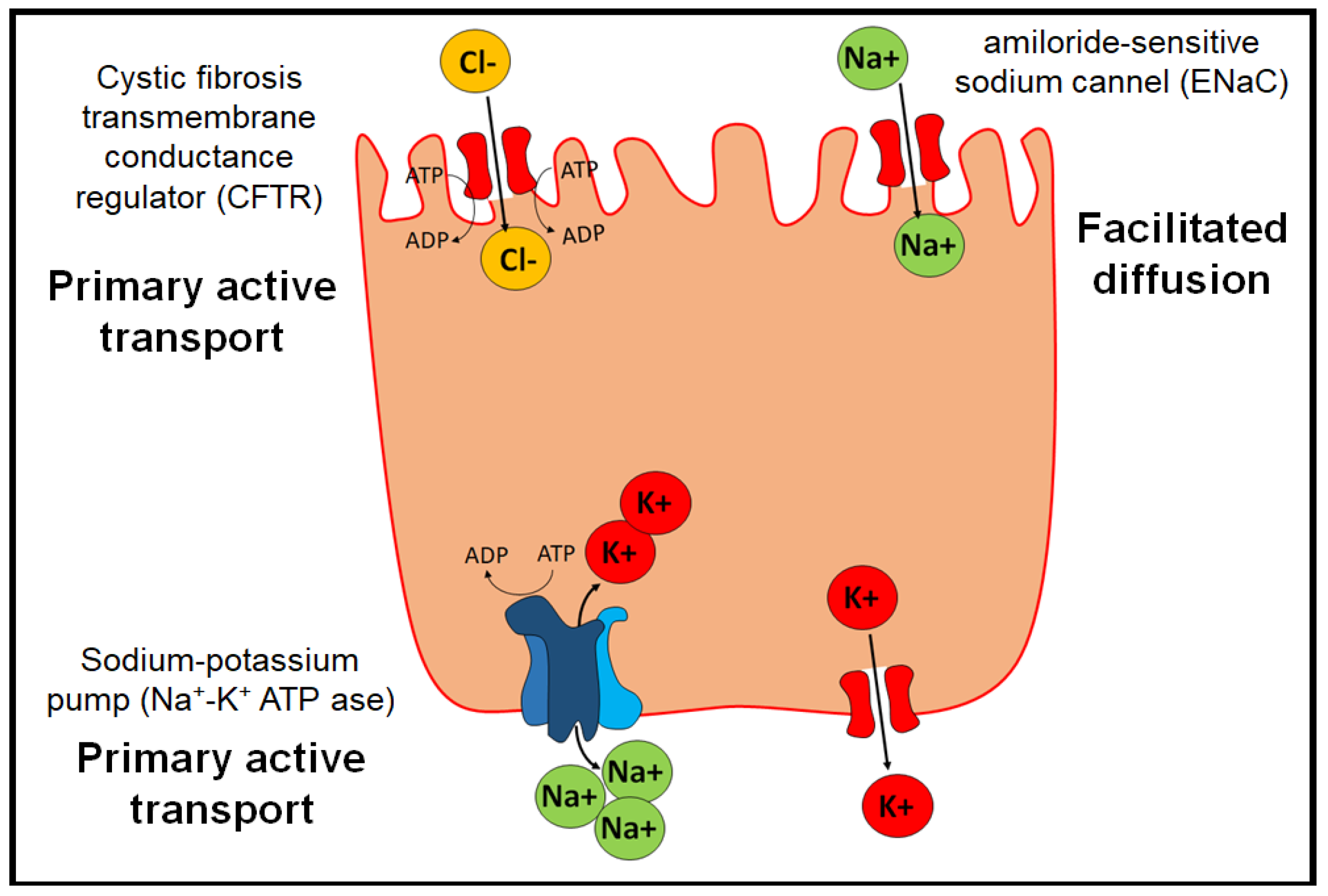
Under physiological conditions, edema in the pulmonary alveoli is resolved by the action of three proteins (Figure 3) [40]:
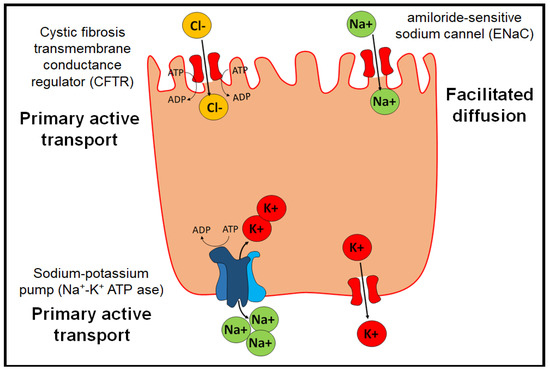
Figure 3.
Physiological transporters that prevent edema in pulmonary alveoli.
- The Na+/K+ ATP-ase pump, which allows two K+ ions to enter intracellularly and three Na+ ions to exit the cell by active transport.
- The epithelial channel of Na+ ions sensitive to amiloride (ENaC) (amiloride-sensitive sodium channel), which allows the transport of Na+ by facilitated diffusion. They are distributed in organs such as the lung, large intestine, kidney, vascular endothelium, and placenta.
- Cystic fibrosis transmembrane conductance regulator (CFTR), which belongs to the ABC transporters and exerts its function through primary active transport.
Given the current SARS-CoV-2 epidemic, a possible therapeutic target for the pathology caused by HCoV could be the identification of drugs that interrupt the PBM–PDZ junctions, as these pathways would be involved in the pathophysiology of the infection. Protein E may be the origin of the future vaccine. Viroporins are viral proteins with ion channel activity that play important roles in various processes, including virus replication and pathogenesis.
8. Therapy in the Viral Response Phase
8.1. Inhibitors of Viral Particle Entry
8.1.1. TMPRSS2 Inhibitors
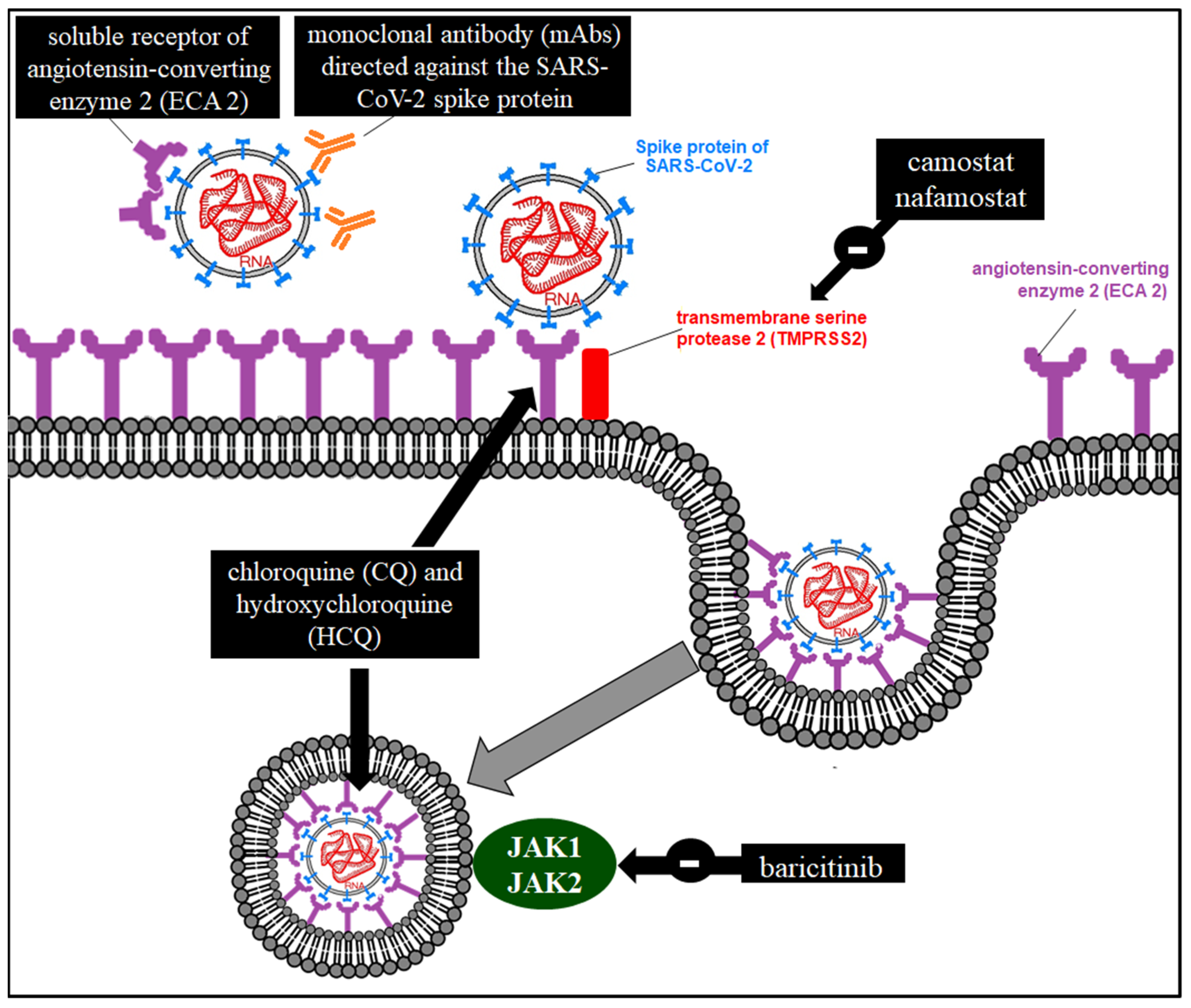
The SARS-CoV-2 spike (S) protein binds to ACE2, which is the entry point for infecting human cells, requiring TMPRSS2 [8,9]. The entry of SARS-CoV-2 into the cell could be blocked by both protein S neutralizing antibodies and TMPRSS2 inhibitors. Among the latter are camostat mesylate [41] (used as a treatment for chronic pancreatitis [42]) and nafamostat [43].
8.1.2. Arbidol
Umifenovir (Arbidol®) binds to the hemagglutinin of the influenza virus [44] and its inhibitory power has been demonstrated in the Zika Virus [45]. Three-dimensional analysis of molecular structure using HADDOCK2.2 (https://haddock.science.uu.nl/ (accessed on 26 March 2023)) and SwissDock (http://swissdock.ch/docking (accessed on 26 March 2023)) servers shows binding of arbidol to trimers of the glycoprotein S of SARS-CoV-2 [46]. The usefulness of this drug has been proven as it interferes with and inhibits membrane fusion with the viral envelope [47].
8.1.3. Antimalarials
In addition to the immunomodulatory effect of the inflammatory cascade that we will see later, antimalarials (hydroxychloroquine (HCQ) and chloroquine (CQ)) have a direct antiviral effect by interfering with the binding of the viral particle to ACE2 (altering the glycosylation of the receptor) [48] or with endocytosis (by increasing the pH of these organelles) [49].
8.1.4. Janus-Associated Kinase (JAK) Inhibitors
Baricitinib (Olumiant®) is a JAK inhibitor used in rheumatoid arthritis [50], which could inhibit endocytosis using a three-dimensional virtual model [51]. In addition, it participates in the immunomodulation of the inflammatory cascade [52,53].
8.1.5. Oseltamivir
Oseltamivir (Tamiflu®) binds to the neuraminidase of the influenza virus [54], which could be useful in patients with SARS-CoV-2 co-infection, although this virus does not require neuraminidase for cell entry.
8.1.6. Monoclonal Antibodies (MAbs) Directed against a Viral Coat Protein
Similarly to palivizumab against respiratory syncytial virus (RSV), the design of a monoclonal antibody (AbMo) directed against SARS-CoV-2 [55,56] could be a therapeutic option in the future.
8.1.7. ACE2 Soluble Receptor
It is based on the design of a recombinant protein similar to ACE2 that contains only the sequence of amino acids to which SARS-CoV-2 binds [57]. The virus would compete for binding to this protein and to ACE.
A scheme of the possible pharmacological mechanisms of action in the interference of SARS-CoV-2 entry is presented in Figure 4.
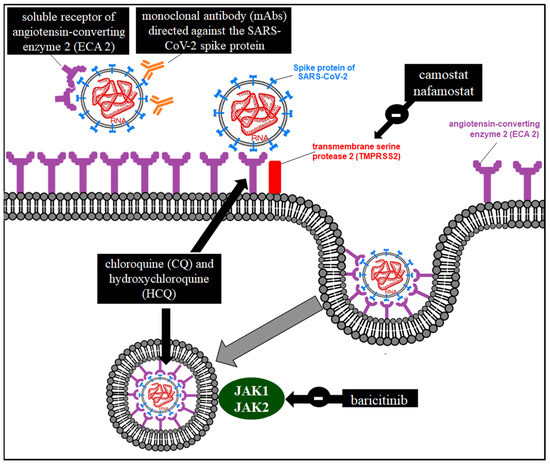
Figure 4.
Possible pharmacological mechanisms of action in the interference of SARS-CoV-2 entry.
8.2. RdRp Inhibitors [58]
Its mechanism of action is by inhibition of RdRp, with some drugs being represented in Figure 4.
Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazinecarboxamide, Avigan®) is a purine nucleotide prodrug (favipiravir ribofuranosyl-5-triphosphate) that inhibits the RdRp of influenza viruses [59,60], Ebola virus, hemorrhagic fever [61], and HCoV. Remdesivir (RDV) is a prodrug of a nucleotide analog (adenosine C nucleoside) developed as a treatment for Ebola virus infection [62] that showed inhibitory power against SARS-CoV and MERS-CoV in vitro [63]. It has been studied in monotherapy [64] and associated with chloroquine [65] in COVID-19. Ribavirin is a guanosine analog used for hepatitis C virus (HCV), which has been used in combination with lopinavir/ritonavir or interferon (IFN) in SARS-CoV1 and MERS [66]. Sofosbuvir is a pharmacologically active uridine nucleotide triphosphate prodrug (GS-461203) that acts as a pan-genotypic inhibitor of HCV RdRp NS5B, indicated for chronic hepatitis in adults. Ledipasvir targets the nonstructural HCV phosphoprotein NS5A, essential for RNA replication and virion assembly. The combination of sofosbuvir with ledipasvir (Harvoni®) or velpatasvir can inhibit both RdRp and PI [67] of SARS-CoV-2. Galidesivir and tenofovir have been shown in molecular studies to inhibit RdRp [68].
8.3. Protease Inhibitors (PIs)
Its mechanism of action is by inhibition of the proteases represented by some drugs in Figure 1.
Velpatasvir is a pan-genotypic inhibitor of the HCV NS3/4A protease described in the previous section [67], while lopinavir is an inhibitor of the protein, similar to 3-chymotrypsin as the protease of the human immunodeficiency virus (HIV), reducing the maturation of viral particles. It is marketed together with ritonavir (Kaletra®), which inhibits the metabolism of lopinavir [69]. It has been used in COVID-19 [70]. Darunavir also acts in a similar way to lopinavir [69,70].
8.4. Inhibitors of Intracellular Transport of Viral Structures
The importin protein is formed by a heterodimer of two subunits (alpha and beta-1) (IMPα/β1) [71] participating in the nuclear transport models of SARS-CoV-2. Studies on its efficacy and safety in COVID-19 are needed. Ivermectin demonstrated inhibition of nuclear transport; either from the non-structural protein 3 (NS3) of flavivirus [72], NS5 of the dengue virus [73], or of the MxA factor of the influenza A virus [74]. In vitro inhibition of SARS-CoV-2 replication has been demonstrated [75].
10. Therapy in the Hyperinflammatory Response Phase with Immunomodulators of the Immune Inflammatory Cascade
10.1. Glucocorticosteroids
Glucocorticosteroids (GCs) regulate the expression of anti-inflammatory proteins in the nucleus (transactivation) and repress the expression of proinflammatory proteins (tranrepression), exerting a potent anti-inflammatory effect [76,77].
10.2. Antimalarials
Antimalarials (hydroxychloroquine (HCQ) and chloroquine (CQ)) have an immunomodulatory effect by increasing the lysosomal pH in antigen-presenting cells (APCs) and by interfering with Toll-like receptor (TLR) signaling (Toll-like receptors) at the level of the innate immune response. They also decrease the production of proinflammatory cytokines (interleukin 1 (IL-1—interleukin-1), interleukin 6 (IL-6—interleukin 6), tumor necrosis factor alpha (TNFα), and interferon-gamma (IFNγ)) [78] of the COVID-19 storm. Its efficacy has been described in COVID-19 either in monotherapy [79] or associated with azithromycin [80,81]. Attention should be paid to cardiovascular effects [82].
10.3. Janus Kinase Inhibitor (JAK 1 and 2)
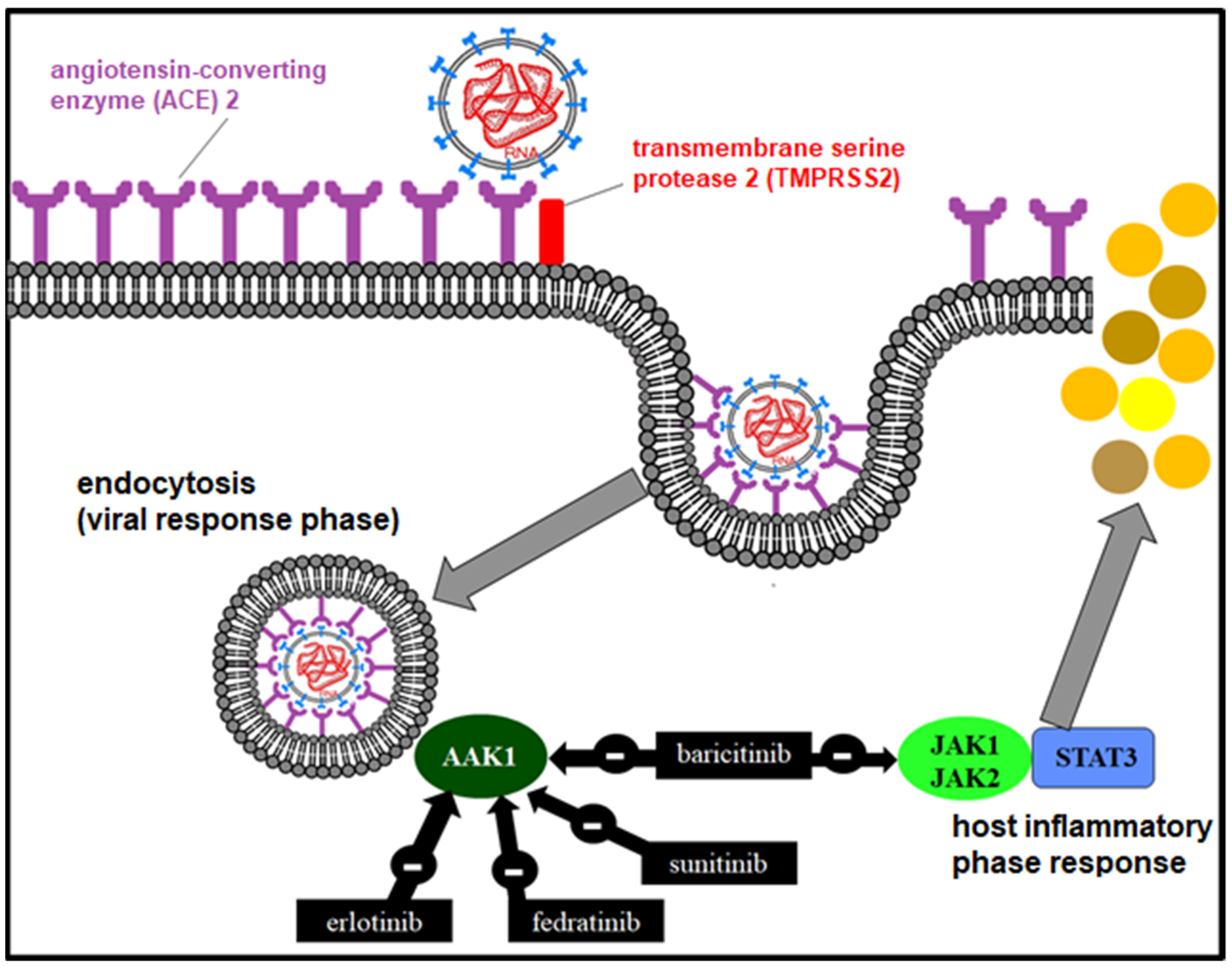
The anti-inflammatory effect of baricitinib (Olumiant®) is due to the reversible inhibition of JAK 1 and 2 through a signal transduction pathway involving STAT proteins [83], which modulates the expression of genes associated with inflammation in immune cells and inhibits IFN production. In addition, it may have a possible antiviral effect by inhibiting AP2-associated protein kinase 1 (AP2-associated protein kinase 1) [84], interrupting the passage of SARS-CoV-2 within the cell and even the intracellular assembly of the viral particles (Figure 5).
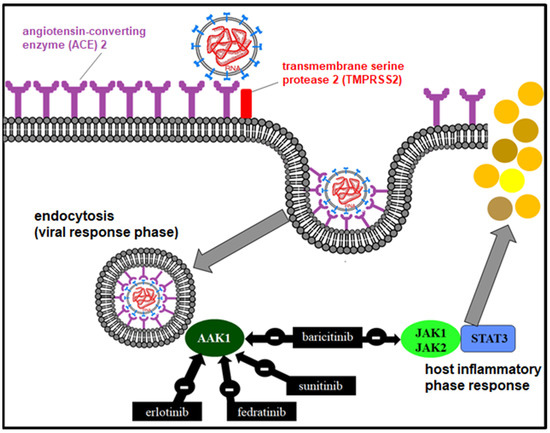
Figure 5.
Mechanism of action of JAK1/2 and AAK1 inhibitors in the viral and inflammatory phase.
Baricitinib is approved in more than 65 countries for the treatment of moderate–severe rheumatoid arthritis (RA). It is useful in reducing mortality in COVID-19 by associating it with different antivirals [85]. RECOVERY included 8156 patients with COVID-19 treated with baricitinib versus usual care between 2 February 2021 and 29 December 2021. Baricitinib significantly reduced the risk of death in hospitalized patients by 20% [86].
Erlotinib, another JAK inhibitor, has shown pharmacokinetic efficacy in combination with ritonavir [87].
10.4. Blockers of the IL-1-Mediated Inflammatory Response
10.4.1. Anakinra (Kineret®)
Monoclonal antibody (mAb) is antagonistic to the human IL-1 receptor (r-metHuIL-1ra), produced in Escherichia coli cells by recombinant DNA technology. It is indicated in rheumatoid arthritis, cryopyrin-associated periodic syndrome (CAPS) (including Muckle-Wells syndrome (MWS), neonatal multisystem inflammatory disease (NOMID) (neonatal-onset multisystem inflammatory disease), chronic infantile neurological cutaneous and articular syndrome (CINCA), and severe manifestations of familial cold autoinflammatory syndrome (FCAS) and familial cold urticaria (FCU) (familial cold autoinflammatory)), and Still’s syndrome. Its usefulness has been described in critical clinical cases where there is a cytokine storm such as macrophage activation syndrome (MAS) and secondary lymphohistiocytic hemophagocytosis (SHLH) (secondary hemophagocytic lymphohistiocytosis). Its use in COVID-19 has been associated with increased survival [88].
10.4.2. Canakinumab (Illaris®)
Recombinant human IgG1κ anti-IL-1 beta mAb [89], listed in CAPS [90] (MWS, NOMID, CINCA, FCAS, and FCU), as well as tumor-necrosis-factor-receptor-associated periodic syndrome (TRAPS) (tumor-necrosis-factor-receptor-associated periodic syndrome), hyperimmunoglobulin D syndrome (HIDS), mevalonate kinase deficiency (MKD), familial Mediterranean fever (FMF), familial Mediterranean fever Still, and arthritic gout.
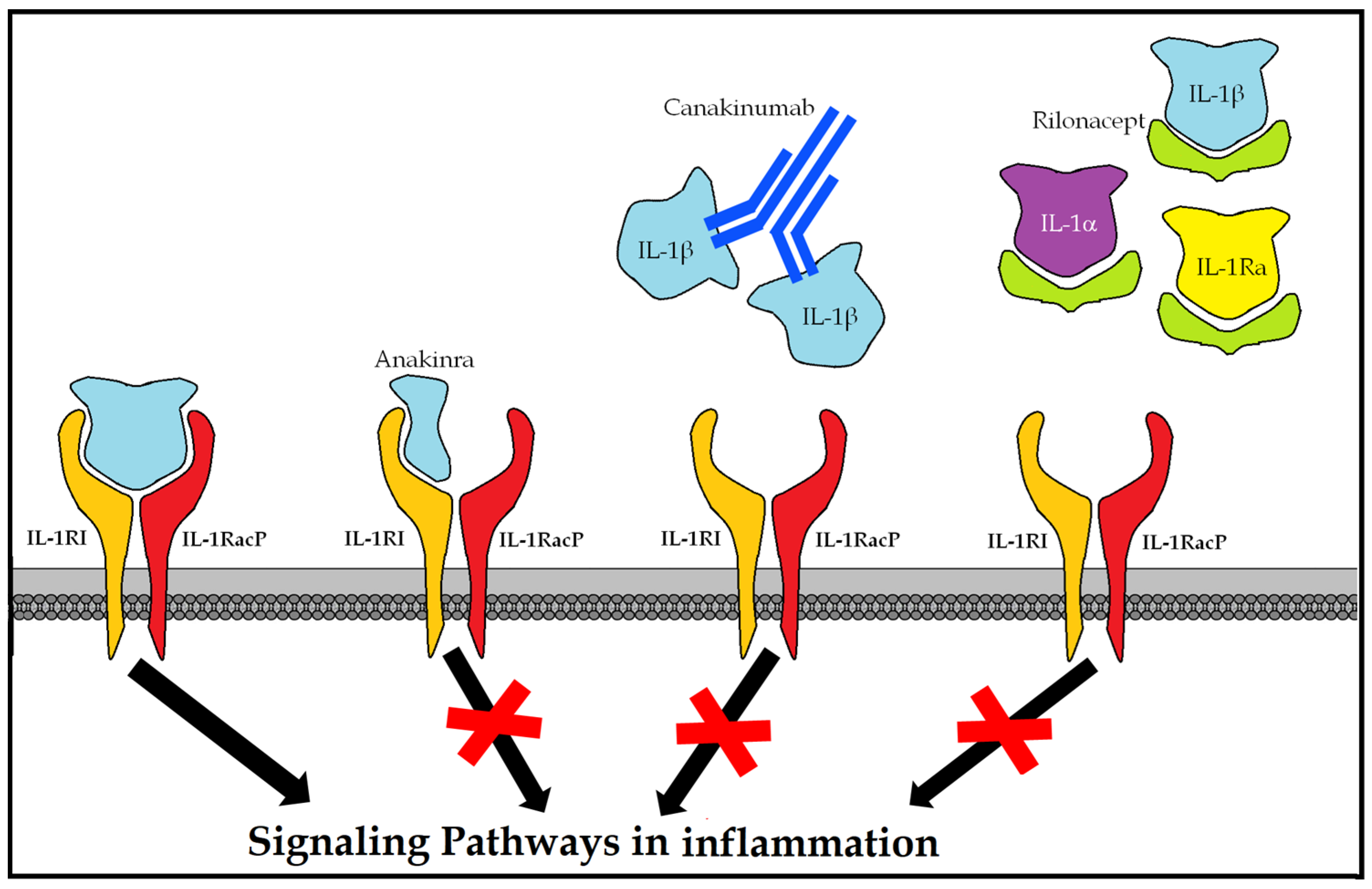
10.4.3. Rilonacept (Arcalyst®)
Dimeric fusion protein with ligand-binding domains of the extracellular portions of the human interleukin 1 receptor type I (IL-1RI) receptor and of the IL-1 receptor accessory protein (IL-1) (1RAcP) (interleukin-1 receptor accessory protein) bound in line to the Fc portion of human IgG1 [89]. By binding to the proinflammatory cytokines IL-1α and IL-1β and antagonizing the endogenous IL-1 receptor (IL-1ra), it blocks the inflammatory cascade (Figure 6).
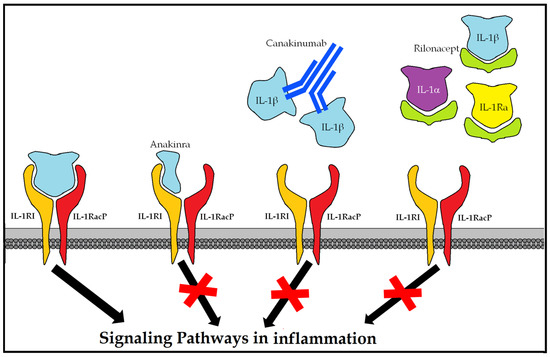
Figure 6.
Therapeutic options for blocking the hyperinflammatory response mediated by IL-1.
10.5. Blockers of the Inflammatory Response Mediated by IL-6
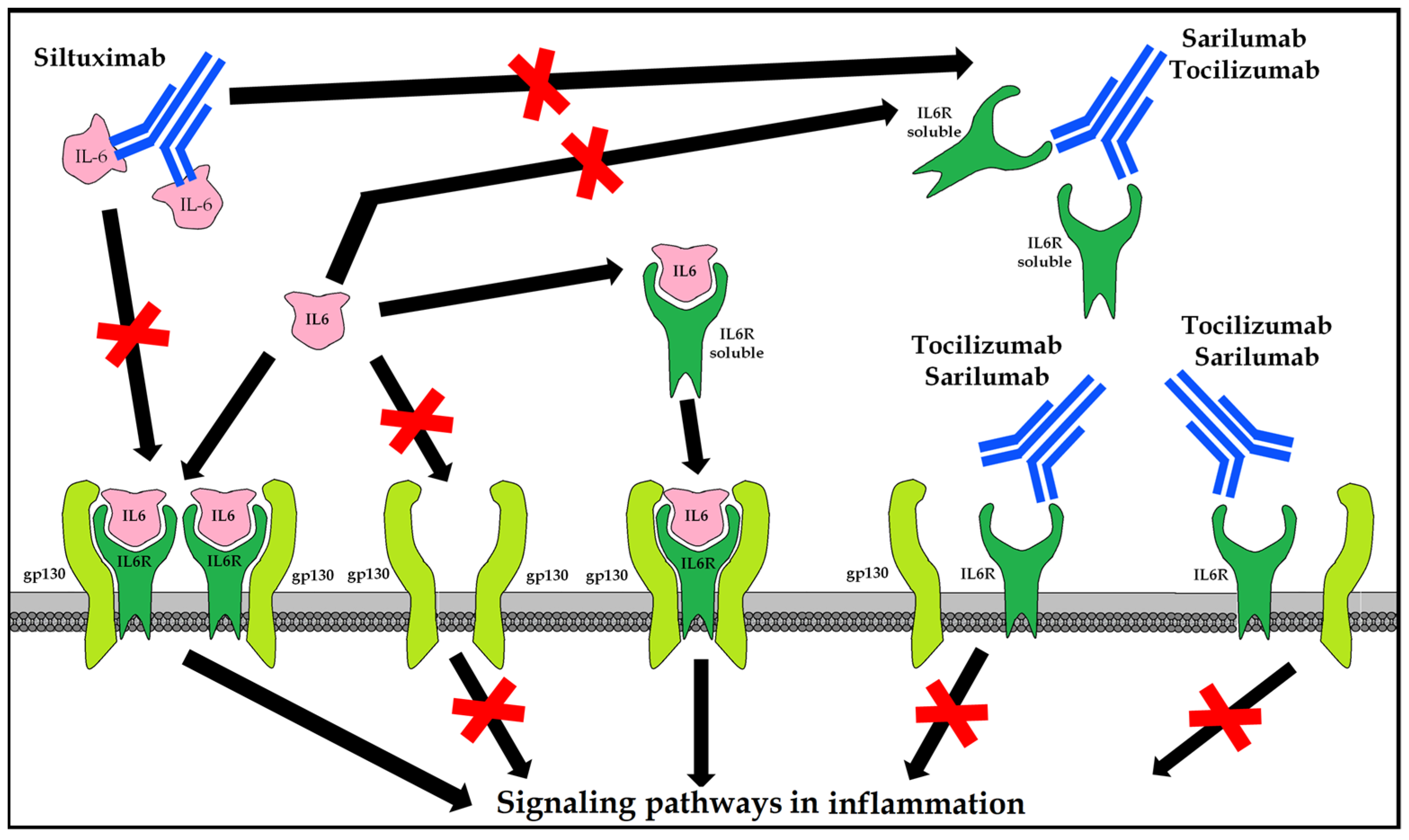
10.5.1. Tocilizumab (Actemra®/RoActemra®)
Recombinant human IgG1 mAb against interleukin 6 receptor (IL-6R) that binds soluble and membrane-bound receptors. It is indicated in combination with methotrexate (MTX) in adults with severe, active, and progressive rheumatoid arthritis (RA) not previously treated with MTX, or in moderate to severe active RA with inadequate response or intolerance to prior treatment with one or more disease-modifying antirheumatic drugs (DMARDs) or tumor necrosis factor (TNF) antagonists; that is, systemic juvenile idiopathic arthritis (sJIA) and polyarticular (pJIA) and giant cell arteritis (GCA) [91].
10.5.2. Sarilumab (Kevzara®)
Recombinant human IgG1 anti-IL 6R mAb binds both soluble and membrane-bound receptors, inhibiting IL-6 cell signaling transmission measured as STAT-3 inhibition. It is indicated in moderate to severe active rheumatoid arthritis (RA) in adults who are inadequate responders to, or intolerant to, one or more disease-modifying antirheumatic drugs (DMARDs) [92].
10.5.3. Siltuximab (Sylvant®)
Chimeric human-murine IgG1κ anti-IL-6 mAb forms stable, high-affinity complexes with soluble forms of IL-6. It is indicated for the treatment of multicentric Castleman’s disease (MCD) in adults negative for human immunodeficiency virus (HIV) [93] and human herpesvirus-8 (HVH8) (Figure 7).
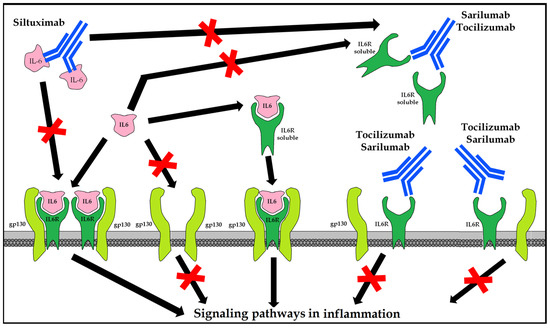
Figure 7.
Therapeutic options for blocking the hyperinflammatory response mediated by IL-6.
10.6. Colchicine
The most studied pharmacological mechanism is the binding to tubulin, blocking the polymerization of microtubules, achieving an antimitotic effect [94,95]. It inhibits chemotaxis in phagocytosis in urate crystals in gouty arthritis [96]. The COLCORONA (Colchicine Coronavirus SARS-CoV-2) clinical trial (NCT04322682) studies the effect of colchicine on the inhibition of IL-1 production in COVID-19 [97].
10.7. Interferons
IFNs are divided into the following: type I (IFNα, IFNβ, IFN-ε, IFN-κ, and IFN-ω) [98], type II (IFNγ), and type III (λ). These have an antiviral effect, although types I and II induce the production of proinflammatory cytokines [99]. IFNα and IFNβ could be useful in early stages but would worsen survival in advanced stages.
Emapalumab (Gamifan®) is an anti-IFNγ mAb indicated for hyperinflammation in primary hemophagocytic lymphohistiocytosis (pHLH) [100] that has been tested in critically ill patients with COVID-19, as IFNγ levels are elevated in patients with COVID-19.
IFNλ has a powerful antiviral effect without a proinflammatory effect, so it could be a therapeutic option [101]. The antiviral role of pegylated IFNλ against HCV has been studied. Azithromycin inefficiently stimulates IFNλ production, which could explain its effect on COVID-19.
10.8. Passive Immunity
10.8.1. Sera from Patients Recovered from COVID-19
It was previously used in epidemics of the H1N1 influenza virus [102,103,104], SARS-CoV-1, and MERS-CoV [105,106]. The appearance of new mutant variants of SARS-CoV-2, such as Delta or Ómicron, which are increasingly contagious and escaped the neutralizing antibodies of previous variants and vaccination (active immunization), has produced successive waves of epidemics [106].
10.8.2. Combined Immunoglobulin Preparations
In the future, and similarly to human anticytomegalovirus immunoglobulin (CMVIG, Megalotect®) (cytomegalovirus immune globulin), commercial preparation of immunoglobulins from different donors would be a therapeutic option, providing a higher concentration than plasma from recovered subjects.
10.8.3. mAbs Directed against Any SARS-CoV-2 Protein
Analogous to palivizumab against respiratory syncytial virus (RSV) [107], the design of an mAb directed against SARS-CoV-2 could be a therapeutic option applied to any infection. Bebtelovimab (LY-CoV1404, 1404) [108] is a neutralizing mAb directed against the S protein of SARS-CoV-2, including Ómicron. Tixagevimab and Cilgavimab (AZD7442) are mAbs isolated from B lymphocytes from patients infected with SARS-CoV-2 that neutralize protein S [109].
10.9. Active Immunity
In the race to manufacture the anti-SARS-CoV-2 vaccine [19], there are more than 125 anti-SARS-CoV-2 vaccine candidates [20]. They are divided into six large groups: (a) live attenuated viruses, inactivated viruses, nucleic acids, replicating viral vectors, non-replicating viral vectors, and recombinant protein subunits. There are studies on the immunomodulation achieved with the bacillus Calmette–Guérin (BCG) vaccine [110].
Some of the vaccines based on RNA technology that express part of the SARS-CoV-2 S protein in host cells are as follows: BNT162b2 (Comirnaty®, Pfizer-BioNTech, Mainz, Germany) [111] and mRNA-1273 (Moderna, Cambridge, MA, USA) [112]. As RNA is more easily degraded than DNA, DNA-based vaccines have also been marketed: ChAdOx1/AZD1222 (Oxford University/Astra Zeneca, Cambridge, UK), which uses a similar mechanism but is based on a chimpanzee adenovirus viral vector, as well as Ad26.COV2.S [113] (Janssen, Beerse, Belgium), carrying a non-replicating adenovirus serotype 26 viral vector with SARS-CoV-2 protein S genes.
11. Conclusions
Although efficacy and safety studies in humans are needed, the possible therapeutic targets in the viral response phase and in the hyperinflammatory response phase of COVID-19 have been reviewed. There is no current evidence to recommend any specific treatment. The use of investigational drugs must be carried out under controlled, randomized, and ethically controlled trials. Passive immunity studies have been carried out through the transfusion of plasma from recovered subjects; even so, there is a race to develop a vaccine that generates active immunity.
Author Contributions
Conceptualization, M.d.C.Z.-B.; Data curation, M.d.C.Z.-B. and Á.A.-P.; Formal analysis, Á.A.-P.; Methodology, M.d.C.Z.-B.; Resources, J.J.-P.; Software, J.J.-P.; Supervision, M.d.C.Z.-B. and Á.A.-P.; Writing—review and editing, Á.A.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| mAb | monoclonal antibody |
| RNA | ribonucleic acid |
| COVID-19 | coronavirus disease 2019 (coronavirus disease-2019) |
| CQ | chloroquine |
| ACE2 | angiotensin-converting enzyme type II |
| ERGI | intermediate compartment endoplasmic reticulum–Golgi apparatus |
| HCoV | human coronavirus |
| HCQ | hydroxychloroquine |
| IFN | interferon |
| IL-1 | interleukin 1 (interleukin 1) |
| IL-6 | interleukin 6 (interleukin 6) |
| IMPα/β1 | importin alpha and beta-1 |
| PI | protease inhibitor |
| JAK | Janus kinase (Janus kinase) |
| MERS-CoV | virus causing Middle East respiratory syndrome (Middle East respiratory syndrome coronavirus) |
| NS3 | nonstructural protein 3 |
| RdRp | RNA-polymerase-RNA-dependent |
| RDV | remdesivir |
| SARS-CoV-1 | severe acute respiratory syndrome coronavirus 1 (severe acute respiratory syndrome coronavirus 1) |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 (severe acute respiratory syndrome coronavirus 2) |
| TMPRSS2 | transmembrane protease, serine 2 |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| RSV | respiratory syncytial virus |
References
- Novel Coronavirus (2019-nCoV) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 23 January 2020).
- Haagmans, B.L.; Osterhaus, A.D. Coronaviruses and their therapy. Antivir. Res. 2006, 71, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Marston, H.D.; Fauci, A.S. Coronavirus Infections—More Than Just the Common Cold. JAMA 2020, 323, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.L.; Teoh, K.T.; Lo, J.; Chan, C.M.; Kien, F.; Escriou, N.; Tsao, S.W.; Nicholls, J.M.; Altmeyer, R.; Peiris, J.S.M.; et al. The M, E, and N Structural Proteins of the Severe Acute Respiratory Syndrome Coronavirus Are Required for Efficient Assembly, Trafficking, and Release of Virus-Like Particles. J. Virol. 2008, 82, 11318–11330. [Google Scholar] [CrossRef] [PubMed]
- Kirchdoerfer, R.N.; Cottrell, C.A.; Wang, N.; Pallesen, J.; Yassine, H.M.; Turner, H.L.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Pre-fusion structure of a human coronavirus spike protein. Nature 2016, 531, 118–121. [Google Scholar] [CrossRef]
- Song, H.C.; Seo, M.-Y.; Stadler, K.; Yoo, B.J.; Choo, Q.-L.; Coates, S.R.; Uematsu, Y.; Harada, T.; Greer, C.E.; Polo, J.M.; et al. Synthesis and Characterization of a Native, Oligomeric Form of Recombinant Severe Acute Respiratory Syndrome Coronavirus Spike Glycoprotein. J. Virol. 2004, 78, 10328–10335. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Hasegawa, H.; Takeda, M.; Nagata, N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 2019, 93, e01815-18. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- De Haan, C.A.; Rottier, P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005, 64, 165–230. [Google Scholar]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G.; et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef]
- Venkatagopalan, P.; Daskalova, S.M.; Lopez, L.A.; Dolezal, K.A.; Hogue, B.G. Coronavirus envelope (E) protein remains at the site of assembly. Virology 2015, 478, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Torres, J.L.; DeDiego, M.L.; Álvarez, E.; Jiménez-Guardeño, J.M.; Regla-Nava, J.A.; Llorente, M.; Kremer, L.; Shuo, S.; Enjuanes, L. Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology 2011, 415, 69–82. [Google Scholar] [CrossRef]
- DeDiego, M.L.; Álvarez, E.; Almazán, F.; Rejas, M.T.; Lamirande, E.; Roberts, A.; Shieh, W.-J.; Zaki, S.R.; Subbarao, K.; Enjuanes, L. A Severe Acute Respiratory Syndrome Coronavirus That Lacks the E Gene Is Attenuated In Vitro and In Vivo. J. Virol. 2007, 81, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Masters, P.S. The Small Envelope Protein E Is Not Essential for Murine Coronavirus Replication. J. Virol. 2003, 77, 4597–4608. [Google Scholar] [CrossRef] [PubMed]
- Ortego, J.; Ceriani, J.E.; Patiño, C.; Plana, J.; Enjuanes, L. Absence of E protein arrests transmissible gastroenteritis coronavirus maturation in the secretory pathway. Virology 2007, 368, 296–308. [Google Scholar] [CrossRef]
- Curtis, K.M.; Yount, B.; Baric, R.S. Heterologous Gene Expression from Transmissible Gastroenteritis Virus Replicon Particles. J. Virol. 2002, 76, 10104–10110. [Google Scholar] [CrossRef]
- Ortego, J.; Escors, D.; Laude, H.; Enjuanes, L. Generation of a Replication-Competent, Propagation-Deficient Virus Vector Based on the Transmissible Gastroenteritis Coronavirus Genome. J. Virol. 2002, 76, 11518–11529. [Google Scholar] [CrossRef]
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Kuo, L.; Hurst, K.R.; Masters, P.S. Exceptional Flexibility in the Sequence Requirements for Coronavirus Small Envelope Protein Function. J. Virol. 2007, 81, 2249–2262. [Google Scholar] [CrossRef]
- Arbely, E.; Khattari, Z.; Brotons, G.; Akkawi, M.; Salditt, T.; Arkin, I. A Highly Unusual Palindromic Transmembrane Helical Hairpin Formed by SARS Coronavirus E Protein. J. Mol. Biol. 2004, 341, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Raamsman, M.J.B.; Locker, J.K.; De Hooge, A.; De Vries, A.A.F.; Griffiths, G.; Vennema, H.; Rottier, P.J.M. Characterization of the Coronavirus Mouse Hepatitis Virus Strain A59 Small Membrane Protein E. J. Virol. 2000, 74, 2333–2342. [Google Scholar] [CrossRef]
- Verdiá-Báguena, C.; Nieto-Torres, J.L.; Alcaraz, A.; DeDiego, M.L.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Coronavirus E protein forms ion channels with functionally and structurally-involved membrane lipids. Virology 2012, 432, 485–494. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; DeDiego, M.L.; Verdiá-Báguena, C.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Fernandez-Delgado, R.; Castaño-Rodriguez, C.; Alcaraz, A.; Torres, J.; Aguilella, V.M.; et al. Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis. PLoS Pathog. 2014, 10, e1004077. [Google Scholar] [CrossRef]
- Verdiá-Báguena, C.; Nieto-Torres, J.L.; Alcaraz, A.; DeDiego, M.L.; Enjuanes, L.; Aguilella, V.M. Analysis of SARS-CoV E protein ion channel activity by tuning the protein and lipid charge. Biochim. Biophys. Acta 2013, 1828, 2026–2031. [Google Scholar] [CrossRef]
- Corse, E.; Machamer, C.E. Infectious Bronchitis Virus E Protein Is Targeted to the Golgi Complex and Directs Release of Virus-Like Particles. J. Virol. 2000, 74, 4319–4326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Surya, W.; Claudine, S.; Torres, J. Structure of a Conserved Golgi Complex-targeting Signal in Coronavirus Envelope Proteins. J. Biol. Chem. 2014, 289, 12535–12549. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yuan, Q.; Torres, J.; Tam, J.P.; Liu, D. Biochemical and functional characterization of the membrane association and membrane permeabilizing activity of the severe acute respiratory syndrome coronavirus envelope protein. Virology 2006, 349, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Maheswari, U.; Parthasarathy, K.; Ng, L.; Liu, D.X.; Gong, X. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007, 16, 2065–2071. [Google Scholar] [CrossRef]
- Torres, J.; Wang, J.; Parthasarathy, K.; Liu, D.X. The Transmembrane Oligomers of Coronavirus Protein E. Biophys. J. 2005, 88, 1283–1290. [Google Scholar] [CrossRef]
- Parthasarathy, K.; Ng, L.; Lin, X.; Liu, D.X.; Pervushin, K.; Gong, X.; Torres, J. Structural Flexibility of the Pentameric SARS Coronavirus Envelope Protein Ion Channel. Biophys. J. 2008, 95, L39–L41. [Google Scholar] [CrossRef] [PubMed]
- Pervushin, K.; Tan, E.; Parthasarathy, K.; Lin, X.; Jiang, F.L.; Yu, D.; Vararattanavech, A.; Soong, T.W.; Liu, D.X.; Torres, J. Structure and Inhibition of the SARS Coronavirus Envelope Protein Ion Channel. PLoS Pathog. 2009, 5, e1000511. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Guardeño, J.M.; Nieto-Torres, J.L.; DeDiego, M.L.; Regla-Nava, J.A.; Fernandez-Delgado, R.; Castaño-Rodriguez, C.; Enjuanes, L. The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis. PLoS Pathog. 2014, 10, e1004320. [Google Scholar] [CrossRef]
- Castaño-Rodriguez, C.; Honrubia, J.M.; Gutiérrez-Álvarez, J.; DeDiego, M.L.; Nieto-Torres, J.L.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Fernandez-Delgado, R.; Verdia-Báguena, C.; Queralt-Martín, M.; et al. Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. mBio 2018, 9, e02325-17. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1282, pp. 1–23. [Google Scholar]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Palma, A.G.; Kotsias, B.A.; Marino, G.I. Funciones de los canales iónicos CFTR y ENAC. Medicina 2014, 74, 133–139. [Google Scholar]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R., Jr.; Nunneley, J.W.; Barnard, D.; Pöhlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef]
- Su, S.-B.; Motoo, Y.; Iovanna, J.L.; Xie, M.-J.; Sawabu, N. Effect of Camostat Mesilate on the Expression of Pancreatitis-Associated Protein (PAP), p8, and Cytokines in Rat Spontaneous Chronic Pancreatitis. Pancreas 2001, 23, 134–140. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsuyama, S.; Li, X.; Takeda, M.; Kawaguchi, Y.; Inoue, J.-I.; Matsuda, Z. Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob. Agents Chemother. 2016, 60, 6532–6539. [Google Scholar] [CrossRef]
- Kadam, R.U.; Wilson, I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. USA 2017, 114, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Vojtech, L.; Wagoner, J.; Slivinski, N.S.J.; Jackson, K.J.; Wang, R.; Khadka, S.; Luthra, P.; Basler, C.F.; Polyak, S.J. The Antiviral Drug Arbidol Inhibits Zika Virus. Sci. Rep. 2018, 8, 8989. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int. J. Antimicrob. Agents 2020, 56, 105998. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yao, M.; Fang, Z.; Lv, X.; Deng, M.; Wu, Z. A study on clinical effect of Arbidol combined with adjuvant therapy on COVID-19. J. Med. Virol. 2020, 92, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Di Trani, L.; Donatelli, I.; Cauda, R.; Cassone, A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006, 6, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Taylor, P.C. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology 2019, 58 (Suppl. S1), i17–i26. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Krishnan, V.; De Bono, S.; Ottaviani, S.; Casalini, G.; Richardson, P.J.; Monteil, V.; Lauschke, V.M.; Mirazimi, A.; Youhanna, S.; et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020, 12, e12697. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strtegy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef]
- Askanase, A.D.; Khalili, L.; Buyon, J.P. Thoughts on COVID-19 and autoinmune diseases. Lupus Sci. Med. 2020, 7, e000396. [Google Scholar] [CrossRef]
- Jefferson, T.; Jones, M.A.; Doshi, P.; Del Mar, C.B.; Hama, R.; Thompson, M.J.; Spencer, E.A.; Onakpoya, I.J.; Mahtani, K.R.; Nunan, D.; et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database Syst. Rev. 2014, 2014, CD008965. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Pan, Z.; Qian, C.; Yang, Y.; You, R.; Zhao, J.; Liu, P.; Gao, L.; Li, Z.; et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell. Mol. Immunol. 2020, 17, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Elfiky, A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020, 248, 117477. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Kuno-Maekawa, M.; Sangawa, H.; Uehara, S.; Kozaki, K.; Nomura, N.; Egawa, H.; Shiraki, K. Mechanism of Action of T-705 against Influenza Virus. Antimicrob. Agents Chemother. 2005, 49, 981–986. [Google Scholar] [CrossRef]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef]
- De Clercq, E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem.—Asian J. 2019, 14, 3962–3968. [Google Scholar] [CrossRef]
- Siegel, D.; Hui, H.C.; Doerffler, E.; Clarke, M.O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 2017, 60, 1648–1661. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Shalhoub, S.; Mandourah, Y.; Al-Hameed, F.; Al-Omari, A.; Al Qasim, E.; Jose, J.; Alraddadi, B.; Almotairi, A.; Al Khatib, K.; et al. Ribavirin and Interferon Therapy for Critically Ill Patients with Middle East Respiratory Syndrome: A Multicenter Observational Study. Clin. Infect. Dis. 2020, 70, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Yiu, C.P.; Wong, K.Y. Prediction of the 2019-nCoV 3C-like protease (3CLpro) structure: Virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir Against SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp): A Molecular Docking Study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef]
- Barragan, P.; Podzamczer, D. Lopinavir/ritonavir: A protease inhibitor for HIV-1 treatment. Expert Opin. Pharmacother. 2008, 9, 2363–2375. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Patel, S.K.; Pathak, M.; Tiwari, R.; Singh, B.R.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; Leblebicioglu, H. Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 23. [Google Scholar] [CrossRef]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Tay, M.Y.F.; Fraser, J.E.; Chan, W.K.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef]
- Götz, V.; Magar, L.; Dornfeld, D.; Giese, S.; Pohlmann, A.; Höper, D. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci. Rep. 2016, 6, 23138. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.M.; Gadkari, M.; Howe, K.N.; Sun, J.; Kardava, L.; Kumar, P.; Kumari, S.; Hu, Z.; Fraser, I.D.; Moir, S.; et al. Immune regulation by glucocorticoids can be linked to cell type–dependent transcriptional responses. J. Exp. Med. 2019, 216, 384–406. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Arnaldi, G.; Boscaro, M.; Falorni, A.; Giordano, C.; Giordano, R.; Pivonello, R.; Pofi, R.; Hasenmajer, V.; Venneri, M.A.; et al. COVID-19 infection and glucocorticoids: Update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J. Endocrinol. Investig. 2020, 43, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Van den Borne, B.E.; Dijkmans, B.A.; De Rooij, H.H.; Le Cessie, S.; Verweij, C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997, 24, 55–60. [Google Scholar] [PubMed]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of Hydroxychloroquine in Patients with COVID-19: Results of a Randomized Clinical Trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.-C.; Gautret, P.; Colson, P.; Fournier, P.-E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020, 35, 101738. [Google Scholar] [CrossRef]
- Lane, J.C.E.; Weaver, J.; Kostka, K.; Duarte-Salles, T.; Abrahao, M.T.F.; Alghoul, H.; Alser, O.; Alshammari, T.M.; Biedermann, P.; Burn, E.; et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: A multinational, network cohort and self-controlled case series study. medRXiv 2020. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Plenge, R. JAK and STAT Signaling Molecules in Immunoregulation and Immune-Mediated Disease. Immunity 2012, 36, 542–550. [Google Scholar] [CrossRef]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022, 400, 359–368, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef]
- Boosman, R.J.; de Gooijer, C.J.; Groenland, S.L.; Burgers, J.A.; Baas, P.; van der Noort, V.; Beijnen, J.H.; Huitema, A.D.; Steeghs, N. Ritonavir-Boosted Exposure of Kinase Inhibitors: An Open Label, Cross-over Pharmacokinetic Proof-of-Concept Trial with Erlotinib. Pharm. Res. 2022, 39, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Din, C.T.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325–e331. [Google Scholar] [CrossRef] [PubMed]
- Dubois, E.A.; Rissmann, R.; Cohen, A.F. Rilonacept and canakinumab. Br. J. Clin. Pharmacol. 2011, 71, 639–641. [Google Scholar] [CrossRef]
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Koné-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 2018, 378, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Raimondo, M.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Favalli, E.G. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des. Dev. Ther. 2017, 11, 1593–1603. [Google Scholar] [CrossRef]
- Deisseroth, A.; Ko, C.-W.; Nie, L.; Zirkelbach, J.F.; Zhao, L.; Bullock, J.; Mehrotra, N.; Del Valle, P.; Saber, H.; Sheth, C.; et al. FDA Approval: Siltuximab for the Treatment of Patients with Multicentric Castleman Disease. Clin. Cancer Res. 2015, 21, 950–954. [Google Scholar] [CrossRef]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, A.; Coluccia, A.; Silvestri, R.; Sorba, G.; Brancale, A. The Tubulin Colchicine Domain: A Molecular Modeling Perspective. ChemMedChem 2012, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Molad, Y.; Reibman, J.; Balakhane, E.; Levin, R.I.; Weissmann, G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Investig. 1995, 96, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.; Elefante, E.; Baldini, C.; Bartoloni, E.; Puxeddu, I.; Talarico, R.; Mosca, M.; Bombardieri, S. COVID-19: The new challenge for rheumatologists. First update. Clin. Exp. Rheumatol. 2020, 38, 175–180. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Hensley, L.E.; Fritz, L.E.; Jahrling, P.B.; Karp, C.L.; Huggins, J.W.; Geisbert, T.W. Interferon-beta 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004, 10, 317–319. [Google Scholar] [CrossRef]
- Prencipe, G.; Bracaglia, C.; Caiello, I.; Pascarella, A.; Francalanci, P.; Pardeo, M.; Meneghel, A.; Martini, G.; Rossi, M.N.; Insalaco, A.; et al. The interferon-gamma pathway is selectively up-regulated in the liver of patients with secondary hemophagocytic lymphohistiocytosis. PLoS ONE 2019, 14, e0226043. [Google Scholar] [CrossRef]
- Prokunina-Olsson, L.; Alphonse, N.; Dickenson, R.; Durbin, J.E.; Glenn, J.S.; Hartmann, R.; Kotenko, S.V.; Lazear, H.M.; O’Brien, T.R.; Odendall, C.; et al. COVID-19 and emerging viral infections: The case for interferon lambda. J. Exp. Med. 2020, 217, e20200653. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Roback, J.D.; Guarner, J. Convalescent Plasma to Treat COVID-19: Possibilities and challenges. JAMA 2020, 323, 1561–1562. [Google Scholar] [CrossRef]
- Hung, I.F.N.; To, K.; Lee, C.-K.; Lee, K.-L.; Chan, K.K.C.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y.; et al. Convalescent Plasma Treatment Reduced Mortality in Patients with Severe Pandemic Influenza A (H1N1) 2009 Virus Infection. Clin. Infect. Dis. 2011, 52, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.Y. Emerging pathogens and their implications for the blood supply and transfusion transmitted infections. Br. J. Haematol. 2012, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Convalescent Plasma Study Group; Nguyen-Van-Tam, J.S.; et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef]
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998, 102 Pt 1, 531–537. [Google Scholar] [CrossRef]
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; Van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. bioRxiv 2022. Update in Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- O’Neill, L.; Netea, M. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).