Abstract
The cytochrome P450 (CYP) enzyme family is the major enzyme system catalyzing the phase I metabolism of xenobiotics, including pharmaceuticals and toxic compounds in the environment. A major part of the CYP-dependent xenobiotic metabolism is due to polymorphic and inducible enzymes, which may, quantitatively or qualitatively, alter or enhance drug metabolism and toxicity. Drug–drug interactions are major mechanisms caused by the inhibition and/or induction of CYP enzymes. Particularly, CYP monooxygenases catalyze hydroxylation reactions to form hydroxylated metabolites. The secondary metabolites are sometimes as active as the parent compound, or even more active. The aim of this review is to summarize some of the significative examples of common drugs used for the treatment of diverse diseases and underline the activity and/or toxicity of their metabolites.
1. Introduction
Cytochrome P450 enzymes (CYPs or P450s) represent a superfamily of heme-containing proteins, predominantly present within the endoplasmic reticulum of hepatocytes, responsible for the oxidative metabolism of a broad range of endogenous substances and xenobiotics. Many members of the CYP class are currently known, and the numbers continue to grow as more genomes are sequenced. Until now, 57 human CYP isoforms have been recognized [1] and progresses in the development of molecular probes for human CYPs have been recently reviewed [2]. CYPs are present in many tissues, such as the liver, intestine, lung, heart, and brain, and catalyze the phase I metabolism of conventional drugs (Figure 1) [3,4]. Indeed, drug metabolism involves the (bio)chemical modification of a drug that may occur in the body [5] and plays a crucial role in pharmaceutical development and clinical practice, and contributes to toxicology, carcinogenesis and endocrinology [6]. Drug metabolism consists mainly of two phases, phase I and phase II [7,8]. Phase I metabolism reactions generally do not determine a significant modification in the molecular weight or aqueous solubility of the substrate. At this stage, a functional group, generally a polar group, such as –OH, –SH or NH2, is unmasked or introduced into the parent molecule, thereby rendering it accessible to further metabolism. Phase II metabolism occurs directly on the parent compounds that incorporate specific structural motifs, or, more often, on the functional groups added or exposed by phase I oxidation. Phase II conjugation generally results in a significant enhancement in molecular weight and aqueous solubility, thus favoring the process of elimination.
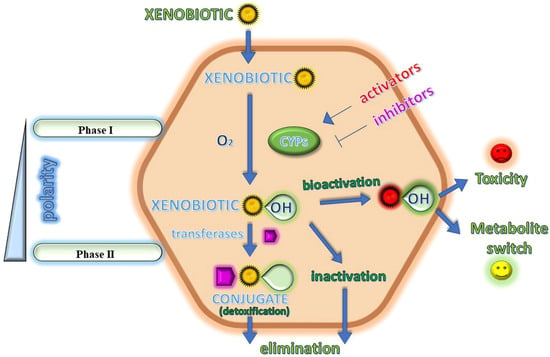
Figure 1.
A cartoon depicting the main intracellular biotransformations in the xenobiotic metabolism.
Most common CYPs are represented by monooxygenases, which bind and reductively activate molecular oxygen, O2, splitting it into its two atoms, one of which is then inserted into the substrate RH that is bound in the P450 active site, contiguous to the heme cofactor. Thus, they are called monooxygenases as they use one oxygen atom to obtain the product ROH and reduce the second oxygen atom to water, following the general reaction:
RH + O2 + 2e− + 2H+ → ROH + H2O
CYP monooxygenases play a pivotal role in drug pharmacokinetics and are frequently involved in drug–drug interactions [9]. CYPs metabolize many xenobiotics including several environmental carcinogens. Particularly, among the 57 known human CYPs, 12 metabolize 90% of xenobiotics [10] and 6 CYP isoenzymes are able to metabolize the most common drugs used in therapy, namely CYP3A4, CYP3A5, CYP2D6, CYP2C9, CYP2C19 and CYP1A2. CYPs are often upregulated by their own substrates, leading to improved metabolism and plasma concentrations of the concurrent drugs. On the contrary, CYP inhibition by several drugs provides high therapeutic drug levels and drug-induced toxicity, preventing CYPs from fulfilling their protective role in detoxification [11]. Moreover, metabolic reactions may be often directly related to food, with some foods exerting their activity by interacting with CYPs. For instance, St. John’s wort deeply induces CYP3A4 [12] and, therefore, it lowers drug bioavailability and exposure. Pomegranate (Punica granatum L.) juice, grapefruit and cranberries effectively inhibit both CYP3A4 and/or CYP2C9 [13,14,15]. Moreover, interindividual variability in CYP-mediated drug metabolism may exist, with some isoforms, including CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4/5, being highly polymorphic [16]. The influence of CYPs on drug toxicity may be mainly exerted by either reducing the exposure to the parent compound or by transforming the drug into a toxic compound [17]. Several factors involved in this mechanism are represented by enzyme induction or inhibition, reversible or irreversible, and pharmacogenetics. Interestingly, in some cases, the metabolites deriving from a drug are even more active than the parent compound. In this case, a “metabolite switch”, i.e., the choice of an active metabolite as a substitute for the parent compound may be proposed, when the former has more advantageous properties than the latter (Figure 1). “Metabolite switch” is a term that resembles the better known ”chiral switch”, which refers to the replacement of a formerly approved racemate with its active enantiomer on the market. The benefits of the chiral switch are a higher therapeutic index related to its higher potency, selectivity and fewer side effects, as well as a faster-action onset and lower drug dosages in patients. Moreover, the chiral switch is an approach that allows manufacturers to keep market exclusiveness for the chiral drugs for which the patent has expired, even though the pure enantiomers have not shown a higher efficiency or better safety profile compared to racemates [18,19]. Recently, the identification of bioactive metabolites, by using activity metabolomics, has been proposed [20], and further studies are also addressed to the gut microbiota and related metabolites in several diseases, such as ulcerative colitis for treatment with mesalamine [21]. Bioinformatic studies are often addressed to develop new compounds with CYP activator or inhibitor profiles. Recently, more attention has been paid to P450 oxidoreductase, which transfers electrons from nicotinamide adenine dinucleotide phosphate-oxidase to CYP enzymes, inducing their expression and affecting the metabolism of various drugs [22]. The regulation of P450 oxidoreductase is related to several diseases, including cancer [23,24], and alcohol intoxication [25] and brain injury [26]. Finally, the expression of CYPs and their activity is considerably influenced by the immune response; indeed, modifications in CYP expression and/or function in COVID-19 [27,28] have likely played a role in the pathophysiology of this disease and in the metabolism of drugs used to treat this disease [29]. In this review, we tried to report some significative examples of metabolites derived from drugs in various fields of therapy, highlighting their activity and/or toxicity.
2. Examples of Drugs Metabolized by CYPs
Some significative examples of drugs metabolized by CYPs with diverse activities were chosen and are summarized in Table 1.

Table 1.
Drugs metabolized by CYPs described in the review.
2.1. Voriconazole
Voriconazole (Figure 2) is a broad-spectrum second-generation antifungal triazole frequently used for the prophylaxis and treatment of invasive fungal infections [30]. It is widely metabolized via the CYP isoenzyme CYP2C19 and, to a lesser extent, CYP2C9 and CYP3A4, to hydroxylated metabolites. Voriconazole N-oxide is the most abundant circulating metabolite [31], followed by 4-hydroxyvoriconazole and dihydroxy-voriconazole [32], which, in turn, then undergo phase II reactions. Voriconazole N-oxide forms during the fluoropyrimidine N-oxidation reactions via CYP2C19 and CYP3A4, while 4-hydroxyvoriconazole is formed through the methyl hydroxylation via CYP3A4 [33]. Currently, voriconazole is also recommended as a first-line therapy for COVID-19-associated pulmonary aspergillosis, which has recently aroused concerns due to increased mortality [34]. High intra- and interindividual variability in voriconazole’s pharmacokinetics has been demonstrated. This compound has a relatively narrow therapeutic range; nevertheless, its metabolism has not yet been completely elucidated [35]. As a matter of fact, voriconazole overdoses are frequently observed, leading to a high risk of adverse effects. Inflammation may be a possible risk factor of voriconazole overdose in hematological patients, as well [36]. It has also to be considered that grapefruit juice likely increases the oral bioavailability of this drug in children, as it selectively inhibits the intestinal CYP3A4; thus, an efficient quantification of voriconazole and its N-oxide derivative is strongly desired, and an ultra-performance liquid chromatographic method with tandem mass spectrometry detection has recently been developed [37]. Furthermore, much evidence shows cutaneous side effects related to voriconazole, such as photosensitivity and skin cancer [38]. In fact, it has been demonstrated that voriconazole enhances oxidative stress in human keratinocytes and facilitates UV-induced DNA damage through catalase inhibition [39]. Therefore, long-term voriconazole therapy can enhance the risk of cutaneous squamous cell carcinoma (cSCC), mainly in patients receiving immunosuppressive therapy [40]. Some authors suggest that actinic keratosis related to the use of voriconazole may precede the occurrence of cutaneous SCC and voriconazole-related SCC may lead to a worse prognosis in immunosuppressive conditions [41].
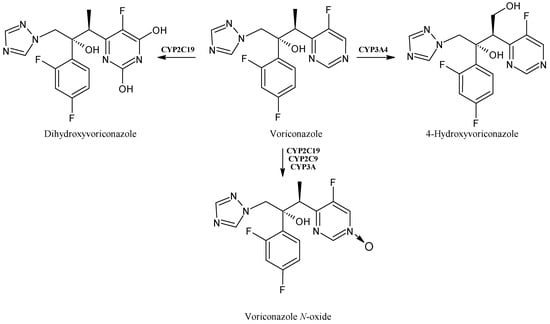
Figure 2.
The structure of voriconazole and its metabolites.
2.2. Mexiletine
Mexiletine (Figure 3) is an antiarrhythmic drug, which belongs to class IB agents and exerts its activity in ventricular arrhythmias, by acting on cardiac voltage-gated sodium channels (hNav1.5) [42], and in myotonic syndromes, given its ability of also blocking the skeletal muscle voltage-dependent sodium channels (hNav1.4) [43]. It is metabolized to hydroxylated metabolites by CYPs, particularly, CYP1A2 and CYP2D6, which are implicated in the formation of para-hydroxymexiletine (PHM) and hydroxy-methylmexiletine (HMM). Together with CYP2E1 and CYP2D6, CYP1A2 induces the N-hydroxylation of mexiletine to form N-hydroxy-mexiletine (NHM). The CYP1A2-mediated hydroxylation is ten times faster for the R-isomer compared to the S-one [44]. These compounds showed activity as skeletal muscle sodium channels blockers [45,46]. Interestingly, the minor metabolite of mexiletine, meta-hydroxy-mexiletine (MHM), was demonstrated to be more active (~2-fold) than the parent mexiletine in displaying a sodium channel blocking activity in cardiac tissues, thus it was suggested for a metabolite switch [47].
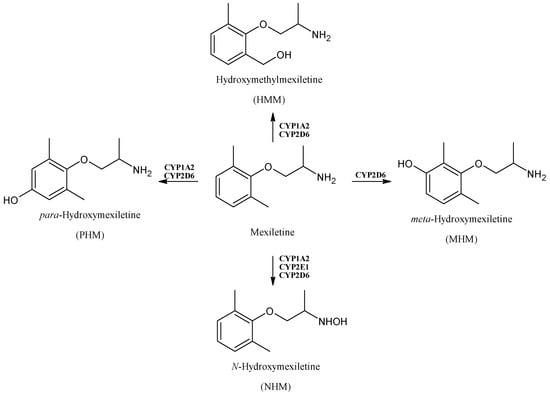
Figure 3.
The structure of mexiletine and its metabolites.
2.3. Sonlicromanol
Sonlicromanol (KH176, Figure 4) is an antioxidant agent targeting the thioredoxin redox systems [48]. Recently, it obtained an orphan drug designation for maternally inherited diabetes and deafness [49] and it is now in phase IIB clinical trials for mitochondrial disease treatments [50]. Sonlicromanol is a chiral drug [51] that, after metabolization, retains the same stereochemistry and it has been suggested to be a substrate for CYP3A4 [52,53]. The in vivo active metabolite of sonlicromanol, KH176m, is also active as an ROS-redox modulator and recent studies evidenced that KH176m may act as an mPGES-1 inhibitor; thus, it has been suggested as a treatment for prostate cancer patients bearing a high mPGES-1 expression [54].
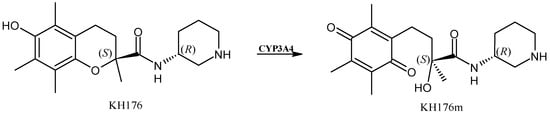
Figure 4.
The structure of sonlicromanol and its active metabolite.
2.4. Sorafenib
Sorafenib (Figure 5) is an oral multikinase inhibitor, involved in tumor proliferation and angiogenesis, belonging to the class of diarylureas [55,56]. It undergoes oxidative biotransformation by human CYP3A4 to its main pharmacologically active metabolite sorafenib N-oxide. It also inhibits several CYP enzymes mediating drug metabolism, such as CYP2C8 [57], which in turn takes part in the elimination of major anticancer drugs, such as paclitaxel and imatinib. Thus, it is being increasingly used in association with other anticancer agents including paclitaxel, and recent studies demonstrated that this co-administration may increase the potential for drug toxicity, probably related to the active sorafenib N-oxide. It has been demonstrated to impair the elimination of co-administered therapeutic agents that are substrates of CYP2C8, and mediate toxic side effects, probably in those individuals where the active metabolite is formed extensively [58].
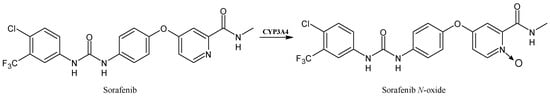
Figure 5.
The structure of sorafenib and its metabolite sorafenib N-oxide.
2.5. Cyclophosphamide
Cyclophosphamide (Figure 6) is a commonly used antitumor drug in clinical practice for different types of cancer, such as breast cancer, lymphoma and myeloma [59]. Particularly, it is a prodrug primarily metabolized by the hepatic CYP2B6, CYP2C9 and CYP3A4 enzymes to its main metabolite 4-hydroxycyclophosphamide, which interconverts with its tautomer aldophosphamide, and is spontaneously transformed into the active agent responsible for alkylation, the phosphoramide mustard. This compound is ionized at a physiological pH and cannot enter the cell, unlike 4-hydroxycyclophosphamide, which can easily diffuse into cells, leading to the plasma concentration responsible for the therapeutic effect [60].
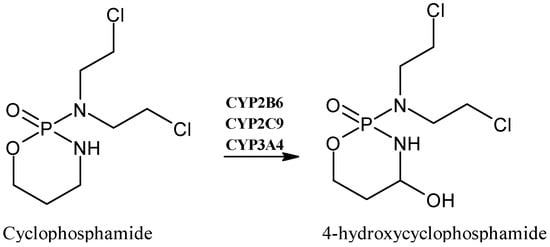
Figure 6.
The structure of cyclophosphamide and its metabolite 4-hydroxycyclophosphamide.
2.6. Triclocarban
Triclocarban (TCC, Figure 7) is a ubiquitous antimicrobial agent found in consumer and personal care products [61,62]. Although its application in over-the-counter hand and body washes was banned by the U.S. Food and Drug Administration (FDA) in 2016, it is still used in other countries and concerns about the widespread emergence of antibiotic-resistant pathogens were raised [63]. TCC is biotransformed by CYPs in hydroxylated species, which can be conjugated with glucuronic acid and sulfate [64]. In mammals, TCC undergoes large oxidative metabolism, leading to three monohydroxylated metabolites (2′-hydroxy-TCC, 3′-hydroxy-TCC, 6-hydroxy-TCC) and the 3,4-dichloro-4′-hydroxy-carbanilide (DHC), resulting from dehalogenation and hydroxylation. DHC, 2′-hydroxy-TCC and 6-hydroxy-TCC can be further converted into the corresponding glucuronides probably by CYP1A1 [65]. The toxicity of TCC may be related to the metabolites, indeed it has been reported that they may bind endogenous macromolecular proteins, acting as a disruptor of physiological homeostasis. Moreover, phase I hydroxylation increases the solubility of TCC in water, leading to the enhancement of hepatotoxicity [66].
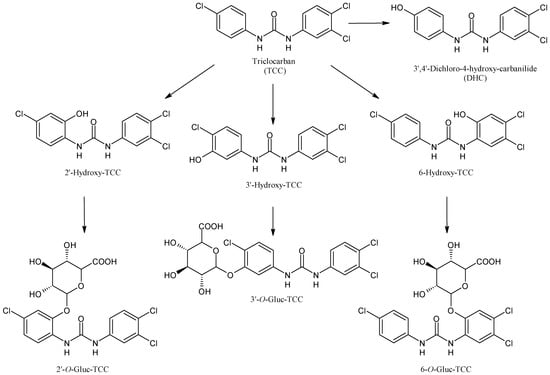
Figure 7.
The structure of triclocarban and its metabolites.
2.7. Sunitinib
Sunitinib (Figure 8) is an effective inhibitor of multiple tyrosine kinase receptors approved by the U.S. FDA on 26 January 2006, for the therapy of metastatic renal cell carcinoma (mRCC) and gastrointestinal stromal tumor (GIST) [67]. The major routes of the oxidative metabolism of sunitinib are represented by N-deethylation, N-oxidation, hydroxylation, oxidative defluorination and conjugation by direct glucuronidation; moreover, the sulfation of hydroxylated metabolites has also been reported [68]. CYP3A4 is the isoform that produces its primary active metabolite, desethyl sunitinib, which possesses the same activity of the parent compound and is then further metabolized by the same isoenzyme. Other metabolites, including mono-oxygenated metabolites, defluorinated sunitinib and glucuronide conjugates, have been described. Given the extreme nature of the toxicity, the CYP3A4 polymorphism was explored but deserves further investigation [69]. Recently, a putative quinoneimine–GSH conjugate, formed by CYP1A2 and CYP3A4, that may be likely responsible for the hepatotoxicity of the drug has been studied [70].
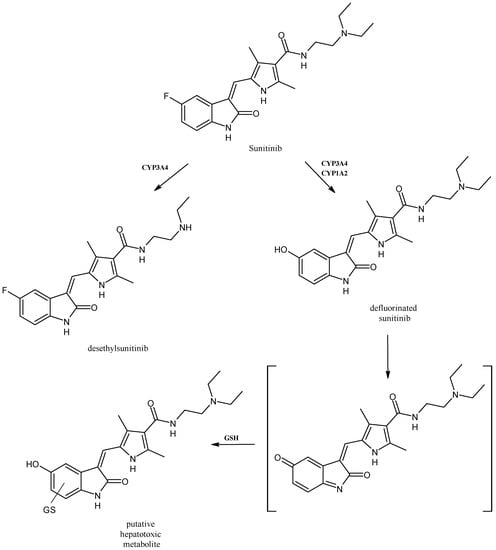
Figure 8.
The structure of sunitinib and its metabolites.
2.8. Hydroxychloroquine
Hydroxychloroquine (HCQ, Figure 9) is a well-known drug used for the therapy of malaria, systemic lupus erythematous, rheumatoid arthritis and for the prevention of thrombosis among patients affected by antiphospholipid antibody syndrome [71,72]. It is mainly biotransformed into three active metabolites in humans: desethylhydroxychloroquine (DHCQ), desethylchloroquine (DCQ) and didesethylchloroquine (DDCQ). CYP3A4, CYP2D6 and CYP2C8 participate in the metabolism of HCQ to various degrees and it has been recently suggested that HCQ and its metabolites are reversible competitive inhibitors of CYP2D6 and that they are mixed-type inhibitors of CYP2J2 and time-dependent CYP3A inhibitors [73]. During the first stages of the COVID-19 pandemic, HCQ was one of the mainly used repositioning drugs [74,75], and the safety and efficacy of this drug may be affected by the co-administration of CYP inhibitors, substrates or inducers [76].
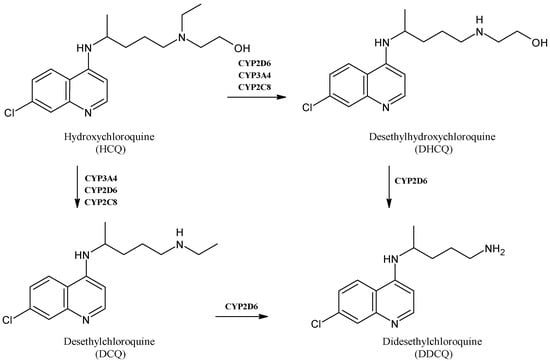
Figure 9.
The structure of hydroxychloroquine and its main metabolites.
2.9. Abrocitinib
Abrocitinib (Figure 10) is an oral selective Janus kinase 1 inhibitor in clinical development for the therapy of moderate to severe atopic dermatitis [77], which has a metabolism mediated by diverse CYP enzymes, such as CYP2C19 (~53%), CYP2C9 (~30%), CYP3A4 (~11%) and CYP2B6 (~6%) [78]. It is converted into four metabolites (M1−M4), two of which are active. Among the two active polar monohydroxylated metabolites, M1 (3-hydroxypropyl) and M2 (2-hydroxypropyl), M2 has the same activity of abrocitinib whereas M1 is less active than the parent drug [79,80]. The biological activity of abrocitinib may be attributed to the parent molecule (about 60%) as well as M1 and M2 (about 10% and 30%, respectively) in the systemic circulation [81,82].
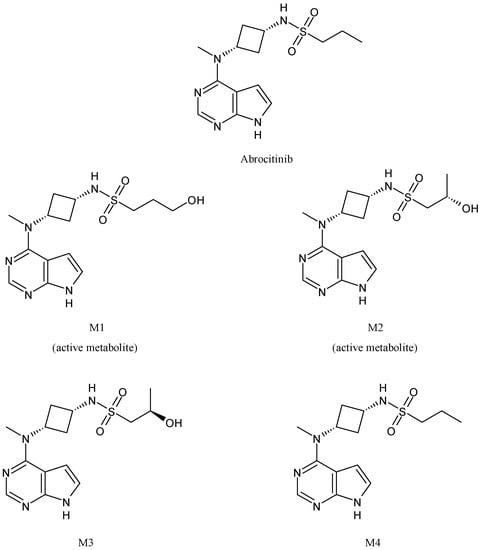
Figure 10.
The structure of abrocitinib and its metabolites.
2.10. Omeprazole
Omeprazole (Figure 11) is a proton-pump inhibitor useful for the treatment of patients with ulcers or gastroesophageal reflux disease [83]. It has a stereogenic center and is used as a racemic mixture of the (S)- and (R)-enantiomers. The (S)-isomer, esomeprazole, is used for the therapy of symptomatic gastroesophageal reflux disease and in the triple-drug regimen for Helicobacter pylori infection [84]. Omeprazole is metabolized both by the CYP2C19 and CYP3A4 pathways to 5-hydroxy-omeprazole, omeprazole sulfone and 5-hydroxy-omeprazole sulfone [85]. (R)- and (S)-omeprazole show stereoselective disposition due to the enzyme-catalyzed metabolism that determines a lower metabolic stability of its (R)-isomer and racemate with respect to esomeprazole [86]. Omeprazole is strongly affected by the CYP2C19 genetic polymorphisms; indeed, several studies have demonstrated that the latter affect the enzyme activity and generate large individual pharmacokinetic variations [87]. Moreover, it is also an inducer of CYPs; specifically, it induces human CYP1A1 and CYP1A2 in human hepatoma cells and hepatocytes [88].
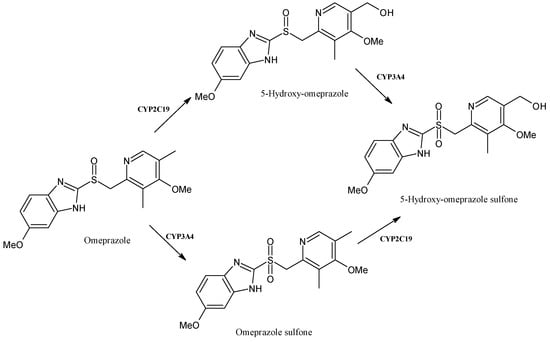
Figure 11.
The structure of omeprazole and its metabolites.
2.11. Tamoxifen
Tamoxifen (Figure 12) is a well-known anticancer drug belonging to selective estrogen-receptor modulators (SERMs) used in the treatment of early and metastatic hormone receptor-positive breast cancer [89]. It is a prodrug, which is metabolized via the CYP enzymes to the more active primary metabolites N-desmethyl-tamoxifen, 4-hydroxy-tamoxifen and the secondary metabolite endoxifen (N-desmethyl-4-hydroxy-tamoxifen) that are responsible for the anticancer activity [90]. The main route of phase I tamoxifen metabolism is its demethylation to N-desmethyl-tamoxifen, catalyzed essentially by CYP3A4/5 [91], whereas the 4-hydroxy-tamoxifen is mainly produced by the action of CYP2D6. However, the formation of N-desmethyl-tamoxifen has also been reported to be catalyzed by CYP2D6 [92]. Single-nucleotide polymorphisms (SNPs) of CYP2D6 play a crucial role in the rate of tamoxifen metabolism, since they have a potential effect in the efficacy of tamoxifen-based therapies. It was recently suggested that pharmacogenomics may play essential roles in the advancement of adjuvant therapies with tamoxifen. The investigation of further SNPs and epigenetic changes of CYP2D6 affecting tamoxifen metabolism may be envisaged, which may enable the design of precision medicine [93]. The involvement of other CYPs, including CYP2C19, CYP1A1, CYP2A6 and CYP3A4, has also been reported [94]. Endoxifen and 4-hydroxy-tamoxifen have 100-fold higher affinity for the target estrogen receptor and 30- to 100-fold higher potency than tamoxifen. The secondary metabolite, endoxifen, is mostly responsible for the tamoxifen’s anticancer activity [95].
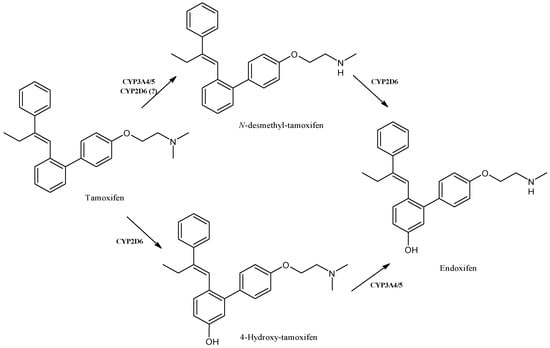
Figure 12.
The structure of tamoxifen and its metabolites.
2.12. Nifedipine
Nifedipine (Figure 13) is a calcium channel antagonist belonging to the 1,4-dihydropyridines, clinically used for the therapy of blood-pressure-related diseases such as angina pectoris, hypertension and Raynaud syndrome [96]. It is metabolized by CYP3A4 to dehydronifedipine in humans [97] and studies on nifedipine in Macaca fascicularis demonstrated its high selectivity for CYP2C76 (a CYP identified in the cynomolgus monkey), leading to its methylhydroxylated metabolite [98]. It was also reported that the CYP2C76 cDNA contains the open reading frame encoding a protein of 489 amino acids that is about 80% identical to any human or monkey P450 cDNAs [99]. It is noteworthy that the nifedipine blood levels can fluctuate due to the interactions with drugs and foods, such as ketoconazole, St. John’s wort and grapefruit juice. The long-term treatment with oral ketoconazole extensively inhibits hepatic CYP3A activity and may dramatically increase the bioavailability of nifedipine [100]. St. John’s wort, acting as an inducer of CYP3A4, in healthy subjects considerably reduced the area under the concentration-time curve (AUC0–∞) for nifedipine, which reflects the body’s exposure to the drug after administering a single dose. A simultaneous significant increase in the AUC0–∞ for dehydronifedipine was observed. This effect was mediated by the induction of CYP3A4 [101]. Moreover, nifedipine pharmacokinetics have been reported to be impaired by the co-ingestion of grapefruit juice, acting as an inhibitor of CYP3A4, leading to the irreversible inactivation of intestinal CYP3A4, reducing the pre-systemic metabolism and enhancing the oral bioavailability of the drugs undergoing this metabolism pathway [102]. The single exposure to grapefruit juice affects intestinal CYP3A4 activity with a half-time of about 24 h, which is completed within 3 days, whereas large doses of this juice may also inhibit hepatic CYP3A4 [103,104]. Grapefruit juice markedly reduces nifedipine metabolism by enhancing its AUC and bioavailability [105], even though this effect was observed following the oral administration of nifedipine, and not after intravenous administration, and appears to be mitigated when smaller amounts of grapefruit juice (250 mL vs. 400 mL or 500 mL) were administered [106].
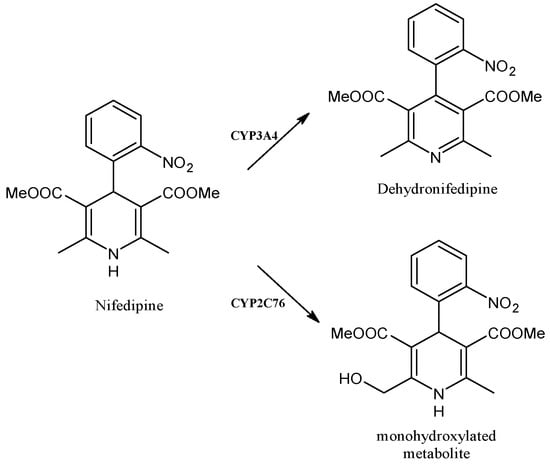
Figure 13.
The structure of nifedipine and its metabolites.
2.13. Vonoprazan
Vonoprazan fumarate (Figure 14) is a potassium-competitive acid blocker that produces a complete acid-inhibition action with a rapid onset, retaining the pH at the targeted level (about 6–7) for over 24 h, and undergoes extensive metabolism. In Japan it has been used for the therapy of H. pylori infection since 2014 [107]. Vonoprazan is metabolized to its inactive metabolites, mainly by cytochrome P450 CYP3A4 and, to some extent, by CYP2B6, CYP2C19, CYP2C9, CYP2D6 and sulfotransferase 2A1 (SULT2A1) [108,109,110]. The biotransformation of vonoprazan fumarate leads to four major metabolites, namely M-I, M-II, M-III and M-IV-Sul. M-I is formed by oxidative elimination of the methylamino moiety; then M-I is metabolized to M-II by further elimination of the pyridinylsulfonyl moiety. M-III derives from the oxidation of the secondary amino group to the nitron form, whereas M-IV-Sul derives from the N-sulfation of the secondary amino group, following hydroxylation of the fluorophenyl ring [111]. Recent molecular modelling studies suggested that CYP2B6 may contribute to vonoprazan metabolism more than CYP3A4 [112]. Moreover, vonoprazan inhibits CYP2B6 and CYP3A4/5 [113].
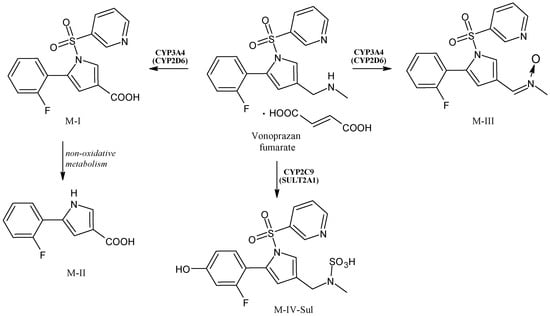
Figure 14.
The structure of vonoprazan and its metabolites.
2.14. Quetiapine
Quetiapine (Figure 15) is a short-acting atypical antipsychotic drug useful in the therapy of schizophrenia or manic episodes of bipolar disorder [114]. Moreover, it is also generally used off-label at low doses as an anxiolytic or hypnotic, even though its cardiovascular safety at these doses is still unknown [115]. The production of the active metabolites quetiapine sulfoxide, N-desalkylquetiapine, O-desalkylquetiapine and 7-hydroxy-quetiapine occurs via CYP3A4/5 biotransformation [116,117,118]. Some authors also described 7-hydroxy-N-desalkyl-quetiapine as a pharmacologically active metabolite [119].
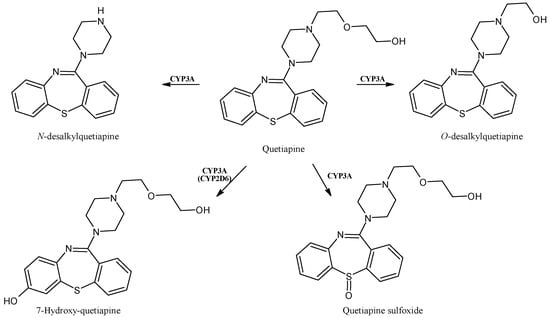
Figure 15.
The structure of quetiapine and its metabolites.
2.15. Atorvastatin
Atorvastatin (Figure 16) belongs to the class of “statins” and acts as a 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) inhibitor, producing an antihyperlipidemic activity. More recently, statins have been proposed, as well, as immunomodulatory and anti-inflammatory drugs [120,121]. Atorvastatin is metabolized by CYP3A4 and CYP3A5 in the gut and liver [122] and its hydroxylated acid metabolites, 2-hydroxy-atorvastatin and 4-hydroxy-atorvastatin, inhibit HMGCR and, consequently, reduce the circulating low-density lipoprotein cholesterol levels, although 2-hydroxy-atorvastatin represents the most abundant metabolite [123,124]. Lactone metabolites, formed from the acid forms of atorvastatin by uridine 5′-diphospho-glucuronosyltransferases (UGTs), do not determine the inhibition of HMGCR, but are unfortunately involved in statin-induced muscle toxicity [125]. In addition, atorvastatin was demonstrated as an inhibitor of CYP3A4 activity, by the means of the 4β-hydroxycholesterol to cholesterol ratio (4βHC: C) measurement, but these data are not in agreement with previous in vitro and in vivo studies [126]. Finally, a single nucleotide polymorphism in the CYP3A5 gene was associated with atorvastatin-induced adverse effects, highlighting the importance of genotyping for predicting its toxicity [127].
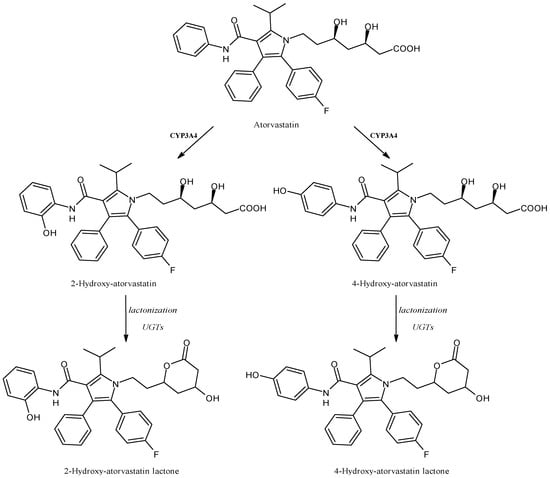
Figure 16.
The structure of atorvastatin and its metabolites.
2.16. Abemaciclib
Abemaciclib (Figure 17) is the third cyclin-dependent kinase 4/6 inhibitor that received approval by the U.S. FDA for the treatment of breast cancer. It was developed by Eli Lilly and Company. Moreover, it is now under investigation for other tumors, including brain cancer [128]. Abemaciclib is mainly metabolized by CYP3A4 into three active metabolites, N-desethylabemaciclib (M2), hydroxy-abemaciclib (M20) and hydroxy-N-desethylabemaciclib (M18), which is subsequently formed by the combination of modifications of M2 and M20. These metabolites are likely relevant for abemaciclib efficacy and toxicity [129]. Abemaciclib regulates several CYPs expression in vitro, but clinical studies using different drugs did not confirm these data [130]. Moreover, even though CYP3A4 was shown as responsible for the transformation of abemaciclib and its metabolites, other recent studies suggested that the drug does not affect the pharmacokinetics of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 substrates in patients with cancer [131].
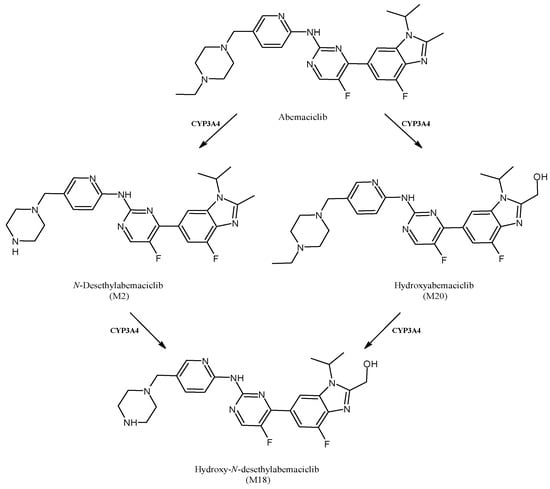
Figure 17.
The structure of abemaciclib and its metabolites.
3. Conclusions
The CYP gene superfamily predominantly resides in the hepatocytes’ endoplasmic reticulum, playing a central role in the metabolism of drugs. The biotransformation of drugs to metabolites catalyzed by CYPs usually starts with the binding of a drug to the enzyme’s active site, then the catalytic turnover of the drug to its metabolite(s) occurs. It is ascertained that the drug binding and orientation depend on the hydrophobic and steric interactions with specific amino acids that are present in the active site of the CYPs. CYP monooxygenases catalyze hydroxylation reactions to form hydroxylated metabolites, which are often responsible for the activity and toxicity of drugs. Very often, scientific studies regarding the therapeutic use and toxic/side effects of drugs lack investigations about the metabolites, which could be much more important than the drugs themselves. This happens with sonlicromanol and mexiletine, where one metabolite is even more active than the parent compound. Additionally, amongst the abrocitinib metabolites, only two are active and, in addition, only one retains the same activity of the parent molecule. Similarly, tamoxifen metabolites show a much higher affinity for the estrogen receptor, on which revolves the anticancer activity. In this view, the “metabolite switch” from the drug to its active metabolite, a phenomenon that is not uncommon and is mainly due to the particular nature of the active site(s) of CYPs, is still rarely mentioned in the scientific literature, although it could shed light on the understanding of the effects in living organisms. It is also important to recall that drug metabolism is a fundamental component of pharmaceutics discovery and development, which contribute to the identification of new molecules and to the benefit of clinical practice. A deeper knowledge about the multifactorial variables that could influence drug metabolism is a fundamental prerequisite for predicting pharmacokinetics and drug response. It should be considered that many drugs are metabolized at clinically relevant concentrations only by one or few CYPs and that the limited data available on the interindividual genetic variability and gene polymorphism together with methodological limitations represent the major issues. Thus, studies directed toward a better understanding of CYPs’ polymorphism and epigenetic regulation influence in drug responses should represent a future trend. Finally, studies on metabolite formation, therapeutic activity and toxicity are needed. The contributions of those studies should be considered to design drugs targeting specific patient populations, in order to highlight the safety and efficacy profiles.
Author Contributions
Conceptualization, M.S.S. and A.C.; writing—original draft preparation, D.I and J.C.; writing—review and editing A.C. and S.A. and C.S.; data curation, E.S., D.S. and M.P.; supervision, M.S.S. and D.I. All authors have read and agreed to the published version of the manuscript.
Funding
PRIN (Progetti di Rilevante Interesse Nazionale) Grant 2020KSY3KL_005 from MUR, Italy (S.A.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Durairaj, P.; Fan, L.; Du, W.; Ahmad, S.; Mebrahtu, D.; Sharma, S.; Ashraf, R.A.; Liu, J.; Liu, Q.; Bureik, M. Functional expression and activity screening of all human cytochrome P450 enzymes in fission yeast. FEBS Lett. 2019, 593, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, X.; Dai, Z.; He, R.; Ding, X.; Yang, L.; Ge, G. Molecular probes for human cytochrome P450 enzymes: Recent progress and future perspectives. Coord. Chem. Rev. 2021, 427, 213600. [Google Scholar] [CrossRef]
- Rendic, S.; Guengerich, F.P. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem. Res. Toxicol. 2015, 28, 38–42. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Human cytochrome P450 enzymes 5–51 as targets of drugs and natural and environmental compounds: Mechanisms, induction, and inhibition–toxic effects and benefits. Drug Metab. Rev. 2018, 50, 256–342. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T.; Novak, R.F.; Halpert, J.R.; Johnson, E.F.; Stevens, J.C. The evolution of drug metabolism and disposition: A perspective from the editors. Drug Metab. Dispos. 2023, 51, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Drug metabolism: A half-century plus of progress, continued needs, and new opportunities. Drug Metab. Dispos. 2023, 51, 99–104. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Drug Metabolism. In The ADME Encyclopedia; Talevi, A., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Hinds, T.D.; Valoti, M.; Lai, Y.; Dorlo, T.P.; Li, Y. Emerging talents in Frontiers in Pharmacology: Drug metabolism and transport 2022. Front. Pharmacol. 2022, 13, 1083163. [Google Scholar] [CrossRef]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme activity of natural products on cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef]
- Becker, D.; Bharatam, P.V.; Gohlke, H. F/g region rigidity is inversely correlated to substrate promiscuity of human cyp isoforms involved in metabolism. J. Chem. Inf. Model. 2021, 61, 4023–4030. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, V.; Parkar, H.; Dasgupta, A. Utility of therapeutic drug monitoring in identifying clinically significant interactions between St. John’s Wort and prescription drugs. Ther. Drug Monit. 2023, 45, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. Clinical potential of fruit in bladder cancer prevention and treatment. Nutrients 2022, 14, 1132. [Google Scholar] [CrossRef]
- Srinivas, N.R. Is pomegranate juice a potential perpetrator of clinical drug-drug interactions? Review of the in vitro, preclinical and clinical evidence. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 223–229. [Google Scholar] [CrossRef]
- Gjestad, C.; Hole, K.; Haslemo, T.; Diczfalusy, U.; Molden, E. Effect of grapefruit juice intake on serum level of the endogenous CYP3A4 metabolite 4β-hydroxycholesterol—An interaction study in healthy volunteers. AAPS J. 2019, 21, 58. [Google Scholar] [CrossRef]
- Tracy, T.S.; Chaudhry, A.S.; Prasad, B.; Thummel, K.E.; Schuetz, E.G.; Zhong, X.-B.; Tien, Y.-C.; Jeong, H.; Pan, X.; Shireman, L.M.; et al. Interindividual Variability in Cytochrome P450-Mediated Drug Metabolism. Drug Metab. Dispos. 2016, 44, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol. Res. 2021, 37, 1–23. [Google Scholar] [CrossRef]
- Tucker, G.T. Chiral switches. Lancet 2000, 355, 1085–1087. [Google Scholar] [CrossRef]
- Hancu, G.; Modroiu, A. Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Dai, L.; Tang, Y.; Zhou, W.; Dang, Y.; Sun, Q.; Tang, Z.; Zhu, M.; Ji, G. Gut microbiota and related metabolites were disturbed in ulcerative colitis and partly restored after mesalamine treatment. Front. Pharmacol. 2021, 11, 2337. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, H.; Yoon, H.Y.; Yee, J.; Gwak, H.S. Association of P450 oxidoreductase gene polymorphism with tacrolimus pharmacokinetics in renal transplant recipients: A systematic review and meta-analysis. Pharmaceutics 2022, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Jiang, G.; Gardner, L.; Xue, G.; Philips, S.; Ly, R.; Wilberforce, O.; Wu, X.; Cantor, E.; Dang, C.; et al. Abstract P1-08-02: Cytochrome P450 reductase gene, POR, associated with paclitaxel induced peripheral neuropathy in patients of European ancestry from the adjuvant breast cancer trial, ECOG-ACRIN E5103. Cancer Res. 2022, 82 (Suppl. 4), P1-08-02. [Google Scholar] [CrossRef]
- Singh, R.D.; Avadhesh, A.; Sharma, G.; Dholariya, S.; Shah, R.B.; Goyal, B.; Gupta, S.C. Potential of cytochrome P450, a family of xenobiotic metabolizing enzymes, in cancer therapy. Antiox. Red. Signal. 2022, 38, 853–876. [Google Scholar] [CrossRef] [PubMed]
- Tice, A.L.; Laudato, J.A.; Gordon, B.S.; Steiner, J.L. Alcohol intoxication stimulates antioxidant gene expression in skeletal muscle in a time dependent manner. FASEB J. 2022, 36 (Suppl. 1). in press. [Google Scholar] [CrossRef]
- Li, Q.S.; Jia, Y.J. Ferroptosis: A critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen. Res. 2023, 18, 506–512. [Google Scholar] [CrossRef] [PubMed]
- McColl, E.R.; Croyle, M.A.; Zamboni, W.C.; Honer, W.G.; Heise, M.; Piquette-Miller, M.; Goralski, K.B. COVID-19 vaccines and the virus: Impact on drug metabolism and pharmacokinetics. Drug Metab. Dispos. 2023, 51, 130–141. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, B.; Deng, J.; Gong, L.; Li, Y.; Li, J.; Zhong, Y. The role of cytochrome P450 enzymes in COVID-19 pathogenesis and therapy. Front. Pharmacol. 2022, 13, 791922. [Google Scholar] [CrossRef]
- Johnson, L.B.; Kauffman, C.A. Voriconazole: A New Triazole Antifungal Agent. Clin. Infect. Dis. 2003, 36, 630–637. [Google Scholar] [CrossRef]
- Hyland, R.; Jones, B.C.; Smith, D.A. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 2003, 31, 540–547. [Google Scholar] [CrossRef]
- Barbarino, J.M.; Owusu-Obeng, A.; Klein, T.E.; Altman, R.B. PharmGKB summary: Voriconazole pathway, pharmacokinetics. Pharmacogenet. Genom. 2017, 27, 201. [Google Scholar] [CrossRef] [PubMed]
- Amsden, J.R.; Gubbins, P.O. Pharmacogenomics of triazole antifungal agents: Implications for safety, tolerability and efficacy. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Le Daré, B.; Boglione-Kerrien, C.; Reizine, F.; Gangneux, J.P.; Bacle, A. Toward the personalized and integrative management of voriconazole dosing during COVID-19-associated pulmonary aspergillosis. Crit. Care 2021, 25, 152. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Kluwe, F.; Mikus, G.; Michelet, R.; Kloft, C. Novel insights into the complex pharmacokinetics of voriconazole: A review of its metabolism. Drug Metab. Rev. 2019, 51, 247–265. [Google Scholar] [CrossRef]
- Gautier-Veyret, E.; Truffot, A.; Bailly, S.; Fonrose, X.; Thiebaut-Bertrand, A.; Tonini, J.; Cahn, J.; Stanke-Labesque, F. Inflammation is a potential risk factor of voriconazole overdose in hematological patients. Fundam. Clin. Pharmacol. 2018, 33, 232–238. [Google Scholar] [CrossRef]
- Moorthy, G.S.; Vedar, C.; Zane, N.; Prodell, J.L.; Zuppa, A.F. Development and validation of a volumetric absorptive microsampling assay for analysis of voriconazole and voriconazole N-oxide in human whole blood. J. Chromatogr. B 2019, 1105, 67–75. [Google Scholar] [CrossRef]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity—An update: Culprit drugs, prevention and management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef]
- Lee, V.; Gober, M.D.; Bashir, H.; O’Day, C.; Blair, I.A.; Mesaros, C.; Weng, L.; Huang, A.; Chen, A.; Tang, R.; et al. Voriconazole enhances UV-induced DNA damage by inhibiting catalase and promoting oxidative stress. Exp. Dermatol. 2020, 29, 29–38. [Google Scholar] [CrossRef]
- Tanaka, H.; Okuma, M.; Ishii, T. Occurrence of voriconazole-induced cutaneous squamous cell carcinoma in Japan: Data mining from different national pharmacovigilance databases. Pharmazie 2022, 77, 307–310. [Google Scholar] [CrossRef]
- Nobeyama, Y.; Umezawa, Y.; Asahina, A. Risk of squamous cell carcinoma in immunosuppressed patients with voriconazole-related actinic keratosis. J. Dermatol. 2022, 49, 1168–1172. [Google Scholar] [CrossRef]
- Zhorov, B.S. Molecular modeling of cardiac sodium channel with mexiletine. Membranes 2022, 12, 1252. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Talon, S.; De Bellis, M.; Desaphy, J.-F.; Franchini, C.; Lentini, G.; Catalano, A.; Corbo, F.; Tortorella, V.; Conte-Camerino, D. Inhibition of skeletal muscle sodium currents by mexiletine analogues: Specific hydrophobic interactions rather than lipophilia per se account for drug therapeutic profile. Naunyn Schmiedeberg’s Arch. Pharmacol. 2003, 367, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.; Fort, R.M.; Rydberg, P.; Harvey, J.N.; Mulholland, A.J. Quantum mechanics/molecular mechanics modeling of drug metabolism: Mexiletine N-hydroxylation by cytochrome P450 1A2. Chem. Res. Toxicol. 2016, 29, 963–971. [Google Scholar] [CrossRef]
- Catalano, A.; Carocci, A.; Fracchiolla, G.; Franchini, C.; Lentini, G.; Tortorella, V.; De Luca, A.; De Bellis, J.; Desaphy, J.-F.; Conte Camerino, D. Stereospecific synthesis of “para-hydroxymexiletine” and sodium channel blocking activity evaluation. Chirality 2004, 16, 72–78. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.; De Luca, A.; Rana, F.; Cavalluzzi, M.M.; Catalano, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Conte Camerino, D. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br. J. Pharmacol. 2006, 149, 300–310. [Google Scholar] [CrossRef]
- Catalano, A.; Desaphy, J.F.; Lentini, G.; Carocci, A.; Di Mola, A.; Bruno, C.; Carbonara, R.; de Palma, A.; Budriesi, R.; Ghelardini, C.; et al. Synthesis and toxicopharmacological evaluation of m-hydroxymexiletine, the first metabolite of mexiletine more potent than the parent compound on voltage-gated sodium channels. J. Med. Chem. 2012, 55, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Beyrath, J.; Pellegrini, M.; Renkema, H.; Houben, L.; Pecheritsyna, S.; van Zandvoort, P.; van den Broek, P.; Bekel, A.; Eftekhari, P.; Smeitink, J.A.M. KH176 safeguards mitochondrial diseased cells from redox stress-induced cell death by interacting with the thioredoxin system/peroxiredoxin enzyme machinery. Sci. Rep. 2018, 8, 6577. [Google Scholar] [CrossRef] [PubMed]
- Khondrion. Khondrion Granted Orphan Drug Designation for KH176 for the Treatment of MIDD from European Commission. 2019. Available online: https://www.khondrion.com/khondrion-granted-orphan-drug-designation-for-kh176-for-the-treatment-of-midd-from-european-commission/ (accessed on 11 July 2020).
- Xiao, Y.; Yim, K.; Zhang, H.; Bakker, D.; Nederlof, R.; Smeitink, J.A.; Renkema, H.; Hollmann, M.W.; Weber, N.C.; Zuurbier, C.J. The redox modulating sonlicromanol active metabolite KH176m and the antioxidant MPG protect against short-duration cardiac ischemia-reperfusion injury. Cardiovasc. Drugs Ther. 2021, 35, 745–758. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A look at the importance of chirality in drug activity: Some significative examples. Appl. Sci. 2022, 12, 10909. [Google Scholar] [CrossRef]
- Janssen, M.C.; Koene, S.; De Laat, P.; Hemelaar, P.; Pickkers, P.; Spaans, E.; Beukema, R.; Beyrath, J.; Groothuis, J.; Verhaak, C.; et al. The KHENERGY study: Safety and efficacy of KH 176 in mitochondrial m.3243A>G spectrum disorders. Clin. Pharmacol. Ther. 2019, 105, 101–111. [Google Scholar] [CrossRef]
- Koene, S.; Spaans, E.; Van Bortel, L.; Van Lancker, G.; Delafontaine, B.; Badilini, F.; Beyrath, J.; Smeitink, J. KH176 under development for rare mitochondrial disease: A first in man randomized controlled clinical trial in healthy male volunteers. Orphanet J. Rare Dis. 2017, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Renkema, H.; Smeitink, J.; Beyrath, J. Sonlicromanol’s active metabolite KH176m normalizes prostate cancer stem cell mPGES-1 overexpression and inhibits cancer spheroid growth. PLoS ONE 2021, 16, e0254315. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as antitumor agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, T.M.; Luo, J.Z.; Lan, C.L.; Wei, Z.L.; Fu, T.H.; Liao, X.W.; Zhu, G.Z.; Ye, X.P.; Peng, T. CYP2C8 suppress proliferation, migration, invasion and sorafenib resistance of hepatocellular carcinoma via PI3K/Akt/p27kip1 axis. J. Hepatocell. Carcinoma 2021, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.C.; Gillani, T.B.; Rawling, T.; Murray, M. Differential inhibition of human CYP2C8 and molecular docking interactions elicited by sorafenib and its major N-oxide metabolite. Chem. Biol. Interact. 2021, 338, 109401. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scumaci, D.; Saturnino, C.; Longo, C.; Sinicropi, M.S. New achievements for the treatment of triple-negative breast cancer. Appl. Sci. 2022, 12, 5554. [Google Scholar] [CrossRef]
- Huang, L.; Winger, B.A.; Cheah, V.; Gingrich, D.; Marzan, F.; Lu, Y.; Cooper, J.C.; Aweeka, F.; Long-Boyle, J. Quantification of N, N’N’’-triethylenethiophosphoramide, N, N’N’’-triethylenephosphoramide, cyclophosphamide, and 4-hydroxy-cyclophosphamide in microvolume human plasma to support neonatal and pediatric drug studies. J. Chromatogr. Open 2022, 2, 100054. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The different facets of triclocarban: A review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; Kong, D.; Li, X.; Wang, A. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Lohmann, W.; Rose, T.; Ahn, K.C.; Hammock, B.D.; Karst, U.; Schebb, N.H. Electrochemistry-mass spectrometry unveils the formation of reactive triclocarban metabolites. Drug Metab. Dispos. 2010, 38, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Muvvala, J.B.; Morin, D.; Buckpitt, A.R.; Hammock, B.D.; Rice, R.H. Metabolic activation of the antibacterial agent triclocarban by cytochrome P450 1A1 yielding glutathione adducts. Drug Metab. Dispos. 2014, 42, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Y.; Liang, Y.; Jiang, L.; Cai, Z. Triclocarban-induced responses of endogenous and xenobiotic metabolism in human hepatic cells: Toxicity assessment based on nontargeted metabolomics approach. J. Hazard. Mater. 2020, 392, 122475. [Google Scholar] [CrossRef]
- Scrivano, L.; Parisi, O.I.; Iacopetta, D.; Ruffo, M.; Ceramella, J.; Sinicropi, M.S.; Puoci, F. Molecularly imprinted hydrogels for sustained release of sunitinib in breast cancer therapy. Polym. Adv. Technol. 2019, 30, 743–748. [Google Scholar] [CrossRef]
- Speed, B.; Bu, H.Z.; Pool, W.F.; Peng, G.W.; Wu, E.Y.; Patyna, S.; Bello, C.; Kang, P. Pharmacokinetics, distribution, and metabolism of [14C] sunitinib in rats, monkeys, and humans. Drug Metab Dispos. 2012, 40, 539–555. [Google Scholar] [CrossRef]
- Patel, N.D.; Chakrabory, K.; Messmer, G.; Krishnan, K.; Bossaer, J.B. Severe sunitinib-induced myelosuppression in a patient with a CYP3A4 polymorphism. J. Oncol. Pharm. Pract. 2018, 24, 623–626. [Google Scholar] [CrossRef]
- Amaya, G.M.; Durandis, R.; Bourgeois, D.S.; Perkins, J.A.; Abouda, A.A.; Wines, K.J.; Mohamud, M.; Starks, S.A.; Daniels, R.N.; Jackson, K.D. Cytochromes P4501A2 and 3A4 catalyze the metabolic activation of sunitinib. Chem. Res. Toxicol. 2018, 31, 570–584. [Google Scholar] [CrossRef]
- Wang, T.F.; Lim, W. What is the role of hydroxychloroquine in reducing thrombotic risk in patients with antiphospholipid antibodies? Hematol. Am. Soc. Hematol. Educ. Progr. 2016, 2016, 714–716. [Google Scholar] [CrossRef]
- Alarcon, G.S.; McGwin, G.; Bertoli, A.M.; Fessler, B.J.; Calvo-Alen, J.; Bastian, H.M.; Vila, L.M.; Reveille, J.D.; Group, L.S. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: Data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 2007, 66, 1168–1172. [Google Scholar] [CrossRef]
- Paludetto, M.N.; Kurkela, M.; Kahma, H.; Backman, J.T.; Niemi, M.; Filppula, A.M. Hydroxychloroquine is metabolized by CYP2D6, CYP3A4, and CYP2C8, and inhibits CYP2D6, while its metabolites also inhibit CYP3A in vitro. Drug Metab. Dispos. 2022, 51, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Skipper, C.; Pastick, K.A.; Engen, N.W.; Bangdiwala, A.S.; Abassi, M.; Lofgren, S.M.; Williams, D.A.; Okafor, E.C.; Pullen, M.F.; Nicol, M.R.; et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: A randomized trial. Ann. Intern. Med. 2020, 173, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An update. Molecules 2022, 27, 8562. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Roy, D.N. Potential clinically significant drug-drug interactions of hydroxychloroquine used in the treatment of COVID-19. Int. J. Clin. Pract. 2021, 75, e14710. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef]
- Dowty, M.; Yang, X.; Lin, J.; Bauman, J.; Doran, A.; Goosen, T.; Wood, L.; Johnson, J.; Banfield, C.; Gupta, P.; et al. P190—The effect of CYP2C9 and CYP2C19 genotype on the pharmacokinetics of PF 04965842, a JAK1 inhibitor in clinical development. Drug Metab. Pharmacokinet. 2020, 35 (Suppl. 1), S80. [Google Scholar] [CrossRef]
- Wang, E.Q.; Le, V.; O’Gorman, M.; Tripathy, S.; Dowty, M.E.; Wang, L.; Malhotra, B.K. Effects of hepatic impairment on the pharmacokinetics of abrocitinib and its metabolites. J. Clin. Pharmacol. 2021, 61, 1311–1323. [Google Scholar] [CrossRef]
- Tripathy, S.; Wentzel, D.; Wan, X.K.; Kavetska, O. Validation of enantioseparation and quantitation of an active metabolite of abrocitinib in human plasma. Bioanalysis 2021, 13, 1477–1486. [Google Scholar] [CrossRef]
- Wang, E.Q.; Le, V.; Winton, J.A.; Tripathy, S.; Raje, S.; Wang, L.; Dowty, M.E.; Malhotra, B.K. Effects of renal impairment on the pharmacokinetics of abrocitinib and its metabolites. J. Clin. Pharmacol. 2022, 62, 505–519. [Google Scholar] [CrossRef]
- Wang, X.; Dowty, M.E.; Wouters, A.; Tatulych, S.; Connell, C.A.; Le, V.H.; Tripathy, S.; O’Gorman, M.T.; Winton, J.A.; Yin, N.; et al. Assessment of the effects of inhibition or induction of CYP2C19 and CYP2C9 enzymes, or inhibition of OAT3, on the pharmacokinetics of abrocitinib and its metabolites in healthy individuals. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 419–429. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, J.; Gao, S.; Zhu, M.X.; Sun, K.; Li, M.; Zhang, G.; Li, Y. Ameliorating cancer cachexia by inhibiting cancer cell release of Hsp70 and Hsp90 with omeprazole. J. Cachexia Sarcopenia Muscle 2021, 13, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Hedge, D.D. Esomeprazole: A clinical review. Am. J. Health Syst. Pharm. 2002, 59, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Shirasaka, Y.; Sager, J.E.; Lutz, J.D.; Davis, C.; Isoherranen, N. Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions. Drug Metab. Dispos. 2013, 41, 1414–1424. [Google Scholar] [CrossRef]
- Li, X.Q.; Weidolf, L.; Simonsson, R.; Andersson, T.B. Enantiomer/enantiomer interactions between the S- and R- isomers of omeprazole in human cytochrome P450 enzymes: Major role of CYP2C19 and CYP3A4. J. Pharmacol. Exp. Ther. 2005, 315, 777–787. [Google Scholar] [CrossRef]
- Park, S.; Hyun, Y.J.; Kim, Y.R.; Lee, J.H.; Ryu, S.; Kim, J.M.; Oh, W.-Y.; Na, H.S.; Lee, J.G.; Seo, D.W. Effects of CYP2C19 genetic polymorphisms on PK/PD responses of omeprazole in Korean healthy volunteers. J. Korean Med. Sci. 2017, 32, 729. [Google Scholar] [CrossRef]
- Yoshinari, K.; Ueda, R.; Kusano, K.; Yoshimura, T.; Nagata, K.; Yamazoe, Y. Omeprazole transactivates human CYP1A1 and CYP1A2 expression through the common regulatory region containing multiple xenobiotic-responsive elements. Biochem. Pharmacol. 2008, 76, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting breast cancer: An overlook on current strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.C.; Winter, S.; Sutiman, N.; Mürdter, T.E.; Chen, S.; Lim, J.S.L.; Li, Z.; Li, J.; Sim, K.S.; Ganchev, B.; et al. Cross-ancestry GWAS defines the extended CYP2D6 locus as the principal genetic determinant of endoxifen plasma concentrations. Clin. Pharmacol. Ther. 2023, 113, 712–723. [Google Scholar] [CrossRef]
- Helland, T.; Alsomairy, S.; Lin, C.; Søiland, H.; Mellgren, G.; Hertz, D.L. Generating a precision endoxifen prediction algorithm to advance personalized tamoxifen treatment in patients with breast cancer. J. Personal. Med. 2021, 11, 201. [Google Scholar] [CrossRef]
- Madlensky, L.; Natarajan, L.; Tchu, S.; Pu, M.; Mortimer, J.; Flatt, S.W.; Nikoloff, D.M.; Hillman, G.; Fontecha, M.R.; Lawrence, H.J.; et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin. Pharmacol. 2011, 89, 718–725. [Google Scholar] [CrossRef]
- Chan, C.W.H.; Law, B.M.H.; So, W.K.W.; Chow, K.M.; Waye, M.M.Y. Pharmacogenomics of breast cancer: Highlighting CYP2D6 and tamoxifen. J. Cancer Res. Clin. Oncol. 2020, 146, 1395–1404. [Google Scholar] [CrossRef]
- Klein, D.J.; Thorn, C.F.; Desta, Z.; Flockhart, D.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Tamoxifen pathway, pharmacokinetics. Pharm. Genom. 2013, 23, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kanji, C.R.; Nyabadza, G.; Nhachi, C.; Masimirembwa, C. Pharmacokinetics of tamoxifen and its major metabolites and the effect of the African ancestry specific CYP2D6* 17 variant on the formation of the active metabolite, endoxifen. J. Personal. Med. 2023, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Schaefer, T.J. Nifedipine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537052/ (accessed on 14 February 2023).
- Mokhtari, B.; Nematollahi, D.; Salehzadeh, H. Electrochemical simultaneous determination of nifedipine and its main metabolite dehydronifedipine using MWCNT modified glassy carbon electrode. J. Mol. Liq. 2018, 264, 543–549. [Google Scholar] [CrossRef]
- Hosaka, S.; Murayama, N.; Satsukawa, M.; Shimizu, M.; Uehara, S.; Fujino, H.; Iwasaki, K.; Iwano, S.; Uno, Y.; Yamazaki, H. Evaluation of 89 compounds for identification of substrates for cynomolgus monkey CYP2C76, a new bupropion/nifedipine oxidase. Drug Metab. Dispos. 2015, 43, 27–33. [Google Scholar] [CrossRef]
- Uno, Y.; Yamazaki, H. mRNA levels of drug-metabolizing enzymes in 11 brain regions of cynomolgus macaques. Drug Metab. Pharmacokinet. 2020, 35, 248–252. [Google Scholar] [CrossRef]
- Kuroha, M.; Kayaba, H.; Kishimoto, S.; Khalil, W.F.; Shimoda, M.; Kokue, E. Effect of oral ketoconazole on first-pass effect of nifedipine after oral administration in dogs. J. Pharm. Sci. 2002, 91, 868–873. [Google Scholar] [CrossRef]
- Moore, L.B.; Goodwin, B.; Jones, S.A.; Wisely, G.B.; Serabjit-Singh, C.J.; Willson, T.M.; Collins, J.L.; Kliewer, S.A. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 7500–7502. [Google Scholar] [CrossRef]
- Dahan, A.; Altman, H. Food-drug interaction: Grapefruit juice augments drug bioavailability—Mechanism, extent and relevance. Eur. J. Clin. Nutr. 2004, 58, 1–9. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; von Moltke, L.L.; Harmatz, J.S.; Chen, G.; Weemhoff, J.L.; Jen, C.; Kelley, C.J.; LeDuc, B.W.; Zinny, M.A. Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin. Pharmacol. Ther. 2003, 74, 121–129. [Google Scholar] [CrossRef]
- Veronese, M.L.; Gillen, L.P.; Burke, J.P.; Dorval, E.P.; Hauck, W.W.; Pequignot, E.; Waldman, S.A.; Greenberg, H.E. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J. Clin. Pharmacol. 2003, 43, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.J.; Martin, U.; Clarke, H.; Waller, D.G.; Renwick, A.G.; George, C.F. Factors affecting the absolute bioavailability of nifedipine. Br. J. Clin. Pharmacol. 1995, 40, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Odou, P.; Ferrari, N.; Barthelemy, C.; Brique, S.; Lhermitte, M.; Vincent, A.; Libersa, C.; Robert, H. Grapefruit juice-nifedipine interaction: Possible involvement of several mechanisms. J. Clin. Pharm. Ther. 2005, 30, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P. Vonoprazan: First global approval. Drugs 2015, 75, 439–443. [Google Scholar] [CrossRef]
- Sugano, K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: Safety and clinical evidence to date. Ther. Adv. Gastroenterol. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Wang, S.; Zhou, Q.; Dai, D.; Shi, J.; Xu, X.; Luo, Q. Cytochrome P450-based drug-drug interactions of vonoprazan in vitro and in vivo. Front. Pharmacol. 2020, 11, 53. [Google Scholar] [CrossRef]
- Echizen, H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: Pharmacokinetic and pharmacodynamic considerations. Clin. Pharmacokinet. 2016, 55, 409–418. [Google Scholar] [CrossRef]
- Yoneyama, T.; Teshima, K.; Jinno, F.; Kondo, T.; Asahi, S. A validated simultaneous quantification method for vonoprazan (TAK-438F) and its 4 metabolites in human plasma by the liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2016, 1015, 42–49. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Dai, D.; Cai, J.; Wang, S.; Hong, Y.; Zhou, S.; Zhao, F.; Zhou, Q.; Geng, P.; et al. Evaluation of commonly used cardiovascular drugs in inhibiting vonoprazan metabolism in vitro and in vivo. Front. Pharmacol. 2022, 13, 909168. [Google Scholar] [CrossRef]
- Graham, D.Y.; Dore, M.P. Update on the use of vonoprazan: A competitive acid blocker. Gastroenterology 2018, 154, 462–466. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A.; Alsaif, N.A.; Khayyat, A.I.A. A comprehensive investigation of interactions between antipsychotic drug quetiapine and human serum albumin using multi-spectroscopic, biochemical, and molecular modeling approaches. Molecules 2022, 27, 2589. [Google Scholar] [CrossRef] [PubMed]
- Højlund, M.; Andersen, K.; Ernst, M.T.; Correll, C.U.; Hallas, J. Use of low-dose quetiapine increases the risk of major adverse cardiovascular events: Results from a nationwide active comparator-controlled cohort study. World Psychiatry 2022, 21, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Bakken, G.V.; Rudberg, I.; Christensen, H.; Molden, E.; Refsum, H.; Hermann, M. Metabolism of quetiapine by CYP3A4 and CYP3A5 in presence or absence of cytochrome B5. Drug Metab. Dispos. 2009, 37, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Y.; Li, X.; Cheng, Z.N.; Zhang, B.K.; Peng, W.X.; Li, H. De Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia. Eur. J. Clin. Pharmacol. 2005, 60, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Babu, D.; Tran, N.; Reiz, B.; Siraki, A.G. Neutrophil myeloperoxidase-mediated n-demethylation of quetiapine leads to N-desalkylquetiapine, a pharmacologically active cytochrome P450 metabolite. Chem. Res. Toxicol. 2022, 35, 1001–1010. [Google Scholar] [CrossRef]
- Castberg, I.; Skogvoll, E.; Spigset, O. Quetiapine and drug interactions: Evidence from a routine therapeutic drug monitoring service. J. Clin. Psychiatry 2007, 68, 1540–1545. [Google Scholar] [CrossRef]
- Sheridan, A.; Wheeler-Jones, C.P.; Gage, M.C. The immunomodulatory effects of statins on macrophages. Immuno 2022, 2, 317–343. [Google Scholar] [CrossRef]
- Motoji, Y.; Fukazawa, R.; Matsui, R.; Nagi-Miura, N.; Miyagi, Y.; Itoh, Y.; Ishii, Y. Kawasaki disease-like vasculitis facilitates atherosclerosis, and statin shows a significant antiatherosclerosis and anti-inflammatory effect in a Kawasaki disease model mouse. Biomedicines 2022, 10, 1794. [Google Scholar] [CrossRef]
- Palleria, C.; Roberti, R.; Iannone, L.F.; Tallarico, M.; Barbieri, M.A.; Vero, A.; Manti, A.; De Sarro, G.; Spina, E.; Russo, E. Clinically relevant drug interactions between statins and antidepressants. J. Clin. Pharm. Ther. 2019, 45, 227–239. [Google Scholar] [CrossRef]
- Partani, P.; Verma, S.M.; Gurule, S.; Khuroo, A.; Monif, T. Simultaneous quantitation of atorvastatin and its two active metabolites in human plasma by liquid chromatography electrospray tandem mass spectrometry. J. Pharm. Anal. 2014, 4, 26–36. [Google Scholar] [CrossRef]
- Turner, R.M.; Fontana, V.; Bayliss, M.; Whalley, S.; Castelazo, A.S.; Pirmohamed, M. Development, validation and application of a novel HPLC-MS/MS method for the quantification of atorvastatin, bisoprolol and clopidogrel in a large cardiovascular patient cohort. J. Pharm. Biomed. Anal. 2018, 159, 272–281. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Statin-related myotoxicity: A comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, J.; Puurunen, J.; Hyötyläinen, T.; Savolainen, M.J.; Ruokonen, A.; Morin-Papunen, L.; Orešič, M.; Piltonen, T.T.; Tapanainen, J.S. The effect of atorvastatin treatment on serum oxysterol concentrations and cytochrome P450 3A4 activity. Br. J. Clin. Pharmacol. 2015, 80, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Kim, H.; Heo, Y.; Yoon, H.Y.; Song, G.; Gwak, H.S. Association between CYP3A5 polymorphism and statin-induced adverse events: A systemic review and meta-analysis. J. Pers. Med. 2021, 11, 677. [Google Scholar] [CrossRef]
- Martinez-Chavez, A.; Loos, N.H.; Lebre, M.C.; Tibben, M.M.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. ABCB1 and ABCG2 limit brain penetration and, together with CYP3A4, total plasma exposure of abemaciclib and its active metabolites. Pharmacol. Res. 2022, 178, 105954. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Chavez, A.; Tibben, M.; de Jong, K.A.M.; Rosing, H.; Schinkel, A.H.; Beijnen, J.H. Simultaneous quantification of abemaciclib and its active metabolites in human and mouse plasma by UHLC-MS/MS. J. Pharm. Biomed. Anal. 2021, 203, 114225. [Google Scholar] [CrossRef]
- Turner, P.K.; Hall, S.D.; Chapman, S.C.; Rehmel, J.L.; Royalty, J.E.; Guo, Y.; Kulanthaivel, P. Abemaciclib does not have a clinically meaningful effect on pharmacokinetics of CYP1A2, CYP2C9, CYP2D6, and CYP3A4 substrates in patients with cancer. Drug Metab. Dispos. 2020, 48, 796–803. [Google Scholar] [CrossRef]
- Goldwaser, E.; Laurent, C.; Lagarde, N.; Fabrega, S.; Nay, L.; Villoutreix, B.O.; Jelsch, C.; Nicot, A.B.; Loriot, M.A.; Miteva, M.A. Machine learning-driven identification of drugs inhibiting cytochrome P450 2C9. PLoS Comput. Biol. 2022, 18, e1009820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).