Abstract
Feedforward loops (FFLs) are relatively simple network motifs, made of three interacting genes, that have been found in a large number in E. coli and S. cerevisiae. More recently, they have also been discovered in multicellular eukaryotes. FFLs are evolutionary favored motifs because they enable cells to survive critical environmental conditions. Among the eight types of possible FFLs, the so-called coherent 1 and incoherent 1 FFL are the most abundant. The former carries out a sign-sensitive delay in gene expression; the latter is a pulse generator and a response time accelerator. So far, only few synthetic FFLs have been engineered, either in cell-free systems or in vivo. In this work, we review the main experimental works published on FFLs, with particular focus on novel designs for synthetic FFLs. They are, indeed, quite different from the natural ones that arose during the course of evolution.
1. Introduction
Synthetic biology aims at engineering programmable, higher-order, functional entities by designing, assembling, and characterizing biological parts, devices, and circuits into living cells [1]. Pre-existing organisms are re-engineered into new, purpose-driven ones that present a tremendous potential to address a number of demands ranging from environmental care to personalized medicine. Successful engineering of a new organism containing a predefined function depends on the incorporation of orthogonal synthetic genetic circuits [2]. They are organized into transcription units (TUs) that are usually placed onto a variable number of plasmids and encode for proteins or RNA sequences.
Proteins are essential subunits for the survival of an individual cell. Their expression is determined by and performed through coordinated molecular interactions. Furthermore, proteins enable cells to cope with internal and external stimuli. For instance, a complex gene regulation occurs in Escherichia coli when the growth medium changes. If the glucose level becomes low, E. coli switches to lactose as an alternative source of energy via the inducible lac operon [3]. This shift in the carbon source prevents any interruptions in protein synthesis and allows the cells to save energy [4]. Thus, transcription networks operate as a global internal processor that decides the nature and the rate at which proteins (and other molecules) shall be produced [5].
Most biological networks are sparse networks, i.e., their interaction density (or connectivity) is low. This could be due to evolutionary selection such that only useful interactions are maintained [6]. Sparseness in networks was first introduced by Paul Erdos and Alfred Renyi [7] in a systematic study of random graphs [8]. Cellular information-processing interactions are complex and form multilayered connections that influence each other. Among multilayered networks of interactions, are transcriptional regulatory networks (TRNs) that play a key role in cellular signaling, gene expression, metabolism, and protein modifications. Ultimately, TRNs dictate what proteins (or other molecules) shall be expressed in the cells per unit of time [9]. Adler and Alon [10] proposed an approach to dissect complex transcriptional networks by analyzing the basic functional patterns they are made of. They discovered that some architectures, referred to as network motifs, appear more frequently than predicted if the edges (i.e., the connections between two nodes) were randomly distributed. This result was confirmed by studies on neuronal networks [11].
Network motifs were first identified in E. coli and later searched for in other organisms, both prokaryotic and eukaryotic [12]. However, studies in high eukaryotes are still in their infancy. Network motifs broadly cluster into two subset of TRNs: sensory and developmental networks [12]. Sensory networks are responsible for reversible decisions in case of (harsh) environmental stimuli such as stress and nutrient insufficiency. In contrast, developmental networks guide differentiation and cell fate events that usually take place over several cell generations. Common motifs in sensory networks are simple regulation [13], feedback loops [14], single-input modules (SIM) [15], and dense overlapping regulons (DOR) [16]. Feedforward loops (FFLs) [17], transcriptional cascade [18], and interlocked FFLs [19] are predominant motifs in developmental networks. They help the cells make the right decisions under precise conditions.
In the following, we will focus our discussion on FFLs since they are the most abundant motif in natural TRNs. Moreover, canonical FFLs and variants of FFLs have been designed and implemented in vitro and in vivo either independently or as components of larger synthetic gene circuits.
2. FFLs: A General Description
2.1. Structure and Working of FFLs
The FFL is one of the most important genetic network motifs in E. coli and Saccharomyces cerevisiae. In its canonical configuration, an FFL consists of three nodes (i.e., genes, referred to as X, Y, and Z) and three edges, each one representing either activation or repression of transcription (see Figure 1). X and Y are regulatory genes encoding for transcription factors, whereas Z is the target gene, often associated with a reporter protein. X and Y can either activate or repress the transcription of Z.
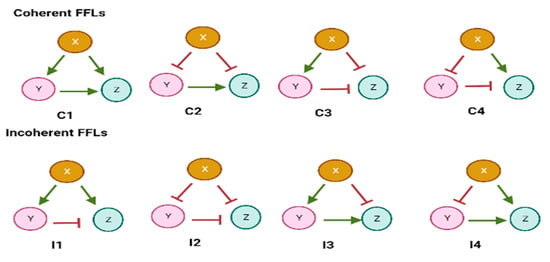
Figure 1.
Coherent and incoherent FFL types. In coherent FFLs, the direct and indirect regulation paths have the same sign. In contrast, the two signs are different in incoherent FFLs.
X acts on Z along two paths: a direct one () and an indirect one along which X regulates Y and Y acts on Z (). Here, the symbol “” stands for either activation or repression of transcription. Thus, there are eight possible different FFLs. They are categorized into two groups: coherent and incoherent FFLs. When the direct and the indirect regulation path have the same sign, the FFL is called coherent (C-FFLs), otherwise it is referred to as incoherent (I-FFLs) [6]. This network motif is the most abundant in E. coli [16], yeast [20], and other organisms including humans [18,21,22,23,24,25]. Interestingly, nearly 40% of E. coli operons are involved in FFLs [16]. Moreover, in the yeast genome [20], 39 transcription factors are involved in 49 FFLs and control over two hundred genes.
Depending on its structure (and possible inputs), an FFL can carry out different tasks including (1) a switch-like device that responds only to a persistent input [16]; (2) temporal controller on gene expression depending on the threshold level of the master (X) and the secondary (Y) regulator [26]; (3) multistep ultra-sensitivity [27] (when minute changes in the level of the master regulator determine an output amplification); (4) noise-filtering [28]; (5) fold-change detection [10]; (6) adaptation [29]; (7) pulse generation [30]; (8) response-time acceleration [31]; and (9) controlling the growth cost of virulence factor [32].
2.2. FFL Is Evolutionary Conserved
Dekel et al. [33] examined the selection of the FFLs over simple regulatory circuits by using a cost–benefit analysis. The selection was calculated by using a dynamic distribution of input signals. An FFL was preferentially chosen where the distribution of signals was adequately broad and characterized by both long and short pulses. Furthermore, according to an evolutionary approach, the dynamic properties of the FFL enable the cells to withstand environmental stress. Hence, FFL functionality in a gene network is crucial to the cell’s survival. As a consequence, the FFL architecture was significantly favored by the evolution [34,35].
2.3. FFL Abundance
Among the eight FFL configurations, some occur more often the others. In both E. coli and Saccharomyces cerevisiae, C1 type is more common than the other three kinds of coherent FFLs. Likewise, I1-FFL occur with the highest frequency among the four incoherent types. The other six types are, therefore, referred to as rare (see Table 1). However, it should be noted that, in S. cerevisiae, C2 and I2 are the next most predominant among the rare FFLs.

Table 1.
The abundance in E. coli and S. cerevisiae of the six rare FFLs according to the previous study [17].
The number of repressor and activator edges was thought to determine the abundance of each FFL. However, this hypothesis turned out to be wrong. In both E. coli and S. cerevisiae, transcription activation is more common than repression. In particular, in E. coli, transcription activation occurs approximately twice more often (66%) than repression (34%). This could give rise to a total of 18 C2 and C4 FFLs, whereas only three have been found (see Table 1). Moreover, type 1, 3 and 4 incoherent FFLs share the same number (2) of activation edges. Nevertheless, they occur in different frequencies in the E. coli genome [16]. Murugan et al. [36] suggested that the robustness against parameter changes (i.e., promoter temporal coupling, perturbation, dissociation constants, mRNA/protein synthesis and degradation rate), could be one of the reasons why type 1 FFLs occur more often than the others. Comparatively, incoherent FFLs are more prevalent in yeast than E. coli.
2.4. Logic Behavior
X and Y transcription factors bind and regulate the promoter upstream of the target gene Z by mimicking either an AND or an OR logic function [31]. In the AND gate configuration, both X and Y proteins should be present to turn on Z, whereas the OR gate requires the action of either transcription factor to return an output. Thus, each type of FFLs can be associated with these two logic functions that imply a distinct behavior.
The scheme of an FFL can be extended by considering external (input) signals that, by interacting with X and Y, determine the expression of the output Z. Represented with the symbols Sx and Sy, input signals are small molecules, proteins or any substance that triggers or shuts down the transcriptional function of X and Y. Sx and Sy are important components of an FFL since they ultimately determine both the dynamics and the steady state of this network motif.
3. Coherent FFLs
3.1. C1-FFL: A Sign-Sensitive Delay Device
3.1.1. AND Logic C1-FFL
In its canonical representation, a C1-FFL is made of three activation edges. Therefore, X activates Z expression both directly and indirectly through Y. Let us suppose that X encodes for an activator whose wild type is inactive (Xi), i.e., unable to bind the DNA. Upon binding the inducer Sx, Xi is activated (Xa) and is thus able to bind the promoter upstream of both Y (Py) and Z (Pz) (see Figure 2). If Xi is constitutively expressed, we can let the system reach a steady state, characterized by a high concentration of Xi, and then add Sx, to the C1-FFL in a sufficiently high amount. By assuming a high affinity between Sx and Xi, Xi switches rapidly to Xa (step-like activation). Xa, then, binds simultaneously Py and Pz. Z expression varies depending on the logic function implemented by Pz. If Pz mimics an AND gate, after the step-like activation, there will be a delay in the synthesis of Z due to the time Y takes to reach the concentration threshold to activate, together with Xa, the transcription of Z. A delay is present only upon induction with Sx (ON step) but not after Sx removal (OFF step). By washing away the inducer Sx, Xa switches rapidly to Xi, which prevents any further production of Z. This behavior is known as sign-sensitive delay since it takes place only at the ON sign of the steps in Sx. The extent of delay depends on the kinetic parameters that regulate the interactions involving Xa, Y, and the DNA. This delay is functional for filtering out fluctuations and spurious pulses, such that only persistent signals lead to the expression of Z [9]. Thus, the C1-FFL is said to work as a persistence detector [6]. In E. coli, the L-arabinose utilization system, which permit the bacterial cells to grow in the absence of glucose, has the structure of a C1-FFL (see Figure 3A). Upon glucose starvation, cAMP (Sx) is produced by the cell and activates CRP. CRP, then, initiates the synthesis of AraC (which, in its turn, is activated by L-arabinose) and binds at the araBAD and araFGH operons where transcription starts upon arrival of AraC, i.e., after 13 and 20 min, respectively. At this point, the consumption of L-arabinose can start [37].
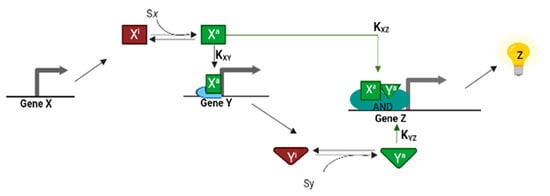
Figure 2.
The dynamics of the coherent type I-FFL with AND logic gate. Sx and Sy activates Xi and Yi, respectively. Hence, both signals are required to turn on Pz that leads the expression of gene Z [6]. Kij represents the affinity between i activator and j promoter.
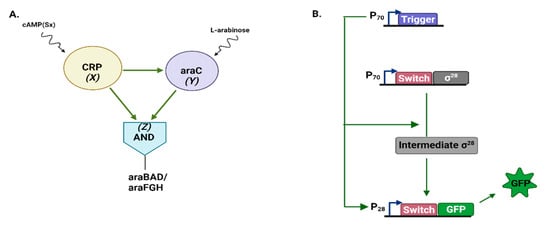
Figure 3.
Natural and synthetic AND logic C1-FFL in E. coli. (A) The bacterial arabinose utilization system requires a second inducer, Sy (L-arabinose), beside the main one, Sx (cAMP). Moreover, Z consists of two targets—the two operons. (B) The synthetic C1-FFL engineered by Pieters et al. [38]. X (the RNA trigger) removes, first, the toehold switch from Y mRNA such that σ28 is produced. Then, X also removes the toehold switch from the green fluorescent protein (GFP) mRNA, which has been transcribed with the help of the σ28 factor. In this way, GFP can be finally synthesized.
In a recent work, Pieters et al. [38] implemented, in an E. coli cell-free transcription-translation (TXTL) system, an AND logic C1-FFL that made use of two RNA molecules (named trigger and toehold switch) and the σ28 factor as regulatory elements (see Figure 3B). Artificial noise signals, generated via microfluidic flow reactors, were used to mimic disturbances on the input that can be met in vivo. This C1-FFL variant decreased, on one hand, the background activity—in comparison to a reference circuit—thus enhancing the fold-change detection [29] but, on the other hand, showed some limitations as a temporal filter.
3.1.2. OR Logic C1-FFL
When Pz implements an OR gate, the C1-FFL shows a delay only when the Sx induction ends (OFF step). An OR gate demands that only one input is equal to 1 to return an output (see Figure 4). Hence, in the presence of Sx, Xa binds Py and Pz and both Y and Z are produced. If Sx is suddenly removed, the production of Z continues until Y is fully degraded. Hence, the OR logic C1-FFL enables sustained expression amid of a temporary loss of the input signal [9]. In E. coli, this kind of C1-FFL controls the self-assembly of the proteins of the flagella motor. The delay that occurs after the loss of Sx spans a time interval of approximately one cell generation. The OR logic C1-FFL guarantees continuous protein expression and protect the system from the abrupt loss of the main regulatory input signal [39].
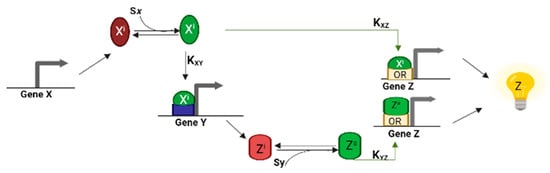
Figure 4.
The dynamics of the coherent type I FFL with OR logic. Gene Z is expressed once a single transcription factor is activated by the corresponding inducer [6]. Kij represents the affinity between i activator and j promoter.
3.1.3. Filtering Out Spurious Signals
C1-FFLs—and, potentially, all types of coherent FFLs—act as sign-sensitive delay circuits. Delays are important because they permit to select the essential signals (filtering) to the FFL working. Signal filtering is asymmetric since it depends on the logic of the FFL and the sign of the inputs. Only persistent (ON sign) stimuli (i.e., longer that TON—the initial time needed by protein Y to build up and activate gene Z) can produce effects on an AND logic gate. In contrast, a delay in the input OFF step permits the OR-gate C1-FFL to maintain Z expression even in front of a sudden loss of one input (as observed in the E. coli flagella system) [6]. The FFL dynamics of protein expression in the presence or absence of the inducers (Sx and Sy) explains the mechanism of filtering out short and redundant noises (see Figure 2 and Figure 4). Xiong et al. [40] confirmed that the AND logic C1-FFL regulation emerges adaptively by filtering short spurious signals. Therefore, the configuration of three node genes with three edges and a logic gates gave the FFLs the capability to “learn” that short, fluctuating input signals shall be cleared out to keep a proper working [6].
3.2. Coherent FFLs in Eukaryotic Cells
It is worth mentioning at the end of this section that coherent FFLs have been discovered in eukaryotic genomes too. A remarkable, well-studied example is given by the C1-FFL that regulates the skeletal muscle differentiation in mouse cells [41,42]. Moreover, as mentioned in Section 2.3, type II coherent FFLs are relatively common in the yeast S. cerevisiae. In particular, a C2-FFL was shown to control the transition of yeast cell morphology from the usual, spherical, egg-shaped one to the filamentous form in case of a shortage of nitrogen supply [43,44].
4. Incoherent FFLs
4.1. I1-FFL as a Pulse Generator and a Response-Time Accelerator
The behavior of genetic circuits and their response to external perturbations depend highly on their connectivity. The dynamics of a circuit consists of a transient phase, during which the species concentrations change at various rates, and one (or more) steady states, the equilibria, at which concentrations are constant since production and degradation cancel out for each species. Often, circuit analysis focuses on steady states such that the experiments neglect the transient phase. In this way, however, an output signal limited in time, such as a pulse, is missed. Moreover, the steady-state analysis does not permit to estimate the circuit response time, i.e., the time a circuit takes to reach one half of the output level at the equilibrium.
Incoherent FFLs—especially type I1—are both pulse generators and response-time accelerators [9]. Hence, these network motifs cannot be understood completely via the sole steady-state analysis.
The height and the amplitude of the peak, as well as the duration of the response time, depend on several FFL kinetic parameters (e.g., the strength of the three promoters and the decay rate of the three proteins) [45]. Since the cells in a population are not synchronized, the peak detection and the response time estimation can be difficult tasks and, in general, require single-cell analysis. This can be carried out via flow cytometry (FACS—fluorescence-activated cell sorting), fluorescent microscopy, or by recording live movies of the synthetic cells [46,47].
The galactose system in E. coli contains an AND logic I1-FFL [31] that has permitted to verify a further feature of this network motif, i.e., the non-monotonic dependence on the input signal [48]. As shown in the simplified scheme in Figure 5, X is the CRP activator that responds to cAMP (Sx), Y corresponds to the repressor galS that senses D-galactose (Sy), Z is the galETK operon that presides over galactose consumption. As we have seen in Section 3.1.1, in the absence of glucose cAMP accumulates in E. coli cells and binds CRP. As a consequence, CRP assumes its active configuration and stimulates the expression of galS and the genes in the galETK operon that make use of galactose. Upon reaching a sufficiently high amount, galS (which is activated by D-galactose) binds the galE promoter and contrasts the expression of the galETK operon leading to a pulse in the concentration of the galETK genes followed by the steady state. Both galE and galP promoter (galP, which is connected to the I1-FFL, acts as a pump to transport galactose into the cell) show the highest activity (a peak) at intermediate concentrations of cAMP, i.e., a non-monotonic response to the input. The I1-FFL confers a rapid response time (one-third of the cell generation time [31]) to the galactose system such that the cells switch quickly to galactose in the absence of glucose.
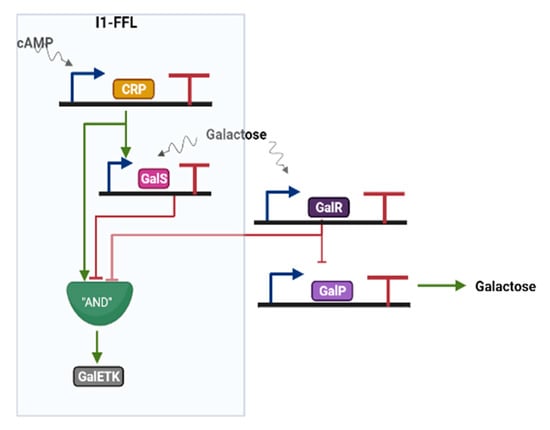
Figure 5.
The I1-FFL in the E. coli galactose system. GalR is a repressor protein that establish the connection between the FFL and GalP. Moreover, GalR is able to bind and downregulate the galE promoter (upstream of GalP).
4.2. Synthetic I1-FFLs in E. coli
In a pioneering work back in 2004, Basu et al. [49] designed an I1-FFL in order to engineer cell–cell communication between the two subpopulations (sender and receiver cells) of an E. coli consortium (see Figure 6A). The I1-FFL was fully contained into the receiver cells and needed a signal from the sender cells to generate a pulse in green fluorescence. In the presence of tetracycline, the sender cells could synthesize LuxI synthase that catalyzed the production of AHL (acyl-homoserine lactone). AHL diffused through the cell membranes and reached the spatially nearby receiver cells. Here, AHL worked as the Sx input since it bound and activated LuxR (X), an activator protein that was constitutively expressed. The activated LuxR triggered the expression of the cI repressor (Y), and GFP (Z), with the consequent generation of a pulse in fluorescence. The amplitude and the time-location of the peak depended on both AHL concentration and increasing rate. Moreover, no communication was possible between too distant (4.5 mm) sender and receiver cells on a solid medium.
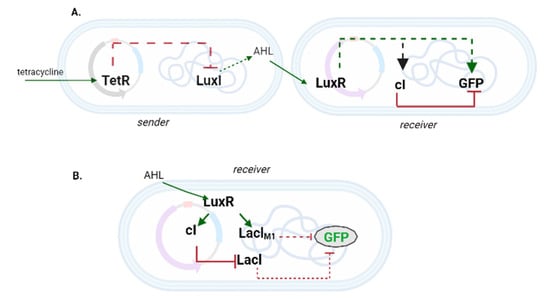
Figure 6.
Incoherent FFLs used in E. coli consortia. (A) A canonical I1-FFL gives a pulse in fluorescence in the presence of its input, AHL. (B) Non-canonical incoherent FFL adopted to achieve pattern formation on solid media.
One year later, Basu et al. [50] realized pattern formation in a similar bacterial consortium by placing the receiver cells at different distances from the sender ones. The receiver cells contained a non-canonical incoherent FFL made of five nodes (see Figure 6B). AHL, produced by the sender cells, activated LuxR in a concentration-dependent fashion. In its turn, the activated LuxR triggered the synthesis of two repressor proteins: cI, which downregulated strongly the expression of LacI, and LacIM1—a variant of LacI—that exerted a mild repression on GFP production. Finally, LacI was able to switch OFF fluorescence expression as well. Receivers close to the senders obtain a high quantity of AHL such that both LacIM1 and cI were highly expressed. The former protein was the responsible for the absence of a fluorescent signal. At lower concentrations of AHL, LacIM1 and cI were rather poorly expressed. However, while LacIM1 failed to lower GFP synthesis, cI completely shut down the production of LacI, which could no longer inhibit GFP expression. Finally, at very low concentration of AHL LuxR was not activated. This resulted in a high amount of LacI in the receivers, which implied absence of fluorescence again. This spatial arrangement of sender and receiver cells led to a so-called bullseye pattern on plate.
In a more recent work, Barone et al. [51] employed the same I1-FFL as in [49] in order to construct an arsenic biosensor. Here, LuxI synthesis was prevented by the action of the arsenic repressor, ArsR, that was constitutively expressed. Molecules of arsenic, thus, triggered AHL production that led to a short-delayed red fluorescence pulse that resented of the arsenic concentration.
Three I1-FFLs, regulated by IPTG and using the T7-RNAP as X node, were presented by Entus et al. [52]. Even though each of them was made of three TUs, their designs were different. The first I1-FFL showed the usual scheme with a competition, at Pz, between T7-RNAP (which worked as an activator) and the MetJ (Y) repressor. Mutations in the met operator allowed to move the fluorescent peak towards lower concentration of IPTG and achieve full output repression around 100 mM IPTG. The second I1-FFL variant (termed gfp:anti-gfp) mixed transcriptional activation and translational repression of GFP. The Y gene corresponded to a fragment of the reverse GFP sequence that, upon transcription, sequestered the mRNA of GFP (Z) and lowered its translation. Stronger fluorescent repression was achieved by transcribing the full GPF reverse sequence. Finally, in the last design, Y encoded for the T7 lysozyme that bound and inhibited T7-RNAP. An increase in the amount of the T7 lysozyme caused a shift of the peak towards lower IPTG concentrations. It should be noted that the fluorescence peak of each I1-FFL was not traced as a function of time but as a function of the IPTG concentrations, i.e., each fluorescence level was the steady-state fluorescence at a different amount of IPTG.
4.3. Incoherent FFLs in Eukaryotes
Biochemical interactions that determine gene expression and lead to the formation of network motifs are well-known in unicellular organisms, whereas there is more uncertainty about higher eukaryotes [53,54]. Nevertheless, some studies have pointed out how incoherent FFLs are present, among multicellular organisms, in gene networks that are involved in complex tasks such as neuron signal regulation and adaptation.
Mammalian skin is known to be innervated with C fibers that transmit itch, pain, heat, and other noxious stimuli. Sensory neurons, which are associated with C-fibers, include VGLUT3+ low-threshold c-mechanoreceptors (CLTMs), MrgprD+ polymodal nociceptors, MrgprA3+ pruriceptors, and MrgprB4+ c-mechanoreceptors. Incoherent FFLs have been reported to control both the segregation and the genesis of mammalian sensory neuron sub-types [55,56]. These results are based on the phenotypic changes observed in knock-out mice. Runx1 (X, activator) and Zfp521 (Y, repressor) form an I1-FFL that controls the molecular identities that belong to the MrgprD+ polymodal nociceptors (Z). Moreover, Runx1 forms (with an unknow factor) an I3-FFLs to regulate the expression of MrgprA3 and MrgpB4. The occurrence of several types of incoherent helps understand how sensory neuron subtypes are formed. Furthermore, it points out that incoherent FFLs have a critical impact in sensory neuron evolution and sensory decoding.
In a different study concerning neuron expression, Abdusselamoglu et al. [57] suggested that intermediate neural progenitors (INPs), in the brain of Drosophila melanogaster, make use of an I1-FFL to express different neurons in a precise chronological order (Dichaete, Grainyhead, Eyeless). The genes Osa, odd-paired (Opa), and Dichaete (D) should be linked via an I1-FFLs where Osa activates directly both D and its repressor Opa. However, the slower activation kinetics of Opa permits the accumulation of D that turn on the downstream cascade prior to its repression by Opa. Thus, the balanced expression of Opa and D seems to regulate the transition between successive temporal identities, though this network topology is not fully understood yet (see Figure 7). Any inconsistencies with this scheme, e.g., the overexpression of Osa, would result in incorrect temporal patterning and immature differentiation.
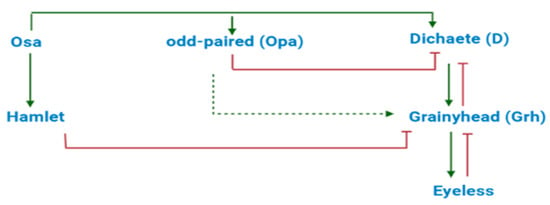
Figure 7.
Drosophila neural stem cell neurogenesis lies, potentially, on an I1-FFLs. The overall network, however, also contains two switches (D/Grh and Grh/Eyeless) and two regulations of Grh: an activation by Opa and a repression by a further gene, Hamlet.
Adaptation in eukaryotic chemotaxis (i.e., the movement along a gradient of a certain substance) has been studied in the social amoeba Dictyostelium discoideum [58]. Adaptation occurs when the output of a system returns to a fixed baseline after a change in the input signal. In the case of amoeba chemotaxis, the concentration of a chemo-attractant (the input) underwent a uniform change that triggered [30] a response from the chemotaxis pathway downstream of a G protein-coupled receptor (GPCR) [59]. In particular, Ras-GTP, i.e., the activated Ras—the protein that regulates signal transduction—showed almost perfect adaptation. This is achieved via a non-canonical incoherent feedforward loop, where the chemoattractant binds GPCR that leads to the activation of both RasGEF and RasGAP. The former activates Ras, the latter represses it. Thus, the simultaneous activation of a Ras activator and a Ras inhibitor is the key for adaptation. Coherent feedforward loops, in contrast, do not appear to be suitable for adaptation, as reported in Ma et al. [60].
Previously, we have seen how an I1-FFL is adopted in E. coli to direct the usage of arabinose or galactose under glucose starvation. Kuttykrishnan et al. [30], in contrast, presented a quantitative model of the glucose signaling pathways in S. cerevisiae. This study revealed how an I2-FFL was capable of generating a transient pulse in the transcription of the HXT2–4 genes, which encode for glucose transporters, in response to a minor increase in the glucose concentration. The repressor Rgt1 (X) downregulates HXT2–4 (Z) on the direct path and upregulates the same target genes by inhibiting the expression of the Mig2 (Y) repressor. It should be noted that this I2-FFL is part of a bigger circuitry called SRM (Snf/Rgt/Mig) network that preside over the expression of the glucose transporters.
4.4. Incoherent FFLs in Cell-Free System
Cell-free transcription–translation system (TXTL) is an emerging technology used to express mRNA and proteins outside of a living organism [61]. In principle, it can be exploited to prototype, tune, and optimize biological circuits, in a relatively short time, before incorporating them into cells [62]. By taking advantage of the simplicity and efficiency of this technique, Guo and Murray [63] designed two novel I1-FFLs that could be adopted as functional modules in complex transcription regulatory networks. In the first circuit, AraC (X), activates the synthesis of TetR (Y) and Z (deGFP, i.e., a GFP variant with a shorter half-life) only in the presence of its inducer, arabinose (Sx). In the second I1-FFL, AraC was replaced by a different activator, LasR, whose inducer is AHL, whereas Y and Z were, again, TetR and deGFP. The size and behavior of each pulse were positively correlated with the amount of the inducer. Both I1-FFLs were built in vivo as well. Theoretical predictions (i.e., in silico simulations) on the dynamics of the two pulse generators matched the experimental results. Moreover, the circuits’ behavior in vivo was essentially the same as in the TXTL system. Therefore, the cell-free system was confirmed to be an effective tool to study and optimize small genetic circuits before their in vivo implementation. However, it is still necessary to understand the limits of this approach, i.e., if it is efficient also when dealing with complex networks containing a larger number of components, connections, and interactions that might be not orthogonal to the chassis chosen to host the circuit.
4.5. Alternative Designs for Incoherent FFLs
All the incoherent FFLs we have described so far are small transcriptional networks. However, different components from transcription factors (namely, RNA molecules) have been used successfully in the C1-FFL implemented in [38]. Short antisense RNA sequences are translational regulators that offer potential advantages with respect to proteins. For instance, they aid RNA conformational arrangements [64]; their structure is easily determined [65]; and they are compatible with the metabolic activity of the host cell [66], which allows fast signal transduction due their short half-life [67]. Furthermore, RNA molecules can also be employed as transcriptional regulators [68]. Westbrook et al. [69] engineered, in a cell-free system, a pulse generator—resembling the I1-FFL—via two modes of transcription regulation based on RNAs: STAR (small transcription activating RNA) and CRISPRi (clustered regularly interspaced short palindromic repeats interference—see Figure 8A). STAR (X) activates GFP (Z) expression by binding a target RNA in the 5′ UTR (untranslated region) of Z and preventing it from folding into a terminator that would force RNA polymerase to fall off the DNA. Besides, CRISPRi (a parallel X) represses GFP synthesis via a synthetic transcription factor made of a nuclease-deficient Cas9 (dCas9) in complex with two RNA sequences: the crRNA (CRISPR RNA) and the tracrRNA (transactivating RNA). It should be noted that this downregulation could have been simplified by means of a single guide RNA (sgRNA [70]) that encompasses the necessary fragments of crRNA and tracrRNA to recognize and bind both dCas9 and the target sequence on the DNA. A pulse in fluorescence arises from the different kinetics of these two regulation systems. STAR demands an RNA-RNA interaction that leads to a quick activation of fluorescence expression (a matter of few minutes). CRISPRi, in contrast, requires the slow coupling of dCas9:tracrRNA with crRNA that can take up to approximately 60 min [71]. In addition, the pulse obtained in this work was in the GFP production rate rather than in the fluorescent signal itself since there is no protein degradation in the TXTL system.
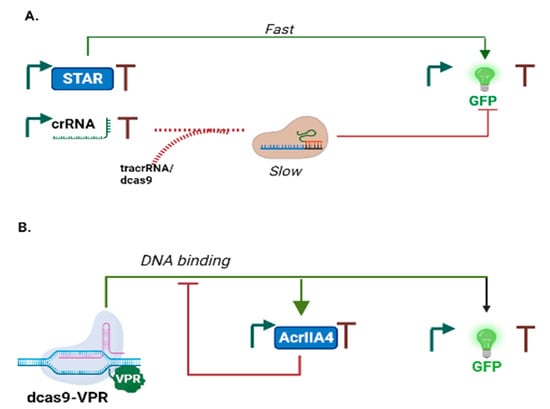
Figure 8.
Alternative designs for a pulse generator. (A) The circuit in [69] is inspired by the I1-FFL architecture. However, GFP activation and repression paths are not connected to each other. (B) Nakamura et al. [72] employed the anti-CRISPR protein AcrIIA4 to prevent the binding of dCas9-VPR to Pz and consequently stop fluorescence expression.
A novel design for a canonical I1-FFL exploited the inhibitory action of a type II anti-CRISPR protein (AcrIIA4) on dCas9 fused to the strong activation domain VPR (dCas9-VPR) [72]. Upon binding a properly designed sgRNA, dCas9-VPR (X) activated both the expression of AcrIIA4 (Y) and GFP (Z—see Figure 8B). The fluorescence signal increased until the amount of AcrIIA4 was high enough to prevent dCas9-VPR:sgRNA from further binding Pz, i.e., the promoter upstream of the GFP gene. This I1-FFL was realized in eukaryotic cells and produced a clear pulse in fluorescence expression.
5. Interlocked FFLs
Interlocked FFLs consist of a variable number of (different type) FFLs that are interconnected within a complex transcriptional network. A well-studied example of interlocked FFLs is the differentiation circuit in the bacterium Bacillus subtilis [19]. In case of starvation or growth difficulty, B. subtilis undergoes sporulation. This process—which involves over three hundred genes—is regulated by a hierarchical cascade of multiple transcription factors named σE, GerE, SpoIIID, σk, and GerR. σE alone acts on over two hundred genes, including GerR and SpoIIID. These two DNA-binding proteins shut down almost half of the genes present in the same σE regulon. The five transcription factors are enclosed in two C1-FFLs interlocked with three I1FFLs (see Figure 9).
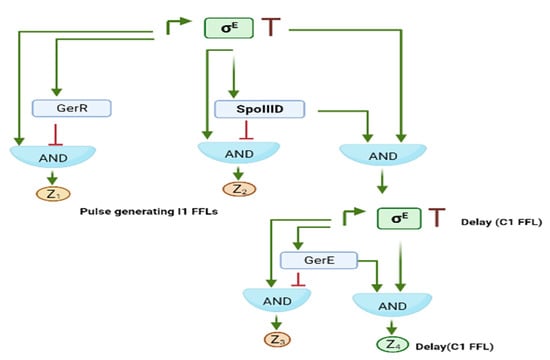
Figure 9.
The sporulation process in B. subtilis is regulated by five interlocked FFLs: two C1-FFLs (responsible for delays in Z3 and Z4 expression) and three I1-FFLs, which generate pulses in Z1, Z2 and σk synthesis.
6. Conclusions
Network motifs analysis is a relatively recent development in the field of Systems Biology. Since most network motifs are associated with precise functions, they have been re-engineered both in cell-free system and in vivo, in the attempt to better understand and optimize their working. Feedforward loops appeared particularly abundant in E. coli and S. cerevisiae because of the role they play in cell survival. Upon detection of particular signals, FFLs regulate the expression of multiple genes by means of transcriptional bursts (pulse generation) or delays. Moreover, the incoherent FFLs accelerate the response time of a genetic network (see Table 2 for an overview of the FFL functionalities). In Synthetic Biology, the implementation of FFLs has often been accompanied by mathematical models, which have permitted to identify new features and delineate design criteria. In particular, retroactivity was shown to have ambivalent effects on FFLs. In general, retroactivity quantifies the changes in the performance of an upstream system when its output establishes a connection with a downstream system [73]. In a TU inside a gene circuit, retroactivity is large when the operators along the promoter have high affinity towards their corresponding transcription factors. An increase in the retroactivity on an incoherent FFL does not always have negative effects on the response time and the pulse amplitude determined by the FFL itself. Thus, increasing retroactivity is a possible strategy to improve the working of an I-FFL [74].

Table 2.
Functions carried out by the eight possible FFLs.
Synthetic FFLs have been mainly engineered for proof of concepts rather than for practical applications. Moreover, they appear as separate devices instead of being integrated into complex networks. This lack of new applications concerns all network motifs, not only the FFLs. In the end, network motifs have been designed and selected by evolution, whereas synthetic gene circuits try to mimic human artifacts such as electronic devices.
At present, synthetic gene circuit schemes shall minimize the number of basic components in order to increase the probability of success in their in vivo implementation. This strategy is antithetical to the way evolution works since it favors redundant solutions. For this reason, natural gene networks have a high fault tolerance that, in contrast, is basically missing in synthetic constructs (biological and not).
Some network motifs, among which are the FFLs, have the capability to make synthetic gene circuits more robust to different kinds of disturbances. Therefore, they could be adopted just to turn a circuit scheme into a more “natural” gene network. This might represent the meeting point between human- and evolution-based design, which could lead to a substantial improvement in the working of synthetic biological networks.
Author Contributions
Conceptualization, M.A.M. and T.W.; writing—original draft preparation, T.W.; writing—review and editing, M.D.A. and M.A.M.; visualization, T.W.; supervision, M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All figures were created at biorender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Endy, D. Foundations for engineering biology. Nature 2005, 438, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.; Badran, A.H. Synthetic Biological Circuits within an Orthogonal Central Dogma. Trends Biotechnol. 2021, 39, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Müller-Hill, B. The Lac Operon: A Short History of a Genetic Paradigm; De Gruyter: Berlin, Germany, 1996. [Google Scholar]
- Balleza, E.; López-Bojorquez, L.N.; Martínez-Antonio, A.; Resendis-Antonio, O.; Lozada-Chávez, I.; Balderas-Martínez, Y.I.; Encarnación, S.; Collado-Vides, J. Regulation by transcription factors in bacteria: Beyond description. FEMS Microbiol. Rev. 2008, 33, 133–151. [Google Scholar] [CrossRef]
- Okano, H.; Hermsen, R.; Kochanowski, K.; Hwa, T. Regulation underlying hierarchical and simultaneous utilization of carbon substrates by flux sensors in Escherichia coli. Nat. Microbiol. 2020, 5, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Alon, U. An Introduction to Systems Biology: Design Principles of Biological Circuits, 2nd ed.; CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Erdős, P.; Rényi, A. On the evolution of random graphs. Publ. Math. Inst. Hung. Acad. Sci. 1960, 5, 17–61. [Google Scholar]
- Marchisio, M.A. Polynomial Observables in the Graph Partitioning Problem. Int. J. Mod. Phys. C 2001, 12, 13–18. [Google Scholar] [CrossRef]
- Alon, U. Network motifs: Theory and experimental approaches. Nat. Rev. Genet. 2007, 8, 450–461. [Google Scholar] [CrossRef]
- Adler, M.; Alon, U. Fold-change detection in biological systems. Curr. Opin. Syst. Biol. 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Song, S.; Sjöström, P.J.; Reigl, M.P.; Nelson, S.M.; Chklovskii, D.B.S. Highly Nonrandom Features of Synaptic Connectivity in Local Cortical Circuits. PLoS Biol. 2005, 3, e68. [Google Scholar] [CrossRef]
- Levine, M.; Davidson, E.H. Gene Regulatory Networks for Development. Proc. Natl. Acad. Sci. USA 2005, 102, 4936–4942. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Nourani, M. Statecharts for Gene Network Modeling. PLoS ONE 2010, 5, e9376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maeda, Y.T.; Sano, M. Regulatory Dynamics of Synthetic Gene Networks with Positive Feedback. J. Mol. Biol. 2006, 359, 1107–1124. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Itzkovitz, S.R.; Kashtan, N.S.; Levitt, R.N.; Alon, U.R. Response to Comment on “Network Motifs: Simple Building Blocks of Complex Networks” and “Superfamilies of Evolved and Designed Networks”. Science 2004, 305, 1107. [Google Scholar] [CrossRef]
- Shen-Orr, S.S.; Milo, R.; Mangan, S.R.; Alon, U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 2002, 31, 64–68. [Google Scholar] [CrossRef]
- Mangan, S.; Alon, U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA 2003, 100, 11980–11985. [Google Scholar] [CrossRef]
- Milo, R.; Itzkovitz, S.; Kashtan, N.; Levitt, R.; Shen-Orr, S.; Ayzenshtat, I.; Sheffer, M.; Alon, U. Superfamilies of Evolved and Designed Networks. Science 2004, 303, 1538–1542. [Google Scholar] [CrossRef]
- Eichenberger, P.; Fujita, M.; Jensen, S.T.; Conlon, E.M.; Rudner, D.Z.; Wang, S.T.; Ferguson, C.; Haga, K.; Sato, T.; Liu, J.S.; et al. The Program of Gene Transcription for a Single Differentiating Cell Type during Sporulation in Bacillus subtilis. PLOS Biol. 2004, 2, e328. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.I.; Rinaldi, N.J.; Robert, F.; Odom, D.T.; Bar-Joseph, Z.; Gerber, G.K.; Hannett, N.M.; Harbison, C.T.; Thompson, C.M.; Simon, I.; et al. Transcriptional Regulatory Networks in Saccharomyces cerevisiae. Science 2002, 298, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Odom, D.T.; Zizlsperger, N.; Gordon, D.B.; Bell, G.W.; Rinaldi, N.J.; Murray, H.L.; Volkert, T.L.; Schreiber, J.; Rolfe, P.A.; Gifford, D.K.; et al. Control of Pancreas and Liver Gene Expression by HNF Transcription Factors. Science 2004, 303, 1378–1381. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core Transcriptional Regulatory Circuitry in Human Embryonic Stem Cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef]
- Saddic, L.A.; Huvermann, B.; Bezhani, S.; Su, Y.; Winter, C.M.; Kwon, C.S.; Collum, R.P.; Wagner, D. The leafy target LMI1 is a meristem identity regulator and acts together with LEAFY to regulate expression of cauliflower. Development 2006, 133, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Swiers, G.; Patient, R.; Loose, M. Genetic regulatory networks programming hematopoietic stem cells and erythroid lineage specification. Dev. Biol. 2006, 294, 525–540. [Google Scholar] [CrossRef]
- Iranfar, N.; Fuller, D.; Loomis, W.F. Transcriptional regulation of post-aggregation genes in Dictyostelium by a feed-forward loop involving GBF and LagC. Dev. Biol. 2006, 290, 460–469. [Google Scholar] [CrossRef]
- Reeves, G.; Bandodkar, P.; Al Asafen, H. Spatiotemporal control of gene expression boundaries using a feedforward loop. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Goldbeter, A.; Koshland, D.E. Ultrasensitivity in biochemical systems controlled by covalent modification. Interplay between zero-order and multistep effects. J. Biol. Chem. 1984, 259, 14441–14447. [Google Scholar] [CrossRef]
- Gui, R.; Liu, Q.; Yao, Y.; Deng, H.; Ma, C.; Jia, Y.; Yi, M. Noise Decomposition Principle in a Coherent Feed-Forward Transcriptional Regulatory Loop. Front. Physiol. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Khetarpal, I.; Sen, S.; Murray, R. Synthetic circuit for exact adaptation and fold-change detection. Nucleic Acids Res. 2014, 42, 6078–6089. [Google Scholar] [CrossRef]
- Kuttykrishnan, S.; Sabina, J.; Langton, L.L.; Johnston, M.; Brent, M.R. A quantitative model of glucose signaling in yeast reveals an incoherent feed forward loop leading to a specific, transient pulse of transcription. Proc. Natl. Acad. Sci. USA 2010, 107, 16743–16748. [Google Scholar] [CrossRef]
- Mangan, S.; Itzkovitz, S.; Zaslaver, A.; Alon, U. The Incoherent Feed-forward Loop Accelerates the Response-time of the gal System of Escherichia coli. J. Mol. Biol. 2006, 356, 1073–1081. [Google Scholar] [CrossRef]
- Pérez-Morales, D.; Nava-Galeana, J.; Rosales-Reyes, R.; Teehan, P.; Yakhnin, H.; Melchy-Pérez, E.I.; Rosenstein, Y.; De la Cruz, M.A.; Babitzke, P.; Bustamante, V.H. An incoherent feedforward loop formed by SirA/BarA, HilE and HilD is involved in controlling the growth cost of virulence factor expression by Salmonella Typhimurium. PLOS Pathog. 2021, 17, e1009630. [Google Scholar] [CrossRef]
- Dekel, E.; Mangan, S.; Alon, U. Environmental selection of the feed-forward loop circuit in gene-regulation networks. Phys. Biol. 2005, 2, 81–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lynch, M. Feedforward loop for diversity. Nature 2015, 523, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Agorio, A.; Durand, S.; Fiume, E.; Brousse, C.; Gy, I.; Simon, M.; Anava, S.; Rechavi, O.; Loudet, O.; Camilleri, C.; et al. An Arabidopsis Natural Epiallele Maintained by a Feed-Forward Silencing Loop between Histone and DNA. PLoS Genet. 2017, 13, e1006551. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R. Theory on the Dynamics of Feedforward Loops in the Transcription Factor Networks. PLoS ONE 2012, 7, e41027. [Google Scholar] [CrossRef][Green Version]
- Mangan, S.; Zaslaver, A.; Alon, U. The Coherent Feedforward Loop Serves as a Sign-sensitive Delay Element in Transcription Networks. J. Mol. Biol. 2003, 334, 197–204. [Google Scholar] [CrossRef]
- Pieters, P.A.; Nathalia, B.L.; van der Linden, A.J.; Yin, P.; Kim, J.; Huck, W.T.S.; de Greef, T.F.A. Cell-Free Characterization of Coherent Feed-Forward Loop-Based Synthetic Genetic Circuits. ACS Synth. Biol. 2021, 10, 1406–1416. [Google Scholar] [CrossRef]
- Kalir, S.; Mangan, S.; Alon, U. A coherent feed-forward loop with a SUM input function prolongs flagella expression in Escherichia coli. Mol. Syst. Biol. 2005, 1, 2005.0006. [Google Scholar] [CrossRef]
- Xiong, K.; Lancaster, A.K.; Siegal, M.L.; Masel, J. Feed-forward regulation adaptively evolves via dynamics rather than topology when there is intrinsic noise. Nat. Commun. 2019, 10, 2418. [Google Scholar] [CrossRef]
- Lluís, F.; Perdiguero, E.; Nebreda, A.R.; Muñoz-Cánoves, P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 2006, 16, 36–44. [Google Scholar] [CrossRef]
- Penn, B.H.; Bergstrom, D.A.; Dilworth, F.J.; Bengal, E.; Tapscott, S.J. A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 2004, 18, 2348–2353. [Google Scholar] [CrossRef]
- Gimeno, C.J.; Ljungdahl, P.O.; Styles, C.A.; Fink, G.R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: Regulation by starvation and RAS. Cell 1992, 68, 1077–1090. [Google Scholar] [CrossRef]
- Wolf, J.J.; Dowell, R.D.; Mahony, S.; Rabani, M.; Gifford, D.K.; Fink, G.R. Feed-Forward Regulation of a Cell Fate Determinant by an RNA-Binding Protein Generates Asymmetry in Yeast. Genetics 2010, 185, 513–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levine, J.H.; Lin, Y.; Elowitz, M.B. Functional Roles of Pulsing in Genetic Circuits. Science 2013, 342, 1193–1200. [Google Scholar] [CrossRef]
- Hao, N.; O’Shea, E.K. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 2012, 19, 31–39. [Google Scholar] [CrossRef]
- Yissachar, N.; Fischler, T.S.; Cohen, A.A.; Reich-Zeliger, S.; Russ, D.; Shifrut, E.; Porat, Z.; Friedman, N. Dynamic Response Diversity of NFAT Isoforms in Individual Living Cells. Mol. Cell 2013, 49, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.; Bren, A.; Dekel, E.; Alon, U. The incoherent feed-forward loop can generate non-monotonic input functions for genes. Mol. Syst. Biol. 2008, 4, 203. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Mehreja, R.; Thiberge, S.; Chen, M.-T.; Weiss, R. Spatiotemporal control of gene expression with pulse-generating networks. Proc. Natl. Acad. Sci. USA 2004, 101, 6355–6360. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Gerchman, Y.; Collins, C.; Arnold, F.H.; Weiss, R. A synthetic multicellular system for programmed pattern formation. Nature 2005, 434, 1130–1134. [Google Scholar] [CrossRef]
- Barone, F.; Dorr, F.; E. Marasco, L.; Mildiner, S.; Patop, I.L.; Sosa, S.; Vattino, L.G.; A. Vignale, F.; Altszyler, E.; Basanta, B.; et al. Design and evaluation of an incoherent feed-forward loop for an arsenic biosensor based on standard iGEM parts. Synth. Biol. 2017, 2, ysx006. [Google Scholar] [CrossRef]
- Entus, R.; Aufderheide, B.; Sauro, H.M. Design and implementation of three incoherent feed-forward motif based biological concentration sensors. Syst. Synth. Biol. 2007, 1, 119–128. [Google Scholar] [CrossRef]
- Xiong, H.; Veedu, R.; Diermeier, S. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.T. The engineering principles of combining a transcriptional incoherent feedforward loop with negative feedback. J. Biol. Eng. 2019, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Pan, X.; Huang, T.; Duan, B.; Yang, F.-C.; Yang, J.; Xiong, M.; Liu, Y.; Ma, Q. Incoherent Feed-Forward Regulatory Loops Control Segregation of C-Mechanoreceptors, Nociceptors, and Pruriceptors. J. Neurosci. 2015, 35, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Duan, B.; Vong, L.; Lowell, B.B.; Ma, Q. Runx1 Controls Terminal Morphology and Mechanosensitivity of VGLUT3-expressing C-Mechanoreceptors. J. Neurosci. 2013, 33, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Abdusselamoglu, M.D.; Eroglu, E.; Burkard, T.R.; A. Knoblich, J. The transcription factor odd-paired regulates temporal identity in transit-amplifying neural progenitors via an incoherent feed-forward loop. eLife 2019, 8, e46566. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Shao, D.; Adler, M.; Charest, P.G.; Loomis, W.F.; Levine, H.; Groisman, A.; Rappel, W.-J.; Firtel, R.A. Incoherent Feedforward Control Governs Adaptation of Activated Ras in a Eukaryotic Chemotaxis Pathway. Sci. Signal. 2012, 5, ra2. [Google Scholar] [CrossRef]
- Janetopoulos, C.; Jin, T.; Devreotes, P. Receptor-Mediated Activation of Heterotrimeric G-Proteins in Living Cells. Science 2001, 291, 2408–2411. [Google Scholar] [CrossRef]
- Ma, W.; Trusina, A.; El-Samad, H.; Lim, W.A.; Tang, C. Defining Network Topologies that Can Achieve Biochemical Adaptation. Cell 2009, 138, 760–773. [Google Scholar] [CrossRef]
- Marshall, R.; Noireaux, V. Quantitative modeling of transcription and translation of an all-E. coli cell-free system. Sci. Rep. 2019, 9, 11980. [Google Scholar] [CrossRef]
- Shin, J.; Noireaux, V. An E. coli Cell-Free Expression Toolbox: Application to Synthetic Gene Circuits and Artificial Cells. ACS Synth. Biol. 2012, 1, 29–41. [Google Scholar] [CrossRef]
- Guo, S.; Murray, R.M. Construction of Incoherent Feedforward Loop Circuits in a Cell-Free System and in Cells. ACS Synth. Biol. 2019, 8, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Anderson, J.; Contreras, L.M. Regulatory RNAs: Charming gene management styles for synthetic biology applications. RNA Biol. 2013, 10, 1778–1797. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Oertner, T.G.; Hegemann, P.; Larkum, M.E. Active cortical dendrites modulate perception. Science 2016, 354, 1587–1590. [Google Scholar] [CrossRef]
- Beisel, C.; Storz, G. Base pairing small RNAs and their roles in global regulatory networks. FEMS Microbiol. Rev. 2010, 34, 866–882. [Google Scholar] [CrossRef]
- Chappell, J.; Takahashi, M.; Lucks, J.B. Creating small transcription activating RNAs. Nat. Chem. Biol. 2015, 11, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lucks, J.B.; Qi, L.; Mutalik, V.K.; Wang, D.; Arkin, A.P. Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc. Natl. Acad. Sci. USA 2011, 108, 8617–8622. [Google Scholar] [CrossRef]
- Westbrook, A.; Tang, X.; Marshall, R.; Maxwell, C.S.; Chappell, J.; Agrawal, D.K.; Dunlop, M.J.; Noireaux, V.; Beisel, C.L.; Lucks, J.; et al. Distinct timescales of RNA regulators enable the construction of a genetic pulse generator. Biotechnol. Bioeng. 2019, 116, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Nakamura, M.; Srinivasan, P.; Chavez, M.; Carter, M.; Dominguez, A.A.; La Russa, M.; Lau, M.B.; Abbott, T.R.; Xu, X.; Zhao, D.; et al. Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat. Commun. 2019, 10, 194. [Google Scholar] [CrossRef]
- Del Vecchio, D.; Ninfa, A.J.; Sontag, E.D. Modular cell biology: Retroactivity and insulation. Mol. Syst. Biol. 2008, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Belta, C.; Isaacson, S.A. How Retroactivity Affects the Behavior of Incoherent Feedforward Loops. iScience 2020, 23, 101779. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).