Abstract
This study aimed to enhance the efficiency of construction waste bricks (PRBPs) in removing diclofenac (DFC) by preparing surfactant-modified waste bricks (CCBPs) as adsorbents. The properties of the adsorbents were analyzed, and the parameters related to the adsorption process were examined. The findings demonstrate that the addition of cetyltrimethyl ammonium bromide (CTAB) significantly improved the ability of the waste bricks to adsorb DCF. The pH values affected the adsorption behavior, with the adsorption decreasing as the pH increased. The adsorption process followed the pseudo-first-order kinetic equation, and the rate of adsorption was fast. The Langmuir model was used to fit the adsorption isotherms. According to the adsorption thermodynamics, the adsorption of DCF onto both adsorbents was exothermic, and it was more favorable at lower temperatures. The adsorption capacity of the CCBPs decreased sharply as the KCl concentration increased. The mechanism of adsorption might be explained by the interplay of the π-π interaction, surface complexation, and electrostatic interaction. This study offers a new method for removing micropollutants from aqueous solutions using waste bricks, thus extending the scope of their reuse applications.
1. Introduction
Due to the significant enhancement in people’s quality of life, there has been an increase in the release of pharmaceuticals and personal care products (PPCPs) into the environment on a global scale. In recent years, PPCPs have been found to accumulate in various aqueous solutions, including solutions from sewage treatment plants, industrial effluents, hospital effluents, reclaimed water irrigation, aquaculture, field runoff and even drinking water [1,2,3]. Despite the low concentration of PPCPs in the environment, they have been found to have a considerable impact on human health and natural ecosystems in both the short term and long term [4]. Diclofenac (DCF) is a common type of PPCP that belongs to the category of nonsteroidal anti-inflammatory drugs, which are frequently prescribed to alleviate pain and inflammation caused by various conditions, such as toothache, headache, osteoarthritis, rheumatoid arthritis, and other related disorders [5,6]. The reports show that more than 1000 tons of DCF are produced and consumed annually, and most of it enters the environment through various pathways [2,7]. Results have confirmed that DCF is not only associated with occasional hepatotoxicity and gastrointestinal effects but also causes renal disease in mammals at low concentrations [7,8,9]. Hence, there is an urgent need to explore and establish novel and efficient approaches to removing DCF from water-based solutions.
Numerous technologies have been developed thus far to eliminate DCF from aqueous solutions, encompassing a wide range of methods such as biological, physical, and chemical processes [10]. However, reports show that biological processes, such as activated sludge processes and constructed wetlands, have little effect on the removal of DCF [11]. Furthermore, studies also show that traditional physical and chemical methods, including coagulation, flocculation, and membrane filtration, are inefficient at eliminating DCF from the environment [12,13]. Advanced oxidation processes (AOPs), such as UV radiation, photocatalysis, ozonation, and Fenton reactions, have been extensively studied as promising techniques to manage micropollutants in drinking water over the past few years [14,15,16]. However, in addition to economic factors, the production of secondary pollutants cannot be neglected as they may exhibit stronger toxicity [14]. Among the various methods available for water treatment applications, adsorption remains a highly effective approach due to its cost-effectiveness, energy efficiency, and ease of operation, particularly for the removal of micropollutants from aqueous solutions [17,18,19,20].
Similar to other types of construction waste, the improper disposal, haphazard accumulation, and landfilling of waste bricks can have negative impacts on the atmosphere, soil, and water environment [21]. However, research has also shown that waste bricks have superior physical and chemical properties when compared to traditional materials such as clay and lime soil. Waste bricks have distinct properties such as specific surface areas, pore volumes, mechanical strengths, and active components, which render them appropriate for eliminating pollutants such as heavy metals and phosphorus from aqueous solutions [22]. However, the practical utilization of waste bricks for removing DCF from aqueous solutions is limited due to their inadequate adsorption sites, resulting in a relatively low removal efficiency.
Table 1 displays the physicochemical characteristics of both CTAB and DCF. Surfactants have strong interactions with pollutants due to their hydrophilic and hydrophobic groups; hence, surfactants are often used as modified materials to enhance the removal efficiencies of different pollutants onto adsorbents [23,24]. Reports have shown that the modification of cationic or anionic surfactants onto adsorbents could increase the adsorption capacity of polycyclic aromatic hydrocarbons, PPCPs, pesticides, and so on [25,26,27,28,29]. As a typical surfactant, hexadecyl trimethyl ammonium bromide (CTAB) can often be used due to its good coordination with anionic pollutants. Results have shown that CTAB can be selected for the elimination of Bisphenol A from aqueous solutions [30]. As the pKa value of DCF is around 4.15, DCF mainly shows electronegativity in various aqueous solutions. Hence, the incorporation of CTAB onto waste bricks could greatly enhance their adsorption of DCF.

Table 1.
The physicochemical properties of CTAB and DCF.
This study presents a method for modifying waste bricks with the surfactant CTAB to remove typical PPCPs, specifically DCF, from aqueous solutions. The rationale for selecting waste bricks was based on their significant usage over the past few decades in China, while the selection of CTAB was motivated by its favorable coordination with anionic pollutants. The prepared materials were subjected to systematic characterization through techniques such as SEM, BET, and XRD. Various parameters were explored to investigate the adsorption behavior of DCF, including adsorption kinetics, isotherms, thermodynamics, pH effect, and ionic strength effect. Additionally, FTIR and XPS were employed to examine the mechanism of the adsorption of DCF onto the waste bricks.
2. Materials and Methods
2.1. Materials and Chemicals
DCF (C14H10Cl2NNaO2) and CTAB (C16H33(CH3)3NBr) were procured from Sigma Aldrich (St. Louis, MO, USA) and utilized without any further purification. Methanol and acetonitrile of UPLC grade were procured from Merck (Darmstadt, Germany), while analytical-grade hydrochloric acid, sodium hydroxide, ethyl alcohol, ammonium chloride, ammonium hydroxide, and formaldehyde were procured from Sinopharm Chemical Reagent Co., Ltd. (Beijng, China). All experiments were performed using ultrapure water with a resistivity of more than 18.2 MΩ/cm (Milli-Q). The waste red bricks were obtained from the Beijing University of Civil Engineering and Architecture.
2.2. Preparation of Adsorbents
Two types of waste red bricks were prepared for this study: pickled red brick particles (PRBPs) and CTAB-modified brick particles (CCBPs). To modify the waste red bricks with CTAB successfully, the cation exchange capacity (CEC) of the CCBPs was measured before the preparation of the CCBPs.
To prepare the PRBPs, waste red bricks were collected, crushed into small particles, and sieved to obtain particles with a size of 0.5 to 1.0 mm. The particles were then submerged in a 6 mol/L HCl solution and kept at a temperature of 363 ± 1 K for 24 h. Afterward, the resulting mixture was filtered using a 0.45 μm polytetrafluoroethylene membrane filter and washed thoroughly with ultrapure water. The obtained products were then dried overnight at 378 ± 1 K.
To determine their cation exchange capacity (CEC), 1.5 g of PRBPs was washed with 50% ethanol solution multiple times. Subsequently, the PRBPs were mixed with 25 mL of a solution containing 0.1 mol/L NH4Cl and 70% ethanol, stirred for 30 min, and left overnight. The resulting solution was then titrated with formaldehyde [31].
To prepare the CCBPs, 1.5 g of PRBPs was added into the solution with different amount of surfactant (0.1 CEC, 0.5 CEC, 1.0 CEC, 1.5 CEC, 2.0 CEC, and 2.5 CEC). Then the mixture was stirred for 24 h, centrifuged, washed several times with ultrapure water, and freeze-dried. The final products were obtained.
2.3. Characterization
The surface characteristics of the adsorbents were analyzed using scanning electron microscopy (SEM, SU8010, Hitachi, Tokyo, Japan). The specific surface area, pore volume, and pore size distribution (PSD) of the adsorbents were determined using the BET method (ASAP2460, American Micron). A Cu Kα radiation X-ray diffractometer (D8ADVANCE, Burker, Germany) was used to obtain the X-ray diffraction (XRD) patterns of the PRBPs and CCBPs within the 2θ range of 10° to 90° and at a scanning rate of 6°/min. FTIR spectra were obtained using KBr disks and analyzed using a FTIR spectrometer (NICOLET 6700, Thermo Fisher Scientific, Waltham, MA, USA). The samples were analyzed within the absorption band range of 500–4500 cm−1 with a resolution of 4 cm−1, using a FTIR spectrometer (NICOLET6700, Thermo Fisher Scientific, Waltham, MA, USA). The zeta potential values of the materials were determined using a Malvern zeta potentiometer (Zetasizer 90, Malvern, UK). The chemical compositions of the materials were analyzed using X-ray photoelectron spectroscopy (XPS) with an X-ray photoelectron spectrometer (Thermo escalab 250Xi, Thermo Fisher Scientific, Waltham, MA, USA).
2.4. The Adsorption Experiments
The batch experiments were conducted under controlled conditions, protected from light, and with single variables. Blank controls and parallel samples were also included in the experiment.
The effectiveness of DCF removal using CCBPs modified with different CTAB concentrations was evaluated by adding 0.06 g of the adsorbent into 30 mL of a DCF solution with an initial concentration of 20 mg/L. The solution and adsorbent were mixed and agitated for 24 h at 150 rpm and 298 K. The pH of the solution was maintained at 7.0 ± 0.1 by adding 0.01 mol/L of HCl or NaOH solutions. After the agitation period, the samples were filtered through a 0.22 μm membrane filter, and the DCF concentration was measured.
To explore the influence of pH values on the adsorption of DCF onto waste bricks, amounts of 0.06 g of both PRBP and CCBP were added separately into 30 mL solutions containing an initial DCF concentration of 20 mg/L. Using HCl and NaOH solutions, the pH values were adjusted and kept constant at around 4.0–9.0 during the entire adsorption process. The mixture solutions were stirred at a speed of 150 rpm for 24 h at a temperature of 298 K. The experimental conditions were the same as those used in the removal efficiency experiments, and both blank control and parallel samples were included in the experiment.
In the adsorption kinetics experiments, 1 g of each adsorbent was added to a 500 mL solution with an initial DCF concentration of 20 mg/L. The pH values were controlled at 4.0 ± 0.1 using HCl and NaOH solutions. Samples were collected at specific time intervals (0–1440 min), filtered, and analyzed for their DCF concentrations. The experimental conditions were kept consistent with the removal efficiency experiments.
To investigate the influence of ionic strength on the adsorption of DCF onto waste bricks, different concentrations of NaCl (ranging from 0 to 0.2 mol/L) were added to the solutions. The pH was adjusted to 4.0 ± 0.1, and all other experimental parameters were kept constant with the removal efficiency experiments.
To conduct adsorption isotherms and thermodynamic experiments, 30 mL of DCF solutions with initial concentrations ranging from 1 to 40 mg/L were prepared, and 0.06 g of each adsorbent was added to each solution. The temperature was maintained at 298, 308, and 318 K, while the pH values were kept constant at 4.0 ± 0.1 using appropriate HCl or NaOH solutions. Other experimental conditions were consistent with those used in the removal efficiency experiments.
The DCF concentrations were determined via ultra performance liquid chromatography (UPLC) using a C18 reverse-phase column (100 mm × 2.1 mm, 1.7 µm particle size) and a wavelength of 265 nm. The mobile phase was a mixture of 20% ultrapure water (0.01% CH3COOH) and 80% methanol with a flow rate of 0.4 mL/min. The UPLC system used was Acquity from Waters (Pleasanton, CA, USA).
3. Results and Discussion
3.1. Characteristics of Waste Bricks
The microstructures of the PRBPs and CCBPs were characterized via SEM. As shown in Figure 1a, the PRBP surface was relatively irregular, with different sizes of micropores before the incorporation of CTAB. However, as shown in Figure 1b, the CCBP surface was much rougher and more uneven while maintaining the main structure of the bricks. Furthermore, compared to PRBP, there were some different microspores on the CCBP surface, indicating that CTAB had successfully assembled on the surfaces of the waste bricks.
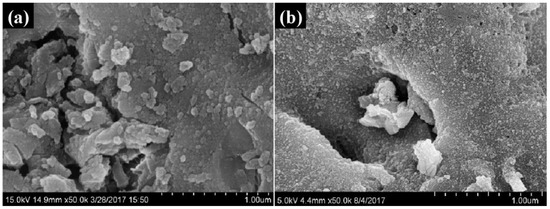
Figure 1.
The scanning electron micrograph images of PRBP (a) and CCBP (b).
Figure 2 shows the N2 adsorption–desorption isotherms and BJH pore size distributions of both the PRBPs and CCBPs. As shown in Figure 2a, the PRBPs and CCBPs have narrow pore size distributions. The adsorption–desorption isotherms of both adsorbents, as shown in Figure 2b, indicate the existence of a porous structure on their surfaces, as evidenced by their type IV with hysteresis loops [32,33]. The pore structural properties of the PRBPs and CCBPs are illustrated in Table 2. Following the addition of CTAB, the surface area of the waste bricks reduced from 10.42 m2/g to 4.33 m2/g, whereas the pore volume and size increased from 0.0059 to 0.0068 cm³/g and 4.51 to 5.16 nm, respectively. These results are the same as those of clay organo-modified with CTAB [31].
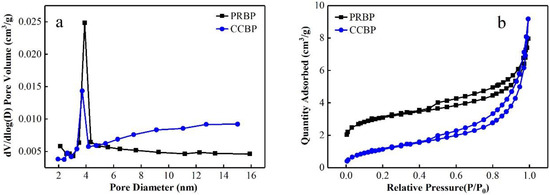
Figure 2.
The pore size distribution (a) and N2 adsorption–desorption isotherms (b) of PRBP and CCBP.

Table 2.
Pore structural properties of samples.
To investigate the characteristics of the waste bricks further, XRD analyses of the PRBPs and CCBPs were conducted, and the results are shown in Figure 3. Significant peaks were observed at 20–26°, which were attributed to the SiO2, consistent with previous studies [34]. After functionalization with CTAB, the main diffraction patterns of the bricks remained virtually unchanged, indicating that the primary structure and composition of the waste red bricks were largely unaltered. The increasing intensity of CCBP suggested that the amount of CTAB incorporated into the samples’ framework was limited and did not lead to significant structural changes.
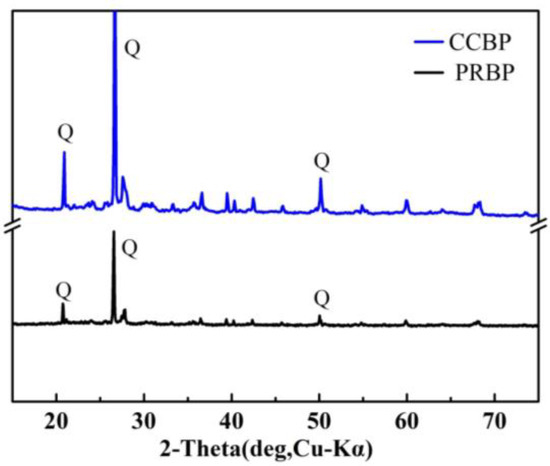
Figure 3.
XRD of PRBP and CCBP.
The zeta potentials of the PRBPs and CCBPs are shown in Figure 4. As the pH values of the solution were increased from 2.0 to 12.0, the zeta potentials of the PRBPs remained negative, indicating that the surface charges of the PRBPs were predominantly negative. On the other hand, for CCBP, the zeta potential increased initially with the increase in pH values, followed by a decrease, and the isoelectric point was observed at approximately pH 7.0. This result suggested that CCBPs mainly demonstrated electropositivity with the solution at a pH ≤ 7.0, while they showed electronegativity with the solution at a pH ≥ 7.0. These results could be attributed to the characteristics of CTAB, which showed good coordination with anionic nonionic amphoteric surfactants [31]. The cation exchange between CTAB and the PRBPs could change the surface characteristics and electrical property of the adsorbent, which would be beneficial for the removal of negatively charged contaminants from aqueous solutions.
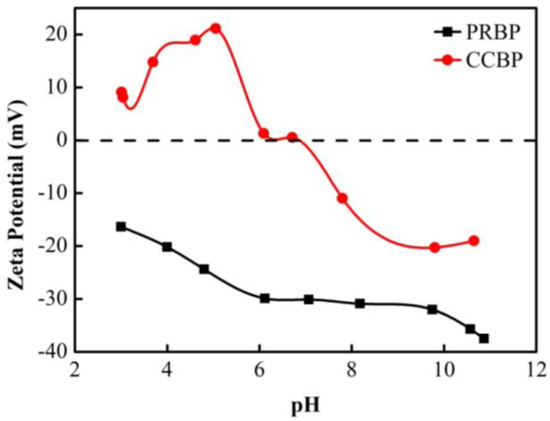
Figure 4.
The zeta potentials of PRBPs and CCBPs.
3.2. Adsorption Performance of Adsorbents
Figure 5 illustrates the adsorption performance of DCF onto PRBPs, CTAB, and CCBPs (modified with different amounts of CTAB). The results indicate that the PRBPs have a low adsorption capacity for DCF removal, whereas CTAB exhibits some adsorption behavior but with a low capacity. However, the capacity of DCF adsorption onto waste bricks is significantly enhanced with the addition of CTAB. As the CEC amount increases from 0 to 2.5, the adsorption capacity of the CCBPs gradually increases from 0.36 to 3.74 mg/g. This improved adsorption capacity is attributed to the strong interaction between CTAB and DCF, which is in line with the adsorption behavior observed for 1-naphthol onto β-cyclodextrin-modified graphene oxide nanosheets [35]. To further investigate the behavior of DCF adsorption onto the CCBPs, the adsorbent modified with 2.5 CEC (CTAB) was selected for subsequent batch experiments.
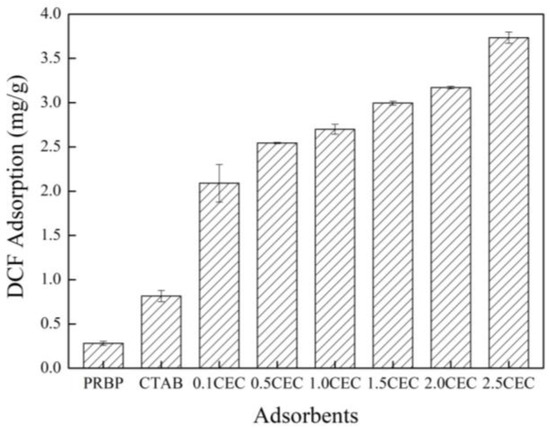
Figure 5.
Effects of DCF adsorption onto PRBPs and CCBPs (experimental conditions: [DCF]0 = 20 mg/L, dosage = 2 g/L, T = 298 K, pH = 7.0 ± 0.1).
3.3. Effect of pH on the Adsorption of DCF
The effect of solution pH on the adsorption of DCF onto PRBPs and CCBPs was examined, and the results are presented in Figure 6. The pH values had a slight impact on the adsorption efficiency of the PRBPs, with the adsorption capacity of DCF decreasing from 0.3 to 0.01 mg/g as the pH values increased from 4.0 to 9.0. In contrast, for the CCBPs, the adsorption capacity of the DCF decreased significantly from 4.5 to 1.0 mg/g as the pH values increased. Moreover, the CCBPs exhibited a higher adsorption capacity than the PRBPs at all pH values. These findings may be attributed to the properties of DCF in an aqueous solution and the surface characteristics of the waste bricks. In the DCF aqueous solution, numerous anions exist, and CTAB has excellent coordination with anions [1,31].
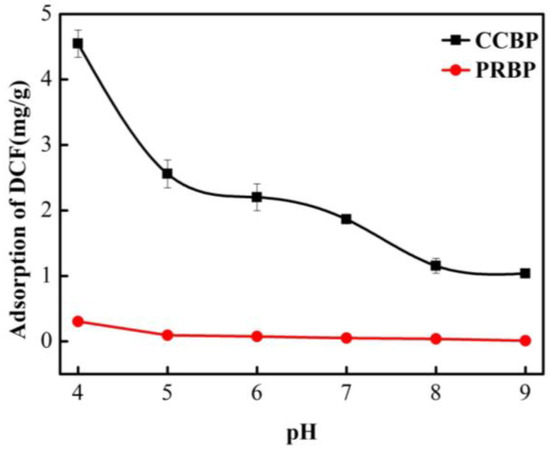
Figure 6.
Effect of pH on the adsorption of DCF onto PRBP and CCBP (experimental conditions: [DCF]0 = 20 mg/L, dosage = 2 g/L, T = 298 K).
As shown in Figure 4, the zeta potential of the PRBPs is mainly negative for the experimental pH values. As the pKa value of DCF was around 4.15 [36], the DCF mainly demonstrated electronegativity at the experimental pH values. The increase in the pH values led to a gradual increase in electronegativity, resulting in enhanced repulsion between DCF and the PRBPs, leading to the decreased adsorption of DCF onto the waste bricks. Similarly, for the CCBPs, the zeta potential was mainly negative when the pH was above 7.0 (Figure 4), and an increase in pH value from 4.0 to 9.0 caused a shift in the charge of the CCBPs from electropositive to electronegative. This reduced the electrostatic attraction between the CCBPs and the DCF, resulting in a significant decrease in the capacity to adsorb DCF. These findings are consistent with previous research on the adsorption of DCF onto various adsorbents [1].
3.4. Adsorption Kinetics
Figure 7 illustrates the adsorption kinetics of the adsorption of DCF onto the PRBPs and the CCBPs. The initial adsorption rate of DCF onto the PRBPs was rapid, reaching equilibrium at around 6 h, while the CCBPs reached equilibrium faster, at approximately 2 h. The rapid adsorption process of the CCBPs is attributed to the plentiful and efficient adsorption sites provided by the structure of the waste bricks. Furthermore, the adsorption capacity and rate of the CCBPs were higher than those of the PRBPs, implying that the introduction of CTAB enhanced the process of the adsorption of DCF onto the waste bricks.
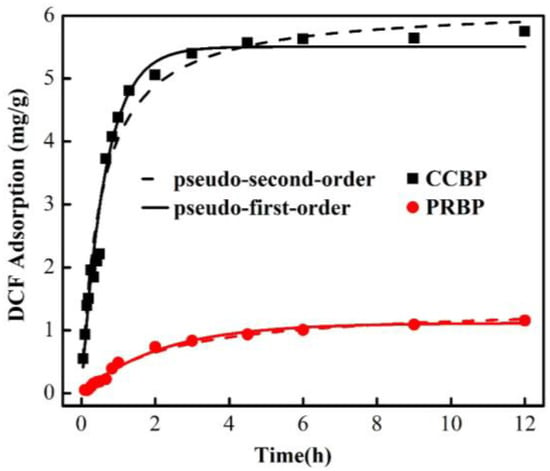
Figure 7.
Adsorption kinetics of DCF onto PRBPs and CCBPs (experimental conditions: [DCF]0 = 20 mg/L, dosage = 2 g/L, T = 298 K, pH= 4.0 ± 0.1).
To investigate the adsorption kinetics of the adsorption of DCF onto the PRBPs and CCBPs further, the pseudo-first-order and pseudo-second-order equations were used to fit the kinetics data, and the models are expressed as Equations (1) and (2) (Zhu et al. 2016):
where Qe (mg/g) represents the adsorbed amounts of DCF at the equilibrium time and Qt (mg/g) represents it at any time t (min), and k1 (mg/h) and k2 (mg/h) are the rate constants for the pseudo-first-order and pseudo-second-order equations, respectively.
The results of the calculated parameters and the correlations of the adsorption kinetics are presented in Table 3. It is evident that both models fit the adsorption process of DCF onto waste bricks well. However, the pseudo-second-order model is more suitable than the pseudo-first-order model in describing the adsorption of DCF onto the CCBPs, indicating that the adsorption process may be primarily controlled by chemical adsorption.

Table 3.
The parameters and correlation coefficients of adsorption kinetic models.
3.5. Effect of Ionic Strength
In Figure 8, the impact of ionic strength on the adsorption of DCF onto the CCBPs and PRBPs is illustrated. The results indicate that the concentration of KCl significantly affects the capacity to adsorb DCF onto the waste bricks. Specifically, as the ionic strength increases from 0 to 0.02 mol/L, the adsorption capacity of DCF onto the CCBPs and PRBPs decreases from 1.3 to 0.05 mg/g and 0.14 to 0.03 mg/g, respectively. These observations may be attributed to the competitive adsorption between the adsorbate and adsorbent. CTAB has good coordination with anionic surfactants [31], and with an increase in the KCl concentration, the abundance of Cl−ions in the solution can be adsorbed onto the surface of the adsorbents, thereby occupying some of the useful adsorption sites. This results in a significant reduction in the adsorption capacity of the DCF. This finding is similar to the adsorption of sulfamethazine on poly (β-cyclodextrin)-conjugated magnetic graphene oxide [37].
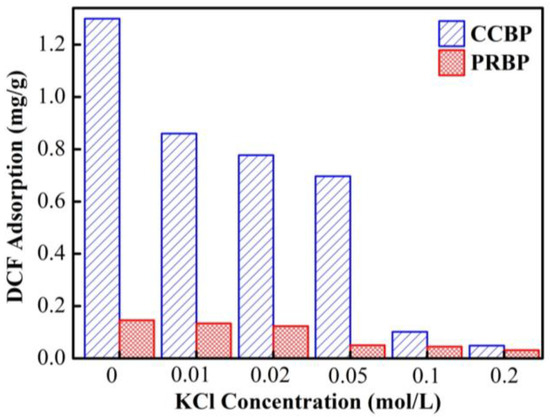
Figure 8.
Effect of ionic strength on the adsorption of DCF onto PRBPs and CCBPs (experimental conditions: [DCF]0 = 20 mg/L, dosage = 2 g/L, T = 298 K, pH = 4.0 ± 0.1).
3.6. Adsorption Isotherms
The isotherms of the adsorption of DCF onto the PRBPs and CCBPs are presented in Figure 9. The results show that the adsorption capacity of DCF increases as the equilibrium concentration increases for both adsorbents. However, the CCBPs exhibit a significantly higher adsorption capacity than the PRBPs at all equilibrium concentrations, highlighting the considerable impact of CTAB modification on the removal of DCF from aqueous solutions.
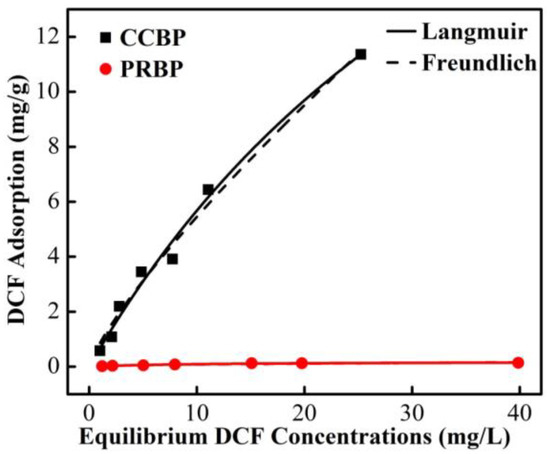
Figure 9.
Adsorption isotherms of DCF onto PRBPs and CCBPs (experimental conditions: dosage = 2 g/L, T = 298 K, pH = 4.0 ± 0.1).
To investigated the isotherms of DCF adsorption onto waste bricks further, the Langmuir isotherm model and Freundlich isotherm model were used to fit the data and are expressed as follows [38]:
where Ce (mg/g) represents the maximum adsorption amount and b (L/mg) represent adsorption equilibrium constant, n represents the Freundlich constant, and KF (mg/g (L/mg)1/n) represents the adsorption performance, which indicates the affinity of the sorbent and the possibility of adsorption.
The isotherms of the adsorption of DCF onto the CCBPs and PRBPs were analyzed using the Langmuir and Freundlich models, and the parameters obtained are shown in Table 4. The Langmuir model provided a better fit to the experimental data than the Freundlich model, with R2 values greater than 0.97 for both adsorbents. This indicates that the adsorption of DCF onto the waste bricks occurs through monolayer molecular adsorption rather than multilayer or heterogeneous adsorption. [39]. Compared with the qmax of the PRBPs (0.19 mg/g), the CCBPs had a higher capacity for the adsorption of DCF (33 mg/g), which was mainly determined by the physical and chemical properties of the CCBPs. In addition, the values of 1/n obtained from the Freundlich model for both the CCBPs and PRBPs were found to be less than 1.0, indicating that the adsorption of DCF onto the two adsorbents is favorable. These findings further support the potential application of CCBPs as an effective adsorbent for the removal of micropollutants from aqueous solutions.

Table 4.
The parameters and correlation coefficients of adsorption isotherm models.
3.7. Adsorption Thermodynamics
The experiments were carried out at various temperatures (298, 308, and 318 K) to investigate the thermodynamics of the adsorption of DCF onto the PRBPs and CCBPs. The results in Figure 10 demonstrate that there was an inverse relationship between the capacity to adsorb DCF onto the waste bricks and the temperature. Specifically, as the temperature increased from 298 K to 318 K, the capacity to adsorb DCF onto both the PRBPs and the CCBPs decreases. This finding suggests that the process of adsorbing DCF onto the waste bricks is exothermic. Additionally, the results indicate that the adsorption of DCF onto waste bricks is more favorable at lower temperatures. This finding is consistent with the results of previous studies in this area [40].
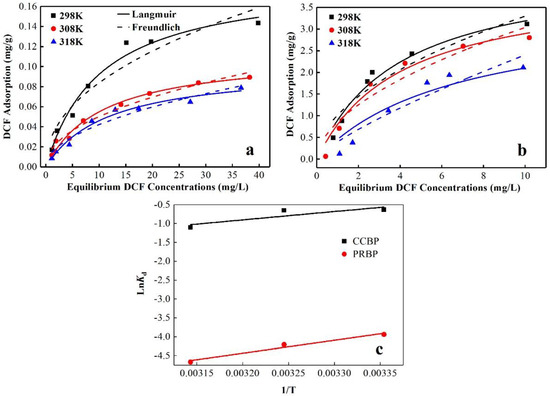
Figure 10.
Adsorption thermodynamic of DCF onto (a) PRBPs and (b) CCBPs, (c) Van’t Hoff plot of PRBPs and CCBPs (experimental conditions: dosage = 2 g/L, T = 298 K, pH = 4.0 ± 0.1).
To investigate the adsorption thermodynamics process further, the thermodynamic parameters of the adsorption of DCF onto the PRBPs and CCBPs can be calculated by the following equations [41,42]:
where H is the adsorption enthalpy change (kJ/mol), S is the adsorption entropy change (J/mol K), R is the ideal gas state constant (8.314 J/mol K), T is the absolute temperature (K), n is the Freundlich adsorption exponent, G is the adsorption free energy change (kJ/mol), and Kd is the adsorption rate constant.
The thermodynamic parameters of the adsorption of DCF onto the PRBPs and CCBPs are listed in Table 5. The estimates of ΔH and ΔS could be obtained from the slope and intercept of the Van’t Hoff plot, ln Kd vs. 1/T, as shown in Figure 10c. The negative value of ΔH of the PRBPs (–28.64 kJ/mol) and CCBPs (–18.49 kJ/mol) confirms that the adsorption of DCF is an exothermic process. The positive value of ΔS for both adsorbents (112.37 and 80.06 J/(mol·K)) indicates that the randomness increases at the solid–liquid interface during adsorption. The negative values of ΔG for the PRBPs and CCBPs indicate that the adsorption is spontaneous.

Table 5.
Thermodynamic parameters of adsorption of DCF onto PRBPs and CCBPs.
3.8. Adsorption Mechanism
To explore the mechanism of DCF adsorption onto waste bricks, FTIR and XPS analyses were carried out before and after the adsorption. Figure 11 presents the FTIR spectra of the CCBPs and the DCF-adsorbed CCBPs. The absorption peaks observed at 2920 and 2850 cm−1 correspond to the symmetric and asymmetric stretching vibrations, respectively, of the -CH2- groups in the alkyl chain [26]. Furthermore, the intensity of the alkyl chain is related to the content of CTAB, suggesting the successful modification of CTAB onto the surface of the waste bricks. After the adsorption of DCF onto the CCBPs, the main peaks of the CCBPs remained unaffected, and the new peaks of the alkyl chain were also maintained, indicating that the adsorption process did not alter the main structure of the CCBPs. However, after DCF adsorption, the spectral band at 1573 cm−1 (stretching vibration of -N=N=) deformed and moved to 1554 cm−1 (stretching vibration of -NO2), which suggests that one of the adsorption mechanism of DCF may be the π-π interaction [35,43].
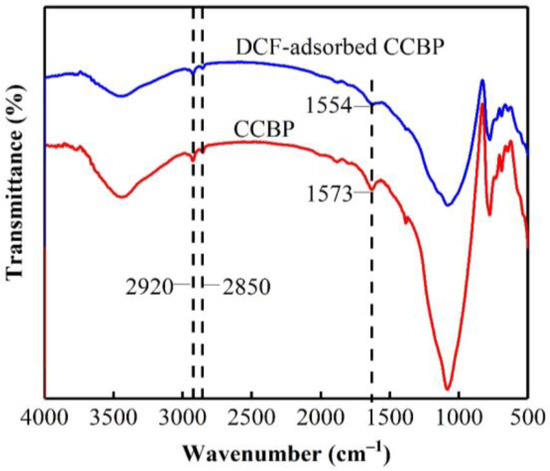
Figure 11.
FTIR spectra of CCBPs and DCF-adsorbed CCBPs.
The XPS of CCBPs before and after the adsorption of DCF are shown in Figure 12. The spectra of C 1s can be divided into three parts: 284.7–284.8 eV, reflecting the C-C or C-H bond, 286.1–286.2 eV, reflecting the C-O or C-N bond, and 288.6–288.7 eV, reflecting the C=O or O-C-O bond [44]. As shown in Figure 12a,b, after the adsorption of DCF, the binding energy of C-O or C-N increased from 286.10 to 286.25 eV, and the binding energy of C=O or O-C-O increased from 288.60 to 288.70 eV, indicating a decrease in the electron density in the outer layer of the C element. These results suggest that the adsorption process is mainly attributed to the chemical reaction. Furthermore, the relative content of C-O or C-N increased from 17.18% to 20.7% and the relative content of C=O or O-C-O decreased from 8.81% to 5.18%, indicating that there is a combination between the oxygen functional groups on the adsorbent and DCF.
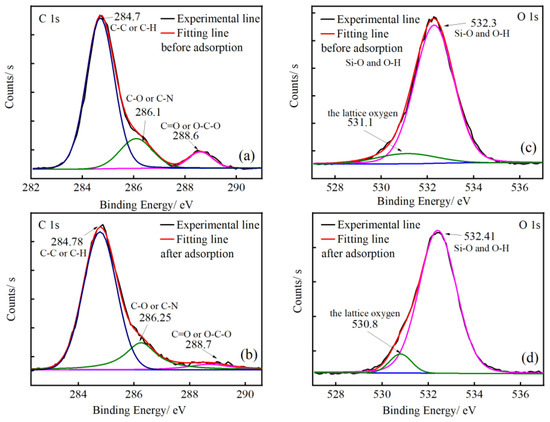
Figure 12.
The XPS spectra of (a) C 1s before adsorption, (b) C 1s after adsorption, (c) O 1s before adsorption, and (d) O 1s after adsorption.
As shown in Figure 12c,d, the O 1s spectrum can be divided into two peak sections: 530.8–531.1 eV could reflect the lattice oxygen in the adsorbent, and 532.3–532.4 eV could reflect the Si-O and O-H bonds in water [45]. After the adsorption of DCF, the relative content of lattice oxygen in the adsorbents before and after adsorption decreased from 9.62% to 6.93%, suggesting that a complexation took place between the oxygen functional groups on the adsorbent and the DCF. The binding energy decreased from 531.1 eV to 530.8 eV, which suggests there may be an electrostatic interaction between the CCBPs and DCF during the adsorption process [35].
4. Conclusions
The study presented in this paper aimed to enhance the efficiency of removing of micropollutants from aqueous solutions by modifying waste red bricks with the cationic surfactant CTAB. The results showed that the CTAB could be successfully incorporated onto the waste red bricks using a simple method while maintaining the structural integrity of the bricks. As the amount of CTAB increased, the efficiency of removing DCF by the bricks also gradually increased. The adsorption process was found to be pH-dependent, with higher pH values leading to a lower adsorption capacity. The fast adsorption of DCF onto the waste bricks was a result of the specific structural properties of the materials as well as the incorporation of CTAB. The adsorption mechanism was suggested to involve mono-layer molecular adsorption, with the ionic strength affecting the process significantly. The adsorption thermodynamics results reveal that the process was exothermic and spontaneous, with a higher favorability at lower temperatures. The adsorption mechanism was proposed to be a combination of the π-π interaction, surface complexation, and electrostatic interaction.
Author Contributions
Z.Z.: conceptualization, methodology, writing—review and editing, and funding acquisition; X.J.: methodology, investigation, data curation, formal analysis, and writing—original draft; H.C.: writing—review and editing, supervision, and project administration; X.Z.: writing—review and editing and supervision; C.T.: writing—review and editing and validation; X.B.: writing—review & editing; Y.G.: writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Pyramid Talent Training Project of the Beijing University of Civil Engineering and Architecture (JDYC20200315) and the Science and Technology General Project of the Beijing Municipal Education Commission (KM202110016009). The authors are also grateful to the Fundamental Research Funds from the Beijing University of Civil Engineering and Architecture (X20140), a joint project of the Beijing Municipal Education Commission and the Municipal Nature Science Foundation (21JH0024).
Data Availability Statement
The article contains all the necessary data to support the findings of the study.
Acknowledgments
We express our gratitude to all individuals who have contributed to this study and whose names are not mentioned here.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- An, H.J.; Bhadra, B.N.; Khan, N.A.; Jhung, S.H. Adsorptive removal of wide range of pharmaceutical and personal care products from water by using metal azolate framework-6-derived porous carbon. Chem. Eng. J. 2018, 343, 447–454. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, O.; Bergheim, M.; Dawick, J.; Kötter, D.; McDonough, K.; Schowanek, D.; Stanton, K.; Wheeler, J.; Willing, A. Comparing the European Union System for the Evaluation of Substances (EUSES) environmental exposure calculations with monitoring data for alkyl sulphate surfactants. Environ. Sci. Eur. 2021, 33, 3. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Yuan, S.; Sun, J.; Zheng, S. Effect of resin charged functional group, porosity, and chemical matrix on the long-term pharmaceutical removal mechanism by conventional ion exchange resins. Chemosphere 2016, 160, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Sorkin, E.M. Diclofenac sodium: A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef]
- Bariguian Revel, F.; Fayet, M.; Hagen, M. Topical diclofenac, an efficacious treatment for osteoarthritis: A narrative review. Rheumatol. Ther. 2020, 7, 217–236. [Google Scholar] [CrossRef]
- Bickley, L.K.; van Aerle, R.; Brown, A.R.; Hargreaves, A.; Huby, R.; Cammack, V.; Jackson, R.; Santos, E.M.; Tyler, C.R. Bioavailability and kidney responses to diclofenac in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 2017, 51, 1764–1774. [Google Scholar] [CrossRef]
- Donati, M.; Conforti, A.; Lenti, M.C.; Capuano, A.; Bortolami, O.; Motola, D.; Moretti, U.; Vannacci, A.; Rafaniello, C.; Vaccheri, A. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: Data from drug-induced liver injury case–control study in Italy. Br. J. Clin. Pharmacol. 2016, 82, 238–248. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Helmreich, B.; Škrbić, B.; Carballa, M.; Papa, M.; Pastore, C.; Emre, Z.; Oehmen, A.; Langenhoff, A.; Molinos, M. Status of hormones and painkillers in wastewater effluents across several European states—Considerations for the EU watch list concerning estradiols and diclofenac. Environ. Sci. Pollut. Res. 2016, 23, 12835–12866. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.; Li, Z.; Lin, S.; Wang, Y.; Pan, S.; Han, J. Promoted elimination of antibiotic sulfamethoxazole in water using sodium percarbonate activated by ozone: Mechanism, degradation pathway and toxicity assessment. Sep. Purif. Technol. 2021, 266, 118543. [Google Scholar] [CrossRef]
- Ali, M.; Song, X.; Ding, D.; Wang, Q.; Zhang, Z.; Tang, Z. Bioremediation of PAHs and heavy metals co-contaminated soils: Challenges and enhancement strategies. Environ. Pollut. 2022, 295, 118686. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Varsha, M.; Kumar, P.S.; Rathi, B.S. A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Lu, J.; Zhou, Y.; Wang, Z.; Zhou, Y. Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review. Chin. Chem. Lett. 2022, 33, 3585–3593. [Google Scholar] [CrossRef]
- Guo, D.; You, S.; Li, F.; Liu, Y. Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation: A review. Chin. Chem. Lett. 2022, 33, 1–10. [Google Scholar] [CrossRef]
- Diotti, A.; Perèz Galvin, A.; Piccinali, A.; Plizzari, G.; Sorlini, S. Chemical and leaching behavior of construction and demolition wastes and recycled aggregates. Sustainability 2020, 12, 10326. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption characteristics of construction waste for heavy metals from urban stormwater runoff. Chin. J. Chem. Eng. 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Gorsky, A.; Racanelli, G.; Belvin, A.; Chambers, R. Greenhouse gas flux from stormwater ponds in southeastern Virginia (USA). Anthropocene 2019, 28, 100218. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Azha, S.F.; Ismail, S. A review of diclofenac occurrences, toxicology, and potential adsorption of clay-based materials with surfactant modifier. J. Environ. Chem. Eng. 2022, 10, 107541. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu (II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Y.; Wang, X.; Li, Z. Sorption and retention of diclofenac on zeolite in the presence of cationic surfactant. J. Hazard. Mater. 2017, 323, 584–592. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-based remediation as an effective approach for removal of environmental pollutants—A review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Tanhaei, B.; Ayati, A.; Ranjbari, S. Substantial improvement in the adsorption behavior of montmorillonite toward Tartrazine through hexadecylamine impregnation. Environ. Res. 2022, 204, 111965. [Google Scholar] [CrossRef]
- Wang, L.-C.; Ni, X.-j.; Cao, Y.-H.; Cao, G.-q. Adsorption behavior of bisphenol A on CTAB-modified graphite. Appl. Surf. Sci. 2018, 428, 165–170. [Google Scholar] [CrossRef]
- Zawrah, M.; Khattab, R.; Saad, E.; Gado, R. Effect of surfactant types and their concentration on the structural characteristics of nanoclay. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yuan, B.; Shen, C.; Fu, M.; Cui, H.; Sun, W. Large scale preparation of Cu-doped α-FeOOH nanoflowers and their photo-Fenton-like catalytic degradation of diclofenac sodium. Chem. Eng. J. 2016, 291, 174–183. [Google Scholar] [CrossRef]
- Jia, J.; Qin, F.; Guo, J.; Liu, Z.; Zhao, Q.; Guan, W. FeOOH coupled Bi2WO6 for efficient photocatalysis-Fenton synergistic degradation of organic pollutants. Mater. Lett. 2023, 336, 133862. [Google Scholar] [CrossRef]
- Labidi, N. Removal of mercury from aqueous solutions by waste brick. Int. J. Environ. Res. 2008, 2, 275–278. [Google Scholar]
- Zheng, H.; Gao, Y.; Zhu, K.; Wang, Q.; Wakeel, M.; Wahid, A.; Alharbi, N.S.; Chen, C. Investigation of the adsorption mechanisms of Pb (II) and 1-naphthol by β-cyclodextrin modified graphene oxide nanosheets from aqueous solution. J. Colloid Interface Sci. 2018, 530, 154–162. [Google Scholar] [CrossRef]
- Oki, J.; Watanabe, D.; Uekusa, T.; Sugano, K. Mechanism of supersaturation suppression in dissolution process of acidic drug salt. Mol. Pharm. 2019, 16, 1669–1677. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wei, J. Fabrication of poly (β-cyclodextrin)-conjugated magnetic graphene oxide by surface-initiated RAFT polymerization for synergetic adsorption of heavy metal ions and organic pollutants. J. Mater. Chem. A 2019, 7, 2055–2065. [Google Scholar] [CrossRef]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of emerging pollutants on activated carbon. Rev. Chem. Eng. 2017, 33, 491–522. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, L.; Zhuang, S. Sorption of polycyclic aromatic hydrocarbons to soils enhanced by heavy metals: Perspective of molecular interactions. J. Soils Sediments 2016, 16, 1509–1518. [Google Scholar] [CrossRef]
- Bernardo, M.; Rodrigues, S.; Lapa, N.; Matos, I.; Lemos, F.; Batista, M.; Carvalho, A.; Fonseca, I. High efficacy on diclofenac removal by activated carbon produced from potato peel waste. Int. J. Environ. Sci. Technol. 2016, 13, 1989–2000. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Huang, Y.; Huang, N.; Ren, C.; Zhang, W. Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J. Alloys Compd. 2015, 633, 338–346. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z.; Zhang, L.; Cheng, B.; Ho, W. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr (VI) ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- Kaur, H.; Bansiwal, A.; Hippargi, G.; Pophali, G.R. Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: Adsorption isotherms, kinetics and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 20473–20485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, J.; Zhang, M.; Zhou, Q.; Liu, F. Insight into the adsorption of PPCPs by porous adsorbents: Effect of the properties of adsorbents and adsorbates. Environ. Pollut. 2016, 214, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhai, Y.; Chen, T.; Yu, L.; Wang, H. Synergistic and antagonistic effects on removal of diclofenac and cadmium onto hydrous manganese dioxide. Nanosci. Nanotechnol. Lett. 2016, 8, 985–992. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).