Halophytes as Medicinal Plants against Human Infectious Diseases
Abstract
1. Introduction
2. Halophytes in Traditional Medicine and Ethnopharmacology
3. Halophytes as a Source of Antimicrobial Compounds
3.1. Antiviral Effects
3.2. Antibacterial Effects
| Bacteria | Plant Species | Reference |
|---|---|---|
| Bacillus cereus | Salicornia europaea | [80] |
| Mesembryanthemum edulis Suaeda monoica Cressa cretica Tamarix gallica | [62,83,84,85,86] | |
| Enterobacter sp. | Exoecaria agallocha | [59] |
| Enterococcus faecalis | Salicornia europaea | [80] |
| Mesembryanthemum edulis Suaeda monoica Tamarix gallica | [86] [87] [86] | |
| Escherichia coli | Arthrocnemum macrostachyum | [88] |
| Salicornia europaea | [80] | |
| Cressa cretica Mesembryanthemum edulis Suaeda monoica Tamarix gallica | [89] [83] [84] [86] | |
| Listeria monocytogenes | Mesembryanthemum edulis Tamarix gallica | [83] [86] |
| Micrococcus luteus | Salicornia europaea | [80] |
| Cressa cretica Mesembryanthemum edulis Retama raetam Retama sphaerocarpa Tamarix gallica | [89] [90] [91] [91] [86] | |
| Mycobacterium tuberculosis | Citrullus colocynthis | [82] |
| Mesembryanthemum edulis Ziziphus spina-christi | [88] [92] | |
| Proteus sp. | Exoecaria agallocha | [34] |
| Pseudomonas sp. | Cressa cretica Mesembryanthemum edulis Suaeda monoica Tamarix gallica | [89] [90] [84] [86] |
| Pseudomonas aeruginosa | Arthrocnemum macrostachyum | [88] |
| Multi-Resistant Pseudomonas aeruginosa | Eryngium barrelieri Eryngium glomeratum | [93] |
| Salmonella sp. | Cressa cretica Mesembryanthemum edulis Suaeda monoica Tamarix gallica | [89] [88] [84] [86] |
| Salmonella typhi | Exoecaria agallocha | [81] |
| Salmonella typhimurium | Salicornia europaea | [80] |
| Serratia marcescens | Salicornia europaea | [80] |
| Staphylococcus aureus | Arthrocnemum macrostachyum | [88] |
| Exoecaria agallocha | [81] | |
| Cressa cretica Mesembryanthemum edulis Suaeda monoica Tamarix gallica | [89] [88] [84] [86] | |
| Methicillin-Resistant Staphylococcus aureus (MRSA) | Acanthus ilicifolius Exoecaria agallocha Rhizophora mucronata Sonneratia caseolaris | [94] [95] [96] [94] |
| Aegiceras corniculatum Avicennia marina Ceriops decandra Eryngium thoraefolium Kochia scoparia Lumnitzera racemosa Rhizophora mucronata Tamarix gallica | [97] [98] [97] [99] [34] [97] [96] [86] | |
| Staphylococcus epidermidis | Salicornia europaea | [80] |
| Vibrio cholerae | Exoecaria agallocha | [81] |
3.3. Antifungal Effects
| Fungi | Plant Species | Reference |
|---|---|---|
| Aspergillus fumigatus | Arthrocnemum indicum Salicornia brachiata Suaeda maritima Suaeda monoica | [111] [84] |
| Cressa cretica | [89] | |
| Myrtus communis | [104] | |
| Eryngium maritimum | [112] | |
| Aspergillus niger | Cakile maritima Crithmum maritimum Eryngium maritimum | [17] |
| Candida albicans | Arthrocnemum indicum Salicornia brachiata Suaeda maritima Suaeda monoica | [111] [84] |
| Salicornia europaea | [80] | |
| Cressa cretica | [89] | |
| Limoniastrum monopetalum Limoniastrum guyonianum | [113] | |
| Puccinellia maritima Spartina maritima Spartina patens | [114] | |
| Salsola kali | [109] | |
| Salsola cyclophylla Suaeda vermiculata | [110] | |
| Myrtus communis Tetraena alba | [104] | |
| Candida glabrata | Arthrocnemum indicum Salicornia brachiata Suaeda maritima Suaeda monoica | [111] |
| Tamarix gallica | [86] | |
| Salicornia europaea | [80] | |
| Cressa cretica | [89] | |
| Limoniastrum monopetalum Limoniastrum guyonianum | [113] | |
| Salsola kali | [109] | |
| Candida holmii | Arthrocnemum indicum Salicornia brachiata Suaeda marítima Suaeda monoica | [111] |
| Salsola kali | [109] | |
| Candida krusei | Arthrocnemum indicum Salicornia brachiata Suaeda marítima Suaeda monoica | [111] |
| Salicornia europaea | [80] | |
| Limoniastrum monopetalum Limoniastrum guyonianum | [113] | |
| Candida parapsilosis | Arthrocnemum indicum Salicornia brachiata Suaeda maritima Suaeda monoica | [111] |
| Limoniastrum monopetalum Limoniastrum guyonianum | [113] | |
| Candida tropicalis | Salicornia europaea | [80] |
| Candida utilis |
4. Current Limitations and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamsutdinov, N.; Shamsutdinova, E.; Orlovsky, N.; Shamsutdinov, Z.S. Halophytes: Ecological features, global resources, and outlook for multipurpose use. Her. Russ. Acad. Sci. 2017, 87, 1–11. [Google Scholar] [CrossRef]
- Perrino, E.; Magazzini, P.; Musarella, C. Management of grazing “buffalo” to preserve habitats by Directive 92/43 EEC in a wetland protected area of the Mediterranean coast: Palude Frattarolo, Apulia, Italy. J. Environ. Integr. 2021, 6, 32. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Han, G.; Guo, J.; Meng, Z.; Chen, M. Progress in the study and use of seawater vegetables. J. Agric. Food Chem. 2020, 68, 5998–6006. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, A.; Iwanaga, F.; Taniguchi, T.; Hara, N.; Matsuo, N.; Mori, N.; Yunus, Q.; Yamanaka, N. Inorganic and organic osmolytes accumulation in five halophytes growing in saline habitats around the Aiding Lake area in Turpan Basin, Northwest China. Arid. Land Res. Manag. 2016, 30, 421–431. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Hasanuzzaman, M.; Lao, M.T. Oxidative stress and antioxidant defense in plants under salinity. React. Oxyg. Nitrogen Sulfur Species Plants Prod. Metab. Signal. Def. Mech. 2019, 12, 291–309. [Google Scholar]
- Abobatta, W.F. Plant responses and tolerance to extreme salinity: Learning from halophyte tolerance to extreme salinity. In Salt and Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 177–210. [Google Scholar]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milanovic, M.; Procopio, A.C.; Spampinato, G.; Maruca, G.; Perrino, E.V.; Mannino, G.C.; Fagoonee, S.; Luzza, F.; Musarella, C.M. Ancient wheats: Beneficial effects on insulin resistance. Minerva Med. 2020, 112, 641–650. [Google Scholar] [CrossRef]
- Faustino, M.V.; Faustino, M.A.; Pinto, D.C. Halophytic grasses, a new source of nutraceuticals? A review on their secondary metabolites and biological activities. Int. J. Mol. Sci. 2019, 20, 1067. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, A.; Papenbrock, J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 2013, 40, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, M.A.; Chaudhary, H.J.; Ullah, A.; Zhang, D. Combating soil salinity with combining saline agriculture and phytomanagement with salt-accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1085–1115. [Google Scholar] [CrossRef]
- Sarker, S.; Ara Hussain, F.; Assaduzzaman, M.; Failler, P. Blue economy and climate change: Bangladesh perspective. J. Ocean. Coast. Econ. 2019, 6, 6. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Oyebode, O.; Kandala, N.-B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Qasim, M.; Gulzar, S.; Khan, M.A. Halophytes as medicinal plants. Urban. Land Use Land Degrad. Environ. 2011, 21, 330–343. [Google Scholar]
- Debez, A.; Belghith, I.; Friesen, J.; Montzka, C.; Elleuche, S. Facing the challenge of sustainable bioenergy production: Could halophytes be part of the solution? J. Biol. Eng. 2017, 11, 1–19. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, J.-W. Dynamics of zonal halophyte communities in salt marshes in the world. J. Mar. Isl. Cult. 2018, 7, 84–106. [Google Scholar] [CrossRef]
- Ksouri, R.; Smaoui, A.; Isoda, H.; Abdelly, C. Utilization of halophyte species as new sources of bioactive substances. J. Arid Land Stud. 2012, 22, 41–44. [Google Scholar]
- Doudach, L.; Meddah, B.; Benbacer, L.; Hammani, K.; El Mzibri, M.; Vérité, P.; Elomri, A.; Cherrah, Y. Ethnopharmacological studies of Mesembryanthemum nodiflorum. Phytopharmacology 2013, 4, 246–258. [Google Scholar]
- Gamal, E.E.-G.; Khalifa, S.A.-K.; Gameel, A.S.; Emad, M.A. Traditional medicinal plants indigenous to Al-Rass province, Saudi Arabia. J. Med. Plants Res. 2010, 4, 2680–2683. [Google Scholar] [CrossRef]
- Alqethami, A.; Hawkins, J.A.; Teixidor-Toneu, I. Medicinal plants used by women in Mecca: Urban, Muslim and gendered knowledge. J. Ethnobiol. Ethnomed. 2017, 13, 62. [Google Scholar] [CrossRef]
- Larhsini, M.; Bousaid, M.; Lazrek, H.; Jana, M.; Amarouch, H. Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia 1997, 68, 371–373. [Google Scholar]
- Musharaf, K.; Shahana, M.; Zabta, K.S. Ethnobotanical importance of halophytes of Noshpho salt mine, District Karak, Pakistan. Res. Pharm. Biotechnol. 2011, 3, 46–52. [Google Scholar]
- Pattanayak, S.; Mandal, T.K.; Bandyopadhyay, S. A study on use of plants to cure enteritis and dysentery in three southern districts of West Bengal, India. J. Medic. Plants Stud. 2015, 3, 277–283. [Google Scholar]
- Al-Snafi, A.E. A review on pharmacological activities of Kochia scoparia-a review. Indo Am. J. Pharm. Sci. 2018, 5, 2213–2221. [Google Scholar]
- Abdul Qadir, M.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. Int. J. Anal. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K. Significance of medicinal plants in human life. In Synthesis of Medicinal Agents from Plants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–24. [Google Scholar]
- Knezevic, P.; Aleksic, V.; Simin, N.; Svircev, E.; Petrovic, A.; Mimica-Dukic, N. Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J. Ethnopharmacol. 2016, 178, 125–136. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778. [Google Scholar] [CrossRef]
- Kefu, Z.; Hai, F.; Ungar, I. Survey of halophyte species in China. Plant Sci. 2002, 163, 491–498. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef]
- Mnafgui, K.; Hamden, K.; Ben Salah, H.; Kchaou, M.; Nasri, M.; Slama, S.; Derbali, F.; Allouche, N.; Elfeki, A. Inhibitory activities of Zygophyllum album: A natural weight-lowering plant on key enzymes in high-fat diet-fed rats. Evid. Based Complement. Altern. Med. 2012, 2012, 620384. [Google Scholar] [CrossRef]
- Gupta, V.K.; Malhotra, S. Pharmacological attribute of Aloe vera: Revalidation through experimental and clinical studies. Ayu 2012, 33, 193. [Google Scholar] [CrossRef]
- Souid, A.; Bellani, L.; Gabriele, M.; Pucci, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B.; Longo, V. Phytochemical and biological activities in Limonium species collected in different biotopes of Tunisia. Chem. Biodivers. 2019, 16, e1900216. [Google Scholar] [CrossRef]
- Saxena, S. Glycyrrhiza glabra: Medicine over the millennium. NIScPR. 2005, 4, 358–367. [Google Scholar]
- Du, Q.; Xin, H.; Peng, C. Pharmacology and phytochemistry of the Nitraria genus. Mol. Med. Rep. 2015, 11, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Altay, V.; Gucel, S.; Guvensen, A. Halophytes in the East Mediterranean–their medicinal and other economical values. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2014; pp. 247–272. [Google Scholar]
- Tounekti, T.; Mahdhi, M.; Khemira, H. Ethnobotanical study of indigenous medicinal plants of Jazan region, Saudi Arabia. Evid. Based Complement. Altern. Med. 2019, 2019, 3190670. [Google Scholar] [CrossRef] [PubMed]
- Pravin, B.; Tushar, D.; Vijay, P.; Kishanchnad, K. Review on Citrullus colocynthis. Int. J. Res. Pharm. Chem. 2013, 3, 46–53. [Google Scholar]
- Lal, B.; Farrukh, H.; Zaman, S. An overview of people plant interaction in the rangeland of District Tank, Pakistan. J. Med. Plants Res. 2012, 6, 2820–2826. [Google Scholar]
- Zhao, K.; Song, J.; Feng, G.; Zhao, M.; Liu, J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2011, 342, 495–509. [Google Scholar] [CrossRef]
- Oratai, N.; Patcharin, S.; Kornkanok, Y.; Narumon, S. A survey of medicinal plants in mangrove and beach forests from sating Phra Peninsula, Songkhla Province, Thailand. J. Med. Plants Res. 2012, 6, 2421–2437. [Google Scholar]
- Kynaston, K.; Sinnott, J. Emerging Infectious Diseases: Clinical Case Studies. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 490. [Google Scholar] [CrossRef][Green Version]
- Steele, G.M.; Franco-Paredes, C.; Chastain, D.B. Noninfectious causes of fever in adults. Nurse Pract. 2018, 43, 38–44. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 1–25. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Papenbrock, J. Changes in secondary metabolites in the halophytic putative crop species Crithmum maritimum L., Triglochin maritima L. and Halimione portulacoides (L.) Aellen as reaction to mild salinity. PLoS ONE 2017, 12, e0176303. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Kumar, A.; Chand, G. Halophytes: The plants of therapeutic medicine. Ecophysiol. Abiotic Stress Responses Util. Halophytes 2019, 271–287. [Google Scholar] [CrossRef]
- Bernal, J.; Mendiola, J.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011, 55, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, M.; Prashanth, S.; Sivaprakash, K.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From wild salt marsh species to sustainable edible crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef]
- Attia-Ismail, S.A. Plant secondary metabolites of halophytes and salt tolerant plants. Halophytic Salt Toler. Feedstuffs 2015, 1, 127–142. [Google Scholar]
- Gahamanyi, N.; Munyaneza, E.; Dukuzimana, E.; Tuyiringire, N.; Pan, C.-H.; Komba, E.V. Ethnobotany, Ethnopharmacology, and Phytochemistry of Medicinal Plants Used for Treating Human Diarrheal Cases in Rwanda: A Review. Antibiotics 2021, 10, 1231. [Google Scholar] [CrossRef]
- Merrouni, I.A.; Elachouri, M. Anticancer medicinal plants used by Moroccan people: Ethnobotanical, preclinical, phytochemical and clinical evidence. J. Ethnopharmacol. 2021, 266, 113435. [Google Scholar] [CrossRef] [PubMed]
- Neiva, V.d.A.; Ribeiro, M.N.S.; Nascimento, F.R.; Cartágenes, M.d.S.S.; Coutinho-Moraes, D.F.; do Amaral, F.M. Plant species used in giardiasis treatment: Ethnopharmacology and in vitro evaluation of anti-Giardia activity. Rev. Bras. De Farmacogn. 2014, 24, 215–224. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Oppong, J.R. Globalization of communicable diseases. Int. Encycl. Hum. Geogr. 2020, 223–228. [Google Scholar]
- Luo, G.G.; Gao, S.J. Global health concerns stirred by emerging viral infections. J. Med. Virol. 2020, 92, 399. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef]
- Premanathan, M.; Kathiresan, K.; Nakashima, H. Mangrove halophytes: A source of antiviral substances. South Pac. Study 1999, 19, 49–57. [Google Scholar]
- Banerjee, M.B.; Ravikumar, S.; Gnanadesigan, M.; Rajakumar, B.; Anand, M. Antiviral, antioxidant and toxicological evaluation of mangrove associate from South East coast of India. Asian Pac. J. Trop. Biomed. 2012, 2, S1775–S1779. [Google Scholar] [CrossRef]
- Medini, F.; Legault, J.; Pichette, A.; Abdelly, C.; Ksouri, R. Antiviral efficacy of Limonium densiflorum against HSV-1 and influenza viruses. S. Afr. J. Bot. 2014, 92, 65–72. [Google Scholar] [CrossRef]
- Chiang, L.; Chiang, W.; Chang, M.; Ng, L.; Lin, C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Lee, H.-W.; Yoon, S.-R.; Lee, H.-M.; Lee, J.Y.; Kim, S.H.; Ha, J.-H. Use of RT-qPCR with combined intercalating dye and sodium lauroyl sarcosinate pretreatment to evaluate the virucidal activity of halophyte extracts against norovirus. Food Control 2019, 98, 100–106. [Google Scholar] [CrossRef]
- Vicente-Soler, J.; Madrid, M.; Franco, A.; Soto, T.; Cansado, J.; Gacto, M. Quorum sensing as target for antimicrobial chemotherapy. In New Weapons to Control Bacterial Growth; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–184. [Google Scholar]
- Musthafa, K.S.; Sahu, S.K.; Ravi, A.V.; Kathiresan, K. Anti-quorum sensing potential of the mangrove Rhizophora annamalayana. World J. Microbiol. Biotechnol. 2013, 29, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Snoussi, M.; Merghni, A.; Nazzaro, F.; Quindós, G.; Akdamar, G.; Mastouri, M.; Al-Sieni, A.; Ceylan, O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb. Pathog. 2017, 109, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Çoban, E.P.; Biyik, H.; Uzun, C. Investigation of antimicrobial activity of some natural plants which are not-cultivated and are sold at bazaars in Aydın vicinity. Int. J. Eng. Sci. 2009, 3, 59–62. [Google Scholar]
- Vadlapudi, V.; Bobbarala, V.; Penumajji, S.; Naidu, K.C. Excoecaria agallocha L. antimicrobial properties against important pathogenic microorganisms. Int. J. ChemTech Res. 2009, 1, 865–867. [Google Scholar]
- Mehta, A.; Srivastva, G.; Kachhwaha, S.; Sharma, M.; Kothari, S. Antimycobacterial activity of Citrullus colocynthis (L.) Schrad. against drug sensitive and drug resistant Mycobacterium tuberculosis and MOTT clinical isolates. J. Ethnopharmacol. 2013, 149, 195–200. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Placines, C.; Rodrigues, M.J.; Pereira, C.; Zengin, G.; Uysal, A.; Jeko, J.; Cziáky, Z.; Reis, C.P.; Gaspar, M.M. If you cannot beat them, join them: Exploring the fruits of the invasive species Carpobrotus edulis (L.) NE Br as a source of bioactive products. Ind. Crops Prod. 2020, 144, 112005. [Google Scholar] [CrossRef]
- Muthazhagan, K.; Thirunavukkarasu, P.; Ramanathan, T.; Kannan, D. Studies on phytochemical screening, antimicrobial and anti radical scavenging effect coastal salt mash plant of a Suaeda monoica. Res. J. Phytochem. 2014, 8, 102–111. [Google Scholar]
- Priyashree, S.; Jha, S.; Pattanayak, S. A review on Cressa cretica Linn.: A halophytic plant. Pharmacogn. Rev. 2010, 4, 161. [Google Scholar] [CrossRef]
- Boulaaba, M.; Snoussi, M.; Saada, M.; Mkadmini, K.; Smaoui, A.; Abdelly, C.; Ksouri, R. Antimicrobial activities and phytochemical analysis of Tamarix gallica extracts. Ind. Crops Prod. 2015, 76, 1114–1122. [Google Scholar] [CrossRef]
- Lincy, M.P.; Paulpriya, K.; Mohan, V. Pharmacochemical characterisation and antibacterial activity of Suaeda monoica leaf Forssk ex Gmel (Chenopodiaceae). Pharma Sci. Monit. 2013, 4, 161–166. [Google Scholar]
- Al-Saleh, G.; Gamal El-Din, A.; Abbas, J.; Saeed, N. Phytochemical and biological studies of medicinal plants in Bahrain: The family Chenopodiaceae—part 2. Int. J. Pharmacogn. 1997, 35, 38–42. [Google Scholar] [CrossRef]
- Sunita, P.; Jha, S.; Pattanayak, S.; Mishra, S. Antimicrobial activity of a halophytic plant Cressa cretica L. J. Sci. Res. 2012, 4, 203–212. [Google Scholar] [CrossRef]
- Falleh, H.; Trabelsi, N.; Bonenfant-Magné, M.; Le Floch, G.; Abdelly, C.; Magné, C.; Ksouri, R. Polyphenol content and biological activities of Mesembryanthemum edule organs after fractionation. Ind. Crops Prod. 2013, 42, 145–152. [Google Scholar] [CrossRef]
- Mariem, S.; Hanen, F.; Inès, J.; Mejdi, S.; Riadh, K. Phenolic profile, biological activities and fraction analysis of the medicinal halophyte Retama raetam. S. Afr. J. Bot. 2014, 94, 114–121. [Google Scholar] [CrossRef]
- Mongalo, N.; Mashele, S.; Makhafola, T. Ziziphus mucronata Willd.(Rhamnaceae): It’s botany, toxicity, phytochemistry and pharmacological activities. Heliyon 2020, 6, e03708. [Google Scholar] [CrossRef] [PubMed]
- Landoulsi, A.; Roumy, V.; Duhal, N.; Skhiri, F.H.; Rivière, C.; Sahpaz, S.; Neut, C.; Benhamida, J.; Hennebelle, T. Chemical Composition and Antimicrobial Activity of the Essential Oil from Aerial Parts and Roots of Eryngium barrelieri Boiss. and Eryngium glomeratum Lam. from Tunisia. Chem. Biodivers. 2016, 13, 1720–1729. [Google Scholar] [CrossRef]
- Prihanto, A.A.; Firdaus, M.; Nurdiani, R. Anti-Methicillin resistant Staphylococcus aureus (MRSA) of methanol extract of mangrove plants leaf: Preliminary report. Drug Invent. Today 2012, 4, 439–440. [Google Scholar]
- Sahoo, G.; Mulla, N.; Ansari, Z.; Mohandass, C. Antibacterial activity of mangrove leaf extracts against human pathogens. Indian J. Pharm. Sci. 2012, 74, 348. [Google Scholar]
- Kusuma, S.; Kumar, P.A.; Boopalan, K. Potent antimicrobial activity of Rhizophora mucronata. J. Ecobiotechnol. 2011, 3, 40–41. [Google Scholar]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V.; Prabhakar, K. Antibacterial activity of some salt marsh halophytes and mangrove plants against methicillin resistant Staphylococcus aureus. World J. Microbiol. Biotechnol. 2009, 25, 155–160. [Google Scholar] [CrossRef]
- Seepana, R.; Perumal, K.; Kada, N.M.; Chatragadda, R.; Raju, M.; Annamalai, V. Evaluation of antimicrobial properties from the mangrove Rhizophora apiculata and Bruguiera gymnorrhiza of Burmanallah coast, South Andaman, India. J. Coast. Life Med. 2016, 4, 475–478. [Google Scholar] [CrossRef]
- Çelik, A.; Aydınlık, N.; Arslan, I. Phytochemical constituents and inhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae). Chem. Biodivers. 2011, 8, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Dubey, O.; Dubey, S.; Schnee, S.; Glauser, G.; Nawrath, C.; Gindro, K.; Farmer, E.E. Plant surface metabolites as potent antifungal agents. Plant Physiol. Biochem. 2020, 150, 39–48. [Google Scholar] [CrossRef]
- Marquez, L.; Quave, C.L. Prevalence and therapeutic challenges of fungal drug resistance: Role for plants in drug discovery. Antibiotics 2020, 9, 150. [Google Scholar] [CrossRef]
- Saïdana, D.; Mahjoub, S.; Boussaada, O.; Chriaa, J.; Mahjoub, M.A.; Chéraif, I.; Daami, M.; Mighri, Z.; Helal, A.N. Antibacterial and antifungal activities of the essential oils of two saltcedar species from Tunisia. J. Am. Oil Chem. Soc. 2008, 85, 817–826. [Google Scholar] [CrossRef]
- Silva, B.; Souza, M.M.; Badiale-Furlong, E. Antioxidant and antifungal activity of phenolic compounds and their relation to aflatoxin B1 occurrence in soybeans (Glycine max L.). J. Sci. Food Agric. 2020, 100, 1256–1264. [Google Scholar] [CrossRef]
- Belmimoun, A.; Meddah, B.; Meddah, A.; Gabaldon, J.; Sonnet, P. Antifungal activity of Myrtus communis and Zygophyllum album extracts against human pathogenic fungi. Eur. J. Biol. Res. 2020, 10, 45–56. [Google Scholar]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Mansour, S.; Djebli, N.; Bouakline, H.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L. Antimicrobial and anti-inflammatory activities of three halophyte plants from Algeria and detection of some biomolecules by HPLC-DAD. Nat. Prod. Res. 2021, 35, 2107–2111. [Google Scholar] [CrossRef]
- Chin, V.K.; Lee, T.Y.; Rusliza, B.; Chong, P.P. Dissecting Candida albicans infection from the perspective of C. albicans virulence and omics approaches on host–pathogen interaction: A review. Int. J. Mol. Sci. 2016, 17, 1643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.; Wang, C.; Shao, J.; Wang, T.; Wu, D.; Ma, K.; Yan, G.; Yin, D. Antifungal evaluation of traditional herbal monomers and their potential for inducing cell wall remodeling in Candida albicans and Candida auris. Biofouling 2020, 36, 319–331. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Boulaaba, M.; Medini, F.; Hajlaoui, H.; Mkadmini, K.; Falleh, H.; Ksouri, R.; Isoda, H.; Smaoui, A.; Abdelly, C. Biological activities and phytochemical analysis of phenolic extracts from Salsola kali L. Role of endogenous factors in the selection of the best plant extracts. S. Afr. J. Bot. 2019, 123, 193–199. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.; Hegazy, M.M. Essential oil constituents and biological activities of the halophytic plants, Suaeda vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J. Essent. Oil Bear. Plants 2019, 22, 82–93. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Für Nat. C 2008, 63, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.A.; Nabavi, S.F.; Orhan, I.E.; Daglia, M.; Izadi, M.; Nabavi, S.M. Blessings in disguise: A review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J. Pharm. Sci. 2015, 23, 53. [Google Scholar] [CrossRef]
- Trabelsi, N.; Waffo-Téguo, P.; Snoussi, M.; Ksouri, R.; Mérillon, J.M.; Smaoui, A.; Abdelly, C. Variability of phenolic composition and biological activities of two Tunisian halophyte species from contrasted regions. Acta Physiol. Plant. 2013, 35, 749–761. [Google Scholar] [CrossRef]
- Faustino, M.V.; Faustino, M.A.; Silva, H.; Cunha, Â.; Silva, A.M.; Pinto, D.C. Puccinellia maritima, Spartina maritima, and Spartina patens halophytic grasses: Characterization of polyphenolic and chlorophyll profiles and evaluation of their biological activities. Molecules 2019, 24, 3796. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
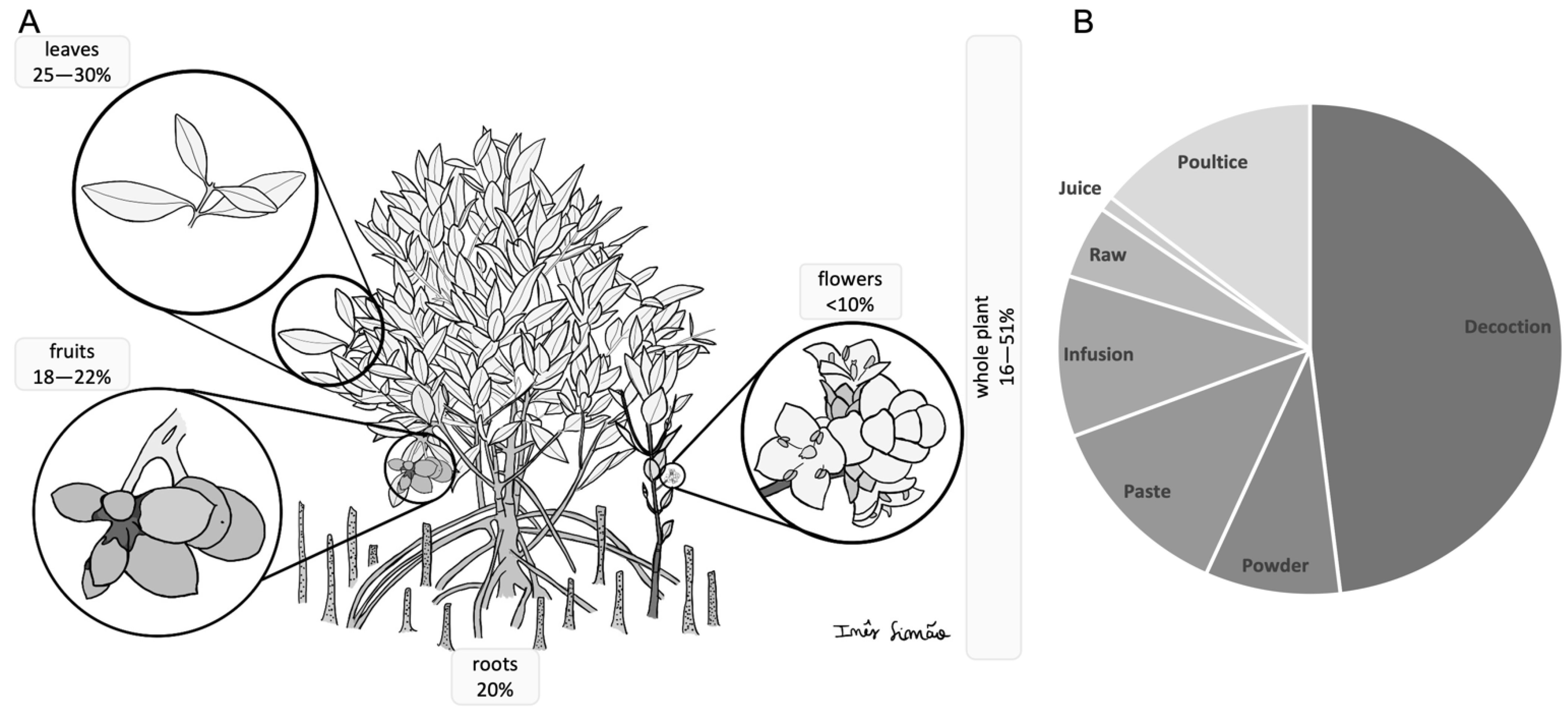
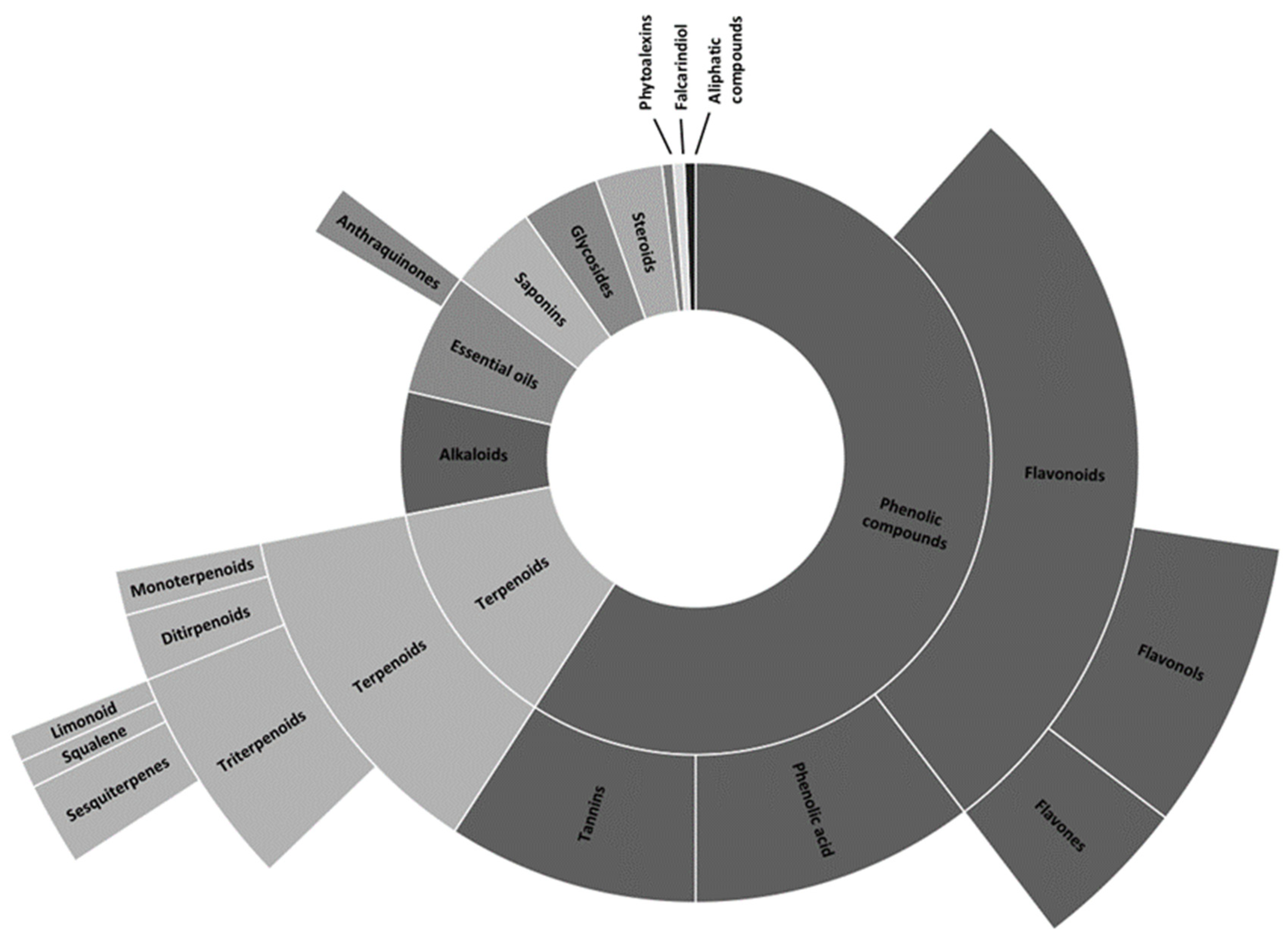
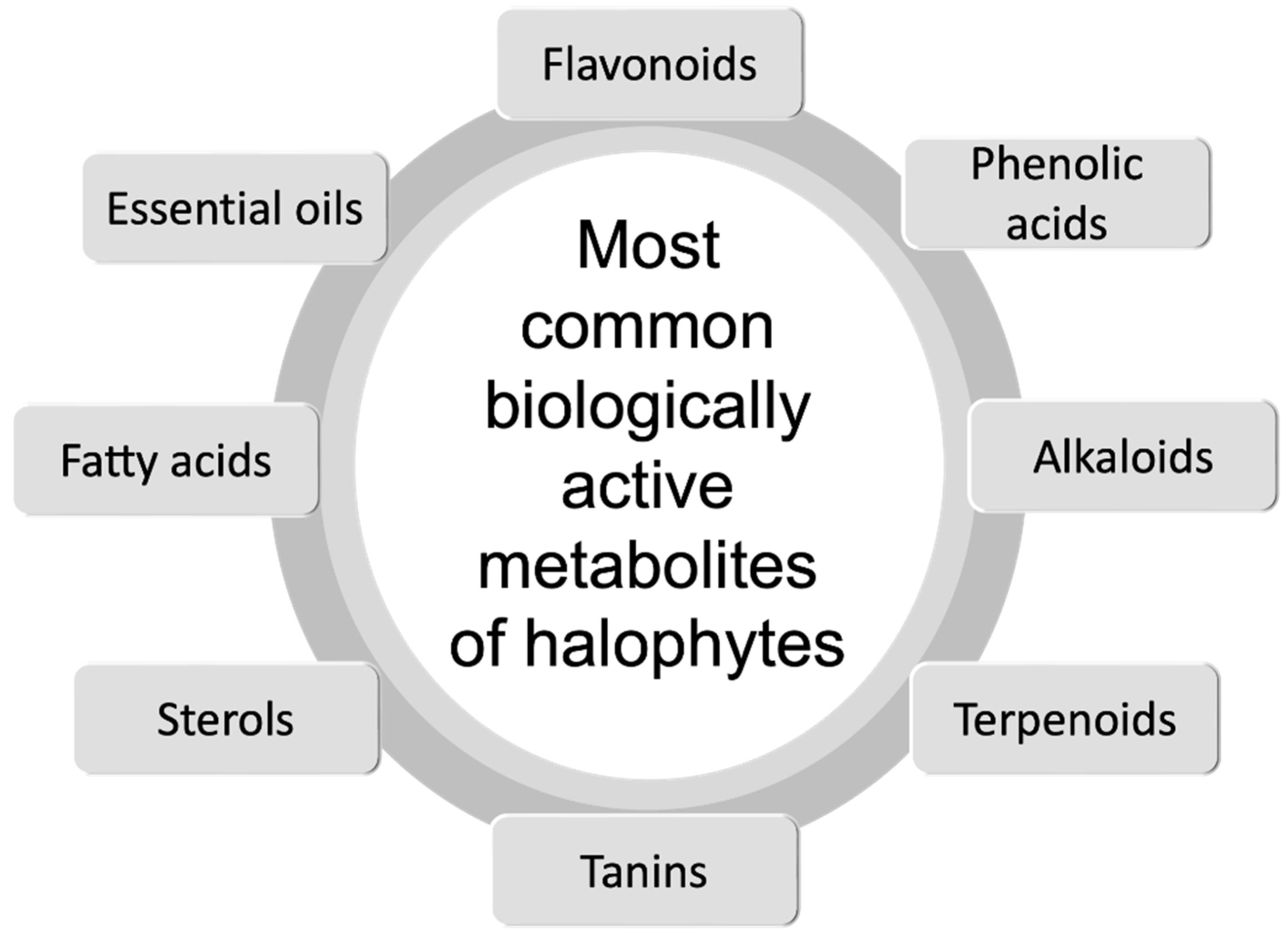
| Familiy | Species | Plant Part | Application | Region | Reference |
|---|---|---|---|---|---|
| Aizoaceae | Mesembryanthemum spp. | aerial parts | fungal and bacterial infections, diarrhea, tuberculosis, antiseptic to treat infections of the mouth and throat | Europe, Africa, Australia and California | [27,28] |
| Amaranthaceae | Aerva javanica | roots, leaves, flowers and seeds | infected wounds, malaria | Saudi Arabia | [29] |
| Apiaceae | Foeniculum vulgare | root and seeds | gastrointestinal, urological, gynecological infections | Saudi Arabia | [30] |
| Apocynaceae | Calotropis procera | leaves and latex | skin infection (antifungal) | Morocco | [31] |
| Calotropis procera | latex | skin infections | Saudi Arabia | [29] | |
| Asteraceae | Blumea lacera | roots | antiseptic, dysentery | Pakistan | [32,33] |
| Xanthium sibiricum | fruits | rhinitis, nasal sinusitis, headache, gastric ulcer, urticaria, rheumatism, bacterial and fungal infections, arthritis and eye diseases | China | [22] | |
| Chenopodiaceae | Kochia scoparia | fruits | dysuria, skin, urinary tract and eye diseases, pruritus and thermal skin lesions | China, Japan and Korea | [34] |
| Beta vulgaris | leaves and stems | digestive disorders, throat inflammation, digestive, diuretic and laxative properties | Mediterranean basin | [23] | |
| Cucurbitaceae | Citrullus colocynthis | fruits and roots | bronchitis, tuberculosis, glands of the neck, throat infection | India and Pakistan, Arabia, West Asia, Tropical Africa and the Mediterranean region | [35] |
| Ephedraceae | Ephedrasinica Ephedramajor | treatment of cold, bronchial asthma, cough, fever, flu, headache, edema, allergies, bacterial infections | China, India | [36] | |
| Myrtaceae | Eucalyptus camaldulensis | leaves | infected wounds | Nigeria | [37] |
| Solanaceae | Lycium barbarum L.chinense | Fruits, leaves, root bark | lung function and eye diseases, cough | China | [38] [39] [40] |
| Tamaricaceae | Tamarix aphylla | leaves and roots | infected wounds | Saudi Arabia | [29] |
| Zygophyllaceae | Tetraena alba | leaves, stems, fruits | antiviral and antifungal | semiarid areas of Saudi Arabia, Africa | [41] |
| Liliaceae | Aloe vera | leaves and roots | fever, constipation, sunstroke, malaria, eczema, psoriasis, hair loss, gastric ulcer, liver pain, diabetes, menstrual troubles, gonorrhea, spleen disorders, nerve pain, rheumatism | [42] | |
| Plumbaginaceae | Limonium spp. | leaves and roots | microbial and viral infections | Tunisia | [43] |
| Fabaceae | Glycyrrhiza spp. | underground unpeeled or peeled stems and roots | upper respiratory tract ailments including coughs, hoarseness, sore throat and bronchitis | China, Japan | [44] |
| Zygophyllaceae | Nitraria spp. | fruits | hypertension, menstrual disorders and gastroenteritis | China | [45] |
| Virus | Plants Species | Reference |
|---|---|---|
| Newcastle disease virus (NDV) | Acanthus ilicifolius Aegiceras corniculatum Bruguiera cylindrica Excoecaria agallocha Lumnitzera racemosa Rhizophora mucronata | [71] |
| Vaccinia virus (VV) | Bruguiera cylindrica Lumnitzera racemosa Rhizophora mucronata Ceriops decandra | |
| Encephalomyocarditis virus (EMCV) | Avicennia marina Bruguiera cylindrica Excoecaria agallocha Lumnitzera racemosa Rhizophora apiculata Rhizophora lamarckii Rhizophora mucronata Salicornia brachiata | |
| Semliki Forest virus (SFV) | Bruguiera cylindrica Ceriops decandra Aegiceras corniculatum Rhizophora lamarckii Rhizophora mucronata | |
| Hepatitis B virus (HBV) | Acanthus ilicifolius Aegiceras corniculatum Avicennia marina Bruguiera cylindrica Ceriops decandra Rhizophora apiculata Rhizophora lamarckii Rhizophora mucronata Salicornia brachiata Sesuvium portulacastrum | |
| Suaeda maritima | [72] | |
| Human immunodeficiency virus (HIV) | Aegiceras corniculatum Ceriops decandra Excoecaria agallocha Rhizophora apiculata Rhizophora lamarckii Rhizophora mucronata | [71] |
| Herpesviruses (HSV-1, HSV-2) | Plantago major | [75] |
| Limonium densiflorum | [73] | |
| Adenoviruses (ADV-3, ADV-8, ADV-11) | Plantago major | [74] |
| Influenza A viruses (H1N1 strain) | Limonium densiflorum | [73] |
| Human norovirus (HuNoV GII.4) | Glehnia littoralis Mesembryanthemum crystallinum Salicornia europaea Spergularia marina Suaeda japonica | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as Medicinal Plants against Human Infectious Diseases. Appl. Sci. 2022, 12, 7493. https://doi.org/10.3390/app12157493
Ferreira MJ, Pinto DCGA, Cunha Â, Silva H. Halophytes as Medicinal Plants against Human Infectious Diseases. Applied Sciences. 2022; 12(15):7493. https://doi.org/10.3390/app12157493
Chicago/Turabian StyleFerreira, Maria João, Diana C. G. A. Pinto, Ângela Cunha, and Helena Silva. 2022. "Halophytes as Medicinal Plants against Human Infectious Diseases" Applied Sciences 12, no. 15: 7493. https://doi.org/10.3390/app12157493
APA StyleFerreira, M. J., Pinto, D. C. G. A., Cunha, Â., & Silva, H. (2022). Halophytes as Medicinal Plants against Human Infectious Diseases. Applied Sciences, 12(15), 7493. https://doi.org/10.3390/app12157493










