Featured Application
Halophytic species can be used to colonize soils with high salinity and shortly become useful for food or pharmaceutical industries.
Abstract
Limonium vulgare Mill. is a plant growing widely in harsh environments, such as salt marshes, for which a chemical profile is still unknown, although some interesting bioactivities were already reported. So, this halophyte chemical profile must be established to find the possible bioactive compounds, valorize the species, and contribute to the salt marsh’s exploitation. This work set the chemical profile of L. vulgare’s aerial parts (leaves and inflorescences) using UHPLC-DAD-ESI/MS2 and GC-MS analysis. The lipophilic profile showed a richness in fatty acids, alkanes, and terpenoids, β-sitosterol being the major compound in inflorescences in the fruiting stage (0.822 ± 0.015 mg/g of the dry plant) and leaves (0.534 ± 0.017 mg/g of the dry plant). In contrast, in the inflorescences in the flowering stage, the major compound is nonacosane (0.228 ± 0.001 mg/g of the dry plant). The polyphenolic profile demonstrates that L. vulgare produces several flavonoids from which quercetin and myricetin can be highlighted; in particular, myricetin derivatives are prevalent in all extracts. Amongst the flavonoids, myricetin 3-rhamnoside is the most abundant in the inflorescences in the flowering stage (6.35 ± 0.05 mg/g of the dry plant), myricetin in leaves (9.69 ± 0.11 mg/g of the dry plant), and in the inflorescences in the fruiting stage baicalin presents the highest amount (5.15 ± 0.07 mg/g of the dry plant). This is the first report on L. vulgare’s chemical profile and the results indicate that this species is an exciting source of bioactive compounds, suggesting it has a use to produce nutraceuticals and/or pharmaceuticals.
1. Introduction
Most halophytes are noteworthy because they tolerate harsh conditions such as salty and windy environments. Limonium vulgare Mill., commonly known as sea lavender, complements this tolerance with its remarkable floral beauty, which is an exception among the halophytic plants. L. vulgare belongs to the Plumbaginaceae family and occurs in salt marshes at the coast side of the European and northwest African Atlantic Ocean, the northern sea, and the Baltic Sea [1]. Plants growing under the harsh conditions in these areas must have a specific adaption. Because of the ability to grow in such a harsh and diverse area, changing from freshwater after heavy rain to high salinity because of the tide could affect their natural combinatorial chemistry [2,3]. L. vulgare is among the accepted species belonging to the Limonium genus [1] of which, until now, the chemical constitution is not thoroughly investigated. Some information regarding the phenolic and carotenoid content was reported, including the total flavonoid content, but there is no detailed identification [4]. Nevertheless, this species’ biological potential was described and interesting results against fungi, bacteria [5], antioxidant [4], and antitumor activities [6] point out that L. vulgare might be a promising source for new drugs or be used as a food additive. Other Limonium species have also revealed important biological activities [7,8,9] and the production of bioactive compounds, such as flavonoids [8,10,11]. Considering the potential of L. vulgare, and the absence of information concerning its quantitative chemical profile, this work aims to establish the chemical profile of its aerial parts and, in this way, contribute to the valorization of an under-explored halophytic species.
2. Materials and Methods
2.1. Chemicals
The solvents used for extraction and sample solubilization were dichloromethane (DCM), ethanol, hexane, and methanol (all VWR, Radnor, PA, USA), of analytical grade. The UHPLC mobile phase eluents, formic acid, and acetonitrile were HPLC grade and purchased from Panreac (Barcelona, Spain). Ultrapure water was obtained by a Direct-Q® water purification system (Merck Life Science, Darmstadt, Germany). Pyridine p.a., N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (99%) and trimethylsilyl chloride (TMSCl) (99%) (Sigma-Aldrich, St. Louis, MO, USA) were applied in the sample derivatization by silylation.
Several chemicals were used as standards for UHPLC-MS or GC-MS analyses (see detailed information in Supplementary Materials).
2.2. Plant Material and Extracts Preparation
Aerial plant parts of Limonium vulgare Mill. were collected in June 2018 in the salt marshes near the University of Aveiro (Coordinates: 40.621801, −8.662621), Portugal. Prof. Helena Silva was responsible for the taxonomic identification and supervised the collection. A voucher specimen (3433 AVE) was deposited in the Herbarium of the Department of Biology, University of Aveiro, Portugal.
Three samples were collected and treated separately: the leaves (LLv), inflorescences in the flowering stage (YFLv), and inflorescences in the fruiting stage (GFLv). The collected plant parts were washed with aqueous ethanol 70% (v/v), air dried, protected from light, and dried in an oven with ventilation at 55 °C until constant weight. Lastly, the plants were ground with a food processor until they became a powder.
Each lipophilic extract was prepared by maceration (1 g plant part: 10 mL hexane, Table S1) at room temperature, under stirring, and in the dark, for three cycles of three days each, with solvent renewal at the end of each cycle. The solvent from the combined extraction steps was first evaporated with a rotary vacuum evaporator at 40 °C and then dried under vacuum at room temperature. Following the extraction with hexane, a Soxhlet extraction with ethanol was prepared (1 g plant part: 20 mL ethanol, Table S1). The plant samples from the first extraction were air dried and then filled into cartridges for Soxhlet devices. The extraction ran three times for 24 h each with fresh solvent until only clear ethanol cycled through the Soxhlet. Subsequently, the ethanol was evaporated with a rotary evaporator. The extracts were dried until mass consistence before further usage. Three replicates of each extract were obtained.
2.3. Gas Chromatography—Mass Spectrometry
The GC-MS analysis of the hexane extracts was done using a Shimadzu GCMS-QP2010Ultra system equipped with a DB-5-J&W capillary column (30 m in length × 0.25 mm in diameter × 0.25 μm thickness of the film). The spectrometric detection from the mass spectrometer utilized 70 eV electron ionization. Helium was used as a carrier gas with a column flow of 1.18 mL/min. GC-injection temperature was set at 320 °C and a split ratio of 50 was applied to an injection volume of 2 μL. The mass spectrometer ion source temperature was at 200 °C and the interface temperature at 300 °C.
Approximately 20 mg of each extract was derivatized using well-established procedures [12,13]. Tetracosane was used as the internal standard and four independent replicates were obtained (see detailed information in Supplementary Materials). The temperature of the column was maintained at 90 °C for 4 min and then increased, first at 16 °C/min until 180 °C, followed by 3 °C/min until 220 °C and by 8 °C/min until 260 °C, and lastly 4 °C/min until achieving 300 °C, which was maintained for 5 min. The start time of record was set at 6.5 min.
Identification of the compounds was mainly done by comparing the results to MS libraries NIST 2014, NIST 2008, and WILEY 2007. If possible, it was also compared with the retention time of pure compounds [14,15,16]. Furthermore, identification of some compounds was done using the retention index [17]. The quantitative analysis was achieved by calibration curves, which were obtained using standards representative of each chemical family present in the samples and using at least six different concentrations. The correlation coefficient (R2) was higher than 0.99 in every curve and the concentrations of the standards were chosen to guarantee the quantification of each compound in the samples by interpolation in the calibration curve (see detailed information in Supplementary Materials).
2.4. Liquid Chromatography—Mass Spectrometry
The ultrahigh performance liquid chromatography-mass spectrometry (UHPLC-DAD-ESI/MSn) analysis was performed using a Thermo Scientific Ultimate 3000RSLC (Dionex, Sunnyvale, CA, USA) equipped with a Dionex UltiMate 3000 RS diode array detector and coupled to a mass spectrometer. The column used was a Thermo Scientific Hypersil GOLD C18 (Part No. 25002-102130; Dim 100 mm × 2.1 mm; Lot 14913; SN 10518298) with a part size of 1.9 µm and its temperature was maintained at 30 °C. The mobile phase was composed of (A) acetonitrile and (B) 0.1% formic acid in water (v/v), both degassed and filtered before use. The flow rate was 0.2 mL/min. For the first 14 min the mobile phase had 5% of (B). Next, the gradient of (B) increased to 40% for 2 min and then to 100% for 7 min. From 23 min to the end it was set back to 5% (B). The injection volume was 2 µL. The equipment was operated in negative-ion mode with an electrospray ionization source of 5.00 kV and ESI capillarity temperature of 275 °C. The full scan covered a mass range of 50 to 2000 m/z. Collision-induced dissociation and MS2 experiments were simultaneously acquired for precursor ions. The quantification of the individual compounds in the plant extracts was performed by the external standard method, using reference compounds for each family. A calibration curve was obtained by the injection of at least six different known concentrations and with a correlation coefficient (R2) higher than 0.99 (see detailed information in Supplementary Materials).
2.5. Statistical Analysis
Data-independent replicates of each sample were analyzed and each aliquot was injected twice. The presented results are the average of four concordant values obtained for each sample (less than 5% variation between injections of the same aliquot and between aliquots of the same sample) and expressed as mean values ± standard deviation (MV ± SD). One-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test were performed using the GraphPad Prism version 7 for Windows (GraphPad Software, Inc., San Diego, CA, USA) to compare the results of each independent replicate. A p-value lower than 0.0001 was considered statistically significant in all analyses.
3. Results and Discussion
3.1. GC-MS Profile
The L. vulgare hexane extract profile was established for the first time, and the aerial plant parts were separated into leaves (LLv) and inflorescences to compare the parts’ chemical profiles. Furthermore, the inflorescences were divided according to their phenology in the flowering stage (YFLv) and fruiting stage (GFLv). The total ion chromatogram (TIC) obtained (Figure 1) demonstrates that the qualitative differences are slight; however, the quantitative analysis, detailed in Table 1, establishes that the flowering stage part is less rich in these specialized metabolites (2.049 ± 0.002 mg/g of the dry plant), whereas the fruiting stage is the richer (6.056 ± 0.016 mg/g of the dry plant). Although leaves are not as rich as inflorescences in the fruiting stage, they are richer than inflorescences in the flowering stage, presenting 4.921 ± 0.019 mg/g of the dry plant.
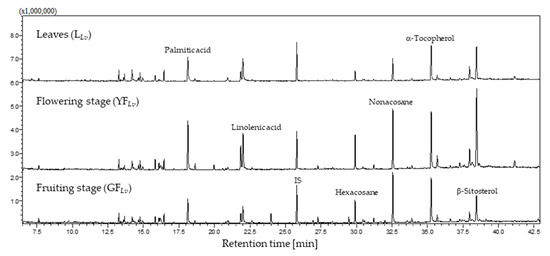
Figure 1.
Total ion chromatogram of the L. vulgare hexane extract.

Table 1.
Chemical composition of hexane extracts of L. vulgare aerial parts (mg g−1 ± SD a).
More detailed analysis evidence that the major chemical families are alkanes, terpenoids, and fatty acids and their derivatives (Table 1; Figure 2). Actually, alkanes account for 41% in YFLv, 36% in GFLv, and 28% in LLv. Terpenoids correspond to 21% in YFLv, 32% in GFLv, and 38% in LLv, and, finally, fatty acids and their derivatives correspond to 30% in YFLv, 25% in GFLv, and 26% in LLv.
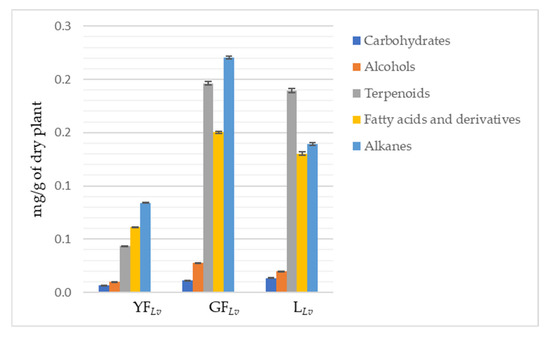
Figure 2.
Graphical representation of the total amount of each class of specialized metabolites for the studied plant parts. All classes are statistically different (Tukey’s test; p < 0.001).
We previously evidenced the high concentration of alkanes in other halophytic species [18]. Their rule in halophyte plants correlates with the necessity to control osmotic stress [19], so they were expected to be found in the L. vulgare hexane extract. Moreover, their higher percentage in YFLv seems logical considering that it is a stage where protection is essential for the plant’s reproductive success [20]. Although carbohydrates, in this study are less important (Figure 2), mainly because the study was focused on other metabolites, they also increase in response to salty environments [21,22,23], and we also found D-psicose in other halophytes [18].
There are pieces of evidence that the amount of terpenoids is not affected by the salty environment [24]; however, in other halophytes, they are also prevalent [18]. In L. vulgare it is obvious that these compounds are predominant in leaves, although their percentage in GFLv is also relevant (Figure 2).
Regarding the fatty acids and their derivatives, it is documented that their production is increased in response to salinity stress [18,25,26]. The other families are much less representative, although it should be highlighted that the methodology applied was not the most appropriate to detect these compounds and can explain their low quantity.
Table 1 detailed analysis that shows that β-sitosterol is the major compound, of the terpenoids family, detected in the L. vulgare hexane extracts, 0.166 ± 0.001 mg/g of the dry plant in YFLv, 0.822 ± 0.015 mg/g of the dry plant in GFLv, and 0.534 ± 0.017 mg/g of the dry plant in LLv. In some parts (GFLv and LLv), it is actually the metabolite found in higher amounts, in fact these plant parts present a good percentage of terpenoids from which phytosterols can be highlighted. Together with β-sitosterol, campesterol and stigmasterol were also present in significant amounts in GFLv (0.218 ± 0.003 and 0.201 ± 0.003 mg/g of the dry plant) and LLv (0.174 ± 0.008 and 0.176 ± 0.012 mg/g of the dry plant). These sterols constitute the major phytosterols found in plants, and, usually, their quantity is not affected by salinity stress [24], but there are examples where the amount of campesterol increases [27]. So, it seems logical that we found more quantity in GFLv and LLv (Table 1). Moreover, campesterol and stigmasterol are among the most relevant phytosterols presenting pharmacological and nutritional effects [28]. Consequently, their occurrence in such interesting amounts enhances the L. vulgare’s economic value; that is, it stimulates the species use to produce nutraceuticals. The reported shreds of evidence of phytosterols’ role in cancer prevention [29] suggest that L. vulgare can be an excellent source from which to isolate these bioactive phytosterols. These phytosterols’ presence was confirmed using standards; however, the typical ion fragments were observed (Figure 3). Of the other terpenoids, tocopherols can also be highlighted, α-tocopherol being the major one (Table 1). Their most typical fragment was observed (Figure 3) [30], although α-tocopherol was also confirmed by the injection of the pure standard in the same conditions. Tocopherols are also recognized for their bioactivities [31], so their occurrence will also contribute to establishing L. vulgare as a health-promoting species.
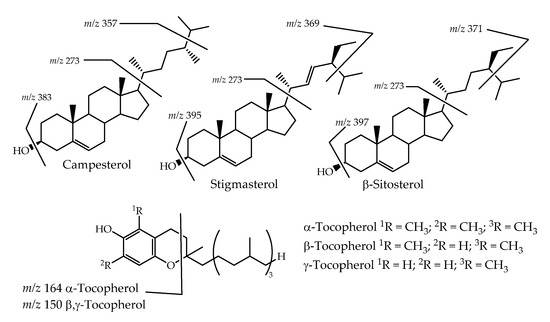
Figure 3.
Chemical structure of the phytosterols and tocopherols, and their most significant fragments observed on GC-MS spectra.
The second and the third most abundant compounds are fatty acids, respectively palmitic and linolenic acids; both acids contribute to the species valorization and, from previous studies, we demonstrate that they can contribute to the species’ survival under salinity stress [18]. Another interesting result is the occurrence of inositol, mainly the myo-inositol isomer. Mahajan and Tuteja [32] described inositol phosphates as messengers to modulate the intracellular calcium level, an essential parameter in reducing osmotic stress. Other studies described the role of myo-inositol in salinity stress and established that it could help the plant defenses by participating in its antioxidant system and also regulate the ionic homeostasis [33].
3.2. LC-MS Profile
The chemical profile of the L. vulgare ethanolic extracts was established using UHPLC-DAD-ESI/MS2 analyses. It was possible to confirm that the aerial parts are quite similar in the type of compounds present (Figure 4), but different in their amounts (Table 2).
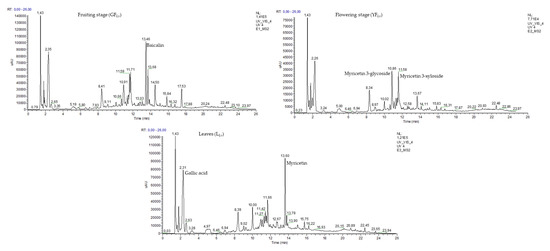
Figure 4.
UHPLC-DAD chromatograms of the L. vulgare ethanol extract, recorded at λ = 280 nm.

Table 2.
Chemical composition of L. vulgare ethanolic extracts. Retention time (tR; min), wavelengths of maximum absorption (λmax; nm), deprotonated molecular ion ([M−H]−; m/z), mass spectral data (MS2; m/z) indicating in parentheses relative intensity, quantification in mg g−1 ± SD a.
The aerial parts of L. vulgare are affluent in flavonoids (56% in YFLv, 67% in GFLv, and 58% in LLv), specialized metabolites associated with several health benefits [34], although the major compound in all plant parts is gallic acid, 9.85 ± 0.06, 9.21 ± 0.09, and 15.44 ± 1.01 mg/g of the dry plant, respectively, in YFLv, GFLv, and LLv. Though, it should be highlighted that this metabolite is recognized for its health-promoting effects [35], contributing, with its presence in L. vulgare aerial parts, to the plant nutritional value.
The main flavonoids present are flavone and flavonol derivatives (Figure 5), with the myricetin derivatives being the most abundant, 66% in YFLv, 51% in GFLv, and 63% in LLv (Table 2). Myricetin is also the major aglycone present in all plant parts analyzed (4.73 ± 0.15 mg/g of the dry plant in YFLv, 4.48 ± 0.05 mg/g of the dry plant in GFLv and 9.69 ± 0.11 mg/g of the dry plant in LLv). This richness in myricetin derivatives, as well as other flavonoids, was detected in other Limonium species although the studies were qualitative [8,36,37]; however, in a previous study with halophytes collected in the same region, we also detected that flavonoids are the major metabolites [38]. Moreover, in some phytochemical studies, using other Limonium species, several myricetin derivatives were isolated [10,11]; these results support our identification.
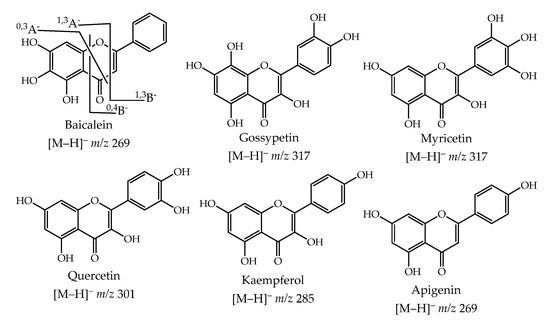
Figure 5.
Chemical structure of the flavonoid aglycones and their most significant fragments.
The aglycones were mainly identified using standard compounds; nevertheless, the specific ion fragments [39,40,41], shown in the baicalein structure (Figure 5), were found to help the identification. In the case of the glycoside derivatives, while the standards are less available, the literature data [39,42,43], as well as our previous experience [44], were the most valuable tools for the identification.
Apigenin 7-(acetylglucoside) was assigned because of the observation of the ion fragment at m/z 430, which can be obtained through a loss of an acetyl moiety (Table 2). Simultaneously, the loss of m/z 221 and 205, suggesting the cleavage of acetylglucose with or without the oxygen atom, confirms the compound structure. Naturally, there are no shreds of evidence where the acetyl group is attached; both apigenin 7-(2″-acetylglucoside) and apigenin 7-(6″-acetylglucoside) were previously found in plants [45] (p. 84). Finally, we can highlight the identification of the myricetin 3-(galloylrhamnoside) three isomers, specialized metabolites previously found in other plants [45] (p. 538). In all of these isomers, the loss of the galloyl and gallate moieties and the galloylrhamnose moiety were detected (Table 2). Again, it was not possible to establish the galloyl moiety position, although the three possible isomers, myricetin 3-(2″-galloylrhamnoside), myricetin 3-(3″-galloylrhamnoside), and myricetin 3-(4″-galloylrhamnoside), could be detected.
The richness of L. Vulgare’s aerial parts in flavonoids herein established agrees with the fact that the salinity stress increases the flavonoid production [36,46]. Moreover, this richness, as well as several of the specialized metabolites herein reported, were detected in another Limonium species [36,37]. However, quantifications concerning these metabolites were not previously reported for Limonium species, making this the first study involving quantification.
Considering the specialized metabolites herein reported, for the first time in L. vulgare, particularly the flavonols, for which several biological properties have been established [47,48], it seems evident that this species can be used as food additives and to produce bioactive compounds. Myricetin and its derivatives can be highlighted because of their amounts in L. vulgare’s aerial parts. Furthermore, this flavonol, to which important pharmaceutical properties have been established [49], contributes to adding value to the species.
4. Conclusions
Overall, the reported results established L. vulgare’s potential in the nutrition or pharmaceutical industry. Toxicological studies are necessary if the application includes the use of the plant. Suppose the application involves the isolation and purification of the bioactive specialized metabolites. In that case, it can be relatively fast because the metabolites, although reported for the first time for this species, are ubiquitous in many plants, and some are already recognized for their pharmacological properties. Actually, L. vulgare is an excellent source of phytosterols and flavonoids, from which β-sitosterol and myricetin can be emphasized. Our findings point out the potential economic value of this underexplored species.
Supplementary Materials
Detailed experimental procedures can be downloaded at https://www.mdpi.com/article/10.3390/app12136384/s1.
Author Contributions
Conceptualization, D.C.G.A.P. and A.M.S.S.; methodology, D.C.G.A.P., B.C. and A.M.S.S.; formal analysis and investigation, B.C., D.C.G.A.P. and A.M.S.S.; resources, H.S. and A.M.S.S.; writing—original draft preparation, B.C. and D.C.G.A.P.; writing—review and editing, D.C.G.A.P., H.S. and A.M.S.S.; supervision, D.C.G.A.P. and A.M.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese National Funds, through FCT (Fundação para a Ciência e a Tecnologia), and as applicable co-financed by FEDER within the PT2020 Partnership agreement by funding the LAQV-REQUIMTE (UIDB/50006/2020) and CESAM (UIDB/50017/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks are due to the University of Aveiro and to Mónica S.G.A. Válega for her support in UHPLC-MS analysis. B.C. also thanks ERASMUS+ for the traineeship support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:687035-1#descriptions (accessed on 4 May 2022).
- Tuteja, N.; Gill, S.S. Climate Change and Plant Abiotic Stress Tolerance; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Chandna, R.; Azooz, M.M.; Ahmad, P. Recent advances of metabolomics to reveal plant response during salt stress. In Salt Stress in Plants: Signalling, Omil and Adaptation; Chandna, R., Azooz, M.M., Ahmad, P., Eds.; Springer: New York, NY, USA, 2013; pp. 1–15. [Google Scholar]
- Souid, A.; Bellani, L.; Gabriele, M.; Pucci, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B.; Longo, V. Phytochemical and biological activities in Limonium species collected in different biotopes of Tunisia. Chem. Biodivers. 2019, 16, e1900216. [Google Scholar] [CrossRef] [PubMed]
- Lellau, T.F.; Liebezeit, G. Activity of ethanolic extracts of salt marsh plants from the lower Saxonian Wadden sea coast against microorganisms. Senckenbergiana Marit. 2003, 32, 177–181. [Google Scholar] [CrossRef]
- Lellau, T.F.; Liebezeit, G. Cytotoxic and antitumor activities of ethanolic extracts of salt marsh plants from the lower Saxonian Wadden sea, southern North Sea. Pharm. Biol. 2008, 41, 293–300. [Google Scholar] [CrossRef]
- Geng, D.; Chi, X.; Dong, Q.; Hu, F. Antioxidants screening in Limonium aureum by optimized on-line HPLC-DPPH assay. Ind. Crops Prod. 2015, 67, 492–497. [Google Scholar] [CrossRef]
- Ruiz-Riaguas, A.; Zengin, G.; Sinan, K.I.; Salazar-Mendías, C.; Llorent-Martínez, E.J. Phenolic profile, antioxidant activity, and enzyme inhibitory properties of Limonium delicatulum (Girard) Kuntze and Limonium quesadense Erben. J. Chem. 2020, 2020, 1016208. [Google Scholar] [CrossRef] [Green Version]
- Senizza, B.; Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Yildiztugay, E.; Elbasan, F.; Jugreet, S.; Mahomoodally, M.F.; Lucini, L. Metabolic profiling and biological properties of six Limonium species: Novel perspectives for nutraceutical purposes. Food Funct. 2021, 12, 3443–3454. [Google Scholar] [CrossRef]
- Lin, L.-C.; Chou, C.-J. Flavonoids and phenolics from Limonium sinense. Planta Med. 2000, 66, 382–383. [Google Scholar] [CrossRef]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Faustino, M.V.; Seca, A.M.L.; Silveira, P.; Silva, A.M.S.; Pinto, D.C.G.A. Gas Chromatography—Mass spectrometry profile of four Calendula L. taxa: A comparative analysis. Ind. Crops Prod. 2017, 104, 91–98. [Google Scholar] [CrossRef]
- Costa, H.R.; Simão, I.; Silva, H.; Silveira, P.; Silva, A.M.S.; Pinto, D.C.G.A. Aglaomorpha quercifolia (L.) Hovenkamp & S. Linds a wild fern used in Timorese cuisine. Foods 2021, 10, 87. [Google Scholar]
- Füzfai, Z.; Boldizsár, I.; Molnar-Perl, I. Characteristic fragmentation patterns of the trimethylsilyl and trimethylsilyl-oxime derivatives of various saccharides as obtained by gas chromatography coupled to ion-trap mass spectrometry. J. Chromatogr. A 2008, 1177, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and tripernoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golm Metabolome Database (GMD). Available online: http://gmd.mpimp-golm.mpg.de/ (accessed on 4 May 2022).
- Nič, M.; Jirát, J.; Košata, B.; Jenkins, A.; McNaught, A. IUPAC Compendium of Chemical Terminology; IUPAC: Research Triagle Park, NC, USA, 2009. [Google Scholar]
- Faustino, M.V.; Faustino, M.A.F.; Silva, H.; Silva, A.M.S.; Pinto, D.C.G.A. Lipophilic metabolites of Spartina maritima and Puccinellia maritima involved in their tolerance to salty environments. Chem. Biodivers. 2020, 17, e2000316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Xiong, L.; Li, W.; Zhu, J.-K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1071–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchoff, B.K.; ClaBen-Bockhoff, R. Inflorescences: Concepts, function, development and evolution. Ann. Bot. 2013, 112, 1471–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue metabolic responses to salt stress in wild and cultivated Barley. PLoS ONE 2013, 8, e55431. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Wu, J.; Seliskar, D.M.; Gallagher, J.L. The response of plasma membrane lipid composition in callus of the halophyte Spartina patens (Poaceae) to salinity stress. Am. J. Bot. 2005, 92, 852–858. [Google Scholar] [CrossRef]
- Duarte, B.; Cabrita, M.T.; Marques, J.C.; Caçador, I. Leaf fatty acid remodelling in the salt-excreting halophytic grass Spartina patens along a salinity gradient. Plant Physiol. Biochem. 2018, 124, 112–116. [Google Scholar] [CrossRef]
- Duarte, B.; Cabrita, M.T.; Gameiro, C.; Matos, A.R.; Godinho, R.; Marques, J.C.; Caçador, I. Disentangling the photochemical salinity tolerance in Aster tripolium L.: Connecting biophysical traits with changes in fatty acid composition. Plant Biol. 2017, 19, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the roles of plant sterols in development and stress responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Saldanha, S.N. Dietary phytochemicals, epigenetics, and colon cancer chemoprevention. In Epigenetics of Cancer Prevention; Bishayee, A., Bhatia, D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 8, pp. 205–229. [Google Scholar]
- Scheppele, S.E.; Mitchum, R.K.; Rudolph, C.J.; Kinneberg, K.F.; Odell, G.V. Mass spectra of tocopherols. Lipids 1972, 7, 297–304. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Li, Y.; Chen, X.; Liu, B.; Li, C.; Gong, X.; Ma, F. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 2018, 133, 116–126. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar]
- Rodrigues, M.J.; Monteiro, I.; Castañed-Loaiza, V.; Placines, C.; Oliveira, M.C.; Reis, C.; Caperta, A.D.; Soares, F.; Pousão-Ferreira, P.; Pereira, C.; et al. Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the irrigation salinity. Ind. Crops Prod. 2020, 143, 111930. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Castañed-Loaiza, V.; Monteiro, I.; Pinela, J.; Barros, L.; Abreu, R.M.V.; Oliveira, M.C.; Reis, C.; Soares, F.; Pousão-Ferreira, P.; et al. Metabolomic profile and biological properties of sea lavender (Limonium algarvense Erben) plants cultivated with aquaculture wastewaters: Implications for its use in herbal formulations and food additives. Foods 2021, 10, 3104. [Google Scholar] [CrossRef] [PubMed]
- Faustino, M.V.; Faustino, M.A.F.; Silva, H.; Cunha, Â.; Silva, A.M.S.; Pinto, D.C.G.A. Puccinellia maritima, Spartina maritima, and Spartina patens halophytic grasses: Characterization of polyphenolic and chlorophyll profiles and evaluation of their biological activities. Molecules 2019, 24, 3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- March, R.E.; Miao, X.-S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias, L.S.; Mendez, A.S.L. LC/ESI-MS method applied to characterization of flavonoids glycosides in B. forficate subsp. pruinose. Quim. Nova 2014, 37, 483–486. [Google Scholar] [CrossRef]
- Yuan, T.; Guo, X.-F.; Shao, S.-Y.; An, R.-M.; Wang, J.; Sun, J. Characterization and identification of flavonoids from Bambusa chungii leaves extract by UPLC-ESI-Q-TOF-MS/MS. Acta Chromtagr. 2021, 33, 281–294. [Google Scholar] [CrossRef]
- Simões, M.A.M.; Pinto, D.C.G.A.; Neves, B.M.R.; Silva, A.M.S. Flavonoid profile of the Genista tridentate L., a species used traditionally to treat inflammatory processes. Molecules 2020, 25, 812. [Google Scholar] [CrossRef] [Green Version]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; John Wiley & Sons Ltd.: Chichester, UK, 1999. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Monjotin, N.; Amiot, M.J.; Fleurentin, J.; Morel, J.M.; Raynal, S. Clinical evidence of the benefits of phytonutrients in human healthcare. Nutrients 2022, 14, 1712. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).