Abstract
The wastewater effluents from textile industries contain highly toxic metal complex dyes. For instance, azo dye has received significant attention owing to its toxicity and environmental stability. This study investigated the oxidation and coagulation processes to effectively remove azo dye from wastewater effluents. Potassium ferrate (K2FeO4) was selected as an oxidant because it has a high oxidation potential, is environmentally stable, and does not generate toxic byproducts. Moreover, it has a combination effect of coagulation and oxidation. Its performance was compared with a single oxidation process (using NaOCl) and a single coagulation process (using FeCl3·6H2O). Based on the jar test experiment, the optimized pH was estimated to be 3 and the optimal dosage was 56.4 mg/L for K2FeO4, and it removed nearly 100% of orange II azo dye (OD) and lissamine green B dye (LGB). However, its removal efficiency decreased when the pH increased to 12. In all processes, dye removal was completed in 5 min of the reaction. Overall, OD and LGB were effectively removed by K2FeO4, compared to the NaOCl and FeCl3·6H2O. This indicates that the combination of oxidation and coagulation of K2FeO4 outperformed the single treatment process without toxic byproduct production.
1. Introduction
The textile industry produces a significant amount of dye effluent, which contains highly toxic metal complex dyes []. As materials can absorb only 20–80% of the dye from dye mixtures, depending on their limited absorption capacity, approximately 10–15% of the dyes are released into effluents [,]. With the increasing use of synthetic chemical dyes over the past few years, a considerable amount of dye-containing industrial wastewater effluent is discharged into aquatic and soil environments [,]. Azo dyes, containing one or more azo groups (-N═N-) in their chemical structure, account for 50–70% of the synthetic dyes that are used in textiles, papers, food, cosmetics, and pharmaceuticals [,]. Given their abundant structural diversity, high molar extinction coefficient, sensitivity to light, and wetness, azo dyes are toxic and mutagenic; moreover, they lead to the discoloration of natural water.
Several approaches have been used for the removal of azo dyes from industrial wastewater. A combination of aerobic and anaerobic processes is carried out on dye effluent because it is cheap and easy to process; however, it is insufficient to remove hazardous particles from dye wastewater [] Biological processes, including adsorption by microbial biomass and microbial degradation [] are not effective for all dyes because of the stability, high water solubility, and high molecular weight of azo compounds []; furthermore, they tend to generate toxic byproducts [,]. In particular, azo dyes cannot be effectively treated by conventional biological processes because they are chemically stable []. Adsorption exhibits excellent removal efficiency, but the cost of adsorbents is significant. Coagulation–flocculation is widely used, but it leads to the generation of sulfur and vat dye effluents. In addition, it produces a considerable amount of sludge. Ion exchange shows good dye removal efficiency, but is limited to certain dyes. Membrane filtration is applied for water recovery; however, the membrane is easily fouled and the initial investment is high []. Chemical oxidation, including ozonation, is also applied for chemical dye removal, but it produces toxic byproducts and has a short half-life [,,]. Wastewater containing azo dyes reduces the amount of light that can penetrate aquatic environments. Moreover, it produces different amines under anaerobic conditions, resulting in serious environmental problems. A recent study reported that H2O2 assisted photoelectrocatalysis for the decolorization and biodegradation of pharmaceutical wastewater [].
Potassium ferrate (K2FeO4), which is an iron (VI) derivative, is a powerful oxidant over a wide pH range; its standard reduction potential varies from +2.2 V to +0.7 V in acidic and basic conditions, respectively [,,]. As compared to chlorine, chlorine dioxide, hydrogen peroxide, and ozone, K2FeO4 works as a strong oxidant for water and wastewater treatment in acidic solutions. The ferrate ion (FeO42−) reduces rapidly and exothermally to Fe (III) and oxygen under strongly acidic conditions. Meanwhile, in strong alkali solutions, it tends to be stable and has a low reduction rate at pH 9.4–9.7. Its decomposition rate dramatically decreases as the pH increases from 7.1 to 11.9 []. In aqueous solution, the ferrate ion (FeO42−) is a monomer with a tetrahedral structure consisting of a high degree of four covalent character equivalent Fe–O bonds []. It is widely used in water treatment because it is stable for up to one year and is easy to prepare. Furthermore, it is stable in organic solvents, but highly soluble in water. Compared to other oxidants, K2FeO4 generates non-toxic products with relatively low amounts of disinfection byproducts (DBPs) []. Moreover, during aqueous oxidation reactions, ferrate (FeO42−) is reduced to Fe (III) ion (Fe3+) and hydrolyzed to form insoluble iron (III) hydroxide (Fe(OH)2). This works as a conventional coagulant, depending on the pH and dosage. Therefore, K2FeO4 can function as an oxidant and a coagulant in a single-step treatment. This paper hypothesized that K2FeO4 can effectively remove different contaminants by multiple treatment mechanisms, namely, the oxidation and coagulation of azo dye, thus proving to be a new, reliable, and low-cost water reuse technology. It could also be ideal for small communities, given its compactly designed operational unit, economical capital cost, and less-demanding management than a conventional two-step unit process.
This study aimed to investigate the treatment efficiency of K2FeO4 for dye wastewater treatment with an emphasis on removing orange II azo dye (OD) and lissamine green B dye (LGB). The treatment efficiency of K2FeO4 in removing OD and LGB was evaluated by comparing its efficiency with that of the conventional NaOCl oxidant and FeCl3·6H2O coagulant.
2. Materials and Methods
2.1. Materials
Orange II azo dye (OD, C16H11N2NaO4S) was obtained from Sigma-Aldrich. Its molecular mass is 350.32 Da (g/mol) and its maximum absorption wavelength (−λ max) is 483 nm. Lissamine green B dye (LGB, C27H25N2NaO7S2), a synthetically produced organic acid dye with two aminophenol groups, was obtained from Sigma-Aldrich (St. Louis, MO, USA). The molecular mass of this dye is 576.6 Da (g/mol) and its maximum absorption wavelength (−λ max) is 633 nm. K2FeO4 (VI) (92% purity, a purple-colored powder) was obtained from Sigma-Aldrich. Iron (III) chloride hexahydrate (FeCl3·6H2O, a yellow-brown solid) was used as a comparative ferric coagulant. Sodium hypochlorite (NaOCl, 6–14% active chlorine) was used as a comparative chlorine oxidant.
2.2. Experimental Procedure
The performance of K2FeO4 as an oxidant and coagulant was evaluated using the jar test. It was used to simulate a full-scale coagulation–oxidation process and determine the optimum conditions for wastewater treatment. The variables of the experiment were set to K2FeO4 dose, dye concentration, contact time, and pH. The jar tester had six paddles that stirred the contents of six 1 L containers.
To start, an equal volume of water sample was added to each of the six beakers. The first sample was used as a control, while the other five samples were adjusted by changing the pH or coagulant/oxidant dose to determine the optimum operating conditions. The pH was adjusted by adding predetermined volumes of 0.1 N NaOH or 0.1 N HCl solutions to the beakers, prior to adding any oxidant and coagulant. The pH was not adjusted after the coagulant/oxidant because the coagulant/oxidant could start hydrolyzing or even precipitating. After the coagulant and/or oxidant were added to each beaker, rapid mixing was conducted at 120 rpm for 1 min. Then, slow mixing was conducted for 30 min to provide the efficient contact. Next, agitation was stopped to allow the flocs to settle. After the jar testing was completed, an adequate sample volume of supernatant liquor was collected from each jar. Once collected, all samples were filtered through a 0.45 µm filter for dye concentration measurement. Each experiment was conducted in triplicates.
2.3. Performance Evaluation of Dye Removal
For pH optimization, 50 mg/L of the dye solutions were reacted with 200 mg/L K2FeO4 in a jar tester at a pH range of 3–12. The dye removal efficiency was examined as a function of the K2FeO4 dose (25, 50, and 100 mg/L as Fe). The dye concentration was varied to 25, 50, and 100 mg/L.
The removal efficiency of K2FeO4 was then compared with that of NaOCl (oxidant) and FeCl3·6H2O (coagulant). The concentration of NaOCl and FeCl3·6H2O increased from 7.05 to 84.6 mg/L. In this test, the dye concentrations were 25 and 50 mg/L.
The removal efficiency was evaluated using ultraviolet-visible (UV-Vis) spectrometry, and the concentrations of OD and LGB were determined at wavelengths of 483 nm and 633 nm, respectively, using a UV-Vis spectrophotometer (HACH, DR 6000). The concentrations of the two types of dyes were derived from the calibration curves obtained through experiments using standard solutions. The dye removal efficiency (R(%)) was calculated using the following equation:
where C0 is the initial dye concentration (mg/L) and C is the final dye concentration (mg/L).
All experiments were conducted in triplicate for reproducibility and reliability. In each plot, the data are presented with standard deviation (error bars). The standard deviation of the results was less than 3%, thus, error bars are not clearly shown.
2.4. Statistics
An analysis of variance was performed on the dye removal experimental group. Three groups were tested for statistical significance between different pH conditions selected as one of the process parameters.
3. Results and Discussion
3.1. Effect of pH on Azo Dye Removal Efficiency by Potassium Ferrate (K2FeO4)
As the oxidation process is significantly affected by pH conditions, optimized pH conditions in K2FeO4 treatment (oxidation–coagulation) were investigated using the remaining azo dyes as a function of time. Figure 1 shows the remaining dye concentration (mg/L) of OD and LGB after reacting with K2FeO4 as a function of time under different pH values (3, 6, 9, and 12). Overall, the removal efficiencies of both dyes by K2FeO4 were enhanced under acidic conditions. The OD and LGB removal efficiencies increased from 57% to 79% when the pH decreased from 12 to 3. However, an increase in contact time did not enhance the performance of K2FeO4 at all pH conditions after 5 min of reaction. Superior performance under acidic conditions was primarily achieved due to the higher reactivity of the K2FeO4. Under acidic conditions, HFeO4− may be generated and reacted with dyes []. Moreover, K2FeO4 is known to be more reactive when the pH is lower than 7.3. Therefore, the optimized pH condition for the removal of dye by K2FeO4 was confirmed to be pH 3. This indicates that the initial solution pH had little impact on its oxidizing power. Meanwhile, K2FeO4 had a much higher oxidation potential at acidic conditions (E0 = 2.20 V) than at basic conditions (E0 = 0.72 V). At pH < 7, although the oxidative ability of ferrate is high, the ferrate is highly unstable. This resulted in more reactive conditions.
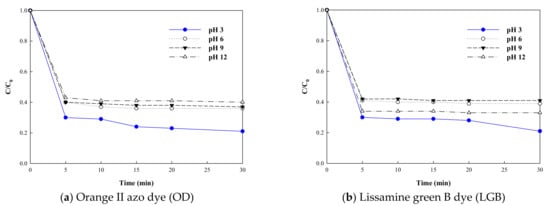
Figure 1.
Effect of pH on the removal efficiency of (a) orange II azo dye (b) and lissamine green B dye by potassium ferrate.
The effect of pH on azo dye removal efficiency was verified by ANOVA. Variation of the pH from 3 to 12 led to statistically significant differences in the removal of OD by K2FeO4. For OD removal by K2FeO4, the F value (46.526) was higher than F-critical (3.239) and the p-value was 3.98E-08 (<0.05). For LGB removal by K2FeO4, the F value (55.94) was higher than F-critical (3.239) and the p-value was 1.06E-08 (<0.05). This indicates that controlling pH in the dye removal experiment led to statistically significant differences in removal.
3.2. Dye Removal Rate by K2FeO4 and NaOCl
Dye removal rates by K2FeO4 and NaOCl treatment were examined by obtaining the slope of ln(Ct) as a function of reaction time (t). First, the dye oxidation behavior of K2FeO4 was compared with that of NaOCl. Figure 2 shows the remaining concentrations of OD and LGB after oxidation as a function of time at pH 3, 6, 9, and 12. As most of the dyes were removed in 5 min, the removal efficiency was compared based on the removal rates at 5 min using the slope (ln(Ct)/t) of ln(Ct) as a function of time. Table 1 lists the reaction rates for all conditions. While K2FeO4 achieved the highest OD removal efficiency (80%) at pH 3, its removal efficiency dropped to 60% as the pH increased to 12, corresponding to a decreasing reaction coefficient (−0.8489). The fastest OD removal rate (−1.1894) was at pH 3. The single oxidation process with NaOCl showed the lowest removal efficiency (~30%) at pH 3 with the slowest reaction rate (-0.6330); however, dye oxidation increased marginally with an increase in pH (to alkali). At pH 12, OD removal using NaOCl was higher than that by K2FeO4. This is because the removal efficiency of K2FeO4 decreases under alkaline conditions (pH 12) [].
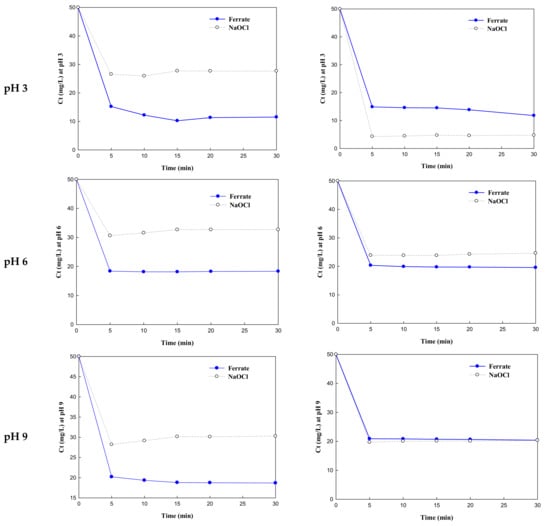
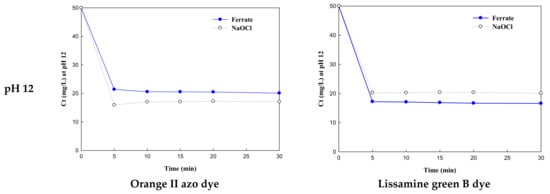
Figure 2.
Reaction rates of potassium ferrate and NaOCl with orange II azo dye and lissamine green B dye under different pH conditions (3, 6, 9, and 12). Dye concentration: 50 mg/L; K2FeO4: 56.40 mg/L as Fe; NaOCl: 56.40 mg/L as Cl2.

Table 1.
Comparison of the removal rates of K2FeO4 vs. NaOCl, and FeCl3·H2O for OD and LGB azo dyes.
Meanwhile, at pH 3, LGB was almost completely removed by NaOCl oxidation; thus, NaOCl performed better than K2FeO4 at pH 3. However, the removal efficiencies of the NaOCl oxidation decreased to 60% at pH 3 with minimal differences between them. There were distinctive differences between OD and LGB, except for the molecular weight. It may be assumed that the decomposition of LGB by hydrochloric acid was proceeded by the injection of hydrochloric acid into the jar tester to adjust the pH to acidic conditions (pH 3). The removal efficiency by oxidation–coagulation using K2FeO4 was slightly higher than that by single oxidation using NaOCl, except at pH 3. Moreover, the marginal decrease in the removal of LGB may be due to the larger molecular weight of LGB in comparison to OD. This indicates that LGB needs additional reaction time for complete removal with K2FeO4, compared to OD.
3.3. Effect of Potassium Ferrate (K2FeO4) Dose on Removal Efficiency
The optimized K2FeO4 dose for effective dye removal was examined at pH 3, which was determined to be the ideal pH condition in Section 3.1. Figure 3 shows the removal efficiency as a function of dosage at different dye (a: OD and b: LGB) concentrations at pH 3. Overall, the removal efficiencies of both dyes increased with increasing K2FeO4 doses. In particular, the OD and LGB concentrations (initial concentration 25 mg/L) decreased rapidly when the K2FeO4 dose was increased from 28 to 42 mg/L. As the K2FeO4 dose was increased to 56.40 mg/L and 70.50 mg/L, the highest OD removal of 99.68% was achieved in 15 min, and the highest LGB removal of 99.68% was achieved in 30 min. This result was better than that observed in previous studies, in which ozonation coupled with a biological aerated filter removed 100% of the azo dye from industrial wastewater in 120 min [] and the advanced oxidation process degraded 100% of the reactive azo dye in 40 min [].
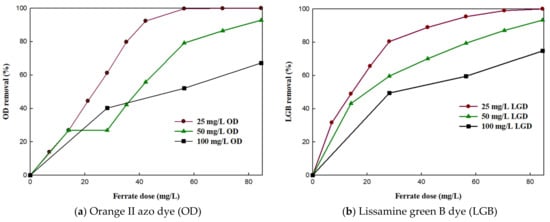
Figure 3.
Effect of potassium ferrate dose on the removal efficiency of (a) orange II azo dye (b) and lissamine green B dye.
The performance of K2FeO4 was compared with that of NaOCl and FeCl3·6H2O. As shown in Figure 4a, K2FeO4 exhibited superior removal efficiency at 25 mg/L of OD. Furthermore, as its dose increased to 60 mg/L, complete removal was achieved. In contrast, FeCl3·6H2O had the lowest removal efficiency (20%), and its performance was not enhanced by an increase in the dose. The removal efficiency of NaOCl increased up to 80% when the OD dose was greater than 80 mg/L; however, it was still lower than that of K2FeO4. Moreover, its performance was reduced when treated with 50 mg/L of OD (Figure 4b). Additionally, 92% of the OD was removed, even though its dose was increased to over 80 mg/L. In contrast, the removal efficiency of NaOCl was reduced to half, even when the dose was increased to 80 mg/L. FeCl3·6H2O showed a marginal increase in the removal of 50 mg/L of OD; however, its removal efficiency was still less than 40%. The superior efficiency of K2FeO4 for dye oxidation was mainly due to the high reactivity and oxidizing capacity of Fe (VI). Based on the oxidizing power, the reduction potential of Fe (VI) (2.20 to 0.70 V) was estimated to be higher than that of ozone (2.076 V), hydrogen peroxide (1.776 V), chlorine (1.358 V), and perchlorate (1.389 V). In addition, ferrate (VI) is quick to oxidize the dye, as compared to permanganate and chromate.
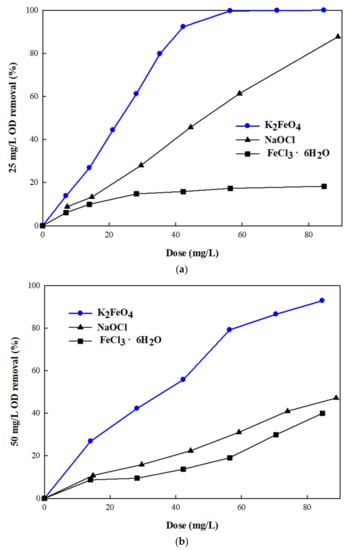
Figure 4.
Comparison of potassium ferrate with NaOCl and FeCl3·6H2O with respect to the removal efficiency of (a) 25 mg/L and (b) 50 mg/L of orange II azo dye at pH 3.
Furthermore, oxidation by K2FeO4 generated subsequent coagulation through its reduction. It synergistically helped in the removal of organic dyes. However, FeCl3·6H2O had a single coagulation effect, resulting in poor removal efficiency.
Similar to OD removal, the combination of oxidation and coagulation exhibited the highest removal efficiency (100%) for LGB. At 25 mg/L, LGB was almost completely removed by K2FeO4 (99.97%) (Figure 5a). The removal efficiency of NaOCl was comparable to that of K2FeO4; that is, it reached a removal efficiency of 96.21% at an LGB dose of 88.81 mg/L. A marginal decrease in removal efficiency was observed for K2FeO4 and NaOCl when the LGB concentration was increased to 50 mg/L, corresponding to 93.26% and 88.57%, respectively. Overall, K2FeO4 and NaOCl exhibited a similar performance for LGB removal. Similar to the OD treatment, FeCl3·6H2O had the poorest removal efficiency (approximately 20%) when treating 25 mg/L and 50 mg/L of LGB (Figure 5b).
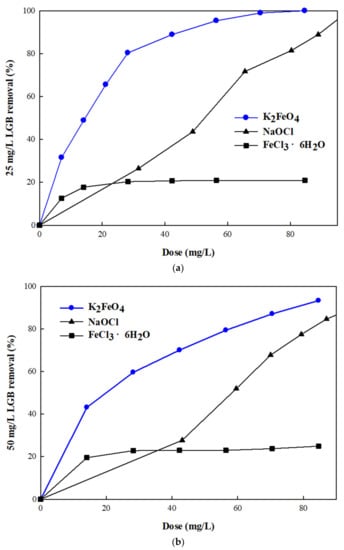
Figure 5.
Comparison of potassium ferrate with NaOCl and FeCl3·6H2O with respect to the removal efficiency of (a) 25 mg/L and (b) 50 mg/L of lissamine green B dye at pH 3.
At the dose of 30 mg/L, K2FeO4 reacted more actively with 25 mg/L of LGB than with 25 mg/L of OD, corresponding to 80% of LGB and 61% of OD removal efficiencies. However, 90% removal of OD (25 mg/L) was achieved at a K2FeO4 dose of 40 mg/L, while 90% of the LGB (25 mg/L) was removed at a K2FeO4 dose of approximately 50 mg/L. A similar trend was observed at 50 mg/L of K2FeO4, indicating that the reaction rate with LGB was faster at the low dose of K2FeO4 (<30 mg/L), while at the higher dose (>30 mg/L) the reaction rate with OD was faster. This is because the relatively large LGB with a higher molecular weight (576.6 Da) reacted more strongly to K2FeO4 at low dosage, as compared to OD (350.32 Da). Meanwhile, the OD removal rate was slowly saturated as a function of the K2FeO4 dose, compared to LGB.
In comparison to OD removal, single oxidation treatment with NaOCl effectively removed LGB, reaching 100% (25 mg/L LGB) and 88.6% (50 mg/L LGB) removal efficiencies as a function of the NaOCl dose, while OD removal efficiency reached 88% (25 mg/L OD) and 40% (50 mg/L OD). Single coagulation treatment with FeCl3·6H2O exhibited the poorest efficiency, removing only 20% of LGB.
The combination of oxidation and coagulation by K2FeO4 occurred in multiple steps, particularly at pH 3, where the highest amount of OD was removed. In contrast, single coagulation by FeCl3·H2O treatment occurred in two steps. The first step of nucleation was completed in 5 min, and most of the OD was removed by coagulation during this period. During the remaining period of nucleation growth, no further OD degradation occurred. Thus, a single coagulation process is not an efficient method for azo dye removal because it takes a longer time and a higher amount of coagulation, as compared to the combination of oxidation and coagulation by ferrate. Oxidation by NaOCl was completed in 5 min at all pH conditions (3–12), and there was no further OD degradation during the remaining period (up to 30 min). In addition, a single coagulation process generates colored coagulated solid wastes, which may result in the toxicity of the sludge produced and an increase in total dissolved solids in the treated wastewater [].
4. Conclusions
Given its high redox potential and simultaneous generation of ferric coagulating species, K2FeO4 was successfully applied to the treatment of dye wastewater. The OD removal efficiency reached 99.7% when 56.4 mg/L of K2FeO4 was used for 15 min of contact time. The LGB removal efficiency was 99.0% using 70.5 mg/L of K2FeO4 over 30 min of contact time. The optimized pH was found to be acidic (pH 3), while contact time had no significant effect on the removal efficiency of NaOCl. Moreover, K2FeO4 exhibited a superior performance in OD and LGB removal, compared to NaOCl as Cl2 and FeCl3·6H2O as Fe (III).
K2FeO4 has the combined effect of oxidation and coagulation, which is useful and important for improving water quality. In comparison with the conventional two-step unit processes (oxidation and coagulation), K2FeO4 is expected to produce a reduced footprint for wastewater treatment. K2FeO4 treatment for azo dye removal has the potential to replace the conventional two-step process as a cost-effective method by synthesizing the K2FeO4 salts on an industrial scale. The K2FeO4 process has a possible application for wastewater treatment plants to treat dye-containing effluent on a real scale.
Author Contributions
Investigation, M.J., S.J.; Formal analysis, M.J.; Methodology, M.J.; Data curation, J.L.; Writing original draft, M.J., J.L.; Funding acquisition, S.J.; Supervision, AJ., S.J.; Writing-review& editing, A.J., S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A3A04004335), the Brain Korea 21 FOUR Project in the Education and Research Center for Infrastructure of Smart Ocean City (i-SOC Center), and Pusan National University Research Grant, 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
OD: orange II azo dye; LGB: lissamine green B dye; K2FeO4: potassium ferrate; ferrate: FeO42−.
References
- Guo, G.; Li, X.; Tian, F.; Liu, T.; Yang, F.; Ding, K.; Liu, C.; Chen, J.; Wang, C. Azo dye decolorization by a halotolerant consortium under microaerophilic conditions. Chemosphere 2020, 244, 125510. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Juang, R.S. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013, 219, 109–117. [Google Scholar] [CrossRef]
- El-Geundi, M.S.; Ismail, H.M.; Attyia, K.M.E. Activated Clay as an Adsorbent for Cationic Dyestuffs. Adsorpt. Sci. Technol. 1995, 12, 109–117. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Labiadh, L.; Barbucci, A.; Cerisola, G.; Gadri, A.; Ammar, S.; Panizza, M. Role of anode material on the electrochemical oxidation of methyl orange. J. Solid State Electrochem. 2015, 19, 3177–3183. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Ludin, N.A.; Mohamad, A.; Kadhum, A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera dentata leaves and Musa acuminata bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta A 2018, 192, 487–498. [Google Scholar] [CrossRef]
- Prasad, A.S.A.; Rao, K.V.B. Aerobic biodegradation of Azo dye by Bacillus cohnii MTCC 3616, an obligately alkaliphilic bacterium and toxicity evaluation of metabolites by different bioassay systems. Appl. Microbiol. Biotechnol. 2013, 97, 7469–7481. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J Env. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Ozkan-Yucel, U.G.; Gokcay, C.F. Effect of Initial Azo Dye Concentration and Biomass Acclimation on Sludge Digestion and Dye Co-treatment. CLEAN Soil Air Water 2010, 38, 387–393. [Google Scholar] [CrossRef]
- Sharma, S.C.D.; Sun, Q.; Li, J.; Wang, Y.; Suanon, F.; Yang, J.; Yu, C.-P. Decolorization of azo dye methyl red by suspended and co-immobilized bacterial cells with mediators anthraquinone-2,6-disulfonate and Fe3O4 nanoparticles. Int. Biodeterior. Biodegrad. 2016, 112, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, X.; Teng, K.; Zhou, B.; Ma, M.; Shan, M.; Jiao, K.; Qian, X.; Fan, J. High flux and rejection of hierarchical composite membranes based on carbon nanotube network and ultrathin electrospun nanofibrous layer for dye removal. J. Membr. Sci. 2017, 535, 94–102. [Google Scholar] [CrossRef]
- Turhan, K.; Turgut, Z. Decolorization of direct dye in textile wastewater by ozonization in a semi-batch bubble column reactor. Desalination 2009, 242, 256–263. [Google Scholar] [CrossRef]
- Lu, X.J.; Yang, B.; Chen, J.H.; Sun, R. Treatment of wastewater containing azo dye reactive brilliant red X-3B using sequential ozonation and upflow biological aerated filter process. J. Hazard. Mater. 2009, 161, 241–245. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abba, A.; MiIno, M.; Arab, H.; Bestetti, M.; Franz, M. Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef]
- Carr, J.D. Kinetics and Product Identification of Oxidation by Ferrate(VI) of Water and Aqueous Nitrogen Containing Solutes. In Ferrates; American Chemical Society: Washington, DC, USA, 2008; Volume 985, pp. 189–196. [Google Scholar]
- Wang, J.; Zheng, T.; Cai, C.; Zhang, Y.; Liu, H. Oxidation of ethanethiol in aqueous alkaline solution by ferrate(VI): Kinetics, stoichiometry and mechanism. Chem. Eng. J. 2019, 361, 1557–1564. [Google Scholar] [CrossRef]
- Sharma, V.K. Potassium ferrate(VI): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Li, C.; Li, X.Z.; Graham, N. A study of the preparation and reactivity of potassium ferrate. Chemosphere 2005, 61, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, M.L.; Schlemper, E.O.; Murmann, R.K. Structure of dipotassium ferrate(VI). Acta Crystallogr. Sect. B 1982, 38, 2237–2239. [Google Scholar] [CrossRef]
- Carr, J.D.; Kelter, P.; Tabatabai, A.; Plichal, D.v.; Erickson, J.; Mclaughlin, C. Properties of Ferrate vi in Aqueous Solution an Alternate Oxidant in Wastewater Treatment. 1985. Available online: https://www.semanticscholar.org/paper/Properties-of-ferrate-vi-in-aqueous-solution-an-in-Carr-Kelter/9fdac66c6273688ce15732940291d5540c8c89e8 (accessed on 21 June 2021).
- Karami, M.; Amin, M.; Nourmoradi, H.; Sadani, M.; Teimouri, F.; Bina, B. Degradation of reactive red 198 from aqueous solutions by advanced oxidation process: O3, O3/H2O2, and persulfate. Int. J. Environ. Health Eng. 2016, 5, 26. [Google Scholar]
- Singh, K.; Arora, S. Removal of Synthetic Textile Dyes From Wastewaters: A Critical Review on Present Treatment Technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).