Strawberry (Fragaria × ananassa) and Kiwifruit (Actinidia deliciosa) Extracts as Potential Radioprotective Agents: Relation to Their Phytochemical Composition and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fruit Samples
2.3. Extracts Preparation
2.4. Chemical Determinations
2.4.1. Water Content
2.4.2. VC
2.4.3. Phenolic Compounds
2.4.4. Total Polyphenols
2.4.5. AOP Evaluation
2.5. Biological Determinations
2.5.1. Culture Conditions
2.5.2. Irradiation Conditions for Radioprotective Activity
2.5.3. Stain Technique
2.5.4. Cytogenetic Analysis
Sister Chromatid Exchange
Evaluation of MI and PI
Chromosomal Aberrations Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Compounds and Antioxidant Activity
| Sample | TP (1) | VC (2) | DPPH (3) | ABTS (3) | FRAP (4) |
|---|---|---|---|---|---|
| K | 349 ± 3 a | 359 ± 10 b | 0.84 ± 0.16 a | 2.2 ± 0.5 a | 4.41 ± 0.08 a |
| KD | 371.6 ± 1.2 b | 322 ± 5 a | 0.90 ± 0.06 a | 2.3 ± 0.2 a | 6.24 ± 0.19 b |
| −6.48% | 10.31% | −7.24% | −4.55% | −41.50% | |
| S | 2237 ± 6 y | 188.5 ± 1.3 z | 5.8 ± 0.4 z | 16.2 ± 0.9 z | 17.2 ± 0.3 z |
| SD | 2435 ± 11 z | 176 ± 3 y | 4.4 ± 0.2 y | 12.6 ± 1.2 y | 13.6 ± 0.5 y |
| −8.85% | 6.63% | 24.14% | 22.22% | 20.43% |
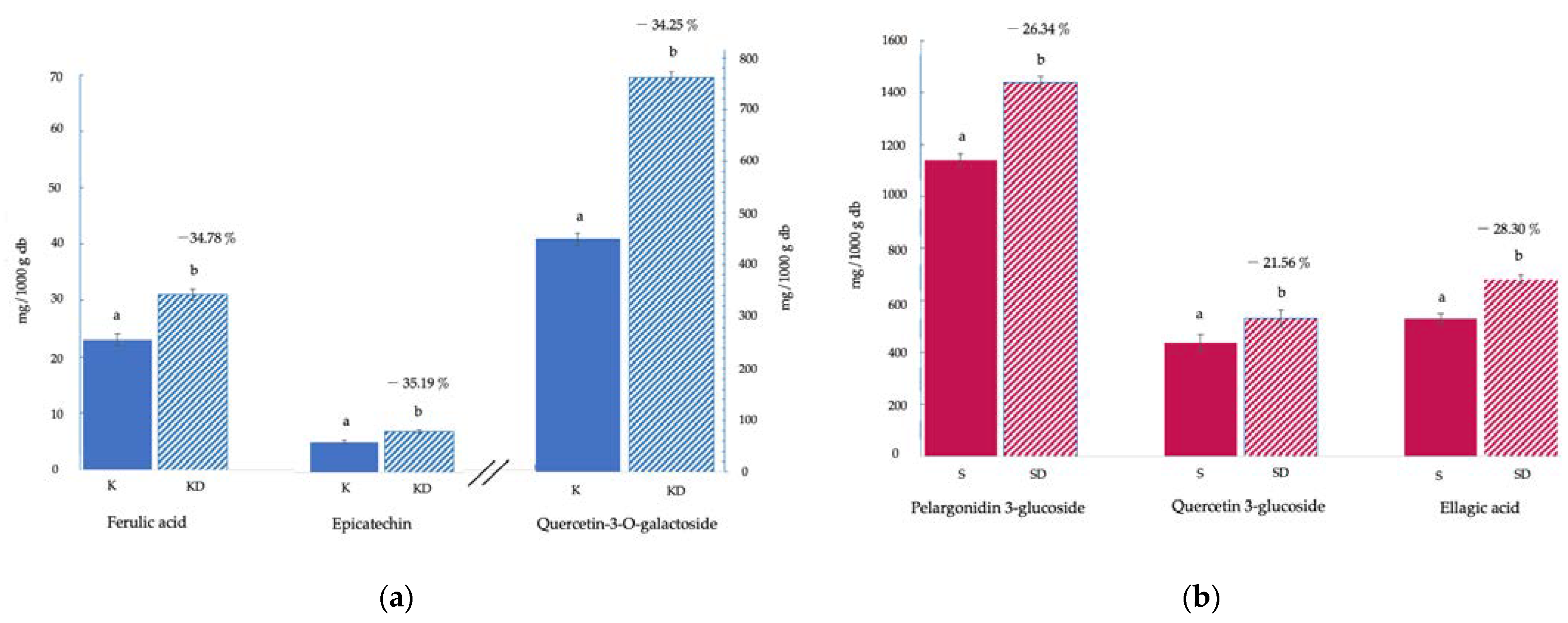
3.2. Genotoxicity and Cytotoxicity Biomarkers
3.2.1. Sister Chromatid Exchanges (SCE)
3.2.2. Mitotic Index and Proliferation Index
3.2.3. Chromosomal Aberrations as Radioprotection Biomarkers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Szeijk, A.; Tomasz, P.; Czubatka-Bienkowska, A.; Olejnik, A.K.; Pawlaczyk-Graja, I.; Gancarz, R.; Zbikowska, H.M. A comparative study on the radioprotective potential of the polyphenolic glycoconjugates from medicinal plants of Rosaceae and Asteraceae families versus their aglycones. J. Photochem. Photobiol. 2017, 171, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Shahani, S.; Rostamnezhad, M.; Ghaffarirad, V.; Ghasemi, A.; Pourfallah, T.A.; Hosseinimehr, S.J. Radioprotective effect of Achillea millefolium L. against genotoxicity induced by ionising radiation in human normal lymphocytes. Dose-Response 2015, 13, 1559325815583761. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubertv, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionising radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef]
- Mettler, F.E.J.; Brenner, D.; Coleman, C.N.; Kaminski, J.M.; Kennedy, A.R. Can radiation risks to patients be reduced without reducing radiation exposure? The status of chemical radioprotectants. Med. Phys. Inf. Clin. Perspect. 2011, 196, 616–618. [Google Scholar] [CrossRef]
- Zbikowska, H.M.; Szejk, M.; Saluk, J.; Gancarz, R.; Olejnik, A.K. Polyphenolic–polysaccharide conjugates from plants of Rosaceae/Asteraceae family as potential radioprotectors. Int. J. Biol. Macromol. 2016, 86, 329–337. [Google Scholar] [CrossRef]
- Mrdanovic, J.; Jungic, S.; Šolajic, S.; Bogdanovic, V.; Jurišic, V. Effects of orally administered antioxidants on micronuclei and sister chromatid exchange frequency in workers professionally exposed to antineoplastic agents. Food Chem. Toxicol. 2012, 50, 2937–2944. [Google Scholar] [CrossRef]
- Bianchi, J.; Fernandes, T.C.C.; Marin-Morales, M.A. Induction of mitotic and chromosomal abnormalities on Allium cepa cells by pesticides imidacloprid and sulfentrazone and the mixture of them. Chemosphere 2016, 144, 475–483. [Google Scholar] [CrossRef]
- Rojas, E.; Herrera, L.A.; Sordo, M.; Gonsebatt, M.E.; Montero, R.; Rodríguez, R.; Ostrosky-Wegman, P. Mitotic index and cell proliferation kinetics for identification of antineoplastic activity. Anti-Cancer Drugs 1993, 4, 599–672. [Google Scholar] [CrossRef]
- Kuntic, V.S.; Stankovic, M.B.; Vujic, Z.B.; Brboric, S.J.; Markovic, S. Radioprotectors—The Evergreen Topic. Chem. Biodivers. 2013, 10, 1791–1803. [Google Scholar] [CrossRef]
- Sebastià, N.; Almonacid, M.; Villaescusa, J.I.; Cervera, J.; Such, E.; Silla, M.A.; Soriano, J.M.; Montoro, A. Radioprotective activity and cytogenetic effect of resveratrol in human lymphocytes: An in vitro evaluation. Food Chem. Toxicol. 2013, 51, 391–395. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef]
- Montoro, A.; Soriano, J.M.; Barquinero, J.F.; Almonacid, M.; Verdú, G.; Sahuquillo, V.; Villaescusa, J.I.; Sebastià, N. Assessment in vitro of cytogenetic and genotoxic effects of propolis on human lymphocytes. Food Chem. Toxicol. 2012, 50, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Ranjan, A.; Kaur, N.; Sur, S.; Tandon, V. Radioprotective Agents: Strategies and Translational Advances. Med. Res. Rev. 2016, 36, 461–493. [Google Scholar] [CrossRef]
- Hlatky, L.; Sachs, R.K.; Vazquez, M.; Cornforth, M.N. Radiation-induced chromosome aberrations: Insights gained from biophysical modelling. BioEssays 2002, 24, 14–723. [Google Scholar] [CrossRef]
- Mazzoni, L.; Perez-Lopez, P.; Giampieri, F.; Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Mezzetti, B.; Battino, M. The genetic aspects of berries: From field to health. J. Sci. Food Agric. 2016, 96, 365–371. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, S46–S59. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmian, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Burillo, P.S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Errico Provenzano, A.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; García-Martínez, E.; Martínez-Navarrete, N. Protective capacity of gum Arabic, maltodextrin, different starches, and fibers on the bioactive compounds and antioxidant activity of an orange puree (Citrus sinensis (L.) Osbeck) against freeze-drying and in vitro digestion. Food Chem. 2021, 357, 129724. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sowndhararajan, K.; Kim, M.; Kim, J.; Kim, D.; Kim, S.; Kim, G.Y.; Kim, S.; Jhoo, J.W. Antioxidant, inhibition of a-glucosidase and suppression of nitric oxide production in LPS-induced murine macrophages by different fractions of Actinidia arguta stem. Saudi J. Biol. Sci. 2014, 21, 532–538. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Gramza-Michałowska, A.; Bueschke, M.; Kulczyński, B.; Gliszczyńska-Świgło, A.; Kmiecik, D.; Bilska, A.; Purłan, M.; Wałęsa, L.; Ostrowski, M.; Filipczuk, M.; et al. Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas. Charact. 2019, 13, 1739–1747. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In vitro safety assessment of the strawberry tree (Arbutus unedo L.) water leaf extract and arbutin in human peripheral blood lymphocytes. Cytotechnology 2018, 70, 1261–1278. [Google Scholar] [CrossRef]
- Iwasawa, H.; Morita, E.; Yui, S.; Yamazaki, M. Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol. Pharm. Bull. 2011, 34, 128–134. [Google Scholar] [CrossRef]
- Weaver, J.; Briscoe, T.; Hou, M.; Goodman, C.; Kata, S.; Ross, H.; McDougall, G.; Stewart, D.; Riches, A. Strawberry polyphenols are equally cytotoxic to tumourigenic and normal human breast and prostate cell lines. Int. J. Oncol. 2009, 34, 777–786. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Biological Dosimetry: Chromosomal Aberration Analysis for Dose Assessment; Technical Reports Series; IAEA: Viena, Austria, 2011; p. 260. [Google Scholar]
- Rojas, E.; Montero, R.; Herrera, L.A.; Sordo, M.; Gonsebatt, M.E.; Rodriguez, R.; Ostrosky-Wegman, P. Are mitotic index and lymphocyte proliferation kinetics reproducible endpoints in genetic toxicology testing? Mutat. Res. Lett. 1992, 282, 283–286. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Tudor, V.; Manole, C.G.; Teodorescu, R.; Asanica, A.; Barbulescu, J.D. Analysis of some phenolic compounds and free radical scavenging activity of strawberry fruits during storage period. Agric. Agric. Sci. Procedia 2015, 6, 157–164. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- García-Martínez, E.; Camacho, M.; Nuria Martínez-Navarrete, N. In Vitro Bioaccessibility of Bioactive Compounds of Freeze-Dried Orange Juice Co-Product Formulated with Gum Arabic and Modified Starch. Molecules 2023, 28, 810. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Hettihewa, S.K.; Hemar, Y.; Rupasinghe. H.P. Flavonoid-Rich Extract of Actinidia macrosperma (AWild Kiwifruit) inhibits Angiotensin-Converting Enzyme In Vitro. Foods 2018, 7, 146. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- Prasad, J.; Pattnaik, S.; Arya, R.; Dash, R.; Sahoo, S.; Pradhan, B.; Bhuyan, P.; Behera, P.; Jena, M.; Sharma, A.; et al. Phytochemicals: A potential next generation agent for radioprotection. Phytomedicine 2022, 106, 154188. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Klimczak, I.; Malecka, M.; Miroslawa, S.; Gliszczynska-Swiglo, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xiaa, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.; Chung, S.; Sung, I.K.; Chun, C.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comps. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Türkez, H.; Aydın, E. The protective role of ascorbic acid on imazalil-induced genetic damage assessed by the cytogenetic tests. Toxicol. Ind. Health 2012, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Montoro, A.; Almonacid, M.; Serrano, J.; Saiz, M.; Barquinero, J.F.; Barrios, L.; Verdu, G.; Pérez, J.; Villaescusa, J.I. Assessment by cytogenetic analysis of the radioprotection properties of propolis extract. Radiat. Prot. Dosim. 2005, 115, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Das, A.B.; Das, P.; Mohanty, P. Effect of a low dose of aluminum on mitotic and meiotic activity, 4C DNA content, and pollen sterility in rice, Oryza sativa L. cv. Lalat. Ecotoxicol. Environ. Saf. 2004, 59, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mourelatos, D. Chromosomes study as predictor of chemo response of tumours. Cancer J. 1996, 9, 136–141. [Google Scholar]
- Brevik, A.; Gaivão, I.; Medin, T.; Jørgenesen, A.; Piasek, A.; Elilasson, J.; Karlsen, A.; Blomhoff, R.; Veggan, T.; Duttaroy, A.K.; et al. Supplementation of a western diet with golden kiwifruits (Actinidia chinensis var ‘Hort 16A’) effects on biomarkers of oxidation damage and antioxidant protection. Nutr. J. 2011, 10, 54. [Google Scholar] [CrossRef]
- Badjatia, N.; Satyam, A.; Singh, P.; Seth, A.; Sharma, A. Altered antioxidant status and lipid peroxidation in Indian patients with urothelial bladder carcinoma. Urol. Oncol. Semin. Orig. Investig. 2009, 28, 360–367. [Google Scholar] [CrossRef]
- Montecinos, V.; Guzman, P.; Barra, V.; Villadgran, M.; Munoz-Motesino, C.; Sotomayor, K.; Escobar, E. Vitamin C is an essential antioxidant that enhances survival of oxidatively stressed human vascular endothelial cells in the presence of a vast molar excess of glutathione. J. Biol. Chem. 2007, 282, 15506–15515. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free. Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rajanikant, G.; Rao, S.K. Evaluation of the effect of ascorbic acid treatment on wound healing in mice exposed to different doses of fractionated gamma radiation. Radiat. Res. 2003, 159, 371–380. [Google Scholar] [CrossRef]
- Prasad, N.R.; Srinivasan, M.; Pugalendi, K.; Menon, V.P. Protective effect of ferulic acid on γ-radiation-induced micronuclei, dicentric aberration and lipid peroxidation in human lymphocytes. Mutat. Res. 2006, 603, 129–134. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. Trends in the development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef]
- Kemertelidze, E.P.; Tsitsishvili, V.G.; Alaniya, M.D.; Sagareishvili, T.G. Structure-function analysis of the radioprotective and antioxidant activity of flavonoids. Chem. Nat. Compd. 2000, 36, 54–59. [Google Scholar] [CrossRef]
| Conditions | YSCE | Y1SCE | Y2SCE | Y3SCE |
|---|---|---|---|---|
| 0 (control) | 8.0 ± 0.5 | 7.2 ± 0.4 | 0.32 ± 0.08 | 0.06 ± 0.03 |
| 0 + methanol/water 70:30 (control + solvent) | 8.2 ± 0.5 | 6.5 ± 0.4 | 0.86 ± 0.13 | 0.00 ± 0.00 |
| KD 50 | 7.5 ± 0.4 | 6.0 ± 0.3 | 0.66 ± 0.11 * | 0.06 ± 0.03 * |
| KD 400 | 7.8 ± 0.5 | 7.0 ± 0.4 | 0.32 ± 0.08 * | 0.04 ± 0.03 * |
| KD 800 | 7.8 ± 0.5 | 6.3 ± 0.3 | 0.60 ± 0.11 * | 0.10 ± 0.04 * |
| SD 50 | 6.4 ± 0.3 | 5.4 ±0.3 * | 0.48 ± 0.10 * | 0.02 ± 0.02 * |
| SD 400 | 6.4 ± 0.5 | 5.5 ± 0.5 * | 0.48 ± 0.14 * | 0.00 ± 0.00 * |
| SD 800 | 5.3 ± 0.3 * | 4.8 ± 0.3 * | 0.26 ± 0.08 * | 0.00 ± 0.00 * |
| Conditions | MI | RMI (%) | PI | RPI (%) |
|---|---|---|---|---|
| 0 (control) | 0.039 ± 0.009 | 100.00 | 1.58 ± 0.05 | 100.00 |
| 0 + methanol/water 70:30 (control + solvent) | 0.039 ± 0.009 | 99.92 | 1.59 ± 0.04 | 100.63 |
| KD 50 | 0.041 ± 0.009 | 103.47 | 1.57 ± 0.05 | 99.37 |
| KD 400 | 0.039 ± 0.009 | 98.39 | 1.59 ± 0.05 | 100.63 |
| KD 800 | 0.039 ± 0.009 | 98.39 | 1.59 ± 0.05 | 100.63 |
| SD 50 | 0.041 ± 0.009 | 105.00 | 1.57 ± 0.05 | 99.37 |
| SD 400 | 0.039 ± 0.009 | 98.39 | 1.57 ± 0.05 | 99.37 |
| SD 800 | 0.035 ± 0.008 * | 88.22 | 1.58 ± 0.05 | 100.58 |
| Conditions | N | Dic | YDic |
|---|---|---|---|
| 0 | 200 | 63 | 0.315 ± 0.037 |
| 0 + methanol/water 70:30 | 175 | 24 | 0.137 ± 0.027 |
| KD 50 | 200 | 23 | 0.115 ± 0.024 |
| KD 400 | 197 | 17 | 0.086 ± 0.020 * |
| KD 800 | 201 | 16 | 0.080 ± 0.019 * |
| SD 50 | 240 | 29 | 0.121 ± 0.022 |
| SD 400 | 175 | 20 | 0.114 ± 0.025 |
| SD 800 | 200 | 23 | 0.115 ± 0.023 |
| Conditions | N | Dic | 0Dic (1) | 1Dic (1) | 2Dic (1) | 3Dic (1) | DI | U |
|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 63 | 137 | 51 | 6 | 0 | 0.87 | −1.30 |
| 0 + methanol/water 70:30 | 175 | 24 | 151 | 22 | 1 | 0 | 0.95 | −0.47 |
| KD 50 | 200 | 23 | 177 | 21 | 1 | 0 | 0.98 | −0.24 |
| KD 400 | 197 | 17 | 180 | 17 | 0 | 0 | 0.92 | −0.83 |
| KD 800 | 201 | 16 | 185 | 16 | 0 | 0 | 0.93 | −0.77 |
| SD 50 | 240 | 29 | 211 | 27 | 1 | 0 | 0.95 | −0.54 |
| SD 400 | 175 | 20 | 155 | 18 | 1 | 0 | 0.99 | −0.09 |
| SD 800 | 200 | 23 | 177 | 23 | 0 | 0 | 0.89 | −1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.d.S.; Sebastià, N.; Montoro, A.; García-Martínez, E. Strawberry (Fragaria × ananassa) and Kiwifruit (Actinidia deliciosa) Extracts as Potential Radioprotective Agents: Relation to Their Phytochemical Composition and Antioxidant Capacity. Appl. Sci. 2023, 13, 8996. https://doi.org/10.3390/app13158996
Ribeiro MdS, Sebastià N, Montoro A, García-Martínez E. Strawberry (Fragaria × ananassa) and Kiwifruit (Actinidia deliciosa) Extracts as Potential Radioprotective Agents: Relation to Their Phytochemical Composition and Antioxidant Capacity. Applied Sciences. 2023; 13(15):8996. https://doi.org/10.3390/app13158996
Chicago/Turabian StyleRibeiro, Margareth da Silva, Natividad Sebastià, Alegría Montoro, and Eva García-Martínez. 2023. "Strawberry (Fragaria × ananassa) and Kiwifruit (Actinidia deliciosa) Extracts as Potential Radioprotective Agents: Relation to Their Phytochemical Composition and Antioxidant Capacity" Applied Sciences 13, no. 15: 8996. https://doi.org/10.3390/app13158996
APA StyleRibeiro, M. d. S., Sebastià, N., Montoro, A., & García-Martínez, E. (2023). Strawberry (Fragaria × ananassa) and Kiwifruit (Actinidia deliciosa) Extracts as Potential Radioprotective Agents: Relation to Their Phytochemical Composition and Antioxidant Capacity. Applied Sciences, 13(15), 8996. https://doi.org/10.3390/app13158996










