Abstract
Nanosponges have shown promising capabilities for efficient removal of organic/inorganic pollutants from water based on absorption/adsorption and disinfection processes. The application of nanosponges (especially cyclodextrin-based nanosponges) can be considered a cost-effective strategy with minimal energy and time requirements in comparison to other routinely deployed water treatment modalities. These polymers with unique physicochemical properties, architectures, and highly cross-linked three-dimensional networks need to be further explored for removing pollutants with simultaneous eliminations of microbial contaminants from wastewater. Additionally, the surface functionalization of these nanosponges utilizing magnetic, titanium dioxide, and silver nanomaterials can significantly improve their properties for water remediation purposes, although nanosponges altered with carbon nanotubes and metallic nanomaterials/nanocatalysts for water treatment appliances are barely explored. Notably, crucial factors such as adsorbent type/dosage, contact time, competing ions, adsorption isotherm models, kinetics, thermodynamics, and reaction/experimental conditions (e.g., molar ratios, temperature, and pH) are important aspects affecting the adsorption and removal of pollutants using nanosponges. Furthermore, the nanotoxicity and biosafety of these nanosponge-based systems utilized for water treatment should be comprehensively evaluated. Herein, recent advancements in the design and deployment of nanosponge-based systems for removing organic/inorganic pollutants from water and wastewater are deliberated with an emphasis on challenges and perspectives.
1. Introduction
One of the most critical aspects in human life is access to safe drinking water. Consequently, water treatment is currently vital, particularly because of population growth and the burden of environmental pollution by domestic waste, sewage, chemical materials, industrial waste, and organic/inorganic pesticides, among others. These pollutants cause toxic effects or generate concerns regarding the color, odor, or taste of water. Microbial contaminations are also of serious trepidation, as has been addressed in the field of nanotechnology and nanomaterials sciences [1]. Improvements on water quality using conventional techniques, such as coagulation/settling treatment methods, membrane-based systems, absorption-based systems, and direct filtration, have been widely deployed, but they encompass several disadvantages/limitations of low efficiency, selectivity, and specificity (Table 1) [2,3]. Thus, there is a vital demand for planning efficient strategies or designing smart and low-cost (nano)systems with high sensitivity/specificity for the efficient removal of pollutants from wastewater and for improving the quality of drinking water [4].

Table 1.
Some salient advantages and limitations of water treatment strategies.
Nanosponges with cavities and mesh-like/colloidal structures comprising solid nanomaterials are suitable for encapsulating various substances/compounds, such as proteins/peptides, drugs, genetic materials, antineoplastic agents, and volatile oils, among others [19]. There are various methods for synthesizing β-cyclodextrin-based nanosponges, including emulsion solvent evaporation, hyper cross-linked cyclodextrin, ultrasound-assisted production, and interfacial phenomenon techniques [20]. Several categories of cyclodextrin-based nanosponges have been introduced, such as plain (urethane-, carbonate-, ester-, and ether-based linkages connecting cyclodextrin to the cross linker), modified (fluorescence and electric charge nanosponges based on specific properties), stimuli-responsive (with the ability of behavior modulation by external environmental changes, such as temperature/pH and reducing/oxidative circumstances), and molecularly-imprinted (with high selectivity/specificity toward molecules) nanosponges [21]. These polymers with unique properties, architectures, and high cross-linked three-dimensional networks are broadly studied in the field of pharmaceutics and biomedicine [22]. Additionally, nanosponges with suitable modifications or functionalizations using carbon nanotubes, TiO2, Ag, etc., have been scrutinized for removing or capturing organic/inorganic pollutants from water and wastewater [23,24]. For instance, cyclodextrin-based nanosponges exhibit promising capacities for removing dissolved organic carbon from water (~84%) [25]. Surfactant-modified zeolite nanosponges are designed for removing nitrate in contaminated water, with the highest nitrate elimination capability (1338 mmol Kg−1/83 mg g−1) [26]. Among the introduced nanosponges, especially derived from cyclodextrin, desirable attributes such as environmental friendliness, sustainability, non-toxicity, cost-effectiveness, and the capability of hosting different molecular agents have shown suitability for water treatment with up-scalable potentials [27]. There have been limited studies regarding the applications of molecular modeling methods for analyzing cyclodextrins, because of the flexibility and size of such molecules. Some limited calculations have been performed based on density functional theory (DFT) methods, with befittingly significant levels of theory and large basis sets. It appears that by uniting the results obtained from experimental and computational studies, the geometry of complexation of these structures can be better analyzed [28]. Notably, some crucial parameters such as adsorbent dosage, pH solution, isotherm, kinetics, thermodynamics, regeneration/desorption studies, recyclability, and adsorption mechanisms need to be further evaluated [29]. Herein, recent developments towards the application of nanosponges for water treatment and pollutant removal are deliberated, focusing on important challenges and future perspectives.
2. Nanosponges for Water Treatment
Cyclodextrin-based nanosponges exhibit excellent potentials as sorbents for removing heavy metal ions from aqueous solutions, providing magnificent opportunities for eliminating organic/inorganic contaminants from water (Table 2) [30]. For instance, cyclodextrin-based nanosponges (0.7–1.2 nm) are synthesized with a strong affinity for absorbing hazardous organic contaminants in wastewater because of the presence of cyclodextrin cavities that provide a hydrophobic environment [31]. Cyclodextrin-based nanosponges can also be applied for fast removal of pollutants from water (~90%), with an utmost adsorption capability of 2 mg g−1. These nanostructures exhibit great potentials for removing some pharmaceutical contaminants from water, namely carbendazim, diclofenac, sulfamethoxazole, and furosemide [32]. Additionally, polymeric nanobiocomposites with multifunctionality are constructed via a cross-linking oligomerization of cyclodextrin deploying phosphorylated multi-walled carbon nanotubes (MWCNTs) trailed by a sol-gel step to incorporate silver (Ag) and titanium dioxide (TiO2) nanoparticles. These composites can be deployed as potential filter nanosponges for eliminating pollutants (e.g., organic/inorganic materials and pathogenic microorganisms) from water and wastewater. Fourier-transform infrared (FTIR) analysis illustrates that oxygen-containing groups are subsisted on these composites, and carbamate bearing characteristic peaks (at 1645 cm−1) can also be recognized because of the polymerization reactions [33].

Table 2.
Some salient advantages and limitations of materials/nanosystems applied for water treatment and pollutant removal from aqueous solutions.
Polymer nanosponges constructed from β-cyclodextrin covalently-cross-linked tannic acid through a condensation reaction can be applied for capturing Pb2+ from wastewater with high selectivity/sensitivity, and phenolic hydroxyl groups of tannic acid with the capability of effective binding to Pb2+ generate stable structures [40]. A significant adsorption saturation competence of ~136.8 mg g−1 for Pb2+ was reported at an original strength of 200 mg L−1, with the highest capability of ~116.5 mg g−1. The efficiency of elimination was ~81% Pb2+ within 3 min, and the adsorption was completed within ~50 min because of plentiful adsorption locations in tannic acid and proficient mass transfer rates maintained by cyclodextrin [40]. Cross-linking polymerization, sol-gel, and amidation reaction techniques are deployed together for producing phosphorylated multi-walled carbon nanotube-cyclodextrin/Ag-doped TiO2 nanosponges for removing Pb2+ and Co2+ metal ions from wastewater [41]. One of the crucial factors with significant effects on the adsorption capacities of nanosponges is the pH solution, which can affect speciation, the ionization degree of pollutants, and the surface charge of adsorbents. By increasing the pH of a solution, the efficacy of Pb2+ and Co2+ removal is enhanced. On the other hand, by temperature increment, adsorption capability is increased with an enhancement in time of contact. The mechanism of adsorption and removal of these metal ions comprises repulsion-ion exchange, physical adsorption, and electrostatic attraction. The maximum elimination results can be obtained for removing Co2+ (~7.812 mg/g) and Pb2+ (~35.86) [41].
Liao et al. [42] designed glycopolymer nanosponges from monosaccharides and β-cyclodextrin by combining a Fischer glycosylation, click reaction, and cross-linking reaction for eliminating boron from water. Secondary bonding, including van der Waals forces and hydrogen bonding, among the incorporated saccharides and the adsorbates, can be accountable for vast improvements in adsorption rates and removal capabilities, providing functional candidates for water treatment and seawater desalination [42]. In addition, cyclodextrin-based nanosponges are synthesized for removing organic pollutants from water, including chlorinated disinfection by-products and 2-methylisoborneol (an odor-causing material in water). High absorption efficiency (~99%) can be attained using these polymers with excellent recyclability [43]. β-cyclodextrin-based nanosponges immobilized with magnetic nanoparticles exhibit excellent potentials for capturing and enriching organic micropollutants (the removal efficiency was ~90% within ~1 min), which can be further evaluated for designing sensitive sensing systems (Figure 1). These ultra-rapid and selective sensing strategies with high efficiency for molecule enrichment can be utilized to design smart nanosystems and devices with portability, flexibility, cost-effectiveness, and simplicity advantages [44].
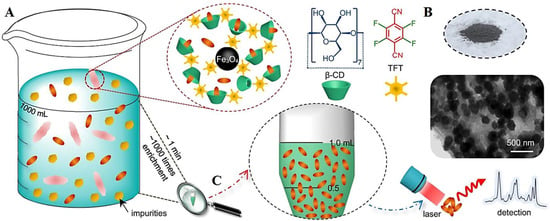
Figure 1.
(A–C) The processes of adsorption/desorption by applying magnetic nanoparticle immobilized β-cyclodextrin-based nanosponges with highly efficient enrichment potentials. Adapted with permission from Ref. [44]. Copyright 2021, Springer Nature, CC BY 4.0.
Cyclodextrin-bas ed nanosponges are designed for the elimination of p-nitrophenol from aqueous streams through the adsorption process [45], and they are prepared via the reaction of β-cyclodextrin with hexamethylenediisocyanate as the cross-linking agent. Notably, the efficiency of adsorption does not noticeably alter at various temperatures; however, adsorption proficiency can be affected by the concentration and type of cross-linking agents. Consequently, the maximum adsorption energy and capacity for these nanosponges, endowed with high porosity and rigidity, are reported to be 1.837 L mg−1 and ~1.0 mg g−1, respectively [45]. In addition, surface-functionalized cis diol comprising mesoporous nanosponges were evaluated for rapid elimination of organic micropollutants and boric acid from water (Figure 2) [46]. These nanosponges exhibit an efficient capacity for boron adsorption, like the commercial resins, in addition to faster adsorption (up to 60 times). Furthermore, bisphenol A can be up-taken by these nanosponges with the equilibrium adsorption achieved in less than two min. The deployment of immobilized polyols can induce synergistic effects because of secondary bonding, simultaneously reducing the time of adsorption and enhancing the capacity of adsorption. Polyol-functionalized mesoporous nanosponges with high sensitivity/selectivity can be considered promising candidates for water treatment appliances [46].
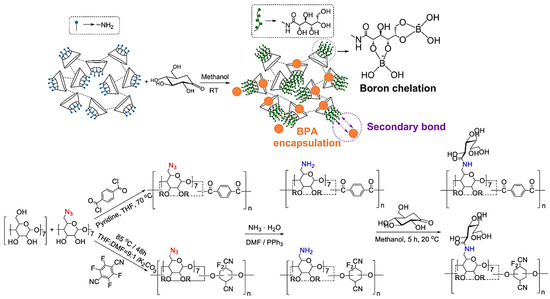
Figure 2.
The preparative process for mesoporous cyclodextrin polymers using a copper-free synthesis technique and functionalization of polyols immobilized on their surfaces. Adapted with permission from Ref. [46], Copyright 2019, American Chemical Society.
Nanosponges constructed from β-cyclodextrin polyurethane with insolubility, recyclability, and great surface area (352.5 m2 g−1) features are additionally altered with phosphorylated MWCNTs and adorned with Ag nanoparticles and TiO2 via the amidation reaction, cross polymerization deploying diisocyanate (as a linker), and the sol-gel process. The designed nanosponges can adsorb Congo red dye and trichloroethylene from wastewater with maximum capacities of 146.96 mg g−1 and 27,507 mg g−1, respectively [29]. Arkas et al. [47] synthesized hyper-branched polymers functionalized with long aliphatic chains for encapsulating/capturing lipophilic polycyclic aromatic pollutants such as pyrene and fluoranthene from water with inclusion formation constants of 2.0 × 108–6.3 × 106 M−1 and 3.8 × 106–4 × 105 M−1, respectively. Notably, the chemical structure of the parent hyper-branched polymers and the type of polycyclic aromatic compounds can affect the loading capacity of nanosponges for fluoranthene (6–31 mg g−1), phenanthrene (15–54 mg g−1), and pyrene (6–35 mg g−1) [47].
Cyclodextrin nanosponges with reusability are designed via cross linking of 1,2,3,4-butanetetracarboxylic acid with β-cyclodextrin in the company of poly(vinyl alcohol) for the adsorptive elimination of cationic contaminants from water [48]. The maximum adsorption was reported for the removal of paraquat (~120.5, 97.0%), safranin (~92.6, 96.7%), and malachite green (~64.9 mg/g, 98.3%) [48]. In addition, biodegradable cyclodextrin-based nanosponges with high biosafety features are constructed using a one-step solvothermal technique using β-cyclodextrin and diphenyl carbonate for removing dyestuffs from the waste stream. The highest adsorption capacity for Basic red 46 and Rhodamine B was ~101.43 mg g−1 and 52.33 mg g−1, respectively. The amount of adsorbent, the molar ratio of β-cyclodextrin and diphenyl carbonate, pH solution, initial concentration, and time of contact can affect the efficiency of nanosponges for pollutant removal [49]. Fenyvesi et al. [50] utilized cyclodextrin bead polymers for the removal of micropollutants with >80% efficiency from effluent. Notably, a correlation between the sorption efficacy and the binding constant of micropollutants and cyclodextrin polymers was reported, revealing that complexes of pollutants with cyclodextrin polymers can play a crucial role in sorption mechanisms [50].
Superparamagnetic Fe3O4 nanoparticles are decorated on the surface of β-cyclodextrin-derived carbonate nanosponges with good reusability for the elimination of dinotefuran from water (Figure 3), and the highest adsorption is ~4.53 × 10−3 mmol g−1 [51]. It appears that these magnetic nanosponges can be considered promising candidates for the removal of neonicotinoids from aqueous solutions, with high efficiency, cost-effectiveness, non-toxicity, and reusability. Poly(vinyl alcohol)-cyclodextrin nanosponges are synthesized via citric acid with β-cyclodextrin in the presence of poly(vinyl alcohol). These nanosponges are employed for removing paraquat from water by the adsorption process. Consequently, the maximum adsorption capacity is ~112.2 mg g−1, and the reuse of these nanosponges is ~90.3% for paraquat remediation after five successive cycles [52]. Cataldo et al. [53] prepared cyclodextrin-calixarene nanosponges as sorbents with high efficiency to eliminate Pb2+ ions from aqueous solutions. These nanosponges can be considered promising candidates for the elimination of inorganic/organic contaminants, especially after suitable surface functionalization/modification to improve sorbent capabilities [53].
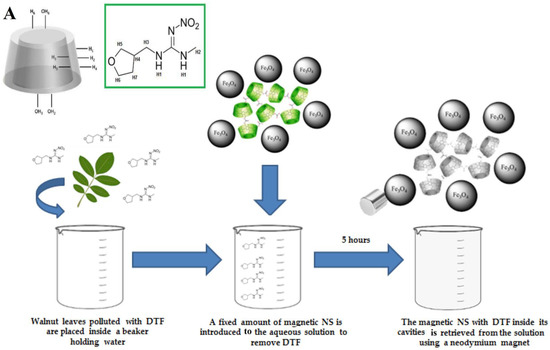
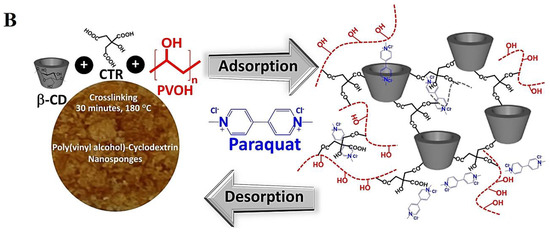
Figure 3.
(A) β-cyclodextrin-based nanosponges (NS) are functionalized by magnetic nanoparticles for the removal of dinotefuran (DTF) pollutant. Adapted with permission from Ref. [51]. Copyright 2020, Multidisciplinary Digital Publishing Institute (CC BY 4.0). (B) Poly(vinyl alcohol)-cyclodextrin nanosponges for the adsorption of paraquat (a hazardous agrochemical) from water. CTR: citric acid; β-CD: β-cyclodextrin. Adapted with permission from Ref. [52]. Copyright 2021, Multidisciplinary Digital Publishing Institute (CC BY 4.0).
Halloysite clay (halloysite nanotubes) and organic cyclodextrin derivatives are utilized to synthesize inorganic–organic nanosponges though microwave irradiation techniques under solvent-free conditions (Figure 4) [54]. These nanosponges are deployed as nanoadsorbents for removing Rhodamine B and some cationic/anionic dyes. Notably, the pH solution and electrostatic interactions can influence the adsorption procedure. High efficiency of adsorption can be attained for cationic dyes relative to anionic ones, offering these nanosponge hybrids for the adsorption of dyes with high selectivity [54]. Additionally, phosphorylated carbon nanotube-β-cyclodextrin nanosponges are fabricated utilizing hexamethylene diisocyanate as a cross linker. These polymers are decorated with Ag and TiO2 nanoparticles via a sol-gel technique for eliminating metal ions pollutants from wastewater [55].
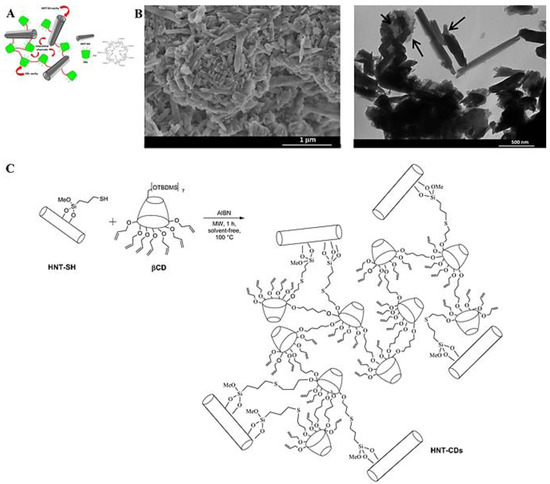
Figure 4.
(A) Halloysite nanotubes (HNT)-cyclodextrin derivatives (CDs) nanosponge hybrids. (B) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the designed nanosponge hybrids. (C) The preparative process of HNT-CDs nanosponge hybrids. Adapted with permission from Ref. [54]. Copyright 2017, American Chemical Society.
Nanosponges with environmentally-benign properties and higher compositions of carboxyl groups are obtained through the cross linking of β-cyclodextrin with linecaps utilizing an aqueous citric acid solution [56]. Pyromellitic nanosponges are fabricated via the reaction of β-cyclodextrin and linecaps with pyromellitic dianhydride in dimethyl sulfoxide. Consequently, pyromellitic nanosponges demonstrate better retention potentials than citrate nanosponges at a metal concentration of 500 ppm. However, these citrate and pyromellitic nanosponges exhibit higher retention potentials (~94%) at low metal concentrations (≤50 ppm). The citrate nanosponge can selectively adsorb significant amounts of heavy metals in the attendance of meddlesome salts from sea water compared to the other constructed nanosponge [56]. Raja et al. [57] synthesized Co3O4/NiO nanosponges through a precipitation method for the photocatalytic degradation of rhodamine B and Congo red dye, wherein they exhibited high photocatalytic efficacy, offering them as attractive candidates with superb photocatalytic performance against organic contaminants [57]. It appears that future works should be envisioned for the optimization of conditions (e.g., temperature, pH, amount of adsorbents used, contact time, competing ions, etc.) to improve the wastewater treatment processes. Moreover, detailed characterizations, simple/cost-effective production techniques, and deep molecular analyses as well as biosafety, commercialization, and nanotoxicological evaluations are still awaiting a thorough investigation, especially for translating the ensuing results from laboratory scales to industrial stages [58,59,60].
3. Conclusions and Future Outlooks
Compared to other nanosystems/materials, nanosponges exhibit high porosity, easy functionalization, simplicity, and cost-effectiveness, which make them attractive candidates for capturing and removing organic/inorganic pollutants/contaminants (e.g., dyes, pharmaceuticals, and heavy metals) from water and wastewater. Notably, cyclodextrin-based nanosponges with unique physicochemical properties and architectures, including good biocompatibility, low/non-toxicity, easy surface functionalization, and bio-absorbent features, can be employed for eliminating a wide variety of pollutants from water via adsorption/inclusion. However, more elaborative studies are warranted to critically evaluate and overcome some crucial aspects/challenges, such as adsorption mechanisms, functionalization processes, removal activities/efficiencies, toxicity/biosafety issues, hydrophobic interactions, electrostatics, and complexation agents for improved targeting of pollutants. Additionally, the type of applied materials (e.g., native/modified polymers), adsorbent dosage, contact time, competing ions, adsorption isotherm models, kinetics, thermodynamics, and reaction/experimental conditions (e.g., molar ratios, temperature, and pH solution) are important factors affecting the adsorption and removal of pollutants using nanosponges. The physicochemical properties, optimization/fabrication processes, complexation behaviors, low-cost production techniques, commercialization, structural variations, and safety evaluations of these nanosystems are essential for their future appliances in water treatment. For antimicrobial and antiviral potentials of these nanosponge-based systems, more elaborative studies are still required for clarifying the mechanism of activities and their efficacy, as well as their long-term biocompatibility and biosafety issues.
Author Contributions
S.I. and R.S.V.: conceptualization, writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was self-funded.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
There are no conflict of interests.
References
- Ihsanullah, A.A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Chhetri, T.; Cunningham, G.; Suresh, D.; Shanks, B.; Kannan, R.; Upendran, A.; Afrasiabi, Z. Wastewater Treatment Using Novel Magnetic Nanosponges. Water 2022, 14, 505. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Lazzara, G. Ecocompatible Halloysite/Cucurbit[8]uril Hybrid as Efficient Nanosponge for Pollutants Removal. ChemistrySelect 2016, 1, 1773–1779. [Google Scholar] [CrossRef]
- Zanetti, M.; Anceschi, A.; Magnacca, G.; Spezzati, G.; Caldera, F.; Rosi, G.P.; Trotta, F. Micro porous carbon spheres from cyclodextrin nanosponges. Microporous Mesoporous Mater. 2016, 235, 178–184. [Google Scholar] [CrossRef]
- Jeong, K.; Son, M.; Yoon, N.; Park, S.; Shim, J.; Kim, J.; Lim, J.-L.; Cho, K.H. Modeling and evaluating performance of full-scale reverse osmosis system in industrial water treatment plant. Desalination 2021, 518, 115289. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Leiknes, T.O. The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review. J. Environ. Sci. 2009, 21, 8–12. [Google Scholar] [CrossRef]
- Lei, Y.; Zhan, Z.; Saakes, M.; Weijden, R.D.V.d.; Buisman, C.J.N. Electrochemical Recovery of Phosphorus from Acidic Cheese Wastewater: Feasibility, Quality of Products, and Comparison with Chemical Precipitation. ACS EST Water 2021, 1, 1002–1013. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.-M.; Zhang, B.-G.; Dai, Y.-R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Trellu, C.; Vargas, H.O.; Mousset, E.; Oturan, N.; Oturan, M.A. Electrochemical technologies for the treatment of pesticides. Curr. Opin. Electrochem. 2021, 26, 100677. [Google Scholar] [CrossRef]
- Urtiaga, A. Electrochemical technologies combined with membrane filtration. Curr. Opin. Electrochem. 2021, 27, 100691. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Matis, K.A. Flotation in Water and Wastewater Treatment. Processes 2018, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Zhang, W. Microwave-enhanced membrane filtration for water treatment. J. Membr. Sci. 2018, 568, 97–104. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Amini, A.; Kim, Y.; Zhang, J.; Boyer, T.; Zhang, Q. Environmental and economic sustainability of ion exchange drinking water treatment for organics removal. J. Clean. Prod. 2015, 104, 413–421. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.J.; Li, W.; Zuberi, Z.; Feng, J.X.; Lin, Q.L.; Ren, J.L.; Luo, F.J.; Ding, Q.M.; Zeng, X.X.; et al. Toward improvements for carrying capacity of the cyclodextrin-based nanosponges: Recent progress from a material and drug delivery. J. Mater. Sci. 2021, 56, 5995–6015. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P. A Comprehensive Patent Review on β-cyclodextrin Cross-linked Nanosponges for Multiple Applications. Recent Pat. Nanotechnol. 2020, 14, 75–89. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Singh, P.; Singhal, A.; Alka. Cyclodextrin-based nanostructured materials for sustainable water remediation applications. Environ. Sci. Pollut. Res. 2020, 27, 32432–32448. [Google Scholar] [CrossRef]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Allabashi, R.; Tsiourvas, D.; Mattausch, E.-M.; Perfler, R. Organic/Inorganic Hybrid Filters Based on Dendritic and Cyclodextrin “Nanosponges” for the Removal of Organic Pollutants from Water. Environ. Sci. Technol. 2006, 40, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Gericke, G.; Sithole, S.P. Cyclodextrin nanosponges in the removal of organic matter for ultrapure water in power generation. J. Water Supply Res. Technol.-AQUA 2009, 58, 299–304. [Google Scholar] [CrossRef]
- El Hanache, L.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Jean Daou, T. Performance of surfactant-modified *BEA-type zeolite nanosponges for the removal of nitrate in contaminated water: Effect of the external surface. J. Hazard. Mater. 2019, 364, 206–217. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Snor, W.; Liedl, E.; Weiss-Greiler, P.; Viernstein, H.; Wolschann, P. Density functional calculations on meloxicam–β-cyclodextrin inclusion complexes. Int. J. Pharm. 2009, 381, 146–152. [Google Scholar] [CrossRef]
- Taka, A.L.; Fosso-Kankeu, E.; Pillay, K.; Yangkou Mbianda, X. Metal nanoparticles decorated phosphorylated carbon nanotube/cyclodextrin nanosponge for trichloroethylene and Congo red dye adsorption from wastewater. J. Environ. Chem. Eng. 2020, 8, 103602. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Li, D.; Ma, M. Nanosponges for water purification. Clean. Prod. Process. 2000, 2, 112–116. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin nanosponges as adsorbent material to remove hazardous pollutants from water: The case of ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Taka, A.L.; Fosso-Kankeu, E.; Mbianda, X.Y.; Klink, M.; Naidoo, E.B. Nanobiocomposite Polymer as a Filter Nanosponge for Wastewater Treatment. Molecules 2021, 26, 3992. [Google Scholar] [CrossRef] [PubMed]
- Gadhban, M.Y.; Abdulmajed, Y.R.; Ali, F.D.; Al-Sharify, Z.T. Preparation of Nano Zeolite and itsApplication in Water Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 870, 012054. [Google Scholar] [CrossRef]
- Awasthi, A.; Jadhao, P.; Kumari, K. Clay nano-adsorbent: Structures, applications and mechanism for water treatment. SN Appl. Sci. 2019, 1, 1076. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Carbon-based Sustainable Nanomaterials for Water Treatment: State-of-art and Future Perspectives. Chemosphere 2021, 263, 128005. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef]
- Shafiei, N.; Nasrollahzadeh, M.; Iravani, S. Green Synthesis of Silica and Silicon Nanoparticles and Their Biomedical and Catalytic Applications. Comments Inorg. Chem. 2021, 41, 317–372. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Trimetallic Nanoparticles: Greener Synthesis and Their Applications. Nanomaterials 2020, 10, 1784. [Google Scholar] [CrossRef]
- Yang, G.; Fang, D.; Yang, L.; Wei, Z.; Tu, Y.; Shao, P.; Hua, Z.; Wang, Z.; Luo, X. Tailored construction of β-cyclodextrin covalently-supported tannic acid polymer nanosponge towards highly selective lead recovery. J. Clean. Prod. 2022, 330, 129882. [Google Scholar] [CrossRef]
- Taka, A.L.; Fosso-Kankeu, E.; Pillay, K.; Mbianda, X.Y. Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: Adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ. Sci. Pollut. Res. 2018, 25, 21752–21767. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, B.; Zhang, Q. Synthesis of glycopolymer nanosponges with enhanced adsorption performances for boron removal and water treatment. J. Mater. Chem. A 2018, 6, 21193–21206. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J. Removal of organic contaminants from water using nanosponge cyclodextrin polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Hao, R.; Shi, Y.; You, H.; Nan, H.; Dai, Y.; Liu, D.; Lei, D.; Fang, J. Ultra-rapid and highly efficient enrichment of organic pollutants via magnetic mesoporous nanosponge for ultrasensitive nanosensors. Nat. Commun. 2021, 12, 6849. [Google Scholar] [CrossRef]
- Salgın, S.; Salgın, U.; Vatansever, Ö. Synthesis and Characterization of β-Cyclodextrin Nanosponge and Its Application for the Removal of p-Nitrophenol from Water. Clean. Soil Air Water 2017, 45, 1500837. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Q. Mesoporous Polymer Nanosponges Immobilized with Functional Polyols for Rapid Removal of Boric Acid and Organic Micropollutants. ACS Appl. Polym. Mater. 2019, 1, 2089–2098. [Google Scholar] [CrossRef]
- Arkas, M.; Eleades, L.; Paleos, C.M.; Tsiourvas, D. Alkylated hyperbranched polymers as molecular nanosponges for the purification of water from polycyclic aromatic hydrocarbons. J. Appl. Polym. Sci. 2005, 97, 2299–2305. [Google Scholar] [CrossRef]
- Martwong, E.; Chuetor, S.; Junthip, J. Adsorption of Cationic Contaminants by Cyclodextrin Nanosponges Cross-Linked with 1,2,3,4-Butanetetracarboxylic Acid and Poly(vinyl alcohol). Polymers 2022, 14, 342. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Li, W.; Liu, K.; Tang, T.; Liu, J.; Jiang, W. One-step synthesis of an environment-friendly cyclodextrin-based nanosponge and its applications for the removal of dyestuff from aqueous solutions. Res. Chem. Intermed. 2020, 46, 1715–1734. [Google Scholar] [CrossRef]
- Fenyvesi, É.; Barkács, K.; Gruiz, K.; Varga, E.; Kenyeres, I.; Záray, G.; Szente, L. Removal of hazardous micropollutants from treated wastewater using cyclodextrin bead polymer—A pilot demonstration case. J. Hazard. Mater. 2020, 383, 121181. [Google Scholar] [CrossRef]
- Salazar, S.; Yutronic, N.; Jara, P. Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. Int. J. Mol. Sci. 2020, 21, 4079. [Google Scholar] [CrossRef] [PubMed]
- Martwong, E.; Chuetor, S.; Junthip, J. Adsorption of Paraquat by Poly(Vinyl Alcohol)-Cyclodextrin Nanosponges. Polymers 2021, 13, 4110. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, S.; Lo Meo, P.; Conte, P.; Di Vincenzo, A.; Milea, D.; Pettignano, A. Evaluation of adsorption ability of cyclodextrin-calixarene nanosponges towards Pb2+ ion in aqueous solution. Carbohydr. Polym. 2021, 267, 118151. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Guernelli, S.; Noto, R.; Riela, S. Synthesis and Characterization of Halloysite–Cyclodextrin Nanosponges for Enhanced Dyes Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 3346–3352. [Google Scholar] [CrossRef] [Green Version]
- Leudjo Taka, A.; Pillay, K.; Mbianda, X.Y. Synthesis and Characterization of a Novel Bio Nanosponge Filter (pMWCNT-CD/TiO2-Ag) as Potential Adsorbent for Water Purification. In Emerging Trends in Chemical Sciences, Proceedings of the ICPAC 2016, Flic en Flac, Mauritius, 18–22 July 2016; Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S.H.L.K.W., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Smarra, A.; Caldera, F.; Musso, G.; Dhakar, N.K.; Cecone, C.; Hamedi, A.; Corsi, I.; Trotta, F. Eco-Friendly β-cyclodextrin and Linecaps Polymers for the Removal of Heavy Metals. Polymers 2019, 11, 1658. [Google Scholar] [CrossRef] [Green Version]
- Raja, V.; Puvaneswaran, S.K.; Swaminathan, K. Unique and hierarchically structured novel Co3O4/NiO nanosponges with superior photocatalytic activity against organic contaminants. Front. Mater. Sci. 2017, 11, 375–384. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of classical and emerging organic pollutants: An extensive review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Ojstršek, A.; Vouk, P.; Fakin, D. Adsorption of pollutants from colored wastewaters after natural wool dyeing. Materials 2022, 15, 1488. [Google Scholar] [CrossRef]
- Zaman, H.G.; Baloo, L.; Pendyala, R.; Singa, P.K.; Ilyas, S.U.; Kutty, S.R.M. Produced water treatment with conventional adsorbents and MOF as an alternative: A review. Materials 2021, 14, 7607. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).