Abstract
Ionising radiation is an important form of treatment for human cancer; however, the side effects associated with oxidative damage caused by radiation compromise its effectiveness. This work aimed to quantify the major bioactive components of freeze-dried kiwifruit (KD) and strawberry (SD) extracts and assess their potential efficacy as radioprotective agents in human blood lymphocytes. Their possible genotoxic and cytotoxic effects were also evaluated. The study was conducted by pre-treating human lymphocytes with KD and SD (50, 400, and 800 µg/mL) before radiation at 2 Gy. The results showed that SD presented a higher antioxidant capacity (12.6 mmol Trolox equivalents/100 g db) and higher values of total phenolic compounds (2435 mg of gallic acid equivalents/100 g db), while KD had the highest vitamin C content (322 mg ascorbic acid/100 g db). Regarding phenolic compounds, pelargonidin-3-glucoside was the most abundant in SD (1439 mg/1000 g db) and quercetin-3-O-galactoside in KD (635 mg/1000 g db). None of the tested concentrations of both fruit extracts showed a genotoxic effect. SD (800 µg/mL) reduced the sister chromatid exchange frequency and mitotic index. The efficacy of KD (400 and 800 µg/mL) in lowering the dicentric chromosome frequency demonstrated its radioprotective activity.
1. Introduction
The term ionising radiation refers to electromagnetic and particle radiation that is powerful enough to ionise atoms or molecules. For this reason, when passing through living tissues, a certain degree of damage may be produced. Both the well-known direct and indirect effects of ionising radiation cause this damage. The indirect impact is mainly responsible for the formation of reactive free radicals through the radiolysis of water, which react with macromolecules like DNA, proteins, and membranes so that they eventually induce cell damage or even dysfunction and death [1,2]. Since DNA is considered the primary target for ionising radiation damage, special attention has been paid to the effects on this molecule. If endogenous defence systems cannot effectively repair DNA lesions, this leads to genome instability and chromosome abnormalities. Radiation-induced genome aberrations play an important role in carcinogenesis caused by radiation.
Concerning radiation damage to humans, it is crucial to protect biological systems from radiation-induced damage [3]. To protect organisms from cellular and molecular damage during irradiation, radioprotectors agents act predominantly by enhancing antioxidant defence mechanisms by scavenging free radicals [2]. It has been suggested that administering radioprotective agents might lower radiation’s harmful effects on cells. Antioxidants have the potential to function as radioprotectors by preventing the propagation of chain reactions initiated by free radicals and thus reducing certain DNA damage caused by ionising radiation [4].
Many natural and synthetic compounds have been examined in the recent past as radioprotector agents. However, some synthetic agents’ inherent toxicity at radioprotective concentrations warrant further search for safer and more effective compounds [5]. Nowadays, attention is focused on natural compounds with a lower toxicity, more favourable administration routes, and improved pharmacokinetics than synthetic radioprotectors [6]. The genotoxic effects of natural compounds may be evaluated by using biomarkers. The sister chromatid exchange (SCE) test permits assessing the consequences of direct DNA damage. This assay also provides information on the genome’s instability when exposed to potentially genotoxic substances. [7]. The mitotic index (MI) reflects the frequency of cell division, and is a reliable parameter to identify induced cytotoxicity [8]. For the analysis of proliferation kinetic index data (PI), the proportion of cells in the first, second, and third division in cell treatment cultures is commonly used [9].
As a result of their low toxicity and minimum adverse effects at the effective dosage, fruits or plants with antioxidant properties have been considered as an appropriate source of radioprotector agents [10]. The radioprotective effects of various phytochemicals such as resveratrol [11], chlorogenic and quinic acids [12], and phenolic compounds [2] have already been analysed. Additionally, some studies have assessed the radioprotective effects of extracts, for example, the ethanolic extract of propolis [13] and phenolic extracts of fruits [14].
The radioprotective efficiency of fruits is generally tested based on the reduction in ionising radiation damage on the peripheral blood lymphocytes as in vitro models. Enzymatic incorrect repair of ionising-radiation-induced DNA damage can result in large-scale rearrangements of the genome, such as translocations and dicentrics. Cell death, mutation, and neoplasia are just a few examples of the major phenotypic changes that these and other chromosomal exchange abnormalities may bring about. Radiation-induced chromosome aberrations (CAs) in lymphocytes are considered the most sensitive biological technique for measuring radiation exposure at doses over 0.1 Gy if an adequate number of metaphases are analysed [15].
Strawberries (Fragaria × ananassa) and kiwifruits (Actinidia sp.) have been thoroughly investigated for their health benefits [16]. Both fruits are an important source of micronutrients and bioactive compounds with antioxidant and health-promoting properties, including minerals, vitamins C (VC), and phenolic compounds [17,18]. In recent years, VC and polyphenols have been suggested to be the most important chemical species contributing to these fruits’ functional properties, particularly those related to the antioxidant potential (AOP) [16,19]. The prevention of degenerative diseases such as various cancers, cardiovascular and neurological conditions, obesity, diabetes, and oxidative stress dysfunctions appears to be intimately linked to this antioxidant action [20]. Nevertheless, data concerning the radioprotective potential of kiwifruits and strawberries related to their antioxidant activity are limited.
With these considerations, the present work aimed to characterise the VC content, main phenolic compound content, and total polyphenol content (TPC) as foremost responsible for the AOP of kiwifruits and strawberries, and to evaluate the antioxidant effect of these fruits, as well as their genotoxic and radioprotective activities in human blood lymphocytes.
2. Materials and Methods
2.1. Chemicals and Reagents
Folin–Ciocalteau reagent, DPPH·(2,2-diphenyl-1-picryl-hydrazyl), ABTS (2,2-azinobis (3-ethylbenzothiaoline-6-sulfonic acid)), gallic acid, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), iron (III) chloride (FeCl3·6H2O), iron (II) sulfate (FeSO4·7H2O), potassium persulfate, ellagic acid, and ferulic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pelargonidin-3-glucoside, Quercetin-3-glucoside, Quercetin-3-galactoside, and epicatechin were from Extrasynthese (Lyon, France). TPTZ (2,4,6-tripyridyl-s-triazine) was purchased from Fluka Chemie (Buchs, Switzerland) and sodium carbonate was from J.T. Baker (Center Valley, PA, USA). DL-dithiothreitol (DTT), oxalic acid, and (+) ascorbic acid were from Scharlab S.L. (Barcelona, Spain). Unless otherwise stated, all chemicals employed were of analytical or superior quality. Methanol, ethanol, formic acid, and acetonitrile were of HPLC grade (Merck, Darmstadt, Germany). For cytogenetic analysis, Carnoy’s lymphocyte fixative solution was prepared with methanol (Merck), acetic acid (Panreac, Barcelona, Spain) (3:1 v/v), bromodeoxyuridine (Sigma-Aldrich, Saint Louis, MO, USA), potassium chloride (Merck), antimitotic Colcemid (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), bisbenzimideHoechst 33258 (Sigma), and Leishman dye (Merck).
2.2. Fruit Samples
Kiwifruit (Actinidia deliciosa cv.Hayward) and strawberry (Fragaria × ananassa) were bought in a local supermarket in Valencia, Spain. Fruit pieces were selected by visual appreciation based on a better physical appearance and similar colour, size, and firmness. The fruits were washed, and the navel and the peduncle parts were discarded. Then, they were cut into slices and triturated using a food processor (Thermomix TM 21 Vorwerk, Madrid, Spain). Kiwifruit was triturated with the peel. The pulp was homogenised and then freeze-dried. The obtained kiwifruit and strawberry puree were named K and S. They were characterised by the biochemical parameters and antioxidant activity, as described in Section 2.4, to be taken as control.
The purees were placed on aluminium pans (200 g in 0.5 cm thickness by pan) and frozen (CVF 545/86, Ing. Climas, Barcelona, Spain) at −40 °C for 48 h before freeze-drying (Telstar Lioalfa-6 Lyophyliser, Barcelona, Spain) at 10−2 Pa and −40 °C for 48 h. To obtain the powder, the freeze-dried fruit was ground in an electric mincer (Moulinette:320, Moulinex, Barcelona, Spain). The products thus obtained were vacuum packaged (Tecnotrip EVO86154, Barcelona, Spain) and stored in a desiccator with silica gel and silicone sealing protected from light until the preparation of the extracts (no more than 24 h). Freeze-dried kiwi and strawberry samples were designated as KD and SD, respectively.
2.3. Extracts Preparation
Fruit extracts used for quantifying bioactive compounds and for determining the AOP and genotoxic and radioprotective activities were obtained using a method adapted from Silva-Espinoza et al. (2022) [21]. Briefly, 0.5 g of sample mixed with a 9.5 mL mixture of methanol/water 70:30 (v/v) and homogenised at 400 rpm (Velp Scientifica, Usmate Velate, Italy) in the darkness for 20 min was centrifuged at 5867× g for 10 min at 10 °C (Selecta Medifriger-BL, Barcelona, Spain). The supernatants were collected, filtered (0.45 µm nylon filter, VWR, Radnor, PA, USA), and dried in a rotary evaporator (35 °C under vacuum, 20 min) to remove the organic solvent and then freeze-dried. Afterwards, the extract powder was collected and stored in hermetic opaque vials.
For analysis, dry extracts were reconstituted with the extraction solvent. For genotoxic and radioprotective activities, KD and SD extracts were screened at different concentrations based on previous studies [22]. In this case, dry extracts were reconstituted with methanol/water (70:30, v/v) to obtain the final concentrations in blood of 50, 400, and 800 µg/mL. For the rest of the analyses, reconstitution was until the initial volume before dehydration.
2.4. Chemical Determinations
Data are expressed as means and standard deviation of nine replicates (extractions in triplicate and measurements in triplicate) for chemical analysis. TP, individual phenolic compounds, VC, and AOP measured by the DPPH, FRAP, and ABTS methods were determined in kiwifruit samples (K and KD) and in strawberry samples (S and SD) before and after the freeze-drying process. Results were expressed per 100 g dry basis (db) for comparative purposes. Moreover, the changes in bioactive compounds caused by freeze-drying were calculated in reference to the fresh fruit’s bioactive content (Equation (1), Table 1, and Figure 1).
where BcF is the bioactive compound content in the fresh fruit, K or S (mg/100 g db) and BcEx is the bioactive compound content in each freeze-dried fruit extract (mg/100 g db).
2.4.1. Water Content
The water content (xw) of fresh fruit was determined by vacuum drying (60 ± 1 °C, <100 mm Hg pressure, Vacioterm, J.P. Selecta, Barcelona, Spain) until constant weight [23]. For freeze-dried samples, xw was evaluated using an automatic Karl Fischer titrator (C10S Mettler Toledo Compact Coulometric KF Titrator, Columbus, OH, USA).
2.4.2. VC
The process for determining VC involved converting dehydroascorbic acid to ascorbic acid (AA) using DTT as a reducing agent and ultra-high-performance liquid chromatography determination (UHPLC, Jasco equipment, Cremella, Italy) [21]. The conditions were Kromaphase 100-C18, 5 μm (4.6 × 250 mm) column (Scharlab S.L, Barcelona, Spain); mobile phase 0.1% oxalic acid, 20 μL volume injection, flow rate 1 mL/min, and detection at 243 nm and 25 °C (UV-visible detector MD-1510). An L (+) ascorbic acid calibration curve was used at 10–530 ppm intervals. The results were expressed in mg AA/100 g (db).
2.4.3. Phenolic Compounds
Three major phenolic compounds were identified and quantified using UHPLC (Jasco equipment, Cremella, Italy). Quercetin-3-O-galactoside, epicatechin, and ferulic acid were determined in kiwifruit and quercetin-3-glucoside, pelargonidin-3-glucoside, and ellagic acid were determined in strawberries.
Compound separations were achieved on a Synergi-C18, 4 μm, 150 × 4.6 mm, Hydro-RP column (Phenomenex, Madrid, Spain) (volume injected 10 μL, flow rate 1 mL/min) with 0.2% formic acid (A) and acetonitrile (B) used as mobile phases. The elution conditions were 10% B for 5 min and a linear gradient to 30% B in the subsequent 30 min [24].
Chromatograms were recorded at 280, 320, 360, and 510 nm (UV-visible detector MD-1510) at 25 °C. Anthocyanin (pelargonidin-3-glucoside) was quantified at 510 nm, flavonols (quercetins) at 360 nm, phenolic acids (ferulic acid and ellagic acid) at 320 nm, and flavan-3-ols (epicatechin) at 280 nm. Phenolics were identified by their retention time and they were quantified through the integration of the peak areas obtained from the corresponding standards chromatograms. The results were expressed as mg/1000 g db for each phenolic compound.
2.4.4. Total Polyphenols
TP content was determined spectrophotometrically according to the adapted Folin–Ciocalteau method [21]. The results were presented as mg gallic acid equivalent (GAE)/100 g db. All measurements were taken with a UV-visible spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA).
2.4.5. AOP Evaluation
The AOP was determined by three complementary assays: ABTS, DPPH, and FRAP. Results of ABTS and DPPH were expressed as mmol Trolox Equivalent (TE)/100 g db using the corresponding Trolox calibration curves. For FRAP methanolic solutions of known Fe(II) concentrations, FeSO4·7H2O was used for calibration and results were expressed as mmol Fe2+/100 g db. All measurements were taken with a UV-visible spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA).
Firstly, the AOP was assessed by using the free radical scavenging activity of the stable radical 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) [21]. Absorbance was recorded at 517 nm at the initial time (Ainitial) and 15 min later (Asample) once the reaction had achieved a steady state.
The percentage of DPPH·(%DPPH·) was calculated by Equation (2):
For the ABTS assay, the procedure followed the spectrophotometric method of Re et al. (1999) [25] and the percentage inhibition of absorbance at 734 nm was calculated.
The FRAP assay was performed according to Silva-Espinoza et al. [21] with some modifications. The FRAP reagent was prepared by combining 25 mL of 0.3 M acetate buffer (pH 3.6), 2.5 mL 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, and 2.5 mL of 20 mM FeCl3·6H2O. The resulting mixture was heated at 37 °C before use. Fruit extracts (30 µL) were allowed to react with 900 µL of the FRAP solution. The absorbance was measured at 593 nm.
2.5. Biological Determinations
2.5.1. Culture Conditions
Culture conditions were implemented for the study of both genotoxicity and radioprotection biomarkers in accordance with Sebastià et al. (2013) [11] and Montoro et al. (2012) [13]. Heparinised human peripheral blood samples were used for both biomarkers of genotoxicity and radioprotection, and were treated with 250 µL of a methanol/water solution of KD and SD to obtain final concentrations in blood of 50, 400, and 800 µg/mL. The selection of the tested concentrations was made based on previous research work and other in vitro studies of the fruit extracts selected in this study [11,13,26,27,28]. For each treatment, separate cultures were established by mixing 0.75 mL of whole blood with 5 mL of PB-MaxTM Karyotiping medium and incubating for 48 and 72 h at 37 °C. To exclusively analyse first-division metaphases, a final concentration of 12 µg/mL of bromodeoxyuridine was present since the setting up of the cultures. An amount of 150 µL of Colcemid was added 2 h before harvesting the 48 h and 72 h cultures to stop the cell culture in the metaphase.
The study received approval from the Ethics Committee of the Hospital Universitario y Politécnico La Fe, and samples of human peripheral blood were collected following the consent exemption report.
2.5.2. Irradiation Conditions for Radioprotective Activity
Human peripheral blood samples containing lithium heparin as an anticoagulant were obtained using sterile Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). An amount of 250 µL of different concentrations of KD and SD was added to 12 mL of blood samples, which were collected 1 h prior to irradiation and kept at 37 °C in a water bath. The samples were then irradiated with a dose of 2 Gy using a linear accelerator (LINACC linaciX, Varian Medical System, Palo Alto, CA, USA) located at the Hospital Universitario y Politécnico La Fe (Valencia, Spain). To facilitate the repair mechanisms, incubation at 37 °C for 1 h immediately after irradiation was necessary. As controls, two tubes were irradiated with a dose of 2 Gy: one containing only peripheral blood and the other containing peripheral blood along with 250 µL of methanol/water (70:30, v/v) (solvent control). The irradiation conditions were established in accordance with the recommendations of the International Atomic Energy Agency [29].
2.5.3. Stain Technique
The stain technique was previously described by Sebastià et al. (2013) [11] and Montoro et al. (2012) [13]. Two- to three-day-old slides were stained with Fluorescence plus Giemsa stain technique. In summary, the old slides were treated with Hoechst 33,258 for 20 min at room temperature. Subsequently, the slides were washed with distilled water and covered with 2× saline-sodium citrate (SSC) before being exposed to UV light (300 W) for 2 min. After washing the slides with water and allowing them to dry for 30 min, they were stained with Leishman (Merck) for 5 min, enabling the identification of first-, second-, and third-division metaphases.
2.5.4. Cytogenetic Analysis
Sister Chromatid Exchange
The frequency of sister chromatid exchanges (SCE), as a genotoxicity biomarker, was determined by analysing 50 s division metaphases for each treatment (Figure S1, Supplementary Material). The assessment of SCE scores in lymphocytes involved recording the total number of exchanges in the analysed cells for each treatment to establish their frequency (YSCE). Additionally, we recorded the number of chromosomes with one (Y1SCE), two (Y2SCE), or three (Y3SCE) chromatid exchanges in the total number of analysed cells for each treatment.
Evaluation of MI and PI
For each concentration tested, MI and PI as cytotoxicity biomarkers were analysed. According to Rojas et al. (1992) [30], the mitotic index was determined by exclusively considering the proportion of metaphases in 500 cells during the first-division metaphases.
Equation (3) was used to evaluate the relative mitotic index (RMI) [30]:
To evaluate the PI, the proportion of cells in the first (M1), second (M2), and third (M3) division was obtained from 100 consecutive metaphases for each concentration (Figure 1). The proliferation index (PI) was calculated using Equation (4), and the relative proliferation index (RPI) was evaluated using Equation (5) [30].
Chromosomal Aberrations Assays
The analysis of CAs for assessing radioprotective capacity was carried out via the dicentric chromosomes assay since this biomarker is the gold standard technique for this purpose [29].
Chromosomal analysis was performed on first-division metaphases, which consisted of 46 centromeres in a total of two hundred cells (Figure S1, Supplementary Material). Chromosomal aberrations were categorized into dicentric chromosomes (Dic) and rings, with the latter being considered only when an acentric fragment was present. Extra acentric fragments were classified as acentric fragments that were not associated with dicentric and ring chromosomes. Other abnormalities such as chromatid breaks and gaps were also taken into account.
2.6. Statistical Analysis
All the results were expressed as mean ± standard deviation values. An analysis of variance was used to calculate significant differences among samples (one way ANOVA). Differences of p < 0.05 were considered as significant. Means separation was performed according to a Student’s t-test. Correlation was assessed using Spearman’s rank correlation coefficient for genotoxicity and radioprotection analyses. All statistical analyses were performed using SPSS (Statistical Package for Social Sciences) version 10.0 for Windows. The Poisson distribution was assessed using the dispersion index test statistic U (variance/mean). A value of U greater than 1.96 indicates over-dispersion at the 5% significance level.
3. Results and Discussion
3.1. Bioactive Compounds and Antioxidant Activity
The water content of the fruit batch used in this study was xw 85.8 ± 0.3 g/100 g for K and 89.9 ± 1.3 g/100 g for S and decreased to around 2.5 ± 0.1 g/100 g for the corresponding freeze-dried samples. The concentration of each phytochemical component analysed and the AOP of kiwifruit and strawberry samples are shown in Table 1 and Figure 1. In general, the TP of kiwifruit samples was lower than in strawberries. The initial TP of fresh fruit samples varied from 349 to 2237 mg GAE/100 g db in K and S, respectively. K presented values similar to those reported by Du et al. (2009) [31], and S showed higher TP than those obtained in other studies with different strawberry varieties [32]. This variability can be attributed to the different fruit variety or states of maturity and the different solvents used in the extraction process.

Table 1.
Total phenolic content (TP), vitamin C (VC), and antioxidant activity (measured by DPPH, ABTS, and FRAP methods) of fresh kiwifruit (K) and strawberry (S) samples and freeze-dried kiwifruit (KD) and strawberry (SD) samples.
Table 1.
Total phenolic content (TP), vitamin C (VC), and antioxidant activity (measured by DPPH, ABTS, and FRAP methods) of fresh kiwifruit (K) and strawberry (S) samples and freeze-dried kiwifruit (KD) and strawberry (SD) samples.
| Sample | TP (1) | VC (2) | DPPH (3) | ABTS (3) | FRAP (4) |
|---|---|---|---|---|---|
| K | 349 ± 3 a | 359 ± 10 b | 0.84 ± 0.16 a | 2.2 ± 0.5 a | 4.41 ± 0.08 a |
| KD | 371.6 ± 1.2 b | 322 ± 5 a | 0.90 ± 0.06 a | 2.3 ± 0.2 a | 6.24 ± 0.19 b |
| −6.48% | 10.31% | −7.24% | −4.55% | −41.50% | |
| S | 2237 ± 6 y | 188.5 ± 1.3 z | 5.8 ± 0.4 z | 16.2 ± 0.9 z | 17.2 ± 0.3 z |
| SD | 2435 ± 11 z | 176 ± 3 y | 4.4 ± 0.2 y | 12.6 ± 1.2 y | 13.6 ± 0.5 y |
| −8.85% | 6.63% | 24.14% | 22.22% | 20.43% |
Values are expressed as means ± standard deviation. For each fruit, the same letters in a column indicate homogeneous groups according to a Student’s t-test at p > 0.05 (ANOVA). Percentages indicate the changes caused by the freeze-drying process (Equation (1)). (1) mg of gallic acid equivalents/100 g dry basis (db), (2) mg ascorbic acid/100 g db, (3) mmol trolox equivalents/100 g db, (4) mmol Fe2+/100 g db.
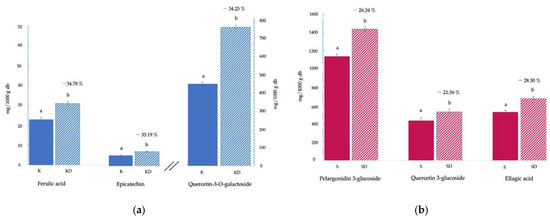
Figure 1.
Polyphenol compound content (mg/100 g dried basis): (a) kiwifruit: fresh (K) and freeze-dried (KD); (b) strawberry: fresh (S) and freeze-dried (SD). Results are expressed as mg per 1000 g dry basis (db). For each fruit and each compound, different letters indicate different homogeneous groups according to a Student’s t-test at p > 0.05 (ANOVA) between samples. Percentages indicate the changes in polyphenol compounds caused by the freeze-drying process (Equation (1)).
Generally, plant preparations are marketed in the form of liquid extracts or as powders resulting from the drying of plant material or the liquid extracts themselves. Solid forms (powders) of plants are being used increasingly because they offer numerous advantages over liquid extracts: ease of standardization; greater physical, chemical, and biological stability; lower transport and storage costs; and the possibility of achieving higher concentrations. For all these reasons, dehydration of samples is a crucial step in order to achieve a product that is suitable for industrial use. However, it is important to bear in mind that dehydration, to some extent, can affect the activity and stability of bioactive compounds due to chemical, enzymatic, and enzymatic degradation and losses through volatilisation and/or thermal degradation. The freeze-drying technique was chosen in this study because of its benefits over other dehydration techniques [21]. The comparison of K and S data with the corresponding KD and SD extracts showed that the freeze-drying process favoured (p < 0.05) the extraction of phenolic compounds. An increase in TP up to 6.48% and 8.85% was observed for kiwi and strawberry, respectively (Equation (1)). This rise could be explained based on the breaking of the remaining cellular structure of the fruit puree due to the ice crystals formed during the freezing step. In this way, the subsequent entry of the solvent is facilitated, and consequently, the extraction of the TP may be improved. The high porosity characteristic of freeze-dried products can also contribute in this sense. Other studies have also shown that the freeze-drying process increases the amount of phenolic compounds [33,34]. Freeze drying caused a reduction in the particle size of the sample, which favoured the contact surface with the solvent and could cause an increase in yield extraction. TP are considered important contributors to AOP in fruits. This activity may be related to several properties such as redox characteristics, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, and even metal chelators [35].
Spectrophotometric techniques are helpful for estimating the TP and the antioxidant capacity. However, more selective techniques, such as chromatographic ones, must be utilised to determine the individual polyphenolic profile. Figure S2 (Supplementary Material) illustrates an example of an obtained chromatogram, and Figure 1 shows the content of each of the phenolic compounds identified in the fresh fruits and in the corresponding KD (Figure 1a) and SD (Figure 1b) methanolic extracts. The percentage of changes due to the freeze-drying process (Equation (1)) has also been included. The molecules were classified as different phenolic compounds: phenolic acids (ferulic and ellagic acid), flavonols (quercetin and epicatechin), and anthocyanins (pelargonidin-3-glucoside). Specifically, the most abundant phenolic compounds found in K were quercetin-3-O-galactoside (473 ± 7 mg/1000 g db), ferulic acid (23 ± 2 mg/1000 g db), and epicatechin (5.4 ± 0.9 mg/1000 g db) and in S, they were pelargonidin-3-glucoside (1139 ± 24 mg/1000 g db), ellagic acid (530 ± 17 mg/1000 g db), and quercetin-3-glucoside (436 ± 33 mg/1000 g db). Generally, the phenolic quantities obtained are in accordance with those described in other studies [24,36]. As described for TP, freeze-dried fruit extracts showed higher concentrations of these components than fresh fruits (p < 0.05). Particularly, phenolics increased by ≈35% for kiwifruit and 21–28% for strawberry in relation to fresh samples (Equation (1)). Concerning the kiwifruit phenolic composition, Jiao et al. (2018) [37] established the significance of certain phenolic compounds in kiwifruit methanolic extracts as antioxidants. According to their study, the sequence of the phenolic components that contribute to reducing power is as follows: epicatechin > vanillic acid > p-coumaric acid > ferulic acid > syringic acid > caffeic acid > catechin. The chemical antioxidant activity of kiwifruit measured in vitro is mainly due to compounds such as phenolic acids and flavonoids which can easily donate electrons by virtue of their aromatic structures [37]. Among the major phenolic components of kiwifruit, quercetin has been reported as a potent antioxidant with radioprotective abilities [38]. Additionally, ferulic acid has been shown to be a powerful antioxidant capable of scavenging hydroxyl and peroxyl radicals as well as nitric oxide radicals. It was revealed that ferulic acid pre-treatment significantly reduced the inflammatory response associated with ionising radiation in murine models [38]. On the other hand, strawberries are also considered a rich source of polyphenols, among them, anthocyanins are quantitatively the most significant ones [20].
Kiwifruits and strawberries may also be highlighted as important sources of VC [20,31]. Along with polyphenolic compounds, VC is considered the most significant water-soluble antioxidant and it intervenes in many physiological reactions. It is effective in scavenging superoxide radical anions, hydrogen peroxide, hydroxyl radicals, singlet oxygen, and reactive nitrogen oxide [35]. The physiological and biochemical effects of VC are a result of its role as an electron donor. Specifically, AA donates two electrons from a double bond between the second and third carbons of the 6-C molecule. In the present study, VC values of K were higher than those of S (Table 1). Some authors reported that the VC in kiwifruit is higher than other fruits such as oranges, strawberries, lemons, apples, peaches, or grapes [39]. In this case, freeze-drying caused significant losses (p < 0.05) of VC by 10.31% and 6.63% in KD and SD, respectively, compared to fresh fruit (Equation (1)). When evaluating the VC content, it is important to consider that the molecule is very labile, thermosensitive, and easily oxidisable, and thus it is easily degraded [40]. In this sense, the freeze-dried sample’s increased porosity makes VC more exposed to oxygen, which could adversely affect its stability [34]. Amounts of VC in KD and SD found in this study were comparable to those described by Leontowicz et al. (2016) [41] and Nemzer et al. (2018) [42], respectively.
The AOP of fruits is crucial for determining their functional value. Over the last few decades, fruit antioxidants have attracted growing interest, suggesting that their consumption may help prevent and reduce oxidative reactions that adversely affect human health, contributing to the development of chronic diseases and cancers in various ways. Since the AOP is due to synergistic reactions between different compounds, using more than one method to measure this activity correctly is recommended. In our study, AOP was evaluated by the free radical scavenging capacity (DPPH and ABTS) and the ferric reducing antioxidant power (FRAP) assays (Table 1). With DPPH and ABTS methods, no significant differences (p > 0.05) were detected among the scavenging activity of kiwifruit samples, so neither the freeze-drying process nor the operations to obtain the dry extract affected this functional property. Our results agree with those reported in the literature [18]. However, the AOP of the kiwifruit samples determined using the FRAP assay showed that KD had significantly (p < 0.05) more ability to reduce Fe3+ to Fe2+ than K (gains of 41.5%). The FRAP reducing power appears to be correlated with the degree of hydroxylation and amount of conjugation in polyphenols [39]. The increase in KD antioxidant activity measured by FRAP may be caused by the formation of novel antioxidant substances, such as polymeric structures of polyphenols with greater antioxidant capacities. In any case, when phenol extracts contain small molecule reducing agents like ascorbic acid, interpretation becomes challenging. For strawberry, S showed the greater AOP (p < 0.05) for all three methods. Freeze drying caused losses (p < 0.05) of 20–24% depending on the analysis method (Equation (1)). Although a significant decrease in the AOP of freeze-dried strawberries was observed, these samples still retain appropriate quality concerning the original content. In general, our results were consistent with the observations reported by Floegel et al. (2011) [43].
3.2. Genotoxicity and Cytotoxicity Biomarkers
3.2.1. Sister Chromatid Exchanges (SCE)
Regarding the results of the SCE analysis, YSCE, Y1SCE, Y2SCE, and Y3SCE values for each concentration of KD and SD are shown in Table 2. All tested concentrations of fruit extracts produced a decrease in the YSCE when the concentration–response data were analysed. In contrast, only the SD with the highest concentration (800 µg/mL) showed a significant reduction (p < 0.05) compared to the blood control and the treatment with methanol/water (70:30). The general reasons can be attributed to antioxidants, including VC and phenolic compounds, that can prevent genotoxic damage, as reported by Mrdanovic et al. (2012) [7]. The treatment with methanol/water (70:30) slightly increased YSCE; however, no significant differences (p > 0.05) were observed compared to the blood control.

Table 2.
The frequency of sister chromatid exchange (YSCE) in human lymphocytes exposed to methanol/water (70:30 v/v) and different concentrations of freeze-dried kiwifruit (KD) and strawberry (SD) extracts (50, 400, and 800 µg/mL) and frequencies of chromosomes with one (Y1SCE), two (Y2SCE), or three (Y3SCE) sister chromatid exchanges assessed in a total of 50 cells.
As shown in Table 2, regarding Y1SCE, SD was the only extract that presented significant differences at any concentration tested (p < 0.05). Our data also revealed that all concentrations of SD and KD presented a statistically significant Y2SCE (p < 0.05) when compared to the treatment with methanol/water (70:30). In our study, it was observed that SD at 800 µg/mL concentration was the only extract that presented statistical differences in chromosomes with one, two, or three exchanges, implicating the non-genotoxicity of this extract at high concentrations. In their study of the protective role of VC, Türkez and Aydın (2012) [44] concluded that ascorbic acid was not genotoxic and reduced the frequencies of SCEs. Mrdanovic et al. (2012) [7], in their study about the effects of orally administered antioxidants on the frequency of sister chromatid exchanges (SCEs) conducted on workers professionally exposed to antineoplastic agents, concluded that the combination of antioxidants could effectively prevent genotoxic damage induced by cytotoxic drugs. In this sense, the AOP of the studied extracts may have contributed to the decreased YSCE.
3.2.2. Mitotic Index and Proliferation Index
An increase or decrease in the MI can determine cytotoxicity levels induced by exposure to a wide range of compounds. An MI study of KD and SD on in vitro lymphocyte cultures was performed in a total of 500 cells for each experiment. Data concerning the MI are summarised in Table 3. Lymphocytes cultured in the presence of 50, 400, and 800 µg/mL of KD exhibited a similar MI to those recorded in lymphocytes without the fruit extracts (control) and with solvent (solvent control). However, a significant decrease (p < 0.05) in MI was observed in cultures treated with 800 µg/mL of SD. In addition, other natural extracts have demonstrated cytotoxic activity against human cells in vitro [11,45]. The observed decrease in mitotic activity could be attributed to various factors, such as the interruption of the mitotic cell cycle during interphase, inhibition of crucial nuclear protein synthesis required for normal mitotic progression, or suppression of DNA synthesis [46].

Table 3.
Mitotic (MI) and proliferation (PI) indices determined in human peripheral lymphocytes cultured for 72 h in the presence of methanol/water (70:30 v/v) solvent and various concentrations of freeze-dried kiwifruit (KD) and strawberry (SD) extracts (50, 400, and 800 µg/mL). The table also shows each concentration’s relative mitotic index (RMI, %) and relative proliferation index (RPI, %).
Data concerning the PI of KD and SD in lymphocytes are summarised in Table 3. Lymphocyte cultures treated only with the solvent or with the different concentrations of KD and SD showed PI values similar to the control value (p > 0.05). Therefore, it can be concluded that these extracts do not exhibit cytostatic effects on human peripheral blood lymphocytes in in vitro cultures and, consequently, are not genotoxic. Other natural extracts have also shown results similar to our in vitro study of PI [45].
The relative indexes RMI and PRI were similar to the control (p > 0.05) (Table 3). Studies examining the relationship between SCE actions and various expressions of cytotoxicity have indicated that an increase in SCE frequency is often accompanied by decreased cell survival and alterations in cell cycle kinetics, resulting in lower values of the mitotic index (MI) [47]. In preclinical screening of natural products for potential antitumor activity, it is common to evaluate a single parameter, typically cell death. However, more comprehensive information can be obtained by assessing two biological endpoints, such as the MI and proliferation index (PI) assays [30]. For example, a reduction in the MI can indicate cell death or arrest of the cell cycle at any stage during interphase.
Our results have demonstrated that the highest concentration of SD reduced the MI, accompanied by a reduction in the YSCE; this might be because SD at 800 µg/mL was an antineoplastic and protective agent against SCE induction, therefore preventing the development, growth, or proliferation of malignant tumour cells.
3.2.3. Chromosomal Aberrations as Radioprotection Biomarkers
The cytogenetic results obtained from the analysis of human lymphocytes exposed to 2 Gy radiation in the presence of varying concentrations of KD and SD are presented in Table 4. After 2 Gy irradiation, the results revealed a decrease in the frequency of dicentric chromosomes (YDic) with an increase in the concentration of KD and SD. The only concentrations for which the reduction in YDic (and therefore reduction in damage) was statistically significant (p < 0.05) were KD at medium and high concentrations (400 μg/mL and 800 μg/mL) when compared to the control treated with methanol/water at 70:30. In our study, other types of CAs induced by the extracts were found (acentric fragments).

Table 4.
Chromosomal aberrations in human lymphocytes exposed to a radiation dose of 2 Gy under different conditions: pre-treated with methanol/water (70:30 v/v) solvent and pre-treated with varying concentrations of kiwifruit (KD) and strawberry (SD) extracts (50, 400, and 800 µg/mL).
The distribution of cells treated and untreated with KD and SD containing a different number of dicentrics is shown in Table 5. After a uniform radiation exposure, the intercellular distribution of dicentrics followed a Poisson distribution in all cases, with or without extracts (U = ±1.96). A value of U > 1.96 indicates overdispersion at the 5% level of significance.

Table 5.
Distribution of dicentric cells (Dic), dispersion index (DI), and normalised unit of this index (U) for each condition treatment with 2 Gy radiation. Samples were pre-treated with methanol/water (70:30 v/v) solvent and pre-treated with different concentrations of freeze-dried kiwifruit (KD) and strawberry (SD) extracts (50, 400, and 800 µg/mL).
Several studies have confirmed that the health protective effect associated with kiwifruit is mainly related to their phytochemicals, which may prevent oxidative damage to biological systems by lowering oxidative stress and altering signal transduction pathways involved in cell survival and proliferation [48]. A possible explanation for the irradiation protective effects of antioxidants is a model based on electron spin resonance studies. According to this model, radiation exposure leads to the formation of short-lived radicals like +OH, which are responsible for cell death, as well as long-lived radicals that have the potential to induce mutations and transformations [38]. Our data showed that kiwifruit extracts reduced the chromosomal aberration frequency after irradiation, probably due to its AOP. Regarding biochemicals contributing towards radioprotection, it is known that some phenolics and VC are able to initiate a wide range of physiological responses that reduce severe chromosomal damage [49]. In this sense, VC acted effectively in the sequestration of ROS and, consequently, in the significant reduction in YDic. This antioxidant property of VC plays a significant role in safeguarding cellular macromolecules, particularly DNA, from oxidative damage [50]. Its protective effect has also been attributed to enhancing other antioxidant compounds’ repair and recovery processes [51]. In this sense, the antioxidant and radioprotective properties of AA have been described by Jagetia et al. [52]. The radio protective activity of this component depends on its antioxidant and free radical scavenging property. Other studies also described that AA was radioprotective in reducing radiation-induced skin excisions in Swiss albino mice exposed to gamma radiation, and also increased their wound healing [53]. On the other hand, the radioprotective effect of phenolics may be mediated through several mechanisms besides free radical scavenging, such as improvements in the antioxidant state and anti-lipid peroxidation potential. These biological properties are conferred due to the presence of a variety of phenolic hydroxyl groups linked to the ring structure. Some studies attribute the protective effect of flavonoids to their direct hydroxyl radical scavenging potency and thus behave like an endogenous enzyme [54]. Polyphenols, especially flavonoid glycosides and their derivatives, contain ketone groups conjugated to aromatic rings activated by electron donor constituents. This prevents energy transfer, reduces oxidative stress, and stabilises redox processing in cells. Polyphenols may up-regulate mRNAs of antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase), thus mitigating the oxidative damage induced by ionising radiation. Up-regulation of DNA repair genes and inhibition of nitric oxide may also protect against radiation-induced DNA damage [55]. Although the mechanisms of radioprotection differ among groups, most compounds act primarily via scavenging of radiation-induced free radicals or by quenching of secondary biomolecular reactive species [53].
Regarding SD, it was observed that YDic was reduced but did not present a significant difference (p ˃ 0.05) compared to the methanol/water control. This means that strawberry extracts at all studied concentrations did not reduce the damage caused by the effect of ionising radiation. Despite the high AOP, phenolic compounds and TP, SD did not act effectively in reducing the YDic. The different radioprotective responses of both fruits may indicate that radioprotection is more related to the type of phenolic compound than to the amount of them, and as it has already been commented, their biological action depends on their chemical structure.
Altogether, our findings encourage further studies to fully understand which molecules are effective and what mechanisms are involved in their radioprotective action. Research on human bioavailability of the phytochemicals involved is also advised.
4. Conclusions
Rapid technological development, radiation exposure from natural or artificial sources, radiotherapy, medical imaging, etc., cause unintended toxicity and contribute to severe radiation-related pathologies. It is crucial to safeguard the biological system from such a barrage of detrimental effects from radiation. Scientific research has led to the creation of several synthetic chemicals to combat radiation’s potential dangers. However, the need for alternative natural sources is vital due to its toxicity and adverse effects. In this report, methanolic extracts were obtained from freeze-dried kiwifruit and strawberries. Comparing both fruits, kiwifruit stood out for its vitamin C, ferulic acid, epicatechin, and quercetin-3-O-galactoside content, and strawberry for its total phenol content, ellagic acid, pelargonidin-3-glucoside, quercetin-3-glucoside, and antioxidant activity. The freeze-drying process resulted in gains in the phenolic compounds of both samples and in the antioxidant activity of the kiwifruit. Then, the radioprotective effect of methanolic extracts from freeze-dried kiwifruit and strawberries against the damage induced by γ-irradiation in human peripheral blood lymphocytes was investigated. The results showed that kiwifruit methanolic extracts at concentrations of 400 and 800 µg/mL possessed radioprotective ability, as evidenced by the significant reduction in the chromosomal aberration frequency after irradiation at a dose of 2 Gy. However, freeze-dried strawberry methanolic extracts did not reduce the damage caused by the effect of ionising radiation. None of the samples were found to exhibit genotoxic or cytotoxic effects. Although the free radical scavenging capacity of the extracts represents a possible mechanism for their radioprotective effect, it cannot be concluded that a greater antioxidant activity implies radioprotective power. The different responses of both fruits may indicate that radioprotection may be related to the different antioxidants present, such as vitamin C, and to the type of phenolic compound rather than to the amount of them, as their biological action depends on their chemical structure. Additionally, the synergistic effect between antioxidant substances must also be taken into account. Further investigation on radioprotection mechanisms is worth conducting in the future. Although potentially protective agents against harmful radiation have been investigated for decades, there is currently no ideal radioprotectant available. Therefore, it is very important that natural compounds with a potential radioprotective effect are studied for medical applications and in radiological and nuclear emergencies. The next step carried out by our research group will be in vivo studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13158996/s1, Figure S1: Metaphases with radioprotective and genotoxic biomarkers used in this study. Figure S2: Representative HPLC chromatogram of polyphenols of strawberries. (1) Pelargonidin-3-Glucoside, (2) quercetin-3-Glucoside, (3) ellagic acid.
Author Contributions
Conceptualisation, A.M. and E.G.-M.; methodology, N.S., A.M. and E.G.-M.; formal analysis, N.S., A.M. and E.G.-M.; data curation, M.d.S.R.; writing—original draft preparation, N.S., A.M. and E.G.-M.; writing—review and editing, E.G.-M.; supervision, A.M. and E.G.-M.; project administration, A.M. and E.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vicerrectorado de Investigación, Universitat Politècnica de València (UPV-FE-2013-56) (Spain).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hospital Universitario y Politécnico La Fe (date of approval: 4 April 2018) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Alberto Yuste del Carmen for the technical support. M.d.S.R. thanks CAPES for the financial support (no. 99999.013619/2013-06-DOC-PLENO; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Coordination for the Improvement of Higher Education of Brazil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Szeijk, A.; Tomasz, P.; Czubatka-Bienkowska, A.; Olejnik, A.K.; Pawlaczyk-Graja, I.; Gancarz, R.; Zbikowska, H.M. A comparative study on the radioprotective potential of the polyphenolic glycoconjugates from medicinal plants of Rosaceae and Asteraceae families versus their aglycones. J. Photochem. Photobiol. 2017, 171, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Shahani, S.; Rostamnezhad, M.; Ghaffarirad, V.; Ghasemi, A.; Pourfallah, T.A.; Hosseinimehr, S.J. Radioprotective effect of Achillea millefolium L. against genotoxicity induced by ionising radiation in human normal lymphocytes. Dose-Response 2015, 13, 1559325815583761. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubertv, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionising radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef]
- Mettler, F.E.J.; Brenner, D.; Coleman, C.N.; Kaminski, J.M.; Kennedy, A.R. Can radiation risks to patients be reduced without reducing radiation exposure? The status of chemical radioprotectants. Med. Phys. Inf. Clin. Perspect. 2011, 196, 616–618. [Google Scholar] [CrossRef]
- Zbikowska, H.M.; Szejk, M.; Saluk, J.; Gancarz, R.; Olejnik, A.K. Polyphenolic–polysaccharide conjugates from plants of Rosaceae/Asteraceae family as potential radioprotectors. Int. J. Biol. Macromol. 2016, 86, 329–337. [Google Scholar] [CrossRef]
- Mrdanovic, J.; Jungic, S.; Šolajic, S.; Bogdanovic, V.; Jurišic, V. Effects of orally administered antioxidants on micronuclei and sister chromatid exchange frequency in workers professionally exposed to antineoplastic agents. Food Chem. Toxicol. 2012, 50, 2937–2944. [Google Scholar] [CrossRef]
- Bianchi, J.; Fernandes, T.C.C.; Marin-Morales, M.A. Induction of mitotic and chromosomal abnormalities on Allium cepa cells by pesticides imidacloprid and sulfentrazone and the mixture of them. Chemosphere 2016, 144, 475–483. [Google Scholar] [CrossRef]
- Rojas, E.; Herrera, L.A.; Sordo, M.; Gonsebatt, M.E.; Montero, R.; Rodríguez, R.; Ostrosky-Wegman, P. Mitotic index and cell proliferation kinetics for identification of antineoplastic activity. Anti-Cancer Drugs 1993, 4, 599–672. [Google Scholar] [CrossRef]
- Kuntic, V.S.; Stankovic, M.B.; Vujic, Z.B.; Brboric, S.J.; Markovic, S. Radioprotectors—The Evergreen Topic. Chem. Biodivers. 2013, 10, 1791–1803. [Google Scholar] [CrossRef]
- Sebastià, N.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M.; Montoro, A. Radioprotective activity and cytogenetic effect of resveratrol in human lymphocytes: An in vitro evaluation. Food Chem. Toxicol. 2013, 51, 391–395. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef]
- Montoro, A.; Soriano, J.M.; Barquinero, J.F.; Almonacid, M.; Verdú, G.; Sahuquillo, V.; Villaescusa, J.I.; Sebastià, N. Assessment in vitro of cytogenetic and genotoxic effects of propolis on human lymphocytes. Food Chem. Toxicol. 2012, 50, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Ranjan, A.; Kaur, N.; Sur, S.; Tandon, V. Radioprotective Agents: Strategies and Translational Advances. Med. Res. Rev. 2016, 36, 461–493. [Google Scholar] [CrossRef]
- Hlatky, L.; Sachs, R.K.; Vazquez, M.; Cornforth, M.N. Radiation-induced chromosome aberrations: Insights gained from biophysical modelling. BioEssays 2002, 24, 14–723. [Google Scholar] [CrossRef]
- Mazzoni, L.; Perez-Lopez, P.; Giampieri, F.; Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Mezzetti, B.; Battino, M. The genetic aspects of berries: From field to health. J. Sci. Food Agric. 2016, 96, 365–371. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, S46–S59. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmian, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Burillo, P.S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Errico Provenzano, A.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; García-Martínez, E.; Martínez-Navarrete, N. Protective capacity of gum Arabic, maltodextrin, different starches, and fibers on the bioactive compounds and antioxidant activity of an orange puree (Citrus sinensis (L.) Osbeck) against freeze-drying and in vitro digestion. Food Chem. 2021, 357, 129724. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sowndhararajan, K.; Kim, M.; Kim, J.; Kim, D.; Kim, S.; Kim, G.Y.; Kim, S.; Jhoo, J.W. Antioxidant, inhibition of a-glucosidase and suppression of nitric oxide production in LPS-induced murine macrophages by different fractions of Actinidia arguta stem. Saudi J. Biol. Sci. 2014, 21, 532–538. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Gramza-Michałowska, A.; Bueschke, M.; Kulczyński, B.; Gliszczyńska-Świgło, A.; Kmiecik, D.; Bilska, A.; Purłan, M.; Wałęsa, L.; Ostrowski, M.; Filipczuk, M.; et al. Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas. Charact. 2019, 13, 1739–1747. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In vitro safety assessment of the strawberry tree (Arbutus unedo L.) water leaf extract and arbutin in human peripheral blood lymphocytes. Cytotechnology 2018, 70, 1261–1278. [Google Scholar] [CrossRef]
- Iwasawa, H.; Morita, E.; Yui, S.; Yamazaki, M. Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol. Pharm. Bull. 2011, 34, 128–134. [Google Scholar] [CrossRef]
- Weaver, J.; Briscoe, T.; Hou, M.; Goodman, C.; Kata, S.; Ross, H.; McDougall, G.; Stewart, D.; Riches, A. Strawberry polyphenols are equally cytotoxic to tumourigenic and normal human breast and prostate cell lines. Int. J. Oncol. 2009, 34, 777–786. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Biological Dosimetry: Chromosomal Aberration Analysis for Dose Assessment; Technical Reports Series; IAEA: Viena, Austria, 2011; p. 260. [Google Scholar]
- Rojas, E.; Montero, R.; Herrera, L.A.; Sordo, M.; Gonsebatt, M.E.; Rodriguez, R.; Ostrosky-Wegman, P. Are mitotic index and lymphocyte proliferation kinetics reproducible endpoints in genetic toxicology testing? Mutat. Res. Lett. 1992, 282, 283–286. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Tudor, V.; Manole, C.G.; Teodorescu, R.; Asanica, A.; Barbulescu, J.D. Analysis of some phenolic compounds and free radical scavenging activity of strawberry fruits during storage period. Agric. Agric. Sci. Procedia 2015, 6, 157–164. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- García-Martínez, E.; Camacho, M.; Nuria Martínez-Navarrete, N. In Vitro Bioaccessibility of Bioactive Compounds of Freeze-Dried Orange Juice Co-Product Formulated with Gum Arabic and Modified Starch. Molecules 2023, 28, 810. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Hettihewa, S.K.; Hemar, Y.; Rupasinghe. H.P. Flavonoid-Rich Extract of Actinidia macrosperma (AWild Kiwifruit) inhibits Angiotensin-Converting Enzyme In Vitro. Foods 2018, 7, 146. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- Prasad, J.; Pattnaik, S.; Arya, R.; Dash, R.; Sahoo, S.; Pradhan, B.; Bhuyan, P.; Behera, P.; Jena, M.; Sharma, A.; et al. Phytochemicals: A potential next generation agent for radioprotection. Phytomedicine 2022, 106, 154188. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Klimczak, I.; Malecka, M.; Miroslawa, S.; Gliszczynska-Swiglo, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xiaa, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.; Chung, S.; Sung, I.K.; Chun, C.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comps. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Türkez, H.; Aydın, E. The protective role of ascorbic acid on imazalil-induced genetic damage assessed by the cytogenetic tests. Toxicol. Ind. Health 2012, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Montoro, A.; Almonacid, M.; Serrano, J.; Saiz, M.; Barquinero, J.F.; Barrios, L.; Verdu, G.; Pérez, J.; Villaescusa, J.I. Assessment by cytogenetic analysis of the radioprotection properties of propolis extract. Radiat. Prot. Dosim. 2005, 115, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Das, A.B.; Das, P.; Mohanty, P. Effect of a low dose of aluminum on mitotic and meiotic activity, 4C DNA content, and pollen sterility in rice, Oryza sativa L. cv. Lalat. Ecotoxicol. Environ. Saf. 2004, 59, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mourelatos, D. Chromosomes study as predictor of chemo response of tumours. Cancer J. 1996, 9, 136–141. [Google Scholar]
- Brevik, A.; Gaivão, I.; Medin, T.; Jørgenesen, A.; Piasek, A.; Elilasson, J.; Karlsen, A.; Blomhoff, R.; Veggan, T.; Duttaroy, A.K.; et al. Supplementation of a western diet with golden kiwifruits (Actinidia chinensis var ‘Hort 16A’) effects on biomarkers of oxidation damage and antioxidant protection. Nutr. J. 2011, 10, 54. [Google Scholar] [CrossRef]
- Badjatia, N.; Satyam, A.; Singh, P.; Seth, A.; Sharma, A. Altered antioxidant status and lipid peroxidation in Indian patients with urothelial bladder carcinoma. Urol. Oncol. Semin. Orig. Investig. 2009, 28, 360–367. [Google Scholar] [CrossRef]
- Montecinos, V.; Guzman, P.; Barra, V.; Villadgran, M.; Munoz-Motesino, C.; Sotomayor, K.; Escobar, E. Vitamin C is an essential antioxidant that enhances survival of oxidatively stressed human vascular endothelial cells in the presence of a vast molar excess of glutathione. J. Biol. Chem. 2007, 282, 15506–15515. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free. Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rajanikant, G.; Rao, S.K. Evaluation of the effect of ascorbic acid treatment on wound healing in mice exposed to different doses of fractionated gamma radiation. Radiat. Res. 2003, 159, 371–380. [Google Scholar] [CrossRef]
- Prasad, N.R.; Srinivasan, M.; Pugalendi, K.; Menon, V.P. Protective effect of ferulic acid on γ-radiation-induced micronuclei, dicentric aberration and lipid peroxidation in human lymphocytes. Mutat. Res. 2006, 603, 129–134. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. Trends in the development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef]
- Kemertelidze, E.P.; Tsitsishvili, V.G.; Alaniya, M.D.; Sagareishvili, T.G. Structure-function analysis of the radioprotective and antioxidant activity of flavonoids. Chem. Nat. Compd. 2000, 36, 54–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).