Abstract
The pyrolysis of pine sawdust was carried out in a fixed bed reactor heated from 30 °C to a maximum of 700 °C in atmospheric nitrogen and pressurized hydrogen (5 MPa). The yield, elemental composition, thermal stability, and composition of the two pyrolysis bio-oils were analyzed and compared. The result shows that the oxygen content of the bio-oil (17.16%) obtained under the hydrogen atmosphere was lower while the heating value (31.40 MJ/kg) was higher than those of bio-oil produced under nitrogen atmosphere. Compounds with a boiling point of less than 200 °C account for 63.21% in the bio-oil at pressurized hydrogen atmosphere, with a proportion 14.69% higher than that of bio-oil at nitrogen atmosphere. Furthermore, the hydrogenation promoted the formation of ethyl hexadecanoate (peak area percentage 19.1%) and ethyl octadecanoate (peak area percentage 15.42%) in the bio-oil. Overall, high pressure of hydrogen improved the bio-oil quality derived from the pyrolysis of pine biomass.
1. Introduction
Biomass is one of the most potential renewable resources [1], which can replace fossil feedstocks to produce chemicals [2]. Biomass is renewable and widely distributed, causing low pollution [3]. The production of various chemicals from biomass is essential to alleviate the resource shortage and environmental problems currently [4].
In recent years, much research has focused on pyrolysis, gasification, liquefaction, combustion, and carbonization of biomass [5]. Among these research topics, pyrolysis has attracted extensive attention [6]. With a low-cost and continuous production process, pyrolysis usually costs energy and produces renewables [7].
Bio-oil, the liquid product of pyrolysis of biomass, has some similar properties to petroleum. The composition of bio-oil obtained from pyrolysis is very complex, and it has characteristics of high moisture content, oxygen content, and acidity, low heating value, poor thermal stability, and low content of each component. The method of refining this pyrolysis bio-oil to obtain specific chemicals is very inefficient [8]. The pyrolysis under different atmospheres and pressures can regulate the thermochemical conversion process of biomass, promote some reactions, and inhibit other reactions to realize the high enrichment of high value-added chemicals in pyrolysis bio-oil [9]. Then, chemicals with high purity are obtained through conventional separation treatment, and the remaining part of pyrolysis bio-oil can also be upgraded into fuel [10]. Therefore, pyrolysis could produce chemicals or fuels, thus remarkably increasing the utilization rate of biomass and improving the economy of the whole process [11].
At present, hydrogasification of biomass involves a reaction under high temperature and pressure. The energy utilization rate of hydrogasification of biomass is high, and its thermal efficiency is close to 80%. Hydrogasification of biomass is an ideal technology that can convert coal to natural gas, and this method has attracted much researcher interest [12]. In recent years. The development and application of biomass conversion technologies (e.g., pyrolysis and catalytic pyrolysis) have also attracted extensive attention all over the world [13]. Among various biomass technologies, thermochemical conversion and hydro pyrolysis are considered relatively new methods to convert biomass into substitutes [14]. During hydrogasification of biomass, hydrogen may inhibit the re-polymerization of free radical fragments into carbon. Therefore, compared with ordinary pyrolysis, hydropyrolysis has the advantages of high carbon content, hydrocarbon yield, and bio-oil quality [15]. In addition, the hydropyrolysis process is self-heating and does not require additional heat, which is easier to expand to the commercial scale [16].
In some studies, the pyrolysis bio-oil was upgraded through catalytic technology [17]. Zheng et al. [18] studied the hydrogasification of biomass and found that hydrogenolysis can directly generate high-quality and high-yield pyrolysis bio-oil and gaseous hydrocarbons without catalysts. The obtained pyrolysis bio-oil has low oxygen content and a high yield of light aromatics. The yields of benzene, toluene, and xylene (BTX) can reach 2.6%.
In recent years, the study on pyrolysis mostly concentrated in the nitrogen atmosphere, and limited studies have focused on the pyrolysis of biomass for bio-oil production in the hydrogen atmosphere. In this paper, the pyrolysis bio-oil in a hydrogen atmosphere at a certain temperature and pressure was analyzed, characterized, and compared with the results of pyrolysis bio-oil in a nitrogen atmosphere. The pyrolysis mechanism of bio-oil in a hydrogen atmosphere is discussed, and the composition of the obtained pyrolysis bio-oil is preliminarily explored, thus providing support for the quality improvement of pyrolysis bio-oil from nuclear energy. This work improves the bio-oil quality of pine biomass pyrolysis in pressurized hydrogen gas and is an interesting experimental scheme for wood pyrolysis.
2. Materials and Methods
2.1. Experimental Raw Materials
Pine wood was from pine trees converted to sawdust and was between 20 and 100 mesh by grinding through sieves. The crushed raw materials were placed in a constant temperature drying oven at 110 °C for 48 h to reduce the influence of moisture in biomass on the heating value of pyrolysis bio-oil. It was used as the experimental feedstock. The industrial and elemental analysis of pine sawdust is shown in Table 1. It shows the average of the results of three samples.

Table 1.
Elemental and industrial analysis of pine sawdust.
2.2. Experimental Device and Process
The experimental conditions of this experiment are from room temperature of 30 °C to pyrolysis temperature of 700 °C, hydrogen atmosphere pressure of 5 MPa, and flow rate of 50 mL/min. It is based on the experimental parameters of two-stage hydrogenolysis of pine leaves by Zheng et al. [19].
The experimental process is shown in Figure 1. Before the experiment, 2 g biomass raw material was wrapped with gauze and put into the pyrolysis reactor. After passing through the drying device, hydrogen entered the reactor at a flow rate of 50 mL/min from bottom to top for purging for 5 min, and then the pressure was slowly increased to 5 MPa. After ensuring that there was no gas leakage in the system, the pyrolysis reactor heated from room temperature 30 °C to a maximum of 700 °C. There are thermocouples at the top and bottom of the reactor for temperature measurement. At the same time, the pipes were wrapped from the inlet and outlet of the reactor to the condenser with a heating belt (300 °C) to prevent the liquid from condensing in advance. Multistage condensation is the gradient condensation of normal temperature, ice saturated salt water, and liquid nitrogen. When the temperature of the pyrolysis furnace reached 700 °C, the reaction was stopped, and the yield of the liquid product was determined by weighing the condensing tube before and after the experiment. The liquid product was washed with acetone three times, dissolved in a test tube for storage, sealed with candle oil, and placed in a refrigerator for refrigeration. After the reaction, the reactor and pipeline were cleaned with dichloromethane three times, and the pyrolysis reactor was calcined at 700 °C in air for 2 h for the next experiment.
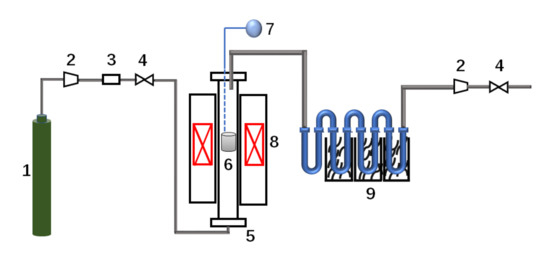
Figure 1.
Schematic diagram of experimental device. 1. Gas cylinder; 2. Pressure-regulating device; 3. Gas drying device; 4. Mass flowmeter; 5. Pyrolysis reactor; 6. Biomass; 7. Explosion-proof alarm device; 8. Heating device; 9. Condenser pipe.
During the pyrolysis experiment in nitrogen, after purging the reactor with nitrogen for 5 min, the weighed biomass samples were placed into the pyrolysis reactor to maintain the nitrogen flow rate, and the pyrolysis furnace was heated to 700 °C at a certain rate under normal pressure. The solid and liquid products were collected, and the steps were consistent with the hydropyrolysis experiment.
2.3. Detection and Characterization of Bio-Oil
2.3.1. Calculation of Yield
After biomass pyrolysis, the product was divided into the gas phase, liquid phase, and solid phase. The gas phase is directly discharged through the gas path. The liquid phase is the oil phase. The solid phase exists in the gauze in the form of coke. The yield was determined by weighing before and after the reaction. The yield of the solid phase and liquid phase is calculated as follows:
where MS is the solid mass (g), ML is the liquid mass (g), WS is the solid phase yield (%), WL is the liquid phase yield (%), and Mm is mass of biomass raw materials.
2.3.2. Elemental Analysis, Determination of H/C, and Heating Value
Carbon, hydrogen, nitrogen, and sulfur in two pyrolysis bio-oil were determined by elemental analysis. Firstly, excess anhydrous copper sulfate was added to remove water, and then copper sulfate was separated by filtration. The bio-oil acetone solution, after water removal, was placed in rotary evaporation at 60 °C to remove acetone. The contents of carbon, hydrogen, nitrogen, and sulfur are all dry basis elements. The oxygen content is obtained by subtraction, and the sulfur content is almost negligible. H/C is the molar ratio of hydrogen to carbon, and the equtaion is as follows:
N1, N2, and N3 are the total moles of hydrogen, oxygen, and carbon in the bio-oil, respectively.
H/C = (N1 − 2 × N2)/N3
The heating value of pyrolysis bio-oil is calculated by Dulong–Berthelot formula [20], as shown in Equation (4):
HHV = 0.338 × C + 1.428 × (H − O/8) + 0.095 × S
HHV is the high heating value (MJ/kg), C is the carbon content, H is the hydrogen content, and O is the oxygen content.
2.3.3. Thermogravimetry (TG) Analysis
TG was performed at a thermogravimetry analyzer (SDTQ 600). Nitrogen was used as a carrier gas, the temperature range was 30–700 °C, and the heating rate was 15 °C/min.
2.3.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The components of pyrolysis bio-oil in two atmospheres were analyzed by GC-MS. The inlet temperature of gas chromatography was 320 °C, the carrier gas was helium, the carrier gas flow rate was 1.0 mL/min, the split ratio was 35:1, the temperature raised to 300 °C at 5 °C/min and maintained for 5 min at 300 °C, the interface temperature was 300 °C, the ion source temperature was 260 °C, the electron energy of EI source was 70 EV, and the scanning range was 20~400 amu.
3. Results and Discussion
3.1. Yield of Pyrolysis Products
The yield of pyrolysis bio-oil is an important index to determine the pyrolysis effect. Based on material balance, the experiments were repeated three times, and the average values of the three experiments were used. The error of pyrolysis bio-oil yield was controlled within ±2.00%. The yield of pyrolysis bio-oil in the hydrogen atmosphere was 59.23%, which was higher than that (40.88%) in the nitrogen atmosphere. Considering that the volatile free radicals and molecular fragments produced by the thermal decomposition of biomass in a hydrogen atmosphere are stabilized in combination with hydrogen, it easily escapes from the inside of the particles, thus remarkably improving the yield of pyrolysis bio-oil. In addition, in a nitrogen atmosphere, the pyrolysis bio-oil is a dark brown liquid with a unique smoke smell and free fluidity [21]. In a hydrogen atmosphere, the color of pyrolysis bio-oil is dark yellow and clearer because hydrodeoxygenation and hydrogenation occur in biomass pyrolysis under hydrogen.
The solid product of pyrolysis in the nitrogen atmosphere is 26.02%, while the solid product in the hydrogen atmosphere is 20.41%. In the raw material, the lignin methoxy group of pine wood can be decomposed into small molecular free radicals to further stabilize the macromolecular fragments produced during lignin pyrolysis to prevent them from polymerizing into carbon [22]. In a hydrogen atmosphere, the decomposition of the lignin methoxy group is inhibited from producing a large amount of polymerized carbon. Moreover, the methanation reaction of pyrolytic carbon is promoted in the hydrogen atmosphere to improve the carbon conversion rate. The oxygen in biomass is also removed in the form of water, thus reducing the conversion of carbon into carbon monoxide or carbon dioxide and improving the utilization rate of carbon.
3.2. Elemental Analysis of Pyrolysis Oil
The oxygen content in pyrolysis oil is an important index of the reaction pyrolysis effect. The elemental analysis of pyrolysis oil under different atmospheres is shown in Figure 2. The experiments were conducted in triplicate for each condition, and the mean values were used.
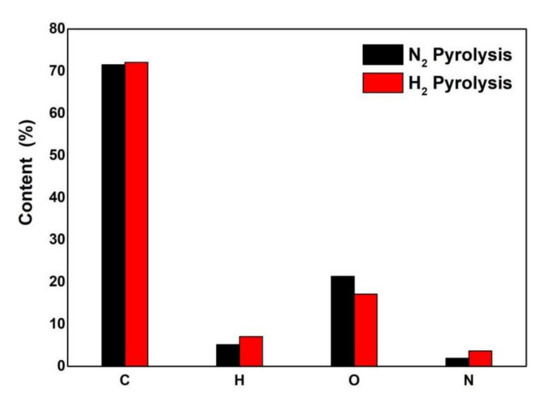
Figure 2.
Element content of pyrolysis bio-oil under different atmospheres.
The oxygen content of pyrolyzed bio-oil in the hydrogen atmosphere was 17.16%, which was much lower than that in the nitrogen atmosphere. The most direct effect of hydrogenation is the reduction in oxygen content in pyrolyzed bio-oil caused by a large amount of oxygen bridge (C–O–C) and hydroxyl (O–H) bonds present in biomass. Oxygen bridge bonds and hydroxyl groups were destroyed during pyrolysis in the hydrogen atmosphere, decreasing the oxygen content of pyrolyzed bio-oil. Moreover, pyrolysis in the hydrogen atmosphere is focused on the hydrodeoxygenation reaction. The hydrogen element content was 7.06%, which was 5.17% higher than that in a nitrogen atmosphere because, in a hydrogen atmosphere, hydrogen combines with pyrolysis steam, while some enter the liquid product to form more stable species. The carbon content is basically unchanged from 71.55% to 72.11%, and biomass carbon content is not easy to lose. The nitrogen content increased from 1.94% to 3.66%, which was higher than that in the nitrogen atmosphere, indicating that the nitrogen volatiles in biomass samples are likely to combine with hydrogen to form stable nitrides (amines) in the hydrogen atmosphere. The subsequent GC-MS results also confirmed the formation of amines.
3.3. H/C and Heating Value
The H/C of pyrolysis bio-oil in a hydrogen atmosphere is 0.098, which is 36% higher than that in a nitrogen atmosphere, confirming the improved fuel performance of pyrolysis bio-oil in a hydrogen atmosphere. Besides, the heating value of pyrolysis bio-oil in a nitrogen atmosphere is 27.75 MJ/kg, while the heating value of pyrolysis bio-oil in a hydrogen atmosphere reached 31.40 MJ/kg. Hydrogen can consume more oxygen in pyrolysis bio-oil at high temperatures and pressure. The addition of more hydrogen to pyrolysis bio-oil increases the chemical properties and heating value of pyrolysis bio-oil.
3.4. TGA Analysis of Bio-Oil
The thermal stability of pyrolysis bio-oil was determined, and the results are shown in Figure 3. The experiments were conducted in triplicate for each condition, and the mean values were used. The overall weight loss rate of pyrolysis bio-oil in the hydrogen atmosphere is higher than that in the nitrogen atmosphere. The maximum weight loss rate of pyrolysis bio-oil in hydrogen and nitrogen atmosphere was observed at 0–200 °C. The weight loss percentage of bio-oil in a nitrogen atmosphere at 0–150 °C is higher than that in a hydrogen atmosphere, indicating that the amount of bio-oil substances in a nitrogen atmosphere is more than that in a hydrogen atmosphere. At 150–300 °C, the weight loss rate in the hydrogen atmosphere is very obvious, which is more than that in the nitrogen atmosphere. The starting point of mass loss of pyrolysis bio-oil in a hydrogen atmosphere is slightly high, but above 180 °C, its characteristic curve shifted to the low-temperature zone, and the weight loss rate is faster than that in a nitrogen atmosphere. This phenomenon occurs mainly because the pyrolysis of bio-oil esters in the hydrogen atmosphere improves the compatibility of each component of the system, and aldehyde and sugar polymerize in the system in the hydrogen atmosphere. Figure 4 shows that the maximum weight loss rate of pyrolysis oil under hydrogen is 146 °C under hydrogen and 100 °C under nitrogen atmosphere, indicating that the loss of pyrolysis bio-oil components under hydrogen atmosphere is the most at 146 °C, which is 46 °C higher than that of pyrolysis bio-oil under nitrogen. This finding is consistent with the results in Figure 3.
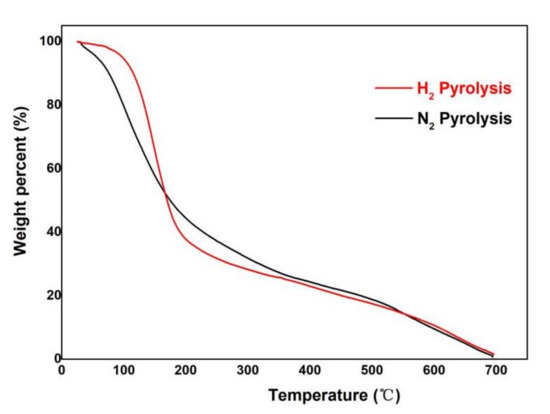
Figure 3.
TG of pyrolysis oil under different atmospheres.
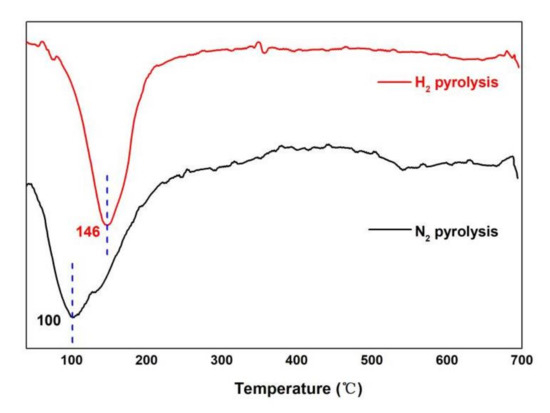
Figure 4.
DTG of pyrolysis oil under different atmospheres.
TG analysis can be used to estimate the boiling point range of pyrolysis bio-oil [23]. According to the TG analysis results, the boiling point range distribution of pyrolysis bio-oil is shown in Figure 5. The results show that the content of compounds with a boiling point of 0–200 °C in the pyrolysis bio-oil obtained in a hydrogenation atmosphere is 63.21%, while the content of compounds with a boiling point of 0–200 °C of pyrolysis bio-oil in a nitrogen atmosphere is 55.11%. Therefore, the low boiling point fraction of pyrolysis bio-oil can be increased in a hydrogen atmosphere, which is conducive to the fueling of pyrolysis bio-oil. The fraction range of gasoline is 30–220 °C. Therefore, pyrolysis bio-oil in the hydrogen atmosphere is conducive to the formation of low boiling point fractions.
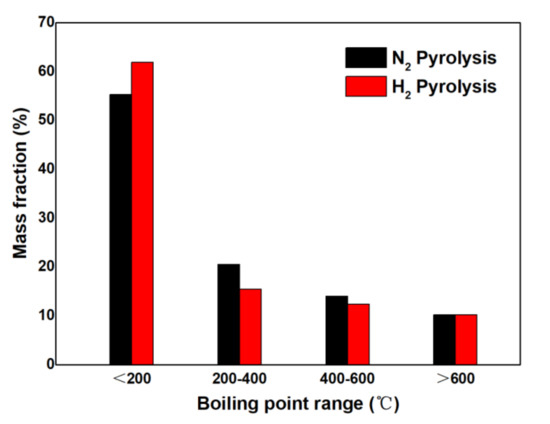
Figure 5.
Weight loss rate of pyrolysis bio-oils in different atmospheres at different temperature ranges.
3.5. GC-MS Analysis of the Bio-Oil
The characteristics of pyrolysis bio-oils in nitrogen and hydrogen atmosphere were further compared. The pyrolysis oil under different atmospheres was analyzed by GC-MS (The peak area of bio-oil components is the percentage of the peak area based on the total components identified), and the results are shown in Figure 6 and Table 2. It should be noted that GC-MS analysis cannot identify all the compounds present in the bio-oil because only volatile compounds could pass through the GC column and be detected. The compounds with a boiling point lower than 400 °C accounts for about 80% of the bio-oil (Figure 5); based on the analysis conditions of GC-MS used, about 80% of the bio-oil components may be detected via GC-MS.
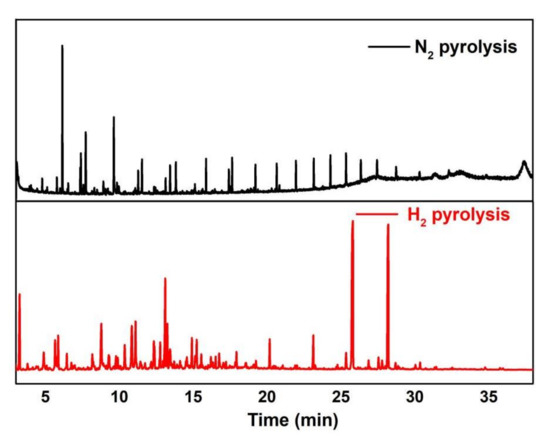
Figure 6.
GC-MS analysis of the pyrolysis bio-oils under different atmospheres.

Table 2.
Composition of bio-oils in different atmospheres.
Based on the analysis and comparison of GC-MS results, the composition of pyrolysis bio-oil obtained from pine wood in a hydrogen atmosphere is significantly different from that in a nitrogen atmosphere. The sum of ethyl acetate (peak area percentage 19.1%) and methyl octadecanoate (peak area percentage 15.42%) generated in 25.80 and 28.19 min accounts for approximately 34.5% of the peak area of pyrolysis bio-oil in hydrogen. This ester has good flammability and is more conducive to fuel ignition and complete oxidation. In addition, long-chain esters are mainly used as lubricants, anti-adhesion agents, and flow aids. It can be used as flow aid direct tablet pressing, filter aid, clarifier and defoamer, and as suspension aid and thickener or liquid preparations. The alkali and water resistance are improved by adding coatings and painting [8]. In industry, the industrial prices of ethyl hexadecanoate and methyl octadecanoate in 2020 are 18,000 and 10,500 yuan/ton, respectively. Therefore, the pyrolysis bio-oil prepared via hydropyrolysis has a certain industrial value. Generally, it is not economically feasible to recover such compounds because of the difficulty of separating such compounds from bio-oil. Accordingly, an economical and effective method will be developed to separate and recover these compounds in the next experiment for an acceptable production cost in the current market.
Table 2 shows that the first three kinds of pyrolysis bio-oil with high peak area under nitrogen atmosphere are phenol, 2-methylphenol, and azulene, but the same substances also appear in pyrolysis bio-oil under hydrogen atmosphere, and the peak area at the corresponding time decreases. The peak area percentage of phenol decreased by 68.34% from 12.73% to 4.03%, the peak area percentage of 2-methylphenol decreased by 84.68% from 6.79% to 1.04%, and the peak area percentage of azulene decreased by 42.60% from 7.84% to 4.50%. The top three peak area percentages of pyrolysis bio-oil in hydrogen atmosphere are methyl octadecanoate (15.42%), aethyl cetate (19.1%), and toluene (9.04%). In a hydrogen atmosphere, the free radicals of hydrogen combine with the phenylpropane unit of lignin, the main raw material in pine, and destroy the connection of different ether bonds or C-C bonds. In this way, lignin pyrolysis is not easy to cut into phenolic products, thus decreasing the phenol peak area. The appearance of methyl octadecanoate and ethyl hexadecanoate may be the esterification of long-chain acids and alcohols of pyrolysis bio-oil to form a large number of long-chain esters.
To systematically and comprehensively analyze the content changes in various substances in the two pyrolysis bio-oils, we further classified the components of pyrolysis bio-oils in different atmospheres. Compounds are divided into eight groups, including nitrogen compounds, esters, acids, hydrocarbons, ketones, phenols, furans, alcohols, and ethers. Hydrocarbons are subdivided into aromatic compounds and aromatics for an accurate analysis. If a compound can be divided into multiple categories, it was classified according to the following priority: phenol > aromatic compound > ester > acid > ketone > alcohol. The results are shown in Figure 7. The peak area cannot represent the exact amount of compounds. The peak area of aromatics in pyrolysis bio-oil under nitrogen atmosphere is the largest (35.76%). In pyrolysis bio-oil under hydrogen, the peak area of aromatics is 30.01%. In addition to the relatively weak oxygen bridge bond C-O-C, side-chain bonds are present on the monomer benzene ring in a large amount of lignin in the sample, and these bonds are easily broken by heating to form active benzene ring groups, which may form large macromolecules with other free radicals [24]. However, inhibiting the formation of active benzene ring groups in the hydrogen atmosphere is consistent with the reduction in aromatic compounds from 7.2% to 0% in the hydrogen atmosphere. It mainly inhibits the formation of active benzene ring groups. Acids, phenols, aldehydes, alcohols, ketones, and furans in pyrolysis gas undergo deoxylysis, oligomerization, hydrogen transfer, and other reactions in the hydrogen atmosphere, and these compounds are difficult to aromatize. In the hydrogen atmosphere, the phenols of pyrolysis bio-oil decreased by 15.6% from 33.66% to 28.38%. It also shows that aromatization is inhibited. Other types of oxygen-containing groups are present in the lignin structure in the sample, such as carbonyl and hydroxyl, which are decomposed into small molecular chemicals and remain in the side chain of the benzene ring. However, in the hydrogen atmosphere, the free radicals of hydrogen inhibit the binding of other groups and are not easy to bind to the side chain of the benzene ring.
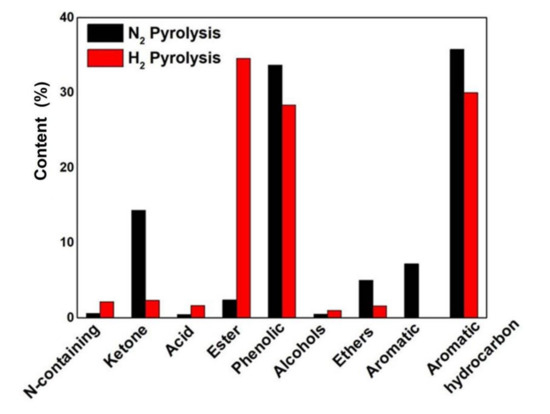
Figure 7.
Comparative analysis of components of pyrolysis bio-oil under different atmospheres.
The ester peak area content of pyrolysis bio-oil in a hydrogen atmosphere is 34.56%, which is approximately 14 times higher than that of pyrolysis bio-oil in a nitrogen atmosphere, and that of alcohol is from 0.48% to 0.97%. It shows that alcohol in pyrolysis bio-oil is doubled in the hydrogen atmosphere. Approximately 40% of alcohols are ethanol, and 35% are methanol, while the content of acids in pyrolysis bio-oil in hydrogen atmosphere ranges from 0.45% to 1.65% of oleic acid in nitrogen. Among these compounds, the acid substances are 19 acids, accounting for 35% and 16 acids, accounting for 25%, which explains that the formation of a large number of esters is caused by the esterification of long-chain acids and esters in pyrolysis products in a hydrogen atmosphere at high temperature, which significantly reduces the acidity of pyrolysis bio-oil, and the average relative molecular weight of pyrolysis bio-oil after hydrogenation is reduced.
The existence of carbonyl compounds such as ketones causes the poor stability of pyrolysis bio-oil [25]. However, the ketone content of 2.35% in pyrolysis oil in the hydrogen atmosphere is 83.61% lower than that (14.34%) of the pyrolysis oil in the nitrogen atmosphere, and the stability is improved. This heating value is the same as the calculated value by using elemental analysis, which may be more effective hydrodeoxygenation on carbonyl in a hydrogen atmosphere. Ether compounds decreased from 5.01% to 1.61%. In biomass samples, cellulose is mainly connected by D-glucopyranose units, and the connecting bond is mainly oxygen bridge bond C-O-C. In comparison with the C-C bond, the activation energy required for the C-O-C bond cleavage is weaker, which is easier to break and combine with hydrogen [26]. As a result, ether compounds are reduced. The increase in N-containing compounds proves that nitrogen combines with hydrogen and forms N-containing compounds in the hydrogen atmosphere, but stable compounds hardly form in the nitrogen atmosphere. Considering all the changes, the quality of pyrolysis bio-oil obtained from biomass pyrolysis in a hydrogen atmosphere is better than that in a nitrogen atmosphere.
4. Conclusions
- The yield of pyrolysis oil in a hydrogen atmosphere is 59.23%, which is higher than that (40.88%) in a nitrogen atmosphere, and the solid product is reduced from 26.02% in a nitrogen atmosphere to 20.41% in a hydrogen atmosphere. The carbon content of pyrolysis bio-oil obtained under hydrogen is basically unchanged. Oxygen decreased from 21.34% to 17.16%, hydrogen increased from 5.17% to 7.06%, the H/C ratio increased from 0.072 to 0.098, and the heating value increased from 27.75 MJ/kg to 31.40 MJ/kg;
- In the hydrogenated pyrolysis of bio-oil, the content of compounds with a boiling point of 0–200 °C is 63.21%, while in a nitrogen atmosphere, the content of components within the boiling point range is 55.11%. Therefore, the low boiling point fraction of pyrolysis bio-oil can be improved in a hydrogen atmosphere, which is conducive to improving the combustion characteristics of pyrolysis oil;
- By comparison, the biomass pyrolysis oil changed obviously in the hydrogen atmosphere. Ethyl hexadecanoate (peak area percentage 19.1%) and methyl octadecanoate (peak area percentage 15.42%) were obtained. The number of phenols decreased from 33.66% to 28.38%. Pyrolysis in a hydrogen atmosphere inhibited its aromatization and reduced the side chain reaction of the benzene ring. The content of esters increased significantly in the hydrogen atmosphere, and acids increased from 0.45% in the nitrogen atmosphere to 1.65% in the hydrogen atmosphere. It is mainly hexadecanoic acid, nineteenth acid, ethanol, and methanol. Therefore, esterification occurred in the pyrolysis process, resulting in the formation of long-chain esters and reducing the acidity of pyrolysis bio-oil.
Author Contributions
J.W.: Investigation, visualization, original draft preparation; S.W.: Data curation, reviewing and editing; J.L.: Methodology, reviewing and editing; M.Y.: Project administration, reviewing and editing; Y.W.: Supervision, conceptualization, reviewing and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Research Project of Shanxi Provincial Department of Science and Technology (No. 202003D311001), National Natural Science Foundation of China (No. 21838006) and the National Key R&D Program of China (No. 2018YFC1902101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, J.; Wat, J.; Liu, Z.; Wu, Y.; Yang, M. Elemental migration and transformation during hydrothermal liquefaction of biomass. J. Hazard. Mater. 2021, 423, 126961. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, M.; Li, W.; Wang, X.; Cui, M.; Yang, Y. Catalytic fast pyrolysis of biomass with noble metal-like catalysts to produce high-grade bio-oil. Analytical Py-GC/MS study. Catal. Today 2018, 302, 169–179. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.; Singh, R. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Wang, T.; Li, B.; Xu, Y.; Ma, L. Efficient upgrading process for production of low quality fuel from bio-oil. Fuel 2016, 179, 312–321. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Norbeck, J.; Park, C.S. A simple kinetic analysis of syngas during steam hydrogasification of biomass using a novel inverted batch reactor with instant high pressure feeding. Bioresour. Technol. 2016, 200, 731–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiang, S.; Wang, W. Experimental and techno-economic studies of upgrading heavy pyrolytic oils from wood chips into valuable fuels. J. Clean. Prod. 2020, 277, 124136. [Google Scholar] [CrossRef]
- Figueirêdoa, M.; Venderboschb, R.; Heeresa, H.; Deuss, P. In-depth structural characterization of the lignin fraction of a pine-derived pyrolysis oil. J. Anal. Appl. Pyrol. 2020, 149, 104837. [Google Scholar] [CrossRef]
- Staš, M.; Auersvald, M.; Kejla, L.; Vrtiška, D.; Kroufek, J.; Kubička, D. Quantitative analysis of pyrolysis bio-oils. A review. TrAC Trends Anal. Chem. 2020, 126, 115857. [Google Scholar] [CrossRef]
- Jaffar, M.; Nahil, M.; Williams, P. Methane production from the pyrolysis-catalytic hydrogenation of waste biomass: Influence of process conditions and catalyst type. Energy Fuels 2019, 33, 7443–7457. [Google Scholar] [CrossRef]
- Ahamed, T.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass—Mechanism, challenges and opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Han, D.; Yang, X.; Li, R.; Wu, Y. Environmental impact comparison of typical and resource-efficient biomass fast pyrolysis systems based on LCA and Aspen Plus simulation. J. Clean. Prod. 2019, 231, 254–267. [Google Scholar] [CrossRef]
- Chandler, D.; Resende, F. Comparison between catalytic fast pyrolysis and catalytic fast hydropyrolysis for the production of liquid fuels in a fluidized bed reactor. Energy Fuels 2019, 33, 3199–3209. [Google Scholar] [CrossRef]
- Saidi, M.; Moradi, P. Catalytic hydrotreatment of lignin-derived pyrolysis bio-oils using Cu/γ-Al2O3 catalyst. Reaction network development and kinetic study of anisole upgrading. Int. J. Energy Res. 2021, 45, 8267–8284. [Google Scholar] [CrossRef]
- Chaihad, N.; Situmorang, Y.; Anniwaer, A.; Kurnia, I.; Karnjanakom, S.; Kasai, Y.; Abudula, A.; Reubroycharoen, P.; Guan, G. Preparation of various hierarchical HZSM-5 based catalysts for in-situ fast upgrading of bio-oil. Renew. Energy 2021, 169, 283–292. [Google Scholar] [CrossRef]
- Arazo, R.; Capareda, S.; Ofrasio, B.; Ido, A.; Chen, W.; Luna, M. Low-temperature catalytic conversion of alkaline sewage sludge bio-oil to biodiesel: Product characteristics and reaction mechanisms. Environ. Technol. Innov. 2021, 21, 101266. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.; Zhang, S.; Xu, Y.; Duan, P. Hydrothermal liquefaction of crop straws: Effect of feedstock composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Yang, X.; Han, D.; Zhao, Y.; Li, R.; Wu, Y. Environmental evaluation of a distributed-centralized biomass pyrolysis system. Total Environ. 2020, 716, 136915. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Zhang, J.; Wang, J. Parametric study of two-stage hydropyrolysis of lignocellulosic biomass for production of gaseous and light aromatic hydrocarbons. Bioresour. Technol. 2017, 244, 142–150. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Wang, J. Comparative investigation of rice husk, thermoplastic bituminous coal and liquid products during hydropyrolysis/co-hydropyrolysis. Bioresour. Technol. 2018, 268, 445–453. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Zhang, L.; Liu, Z.; Wu, Y. Catalytic hydrothermal liquefaction of microalgae over mesoporous silicabased materials with site-separated acids and bases. Fuel 2020, 279, 118–127. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Wan, S.; Ru, B.; Lin, H.; Sun, W.; Luo, Z. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour. Technol. 2015, 182, 120–127. [Google Scholar]
- Zhou, D.; Zhang, S.; Fu, H.; Chen, J. Liquefaction of macroalgae enteromorpha prolifera in sub-/supercritical alcohols: Direct production of ester compounds. Energy Fuels 2012, 26, 2342–2351. [Google Scholar] [CrossRef]
- Neutelings, G. Lignin variability in plant cell walls: Contribution of new models. Plant Sci. 2011, 181, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Haruo, K.; Sunao, H.; Shiro, S. Effects of side-chain hydroxyl groups on pyrolytic β-ether cleavage of phenolic lignin model dimer. J. Wood Sci. 2007, 53, 268–271. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).