Abstract
Canola (Brassica napus L.) is cultivated worldwide and utilized as a vegetable oil, biodiesel, and livestock feed. It is also a major living modified (LM) crop alongside corn, soybean, and cotton. Many canola events have been authorized for food, feed, and processing use in South Korea. Concerns about the unintentional release of LM canola into the natural environment have increased environmental monitoring and post-management of living modified organisms (LMOs) is on the rise. The Ministry of Environment (MOE) and the National Institute of Ecology (NIE) conducted an environmental LMO monitoring and post-management project for LM canola from 2014 to 2017. The number of suspicious LM samples gradually increased each year. In this study, a multiplex PCR method was established to detect seven single LM canola events (Topas 19/2, Rf3, Dp-73496-4, Ms8, GT73, Mon88032, and T45) to cover 14 approved LM canola events. This method was utilized to detect 22 LMs out of 260 suspicious canola samples. Thus, this new method is more efficient in terms of time and cost than conventional PCR methods for the identification and monitoring of LMOs.
1. Introduction
The prevalence of living modified organisms (LMOs) that result from advances in biotechnology, especially in modern agriculture, has been rapidly increasing. However, the extent to which LMOs could potentially have adverse effects on the natural environment or human health, remains both unclear and controversial. To address these potential risks, the Convention on Biological Diversity (CBD), which entered into force in December 1993, provided information on the safety of modern biotechnological advancements and this included LMOs [1]. Accordingly, the Cartagena Protocol on Biosafety (CPB) from the CBD was adopted in January 2000 and aimed to maintain an appropriate level of safety for the transfer, handling, and use of LMOs that may negatively affect biodiversity [2].
South Korea signed the CPB in September 2000 and legalized the “Transboundary Movement, Etc. of LMO Act” (herein referred to as LMO Act) in March 2001, to implement the protocol [3]. The CPB and LMO Act in South Korea came into effect in January 2008. The LMO Act designates the national authority for the safe management of LMOs to seven administrative agencies of the South Korean government. The Ministry of Environment (MOE) is in charge of the jurisdiction over LMOs when they are “used to reduce or remove environmental pollutants or to restore the environment,” and when there are “effects on natural ecosystems” [4].
As an affiliated organization of the MOE, the National Institute of Ecology (NIE) has been conducting LMO safety management practices, such as monitoring them in natural environments, the development of detection methods, and risk review/assessments. In particular, due to the concerns about the risks to natural ecosystems from the unintentional release of LMOs, environmental LMO monitoring and post-management projects have been conducted worldwide and have included the primary removal of spilled LMOs, the prevention of their further spread, and successful post-safety management, accompanied by risk assessments [5,6,7]. In 2009, the MOE initiated the natural environmental LMO monitoring and post-management project, to manage those LMOs that may affect ecosystem biodiversity. Since 2014, the NIE has used molecular-based detection methods to monitor and identify unintentionally released LM plants, including canola, corn, cotton, soybean, and alfalfa, in natural environments near import ports, roadsides, stockbreeding farmhouses, and feed factories [8,9].
Canola (Brassica napus), which belongs to the genus Brassica L., in the family Brassicaceae, is used worldwide as a vegetable oil, for biodiesel, and as a livestock feed [10]. It accounts for 12% of the world’s total vegetable oil production after soybean [11]. Together with corn, soybean, and cotton, canola is one of the major LM crops [12]. Furthermore, the total cultivation area and yield of LM crops has been increasing gradually every year since their commercialization. In 2017, it was estimated that there were approximately 33.7 million hectares of global canola cultivation and around 30% (10.2 million hectares) of this was LM canola. Canada has the largest LM canola cultivation area (8.83 million hectares) in the world, followed by the United States at 876,000 hectares, Australia at 492,000 hectares, and Chile at 4051 hectares [13]. Many countries, such as South Korea, Iran, Japan, Malaysia, New Zealand, Singapore, Taiwan, and the European Union, have been importing LMOs including LM canola for food, feed, and processing but do not grow them [13].
Canola and five other species within the genus Brassica have genetic relationships that can be described using U’s triangle model. The three allotetraploid species, B. juncea (AABB genome), B. napus (AACC genome), and B. carinata (BBCC genome), were derived from the hybridization of the following diploid species B. rapa (AA genome), B. nigra (BB genome), and B. oleracea (CC genome). Interspecific hybrids between canola and plants of the same genus, such as B. juncea, B. carinata, B. rapa, B. nigra, and B. oleracea, are possible [14,15,16]. Canola can also hybridize with some species within the family Brassicaceae, including Raphanus raphanistrum, Sinapis arvensis, and Erucastrum gallicum [17,18,19]. Additionally, a previous study indicated that there was spontaneous growth of unintentionally released LM canola around Japanese harbors [20]. Consequently, there is increasing concern in South Korea, regarding the unintentional release of LM canola into the natural ecosystem.
Since 2017, a total of 14 LM canola events, including single genes and stacked traits, have been authorized for food, feed, and processing in South Korea. Furthermore, every year, the numbers of environmental monitoring sites and suspicious LM samples have increased. In this study, we have established a multiplex PCR method to detect seven single LM canola events that would cover the 15 approved LM canola events in South Korea, and the method was applied to identify suspicious LM samples collected from a monitoring project from 2014 to 2017. This study suggests that our new method is more efficient in terms of time and cost than conventional PCR methods used previously in successful LMO monitoring projects.
2. Materials and Methods
2.1. LMO Monitoring Methods and Plant Materials
Since 2009, the MOE and the NIE in South Korea have implemented environmental LMO monitoring and post-management projects. The monitoring results about LM canola conducted from 2014 to 2017 were analyzed in this study. Environmental LMO monitoring was conducted using information about import routes, such as ports, roadsides, stockbreeding farmhouses, and feed factories. Survey sites were investigated at least twice a year, because suspicious canola plants are found in the environment throughout the year. The distribution of the suspected LM canola within a 100 m radius per each survey site was recorded using a global positioning system (GPS), field photos, and environmental information for each investigation site.
Two hundred and sixty suspicious LM canola samples (e.g., leaf or whole plant) were collected for this study. For the DNA analysis, suspicious plants were collected and they were then placed into a paper capsule and stored at 4 °C, after drying with silica gel.
Certified reference materials (CRM) for the LM canola are DP-073496-4 from the Institute for Reference Materials and Measurements (IRMM) and GT73, MON88302, T45, Ms8, Rf3, Topas 19/2, and non-modified canola from the American Oil Chemists’ Society (AOCS); these were used as control samples in the experiments.
2.2. DNA Extraction
Genomic DNA was extracted from the CRMs and dried tissue samples collected under the monitoring project using the Exgene Plant SV mini kit (GeneAll, Korea), according to the manufacturer’s instruction. The concentration of DNA isolated from each sample was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The final concentration of genomic DNA was adjusted to 100 ng/μL for analysis using the newly developed multiplex PCR; the DNA samples were stored at −20 °C until use.
2.3. Primer Design and Multiplex PCR Analysis
An event-specific primer pair for the LM canola was developed using information for the LMO structures and nucleotide sequence data obtained from the Joint Research Centre-European Commission (JRC-EC), International Service for the Acquisition of Agri-biotech Applications (ISAAA), and the National Center for Biotechnology Information (NCBI) [21,22,23,24,25,26,27,28,29]. The primers were designed using canola genome regions and event-specific regions (Table 1). The primers were synthesized by Biofact Inc. (Daejeon, Korea) and diluted to 100 pmol/μL with sterilized water for further use.

Table 1.
Newly designed oligonucleotide primers used to detect the seven living modified canola events.
Polymerase chain reactions (PCR) were conducted using AccuPower® Gold Multiplex PCR PreMix (Bioneer, Korea), containing 100 ng of each template DNA and 1 μL of each primer (5 pmol for Topas 19/2 and Rf3 and 2.5 pmol for Dp-73496-4, Ms8, GT73, Mon88032, and T45). The multiplex PCR conditions were as follows: one cycle for 30 s at 94 °C (denaturation), 13 cycles for 30 s at 65 °C with a reducing temperature of 0.7 °C per cycle (annealing), and 1 min at 72 °C (extension); followed by 23 cycles for 30 s at 94 °C, 30 s at 56 °C, and 1 min at 72 °C [30]. Genomic DNA was amplified using the ProFlex PCR System (Applied Biosystems, Waltham, MA, USA). The multiplex PCR method was used to analyze 260 suspicious volunteer samples collected under the LMO monitoring project. The amplified products were separated by electrophoresis on a 2.0% agarose gel at 135 V for 25 min and the gel images were visualized using the ChemiDoc XRS+ Imaging System (Bio-Rad, Hercules, CA, USA).
3. Results and Discussion
3.1. The Necessity of the Multiplex PCR Technique for the Environmental LMO Monitoring Project
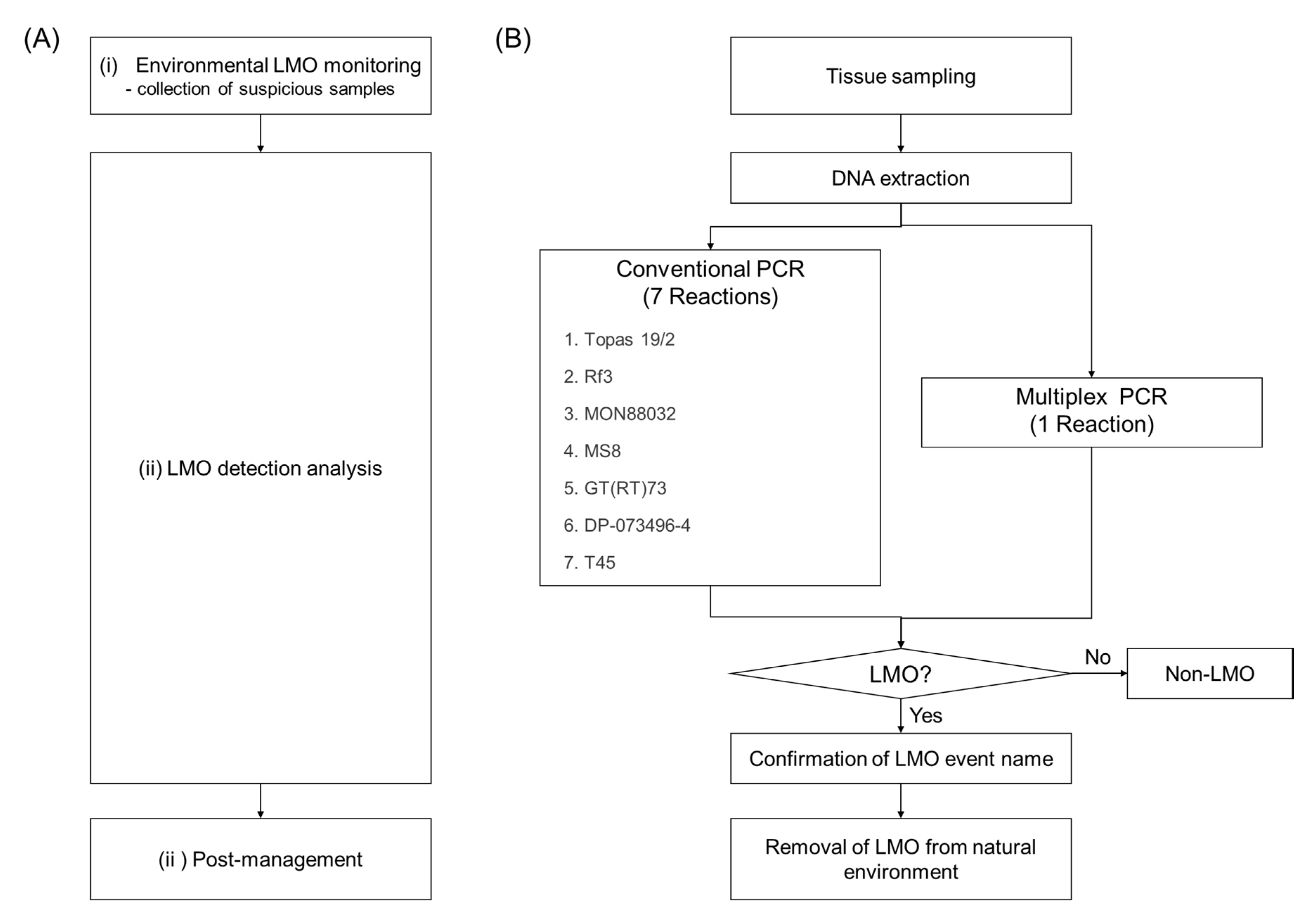
The process of the natural environmental LMO monitoring and post-management project developed by the MOE and NIE was conducted in three steps: (i) environmental LMO monitoring, (ii) LMO detection analysis, and (iii) post-management (Figure 1). LMO environmental monitoring sites were determined according to the information available for the import ports, roadsides, feed factories, and stockbreeding farmhouses. The LMO monitoring was conducted in the surrounding natural environments of the sites that were identified as having a potential risk for LMO release. Tissue samples were collected from suspicious LMOs and they were identified using PCR. Every year, the NIE develops detection methods for single events, to help detect the LMOs. However, these single detection methods require many PCR reactions. To efficiently analyze the many suspicious canola samples collected in four years, a new LMO identification process using the developed multiplex PCR method, which is more efficient than the conventional PCR methods, is necessary. It takes seven reactions to analyze seven LM canola events using the single PCR method, but would take only one reaction using a multiplex PCR system (Figure 1B). The development of multiplex PCR methods is thus necessary to overcome the disadvantages of the current methods, such as high cost and time requirements.
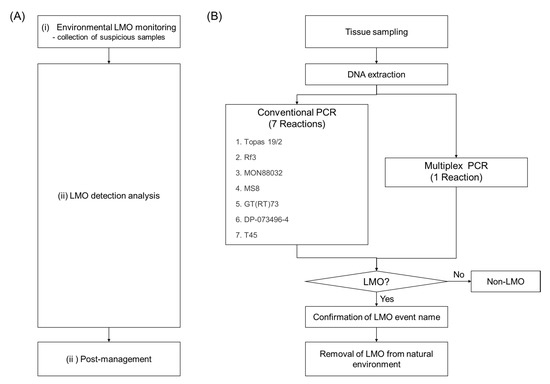
Figure 1.
Processing scheme for the environmental living modified organism (LMO) monitoring and post-management project. (A) Three steps for the LMO monitoring. (B) Flowchart to show the efficiency of the multiplex PCR for the LMO detection analysis.
3.2. LMO Monitoring in the Natural Environment for Canola
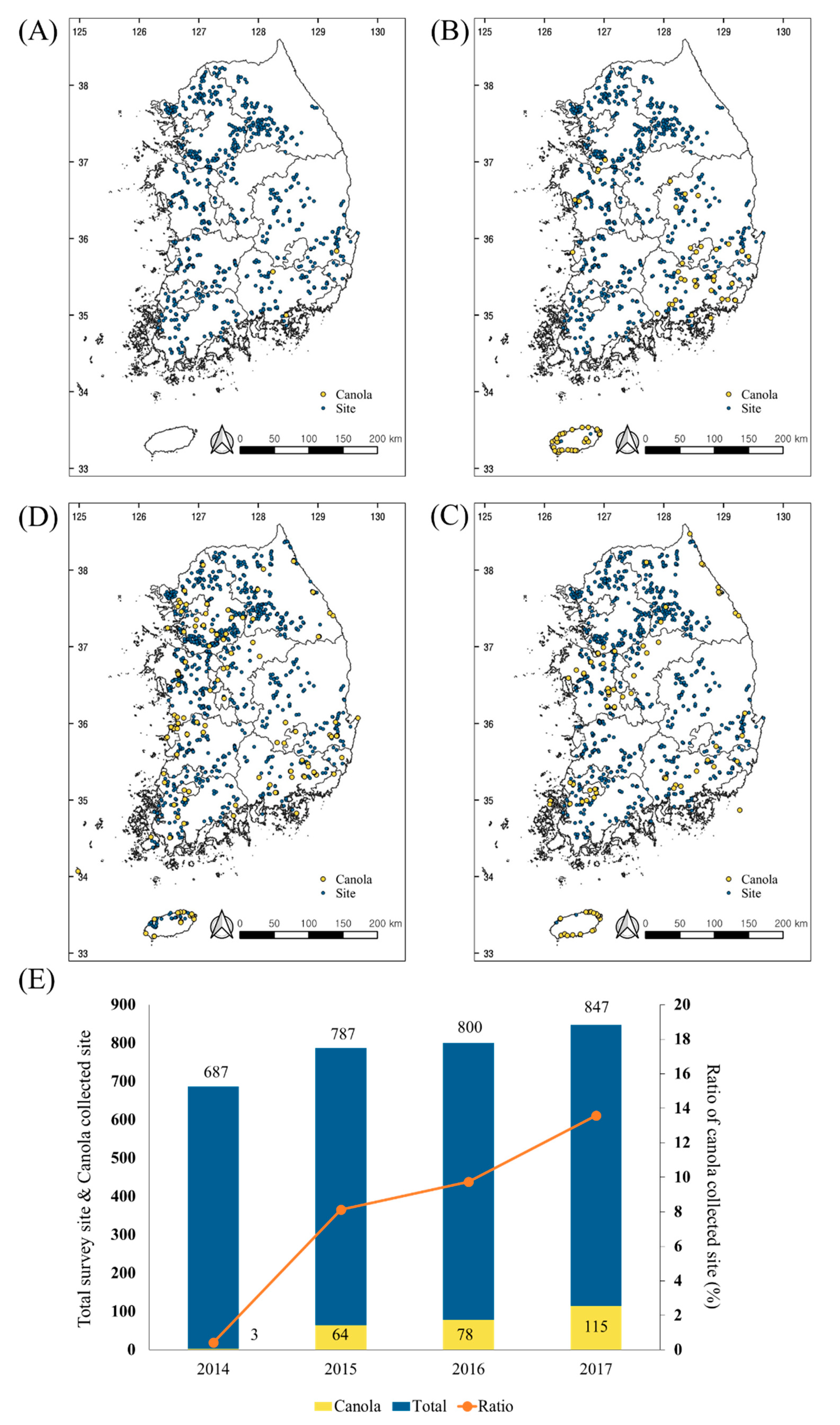
The Environmental LMO Monitoring and Post-Management Project is operated by the NIE, which is part of the MOE. LM canola has been monitored in the environment since 2009, in five provinces, including Gyeonggi-do, Gyeongsang-do, Chungcheong-do, Jeolla-do, and Gangwon-do. Jeju-do has also been included as an investigation site since 2015. Over a four-year period, 3121 environmental sites were investigated: 687 in 2014, 787 in 2015, 800 in 2016, and 847 in 2017 (Figure 2; blue). Suspicious LM canola samples were collected from 260 sites: 3 in 2014, 65 in 2015, 78 in 2016, and 114 in 2017 (Figure 2; yellow). Although suspicious samples were only found at three sites in Gyeongsang-do in 2014, there was a remarkable increase in the suspicious LM canola samples between 2015 and 2017 (Figure 2E). The most suspicious LM canola samples were collected from roadsides along transportation routes for food and feed factories. The number of LM canola volunteers has been increasing annually since 2014. These results suggest the need for a novel multiplex PCR method for Topas 19/2, Rf3, Dp-73496-4, Ms8, GT73, Mon88032, and T45 to identify LM canola of the 260 volunteer samples.
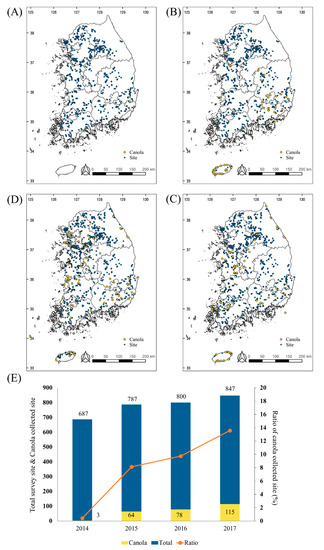
Figure 2.
Living modified organism (LMO) monitoring sites in South Korea from 2014 to 2017. The maps show the monitoring survey sites (blue spot) and collection sites of suspicious canola samples (yellow spot) in 2014 (A), 2015 (B), 2016 (C), and 2017 (D). The bar graph indicates the number of survey sites and collection sites for the suspicious samples. The red line showed the ratio of suspicious canola collection sites compared to the survey sites (E).
3.3. Establishment of a Novel Multiplex PCR Method
A novel multiplex PCR method was developed to detect seven LM canola events, including Topas 19/2, Rf3, Dp-073496-4, Ms8, GT73, Mon88032, and T45. To increase the primer efficiency and accuracy, they were all designed to be contained within the canola genome region and transgenic region. For visible analysis on the agarose gel, primer pairs of various sizes in the range of 95–550 bp were selected. The PCR product sizes for the seven LM canola events were expected to be 95 bp (Topas 19/2), 139 bp (Rf3), 219 bp (Dp-73496-4), 249 bp (Ms8), 317 bp (GT73), 407 bp (Mon88032), and 550 bp (T45) (Table 1).
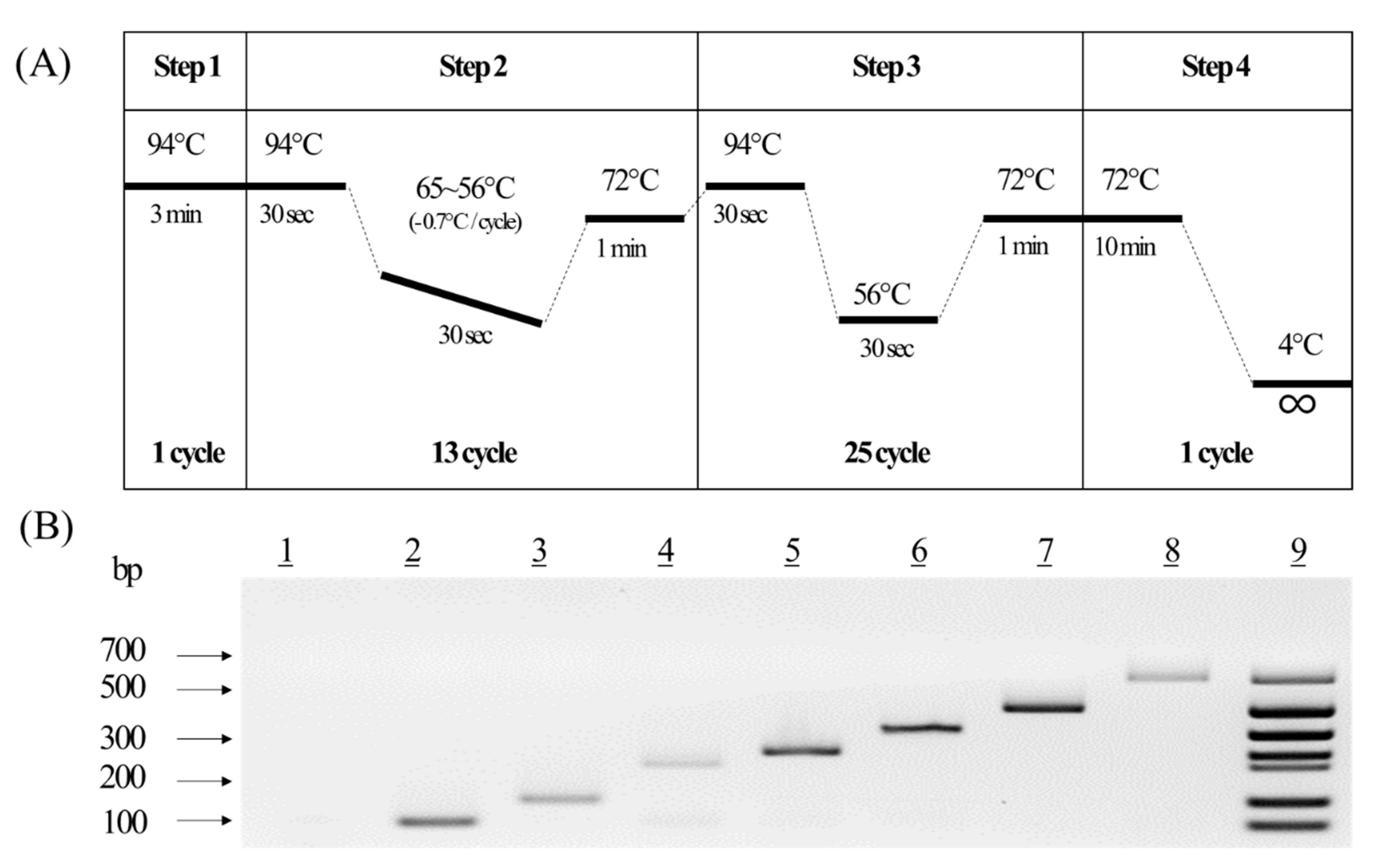
To develop a novel multiplex PCR method to detect the LM canola events, primer pairs of various sizes for each event were designed and tested to confirm the efficiency and accuracy of the PCR analysis. The specificity of the primer pairs was confirmed using one event genomic DNA from each CRM (seven events and one non-LM). The agarose gel image of the results showed a high efficiency for the primer pairs, as they detected only a single band for each event. All the bands for the PCR products were confirmed to be their expected sizes (data not shown). These results indicated that each of the seven designed primer pairs amplified only the desired event. Although the novel primer pairs were confirmed to be able to decipher one event from another using genomic DNA, many stacked LM products, including those with more than one gene, were imported into South Korea. To detect multiple genes simultaneously in the seven LM canola, various PCR parameters, such as primer concentration and Tm value for multiplex PCR analysis, were tested (Supplementary Figure S1). Conclusively, the touchdown PCR method was chosen to increase the efficiency and visibility of the PCR amplification (Figure 3A). The newly developed multiplex PCR methods were confirmed using a mixture of seven primer pairs. The resulting agarose gel image showed a high efficiency by representing only a single band for each event and multiple bands in the DNA mixture (Figure 3B). The developed multiplex PCR method was, thus, determined to be suitable for the quantitative analysis of the LM canola events.
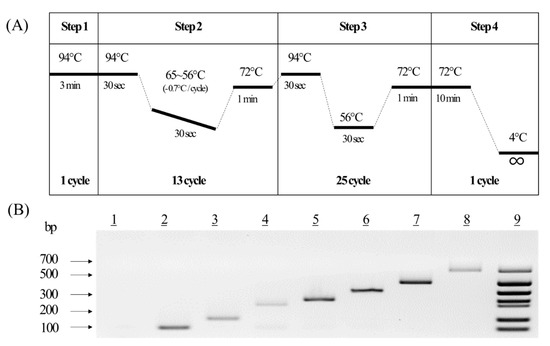
Figure 3.
Establishment of a novel multiplex PCR for seven LM canola events. (A) Schematic diagram of the multiplex PCR conditions. (B) Agarose gel image of the products from the multiplex PCR method for the LM canola, in which each line indicates a non-LM PCR product (line 1), Topas 19/2 (line 2), Rf3 (line 3), Dp-073496-4 (line 4), Ms8 (line 5), GT73 (line 6), Mon88032 (line 7), T45 (line 8), and the multiplex PCR product (line 9).
3.4. Detection of LM Canola Using the Newly Developed Multiplex PCR Method
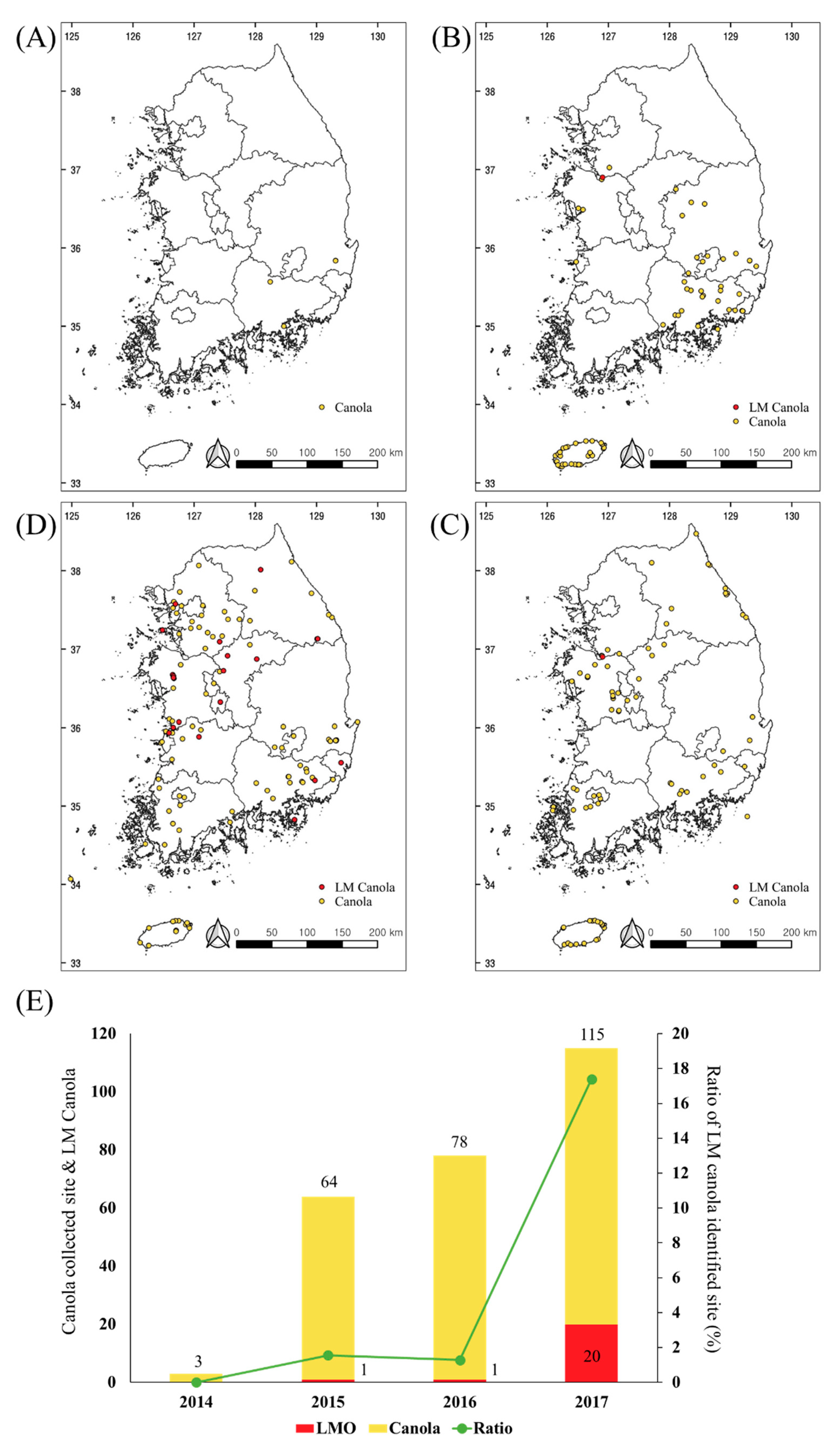
The novel multiplex PCR method was utilized to detect the seven LM canola in 260 monitoring samples, from 2014 to 2017 in South Korea. As a result, 22 samples were identified as being LM canola (Figure 4). All the LM canola identified were GT73. To confirm the detection of the GT73, nucleotide sequencing analysis was performed and all the results indicated that the target region was detected correctly (data not shown). In 2014, there were no LM signals detected in any of the samples, indicating that they were non-LM canola. Positive bands were identified from 2015 to 2017, 1 in 2015, 1 in 2016, and 20 in 2017 (Figure 5). In particular, the LM canola was identified nationwide and dramatically increased in 2017. The increase is possibly because LM canola, unapproved as a cultivation crop, was mixed with non-LM canola and grown in 56 places nationwide in 2017. The canola plants identified as LMO were eradicated and the LMO sites were monitored for the next five years.

Figure 4.
Application of the multiplex PCR method for living modified organism (LMO) identification with the collected suspicious canola samples. The numbers indicate the collection year of the suspicious canola samples (red) and are identified as LM canola (yellow).
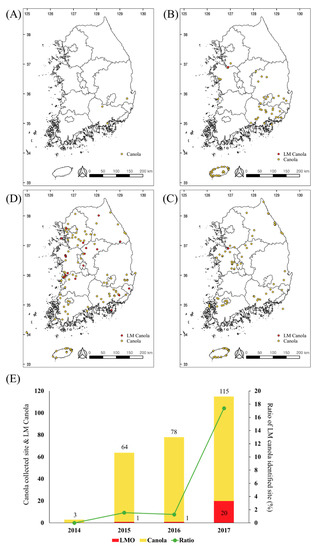
Figure 5.
Living modified organism (LMO) identification in the samples collected from 2014 to 2017. The maps show the collection sites of the suspicious canola samples (yellow spot) and the sites of the LM canola (red spot) in 2014 (A), 2015 (B), 2016 (C), and 2017 (D). The bar graph presents the number of collection sites for the suspicious samples (yellow) and the LM canola samples (red). The green line shows the ratio of the LM canola identified sites per sample collection site (E).
4. Conclusions
LM canola is cultivated worldwide and utilized for vegetable oil, biodiesel, and livestock feed. In South Korea, LM crop cultivation is prohibited, even though LM crops have been approved for use as food and feed, and for processing. Consequently, the LM canola used in South Korea must be imported. However, concern has been increasing regarding the unintentional release of LM canola. In response, since 2009, the MOE in South Korea has regularly conducted environmental LMO monitoring and post-management projects. At present, a total of 17 LM canola events, including stacked traits and single genes, have been authorized for import to South Korea and in the future new LMO events will be developed and approved and, consequently, will require updated detection methods. The monitoring and post-management project should thus be expanded to eradicate the negative effects of LMOs in the ecosystem of the whole nation.
In this study, unintentionally released LM canola events were monitored and detected using a novel multiplex PCR method in natural populations, from 2014 to 2017. Although many single PCR-based detection methods have previously been developed that use sequence information, such as the 35S promoter and T-NOS terminator, this new technique is unique and distinctive, as it allows for the simultaneous detection of several newly approved LMO events. The novel detection method developed by the NIE for identifying LMOs, under the circumstance of continuous release of LM canola into the natural environment, is cost-effective and time saving. Consequently, this method reduces analysis time and costs, increases accuracy, and makes comparative analysis more convenient. The 260 suspicious canola samples identified from the 3121 monitoring sites were analyzed using this newly developed method. The results of its application clearly showed that 22 samples were identified as LMO and were eradicated in the natural environment. Based on these results, environmental LMO monitoring and post-management projects using the newly developed multiplex PCR analysis could improve the management of natural environments and LMO spread in many countries, including South Korea.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/21/7721/s1, Figure S1: Analysis using the novel multiplex PCR under various conditions for seven LM canola events.
Author Contributions
Conceptualization, I.R.K. and J.R.L.; methodology, I.R.K. and J.R.L.; validation, I.R.K. and J.R.L.; formal analysis, I.R.K., H.S.L. and J.R.L.; investigation, I.R.K., H.S.L., W.C., D.I.K. and J.R.L.; resources, I.R.K. and J.R.L.; data curation, I.R.K. and J.R.L.; writing—original draft preparation, I.R.K. and J.R.L.; writing—review and editing, S.Y.L. and J.R.L.; visualization, I.R.K., D.I.K. and J.R.L.; supervision, S.Y.L. and J.R.L.; project administration, J.R.L.; funding acquisition, S.Y.L. and J.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Institute of Ecology (NIE), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIE-A-2020-06 and NIE-A-2020-07); and by the NG-BioGreen 21 Program (SSAC, Grant #PJ013173), RDA, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CBD. Convention on Biological Diversity. Available online: https://www.cbd.int (accessed on 10 October 2020).
- CPB. The Cartagena Protocol on Biosafety. Available online: https://bch.cbd.int/protocol/text/ (accessed on 10 October 2020).
- Jang, H.-M. Guideline for managing research facilities and LMOs for R&D by the Act on transboundary movement of LMOs, etc. J. Plant Biotechnol. 2008, 35, 5–12. [Google Scholar] [CrossRef]
- House, K.B.C. Biosafety White Paper 2017; Korea Biosafety Clearing House: Daejeon, Korea, 2017. [Google Scholar]
- Paulauskas, A.; Jodinskas, G.; Lygis, D.; Jodinskiene, M. GMO monitoring system in Lithuania. J. Verbrauch. Lebensm. 2009, 3, 32–35. [Google Scholar] [CrossRef]
- Kleppin, L.; Schmidt, G.; Schröder, W. Cultivation of GMO in Germany: Support of monitoring and coexistence issues by WebGIS technology. Environ. Sci. Eur. 2011, 23, 1–11. [Google Scholar] [CrossRef]
- Züghart, W.; Benzler, A.; Berhorn, F.; Sukopp, U.; Graef, F. Determining indicators, methods and sites for monitoring potential adverse effects of genetically modified plants to the environment: The legal and conceptual framework for implementation. Euphytica 2008, 164, 845–852. [Google Scholar] [CrossRef]
- Lee, J.R.; Lim, H.S.; Choi, W.; Park, J.H.; Jung, Y.J.; Kim, D.W.; Kim, I.R.; Yoo, S.H.; Seol, M.-A.; Han, S.M. Study on Environmental Monitoring and Post-Management of LMO; National Institute of Ecology: Seocheon-gun, Korea, 2019. [Google Scholar]
- Shin, S.Y.; Moon, J.C.; Choi, W.; Kim, I.R.; Jo, B.-H.; Lee, J.R. Detection and environmental unintentional release monitoring of living modified maize (Zea mays L.) in Gyeonggi-do of South Korea in 2014. J. Plant Biotechnol. 2018, 45, 77–82. [Google Scholar] [CrossRef]
- Myers, R. Growing Canola for Oilseed or Cover Crop Use; University of Missouri Extension: Columbia, MO, USA, 2018; pp. 1–7. [Google Scholar]
- US Department of Agriculture, Agricultural Research Service. Oilseeds: World Markets and Trade; Foreign Agricultural Service, USDA: Washington, DC, USA, 2019.
- Brookes, G.; Barfoot, P. Global income and production impacts of using GM crop technology 1996–2014. GM Crops Food 2016, 7, 38–77. [Google Scholar] [CrossRef] [PubMed]
- Briefs, I. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. 2017; p. 53. Available online: http://www.agi.gov.vn/files/files/ISAAA/ISAAA%20Brief%20No_%2053%20-%202017_compressed.pdf (accessed on 31 October 2020).
- Meng, J.; Shi, S.; Gan, L.; Li, Z.; Qu, X. The production of yellow-seeded Brassica napus (AACC) through crossing interspecific hybrids of B. campestris (AA) and B. carinata (BBCC) with B. napus. Euphytica 1998, 103, 329–333. [Google Scholar] [CrossRef]
- Bing, D.; Downey, R.; Rakow, G. Hybridizations among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the field. Plant Breed. 1996, 115, 470–473. [Google Scholar] [CrossRef]
- Ford, C.S.; Allainguillaume, J.; Grilli-Chantler, P.; Cuccato, G.; Allender, C.J.; Wilkinson, M.J. Spontaneous gene flow from rapeseed (Brassica napus) to wild Brassica oleracea. Proc. R. Soc. B Biol. Sci. 2006, 273, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Warwick, S.; Simard, M.-J.; Légère, A.; Beckie, H.; Braun, L.; Zhu, B.; Mason, P.; Séguin-Swartz, G.; Stewart, C. Hybridization between transgenic Brassica napus L. and its wild relatives: Brassica rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) OE Schulz. Theor. Appl. Genet. 2003, 107, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Lefol, E.; S´eguin-Swartz, G.; Downey, R.K. Sexual hybridisation in crosses of cultivated Brassica species with the crucifers Erucastrum gallicum and Raphanus raphanistrum: Potential for gene introgression. Euphytica 1997, 95, 127–139. [Google Scholar] [CrossRef]
- Snowdon, R.K.; Winter, H.; Diestel, A.; Sacristán, M.D. Development and characterisation of Brassica napus-Sinapis arvensis addition lines exhibiting resistance to Leptosphaeria maculans. Theor. Appl. Genet. 2000, 101, 1008–1014. [Google Scholar] [CrossRef]
- Kawata, M.; Murakami, K.; Ishikawa, T. Dispersal and persistence of genetically modified oilseed rape around Japanese harbors. Environ. Sci. Pollut. Res. 2009, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- JRC. Joint Research Centre. Available online: http://gmo-crl.jrc.ec.europa.eu/gmomethods (accessed on 10 October 2020).
- ISAAA. International Service for the Acquisition of Agri-Biotech Applications. Available online: http://www.isaaa.org/gmapprovaldatabase/ (accessed on 10 October 2020).
- Mazzara, M.; Luque-Perez, E.; Bevilacqua, A.; Van den Eede, G. In-house validation of an Event-specific Method for the Quantification of Oliseed Rape Topas 19/2 using Real-time PCR. JCR Sci. Tech. Rep. 2011, 1–17. [Google Scholar] [CrossRef]
- Jacchia, S.; Bogni, A.; Mazzara, M.; Kreysa, J. Event-Specific Method for the Quantification of Oilseed Rape DP-073496-4 Using Real-Time PCR; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2014. [Google Scholar]
- Mazzara, M.; Bogni, A.; Savini, C.; Van Den Eede, G. Event-specific Method for the Quantification of Oilseed Rape Line Ms8 Using Real-Time PCR; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2007. [Google Scholar]
- Mazzara, M.; Grazioli, E.; Savini, C.; Van Den Eede, G. Event-Specific Method for the Quantification of Oilseed Rape Line RT73 Using Real-Time PCR; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2007; pp. 1–108. [Google Scholar]
- Mazzara, M.; Savini, C.; Bogni, A.; Van Den Eede, G. Event-Specific Method for the Quantification of Oilseed Rape Line Rf3 Using Real-Time PCR v. 1.01; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2013. [Google Scholar]
- Savini, C.; Bogni, A.; Mazzara, M.; Kreysa, J. Event-Specific Method for the Quantification of Oilseed Rape MON88302 by Real-Time PCR; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2013. [Google Scholar]
- Savini, C.; Sacco, M.G.; Mazzara, M.; Kreysa, J. Event-specific Method for the Quantification of Soybean DAS-68416-4 Using Real-Time PCR; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2014. [Google Scholar]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Lee, T.V.d.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).