Abstract
Neovascularization is a complex, multistep process that includes the activation of endothelial cells, degradation of the basement membrane surrounding the blood vessel, formation of tip cells, the sprouting, migration and proliferation of endothelial cells into the interstitial space, and then the generation of space in the matrix to allow for the formation of a new, proper lumen of a newly formed blood vessel. Abundant neovascularization can be found in tendinous tissue obtained from asymptomatic athletes or the meniscus early after the injury. The concept of neovascularization in musculoskeletal system disorders seems to be mainly associated with pain and poor clinical outcomes. On the one hand, this phenomenon allows for tissue regeneration, but on the other, it is present during the degeneration process in connective tissue. Establishing the current concept on neovascularization is also needed. A narrative review of the current literature was conducted using databases including Embase, PubMed and Cochrane. This review aims to investigate the exact role of the neovascularization process in tendon and meniscus lesions and its role as a potential target in clinics, specifically in platelet-rich plasma (PRP) therapy. The stabilization of the neovessels required to achieve the healed tissue, together with the standardization of the PRP injections, can offer an alternative future therapeutic approach for the treatment of tendinopathy and meniscal injuries.
1. Introduction
Neovascularization is the natural formation of new blood vessels that serve as collateral circulation in response to poor local perfusion. Abundant neovascularization can be found in tendinous tissue obtained from asymptomatic athletes or the meniscus early after the injury [1]. The neovascularization process is crucial in the connective tissue healing process. During the regeneration, in the formation phase, intensive neovascularization is observed [2]. However, the neovascularization process is also typical for osteoarthritis, retinopathy, inflammation, tumors as well as tendon and meniscus pathology [3,4]. The concept of neovascularization in musculoskeletal system disorders seems to be associated mainly with pain and poor clinical outcomes [5]. On the one hand, this phenomenon allows for tissue regeneration, but on the other, it is present during the degeneration process in connective tissue. Establishing the current concept on neovascularization is also needed.
Hence, this review aims to investigate the exact role of the neovascularization process in musculoskeletal, sport-related pathologies, such as tendinopathies and meniscus lesions. In addition, the role of platelet-rich plasma (PRP) therapy as a potential target in a clinical therapy was analyzed.
2. Neovascularization in Tendon Disorders and Its Therapeutic Potential
Tendons are structures responsible for the distribution of force generated by muscles. They are designed to contribute to human body movements, stabilize joints, and absorb the shocks [6,7,8]. The special properties of tendinous tissue allow for the high load toleration, which results mainly from the organized structure often described as “synthetic climbing rope” [7]. This unique construction of the tendon allows for the spreading of stress and decreases the risk of rupture [7,9]. Tendinous tissue is classified as dense regular connective tissue formed and supported by specialized fibroblasts called tendon cells (tenocytes). The extracellular matrix (ECM), abundant with densely packed and regularly arranged collagen fibrils, forms a scaffold for tenocytes, capillary vessels, nerve endings, and creates an environment for metabolic reactions [10,11]. Tendons are metabolically active structures, and like other tissues, they require a blood supply. However, its vascular perfusion is relatively weak compared to other types of connective tissue, such as muscles [2,12]. In healthy tendons, vascularization is extremely limited, with a small number of capillaries localized between bundles of collagen. These capillaries arise mainly from the musculotendinous junction, osteotendinous junction, and connective tissue sheath [2].
There is a group of tendons that contain specific hypovascular regions that lead to poor regeneration and predispose the tendons to pathology [4,6,7,13,14,15,16,17,18,19]. Moreover, these specific tendons are usually exposed to increased forces with various vectors of action, e.g., the rotator cuff tendons, the long head of the biceps tendon, Achilles tendon, posterior tibialis tendon, patellar tendon, gluteal tendons, and the tibialis anterior tendon.
The excessive load acting on tendinous tissue from these certain regions, in the presence of risk factors, may lead to the development of pathology—tendinopathy, which manifests microscopically as a degenerative process. The etiopathogenesis of tendinopathy indicates hypoxia and an anaerobic environment within degenerated tendinous tissue lesions [20]. These specific conditions activate the metabolic pathways to save tissue from hypoxia, and the abundant expansion of newly formed capillaries followed by the chaotic production of ECM components is observed [21,22,23] (Figure 1).
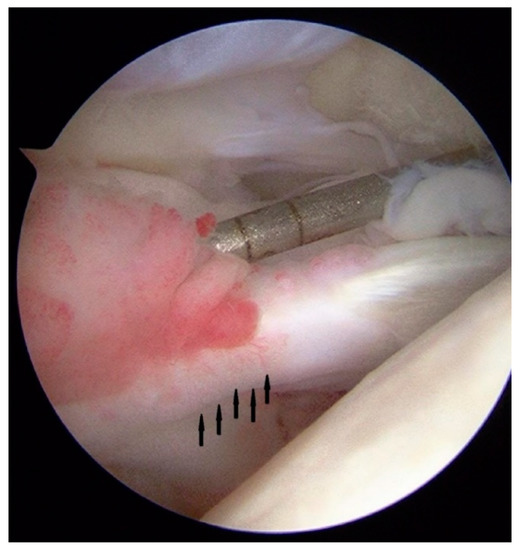
Figure 1.
Arthroscopic view of the long head of the biceps brachii muscle tendon. The neovessels in the proximal part of tendon are seen (arrows) in the patient with symptomatic tendinopathy.
In the 1990s, blood flow in symptomatic tendons assessed by ultrasound (US) and power Doppler modality was described by Newman et al. [24]. Hypothetically, the concomitant nerve ending ingrowth that accompanies new vessel formation is thought to be responsible for pain. Neurovascular ingrowth in painful areas of tendons has been described in a few studies [12,25,26,27]. Moreover, it was supported by the detection of several sensory neurotransmitters within and around the tendons using microdialysis [28]. Additionally, Alfredson and Öhberg presented US-guided techniques to reduce neovascularization by injecting the sclerosants in the most abundant regions of neovascularization [29]. On the other hand, some recent studies revealed that neovascularization in tendon disorders has a mythological status and does not necessarily correspond to the clinical outcome [5,18,20,21,30,31]. Various authors clearly showed that there is no connection between neoangiogenesis and pain [19,30,31]. Neovascularization was also found in Achilles tendons among asymptomatic athletes [1]. Most of the studies which presented the negative impact of neovascularization were related to the Achilles tendon, but more recent research showed that neovessels are detected in 29% of asymptomatic athletes, and in 100% of subjects after strenuous exercise [1,29,32,33,34].
The question arises as to whether the neovascularization found in these studies reflected a physiological or a pathological response. This is especially interesting considering a surprising phenomenon observed in the tendinous tissue of smokers. It indicates that cigarette smoking inhibits the neovascularization process [21,35]. Although neovascularization in smokers was deeply reduced, it did not correlate with the clinical outcomes. It clearly shows that microscopic evaluation of the new vessel formation may not be fully comparable with clinical parameters of patients. Fearon et al. suggested that the complete lack of vascularity in the obtained pathological tendons, as well as the excessive expansion of new capillaries, should be graded as extreme pathology in the histopathological score—the Bonar scoring system [36].
Tendinous tissue after the injury requires the regeneration process, which consists of three main phases, while the most intensive neoangiogenesis is observed in the formation phase [2]. Tendons in response to hypoxia secrete angiogenic growth factors that induce the growth of neovessels [20,37,38]. However, in tendinopathy, we can observe that this mechanism fails, which results in an impaired delivery of oxygen and nutrients, which are required for tissue regeneration.
The ECM regulates the topology and elongation of vessels, using the proteases to form the area for new capillaries [39]. Interestingly, some authors observed that angiogenesis is significantly reduced in the areas of the tendinous tissue with the increased production and aggregation of non-collagenous ECM (glucosaminoglicans and mucopolisacharides) [21,40]. Koehler et al. using a 3D angiogenesis model showed that glycosaminoglycan accumulation impaired the biological activity of vascular endothelial growth factor (VEGF) [41]. Cheng et al. also revealed that non-collagenous components of the ECM inhibit VEGF receptor signaling [42]. VEGF, the major role of which is related to the stimulation of the angiogenesis process, is elevated after the inflammatory phase, during the formation and remodeling phases [7]. Studies in cancer biology or retinopathy revealed that hypoxia-induced newly formed capillaries are hyperpermeable and characterized by impaired perfusion [20,37]. In turn, in tendinopathy, the immaturity of vessels may be responsible for persisting hypoxia within regions of neovascularity [20,37].
From a clinical research perspective, neovascularization can be defined in different ways. The summarized definitions and clinical implications are presented in Table 1.

Table 1.
Different approaches to the neovascularization phenomenon in tendon and meniscus studies.
The experimental evidence that growth factors in platelets enhance the recruitment, proliferation, and differentiation of cells involved in tissue regeneration allowed for the widespread use of PRP in the therapy of tendinopathy [46]. The potential effect of PRP on neovessels has already been extensively studied in Achilles tendinopathy [5].
Based on these studies, it was suggested that the formation of neovessels is related to the release of VEGF as a potential angiogenetic trigger factor. De Vos et al. assessed the role of PRP injection on neovascularization and the echogenicity of the Achilles tendon; however, there were no statistically significant differences between the groups [47].
In turn, Maia et al. presented an animal study showing the effect of PRP injections into an injured flexor tendon [47]. In the PRP-treated group, the flexor tendon was more organized with the proper arrangement of the collagen fibers and fibroblasts in the ECM. The numbers of fibroblasts and blood vessels did not differ between the groups. Similarly, Zabrzynski et al. showed that neovascularization was reduced in the areas of the non-collagenous chaotic matrix formation [21,48].
On the contrary, Kesikburun et al. at a 1-year follow-up after a PRP injection found no effect on improving quality of life, pain, disability, and shoulder range of motion compared to patients with a chronic rotator cuff tear who were treated only with an exercise program [49]. On the other hand, Finnoff et al. presented PRP injection as a safe and effective treatment for chronic recalcitrant tendinopathy. Authors found that 84% of subjects had an improvement in echotexture, 64% had a resolution of intratendinous calcifications, and 82% had a decrease in intratendinous neovascularity [50]. Additionally, Balasubramanian et al., in their systematic review about the clinical effects of PRP therapy in tendinopathy, found that PRP was most effective in patellar and lateral epicondylar tendinopathy [51]. Simultaneously, there was a lack of evidence to support the use of PRP in Achilles and rotator cuff tendinopathy. In turn, Zhang et al. revealed in their meta-analysis that PRP injection with eccentric training did not improve VISA-A scores, reduce tendon thickness, or color Doppler activity in patients with chronic Achilles tendinopathy compared with saline injection [52].
There is a huge discrepancy in clinical outcomes and effects after PRP injection treatment, and there is no sufficient evidence to prove the connection of PRP treatment and its effect on neovascularization. One of the main problems with PRP is the absence of standardization [53]. The large number and variability of the commercially available PRP systems lead to a lack of consistency among studies. The final product can differ between PRP systems. In addition, the content of growth factors can vary between the patients, even if the same PRP system is used. In addition, the potential of a placebo effect can explain the discrepancy in clinical outcomes of randomized, controlled trials.
3. Neovascularization in Meniscal Lesions and Its Therapeutic Potential
Menisci are a vital contributor to proper knee joint congruence and kinematics. Thus, it is suggested that the absence of the meniscus inevitably leads to cartilage lesion and the development of osteoarthritis [54]. Restoration of meniscal integrity is thought to be an essential step in joint preservation surgery. The role of menisci in the knee joint is complex. It is responsible for load transmission, shock absorption, proprioception, articular cartilage nutrition, lubrication, and protection [55,56,57,58]. Meniscal tissue consists of cells suspended in an ECM composed mainly of collagen (type 1), glycoproteins, proteoglycans, and elastin [44]. The disruption in collagen type 1 architecture with progressive loss of proteoglycans are signs of the meniscal degeneration [59]. There is a strong relationship between meniscus degeneration and articular cartilage damage, which inevitably leads to the development of osteoarthritis (OA) [60].
The meniscectomy in osteoarthritis (OA) can lead to a reduction in pain; however, the exact mechanisms are not yet established [44,61]. Day et al. indicated that the nerve fibers and endings were localized in the menisci with concomitant capillary vessels mainly in the outer third portion of the meniscus [62]. Moreover, some studies clearly showed the variations in vascularization and the innervation of the meniscus; however, it is uncertain what is responsible for this phenomenon [63].
The healing potential of the meniscus depends on the blood supply. Blood vessels mainly origin from the medial and lateral geniculate arteries. However, in the mature skeleton, only the peripheral 10–25% of the meniscus receives blood supply from vessels attached to the synovial membrane [45]. This area is called the red-red zone [56]. The inner 10–25% of the meniscus is called the white zone and is nourished by diffusion from the synovial fluid. The transient central region of the meniscus is called the red-white region and has features of each zone. Former recommendation advised suturing menisci only in the red-red zone, but Barber-Westin et al. proved that the meniscus also has regenerative potential in the hypovascular zone, with an 86% rate of healed menisci in the red-white zone [64].
Ashraf et al. investigated the prevalence of vascular and neural ingrowth in OA [44]. The authors observed that in knee joints with more severe arthrosis, there was more abundant collagen degeneration in the outer third of the meniscus and these regions were more vascularized. Moreover, the calcitonin gene-related peptide (CGRP)-immunoreactive nerve profiles were identified alongside blood vessels. Again, it was commonly observed in the outer third part of the meniscus [44]. The inner region of the meniscus was mostly aneural [44]. These results suggest that neoangiogenesis and concomitant nerve ingrowth may contribute to pain in knee OA [44,65]. On the other hand, the neovascularization in the meniscus may be a regenerative response similar to the one observed in the tendinous tissue. The revascularization in the meniscus structure after transplantation is commonly observed and crucial to the healing process [45]. In the last decade, there has been a growing interest in using biological agents to enhance the healing of degenerative tissues in the knee joint [66,67,68]. The efficacy of PRP was examined in meniscal mechanisms under normal and post-traumatic inflammatory conditions in the New Zealand white rabbit model [69]. A reproducible defect on the meniscus was used to implant fibrin glue or PRP. The study showed that PRP treatment increases catabolic molecules, especially those related to inflammation, which may accelerate fibrosis instead of meniscal cartilage regeneration. In human randomized clinical trials, Kamiński et al. showed that PRP augmentation in suturing bucket-handle meniscal tears and trephination for degenerative meniscal tears are more effective than placebo [70,71]. However, Belk et al., in their systematic review, concluded that there is a limited number of high-quality studies comparing outcomes after meniscal repair with or without PRP augmentation [72].
Xue et al. revealed that, after meniscus transplantation, the meniscus body showed new vessel ingrowth mainly at the adhesion margin, while no significant vascular distribution was found at the free margin [45]. Moreover, blood circulation in vessels peaked after 8 weeks in the meniscus, mimicking the VEGF expression, which showed a progressive decrease with time, even though the vascular endothelial cells gradually increased over time. Moreover, there were no statistical differences in the various assessments between the allograft and autograft groups. The authors showed that hypervascular areas are associated with the meniscus attachment and adjoining regions can provide nutritional support for the meniscus body as well as a foundation for the reconstruction of the entire meniscus.
Clinical trials with PRP for tendinopathy and meniscal lesions were summarized in Table 2.

Table 2.
Summarization of therapeutic area and clinical effects of PRP therapy.
4. Conclusions
The rate of tissue turnover is increased in tendinopathic tendons. The persisting hypoxia and anaerobic metabolism lead to the production of poorly organized but highly vascularized tissue. The tissue regeneration requires an abundant supply of oxygen and nutrients. The role of neovascularization in this field should be reconsidered. Detected neovascularization has no additional value for the diagnosis and clinical prognosis. Nevertheless, stabilization of the neovessels is important to achieve the healed tissue. The standardization of PRP systems has not yet been achieved, and clinical outcomes differed between clinical trials. Better knowledge of neovascularization processes is still needed for a future alternative therapeutic approach for the treatment of tendinopathy.
Author Contributions
Conceptualization, D.S. and Ł.J.; methodology, D.S., W.S. and Ł.J.; software, investigation, Ł.J., D.S., M.G. and P.P.; resources, D.S.; data curation; writing—original draft preparation, D.S. and Ł.J.; writing—review and editing, W.S., P.P. and M.G.; visualization, D.S.; supervision M.G.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sengkerij, P.M.; de Vos, R.-J.; Weir, A.; van Weelde, B.J.G.; Tol, J.L. Interobserver Reliability of Neovascularization Score Using Power Doppler Ultrasonography in Midportion Achilles Tendinopathy. Am. J. Sports Med. 2009, 37, 1627–1631. [Google Scholar] [CrossRef]
- Fenwick, S.A.; Hazleman, B.L.; Riley, G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Pękała, P.; Zabrzyński, J.; Kruczyński, J. Genetics in Cartilage Lesions: Basic Science and Therapy Approaches. Int. J. Mol. Sci. 2020, 21, 5430. [Google Scholar] [CrossRef]
- Rees, J.D.; Wilson, A.M.; Wolman, R.L. Current concepts in the management of tendon disorders. Rheumatology (Oxford) 2006, 45, 508–521. [Google Scholar] [CrossRef] [Green Version]
- Tol, J.L.; Spiezia, F.; Maffulli, N. Neovascularization in Achilles tendinopathy: Have we been chasing a red herring? Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1891–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon basic science: Development, repair, regeneration, and healing: Tendon development, injury, and repair. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef] [Green Version]
- Zabrzyński, J.; Łapaj, Ł.; Paczesny, Ł.; Zabrzyńska, A.; Grzanka, D. Tendon-function-related structure, simple healing process and mysterious ageing. Folia Morphol. 2018, 77, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen Structure of Tendon Relates to Function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Murrell, G.A.C. The Basic Science of Tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Zabrzyński, J.; Gagat, M.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D. Electron microscope study of the advanced tendinopathy process of the long head of the biceps brachii tendon treated arthroscopically. Folia Morphol. 2018, 77, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, B. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Kaux, J.-F.; Forthomme, B.; Goff, C.L.; Crielaard, J.-M.; Croisier, J.-L. Current opinions on tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal. Interact. 2006, 6, 181–190. [Google Scholar]
- Gruchow, H.W.; Pelletier, D. An epidemiologic study of tennis elbow: Incidence, recurrence, and effectiveness of prevention strategies. Am. J. Sports Med. 1979, 7, 234–238. [Google Scholar] [CrossRef]
- Grzelak, P.; Polguj, M.; Podgórski, M.; Majos, A.; Krochmalski, M.; Domżalski, M. Patellar ligament hypertrophy evaluated by magnetic resonance imaging in a group of professional weightlifters. Folia Morphol. 2012, 71, 240–244. [Google Scholar]
- Albano, D.; Martinelli, N.; Bianchi, A.; Romeo, G.; Bulfamante, G.; Galia, M.; Sconfienza, L.M. Posterior tibial tendon dysfunction: Clinical and magnetic resonance imaging findings having histology as reference standard. Eur. J. Radiol. 2018, 99, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ditsios, K.; Agathangelidis, F.; Boutsiadis, A.; Karataglis, D.; Papadopoulos, P. Long Head of the Biceps Pathology Combined with Rotator Cuff Tears. Adv. Orthop. 2012, 2012, 405472. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Huri, G.; Gryckiewicz, S.; Çetik, R.M.; Szwedowski, D.; Łapaj, Ł.; Gagat, M.; Paczesny, Ł. Biceps Tenodesis Versus Tenotomy with Fast Rehabilitation Protocol—A Functional Perspective in Chronic Tendinopathy. J. Clin. Med. 2020, 9, 3938. [Google Scholar] [CrossRef]
- Järvinen, T.A. Neovascularisation in tendinopathy: From eradication to stabilisation? Br. J. Sports Med. 2020, 54, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Zabrzynski, J.; Gagat, M.; Paczesny, L.; Grzanka, D.; Huri, G. Correlation between smoking and neovascularization in biceps tendinopathy: A functional preoperative and immunohistochemical study. Ther. Adv. Chronic Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Process of neovascularisation compared with pain intensity in tendinopathy of the long head of the biceps brachii tendon associated with concomitant shoulder disorders, after arthroscopic treatment. Microscopic evaluation supported by immunohistochemical. Folia Morphol. 2018, 77, 378–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.S.; Raza, S.A.; Pilcher, J.; Heron, C.; Poloniecki, J.D. The prevalence of neovascularity in patients clinically diagnosed with rotator cuff tendinopathy. BMC Musculoskelet. Disord. 2009, 10, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, J.S.; Adler, R.S.; Bude, R.O.; Rubin, J.M. Detection of soft-tissue hyperemia: Value of power Doppler sonography. Am. J. Roentgenol. 1994, 163, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Danielson, P. An Emerging Role for Angiogenesis in Tendinopathy. Eur. Musculoskelet. Rev. 2009, 4, 75–76. [Google Scholar]
- Alfredson, H.; Ohberg, L.; Forsgren, S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 334–338. [Google Scholar] [CrossRef]
- Hackett, L.; Millar, N.L.; Lam, P.; Murrell, G.A.C. Are the Symptoms of Calcific Tendinitis Due to Neoinnervation and/or Neovascularization? J. Bone Jt. Surg. 2016, 98, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Knobloch, K. The role of tendon microcirculation in Achilles and patellar tendinopathy. J. Orthop. Surg. 2008, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Alfredson, H.; Öhberg, L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: A double-blind randomised controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 338–344. [Google Scholar] [CrossRef]
- De Jonge, S.; Warnaars, J.L.F.; De Vos, R.J.; Weir, A.; van Schie, H.T.M.; Bierma-Zeinstra, S.M.A.; Verhaar, A.N.; Tol, J.L. Relationship between neovascularization and clinical severity in Achilles tendinopathy in 556 paired measurements. Scand. J. Med. Sci. Sports 2014, 24, 773–778. [Google Scholar] [CrossRef]
- De Marchi, A.; Pozza, S.; Cenna, E.; Cavallo, F.; Gays, G.; Simbula, L.; De Petro, P.; Massè, A.; Massazza, G. In Achilles tendinopathy, the neovascularization, detected by contrast-enhanced ultrasound (CEUS), is abundant but not related to symptoms. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.; Ohberg, L.; Alfredson, H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: Remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1327–1332. [Google Scholar] [CrossRef]
- Ohberg, L.; Alfredson, H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: Pilot study of a new treatment. Br. J. Sports Med. 2002, 36, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Boesen, M.I.; Boesen, A.; Koenig, M.J.; Bliddal, H.; Torp-Pedersen, S. Ultrasonographic investigation of the Achilles tendon in elite badminton players using color Doppler. Am. J. Sports Med. 2006, 34, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.N.; Newton, J.B.; Boorman-Padgett, J.F.; Weiss, S.N.; Nuss, C.A.; Gittings, D.J.; Farber, D.C.; Soslowsky, L.J. Nicotine impairs intra-substance tendon healing after full thickness injury in a rat model. J. Orthop. Res. 2019, 37, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Fearon, A.; Dahlstrom, J.E.; Twin, J.; Cook, J.; Scott, A. The Bonar score revisited: Region of evaluation significantly influences the standardized assessment of tendon degeneration. J. Sci. Med. Sport 2014, 17, 346–350. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, A.; Harris, A.L. Metabolic and hypoxic adaptation to anti-angiogenic therapy: A target for induced essentiality. EMBO Mol. Med. 2015, 7, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef] [PubMed]
- Rivilis, I.; Milkiewicz, M.; Boyd, P.; Goldstein, J.; Brown, M.D.; Egginton, S.; Hansen, F.M.; Hudlicka, O.; Haas, T.L. Differential involvement of MMP-2 and VEGF during muscle stretch-versus shear stress-induced angiogenesis. Am. J. Physiol. Heart. Circ. Physiol. 2002, 283, H1430–H1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, L.T.; Underwood, C.J.; Guilkey, J.E.; Hoying, J.B.; Weiss, J.A. Extracellular Matrix Density Regulates the Rate of Neovessel Growth and Branching in Sprouting Angiogenesis. PLoS ONE 2014, 9, e85178. [Google Scholar] [CrossRef] [Green Version]
- Koehler, L.; Ruiz-Gómez, G.; Balamurugan, K.; Rother, S.; Freyse, J.; Möller, S.; Schnabelrauch, M.; Köhling, S.; Djordjevic, S.; Scharnweber, D.; et al. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci. Rep. 2019, 9, 18143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-J.; Huang, N.-K.; Chang, T.-T.; Ling Wang, D.; Lu, M.-K. Study for anti-angiogenic activities of polysaccharides isolated from Antrodia cinnamomea in endothelial cells. Life Sci. 2005, 76, 3029–3042. [Google Scholar] [CrossRef]
- Ohberg, L.; Lorentzon, R.; Alfredson, H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: An ultrasonographic investigation. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2001, 9, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D.A. Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2011, 70, 523–529. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, L.; Shuang, F.; Zhang, Y.; Zhang, Y.; Luo, D.; Kang, X.; Wang, X.; Hou, S.; Zhong, H. Robust Revascularization, Despite Impaired VEGF Production, After Meniscus Allograft Transplantation in Rabbits. Am. J. Sports Med. 2013, 41, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.J.; Weir, A.; van Schie, H.T.M.; Bierma-Zeinstra, S.M.A.; Verhaar, J.A.N.; Weinans, H.; Tol, J.L. Platelet-Rich Plasma Injection for Chronic Achilles Tendinopathy: A Randomized Controlled Trial. JAMA 2010, 303, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Maia, L.; de Souza, M.V.; Ribeiro Júnior, J.I.; de Oliveira, A.C.; Alves, G.E.S.; dos Anjos Benjamin, L.; Sancler Silva, Y.F.R.; Zandim, B.M.; do Carmo Lopes Moreira, J. Platelet-Rich Plasma in the Treatment of Induced Tendinopathy in Horses: Histologic Evaluation. J. Equine. Vet. Sci. 2009, 29, 618–626. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Gagat, M.; Łapaj, Ł.; Paczesny, Ł.; Yataganbaba, A.; Szwedowski, D.; Huri, G. Relationship between long head of the biceps tendon histopathology and long-term functional results in smokers. A time to reevaluate the Bonar score? Ther. Adv. Chronic Dis. 2021, 12, 204062232199026. [Google Scholar] [CrossRef]
- Kesikburun, S.; Tan, A.K.; Yılmaz, B.; Yaşar, E.; Yazıcıoğlu, K. Platelet-Rich Plasma Injections in the Treatment of Chronic Rotator Cuff Tendinopathy: A Randomized Controlled Trial With 1-Year Follow-up. Am. J. Sports Med. 2013, 41, 2609–2616. [Google Scholar] [CrossRef]
- Finnoff, J.T.; Fowler, S.P.; Lai, J.K.; Santrach, P.J.; Willis, E.A.; Sayeed, Y.A.; Smith, J. Treatment of Chronic Tendinopathy with Ultrasound-Guided Needle Tenotomy and Platelet-Rich Plasma Injection. PM&R 2011, 3, 900–911. [Google Scholar] [CrossRef]
- Balasubramaniam, U.; Dissanayake, R.; Annabell, L. Efficacy of platelet-rich plasma injections in pain associated with chronic tendinopathy: A systematic review. Phys. Sportsmed. 2015, 43, 253–261. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Xu, S.-Z.; Gu, P.-C.; Du, J.-Y.; Cai, Y.-Z.; Zhang, C.; Lin, X.J. Is Platelet-rich Plasma Injection Effective for Chronic Achilles Tendinopathy? A Meta-analysis. Clin. Orthop. 2018, 476, 1633–1641. [Google Scholar] [CrossRef]
- Abate, M.; Di Gregorio, P.; Schiavone, C.; Salini, V.; Tosi, U.; Muttini, A. Platelet Rich Plasma in Tendinopathies: How to Explain the Failure. Int. J. Immunopathol. Pharmacol. 2012, 25, 325–334. [Google Scholar] [CrossRef]
- Lamplot, J.D.; Tompkins, W.P.; Friedman, M.V.; Nguyen, J.T.; Rai, M.F.; Brophy, R.H. Radiographic and Clinical Evidence for Osteoarthritis at Medium-Term Follow-up after Arthroscopic Partial Medial Meniscectomy. Cartilage 2019, 1947603519892315. [Google Scholar] [CrossRef]
- Mordecai, S.C. Treatment of meniscal tears: An evidence based approach. World. J. Orthop. 2014, 5, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.; Koch, M.; Angele, P.; Zellner, J. The role of meniscal repair for prevention of early onset of osteoarthritis. J. Exp. Orthop. 2018, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doral, M.N.; Bilge, O.; Huri, G.; Turhan, E.; Verdonk, R. Modern treatment of meniscal tears. EFORT Open. Rev. 2018, 3, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bryceland, J.K.; Powell, A.J.; Nunn, T. Knee Menisci: Structure, Function, and Management of Pathology. Cartilage 2017, 8, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Mauerhan, D.R.; Honeycutt, P.R.; Kneisl, J.S.; Norton, H.J.; Zinchenko, N.; Gruber, H.E.; Hanley, E.N., Jr. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res. Ther. 2010, 12, R56. [Google Scholar] [CrossRef] [Green Version]
- Englund, M. The role of the meniscus in osteoarthritis genesis. Rheum. Dis. Clin. N. Am. 2008, 34, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Bin, S.-I.; Lee, S.-H.; Kim, C.-W.; Kim, T.-H.; Lee, D.-H. Results of arthroscopic medial meniscectomy in patients with grade IV osteoarthritis of the medial compartment. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 264–268. [Google Scholar] [CrossRef]
- Day, B.; Mackenzie, W.G.; Shim, S.S.; Leung, G. The vascular and nerve supply of the human meniscus. Arthrosc. J. Arthrosc. Relat. Surg. 1985, 1, 58–62. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F. Microvasculature of the human meniscus. Am. J. Sports Med. 1982, 10, 90–95. [Google Scholar] [CrossRef]
- Barber-Westin, S.D.; Noyes, F.R. Clinical Healing Rates of Meniscus Repairs of Tears in the Central-Third (Red-White) Zone. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 134–146. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef] [PubMed]
- Dallo, I.; Szwedowski, D.; Mobasheri, A.; Irlandini, E.; Gobbi, A. A prospective study comparing leukocyte-poor platelet-rich plasma combined with hyaluronic acid and autologous microfragmented adipose tissue in patients with early knee osteoarthritis. Stem Cells Dev. 2021, 30, 651–659. [Google Scholar] [CrossRef]
- Szwedowski, D.; Dallo, I.; Irlandini, E.; Gobbi, A. Osteo-core Plasty: A Minimally Invasive Approach for Subchondral Bone Marrow Lesions of the Knee. Arthrosc. Tech. 2020, 9, e1773–e1777. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Shon, O.-J.; Park, S.-I.; Kim, H.-J.; Kim, S.; Ahn, M.-W.; Do, S.H. Platelet-Rich Plasma Increases the Levels of Catabolic Molecules and Cellular Dedifferentiation in the Meniscus of a Rabbit Model. Int. J. Mol. Sci. 2016, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminski, R.; Maksymowicz-Wleklik, M.; Kulinski, K.; Kozar-Kaminska, K.; Dabrowska-Thing, A.; Pomianowski, S. Short-Term Outcomes of Percutaneous Trephination with a Platelet Rich Plasma Intrameniscal Injection for the Repair of Degenerative Meniscal Lesions. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Int. J. Mol. Sci. 2019, 20, 856. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, R.; Kulinski, K.; Kozar-Kaminska, K.; Wielgus, M.; Langner, M.; Wasko, M.K.; Kowalczewski, J.; Pomianowski, S. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Evaluating Meniscal Healing, Clinical Outcomes, and Safety in Patients Undergoing Meniscal Repair of Unstable, Complete Vertical Meniscal Tears (Bucket Handle) Augmented with Platelet-Rich Plasma. BioMed Res. Int. 2018, 2018, 9315815. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Thon, S.G.; Littlefield, C.P.; Smith, J.H.; McCarty, E.C. Augmentation of Meniscal Repair with Platelet-Rich Plasma: A Systematic Review of Comparative Studies. Orthop. J. Sports Med. 2020, 8, 2325967120926145. [Google Scholar] [CrossRef] [PubMed]
- Boesen, A.P.; Hansen, R.; Boesen, M.I.; Malliaras, P.; Langberg, H. Effect of High-Volume Injection, Platelet-Rich Plasma, and Sham Treatment in Chronic Midportion Achilles Tendinopathy: A Randomized Double-Blinded Prospective Study. Am. J. Sports Med. 2017, 45, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, S.; de Vos, R.J.; Weir, A.; van Schie, H.T.M.; Bierma-Zeinstra, S.M.A.; Verhaar, J.A.N.; Harrie, W.; Tol, J.L. One-Year Follow-up of Platelet-Rich Plasma Treatment in Chronic Achilles Tendinopathy: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Sports Med. 2011, 39, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, M.; Castorina, A.; Vulpiani, M.C.; Baldini, R.; Pavan, A.; Ferretti, A. Platelet-Rich Plasma Versus Focused Shock Waves in the Treatment of Jumper’s Knee in Athletes. Am. J. Sports Med. 2013, 41, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Peerbooms, J.C.; Sluimer, J.; Bruijn, D.J.; Gosens, T. Positive Effect of an Autologous Platelet Concentrate in Lateral Epicondylitis in a Double-Blind Randomized Controlled Trial: Platelet-Rich Plasma Versus Corticosteroid Injection with a 1-Year Follow-up. Am. J. Sports Med. 2010, 38, 255–262. [Google Scholar] [CrossRef]
- Creaney, L.; Wallace, A.; Curtis, M.; Connell, D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: A prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br. J. Sports Med. 2011, 45, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Everhart, J.S.; Cavendish, P.A.; Eikenberry, A.; Magnussen, R.A.; Kaeding, C.C.; Flanigan, D.C. Platelet-Rich Plasma Reduces Failure Risk for Isolated Meniscal Repairs but Provides No Benefit for Meniscal Repairs with Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2019, 47, 1789–1796. [Google Scholar] [CrossRef]
- Pujol, N.; Salle De Chou, E.; Boisrenoult, P.; Beaufils, P. Platelet-rich plasma for open meniscal repair in young patients: Any benefit? Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 51–58. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).