Abstract
The determination of trace pollutants in seawater is challenging, and sampling is a crucial step in the entire analytical process. Passive samplers combine in situ sampling and preconcentration, thus limiting the tedious treatment steps of the conventional sampling methods. Their use to monitor water quality in confined marine environment could bring several advantages. In this work, the presence of organic contaminants at trace and ultra-trace levels was assessed in the Genoa Aquarium supply-and-treated water using Polar Organic Integrative Samplers (POCIS). Both untargeted gas chromatography-mass spectrometry and targeted liquid chromatography-tandem mass spectrometry were employed. The untargeted approach showed the presence of hydrocarbons, diphenyl sulfone and 2,4-di-tert-butyl-phenol. Only hydrocarbons were detected in all the samples. Nineteen emerging contaminants, belonging to different classes (pharmaceuticals, UV-filters, hormones and perfluorinated compounds), were selected for the target analysis. Thirteen analytes were detected, mainly in supply water, even though the majority of them were below the quantitation limit. It is worthy to note that two of the detected UV-filters had never been reported in seawater using the POCIS samplers. The comparison of the analytes detected in supply and treated water indicated a good performance of the Aquarium water treatment system in the abatement of seawater contaminants.
1. Introduction
Coastal marine waters constitute the main receptor of human activities. They are subjected to different anthropogenic stressors, including wastewater treatment plant discharge, shipping traffic, runoff and atmospheric depositions [1,2,3]. The low concentration of seawater contaminants is due to dilution and to the complex hydrodynamics of the marine environment [3]. The detection and quantification of pollutants in marine environments represent an analytical challenge due to the matrix complexity and the trace levels of contaminants that require sensitive methodologies. Sampling is a crucial step in the analytical process. Conventional sampling methods usually require several liters of water, repetitive samplings and treatment steps. On the other hand, passive accumulation devices provide in situ integrative sampling, allowing the determination of time-weighted average (TWA) concentrations. TWA provides an estimation of the contamination level of the considered water environment that can be very advantageous to assess the exposure of living organisms and the associated toxicological risks. Passive samplers are useful for tracking spatial and temporal concentration profiles in waters [4] and to assess the occurrence of episodic events [5]. Moreover, these techniques combine sampling, preconcentration and purification steps, limiting sample manipulation. Among passive sampling devices, the Polar Organic Chemical Integrative Sampler (POCIS) was designed to sample polar compounds [5]. However, POCIS was applied to the sampling of chemicals characterized by a logKow comprised between 0 and 5 [6]. It consists in a sorbent phase sandwiched between two microporous membranes, usually made of polyethersulfone (PES), which are kept together by two stainless rings.
POCIS devices generally show low affinity for highly hydrophobic and ionic compounds [7]. Indeed, low diffusion through the membranes was found for hydrophobic substances because the PES polymer can act as the primary receiving sorption phase for these compounds [8]. Nevertheless, the detection of some lipophilic substances using these samplers is reported [9,10]. The introduction and testing of new sorption materials and membranes in passive techniques are aimed at increasing the range of chemicals that can be sampled [6].
POCIS are mainly employed to monitor contaminants in freshwater and wastewater [10,11,12,13,14], while their use for seawater monitoring remains limited [9,15,16]. Furthermore, the use of this device in a confined marine environment was only reported for the detection of micro-pollutants in a Spanish fish farm located in a coastal area [17]. Nevertheless, their use to monitor water quality of circumscribed environments (such as swimming pools or aquarium tanks) could bring several advantages. Aquarium water is generally collected from the coastal zone and subjected to treatments. Then, renewal water systems allow the water in fish tanks to be replaced to some extent every day, thus making the passive sampling approach useful for verifying water quality over time.
The aim of this work was to identify the presence of polar organic contaminants in Aquarium water (Genoa, Liguria, Italy). POCIS samplers were employed to continuously monitor the quality of supply water (withdrawn from the sea), of treated water (filtered and disinfected) and of shark tank water, verifying the efficacy of the treatment system used before tank filling. Gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) were employed to detect the presence of classical and emerging contaminants.
2. Materials and Methods
2.1. Standard and Reagents
Dichloromethane, 2-propanol, trifluoroacetic acid, acetonitrile (ACN) and methanol (CH3OH), all of analytical or chromatographic grade, were obtained from Merck (Darmstadt, Germany). Water was purified by a Milli-Q system (Millipore, Watford, Hertfordshire, UK). A standard mixture containing even n-alkanes with chains from 10 to 40 carbon atoms (C10–C40) was purchased from Sigma-Aldrich (Milan, Italy). Analytical standards of diphenyl sulfone (DPS), 2,4-ditert butylphenol (2,4-DTBP), ibuprofen (IBU), perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), gemfibrozil (GBZ), estrone (E1), triclosan (TCS), carbamazepine (CBZ), bisphenol-A (BPA), β-estradiol (E2), 17-α-ethinylestradiol (EE2), benzophenone-3 (BP-3), ethylhexyl methoxycinnamate (EHMC), octocrylene (OC), octyl dimethyl p-aminobenzoic acid (OD-PABA), naproxen (NAP), diclofenac (DIC), mefenamic acid (MEF) and caffeine (CAF) were obtained from Sigma-Aldrich (Milan, Italy). All standards were of high purity grade (>97%).
2.2. Genoa Aquarium Water
Supply water, used in all Aquarium oceanic tanks, is directly taken from the sea through a submarine pipeline. This pipe draws marine water at about 10 m from the breakwater and 50 m depth. Before use, raw water is subjected to physico-chemical and microbiological analysis; furthermore, a toxicity test is performed using ephyrae. The ephyra is one of the jellyfish life cycle stages and ephyrae are used as bioindicators for toxic organic substances. If the mentioned tests ensure good water quality, a mechanical treatment is performed by two sand prefilters (each one of 250 m3 per hour). Quarzifer sand granulometry is 0.4–0.8 mm. The filtration step is followed by UV disinfection, and the system (Panaque srl, Capranica, Italy) consists in eight 75-watt lamps of 156 cm height. After these treatments, water is stored in aerated accumulation tanks (treated water).
The multitaxa shark tank hosts various species of sharks from different environments and other fishes. Its size is 9.9 × 23.5 m and contains a water volume of approximately 1200 m3. The tank presents an internal treatment system consisting of four sand filters (each one with a 300 m3 per hour discharge) and O3 disinfection. Every day, 150 m3 of the tank water is replaced (approximately 10% of the total volume), and filters are backwashed.
2.3. Sampling Method
Two studies were carried out to investigate the presence of organic contaminants in raw and treated Aquarium waters using POCIS devices.
The initial monitoring was performed between March 2017 and January 2018. POCISs were used to monitor shark tank water, treated water and supply water.
In particular, the following samplings of two weeks were performed:
- Sampling I (spring): Two POCIS deployed in the shark tank (P1-P2) and two POCIS in a laboratory beaker filled with 4 L of Aquarium treated water (T1-T2);
- Sampling II (winter): Two POCIS deployed in the shark tank (P3-P4), two POCIS in a laboratory beaker filled with 4 L of Aquarium treated water (T3-T4) and two POCIS in a beaker filled with 4 L of supply water (S1-S2).
POCIS deployed in the shark tank were protected by stainless-steel grids, a Zn tablet was fixed on the protective system to avoid corrosion, and the devices were positioned at the bottom of the tank. Treated and supply waters, sampled in laboratory beakers, were subjected to magnetic agitation (Figure S1), and water was replaced as frequently as the shark tank water. This action allowed the minimization of differences between the two water samplings, although agitation conditions may still have an influence on the sampling rate of analytes [18].
The second study, carried out in January 2019, involved a sampling of two weeks:
- Sampling III (winter): Three POCIS deployed in a tank of 150 L (Figure S2) filled with supply water (S3, S4 and S5) or treated water (T5, T6 and T7).
POCISs were placed parallel to the water flow to allow the correct diffusion of analytes into the sampler [19]. Based on the results of the first study, no POCISs were deployed in fish tanks. Indeed, the focus of this last monitoring (sampling III) was to confirm the good performance of the filtration system and to evaluate the presence of emerging contaminants. To take into account the blank signals, for each study two POCISs were submerged in beakers filled with 1.5 L of milli-Q water for 7 days.
The average temperature and salinity of the sampled waters were 23 °C and 36 ng kg−1, respectively.
2.4. POCIS Preparation and Extraction
Both commercial POCIS (E&H Services, Praha, Czech Republic) and devices assembled in our laboratories were used for passive sampling. The laboratory assembly was performed in accordance with the characteristics of commercial ones (mass of the sorbent phase 200 mg and 45.8 cm2 as sampler surface area). PES membranes (0.1 μm pore size) and Oasis HLB sorbent were purchased from Pall Italia (Buccinasco, Italy) and Waters (Vimodrone, Italy), respectively. Before use, PES membranes were washed in a H2O/CH3OH solution (80:20 v/v) for 24 h at room temperature (RT) and 1 h at 40 °C. Membranes were allowed to dry and subsequently washed with CH3OH for 24 h at RT and 1 h at 40 °C. After drying under a fume hood, 200 mg of HLB phase was weighted and placed between two membranes. Then, the membrane-sorbent-membrane layer was compressed between two stainless-steel support rings and held together with three thumbscrews. The prepared POCIS were stored at −20 °C.
After the exposure, samplers were collected, rinsed with Milli-Q water, wrapped in aluminum foil, transported to the laboratory under cooled conditions (4 °C) and kept at −20 °C until analysis.
Prior to processing, the samplers were thawed and rinsed with Milli-Q water to remove fouling present on POCIS membranes, although during all the sampling experiments, no significant biofouling was observed. This is probably due to the daily replacement of a part of the water in the shark tank. POCISs were dismantled and the sorbent phase was transferred using Milli-Q water into a 1 cm diameter glass syringe cartridge fitted with a polytetrafluoroethylene (PTFE) frit and glass wool and dried under a gentle nitrogen stream. Elution was performed with 10 mL of dichloromethane: isopropanol: trifluoroacetic acid (80:19.9:0.1 v/v/v) in a flask and the extract was reduced back down to a small volume [9]. Then, 20 mL of dichloromethane was added, and the solution was evaporated to dryness and redissolved in 1 mL of methanol for GC-MS analysis. The extracts in methanol were diluted 1:1 using Milli-Q water for LC-MS/MS. Samples were filtered through 0.2 μm PTFE filters before chromatographic analysis.
2.5. Analytical Methods
The untargeted analyses of the volatile compounds were performed on an Agilent 7890A gas chromatograph connected to a 5975C mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) with an electron ionization source (EI) set at 70 eV. Chromatographic separation was achieved on a Phenomenex ZB5 capillary column (30 m × 0.25 mm × I.D. 0.25 mm) coated with 5% phenylpolysiloxane at a constant He flow rate of 1.2 mL min−1. The injector was maintained at 250 °C. The temperature program was set as follows: 50 °C kept for 1 min, ramp of 10 °C min−1 up to 300 °C and kept constant for 10 min. The transfer line, ion source and quadrupole were held at 280 °C, 230 °C and 150 °C, respectively. The mass spectrometer was set in full-scan (SCAN) mode for non-target screening, and full-scan spectra were collected from 50 to 550 m/z. The mass spectra obtained in full scan for each chromatographic signal were compared with those contained in the NIST2 library (Version 2.2, June 2014). The selected ion monitoring (SIM) mode was used for the semi-quantitative analysis of chosen compounds. For each analyte, two ions were monitored in SIM mode, using the most abundant one for quantitation.
The presence of target emerging contaminants was evaluated by using liquid chromatography coupled to tandem mass spectrometry [20]. Chromatographic separations were performed by an Agilent Liquid Chromatograph Series 1200SL. HPLC was equipped with a biphenyl Raptor column (2.1 mm × 50 mm, 1.8 µm by Restek, Milan, Italy) held at 60 °C. An Agilent 6430 MSD triple-quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with an electrospray ion source (ESI) was employed for detection and used both in positive and negative ionization modes. Multiple reaction monitoring (MRM) was set to enhance sensitivity by choosing two transitions for each analyte (Table S1), using the most abundant one for quantification.
Positive acquisition involved a chromatographic run in isocratic conditions with a 0.2 mL min−1 flow rate and mobile phase composition of Milli-Q water:ACN = 60:40 (v/v). As for the analytes acquired in the negative mode, separation was performed in isocratic conditions as well. The mobile phase was Milli-Q water:ACN = 50:50 (v/v) containing 0.1% of acetic acid, and the flow rate was of 0.3 mL min−1. Three technical replicates were performed for each sample and the injection volume was of 10 μL.
Three points calibration curves were drawn for each analyte in the 0.01–10 μg L−1 range. The performances of the instrumental method are reported in Table 1. The LOD and LOQ were calculated by considering a signal to noise ratio of 3 and 10, respectively; dilution factor and final volume of the extracts were considered to obtain LOD and LOQ values in the POCIS sampler.

Table 1.
Limits of detection and quantitation of the monitored emerging pollutants in solution (µg L−1) and in the POCIS sampler (ng/POCIS), intra-day precision and accuracy.
3. Results and Discussion
3.1. Untargeted Analysis
In both monitoring campaigns, the first step was a general characterization of the most relevant contaminants present in aquarium waters. As already mentioned in the introduction, although POCISs were originally designed to sample polar analytes, the detection of lipophilic compounds in certain deployment conditions has been reported in the literature [9,10].
Therefore, an untargeted analysis by GC-MS was chosen by also considering the probable presence of volatile and semi-volatile contaminants coming from bunker fuel. GC-MS in EI mode allows the tentative identification of several compounds thanks to the match with a spectrum database. Non-targeted analysis highlighted the presence of hydrocarbons and two other chemicals as the most abundant: DPS and 2,4-DTBP. While hydrocarbons and DPS were already detected in Ligurian seawater [9], 2,4-DTBP was identified for the first time using POCIS.
3.1.1. Hydrocarbons
After a first screening in full scan mode, a more sensitive and selective analysis was performed in SIM mode. The ions monitored were at 57, 71 and 85 m/z, which are characteristics of linear hydrocarbons. Procedural blanks were analyzed, and their contribution in hydrocarbons was subtracted from the samples. For each sequence of analyses, an alkanes standard mixture (C10–C40, concentration 1 mg L−1) was employed to verify retention times and normalize chromatographic signals in order to perform a comparative analysis of the extracts obtained from different samplers.
Results obtained in the first monitoring, reported in Figure 1, did not show significant differences among the hydrocarbons sampled in the tank (P1 and P2; P3 and P4) and in the treated water (T1 and T2; T3 and T4). Furthermore, results obtained for the samplings I and II showed a comparable profile regarding the presence of the homologous series of even n-alkanes of petrogenic origin [21]; therefore, no seasonal trend appears evident.
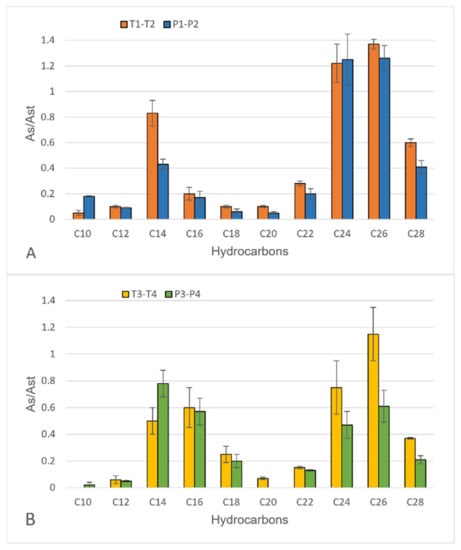
Figure 1.
Results obtained from the semi-quantitative analysis of hydrocarbons (chain length: 10–28 C atoms) in treated water (T1 and T2 in orange) and shark tank water (P1 and P2 in blue) in sampling I (A). Results obtained from the semi-quantitative analysis of hydrocarbons (chain length: 10–28 C atoms) in treated water (T3 and T4 in yellow) and shark tank water (P3 and P4 in green) in sampling II (B). As/Ast represents the ratio between the hydrocarbon peak areas in the sample and in the reference standard.
In sampling II of the first monitoring, an evaluation of the treatment process was carried out by analyzing the POCIS deployed in supply water and treated water (Figure 2).
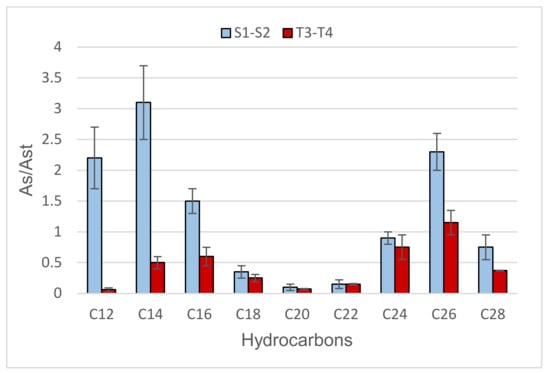
Figure 2.
Results obtained from the semi-quantitative analysis of hydrocarbons (chain length: 12–28 C atoms) in supply water (S1 and S2 in light blue) and treated water (T3 and T4 in red) in sampling II. As/Ast represents the ratio between the hydrocarbon peak areas in the sample and in the reference standard.
T3 and T4 extracts showed a good decrease in hydrocarbon concentration, especially for short chain alkanes (between C12 and C16). This result suggests that sand filtration has a lower impact on long chain analytes or that shorter hydrocarbons tend to undergo degradation onto the filter [22].
Water treatment efficiency was further investigated in the second monitoring. Linear alkanes from C10 to C40 were found in S3, S4 and S5 and in T5, T6 and T7 water extracts. Analogously to the first monitoring, a general decrease was observed for each analyte, especially for short-chain compounds: C12 and C14 hydrocarbons presented the most abundant decrease (84% and 69%, respectively).
As a final remark for hydrocarbons, it is worth highlighting that the profile of supply water presented the highest peaks in the diesel range (C8–C23) [23,24] both in the first and in the second monitoring.
3.1.2. Industrial Compounds
In sampling I and II, the screening in full-scan mode of the extracts allowed the detection of DPS and 2,4-DTBP. This untargeted procedure was employed also in sampling III, where, in addition to hydrocarbons, only 2,4-DTBP was found. Subsequently, semi-quantitative analysis was carried out in SIM mode. The ions at 125 m/z and 191 m/z were monitored for DPS and 2,4-DTBP, respectively. Analogously to hydrocarbons, the ratio between the analytes peak area in the sample and in a reference standard (concentration 1 mg L−1) was used to compare different POCIS extracts.
DPS is a herbicide employed as ovicide and acaricide in the past. Today, it is mainly used as a monomer in the polymer industry and is a transformation product of phenols synthesis. It enters aquatic ecosystems through various sources and Wick et al. affirmed that photochemical and chemical degradations are not significant in surficial water [25].
DPS concentrations estimated in the sampling I extracts were approximately 1 mg L−1, corresponding to 1 µg/POCIS. This value was comparable to that reported in one of our previous studies, where POCISs were deployed in Santa Margherita Harbor (Liguria) [9]. Furthermore, the detection of DPS at low concentrations in seawater was reported during a micro-pollutant monitoring campaign in a fish farm [17]. On the other hand, in sampling II and III, DPS was neither detected in the supply water nor in the treated water. This difference could be related to the sampling period of the last two deployments (winter).
2,4-DTBP is a volatile organic compound used as intermediate for the preparation of UV stabilizers and antioxidants. Migration tests performed on high-density polyethylene pipes (HDPE) used in drinking water distribution networks showed 2,4-DTBP as the major migrating component from pipes into water [26]. It has fungicidal activity [27] and has shown potential endocrine disrupting effects and aquatic toxicity [28].
Regarding first monitoring, higher levels of 2,4-DTBP in treated and shark tank waters were observed in sampling I compared to sampling II (mean value approximately 3 µg/POCIS and 1 µg/POCIS, respectively). Moreover, the concentration of 2,4-DTBP was approximately 6 µg/POCIS for supply water extracts obtained from sampling II, indicating strong abatement during water treatment (6 µg/POCIS and 1 µg/POCIS detected in supply and treated water, respectively).
The second monitoring (sampling III) confirmed the good performances of the treatment system in pollutant removal. 2,4-DTBP was found in the sole supply water at a concentration around 4 µg/POCIS, while there was no evidence of the analyte presence in the treated water. The good performance of aquarium filtration and disinfection systems in 2,4-DTBP removal was an important result due to its endocrine disruptor effect and its aquatic toxicity.
It is worth noting that a lower amount of 2,4-DTBP was sampled in winter (samplings II and III) compared to spring (sampling I), confirming the trend already observed for DPS, which was only detected in POCIS deployed in spring (sampling I).
3.2. Targeted Analysis of Emerging Contaminants
In addition to the evaluation of major volatile and semi-volatile compounds, the second monitoring (sampling III) involved a further investigation on the presence of emerging contaminants (ECs) in aquarium waters (supply and treated). The selected target analytes belong to different classes (pharmaceuticals, UV filters, hormones and perfluorinated compounds); therefore, an LC-MS/MS multi-residual analysis method was used for their determination. The concentration of the analytes was expressed as the amount of analyte per POCIS (ng/POCIS) to obtain a quantitative comparison between supply and treated waters.
Thirteen analytes were identified in POCIS deployed in supply and treated waters (results in Table 2), although many of them were simply detected but not quantifiable. Sampling rates (Rs) of some of the considered chemicals are present in the literature, thus theoretically allowing the estimation of the time-weighted average (TWA) concentration of the contaminants in supply and treated waters. However, the Rs from the literature are generally affected by high variability; thus, analogously to the detected volatile compounds, the TWA concentrations of most ECs were not calculated.

Table 2.
Measured mean concentrations (±standard deviation) of the emerging pollutants detected in supply (S3, S4 and S5) and treated (T5, T6 and T7) water (ng/POCIS).
The data obtained exhibit a general decrease in concentration after treatments, confirming the good performance of filtration and disinfection systems.
The presence of different contaminants in the samples, even though at low concentrations, confirms that these compounds may be considered as semi-persistent in the environment. In fact, they are continuously released from wastewater treatment plants due to incomplete removal [1,15].
The most concentrated chemicals in the supply water were BPA and the UV-filters OC and EHMC.
BPA is an intermediate in the production of epoxy resins and polycarbonate plastics [29]. It is used in many plastic products [30] and is ubiquitous due to its frequent release [31]. BPA represents a potential concern for aquatic organisms, with reproductive and developmental effects on aquatic species [32]. In order to assess the risk for marine organisms, the Risk Characterization Ratio is used, which is the ratio between the Predicted Environmental Concentration (PEC) in water and the provisional predicted no-effect concentration (PNEC). A PNEC value of 0.15 μg L−1 for BPA has been proposed for marine waters by the European Union [33]. Considering the literature sampling rate for BPA obtained in seawater of 0.288 L day−1 [16], the TWA concentration in water results in 0.0015 μg L−1. By assuming TWA as an acceptable approximation of PEC, the Risk Characterization Ratio (PEC/PNEC) should be below 1 in the analyzed waters. This result suggests that adverse effects are not expected on seawater organisms for this substance [34].
UV filter compounds are integrated in many cosmetic formulations and can reach surface waters through the release from the skin during recreational activity or through wastewater [35]. Relatively high levels of EHMC and OC and the presence of BP-3 and OD-PABA were detected in POCIS extracts. Due to hormonal activity shown by several UV filters at very low concentrations [10], these results underline the importance of their monitoring. No Rs are present in the literature regarding standard POCIS sampling of UV-filters [36]. Hence, it was not possible to estimate TWA concentrations. Nonetheless, the detection of two UV-filters at considerable concentrations in the samplers represent the first report regarding POCIS uptake of these substances from seawater. This result highlights the usefulness of this approach in pre-concentrating contaminants from large water volumes where some contaminants are subjected to huge dilution factors.
4. Conclusions
In this study, an assessment of the contamination of Genoa Aquarium supply, treated waters and shark tank waters was performed.
The untargeted analysis resulted in the identification of hydrocarbons in all samples, and chromatogram profiles showed their petrogenic origins. The industrial pollutants DPS and 2,4-DTBP were found in some samples using the non-targeted approach. Moreover, the presence of different emerging contaminants was investigated by using target analysis: the UV filters OC and EHMC were the most concentrated among ECs both in supply and treated waters. BPA, Gemfibrozil and Carbamazepine were also detected in quantifiable amounts in the supply water. Water treatment system efficacy was evaluated by comparing supply and treated waters: results generally showed a decrease in the concentration of contaminants after treatment.
The passive sampling approach permitted the enhancement of sensitivity and allowed comparisons of water quality based on time-integrative sampling. The data obtained with POCIS are mediated over the deployment period, considering fluctuations and episodic events. On the contrary, the classical sampling approach provides only a snapshot of the pollution levels at the time of the sampling. In addition, a much larger number of spot samples should be collected during the two weeks of exposure, while also considering the shark tank water volume.
The use of POCIS to monitor Genoa Aquarium water quality allowed the detection of the low concentration presence of some endocrine disruptor compounds and substances with aquatic toxicity. A more in-depth study will be necessary to establish contaminant concentrations in water based on sampling rates. This investigation would hopefully allow a risk assessment related to the health of aquarium fishes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12062951/s1, Figure S1: Deployment of POCIS in beakers during sampling II: on the right the samplers S1 and S2 and on the left T3 and T4; Figure S2: Exposure tank with deployed POCIS during sampling III; Table S1: MRM transitions of the emerging pollutants monitored.
Author Contributions
Conceptualization, E.M., M.D.C., N.P. and E.C.; methodology, E.M. and M.D.C.; data curation, C.S. and B.B.; writing—original draft preparation, C.S. and B.B.; writing—review and editing, C.S., E.M. and M.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author upon request.
Acknowledgments
Tomas Ocelka from E&H Service is gratefully acknowledged for kindly providing commercial POCIS.
Conflicts of Interest
The authors have no relevant financial or nonfinancial interests to disclose.
References
- Comeau, F.; Surette, C.; Brun, G.L.; Losier, R. The Occurrence of Acidic Drugs and Caffeine in Sewage Effluents and Receiving Waters from Three Coastal Watersheds in Atlantic Canada. Sci. Total Environ. 2008, 396, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and Spatial Distribution of 158 Pharmaceuticals, Drugs of Abuse and Related Metabolites in Offshore Seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpin-Pont, L.; Martínez-Bueno, M.J.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the Marine Environment: A Review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.; Perkins, S.; Nilsen, E.; Morace, J. Spatial and Temporal Trends in Occurrence of Emerging and Legacy Contaminants in the Lower Columbia River 2008–2010. Sci. Total Environ. 2014, 484, 322–330. [Google Scholar] [CrossRef]
- Alvarez, D.A.; Petty, J.D.; Huckins, J.N.; Jones-Lepp, T.L.; Getting, D.T.; Goddard, J.P.; Manahan, S.E. Development of a Passive, in Situ, Integrative Sampler for Hydrophilic Organic Contaminants in Aquatic Environments. Environ. Toxicol. Chem. 2004, 23, 1640–1648. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, K.; Stepnowski, P.; Paszkiewicz, M. Pollutant Analysis Using Passive Samplers: Principles, Sorbents, Calibration and Applications. A Review. Environ. Chem. Lett. 2021, 19, 465–520. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Prieto, A.; Zugazua, O.; Zuloaga, O.; Olivares, M.; Usobiaga, A.; Paschke, A.; Etxebarria, N. Evaluation of Polar Organic Chemical Integrative and Hollow Fibre Samplers for the Determination of a Wide Variety of Organic Polar Compounds in Seawater. Talanta 2018, 185, 469–476. [Google Scholar] [CrossRef]
- Vermeirssen, E.L.M.; Dietschweiler, C.; Escher, B.I.; van der Voet, J.; Hollender, J. Transfer Kinetics of Polar Organic Compounds over Polyethersulfone Membranes in the Passive Samplers Pocis and Chemcatcher. Environ. Sci. Technol. 2012, 46, 6759–6766. [Google Scholar] [CrossRef]
- Di Carro, M.; Magi, E.; Massa, F.; Castellano, M.; Mirasole, C.; Tanwar, S.; Olivari, E.; Povero, P. Untargeted Approach for the Evaluation of Anthropic Impact on the Sheltered Marine Area of Portofino (Italy). Mar. Pollut. Bull. 2018, 131, 87–94. [Google Scholar] [CrossRef]
- Fent, K.; Zenker, A.; Rapp, M. Widespread Occurrence of Estrogenic UV-Filters in Aquatic Ecosystems in Switzerland. Environ. Pollut. 2010, 158, 1817–1824. [Google Scholar] [CrossRef]
- Tanwar, S.; Di Carro, M.; Magi, E. Innovative Sampling and Extraction Methods for the Determination of Nonsteroidal Anti-Inflammatory Drugs in Water. J. Pharm. Biomed. Anal. 2015, 106, 100–106. [Google Scholar] [CrossRef]
- Mirasole, C.; Di Carro, M.; Tanwar, S.; Magi, E. Liquid Chromatography–Tandem Mass Spectrometry and Passive Sampling: Powerful Tools for the Determination of Emerging Pollutants in Water for Human Consumption. J. Mass Spectrom. 2016, 51, 814–820. [Google Scholar] [CrossRef]
- Jeong, Y.; Schäffer, A.; Smith, K. A Comparison of Equilibrium and Kinetic Passive Sampling for the Monitoring of Aquatic Organic Contaminants in German Rivers. Water Res. 2018, 145, 248–258. [Google Scholar] [CrossRef]
- Jones, L.; Ronan, J.; McHugh, B.; Regan, F. Passive Sampling of Polar Emerging Contaminants in Irish Catchments. Water Sci. Technol. 2019, 79, 218–230. [Google Scholar] [CrossRef]
- Martínez Bueno, M.J.; Herrera, S.; Munaron, D.; Boillot, C.; Fenet, H.; Chiron, S.; Gómez, E. POCIS Passive Samplers as a Monitoring Tool for Pharmaceutical Residues and Their Transformation Products in Marine Environment. Environ. Sci. Pollut. Res. 2016, 23, 5019–5029. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, J.L.; Zhao, H.; Hou, L.; Yang, Y. Application of Passive Sampling in Assessing the Occurrence and Risk of Antibiotics and Endocrine Disrupting Chemicals in the Yangtze Estuary, China. Chemosphere 2014, 111, 344–351. [Google Scholar] [CrossRef]
- Martínez Bueno, M.J.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Application of Passive Sampling Devices for Screening of Micro-Pollutants in Marine Aquaculture Using LC-MS/MS. Talanta 2009, 77, 1518–1527. [Google Scholar] [CrossRef]
- Harman, C.; Allan, I.J.; Vermeirssen, E.L.M. Calibration and Use of the Polar Organic Chemical Integrative Sampler—A Critical Review. Environ. Toxicol. Chem. 2012, 31, 2724–2738. [Google Scholar] [CrossRef]
- Di Carro, M.; Bono, L.; Magi, E. A Simple Recirculating Flow System for the Calibration of Polar Organic Chemical Integrative Samplers (POCIS): Effect of Flow Rate on Different Water Pollutants. Talanta 2014, 120, 30–33. [Google Scholar] [CrossRef]
- Di Carro, M.; Lluveras-Tenorio, A.; Benedetti, B.; Magi, E. An Innovative Sampling Approach Combined with Liquid Chromatography–Tandem Mass Spectrometry for the Analysis of Emerging Pollutants in Drinking Water. J. Mass Spectrom. 2020, 55, e4608. [Google Scholar] [CrossRef]
- Commendatore, M.G.; Esteves, J.L. Natural and Anthropogenic Hydrocarbons in Sediments from the Chubut River (Patagonia, Argentina). Mar. Pollut. Bull. 2004, 48, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kalmykova, Y.; Moona, N.; Strömvall, A.M.; Björklund, K. Sorption and Degradation of Petroleum Hydrocarbons, Polycyclic Aromatic Hydrocarbons, Alkylphenols, Bisphenol A and Phthalates in Landfill Leachate Using Sand, Activated Carbon and Peat Filters. Water Res. 2014, 56, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Gaines, R.B.; Frysinger, G.S.; Hendrick-Smith, M.S.; Stuart, J.D. Oil Spill Source Identification by Comprehensive Two-Dimensional Gas Chromatography. Environ. Sci. Technol. 1999, 33, 2106–2112. [Google Scholar] [CrossRef]
- Ou, S.; Zheng, J.; Zheng, J.; Richardson, B.J.; Lam, P.K.S. Petroleum Hydrocarbons and Polycyclic Aromatic Hydrocarbons in the Surficial Sediments of Xiamen Harbour and Yuan Dan Lake, China. Chemosphere 2004, 56, 107–112. [Google Scholar] [CrossRef]
- Wick, L.Y.; Gschwend, P.M. By-Products of a Former Phenol Manufacturing Site in a Small Lake Adjacent to a Superfund Site in the Aberjona Watershed. Environ. Health Perspect. 1998, 106, 1069–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skjevrak, I.; Due, A.; Gjerstad, K.O.; Herikstad, H. Volatile Organic Components Migrating from Plastic Pipes (HDPE, PEX and PVC) into Drinking Water. Water Res. 2003, 37, 1912–1920. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-Tert-Butyl Phenol as the Antifungal, Antioxidant Bioactive Purified from a Newly Isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, J.; Li, J.; Zhou, L.; Zhang, H.; Sun, J.; Zhuang, S. The Evaluation of Endocrine Disrupting Effects of Tert-Butylphenols towards Estrogenic Receptor A Androgen Receptor and Thyroid Hormone Receptor Β and Aquatic Toxicities towards Freshwater Organisms. Environ. Pollut. 2018, 240, 396–402. [Google Scholar] [CrossRef]
- Ademollo, N.; Patrolecco, L.; Rauseo, J.; Nielsen, J.; Corsolini, S. Bioaccumulation of Nonylphenols and Bisphenol A in the Greenland Shark Somniosus Microcephalus from the Greenland Seawaters. Microchem. J. 2018, 136, 106–112. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and Its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A Exposure, Effects, and Policy: A Wildlife Perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Miglioli, A.; Balbi, T.; Besnardeau, L.; Dumollard, R.; Canesi, L. Bisphenol A Interferes with First Shell Formation and Development of the Serotoninergic System in Early Larval Stages of Mytilus galloprovincialis. Sci. Total Environ. 2021, 758, 144003. [Google Scholar] [CrossRef] [PubMed]
- Aschberger, K.; Munn, S.; Olsson, H.; Pakalin, S.; Pellegrini, G.; Vegro, S.; Paya Perez, B. Updated European Union Risk Assessment Report: 4,4′-Isopropylidenediphenol (Bisphenol-A): Environment Addendum of February 2008; Publications Office: Luxembourg, 2010. [Google Scholar] [CrossRef]
- Muñoz, I.; Martínez Bueno, M.J.; Agüera, A.; Fernández-Alba, A.R. Environmental and Human Health Risk Assessment of Organic Micro-Pollutants Occurring in a Spanish Marine Fish Farm. Environ. Pollut. 2010, 158, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.N.; Scapolla, C.; Di Carro, M.; Magi, E. Rapid and Selective Determination of UV Filters in Seawater by Liquid Chromatography–Tandem Mass Spectrometry Combined with Stir Bar Sorptive Extraction. Talanta 2011, 85, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- MacKeown, H.; Magi, E.; Di Carro, M.; Benedetti, B. Unravelling the role of membrane pore size in polar organic chemical integrative samplers (POCIS) to broaden the polarity range of sampled analytes. Anal. Bioanal. Chem. 2022, 414, 1963–1972. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).