Abstract
To feed the ever-increasing population under changing climate scenarios, it is imperative to investigate the role of halophytes, which are equipped with special adaptation mechanisms to cope under extreme conditions of salinity. In the current review, we aimed to report newly identified bioactive secondary metabolites that might play a role in establishing rhizosphere microbe associations, elucidate the negative impacts of salt stress, and direct the growth and yield of halophytes. A systematic approach was developed that deciphers those metabolites involved in regulating the physiological, biochemical, and molecular responses of halophytes to salt stress. The mechanism of salinity tolerance, recruitment of beneficial microbes, and signaling role of secondary metabolites were also discussed. The role of halotolerant rhizobacteria’ secondary metabolites in the physiology and growth parameters of halophytes was also discussed.
1. Introduction
1.1. Impact of Climate Change on Biosaline Agriculture and the Role of Halophytes
Current climate change poses a serious threat to agricultural productivity. A rise in the atmospheric CO2 level, heat waves, elevated temperature, soil drought, and salinity are all major outcomes of climate change that adversely affect the growth of glycophytes [1,2]. Halophyte crops are better alternatives for food, fodder, fiber, fuel, essential oil, and medicines, as they have developed special characteristics to cope with environmental extremes. Halophytes possess a greater degree of tolerance than non-halophyte crops. Some parasitic plants develop succulence when growing on halophytic hosts, and an interesting finding is that halophytes growing in their natural habitats do not show signs of oxidative stress [3]. Halogeton glomeratus is an annual herbaceous and succulent halophyte belonging to the family Chenopodiaceae. It is considered a potential source for oilseed production and also assists in phytoremediation. Exposure of H. glomeratus to long-term salinity and drought stress results in decreased chlorophyll and carotenoid content and inhibition of the photosynthetic rate, transpiration rate, stomatal conductance, water potential, and biomass [4].
Halophyte plants survive in soil where salinity is around 200 mM NaCl [5]. Plant ecologists classify halophytes into three main groups. The first is euhalophytes that dilute salt within their stems or leaves and have a strong capability to endure salt stress. Recretohalophytes secrete salt from their leaves and grow widely around the world, inhabiting inland saline lands and seawater. Pseudo-halophytes can not only hold up ions in roots but also minimize their transport to the shoot parts. On the basis of morphology, they are classified into two groups: excretive and succulent. Based on salt demand and tolerance to NaCl, they can be classified as obligate halophytes, facultative halophytes, and habitat-indifferent halophytes. Obligate halophytes are true halophytes, as they need salt for their growth. Members of the family Chenopodiacea belong to this category. Facultative halophytes generally grow in soil where salts are in quite low concentrations, but they can also grow under saline conditions. Monocots belonging to families Poaceae, Cypraceae, and Juncaceae and a large number of dicotyledons belong to this group. Habitat-indifferent halophytes prefer to live in saline-free soil but can also thrive in saline soil. Examples are Festuca rubra, Agrostis stolonifera, and Juncus bufonius. On the basis of habitat, halophytes are classified into hydrophalophytes (grow in saline water or salt marshes) and xerohalophytes (grow in desert). Salinity induces the release of oleanolic acid in the root exudates of Salicornia, which act as chemoattractants for colonizing halophilic siderophores producing Halomonas anticariensis FP35T [6]. In return, colonization of this bacterium enhances the positive effects on the root length, shoot length, germination, and vigor index of S. hispanica [6].
1.2. Halophytes as Crop Plants
For agricultural sustainability under saline conditions, two possible approaches are needed: (i) improving salt tolerance of cultivated crops, or (ii) domestication of halophytes [5]. The majority of crop plants are non-halophytes. Genetically modified crops have been developed for biosaline agriculture, but this is time consuming and involves many genes possessing various pros and cons and causes various allergic reactions, disrupting natural gene flow and increasing the risk factors of human diseases [7].
The cultivation of halophytes for food, fodder, edible oil, biofuel, and medicine seems to be an alternate option, as they are fully equipped with better salt tolerance properties. Many species of halophytes have the potential to be used as gourmet vegetables and salad, i.e., Aster tripolium, Atriplex hortensis, Beta maritime, Crambe maritima, Crithmum maritimum, Inula crithmoides, Salicornia spp., Salsola soda, and Tetragonia tetragonioides. Some wild edible halophytes are in the genera Bassia, Beta, Cakile, Chenopodium, Plantago, Portulaca, and Suaeda. Atriplex halimus, Salicornica fruticosa, and Cakile maritime accumulate Na+ in their leaves, but the content of this ion is lower compared to other culinary halophytes. These plants can be used as sources of green salt (plant-based salt contains 50 % less Na than common salt) due to their nutritional value [8,9]. The seeds and lignocellulosic biomass of several halophyte genera, i.e., Salicornia, Suaeda, Atriplex, Distchlis, and Batis, have been exploited for the production of biofuel (bioethanol). Suaeda is a high salt tolerant C4 species. This genus ameliorates contaminated saline soil and is also used in food, fodder, medicine, and bioenergy.
A wide group of halophyte species (2500–3000) occur naturally in salt marshes, providing an opportunity to use them as crop plants to combat food security in the future. Seven species of plants, namely Suaeda glauca, Bassia scoparia, H. glomeratus, Kalidium foliatum, Medicago falcata, Atriplex canescens, and Artemisia desertorum, can be used as enrichment materials for Zn and Cu. H. glomeratus has medicinal value in traditional Chinese medicine. Wang et al. [10] identified secondary metabolites that included flavones, flavonols, flavandiols, glucosinolates, isoquinolines, pyridines, indoles, amino acids, lipids, carbohydrates, and ATP-binding cassette transporters. These metabolites regulate osmotic adjustment and modulate the adjustment of membrane lipid action in H. glomeratus. These metabolites also have applications in human cardiovascular diseases, cancers, diabetes, and heart diseases. Economic importance of some halophytes are listed below in Table 1.

Table 1.
Economically important halophytes, their bioactive metabolites, and their role in plants and humans.
1.3. Salt Tolerance Mechanism of Halophytes: Role of Secondary Metabolites
According to Meng et al. [27], halophytes have adapted to thrive under high salinity conditions by secreting salt crystals through salt glands, regulating cellular ion homeostasis and osmotic pressure, detoxifying reactive oxygen species (ROS), and bringing alterations in membrane composition. A diverse group of secondary metabolites is involved in regulating these functions. Functionally, they are classified as osmoprotectants, antioxidants, polyamines, and phytohormones. On the basis of their biosynthetic pathway, they are categorized into (i) phenolic groups (composed of single sugar and benzene rings); (ii) terpenes and steroids; and (iii) N-containing compounds. In this section, an effort has been made to explain the role of secondary metabolites in modulating the salt tolerance strategies of halophytes. The following are adaptations of selected halophytes against salt stress.
1.3.1. Modification in Morphology or Anatomy
Salt stress induces anatomical modifications in roots, stems, and leaves of halophytes. Under high salinity, C. cilliaris conserved water by increasing sclerification in the cortex and pith region and decreasing root thickness with a greater proportion of parenchyma cells, and rich density of vesicular hairs and trichomes on leaves might be essential for water conservation and salt excretion [28]. The pattern of adaptation is species specific and variable among different species, i.e., Salicornia perennans (C3 sp.) showed larger variation in leaf functional traits, both at the level of cell morphology and membrane system (chloroplast envelope and thylakoid); however, Climacoptera crassa (C4 sp.) showed an increase of the mesophyll cell surface, expansion of the interface area between mesophyll and bundle sheath cells, and an increased volume of the latter [29]. These modifications were compensated for the scarce CO2 supply and increased salt concentration.
Chloris gayana is a C4 monocot halophyte that tolerates salinity by accumulating the highest level of Na+ and Cl− in xylem parenchyma and epidermal cells but maintaining the lowest level in photosynthetically active mesophyll and bundle sheath cells. Furthermore, it was lowered more than in respective vacuoles [30]. Several genotypes of Trifolium fragiferum were grown at different salinity levels in controlled conditions. The accumulation of mineralome was both genotypic and organ specific. Maximum Na+ and Cl− were accumulated in leaf petioles followed by leaf blades and stolons; the Zn concentration increased in all plant parts, and the Ca+2 and Mg+2 concentrations decreased [31]. In another study, it was reported that T. fragiferum showed high competitive ability based on better physiological salinity tolerance [32]. Solanum chilense is a halophyte sp. and wild relative of Solanum lycopersicum that can maintain its reproduction despite the accumulation of Na+ in its floral organs [33].
1.3.2. SOS System Activate Salt Glands to Exclude Na from Cells
The sodium overly sensitive system (SOS) plays a crucial role in excluding Na+ from roots and loading to the xylem to regulate ion homeostasis. The SOS system is composed of SOS1, SOS2, and SOS3. Under salt stress, the increase in cytosolic Ca+2 is perceived by the SOS3 component, which interacts and activates SOS2, which finally activates the SOS1 Na/H antiporter. SOS1 then mediates Na+ efflux to the apoplast through active transport driven by the H gradient across the plasma membrane established by H-ATPase.
Recretohalophytes show higher salt tolerance potential than other halophytes due to the presence of salt glands, which secrete excess salt crystals onto the leaf surface to maintain cellular ion homeostasis. The genera of recretohalophytes that show such mechanisms include Limonium and Tamarix. Ions secreted through salt glands include cations i.e., Na+1, K+1, Ca+2, Mg+2, Mn+2, and Fe+2, and anions (Cl−1, Br−1, I−1, SO42−, PO43−, and NO3−1). Suaeda salsa exhibited greater expression of the S. salsa SOS1 gene in roots, which was correlated with the increased exclusion of Na by roots [34].
1.3.3. Succulence Mechanism and Epidermal Bladder Cells
Halophytes exhibit selective Na+1 transport; if the concentration is high, then it is sequestered in vacuoles. Na+1 sequestration is possible though the NHX family Na/H antiporter in the tonoplast and V-H-ATPase, which create a pH gradient between the cytoplasm and vacuole, allowing Na+1 to enter into the vacuole against the electrochemical gradient. The compartmentalization of Na+1 in the vacuoles contributes not only to ion homeostasis and cell turgidity but also protects metabolic enzymes from ion toxicity. Some halophytes exhibit the succulence property as they have thick leaves and stems to accumulate excess Na+1 and Cl−1 in vacuoles, increasing the size of mesophyll cells, and have smaller intercellular spaces to enhance water content and cause a high turgor pressure.
Duan et al. [35] reported that Glycine max showed a metabolic response to salt stress by producing isoleucine, serine, and aspartic acid. Similarly, three halophytes (Frankenia pulverulenta, Atriplex prostrata, and Plantago coronopus) showed increased content of alkaloid derivative, polyamines (spermidine + spermine ratio) against 400 mM NaCl stress [36]. Epidermal bladder cells, also known as salt bladders, contain vacuoles that store excess Na+ and Cl−. They also store ROS-scavenging metabolites and organic osmoprotectants.
1.3.4. Osmotic Adjustment through Osmolytes
Halophytes synthesize osmolytes in response to low external water potential to adjust osmosis and maintain positive turgor pressure. Some examples of osmolytes are proline, aquaporin, and glycine betaine. Salicornia and Sarcocomia species have a long history of human consumption and are ideal models for developing halophyte crops. Osmotic adjustment in both species is related to sequestration of Na+1, Cl−1, Ca+2 in the shoot that might be achieved via production of high levels of glycine betaine [37]. Yadav et al. [38] reported that amino acids, sugars, organic acids, quinidine acid, kaempferol, and melatonin contents were increased under elevated stress conditions (500 mM NaCl and 5% polyethylene glycol) for 24 h.
1.3.5. Regulation of ROS by Secondary Metabolites
Reactive oxygen species (ROS) play a role in the signaling pathway, but their increased accumulation in cells triggers oxidative stress. Halophytes are equipped with powerful antioxidant systems with enzymatic and non-enzymatic components to regulate ROS in the cell. Cakile maritima exhibited stimulation of amino acid biosynthesis and decreased sugar content and GABA to 400 mM NaCl stress at day 20 [39]. Three wild spp. of Suaeda synthesize betacyanin to regulate ROS and avoid oxidative stress [40]. Salinity also induces the production of various flavonoids and phenolic compounds in halophytes against ROS.
Pungin et al. [41] reported that two halophytes, Spergularia marina and Glaux maritima, inhabiting salt marshes showed an increased content of flavonoids (hesperetin, epicatechin, apigenin derivative, luteolin derivative) and protocatechuic acid, which were also correlated with increased antioxidant activity. Linic et al. [42] reported an increased accumulation of phenolic acids, particularly hydroxycinnamic acids, and decreased accumulation of caffeic, salicylic, and 4-coumaric acid in three Brassica spp., upon short-term exposure to 200 mM NaCl stress. Myo-inositol, a derivative of inositol upregulated stress responsive genes in Chenopodium quinoa, is related to antioxidant enzyme activities and increased accumulation of free amino acids and soluble sugars under salt stress [43].
1.3.6. Activation of Hormonal Signaling
Abscisic acid (ABA), jasmonic acid, and ethylene are the main plant hormones produced in response to salt stress. They act as signaling molecules to activate salt tolerance strategies, i.e., halotropism is the tropic movement of Na in the roots, in which halophytes obtain optimum salt in order to maintain growth and development, while non-halophytes show negative halotropism to avoid salt stress. Limonium bicolor is a recretohalophyte with multicellular salt glands. This plant showed positive halotropism, as evidenced from root elongation when treated with 200 mM NaCl stress. IAA was involved in regulating this phenomenon [44]. Phytohormones also maintain the high vitality of anthers and pollens in some halophytes under salt stress. Guo et al. [45] reported a higher number of pollens and pollen activity in Suaeda salsa under saline conditions. GA signals partially act with JA signals via regulation of JA biosynthesis that participate in stamen development under saline conditions.
1.3.7. Maintaining Biogenetics
Haloxylon salicornicum is a xero-halophyte found in saline and arid regions of the world. Panda et al. [46] used GC-QTOF–MS and HPLC-DAD to analyze the expression of metabolites in shoot samples of H. salicornicum treated with 400 mM NaCl. Out of 56 identified metabolites, 47 were significantly changed in response to salt stress. These metabolites were mainly amino acids, organic acids, amines, sugar alcohols, sugars, fatty acids, alkaloids, and phytohormones. Some amino acids, e.g., alanine, phenylalanine, lysine, and tyramine, were upregulated. These metabolites upregulated the tricarboxylic (TCA) cycle by optimal production of coenzymes (NADH and FADH2) and ample quantity of ATP during stress conditions.
Carix pumila was treated with 200, 300, 400, and 500 mM NaCl for 60 h, and leaf samples were analyzed to identify metabolites involved in the regulation of glycolysis, the pentose phosphate pathway, and the TCA cycle [47]. Heat map of hierarchical cluster analysis revealed changes in 39 metabolites, including 16 organic acids (galactaric acid, succinic acid, benzoic acid, fumaric acid, glyceric acid, malonic acid, gluconic acid, glutaric acid, malic acid, hexadecanoic acid, propanoic acid, octadecanoic acid, phosphoric acid, hexacosanoic acid, and hydroxycarbamic acid), 9 amino acids (alanine, proline, aspartic acid, valine, serine, asparagine, glycine, threonine, isoleucine), 9 kinds of sugars (glucose, mannopyranoside, galactopyranose, glucopyranose, galactose, allose, sucrose, fructose, mannose), 3 sugar alcohols (glycerol, galactinol, myo-inositol), and 2 amines (ethanolamine, hydroxylamine) [48].
1.4. Halobacteria Diversity
Halobacterium species are obligate aerobic, rod-shaped archaea enveloped by a single lipid bilayer membrane surrounded by an S-layer made from the cell-surface glycoprotein [7]. They can use amino acids for their growth in aerobic conditions, but they can also grow in an anaerobic environment, given the correct conditions [7]. Halobacteria can be found in highly saline lakes, such as the Great Salt Lake, the Dead Sea, and Lake Magadi. Halobacteria are candidates for a life form present on Mars. These microorganisms develop a thin crust of salt that can moderate some of the ultraviolet light and make it opaque through their photosynthetic pigment bacteriorhodopsin [7].
Halobacteria are able to survive under a wide range of salinities. According to their survival potential, they are classified as halotolerant or halophilic bacteria. Halotolerant bacteria can survive in media up to 25% NaCl, whereas halophile bacteria require salt to grow. They have been isolated from different halophyte plants across the world, i.e., the rhizosphere of Leymus chinensis, Puccinellia tenuiflora, and Suaeda glauca, are highly enriched with the phyla of bacteria Actinobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Gemmatimonadetes, Haloarchaea, and Proteobacteria [49,50,51]. The Firmicutes phylum consisted of genera Bacillus, Virgibacillus, Salincoccus, Marinococcus, Halobacillus, Planococcus, Thalassobacillus, and Salimicrobium; in phylum Actinobacteria, Nocardiopsis was the representative genus; in phylum Proteobacteria, Halomonas, Idiomarina, and Psychrobacter were representative genera [48]. Halobacteria exhibit a highly complex network of diversity than endophytic bacteria and bacteria from bulk soil. Gao et al. [52] reported Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria in the rhizosphere of Salicornica europaea, Kalidium foliatum, and Borsczowia aralocaspica. These bacteria exhibit a variety of mechanisms that have multiple roles, e.g., safeguard halophytes from salt stress, promote their growth, and remediate soil contamination (Figure 1).
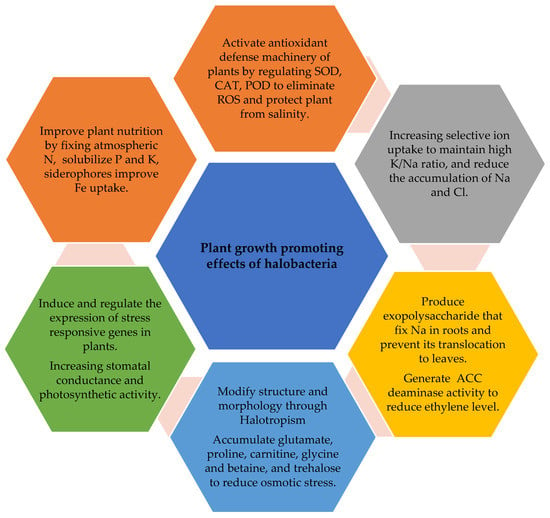
Figure 1.
Mechanism of halobacteria in ameliorating salt stress and improving growth and yield of halophytes under saline conditions.
1.5. Climate Change Modulates Rhizosphere Microbial Community
The main factors governing the diversity of the rhizosphere microbiome are (1) climatic factors (precipitation, temperature, salinity, drought, etc.), (2) soil physicochemical properties (pH, EC, cation exchange capacity, organic C content), and (3) root exudation pattern and composition [45]. Lashini et al. [53] isolated rhizobacteria from the rhizosphere soil of olive trees grown in semiarid and arid areas of Morocco. The strains were 85% halotolerant and 65% thermotolerant, and they were able to overcome high salinity (≥4%) and temperature stress (≥45 °C). They were identified as Bacillus licheniformis, Arthrobacter globiformis, and Bacillus megaterium. About 21% of photosynthetically fixed C is transferred into plant roots and root exudates in the form of soluble sugars, phenolic acids, amino acids, and organic acids. The elevated level of such compounds alters the community composition and structure of active bacteria [54]. The greater input of liable C via root exudates may increase microbial N demand, as competition occurred between microbes and plants for available N. Thus, N dynamics are likely to change under elevated CO2. It also affects the community of N-fixing bacteria [54]. Salinity influences the structure of the rhizosphere microbiome of halophytes. Mukhtar et al. [55] conducted a study to compare the composition of the rhizosphere microbiome of halophytes Urochloa, Kochia, Salsola, and Atriplex inhabiting the moderate and high saline environment of Khewra salt rang, Pakistan, with that of non-halophyte Triticum. Analysis of the 16S rRNA gene showed that Actinobacteria were dominant in the saline soil. Other identified groups were Euryarchaeota, Ignavibacteriae, and Nanohaloarchaeota. Soil physicochemical properties, i.e., pH, EC, and SOC, also influence the microbe community. For example, TS, Cl−, SO42−, HCO3−1, Na+, and Mg+ are the main factors influencing the rhizosphere microbiome structure [56]. Soil pH is one of the key factors shaping halophytic rhizosphere soil bacterial community composition and diversity, and SWC content is a possible factor affecting bacterial community functions [57].
1.6. Secondary Metabolites Recruit Beneficial Rhizospheric Microbes
Root exudates are crucial in modulating the composition and functional diversity of rhizosphere microbes. Mucilage is actively released from the roots, while diffusates are passively released due to osmotic differences between the soil solution and the cell. Both types include organic compounds, which are classified into low and high molecular weight compounds. Heavy molecular weight compounds (i.e., mucilage, cellulose) are not easily used by microbes and make up the majority of C released from roots. Low molecular weight compounds are highly diverse and have a wide array of functions. These consist of organic acids, amino acids, proteins, sugar, phenolics, and other secondary metabolites (including benzoxazinoids, coumarins, flavonoids, indole compounds, and terpenes) that shape the rhizosphere microbiome [58].
Plant secondary metabolites have differential effects on soil microbiota. Two indole metabolites, benzoxazolinone and gramine, produced by different Graminae species, and quercetin, a flavonoid synthesized by many dicot species, revealed significant effects on soil bacteria, with benzoxazolinone showing a predominantly inhibitory effect preventing the accumulation of many predominantly harmful taxa, while gramine and quercetin mostly exert their function by attracting beneficial bacteria [58]. Oleanolic acid possesses chemoattractant properties and is the principal constituent of Salicornia root exudates that have the potential to expand the colonization of salt-tolerant Salicornia hispanica by halophilic siderophore-producing bacteria Halomonas anticariensis FP35T [6].
Terpene is a highly diverse group of secondary metabolites that play an important role in plant microbe interaction. Arabidopsis thaliana root exudates contain triterpenes, namely thalianin, thalianyl medium-chain fatty acid esters (three steps), and arabidin, that are involved in belowground communication and able to directly modulate A. thaliana-specific root bacterial communities in a very selective manner [59]. Xiong et al. [60] reported that organic acids (2-methylbutyric acid, stearic acid, palmitic acid, palmitoleic acid, and oleic acid) found in the root exudates of Limonium sinesis promoted the chemotaxis of the rhizosphere PGPR strain Bacillus flexus KLBMP 4941. Strigolactone is a new class of phytohormone that acts as a signaling molecule in symbiosis to recruit root- and rhizosphere-associated microbiomes. Kim et al. [61] analyzed the bacterial and fungal microbial communities of 16 rice genotypes differing in exudation of root strigolactone. The results showed that structural differences in the exuded strigolactones affected different sets of microbes, i.e., the relative abundance of phosphate solubilizing microbes; Burkholderia, Caballeronia, Paraburkholderia and Acidobacteria were linked to orobanchol strigolactone, whereas 4-deoxyorobanchol was associated with genera Dyella and Umbelopsis [61].
1.7. Halobacteria for Biosaline Agriculture
Halobacteria possess plant growth promoting characteristics, i.e., Reang et al. [62] isolated halotolerant and halophilic bacteria belonging to Halomonas pacifca, H. stenophila, Bacillus haynesii, B. licheniformis, and Oceanobacillus aidingensis. These isolates were able to produce indole acetic acid, solubilized phosphate, and potash and showed N-fixing capacity. There is a growing number of publications on plant growth promotion by halophilic rhizobacteria isolated from the roots of halophyte species [63,64].
Endophytic halophiles (Halomonas and Bacillus) isolated from the roots of halophiles showed maximum salt tolerance up to 4 mM NaCl [65]. When these isolates were used to inoculate alfalfa seedlings, they stimulated plant growth in the presence of 1% NaCl, a level that significantly inhibited the growth of uninoculated plants. Exopolysaccharides (EPS) are commonly produced metabolites by halotolerant bacteria, which exhibit 40–90% extracellular matrix of bacteria under stress conditions [66]. EPS exhibits antioxidant activity and confers tolerance to bacteria against reactive oxygen species. This property can be exploited to alleviate salt stress damage in crops [67].
Robinia pseudoacacia seedlings exposed to VOCs of the JZ-GX1 strain showed increases in biomass, plant development, and lateral root numbers. Additionally, decreases in malondialdehyde, superoxide anion (O2−) and hydrogen peroxide (H2O2) contents and increases in proline contents and superoxide dismutase, peroxidase, and glutathione reductase activities were observed in Acacia leaves. Notably, the sodium–potassium ratio in the roots, stems, and leaves of Acacia exposed to VOCs of the JZ-GX1 strain were significantly lower than those in the control samples [68]. Swiss Chard inoculated with halotolerant PGPR and watered with 85 nmol L−1 NaCl showed higher values of leaf dry weight than control plants [69]. Furthermore, PGPR inoculation reduced electrolyte leakage and Na+ uptake and improved the chlorophyll a fluorescence parameters, chlorophyll, and carotenoid concentrations, stomatal conductance, and antioxidant capacity of Swiss chard. Nijafi Zilaie et al. [70] reported that two halotolerant bacteria Bacillus pumilus and Zhihengliuella halotolerans were able to reduce the content of ascorbic acid, flavonoid, total phenol, proline, malondialdehyde, and catalase activity, and ultimately improved the antioxidant capacity of Haloxylon aphyllum (Table 2).
Ullah and Bano [71] reported that methanolic extracts of Suaeda fruticosa leaves at a concentration of 0.5 mg/mL and 0.35 mg/mL were found active against B. subtilis, S. aureus, E. coli, P. aeruginosa, Candida tropicalis, and Candida albicans. Suaeda fruticosa aqueous extract showed hypoglycemic (41%) and anti-hyperglycemic (53%) effects in the hypercholesterolemic and insulin-resistant sand rats. The endophytes residing in the roots of halophytes have a better adaptation to saline conditions. Hassan et al. [72] demonstrated that application of root powder of Cenchrus ciliaris, a halophytic weed grown in Khewra salt range, induces salt tolerance in wheat, which was mediated by 3 PGPR, B. cereus, P. moraviensis, and Stenotrophomonas maltophilia, inhabiting the roots of the halophytic weed.
The Glutamicibacter genus is a promising candidate for phytoremediation of saline soil due to multiple potential plant growth promotion traits and tolerated a high concentration of NaCl (Table 2). Glutamicibacter halophytocola isolated from roots of Limonium sinesis significantly promoted the growth of L. sinesis under NaCl stress. Inoculation of L. sinesis with this bacteria increased the concentrations of total chlorophyll, proline, antioxidative enzymes, flavonoids, K+, and Ca2+ in the leaves; the concentrations of malondialdehyde (MDA) and Na+ were reduced [73].

Table 2.
Effect of halobacteria on the growth and physiology of some halophytes.
Table 2.
Effect of halobacteria on the growth and physiology of some halophytes.
| Halobacteria | Host Plant | Effect on Host Plant | Reference |
|---|---|---|---|
| Alcaligenes faecalis SBN01 and SBN02 | Wheat | Plant biomass increased at 600 mM NaCl, accumulation of Total Chl, and Carotenoid | [74] |
| Glutamicibacter halophytcola | Tomato seeds | At 200 mM NaCl, Root biomass increased, K+/Na+ ratio changed | [75] |
| Bacillus pumilus FAB10 | Wheat | At 250 mM NaCl, internal CO2 increased, reduced CAT, SOD, and glutathione reductase | [76] |
| Aneurinibacillus aneurinilyticus, Paenibacillus sp. | Phaseolus vulgaris | root (220%) and shoot (425%) biomass and total chlorophyll content (57%) increased | [77] |
| Klebsiella sp. | Avena sativa | Increased relative water content, proline content, electrolyte loss, MDA content in shoots, and decreased SOD and POD | [78] |
| Bacillus tequilensis, Bacillus aryabhattai | Rice | Increase photosynthetic rate, transpiration, stomatal conductance, grain yield | [79] |
| B. marisflavi, Zhihengliuella flava and H. nanhaiensis | Zea mays L. | Root and shoot length increased in 200 to 400 mM NaCl, high accumulation of proline compared with the non-inoculated plants | [80] |
| S. chartreusis, S. tritolerans, and S. rochei | Salicornia bigelovii | enhanced shoot and root dry biomass by 32.3–56.5% and 42.3–71.9%, respectively, 69.1% increase in seed yield | [81] |
| Alcaligenes sp. AF7 | Rice | enhanced the fresh and dry biomass of rice at 170 mM NaCl (EC 9 dS/m) | [82] |
| Halomonas sp. Exo1 | Rice | enhanced germination index upto 83%, enhanced length and weight of vegetative parts | [83] |
| P. plecoglossicida, B. flexus, and B. safensis | Bacopa monnieri | Increased in shoot Na+/K+ ratio, increased the growth, and increased bacoside A yield | [84] |
| Staphylococcus sp., | Salicornia sp. | At 200 MNaCl, plant growth index was increased by 13.9–47.0% | [85] |
| Halomonas, Bacillus | Alfalfa | Increased shoot fresh weight, increased biomass by 2.4 times higher than control | [65] |
2. Conclusions and Future Perspective
Halophytes offer an ecofriendly alternative to meet the food demand of growing populations by re-vegetating saline lands. They can be utilized as an excellent source of biofuel because they contribute to C balance and inhibit greenhouse gas emission problems. Their root exudates contain some metabolites that recruit beneficial microbes in the rhizosphere. Halophytes synthesize phenolics, flavonoids, and a plethora of bioactive compounds that exhibit antioxidant and antimicrobial properties. The microbes associated with halophytes also produce a diverse group of bioactive secondary metabolites that modulate the growth and yield of crops under stress. Halophyte root pieces habilitating halotolerant bacteria may comprise a good source of biofertilizer or a carrier for biofertilizers. Halophytes are tools for understanding the salt tolerance mechanism of plants and for adapting agriculture to climate change. They can be used as cash crops for biosaline agriculture and also for rehabilitation of arid and semiarid regions. Halophytes also act as good phytoremediators for contaminated lands. The need is to have a deeper insight into the cross communication between halophytes and microbe’s secondary metabolites in rhizosphere to understand and decipher mechanism that inculcate biosaline agriculture.
Author Contributions
A.B. conceptualization, Editing, and supervision A. in write up validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andjelkovic, V. Introductory chapter: Climate changes and abiotic stress in plants. In Plant, Abiotic Stress and Responses to Climate Change; IntechOpen: London, UK, 2018; p. 4. [Google Scholar]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 161. [Google Scholar] [CrossRef]
- Flowers, T.J.; Muscolo, A. Introduction to the special issue: Halophytes in a changing world. AoB Plants 2015, 7, plv020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, B.; Li, L.; Zeng, F.; Li, X. Negative effects of long-term exposure to salinity, drought, and combined stresses on halophyte Halogeton glomeratus. Physiol. Plant. 2021, 173, 2307–2322. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.Y.; Yuan, F.; Dong, X.X.; Wang, J.; Wang, B.S. Distribution pattern and salt excretion rate of salt glands in two recretohalophyte species of Limonium (Plumbaginaceae). S. Afr. J. Bot. 2018, 115, 74–80. [Google Scholar] [CrossRef]
- Sampedro, I.; Pérez-Mendoza, D.; Toral, L.; Palacios, E.; Arriagada, C.; Llamas, I. Effects of halophyte root exudates and their components on chemotaxis, biofilm formation and colonization of halophilic bacterium Halomonas anticariensis FP35T. Microorganisms 2020, 8, 575. [Google Scholar] [CrossRef]
- Bawa, A.S.; Anilakumar, K.R. Genetically modified foods: Safety, risks and public concerns—A review. J. Food Sci. Technol. 2013, 50, 1035–1046. [Google Scholar] [CrossRef]
- Hamed, K.B.; Castagna, A.; Ranieri, A.; Garcia-Caparros, P.; Santin, M.; Hernandez, J.A.; Espin, G.B. Halophyte based Mediterranean agriculture in the contexts of food insecurity and global climate change. Environ. Exp. Bot. 2021, 191, 104601. [Google Scholar] [CrossRef]
- Agudelo, A.; Carvajal, M.; Martinez-Ballesta, M.D.C. Halophytes of the Mediterranean Basin-Underutilized Species with the Potential to be Nutritious Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Yao, L.; Ma, Z.; Li, C.; Si, E.; Wang, H. Metabolomics Analyses Provide Insights into Nutritional Value and Abiotic Stress Tolerance in Halophyte Halogeton glomeratus. Front. Plant Sci. 2021, 12, 1336. [Google Scholar] [CrossRef]
- Fu, H.M.; Yin, C.L.; Shen, Z.Y.; Yang, M.H. Flavonoids from the leaves of Apocynum venetum and their anti-inflammatory activity. J. Chem. Res. 2022, 46, 17475198211073871. [Google Scholar] [CrossRef]
- Ali, B.; Musaddiq, S.; Iqbal, S.; Rehman, T.; Shafiq, N.; Hussain, A. The Therapeutic Properties, Ethno pharmacology and Phytochemistry of Atriplex Species: A review. Pak. J. Biochem. Biotechnol. 2021, 2, 49–64. [Google Scholar] [CrossRef]
- Basyuni, M.; Illian, D.N.; Istiqomah, M.A.; Sari, D.P.; Nuryawan, A.; Hasibuan, P.A.Z.; Siregar, E.S. Prominent Secondary Metabolites from Selected Genus of Avicennia Leaves. Maced. J. Med. Sci. 2019, 7, 3765. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Samiullah, A.B. Antimicrobial Potential of Various Extract of Suaeda fruticosa Arial Parts against Some Infectious Microbes Used. Asian Pac. J. Trop. Biomed. 2012, 1, 4. [Google Scholar]
- Sunita, A.; Ganesh, K.; Sonam, M. Screening and evaluation of bioactive components of Cenchrus ciliaris L. by GC-MS analysis. Int. Res. J. Pharm. 2017, 8, 69–76. [Google Scholar]
- Zorin, S.N.; Sidorova, Y.S.; Petrov, N.A.; Perova, I.B.; Malinkin, A.D.; Bokov, D.O.; Mazo, V.K. A new functional food ingredient enriched by Phytoecdisteroids and Polyphenols from quinoa grains (Chenopodium quinoa). Res. J. Pharm. Technol. 2021, 14, 4321–4328. [Google Scholar]
- Tarbeeva, D.V.; Krylova, N.V.; Iunikhina, O.V.; Likhatskaya, G.N.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Fedoreyev, S.A. Biologically active polyphenolic compounds from Lespedeza bicolor. Fitoterapia 2022, 157, 105121. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.; Hegazy, M.M. Essential oil constituents and biological activities of the halophytic plants, Suaeda vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J. Essent. Oil Bear. Plants 2019, 22, 82–93. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and biological properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two wild officinal species of conservation concern in Apulia (Italy). A preliminary survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Mansour, S.; Djebli, N.; Bouakline, H.; Atik-Bekkara, F. Antimicrobial and anti-inflammatory activities of three halophyte plants from Algeria and detection of some biomolecules by HPLC-DAD. Nat. Prod. Res. 2021, 35, 2107–2111. [Google Scholar] [CrossRef]
- Srivarathan, S.; Phan, A.D.T.; Hong, H.T.; Chua, E.T.; Wright, O.; Sultanbawa, Y.; Netzel, M.E. Tecticornia sp. (Samphire): A promising underutilized Australian indigenous edible halophyte. Front. Nutr. 2021, 8, 607799. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Locatelli, M.; Innosa, D.; Cacciagrano, F.; Polesná, L.; Santos, T.F.; Custódio, L. Unravelling the potential of the medicinal halophyte Eryngium maritimum L.: In vitro inhibition of diabetes-related enzymes, antioxidant potential, polyphenolic profile and mineral composition. S. Afr. J. Bot. 2019, 120, 204–212. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Marques, C.; Santos, T.F.; Rodrigues, M.; Custódio, L. Health promoting potential of herbal teas and tinctures from Artemisia campestris subsp. maritima: From traditional remedies to prospective products. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Monteiro, I.; Castañeda-Loaiza, V.; Placines, C.; Oliveira, M.C.; Reis, C.; Caperta, A.D.; Soares, F.; Pousão-Ferreira, P.; Pereira, C.; et al. Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the irrigation salinity. Ind. Crops Prod. 2020, 143, 111930. [Google Scholar] [CrossRef]
- Carius, B.; Silva, H.; Silva, A.M.; Pinto, D.C. Chemical Profiling of Limonium vulgare Mill. Using UHPLC-DAD-ESI/MS2 and GC-MS Analysis. Appl. Sci. 2022, 12, 6384. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Wasim, M.A.; Naz, N. Anatomical adaptations of tolerance to salt stress in Cenchrus ciliaris L., a saline desert grass. JAPS J. Anim. Plant Sci. 2020, 30, 24. [Google Scholar]
- Rozentsvet, O.; Shuyskaya, E.; Bogdanova, E.; Nesterov, V.; Ivanova, L. Effect of salinity on leaf functional traits and chloroplast lipids composition in Two C3 and C4 Chenopodiaceae Halophytes. Plants 2022, 11, 2461. [Google Scholar] [CrossRef]
- Oi, T.; Clode, P.L.; Taniguchi, M.; Colmer, T.D.; Kotula, L. Salt tolerance in relation to elemental concentrations in leaf cell vacuoles and chloroplasts of a C4 monocotyledonous halophyte. Plant Cell Environ. 2022, 45, 1490–1506. [Google Scholar] [CrossRef]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Romanovs, M.; Ievinsh, G. Effect of salinity on growth, ion accumulation and mineral nutrition of different accessions of a crop wild relative legume species, Trifolium Fragiferum. Plants 2022, 11, 797. [Google Scholar] [CrossRef]
- Dumiņs, K.; Andersone-Ozola, U.; Samsone, I.; Elferts, D.; Ievinsh, G. Growth and physiological performance of a coastal species Trifolium fragiferum as affected by a coexistence with Trifolium repens, NaCl treatment and inoculation with rhizobia. Plants 2021, 10, 2196. [Google Scholar] [CrossRef]
- Bigot, S.; Pongrac, P.; Šala, M.; Van Elteren, J.T.; Martínez, J.P.; Lutts, S.; Quinet, M. The Halophyte Species Solanum chilense Dun. Maintains Its Reproduction despite Sodium Accumulation in Its Floral Organs. Plants 2022, 11, 672. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, R.; Ma, Y.; Song, J. Physiological and molecular evidence for Na+ and Cl− exclusion in the roots of two Suaeda salsa populations. Aquat. Bot. 2018, 146, 1–7. [Google Scholar] [CrossRef]
- Duan, X.; Pan, T.; Pu, Y.; Hou, X.; Lei, X.; Gou, M. Characterization of metabolic responses to salt stress in soybean seedling using gas chromatography-mass spectrometry. Emir. J. Food Agric. 2022, 34, 642–649. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Calero, J.; Del Pilar Cordovilla, M. Salinity responses of three halophytes from inland salt marshes of Jaén (southern Spain). Flora 2020, 266, 151589. [Google Scholar] [CrossRef]
- Calone, R.; Mircea, D.M.; González-Orenga, S.; Boscaiu, M.; Lambertini, C.; Barbanti, L.; Vicente, O. Recovery from Salinity and Drought Stress in the Perennial Sarcocornia fruticosa vs. the Annual Salicornia europaea and S. veneta. Plants 2022, 11, 1058. [Google Scholar] [CrossRef]
- Yadav, S.; Elansary, H.O.; Mattar, M.A.; M-Elhindi, K.; Alotaibi, M.A.; Mishra, A. Differential accumulation of metabolites in Suaeda sp. provides new insights into abiotic stress tolerance in C4 halophytic species in elevated CO2 conditions. Agronomy 2021, 11, 31. [Google Scholar] [CrossRef]
- Arbelet-Bonnin, D.; Blasselle, C.; Palm, E.R.; Redwan, M.; Ponnaiah, M.; Laurenti, P.; Meimoun, P.; Gilard, F.; Gakière, B.; Mancuso, S.; et al. Metabolism regulation during salt exposure in the halophyte Cakile maritima. Environ. Exp. Bot. 2020, 177, 104075. [Google Scholar] [CrossRef]
- Ibraheem, F.; Al-Zahrani, A.; Mosa, A. Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity. Plants 2022, 11, 537. [Google Scholar] [CrossRef]
- Pungin, A.; Lartseva, L.; Loskutnikova, V.; Shakhov, V.; Krol, O.; Popova, E.; Volodina, A. The Content of Certain Groups of Phenolic Compounds and the Biological Activity of Extracts of Various Halophyte Parts of Spergularia marina (L.) Griseb. and Glaux maritima L. at Different Levels of Soil Salinization. Plants 2022, 11, 1738. [Google Scholar] [CrossRef]
- Linic, I.; Samec, D.; Gruz, J.; Vujcic Bok, V.; Strnad, M.; Salopek-Sondi, B. Involvement of phenolic acids in short-term adaptation to salinity stress is species-specific among Brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Al-Mushhin, A.A.; Qari, S.H.; Fakhr, M.A.; Alnusairi, G.S.; Alnusaire, T.S.; ALrashidi, A.A.; Soliman, M.H. Exogenous Myo-Inositol Alleviates Salt Stress by Enhancing Antioxidants and Membrane Stability via the Upregulation of Stress Responsive Genes in Chenopodium quinoa L. Plants 2021, 10, 2416. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Geng, F.; Dong, X.; Yuan, F.; Wang, B. Sodium is the critical factor leading to the positive halotropism of the halophyte Limonium bicolor. Plant Biosyst. 2019, 153, 544–551. [Google Scholar] [CrossRef]
- Guo, J.; Dong, X.; Li, Y.; Wang, B. NaCl treatment markedly enhanced pollen viability and pollen preservation time of euhalophyte Suaeda salsa via up regulation of pollen development-related genes. J. Plant Res. 2020, 133, 57–71. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Parida, A.K. Unraveling salt responsive metabolites and metabolic pathways using non-targeted metabolomics approach and elucidation of salt tolerance mechanisms in the xero-halophyte Haloxylon salicornicum. Plant Physiol. Biochem. 2021, 158, 284–296. [Google Scholar] [CrossRef]
- Wang, S.W.; Xu, F.F.; Guo, L.J.; He, T.T.; Li, X.L.; Yuan, L.; Liu, H.T. Different responses of the halophyte Carex pumila to salt stress. Biol. Plant. 2020, 64, 519–528. [Google Scholar] [CrossRef]
- Colchado-López, J.; Rougon-Cardoso, A.; Vélez, P.; Rosas, U. Meta-analysis of community composition patterns of halophyte and xerophyte rhizosphere associated bacteria. Rhizosphere 2022, 24, 100588. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, F.; Cheng, Z.; Wang, H. Drivers on the Halophytes’ Rhizosphere Bacteria Community and Functions in North China Salinized Areas. Research Square, 22 February 2021. [Google Scholar] [CrossRef]
- Ruginescu, R.; Gomoiu, I.; Popescu, O.; Cojoc, R.; Neagu, S.; Lucaci, I.; Batrinescu-Moteau, C.; Enache, M. Bioprospecting for Novel Halophilic and Halotolerant Sources of Hydrolytic Enzymes in Brackish, Saline and Hypersaline Lakes of Romania. Microorganisms 2020, 8, 1903. [Google Scholar] [CrossRef]
- Gao, L.; Huang, Y.; Liu, Y.; Mohamed, O.A.A.; Fan, X.; Wang, L.; Ma, J. Bacterial community structure and potential microbial coexistence mechanism associated with three halophytes adapting to the Extremely hypersaline environment. Microorganisms 2022, 10, 1124. [Google Scholar] [CrossRef]
- Lahsini, A.I.; Sallami, A.; Obtel, M.; Douira, A.; El Modafar, C.; Benkerroum, N.; Filali-Maltouf, A. Isolation and molecular identification of an indigenous abiotic stress-tolerant plant growth-promoting rhizobacteria from the rhizosphere of the olive tree in southern Morocco. Rhizosphere 2022, 23, 100554. [Google Scholar] [CrossRef]
- Rosado-Porto, D.; Ratering, S.; Cardinale, M.; Maisinger, C.; Moser, G.; Deppe, M.; Schnell, S. Elevated atmospheric CO2 modifies mostly the metabolic active rhizosphere soil microbiome in the Giessen FACE experiment. Microb. Ecol. 2022, 83, 619–634. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mirza, B.S.; Mehnaz, S.; Mirza, M.S.; Mclean, J.; Malik, K.A. Impact of soil salinity on the microbial structure of halophyte rhizosphere microbiome. World. J. Microbiol. Biotechnol. 2018, 34, 1–17. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, J.; Xue, J. Salinity drives shifts in soil microbial community composition and network complexity along vegetation community succession in coastal tidal flats. Estuar. Coast. Shelf Sci. 2022, 276, 108005. [Google Scholar] [CrossRef]
- Korenblum, E.; Massalha, H.; Aharoni, A. Plant–microbe interactions in the rhizosphere via a circular metabolic economy. Plant Cell 2022, 34, 3168–3182. [Google Scholar] [CrossRef]
- Schütz, V.; Frindte, K.; Cui, J.; Zhang, P.; Hacquard, S.; Schulze-Lefert, P.; Dörmann, P. Differential impact of plant secondary metabolites on the soil microbiota. Front. Microbiol. 2021, 12, 1267. [Google Scholar] [CrossRef]
- Huang, A.C.; Jiang, T.; Liu, Y.X.; Bai, Y.C.; Reed, J.; Qu, B.; Goossens, A.; Nutzmann, H.W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 10, eaau6389. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Li, X.W.; Wang, T.T.; Gong, Y.; Zhang, C.M.; Xing, K.; Qin, S. Root exudates-driven rhizosphere recruitment of the plant growth-promoting rhizobacterium Bacillus flexus KLBMP 4941 and its growth-promoting effect on the coastal halophyte Limonium sinense under salt stress. Ecotoxicol. Environ. Saf. 2020, 194, 110374. [Google Scholar] [CrossRef]
- Kim, B.; Westerhuis, J.A.; Smilde, A.K.; Flokova, K.; Suleiman, A.K.; Kuramae, E.E.; Zancarini, A. Effect of strigolactones on recruitment of the rice root-associated microbiome. FEMS Microbiol. Ecol. 2022, 98, fiac010. [Google Scholar] [CrossRef]
- Reang, L.; Bhatt, S.; Tomar, R.S.; Joshi, K.; Padhiyar, S.; Vyas, U.M.; Kheni, J.K. Plant growth promoting characteristics of halophilic and halotolerant bacteria isolated from coastal regions of Saurashtra Gujarat. Sci. Rep. 2022, 12, 4699. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; Nielsen, B.L. Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Arora, N.K. Plant growth-promoting rhizospheric microbes for remediation of saline soils. In Phyto and Rhizo Remediation; Arora, N.K., Kumar, N., Eds.; Springer: Singapore, 2019; Volume 9, pp. 121–146. [Google Scholar]
- Xiong, Y.; Ju, X.; Li, X.; Gong, Y.; Xu, M.; Zhang, C.; Qin, S. Fermentation conditions optimization, purification, and antioxidant activity of exopolysaccharides obtained from the plant growth-promoting endophytic Actinobacteria Glutamicibacter halophytocola KLBMP 5180. Int. J. Biol. Macromol. 2020, 153, 1176–1185. [Google Scholar] [CrossRef]
- Li, P.S.; Kong, W.L.; Wu, X.Q.; Zhang, Y. Volatile organic compounds of the plant growth-promoting rhizobacteria JZ-GX1 enhanced the tolerance of Robinia pseudoacacia to salt stress. Front. Plant Sci. 2021, 12, 2303. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Romano-Rodríguez, E.; Mesa-Marín, J.; Sola-Elías, C.; Mateos-Naranjo, E. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization. Agronomy 2022, 12, 468. [Google Scholar] [CrossRef]
- Najafi Zilaie, M.; Mosleh Arani, A.; Etesami, H.; Dinarvand, M. Improved salinity and dust stress tolerance in the desert halophyte Haloxylon aphyllum by halotolerant plant growth-promoting rhizobacteria. Front. Plant Sci. 2022, 13, 2625. [Google Scholar] [CrossRef]
- Ullah, S.; Bano, A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015, 61, 307–313. [Google Scholar] [CrossRef]
- Hassan, T.U.; Bano, A.; Naz, I. Halophyte root powder: An alternative biofertilizer and carrier for saline land. Soil Sci. Plant Nutr. 2018, 64, 653–661. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Zhang, Y.J.; Wang, T.T.; Xiong, Y.W.; Xing, K. Diversity of bacterial microbiota of coastal halophyte Limonium sinense and amelioration of salinity stress damage by symbiotic plant growth-promoting actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 2018, 84, e01533-18. [Google Scholar] [CrossRef]
- Babar, M.; Rasul, S.; Aslam, K.; Abbas, R.; Manzoor, I.; Hanif, M.K.; Naqqash, T. Mining of halo-tolerant plant growth promoting rhizobacteria and their impact on wheat (Triticum aestivum L.) under saline conditions. J. King Saud Univ. Sci. 2021, 33, 101372. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Gong, Y.; Li, X.W.; Chen, P.; Ju, X.Y.; Zhang, C.M.; Yuan, B.; Lv, Z.P.; Xing, K.; Qin, S. Enhancement of Growth and Salt Tolerance of Tomato Seedlings by a Natural Halotolerant Actinobacterium Glutamicibacter halophytocola KLBMP 5180 Isolated from a Coastal Halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth Stimulation and Alleviation of Salinity Stress to Wheat by the Biofilm Forming Bacillus pumilus Strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. Confers Enhanced Tolerance to Salinity and Plant Growth Promotion in Oat Seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of Salt-Tolerant Plant Growth-Promoting Rhizobacteria and the Effect on Growth and Yield of Saline-Affected Rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef]
- Aslam, F.; Ali, B. Halotolerant Bacterial Diversity Associated with Suaeda fruticosa (L.) Forssk. Improved Growth of Maize under Salinity Stress. Agronomy 2018, 8, 131. [Google Scholar] [CrossRef]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.H.I.; Ayyash, M.M.; El-Keblawy, A.; El-Tarabily, K.A. Halotolerant marine rhizosphere-competent Actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. Biotech 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas rhizobacteria of Avicennia marina of Indian Sundarbans promote rice growth under saline and heavy metal stresses through exopolysaccharide production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Pankaj, U.; Singh, D.N.; Mishra, P.; Pooja, G.A.U.R.; Babu, C.V.; Shanker, K.; Verma, R.K. Autochthonous halotolerant plant growth-promoting rhizobacteria promote bacoside A yield of Bacopa monnieri (L.) Nash and phytoextraction of salt-affected soil. Pedosphere 2020, 30, 671–683. [Google Scholar] [CrossRef]
- Komaresofla, B.R.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved growth and salinity tolerance of the halophyte Salicornia sp. by co–inoculation with endophytic and rhizosphere bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).