Abstract
Periodontal regeneration is a complex goal, which is commonly pursued with a combination of surgical techniques, biomaterials, and bioactive compounds. One such compound is enamel matrix derivative (EMD), a medical substance that is extracted from porcine tooth germs and which contains several protein fractions with BMP- and TGF-β-like action. Activation of TGF-β signaling is required for EMD activity on cells and tissues, and a growing body of evidence indicates that EMD largely relies on this pathway. As low frequency electromagnetic fields (EMFs) have long been investigated as a tool to promote bone formation and osteoblast activity, and because recent studies have reported that the effects of EMFs on cells require primary cilia, by modulating the presence of membrane-bound receptors (e.g., for BMP) or signal mediators, it can be hypothesized that the application of EMFs may increase cell sensitivity to EMD: as TGFBR receptors have also been identified on primary cilia, EMFs could make cells more responsive to EMD by inducing the display of a higher number of receptors on the cellular membrane.
1. Introduction
The regeneration of compromised periodontal tissues is a complex endeavor, and has been tackled from different angles over the years, including through the use of surgery, membranes, and growth factors [1]. In contrast to bone, the periodontal ligament does not spontaneously regenerate following periodontitis or trauma, and surgery alone has shown inconsistent results. Enamel matrix derivative (EMD), which is an enamel protein matrix obtained from porcine tooth germs, has yielded promising results, promoting the regeneration of both bone and ligament. Considering the still only partially predictable results of periodontal regeneration, solutions that increase its clinical success are sorely needed. The present paper focuses on the adjunctive use of low frequency electromagnetic fields (EMFs), which can act synergically with EMD to improve tissue regeneration.
2. Hypothesis
EMFs have long been explored as a tool to improve bone regeneration, and their ability to promote bone growth in bone wounds and under bone loss conditions has been investigated in many studies. The hypothesis of the present paper, however, focuses on exploring whether EMFs can synergize with EMD, increase cell and tissue sensitivity to EMD, and thus aid it in promoting the regeneration of periodontal tissues (Figure 1), based on the findings of recent research on these treatments.
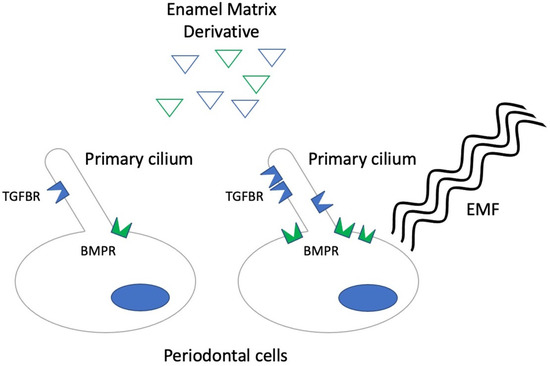
Figure 1.
Diagram describing the hypothesized synergic effect of low frequency electromagnetic fields (EMF) with enamel matrix derivative (EMD). Blue triangles represent the EMD protein fraction with TGF-β-like activity, while the green triangles represent the fraction with BMP-like activity. In the presence of EMFs, higher levels of BMP receptor (BMPR) expression in cells and, possibly, TGF-β receptor (TGFBR) expression on cilia and in the periciliary area is observed, thus making cells more responsive to EMD.
3. Enamel Matrix Derivative
Enamel matrix derivative is a popular therapeutic aid that has been successful in promoting the regeneration of periodontal tissues, including bone, periodontal ligament, and soft tissues [2], while displaying a cytostatic effect toward epithelial cells [3]. Its use is supported by a vast literature [1,4,5]. EMD is obtained from porcine tooth germs and, as such, is a complex compound, containing proteins with different activity [6]. However, it has been repeatedly shown that, besides stimulating BMP-2 and TGF-β1 expression [7], some protein fractions of EMD actually exert a direct BMP- (fractions 4–6) or TGF-β-like (fractions 8–13) action [8,9], such as promoting Smad2 translocation [10]. A micro-array characterization of gene patterns in gingival and palatal fibroblasts confirmed, strikingly, that EMD was capable of regulating TGF-β target genes [11]. The activation of TGFBRI receptors—possibly by direct or paracrine action—has actually been shown to be required for EMD activity on palatal fibroblasts [12], periodontal ligament cells [13], and pre-adipocytes [14], for its pro-osteoclastogenic effect on bone marrow [15], and it has been reported that anti-TGF-β antibodies were able to block EMD effects on epithelial cells [16]. Although other components of EMD have been proven to possess distinct biological activities [17,18], it appears that a substantial part of its effectiveness may be due to its capability to stimulate the BMP and TGF-β signaling pathways. Thus, it may find an unexpected ally in low frequency electromagnetic field therapy.
4. Electromagnetic Fields (EMFs) and Cellular Responses
4.1. General Considerations
Electromagnetic fields (EMFs) are a common occurrence, as most man-made electrical appliances generate a fair amount of them. These EMFs compound the EMFs that are intentionally produced and used to transmit signals such radio-waves, Wi-Fi, and cell phones signals, and also natural EMFs [19]. Although ionizing radiations, e.g., X-rays, are prime examples of electromagnetic waves interacting with living matter [20,21], a staggering amount of evidence has consistently shown over the years that non-ionizing radiations, i.e., radiations incapable of ionizing matter, can actually affect cells and tissues [22,23]. After realizing that the EMFs generated by peculiar electrical wiring or high-power radars could be associated with neoplastic disease [24,25], increasing efforts have been progressively devoted to both reduce the risks associated with the overwhelming EMF pollution that permeates our society [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], and use EMFs to benefit patients’ health.
4.2. EMFs and Bone
The new application of EMFs in medicine is best characterized by its use in bone healing, as pre-clinical studies have shown that EMFs can increase bone mass in several models of bone loss [42,43,44,45,46,47,48,49,50,51,52], disuse osteoporosis [53,54,55], diabetes-associated osteoporosis [56], and hyperthyroidism-related bone loss [57]. Its use can also improve bone healing [58,59,60,61,62,63,64,65] in complex clinical situations, such as osteoporotic fractures [66] or arthritis [67]. Numerous clinical investigations have also reported benefits in osteoporosis patients [68,69,70], and as an adjunctive therapy for non-unions or osteotomies [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86], although no consensus has thus far been reached on consistent sets of parameters [87]. To further add to the complexity of the issue, EMFs can be generated in a vast range of waveforms, including square, trapezoidal, triangular shape (often known as Pulsed EMFs or PEMFs), and even sinusoidal waves—known as SEMFs [88]—and are often generated as bursts of shorter impulses (PRF EMFs). Their frequencies can range from 0.2 Hz up to 1 kHz [89,90,91,92], though in the case of PRF PEMFs, the carrier frequency can be several kHz, and can be applied at different intensities, from a few milli-Tesla up to 1 Tesla and for varying durations.
4.3. EMFs and Cell Signaling
Although EMFs have been shown to induce complex reactions from bone cells, including the activation of several signaling pathways [93], there is a solid body of evidence pointing to the importance of BMP and TGF-β signaling in cell responses to electromagnetic fields. It has long been known that 15 Hz PRF PEMF pulses enhanced TGF-β1 expression in osteosarcoma cells [94], while a similar stimulation promoted TGF-β1 secretion in serum-starved MC3T3 cells (Patterson et al. [95]) and in tendon cells [96]. This method also elevated serum TGF-β levels in a rat model of disuse osteoporosis [55]. Complex networks of paracrine feed-forward loops have also been outlined by several works, such as Schwartz et al., who showed that 15 Hz PEMF bursts synergized with BMP to stimulate the expression of TGF-β, both in its latent and active forms [97]. More recently, Selvamurugan et al. have shown that TGF-β signaling is required to gain the effects of 15 Hz, 67 ms-long PRF PEMFs in human bone marrow cells, supporting the activity of miRNA21, a microRNA that suppresses the TGF-β signaling inhibitor Smad-7 [98]. Recent studies, however, have reported exciting new findings on the mechanisms of cell responses to EMFs, including the involvement of primary cilia, which sheds new light on the biological action of electromagnetic fields and may also provide a rationale as to why EMFs could enhance EMD action.
4.4. EMFs and Primary Cilia
Primary cilia are solitary organelles composed of a peculiar inner microtubular structure [99] that can be found on a vast range of cell types, at least in certain differentiation stages [100]. Primary cilia appear as long cytoplasmic extroflections, which, unlike flagelles, are non-motile and possess an axoneme that is supported by a basal body, which forms when the mother centriole is docked at the cytoplasmic membrane [101]. The periciliary area may therefore act as a microtubule organizing center, and the role of primary cilia in cells is often, maybe unsurprisingly so, to act as a sensor for stimuli of different nature, including mechanical stress, e.g., fluid shear stress in kidney epithelial cells or in osteocytes [102].
The first evidence that cilia are required for EMF stimulation dates back to the report by Yan et al. [103], who showed that 0.6 mT, 50 Hz pulsed EMFs increased the proliferation, mineralization, and expression of differentiation markers in primary rat calvaria cells, but these effects were not observed when primary cilia were disrupted by siRNA inhibition of the intraflagellar transport protein 88 (IFT88), a molecule that controls the movement of cargo along the axoneme [104]. These findings were then confirmed by Wang et al. [105], who were able to inhibit the response of osteocyte-like MLO-Y4 cells and the expression of Rankl and Opg—two key controllers of bone turnover—to 0.5 mT, 15 Hz PRF PEMFs by silencing the Polaris protein, which is required for cilia formation [106].
Several signaling pathways have thus far been associated with these primary cilia-mediated cell responses to electromagnetic fields: PI3K/AKT has been shown to be activated by 50 Hz, 0.6 mT PEMFs in rat calvaria osteoblasts, which require the presence of primary cilia [107]. There is also evidence indicating that the cAMP/PKA/CREB signaling pathway is activated by PEMF in the same cell model [108] and that Wnt10b/β-catenin is also required for osteoblast responses to sinusoidal EMFs [109]. What is most interesting is that, in this latter study, Wnt10b appeared to co-localize at the base of the cilia, only to be recruited and disappear upon EMF stimulation. This is also consistent with an important study by Xie et al., which demonstrates that BMP receptor II (BMPRII) was required for the increased BMP-Smad1/5/8 signaling in primary rat calvaria cells after PEMF stimulation, and that PEMFs up-regulated the expression of BMPRII at the base of the cilia, which in turn were required for BMPRII signaling [110].
4.5. Primary Cilia and Membrane Trafficking
Primary cilia have also been singled out as key structures for controlling vesicle trafficking and membrane receptor expression. The base of the cilia in many cell types, including fibroblasts, are normally surrounded by a special membrane domain known as the cilium pocket region (CiPo), which has a peculiar biomolecular composition and is a hotspot for both exo- and endocytosis [111], while the tip of the cilia is a source of extracellular vesicles [101]. Cilia and the surrounding membrane area thus appear to be rich in clathrin-coated pits, clathrin-coated vesicles [112], endosomes [100,113,114,115], and possibly caveolae [116], which are controlled by the complex cytoskeletal network that underlies and surrounds cilia [101]. Vesicle endocytosis is a power tool that cells use to control the array of membrane-bound receptors exhibited on their surface. Cells can actually tune their signaling capabilities by removing available receptors from the membrane or by providing more free receptors for ligand binding [115]. Somewhat unsurprisingly, then, cilia and the periciliary area are rich with several kinds of receptors, including the BMP receptor, but also PDGFR and TGFBR [113,117]. TGF-β receptors, in particular, have been shown to be localized at the tip of the primary cilia in fibroblasts, and their signaling has been proven to be regulated by vesicle trafficking, as clathrin-mediated endocytosis promotes their activation [116], whereas caveolin-mediated endocytosis downregulates TGF-β signaling [118].
Although no published evidence has addressed the effect of electromagnetic fields on the turnover of TGF-β receptors in the ciliary and periciliary area yet, there is evidence supporting the hypothesis that EMFs can actually control clathrin-mediated endocytosis in murine B16F10 melanoma cells [119,120,121], autophagy in human neuroblastoma SH-SY5Y cells [122], phagocytosis in mouse macrophages [123], and endocytosis at synapses [124]. There is also solid evidence that 75 Hz, 2.5 mT PEMFs upregulate the expression and membrane density of adenosine receptors in osteoblastic cells [125], as well as in other cell models [126], and this could provide a clue as to one way that EMFs could also control TGF-β signaling.
5. Hypothesis Testing
Although the pre-clinical in vitro and in vivo testing of this hypothesis may appear straightforward, when it comes to the chosen model, the most challenging factor is possibly the choice of the optimal parameters for EMF stimulation, most noticeably EMF frequency, waveform, and intensity, but also the duration of treatment. The plethora of experimental conditions proposed in the literature has often relied more on technological availability, convenience, or the need to use distinctive instruments and protocols for commercial reasons than purely scientific rationales, but recent attempts to apply machine learning algorithms to pinpoint the most promising experimental parameters [127] may prove a first step in the right direction. The most common stimulation regimes supported by the literature are 75 Hz EMFs with trapezoidal waves, with treatment intensities in the range of 1.5–2.5 mT and 15 Hz PRF PEMF bursts with intensities of 0.3–1.8 mT. In the case of sinusoidal waves, however, 50 or 60 Hz waves with intensities between 0.6 and 1.8 mT are possibly the most common parameters in the literature.
6. Conclusions
Electromagnetic fields may prove to be a relatively inexpensive support treatment to enhance the effectiveness of EMD in restoring periodontal tissues, such as periodontal ligament, as they have been shown to act on several signaling pathways, and because there is mounting evidence that they can regulate the membrane receptor availability on and around primary cilia. Their action is known to affect BMP receptors, and we hypothesize that it might also affect TGF-β receptors, by controlling membrane trafficking. This, in turn, would make cells more sensitive to the action of BMP and TGF-β growth factors (Figure 1), which are pivotal to the action of EMD, either through paracrine stimulation or through the direct action of some of the protein fractions contained in EMD.
Author Contributions
Conceptualization, C.G., G.P. and S.G.; writing—original draft preparation, C.G.; writing—review and editing, G.P.; supervision, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsai, S.J.; Ding, Y.W.; Shih, M.C.; Tu, Y.K. Systematic Review and Sequential Network Meta-Analysis on the Efficacy of Periodontal Regenerative Therapies. J. Clin. Periodontol. 2020, 47, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Biologics-Based Regenerative Technologies for Periodontal Soft Tissue Engineering. J. Periodontol. 2020, 91, 147–154. [Google Scholar] [CrossRef]
- Grandin, H.M.; Gemperli, A.C.; Dard, M. Enamel Matrix Derivative: A Review of Cellular Effects In Vitro and a Model of Molecular Arrangement and Functioning. Tissue Eng. Part B Rev. 2012, 18, 181–202. [Google Scholar] [CrossRef]
- Sculean, A.; Alessandri, R.; Miron, R.; Salvi, G.E.; Bosshardt, D.D. Enamel Matrix Proteins and Periodontal Wound Healing and Regeneration. Clin. Adv. Periodontics 2011, 1, 101–117. [Google Scholar] [CrossRef]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal Regeneration with Enamel Matrix Derivative in Reconstructive Periodontal Therapy: A Systematic Review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Wyganowska-Świątkowska, M.; Urbaniak, P.; Nohawica, M.M.; Kotwicka, M.; Jankun, J. Enamel Matrix Proteins Exhibit Growth Factor Activity: A Review of Evidence at the Cellular and Molecular Levels. Exp. Ther. Med. 2015, 9, 2025–2033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miron, R.J.; Chandad, F.; Buser, D.; Sculean, A.; Cochran, D.L.; Zhang, Y. Effect of Enamel Matrix Derivative Liquid on Osteoblast and Periodontal Ligament Cell Proliferation and Differentiation. J. Periodontol. 2016, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nagano, T.; Yamakoshi, Y.; Gomi, K.; Arai, T.; Fukae, M.; Katagiri, T.; Oida, S. Enamel Matrix Derivative Gel Stimulates Signal Transduction of BMP and TGF-β. J. Dent. Res. 2005, 84, 510–514. [Google Scholar] [CrossRef]
- Van der Pauw, M.T.; Van den Bos, T.; Everts, V.; Beertsen, W. Enamel Matrix-Derived Protein Stimulates Attachment of Periodontal Ligament Fibroblasts and Enhances Alkaline Phosphatase Activity and Transforming Growth Factor β 1 Release of Periodontal Ligament and Gingival Fibroblasts. J. Periodontol. 2000, 71, 31–43. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Momose, M.; Kato, Y.; Yoshie, H.; Burns, D.M. Enamel Matrix Derivative (EMDOGAIN®) Rapidly Stimulates Phosphorylation of the MAP Kinase Family and Nuclear Accumulation of Smad2 in Both Oral Epithelial and Fibroblastic Human Cells. J. Periodontal Res. 2001, 36, 367–376. [Google Scholar] [CrossRef]
- Gruber, R.; Stähli, A.; Miron, R.J.; Bosshardt, D.D.; Sculean, A. Common Target Genes of Palatal and Gingival Fibroblasts for EMD: The Microarray Approach. J. Periodontal Res. 2015, 50, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Bosshardt, D.; Sculean, A.; Gruber, R. Emdogain-Regulated Gene Expression in Palatal Fibroblasts Requires TGF-ΒRI Kinase Signaling. PLoS ONE 2014, 9, e105672. [Google Scholar] [CrossRef] [PubMed]
- Heng, N.H.M.; Zahlten, J.; Cordes, V.; Ong, M.M.-A.; Goh, B.T.; N’Guessan, P.D.; Pischon, N. Effects of Enamel Matrix Derivative and Transforming Growth Factor-Β1 on Connective Tissue Growth Factor in Human Periodontal Ligament Fibroblasts. J. Periodontol. 2015, 86, 569–577. [Google Scholar] [CrossRef]
- Gruber, R.; Bosshardt, D.D.; Miron, R.J.; Gemperli, A.C.; Buser, D.; Sculean, A. Enamel Matrix Derivative Inhibits Adipocyte Differentiation of 3T3-L1 Cells via Activation of TGF-ΒRI Kinase Activity. PLoS ONE 2013, 8, e71046. [Google Scholar] [CrossRef][Green Version]
- Gruber, R.; Roos, G.; Caballé-Serrano, J.; Miron, R.; Bosshardt, D.D.; Sculean, A. TGF-ΒRI Kinase Activity Mediates Emdogain-Stimulated in Vitro Osteoclastogenesis. Clin. Oral Investig. 2014, 18, 1639–1646. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Yoshie, H.; Burns, D.M. Anti-TGF-β Antibody Blocks Enamel Matrix Derivative-Induced Upregulation of P21WAF1/Cip1 and Prevents Its Inhibition of Human Oral Epithelial Cell Proliferation. J. Periodontal Res. 2002, 37, 255–262. [Google Scholar] [CrossRef]
- Hoang, A.M.; Klebe, R.J.; Steffensen, B.; Ryu, O.H.; Simmer, J.P.; Cochran, D.L. Amelogenin Is a Cell Adhesion Protein. J. Dent. Res. 2002, 81, 497–500. [Google Scholar] [CrossRef]
- Warotayanont, R.; Frenkel, B.; Snead, M.L.; Zhou, Y. Leucine-Rich Amelogenin Peptide Induces Osteogenesis by Activation of the Wnt Pathway. Biochem. Biophys. Res. Commun. 2009, 387, 558–563. [Google Scholar] [CrossRef]
- Olson, P.; Amit, H. Changes in Earth’s Dipole. Naturwissenschaften 2006, 93, 519–542. [Google Scholar] [CrossRef]
- Squillaro, T.; Galano, G.; De Rosa, R.; Peluso, G.; Galderisi, U. Concise Review: The Effect of Low-Dose Ionizing Radiation on Stem Cell Biology: A Contribution to Radiation Risk. Stem Cells 2018, 36, 1146–1153. [Google Scholar] [CrossRef]
- Halls, N. The Microbiology of Irradiation Sterilization. Med. Device Technol. 1992, 3, 37–45. [Google Scholar]
- Yost, M.G. Occupational Health Effects of Nonionizing Radiation. Occup. Med. 1992, 7, 543–566. [Google Scholar]
- Hardell, L.; Sage, C. Biological Effects from Electromagnetic Field Exposure and Public Exposure Standards. Biomed. Pharmacother. 2008, 62, 104–109. [Google Scholar] [CrossRef]
- Wertheimer, N.; Leeper, E. Electrical Wiring Configurations and Childhood Cancer. Am. J. Epidemiol. 1979, 109, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Robinette, C.D.; Silverman, C.; Jablon, S. Effects upon Health of Occupational Exposure to Microwave Radiation (Radar). Am. J. Epidemiol. 1980, 112, 39–53. [Google Scholar] [CrossRef]
- Wertheimer, N.; Leeper, E. Adult Cancer Related to Electrical Wires near the Home. Int. J. Epidemiol. 1982, 11, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Budinger, T.F. Nuclear Magnetic Resonance (NMR) in Vivo Studies: Known Thresholds for Health Effects. J. Comput. Assist. Tomogr. 1981, 5, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Kavet, R.I.; Banks, R.S. Emerging Issues in Extremely-Low-Frequency Electric and Magnetic Field Health Research. Environ. Res. 1986, 39, 386–404. [Google Scholar] [CrossRef]
- Brown, H.D.; Chattopadhyay, S.K. Electromagnetic-Field Exposure and Cancer. Cancer Biochem. Biophys. 1988, 9, 295–342. [Google Scholar]
- Kavet, R. An Alternate Hypothesis for the Association between Electrical Wiring Configurations and Cancer. Epidemiology 1991, 2, 224–229. [Google Scholar] [CrossRef]
- Tenforde, T.S. Biological Interactions and Potential Health Effects of Extremely-Low-Frequency Magnetic Fields from Power Lines and Other Common Sources. Annu. Rev. Public Health 1992, 13, 173–196. [Google Scholar] [CrossRef]
- Habash, R.W.Y.; Brodsky, L.M.; Leiss, W.; Krewski, D.; Repacholi, M. Health Risks of Electromagnetic Fields. Part I: Evaluation and Assessment of Electric and Magnetic Fields. Crit. Rev. Biomed. Eng. 2003, 31, 141–195. [Google Scholar] [CrossRef] [PubMed]
- Bellieni, C.V.; Bagnoli, F.; Pinto, I.; Stacchini, N.; Buonocore, G. Reduction of Exposure of Newborns and Caregivers to Very High Electromagnetic Fields Produced by Incubators. Med. Phys. 2004, 32, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-De la Fuente, A.O.; Alcocer-González, J.M.; Heredia-Rojas, J.A.; Rodríguez-Padilla, C.; Rodríguez-Flores, L.E.; Santoyo-Stephano, M.A.; Castañeda-Garza, E.; Taméz-Guerra, R.S. Effect of 60 Hz Electromagnetic Fields on the Activity of Hsp70 Promoter: An in Vivo Study. Cell Biol. Int. Rep. 2012, 19, e00014. [Google Scholar] [CrossRef][Green Version]
- Carpenter, D.O. Human Disease Resulting from Exposure to Electromagnetic Fields1). Rev. Environ. Health 2013, 28, 159–172. [Google Scholar] [CrossRef]
- Monazzam, M.; Hosseini, M.; Matin, L.; Aghaei, H.; Khosroabadi, H.; Hesami, A. Sleep Quality and General Health Status of Employees Exposed to Extremely Low Frequency Magnetic Fields in a Petrochemical Complex. J. Environ. Health Sci. Eng. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Lasalvia, M.; Scrima, R.; Perna, G.; Piccoli, C.; Capitanio, N.; Biagi, P.F.; Schiavulli, L.; Ligonzo, T.; Centra, M.; Casamassima, G.; et al. Exposure to 1.8 GHz Electromagnetic Fields Affects Morphology, DNA-Related Raman Spectra and Mitochondrial Functions in Human Lympho-Monocytes. PLoS ONE 2018, 13, e0192894. [Google Scholar] [CrossRef]
- Pall, M.L. Wi-Fi Is an Important Threat to Human Health. Environ. Res. 2018, 164, 405–416. [Google Scholar] [CrossRef]
- Pall, M.L. Microwave Frequency Electromagnetic Fields (EMFs) Produce Widespread Neuropsychiatric Effects Including Depression. J. Chem. Neuroanat. 2016, 75, 43–51. [Google Scholar] [CrossRef]
- Belyaev, I.; Dean, A.; Eger, H.; Hubmann, G.; Jandrisovits, R.; Kern, M.; Kundi, M.; Moshammer, H.; Lercher, P.; Müller, K.; et al. EUROPAEM EMF Guideline 2016 for the Prevention, Diagnosis and Treatment of EMF-Related Health Problems and Illnesses. Rev. Environ. Health 2016, 31, 363–397. [Google Scholar] [CrossRef]
- Blank, M.; Havas, M.; Kelley, E.; Lai, H.; Moskowitz, J. International Appeal: Scientists Call for Protection from Non-Ionizing Electromagnetic Field Exposure. Eur. J. Oncol Environ Health 2015, 20, 180–182. [Google Scholar]
- Jing, D.; Shen, G.; Huang, J.; Xie, K.; Cai, J.; Xu, Q.; Wu, X.; Luo, E. Circadian Rhythm Affects the Preventive Role of Pulsed Electromagnetic Fields on Ovariectomy-Induced Osteoporosis in Rats. Bone 2010, 46, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gou, H.; Wang, S.; Zhu, J.; Tian, S.; Yu, L. Effect of Pulsed Electromagnetic Field on Bone Formation and Lipid Metabolism of Glucocorticoid-Induced Osteoporosis Rats through Canonical Wnt Signaling Pathway. Evid.-Based Complement. Altern. Med. 2016, 2016, 4927035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, H.; Yang, L.; Chen, S.; Guo, H.; Xia, L.; Liu, H.; Qin, Y.; Liu, C.; Wei, X.; et al. Effects of Pulsed Electromagnetic Fields on Bone Mass and Wnt/β-Catenin Signaling Pathway in Ovariectomized Rats. Arch. Med. Res. 2012, 43, 274–282. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Guo, H.; Xia, L.; Liu, H.; Qin, Y.; He, C. Pulsed Electromagnetic Field Stimulates Osteoprotegerin and Reduces RANKL Expression in Ovariectomized Rats. Rheumatol. Int. 2013, 33, 1135–1141. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, Y.; Zeng, Y.; Xie, H.; Fu, C.; Li, N. Effect of Intervention Initiation Timing of Pulsed Electromagnetic Field on Ovariectomy-Induced Osteoporosis in Rats. Bioelectromagnetics 2017, 38, 456–465. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, Y.; Xie, H.; Liao, Y.; Zeng, Y.; Li, N.; Sun, G.; Wu, Q.; Zhou, G. Effects of Combined Treatment with Ibandronate and Pulsed Electromagnetic Field on Ovariectomy-Induced Osteoporosis in Rats. Bioelectromagnetics 2017, 38, 31–40. [Google Scholar] [CrossRef]
- Jing, D.; Li, F.; Jiang, M.; Cai, J.; Wu, Y.; Xie, K.; Wu, X.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed Electromagnetic Fields Improve Bone Microstructure and Strength in Ovariectomized Rats through a Wnt/Lrp5/β-Catenin Signaling-Associated Mechanism. PLoS ONE 2013, 8, e79377. [Google Scholar] [CrossRef]
- Lei, T.; Liang, Z.; Li, F.; Tang, C.; Xie, K.; Wang, P.; Dong, X.; Shan, S.; Jiang, M.; Xu, Q.; et al. Pulsed Electromagnetic Fields (PEMF) Attenuate Changes in Vertebral Bone Mass, Architecture and Strength in Ovariectomized Mice. Bone 2018, 108, 10–19. [Google Scholar] [CrossRef]
- Chang, K.; Chang, W.H.-S. Pulsed Electromagnetic Fields Prevent Osteoporosis in an Ovariectomized Female Rat Model: A Prostaglandin E2-Associated Process. Bioelectromagnetics 2003, 24, 189–198. [Google Scholar] [CrossRef]
- Bilotta, T.W.; Zati, A.; Gnudi, S.; Figus, E.; Giardino, R.; Fini, M.; Pratelli, L.; Mongiorgi, R. Electromagnetic Fields in the Treatment of Postmenopausal Osteoporosis: An Experimental Study Conducted by Densitometric, Dry Ash Weight and Metabolic Analysis of Bone Tissue. Chir. Degli Organi Mov. 1994, 79, 309–313. [Google Scholar]
- Zati, A.; Gnudi, S.; Mongiorgi, R.; Giardino, R.; Fini, M.; Valdrè, G.; Galliani, I.; Montagnani, A.M. Effects of Pulsed Magnetic Fields in the Therapy of Osteoporosis Induced by Ovariectomy in the Rat. Boll. Della Soc. Ital. Biol. Sper. 1993, 69, 469–475. [Google Scholar]
- Jing, D.; Cai, J.; Wu, Y.; Shen, G.; Li, F.; Xu, Q.; Xie, K.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed Electromagnetic Fields Partially Preserve Bone Mass, Microarchitecture, and Strength by Promoting Bone Formation in Hindlimb-Suspended Rats. J. Bone Miner. Res. 2014, 29, 2250–2261. [Google Scholar] [CrossRef]
- Li, B.; Bi, J.; Li, W.; Huang, S.; Zhang, S.; Zhao, J.; Meng, Q.; Fei, J. Effects of Pulsed Electromagnetic Fields on Histomorphometry and Osteocalcin in Disuse Osteoporosis Rats. Technol. Health Care 2017, 25, 13–20. [Google Scholar] [CrossRef]
- Shen, W.-W.; Zhao, J.-H. Pulsed Electromagnetic Fields Stimulation Affects BMD and Local Factor Production of Rats with Disuse Osteoporosis. Bioelectromagnetics 2009, 31, 113–119. [Google Scholar] [CrossRef]
- Li, J.; Zeng, Z.; Zhao, Y.; Jing, D.; Tang, C.; Ding, Y.; Feng, X. Effects of Low-Intensity Pulsed Electromagnetic Fields on Bone Microarchitecture, Mechanical Strength and Bone Turnover in Type 2 Diabetic Db/Db Mice. Sci. Rep. 2017, 7, 10834. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Fu, T.; Liu, Y.; Wei, S.; Yang, Y.; Zhao, D.; Zhao, W.; Song, M.; Tang, X.; et al. Effects of Electromagnetic Fields on Bone Loss in Hyperthyroidism Rat Model. Bioelectromagnetics 2017, 38, 137–150. [Google Scholar] [CrossRef]
- Huegel, J.; Choi, D.S.; Nuss, C.A.; Minnig, M.C.C.; Tucker, J.J.; Kuntz, A.F.; Waldorff, E.I.; Zhang, N.; Ryaby, J.T.; Soslowsky, L.J. Effects of Pulsed Electromagnetic Field Therapy at Different Frequencies and Durations on Rotator Cuff Tendon-to-Bone Healing in a Rat Model. J. Shoulder Elb. Surg. 2018, 27, 553–560. [Google Scholar] [CrossRef]
- Bilgin, H.M.; Çelik, F.; Gem, M.; Akpolat, V.; Yıldız, İ.; Ekinci, A.; Özerdem, M.S.; Tunik, S. Effects of Local Vibration and Pulsed Electromagnetic Field on Bone Fracture: A Comparative Study. Bioelectromagnetics 2017, 38, 339–348. [Google Scholar] [CrossRef]
- Sarker, A.B.; Nashimuddin, A.N.; Islam, K.M.; Rabbani, K.S.; Rahman, M.; Mushin, A.U.; Hussain, M. Effect of PEMF on Fresh Fracture-Healing in Rat Tibia. Bangladesh Med. Res. Counc. Bull. 1993, 19, 103–112. [Google Scholar]
- Taylor, K.F.; Inoue, N.; Rafiee, B.; Tis, J.E.; McHale, K.A.; Chao, E.Y.S. Effect of Pulsed Electromagnetic Fields on Maturation of Regenerate Bone in a Rabbit Limb Lengthening Model. J. Orthop. Res. 2006, 24, 2–10. [Google Scholar] [CrossRef]
- Fredericks, D.C.; Nepola, J.V.; Baker, J.T.; Abbott, J.; Simon, B. Effects of Pulsed Electromagnetic Fields on Bone Healing in a Rabbit Tibial Osteotomy Model. J. Orthop. trauma 2000, 14, 93–100. [Google Scholar] [CrossRef]
- Midura, R.J.; Ibiwoye, M.O.; Powell, K.A.; Sakai, Y.; Doehring, T.; Grabiner, M.D.; Patterson, T.E.; Zborowski, M.; Wolfman, A. Pulsed Electromagnetic Field Treatments Enhance the Healing of Fibular Osteotomies. J. Orthop. Res. 2005, 23, 1035–1046. [Google Scholar] [CrossRef]
- Landry, P.S.; Sadasivan, K.K.; Marino, A.A.; Albright, J.A. Electromagnetic Fields Can Affect Osteogenesis by Increasing the Rate of Differentiation. Clin. Orthop. Relat. Res. 1997, 338, 262–270. [Google Scholar] [CrossRef]
- Takano-Yamamoto, T.; Kawakami, M.; Sakuda, M. Effect of a Pulsing Electromagnetic Field on Demineralized Bone-Matrix-Induced Bone Formation in a Bony Defect in the Premaxilla of Rats. J. Dent. Res. 1992, 71, 1920–1925. [Google Scholar] [CrossRef]
- Androjna, C.; Fort, B.; Zborowski, M.; Midura, R.J. Pulsed Electromagnetic Field Treatment Enhances Healing Callus Biomechanical Properties in an Animal Model of Osteoporotic Fracture. Bioelectromagnetics 2014, 35, 396–405. [Google Scholar] [CrossRef]
- Yang, X.; He, H.; Zhou, Y.; Zhou, Y.; Gao, Q.; Wang, P.; He, C. Pulsed Electromagnetic Field at Different Stages of Knee Osteoarthritis in Rats Induced by Low-Dose Monosodium Iodoacetate: Effect on Subchondral Trabecular Bone Microarchitecture and Cartilage Degradation. Bioelectromagnetics 2017, 38, 227–238. [Google Scholar] [CrossRef]
- Garland, D.E.; Adkins, R.H.; Matsuno, N.N.; Stewart, C.A. The Effect of Pulsed Electromagnetic Fields on Osteoporosis at the Knee in Individuals with Spinal Cord Injury. J. Spinal Cord Med. 1999, 22, 239–245. [Google Scholar] [CrossRef]
- Tabrah, F.L.; Ross, P.; Hoffmeier, M.; Gilbert, F. Clinical Report on Long-Term Bone Density after Short-Term EMF Application. Bioelectromagnetics 1998, 19, 75–78. [Google Scholar] [CrossRef]
- Tabrah, F.; Hoffmeier, M.; Gilbert, F.; Batkin, S.; Bassett, C.A.L. Bone Density Changes in Osteoporosis-Prone Women Exposed to Pulsed Electromagnetic Fields (PEMFs). J. Bone Miner. Res. 1990, 5, 437–442. [Google Scholar] [CrossRef]
- Bassett, C.A.; Pilla, A.A.; Pawluk, R.J. A Non-Operative Salvage of Surgically-Resistant Pseudarthroses and Non-Unions by Pulsing Electromagnetic Fields. A Preliminary Report. Clin. Orthop. Relat. Res. 1977, 124, 128–143. [Google Scholar]
- Bassett, C.A.; Mitchell, S.N.; Gaston, S.R. Treatment of Ununited Tibial Diaphyseal Fractures with Pulsing Electromagnetic Fields. J. Bone Jt. Surgery. Am. Vol. 1981, 63, 511–523. [Google Scholar] [CrossRef]
- Assiotis, A.; Sachinis, N.P.; Chalidis, B.E. Pulsed Electromagnetic Fields for the Treatment of Tibial Delayed Unions and Nonunions. A Prospective Clinical Study and Review of the Literature. J. Orthop. Surg. Res. 2012, 7, 24. [Google Scholar] [CrossRef]
- Shi, H.; Xiong, J.; Chen, Y.; Wang, J.; Qiu, X.; Wang, Y.; Qiu, Y. Early Application of Pulsed Electromagnetic Field in the Treatment of Postoperative Delayed Union of Long-Bone Fractures: A Prospective Randomized Controlled Study. BMC Musculoskelet. Disord. 2013, 14, 35. [Google Scholar] [CrossRef]
- Streit, A.; Watson, B.C.; Granata, J.D.; Philbin, T.M.; Lin, H.-N.; O’Connor, J.P.; Lin, S. Effect on Clinical Outcome and Growth Factor Synthesis with Adjunctive Use of Pulsed Electromagnetic Fields for Fifth Metatarsal Nonunion Fracture. Foot Ankle Int. 2016, 37, 919–923. [Google Scholar] [CrossRef]
- Refai, H.; Radwan, D.; Hassanien, N. Radiodensitometric Assessment of the Effect of Pulsed Electromagnetic Field Stimulation versus Low Intensity Laser Irradiation on Mandibular Fracture Repair: A Preliminary Clinical Trial. J. Maxillofac. Oral Surg. 2014, 13, 451–457. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Hassanein, H.R.; Dahaba, M. Effect of Pulsed Electromagnetic Field on Healing of Mandibular Fracture: A Preliminary Clinical Study. J. Oral Maxillofac. Surg. 2011, 69, 1708–1717. [Google Scholar] [CrossRef]
- Barker, A.T.; Dixon, R.A.; Sharrard, W.J.; Sutcliffe, M.L. Pulsed Magnetic Field Therapy for Tibial Non-Union. Interim Results of a Double-Blind Trial. Lancet 1984, 1, 994–996. [Google Scholar] [CrossRef]
- Scott, G.; King, J.B. A Prospective, Double-Blind Trial of Electrical Capacitive Coupling in the Treatment of Non-Union of Long Bones. J. Bone Jt. Surg. Am. Vol. 1994, 76, 820–826. [Google Scholar] [CrossRef]
- Sharrard, W.J. A Double-Blind Trial of Pulsed Electromagnetic Fields for Delayed Union of Tibial Fractures. J. Bone Jt. Surg. Br. Vol. 1990, 72, 347–355. [Google Scholar] [CrossRef]
- Simonis, R.B.; Parnell, E.J.; Ray, P.S.; Peacock, J.L. Electrical Treatment of Tibial Non-Union: A Prospective, Randomised, Double-Blind Trial. Injury 2003, 34, 357–362. [Google Scholar] [CrossRef]
- Lazović, M.; Kocić, M.; Dimitrijević, L.; Stanković, I.; Spalević, M.; Cirić, T. Pulsed Electromagnetic Field during Cast Immobilization in Postmenopausal Women with Colles’ Fracture. Srp. Arh. Celok. Lek. 2012, 140, 619–624. [Google Scholar] [CrossRef]
- Cheing, G.; Wan, J.; Kai Lo, S. Ice and Pulsed Electromagnetic Field to Reduce Pain and Swelling after Distal Radius Fractures. J. Rehabil. Med. 2005, 37, 372–377. [Google Scholar] [CrossRef]
- Hannemann, P.F.W.; Essers, B.A.B.; Schots, J.P.M.; Dullaert, K.; Poeze, M.; Brink, P.R.G. Functional Outcome and Cost-Effectiveness of Pulsed Electromagnetic Fields in the Treatment of Acute Scaphoid Fractures: A Cost-Utility Analysis. BMC Musculoskelet. Disord. 2015, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Faldini, C.; Cadossi, M.; Luciani, D.; Betti, E.; Chiarello, E.; Giannini, S. Electromagnetic Bone Growth Stimulation in Patients with Femoral Neck Fractures Treated with Screws: Prospective Randomized Double-Blind Study. Curr. Orthop. Pract. 2010, 21, 282–287. [Google Scholar] [CrossRef]
- Adie, S.; Harris, I.A.; Naylor, J.M.; Rae, H.; Dao, A.; Yong, S.; Ying, V. Pulsed Electromagnetic Field Stimulation for Acute Tibial Shaft Fractures. J. Bone Jt. Surg.-Am. 2011, 93, 1569–1576. [Google Scholar] [CrossRef]
- Daish, C.; Blanchard, R.; Fox, K.; Pivonka, P.; Pirogova, E. The Application of Pulsed Electromagnetic Fields (PEMFs) for Bone Fracture Repair: Past and Perspective Findings. Ann. Biomed. Eng. 2018, 46, 525–542. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.Q.; Ge, B.F.; Ma, X.N.; Ma, H.P.; Xian, C.J.; Chen, K.M. Different Electromagnetic Field Waveforms Have Different Effects on Proliferation, Differentiation and Mineralization of Osteoblasts in Vitro. Bioelectromagnetics 2014, 35, 30–38. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Yang, Y.; Tang, X.; Zhao, D.; Zhao, W.; Wu, H. Effect of 1 MT Sinusoidal Electromagnetic Fields on Proliferation and Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stromal Cells. Bioelectromagnetics 2013, 34, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ma, S.; Li, Y.; Ding, G.; Teng, Z.; Liu, J.; Ren, D.; Guo, Y.; Ma, L.; Guo, G. Effects of PEMF Exposure at Different Pulses on Osteogenesis of MC3T3-E1 Cells. Arch. Oral Biol. 2014, 59, 921–927. [Google Scholar] [CrossRef]
- Markov, M.S. Magnetic Field Therapy: A Review. Electromagn. Biol. Med. 2007, 26, 1–23. [Google Scholar] [CrossRef]
- Lei, T.; Li, F.; Liang, Z.; Tang, C.; Xie, K.; Wang, P.; Dong, X.; Shan, S.; Liu, J.; Xu, Q.; et al. Effects of Four Kinds of Electromagnetic Fields (EMF) with Different Frequency Spectrum Bands on Ovariectomized Osteoporosis in Mice. Sci. Rep. 2017, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Pedrazzi, G.; Guizzardi, S. The Cellular Effects of Pulsed Electromagnetic Fields on Osteoblasts: A Review. Bioelectromagnetics 2019. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.H.; Schwartz, Z.; Liu, Y.; Guerkov, H.; Dean, D.D.; Simon, B.; Boyan, B.D. Pulsed Electromagnetic Field Stimulation of MG63 Osteoblast-like Cells Affects Differentiation and Local Factor Production. J. Orthop. Res. 2000, 18, 637–646. [Google Scholar] [CrossRef]
- Patterson, T.E.; Sakai, Y.; Grabiner, M.D.; Ibiwoye, M.; Midura, R.J.; Zborowski, M.; Wolfman, A. Exposure of Murine Cells to Pulsed Electromagnetic Fields Rapidly Activates the MTOR Signaling Pathway. Bioelectromagnetics 2006, 27, 535–544. [Google Scholar] [CrossRef]
- de Girolamo, L.; Viganò, M.; Galliera, E.; Stanco, D.; Setti, S.; Marazzi, M.G.; Thiebat, G.; Corsi Romanelli, M.M.; Sansone, V. In Vitro Functional Response of Human Tendon Cells to Different Dosages of Low-Frequency Pulsed Electromagnetic Field. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Simon, B.J.; Duran, M.A.; Barabino, G.; Chaudhri, R.; Boyan, B.D. Pulsed Electromagnetic Fields Enhance BMP-2 Dependent Osteoblastic Differentiation of Human Mesenchymal Stem Cells. J. Orthop. Res. 2008, 26, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Selvamurugan, N.; He, Z.; Rifkin, D.; Dabovic, B.; Partridge, N.C. Pulsed Electromagnetic Field Regulates MicroRNA 21 Expression to Activate TGF-β Signaling in Human Bone Marrow Stromal Cells to Enhance Osteoblast Differentiation. Stem Cells Int. 2017, 2017, 2450327. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.B.; Schrøder, J.M.; Satir, P.; Christensen, S.T. The Ciliary Cytoskeleton. Compr. Physiol. 2012, 2, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Satir, P.; Pedersen, L.B.; Christensen, S.T. The Primary Cilium at a Glance. J. Cell Sci. 2010, 123, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.L.; Lake, A.V.R.; Johnson, C.A. Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, A Little More Actin Please. Front. Cell Dev. Biol. 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Spasic, M.; Jacobs, C.R. Primary Cilia: Cell and Molecular Mechanosensors Directing Whole Tissue Function. Semin. Cell Dev. Biol. 2017, 71, 42–52. [Google Scholar] [CrossRef]
- Yan, J.L.; Zhou, J.; Ma, H.P.; Ma, X.N.; Gao, Y.H.; Shi, W.G.; Fang, Q.Q.; Ren, Q.; Xian, C.J.; Chen, K.M. Pulsed Electromagnetic Fields Promote Osteoblast Mineralization and Maturation Needing the Existence of Primary Cilia. Mol. Cell. Endocrinol. 2015, 404, 132–140. [Google Scholar] [CrossRef]
- Serra, R. Role of Intraflagellar Transport and Primary Cilia in Skeletal Development. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2008, 291, 1049–1061. [Google Scholar] [CrossRef]
- Wang, P.; Tang, C.; Wu, J.; Yang, Y.; Yan, Z.; Liu, X.; Shao, X.; Zhai, M.; Gao, J.; Liang, S.; et al. Pulsed Electromagnetic Fields Regulate Osteocyte Apoptosis, RANKL/OPG Expression, and Its Control of Osteoclastogenesis Depending on the Presence of Primary Cilia. J. Cell. Physiol. 2018, 234, 10588–10601. [Google Scholar] [CrossRef]
- Taulman, P.D.; Haycraft, C.J.; Balkovetz, D.F.; Yoder, B.K. Polaris, a Protein Involved in Left-Right Axis Patterning, Localizes to Basal Bodies and Cilia. Mol. Biol. Cell 2001, 12, 589–599. [Google Scholar] [CrossRef]
- Ren, Q.; Zhou, J.; Wang, M.G.; Chen, K.M. Pulsed Electromagnetic Fields Stimulating Osteogenic Differentiation and Maturation Involves Primary Cilia-PI3K/AKT Pathway. J. Peking Univ. 2019, 51, 245–251. [Google Scholar]
- Wang, Y.Y.; Xi, H.R.; Shi, W.G.; Zhou, J.; Chen, K.M. Effect of Low-Frequency Pulsed Electromagnetic Fields on Bone Formation in Rat Osteoblasts and Its Mechanism. Acta Acad. Med. Sin. 2019, 41, 21–27. [Google Scholar]
- Zhou, J.; Gao, Y.; Zhu, B.; Shao, J.; Ma, H.; Xian, C.J.; Chen, K. Sinusoidal Electromagnetic Fields Increase Peak Bone Mass in Rats by Activating Wnt10b/Β-Catenin in Primary Cilia of Osteoblasts. J. Bone Miner. Res. 2019, 34, e3704. [Google Scholar] [CrossRef]
- Xie, Y.F.; Shi, W.G.; Zhou, J.; Gao, Y.H.; Li, S.F.; Fang, Q.Q.; Wang, M.G.; Ma, H.P.; Wang, J.F.; Xian, C.J.; et al. Pulsed Electromagnetic Fields Stimulate Osteogenic Differentiation and Maturation of Osteoblasts by Upregulating the Expression of BMPRII Localized at the Base of Primary Cilium. Bone 2016, 93, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Benmerah, A. The Ciliary Pocket. Curr. Opin. Cell Biol. 2013, 25, 78–84. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.A.; Ajbro, K.D.; Koefoed, K.; Vestergaard, M.L.; Veland, I.R.; HenriquesdeJesus, M.P.R.; Pedersen, L.B.; Benmerah, A.; Andersen, C.Y.; Larsen, L.A.; et al. TGF-β Signaling Is Associated with Endocytosis at the Pocket Region of the Primary Cilium. Cell Rep. 2013, 3, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Rattner, J.B.; Sciore, P.; Ou, Y.; Van Der Hoorn, F.A.; Lo, I.K.Y. Primary Cilia in Fibroblast-like Type B Synoviocytes Lie within a Cilium Pit: A Site of Endocytosis. Histol. Histopathol. 2010, 25, 865–875. [Google Scholar]
- Sorkin, A.; von Zastrow, M. Endocytosis and Signalling: Intertwining Molecular Networks. Nat. Rev. Mol. Cell Biol. 2009, 10, 609–622. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Mogensen, J.B.; Christensen, S.T. Endocytic Control of Cellular Signaling at the Primary Cilium. Trends Biochem. Sci. 2016, 41, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Clement, C.A.; Teilmann, S.C.; Pazour, G.J.; Hoffmann, E.K.; Satir, P.; Christensen, S.T. PDGFRαα Signaling Is Regulated through the Primary Cilium in Fibroblasts. Curr. Biol. 2005, 15, 1861–1866. [Google Scholar] [CrossRef]
- Balogh, P.; Katz, S.; Kiss, A.L. The Role of Endocytic Pathways in TGF-β Signaling. Pathol. Oncol. Res. 2013, 19, 141–148. [Google Scholar] [CrossRef]
- Moisescu, M.G.; Leveque, P.; Bertrand, J.R.; Kovacs, E.; Mir, L.M. Microscopic Observation of Living Cells during Their Exposure to Modulated Electromagnetic Fields. Bioelectrochemistry 2008, 74, 9–15. [Google Scholar] [CrossRef]
- Moisescu, M.G.; Leveque, P.; Verjus, M.A.; Kovacs, E.; Mir, L.M. 900 MHz Modulated Electromagnetic Fields Accelerate the Clathrin-Mediated Endocytosis Pathway. Bioelectromagnetics 2009, 30, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Mahrour, N.; Pologea-Moraru, R.; Moisescu, M.G.; Orlowski, S.; Levêque, P.; Mir, L.M. In Vitro Increase of the Fluid-Phase Endocytosis Induced by Pulsed Radiofrequency Electromagnetic Fields: Importance of the Electric Field Component. Biochim. Biophys. Acta-Biomembr. 2005, 1668, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Osera, C.; Fassina, L.; Amadio, M.; Angeletti, F.; Morini, M.; Magenes, G.; Venturini, L.; Biggiogera, M.; Ricevuti, G.; et al. Autophagy Is Modulated in Human Neuroblastoma Cells through Direct Exposition to Low Frequency Electromagnetic Fields. J. Cell. Physiol. 2014, 229, 1776–1786. [Google Scholar] [CrossRef]
- Frahm, J.; Lantow, M.; Lupke, M.; Weiss, D.G.; Simkó, M. Alteration in Cellular Functions in Mouse Macrophages after Exposure to 50 Hz Magnetic Fields. J. Cell. Biochem. 2006, 99, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ge, J.; Guo, B.; Guo, J.; Hao, M.; Wu, Y.; Lin, Y.; La, T.; Yao, P.; Mei, Y.; et al. Extremely Low Frequency Electromagnetic Fields Facilitate Vesicle Endocytosis by Increasing Presynaptic Calcium Channel Expression at a Central Synapse. Sci. Rep. 2016, 6, 21774. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Targa, M.; Corciulo, C.; Gessi, S.; Merighi, S.; Setti, S.; Cadossi, R.; Goldring, M.B.; Borea, P.A.; Varani, K. Pulsed Electromagnetic Fields Increased the Anti-Inflammatory Effect of A2A and A3 Adenosine Receptors in Human T/C-28a2 Chondrocytes and HFOB 1.19 Osteoblasts. PLoS ONE 2013, 8, e65561. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R. Adenosine Receptors as a Biological Pathway for the Anti-Inflammatory and Beneficial Effects of Low Frequency Low Energy Pulsed Electromagnetic Fields. Mediat. Inflamm. 2017, 2017, 2740963. [Google Scholar] [CrossRef]
- Halgamuge, M.N. Supervised Machine Learning Algorithms for Bioelectromagnetics: Prediction Models and Feature Selection Techniques Using Data from Weak Radiofrequency Radiation Effect on Human and Animals Cells. Int. J. Environ. Res. Public Health 2020, 17, 4595. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).