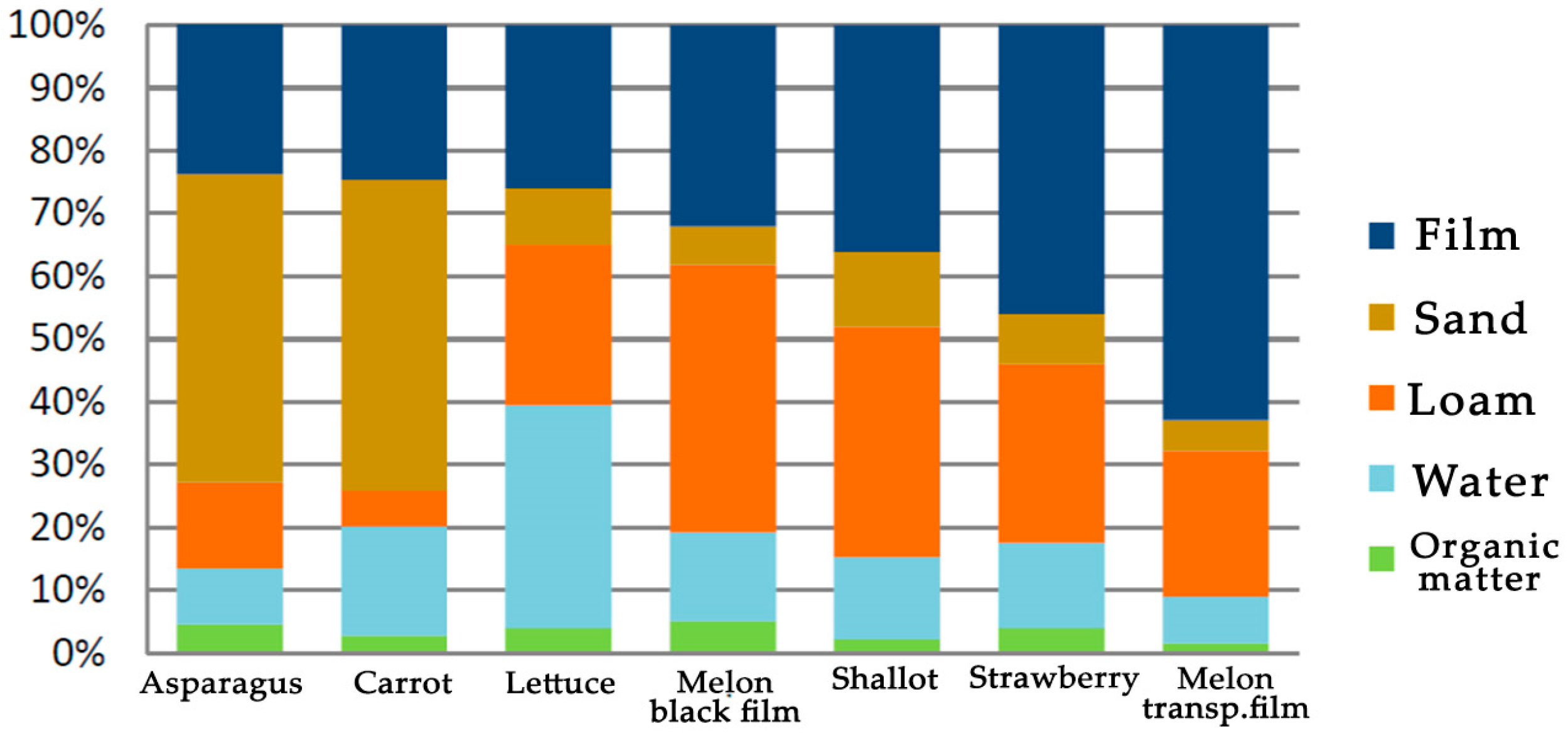
Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives
Abstract
1. Introduction
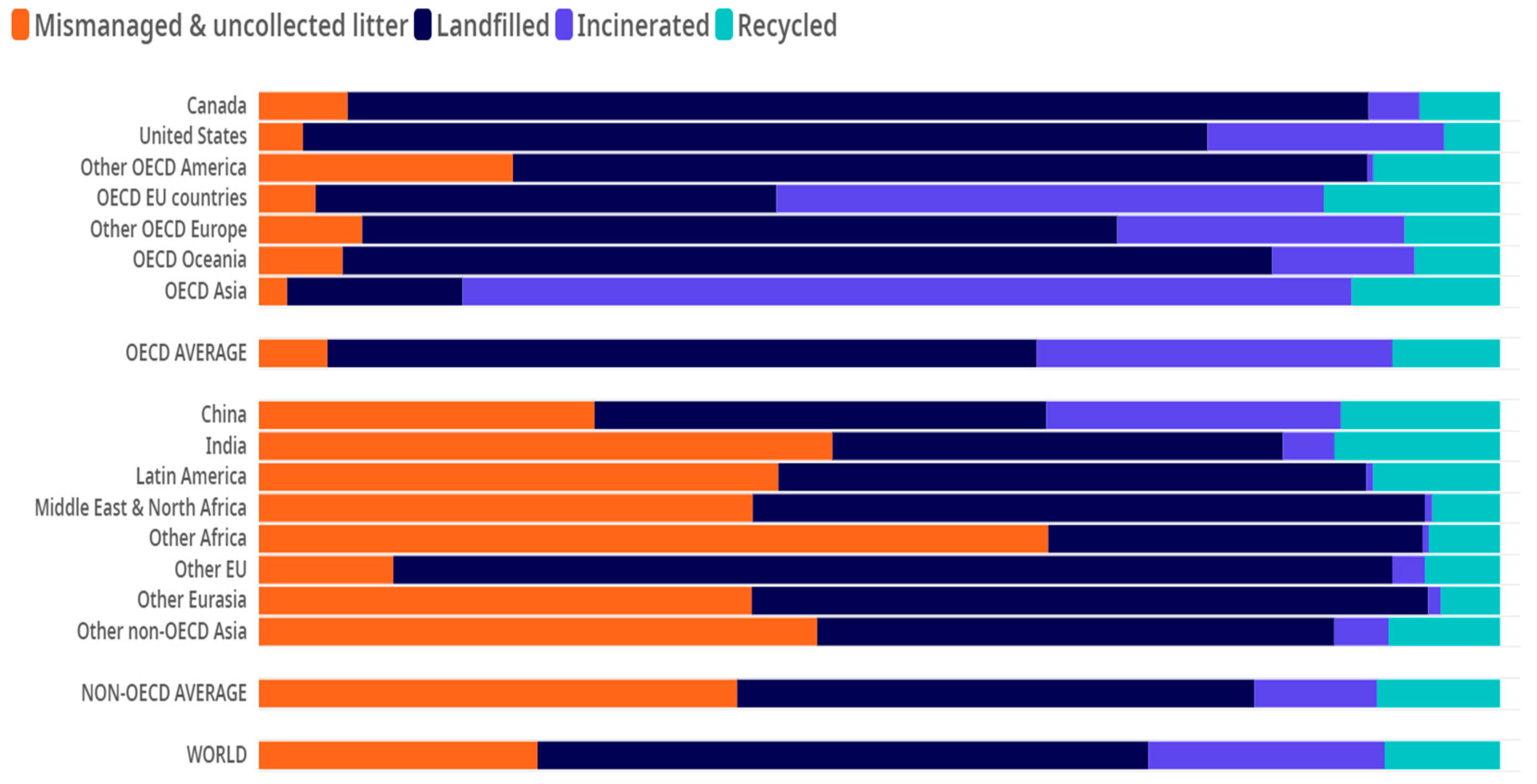
2. Plastic in the Environment
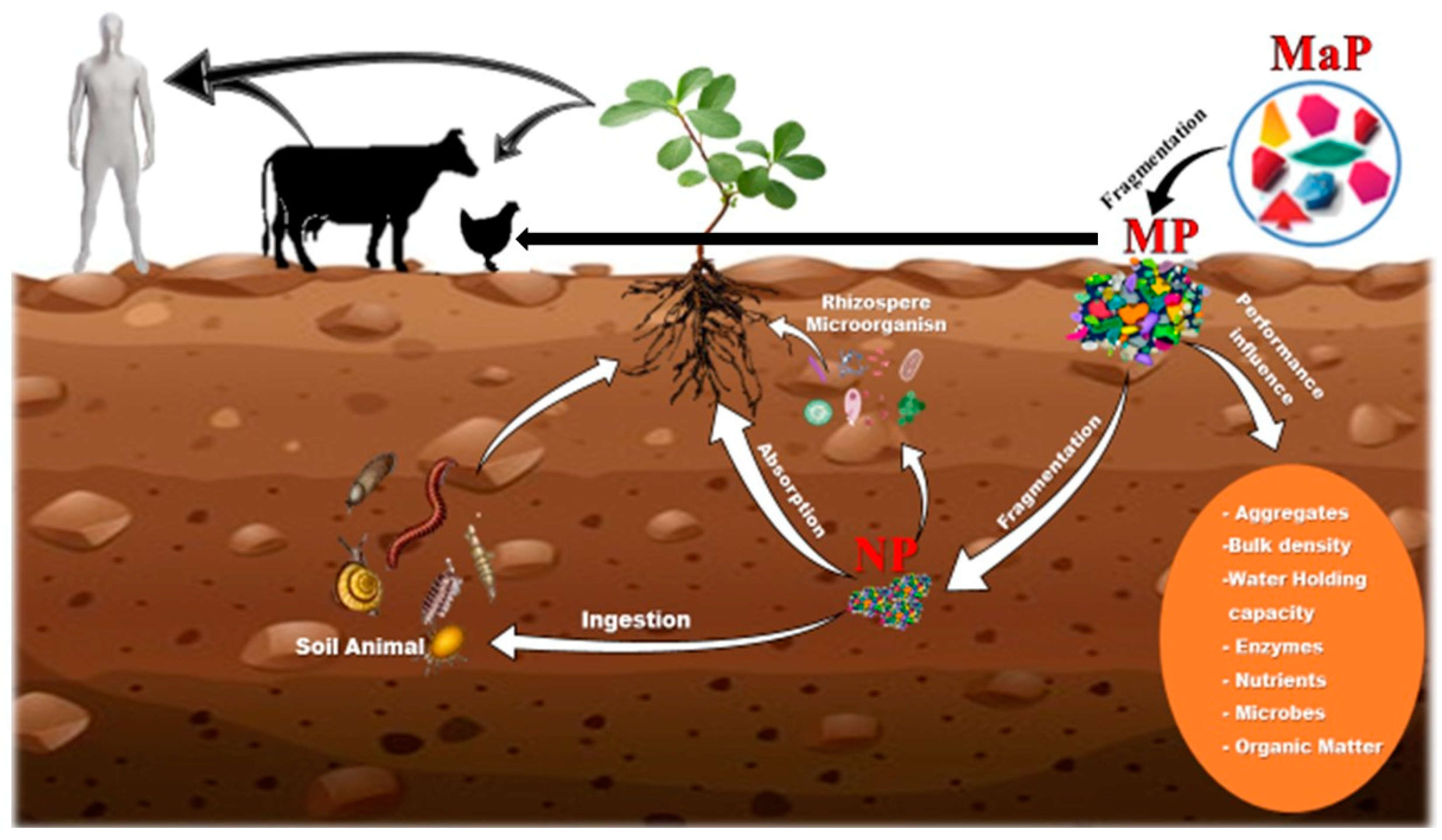
3. Plastics in the Soil
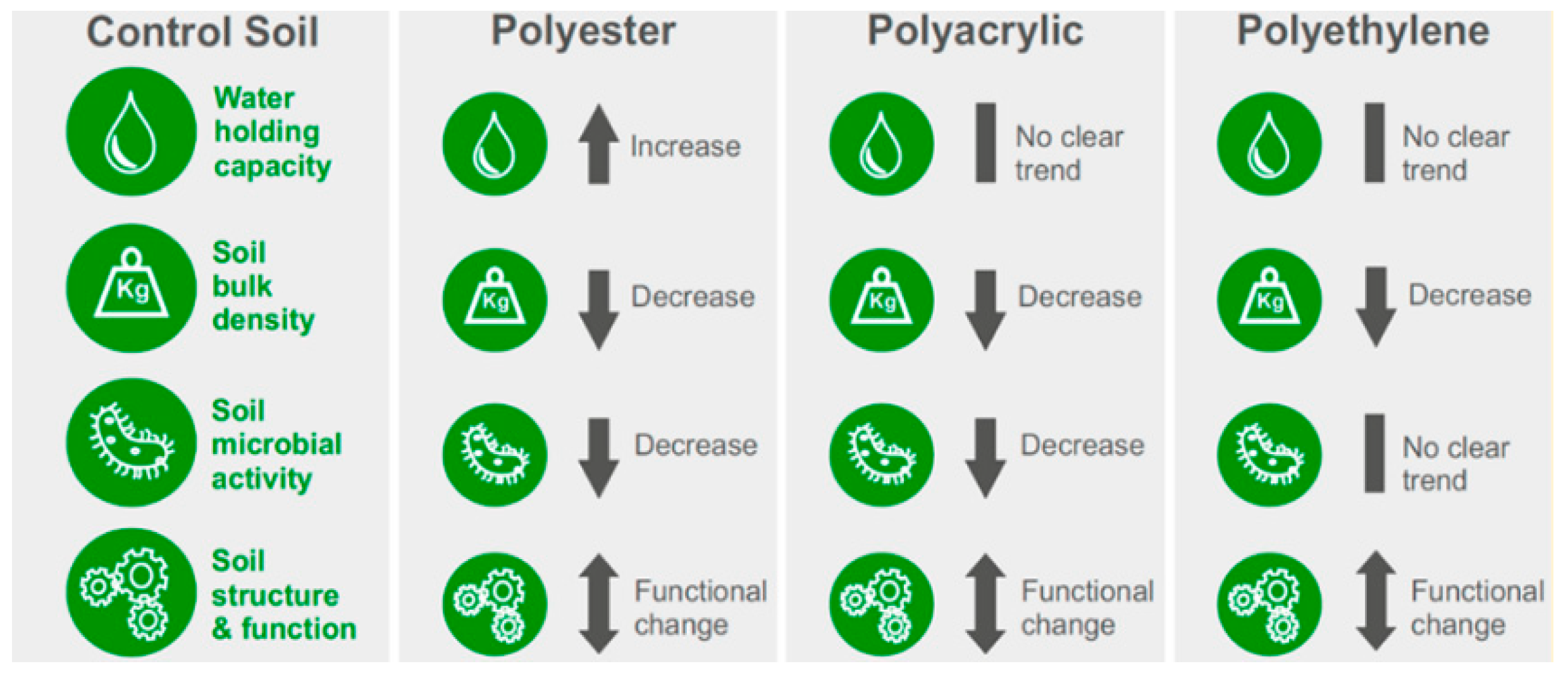
3.1. Impact of MPs on Soil Physical Properties
3.2. Impact of MPs on Soil Fertility, Soil Carbon, and Plant Growth
3.3. Impact of MP and NP on Human Health
4. Conventional Disposal Processes of Plastic Mulch
5. Recycling of Plastic Mulch
6. Alternatives to Plastic Mulch
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vox, G.; Loisi, R.V.; Blanco, I.; Mugnozza, G.S.; Schettini, E. Mapping of Agriculture Plastic Waste. Agric. Agric. Sci. Procedia 2016, 8, 583–591. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crops Res. 2006, 95, 115–125. [Google Scholar] [CrossRef]
- Zribi, W.; Aragüés, R.; Medina, E.; Faci, J.M. Efficiency of inorganic and organic mulching materials for soil evaporation control. Soil Tillage Res. 2015, 148, 40–45. [Google Scholar] [CrossRef]
- Santosh, D. Implication of Plastic Mulch in Soil and Plant Nutrition—A Review. Indian J. Nat. Sci. 2022, 13, 0976–0997. [Google Scholar]
- Prem, M.; Ranjan, P.; Seth, N.; Patle, G.T. Mulching Techniques to Conserve the Soil Water and Advance the Crop Production—A Review. Curr. World Environ. 2020, 15, 10–30. [Google Scholar] [CrossRef]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef]
- Baumann, D.T. Intercropping leeks to suppress weeds. Weed Res. 2000, 40, 359. [Google Scholar] [CrossRef]
- Kolota, E.; Adamczewska-Sowinska, K. the effects of living mulches on yield, overwintering and biological value of leek. Acta Hortic. 2004, 638, 209–214. [Google Scholar] [CrossRef]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Hassan, S.A.; Abdin, R.Z.; Abdin, R.Z. Growth and Yield of Chilli (Capsicum annuum L.) in Response to Mulching and Potassium Fertilization. Pertanika J. Trop. Agric. Sci. 1995, 18, 113–117. [Google Scholar]
- Prosdocimi, M.; Tarolli, P.; Cerdà, A. Mulching practices for reducing soil water erosion: A review. Earth-Sci. Rev. 2016, 161, 191–203. [Google Scholar] [CrossRef]
- Gan, Y.; Siddique, K.H.; Turner, N.C.; Li, X.-G.; Niu, J.-Y.; Yang, C.; Liu, L.; Chai, Q. Ridge-Furrow Mulching Systems—An Innovative Technique for Boosting Crop Productivity in Semiarid Rain-Fed Environments. In Advances in Agronomy; Academic Press: Oxford, UK, 2013; Volume 118, pp. 429–476. ISBN 9780124059429. [Google Scholar]
- Biswas, S.K.; AR, A.; MS, R.; MA, H. Effect of drip irrigation and mulching on yield, water-use efficiency and economics of tomato. Plant Soil Environ. 2016, 61, 97–102. [Google Scholar] [CrossRef]
- Haapala, T.; Palonen, P.; Tamminen, A.; Ahokas, J. Effects of different paper mulches on soil temperature and yield of cucumber (Cucumis sativus L.) in the temperate zone. Agric. Food Sci. 2015, 24, 52–58. [Google Scholar] [CrossRef]
- Shiukhy, S.; Raeini-Sarjaz, M.; Chalavi, V. Colored plastic mulch microclimates affect strawberry fruit yield and quality. Int. J. Biometeorol. 2015, 59, 1061–1066. [Google Scholar] [CrossRef]
- Sideman, R.G. Performance of Sweetpotato Cultivars Grown Using Biodegradable Black Plastic Mulch in New Hampshire. HortTechnology 2015, 25, 412–416. [Google Scholar] [CrossRef]
- Freitas, A.R.d.J.; de Freitas, F.C.L.; de Souza, C.M.; Delazari, F.T.; Berger, P.G.; Borges, F.J.G.; Zanuncio, J.C. Biodegradable mulch controls weeds and increases water use efficiency in lettuce crops. Hortic. Bras. 2021, 39, 330–334. [Google Scholar] [CrossRef]
- Westbrook, A.S.; Bhaskar, V.; DiTommaso, A. Weed control and community composition in living mulch systems. Weed Res. 2022, 62, 12–23. [Google Scholar] [CrossRef]
- Acharya, C.L.; Sharma, P.D. Tillage and mulch effects on soil physical environment, root growth, nutrient uptake and yield of maize and wheat on an Alfisol in north-west India. Soil Tillage Res. 1994, 32, 291–302. [Google Scholar] [CrossRef]
- Pervaiz, M.A.; Iqbal, M.; Shahzad, K.; Hassan, A.U. Effect of Mulch on Soil Physical Properties and N, P, K Concentration in Maize (Zea mays) Shoots under Two Tillage Systems. Int. J. Agric. Biol. 2009, 11, 119–124. [Google Scholar]
- Franquera, E.N.; Mabesa, R.C. Colored Plastic Mulch Effects on the Yield of Lettuce (Lactuca sativa L.) and Soil Temperature. J. Adv. Agric. Technol. 2016, 3, 155–159. [Google Scholar] [CrossRef][Green Version]
- Hayes, D.G.; Anunciado, M.B.; DeBruyn, J.M.; Bandopadhyay, S.; Schaeffer, S.; English, M.; Ghimire, S.; Miles, C.; Flury, M.; Sintim, H.Y. Biodegradable Plastic Mulch Films for Sustainable Specialty Crop Production. In Polymers for Agri-Food Applications; Springer: Cham, Switzerland, 2019; pp. 183–213. [Google Scholar]
- Gao, H.; Yan, C.; Liu, Q.; Ding, W.; Chen, B.; Li, Z. Effects of plastic mulching and plastic residue on agricultural production: A meta-analysis. Sci. Total Environ. 2019, 651, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 91001. [Google Scholar] [CrossRef]
- Liu, J.; Bu, L.; Zhu, L.; Luo, S.; Chen, X.; Li, S. Optimizing Plant Density and Plastic Film Mulch to Increase Maize Productivity and Water—Use Efficiency in Semiarid Areas. Agron. J. 2014, 106, 1138–1146. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Barman, M.; De, M.; Chatterjee, D. Agriculture: Polymers in Crop Production Mulch and Fertilizer. In Encyclopedia of Polymer Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bhardwaj, R.L.; Meena, C.B.; Singh, N.; Ojha, S.N.; Dadhich, S.K. Annual Progress Report of Krishi Vigyan Kendrs; MPUAT Udaipur: Sirohi, India, 2011. [Google Scholar]
- Lamont, W.J. Plastic Mulches for the Production of Vegetable Crops. HortTechnology 1993, 3, 45–60. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Löffler, P.; Eichhöfer, S.; David, J.; Muñoz, K.; Schaumann, G.E. Are agricultural plastic covers a source of plastic debris in soil? A first screening study. Soil 2022, 8, 31–47. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Dong, H.; Yang, G.; Zhang, Y.; Yang, Y.; Wang, D.; Zhou, C. Recycling, disposal, or biodegradable-alternative of polyethylene plastic film for agricultural mulching? A life cycle analysis of their environmental impacts. J. Clean. Prod. 2022, 380, 134950. [Google Scholar] [CrossRef]
- Henseler, M. Plastic Emission from Mulch Film and Abatement Measures. SSRN J. 2022, preprint. [Google Scholar] [CrossRef]
- Espí, E.; Salmerón, A.; Fontecha, A.; García, Y.; Real, A.I. PLastic Films for Agricultural Applications. J. Plast. Film Sheeting 2006, 22, 85–102. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Zhao, Z.-Y.; Wang, P.-Y.; Mo, F.; Zhou, R.; Cao, J.; Liu, S.-T.; Zhang, F.; Wesly, K.; Wang, Y.-B.; et al. Aging rate, environmental risk and production efficiency of the low-density polyethylene (LDPE) films with contrasting thickness in irrigated region. Ecotoxicol. Environ. Saf. 2023, 264, 115399. [Google Scholar] [CrossRef]
- Apeeurop. Plasticulture in Europe—Statistics. Available online: https://apeeurope.eu/statistics/ (accessed on 24 March 2022).
- Bertling, J.; Zimmermann, T.; Rödig, L. Kunststoffe in der Umwelt: Emissionen in Landwirtschaftlich Genutzte Böden; Fraunhofer UMSICHT: Oberhausen, Germany, 2021. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2021. 2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 29 March 2022).
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Contat-Rodrigo, L. Thermal characterization of the oxo-degradation of polypropylene containing a pro-oxidant/pro-degradant additive. Polym. Degrad. Stab. 2013, 98, 2117–2124. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- European Parliament. Waste Management in the EU: Infographic with Facts and Figures|News|European Parliament. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20180328STO00751/waste-management-in-the-eu-infographic-with-facts-and-figures (accessed on 14 September 2023).
- Zhang, S.; Bao, A.; Lin, X.; Jia, G.; Zhang, Q. Microplastic Accumulation in Agricultural Soils with Different Mulching Histories in Xinjiang, China. Sustainability 2023, 15, 5438. [Google Scholar] [CrossRef]
- Li, C.; Moore-Kucera, J.; Lee, J.; Corbin, A.; Brodhagen, M.; Miles, C.; Inglis, D. Effects of biodegradable mulch on soil quality. Appl. Soil Ecol. 2014, 79, 59–69. [Google Scholar] [CrossRef]
- Hayes, D.G.; Dharmalingam, S.; Wadsworth, L.C.; Leonas, K.K.; Miles, C.; Inglis, D.A. Biodegradable Agricultural Mulches Derived from Biopolymers. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; Khemani, K., Scholz, C., Eds.; American Chemical Society: Washington, DC, USA, 2012; pp. 201–223. ISBN 9780841228221. [Google Scholar]
- Moreno, M.M.; Cirujeda, A.; Aibar, J.; Moreno, C. Soil thermal and productive responses of biodegradable mulch materials in a processing tomato (Lycopersicon esculentum Mill.) crop. Soil Res. 2016, 54, 207. [Google Scholar] [CrossRef]
- Huckins, J.N.; Manuweera, G.K.; Petty, J.D.; Mackay, D.; Lebo, J.A. Lipid-containing semipermeable membrane devices for monitoring organic contaminants in water. Environ. Sci. Technol. 1993, 27, 2489–2496. [Google Scholar] [CrossRef]
- Nerín, C.; Tornés, A.R.; Domeño, C.; Cacho, J. Absorption of Pesticides on Plastic Films Used as Agricultural Soil Covers. J. Agric. Food Chem. 1996, 44, 4009–4014. [Google Scholar] [CrossRef]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lamont, W.J.; Bartok, J.W.; Berghage, R.D.; Bonanno, A.R.; Fiola, J.A.; Garrison, S.A.; Garthe, J.W.; Hochmuth, G.J.; Hodges, L.; Olson, S.; et al. Production of Vegetables, Strawberries, and Cut Flowers Using Plasticulture (NRAES-133); Natural Resource, Agriculture, and Engineering Service (NRAES): Ithaca, NY, USA, 2004. [Google Scholar]
- Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wen, B.; Shan, X. Survey of phthalate pollution in arable soils in China. J. Environ. Monit. 2003, 5, 649–653. [Google Scholar] [CrossRef]
- Niu, L.; Xu, Y.; Xu, C.; Yun, L.; Liu, W. Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ. Pollut. 2014, 195, 16–23. [Google Scholar] [CrossRef]
- Lü, H.; Mo, C.-H.; Zhao, H.-M.; Xiang, L.; Katsoyiannis, A.; Li, Y.-W.; Cai, Q.-Y.; Wong, M.-H. Soil contamination and sources of phthalates and its health risk in China: A review. Environ. Res. 2018, 164, 417–429. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Xiang, L.; Gu, C.; Redmile-Gordon, M.; Sheng, H.; Wang, Z.; Fu, Y.; Bian, Y.; Jiang, X. Risk Assessment of Agricultural Plastic Films Based on Release Kinetics of Phthalate Acid Esters. Environ. Sci. Technol. 2021, 55, 3676–3685. [Google Scholar] [CrossRef]
- Wang, D.; Xi, Y.; Shi, X.Y.; Zhong, Y.J.; Guo, C.L.; Han, Y.N.; Li, F.M. Effect of plastic film mulching and film residues on phthalate esters concentrations in soil and plants, and its risk assessment. Environ. Pollut. 2018, 164, 417–429. [Google Scholar] [CrossRef]
- OECD. Plastic Pollution is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 14 September 2023).
- Valavanidis, A.; Iliopoulos, N.; Gotsis, G.; Fiotakis, K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. Hazard. Mater. 2008, 156, 277–284. [Google Scholar] [CrossRef]
- Eriksson, O.; Finnveden, G. Plastic waste as a fuel—CO2-neutral or not? Energy Environ. Sci. 2009, 2, 907. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Lonergan, G. Biodegradability of a Selected Range of Polymers and Polymer Blends and Standard Methods for Assessment of Biodegradation. J. Polym. Environ. 2005, 13, 231–251. [Google Scholar] [CrossRef]
- Moore, J.; Wszelaki, A. Plastic Mulch in Fruit and Vegetable Production: Challenges for Disposal FA-2016-02. 2016. Available online: https://extension.tennessee.edu/publications/Documents/W410.pdf (accessed on 29 March 2022).
- Velandia, M.; Smith, A.; Wszelaki, A.; Galinato, S.; Marsh, T.; The Economics of Adopting Biodegradable Plastic Mulch Films. Extension Reports 302940, University of Tennessee, Department of Agricultural and Resource Economics 2018. Available online: https://ideas.repec.org/p/ags/utaeer/302940.html (accessed on 5 August 2023).
- Levitan, L.; Barros, A. Recycling Agricultural Plastics in New York State; Environmental Risk Analysis Program, Cornell University: Ithaca, NY, USA, 2003; Available online: http://www.cfe.cornell.edu/erap/C&ER/PlasticsDisposal/AgPlasticsRecycling/ (accessed on 4 November 2022).
- Hemphill, D.D. Agricultural Plastics as Solid Waste: What are the Options for Disposal? HortTechnology 1993, 3, 70–73. [Google Scholar] [CrossRef]
- Lamont, W.J. Plastics: Modifying the Microclimate for the Production of Vegetable Crops. HortTechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Pradel, A.; Gautier, M.; Bavay, D.; Gigault, J. Micro- and nanoplastic transfer in freezing saltwater: Implications for their fate in polar waters. Environ. Sci. Process. Impacts 2021, 23, 1759–1770. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Bachelier, J.B.; Souza, M.; Chung, W.; Kloas, W.; Bergmann, J.; Faltin, E.; Chung, W.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Tull, T.; Krais, S.; Peschke, K.; Weyrauch, S.; Triebskorn, R.; Köhler, H.-R. Tire and Road Wear Particle-Containing Sediments with High Organic Content Impact Behavior and Survival of Chironomid Larvae (Chironomus riparius). Environments 2023, 10, 23. [Google Scholar] [CrossRef]
- Sommer, F.; Dietze, V.; Baum, A.; Sauer, J.; Gilge, S.; Maschowski, C.; Gieré, R. Tire Abrasion as a Major Source of Microplastics in the Environment. Aerosol Air Qual. Res. 2018, 18, 2014–2028. [Google Scholar] [CrossRef]
- Brandes, E.; Thomas, D. Plastik im Boden. Available online: https://www.thuenen.de/de/thema/boden/plastik-im-boden/ (accessed on 13 July 2021).
- Selonen, S.; Dolar, A.; Jemec Kokalj, A.; Sackey, L.N.; Skalar, T.; Cruz Fernandes, V.; Rede, D.; Delerue-Matos, C.; Hurley, R.; Nizzetto, L.; et al. Exploring the impacts of microplastics and associated chemicals in the terrestrial environment—Exposure of soil invertebrates to tire particles. Environ. Res. 2021, 201, 111495. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Šourková, M.; Adamcová, D.; Vaverková, M.D. The Influence of Microplastics from Ground Tyres on the Acute, Subchronical Toxicity and Microbial Respiration of Soil. Environments 2021, 8, 128. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [PubMed]
- Uwamungu, J.Y.; Wang, Y.; Shi, G.; Pan, S.; Wang, Z.; Wang, L.; Yang, S. Microplastic contamination in soil agro-ecosystems: A review. Environ. Adv. 2022, 9, 100273. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Astner, A.F.; Hayes, D.G.; O’Neill, H.; Evans, B.R.; Pingali, S.V.; Urban, V.S.; Young, T.M. Mechanical formation of micro- and nano-plastic materials for environmental studies in agricultural ecosystems. Sci. Total Environ. 2019, 685, 1097–1106. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the soil environment: Occurrence, risks, interactions and fate—A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Zhou, Y.; Li, Y.; XUE, Y.; Fu, C.; Tu, C.; Luo, Y. Separation of microplastics from a coastal soil and their surface microscopic features. Chin. Sci. Bull. 2016, 61, 1604–1611. [Google Scholar] [CrossRef]
- Büks, F.; van Schaik, N.L.; Kaupenjohann, M. What do we know about how the terrestrial multicellular soil fauna reacts to microplastic? Soil 2020, 6, 245–267. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. J. Ind. Ecol. 2017, 22, 314–326. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Hu, W.; Qin, X.; Ma, X.; Yan, C.; Wanc, H. The status and distribution characteristics of residual mulching film in Xinjiang, China. J. Integr. Agric. 2016, 15, 2639–2646. [Google Scholar] [CrossRef]
- He, H.; Wang, Z.; Guo, L.; Zheng, X.; Zhang, J.; Li, W.; Fan, B. Distribution characteristics of residual film over a cotton field under long-term film mulching and drip irrigation in an oasis agroecosystem. Soil Tillage Res. 2018, 180, 194–203. [Google Scholar] [CrossRef]
- Li, S.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.; Jones, D.L.; Wang, J. Macro- and microplastic accumulation in soil after 32 years of plastic film mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Liu, S.; Li, H.; Chen, X.; Peng, C.; Zhang, P.; Liu, X. Distinct microplastic distributions in soils of different land-use types: A case study of Chinese farmlands. Environ. Pollut. 2021, 269, 116199. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape- and polymer-dependent effects on soil processes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ingraffia, R.; Amato, G.; Bagarello, V.; Carollo, F.G.; Giambalvo, D.; Iovino, M.; Lehmann, A.; Rillig, M.C.; Frenda, A.S. Polyester microplastic fibers affect soil physical properties and erosion as a function of soil type. Soil 2022, 8, 421–435. [Google Scholar] [CrossRef]
- Koskei, K.; Munyasya, A.N.; Wang, Y.-B.; Zhao, Z.-Y.; Zhou, R.; Indoshi, S.N.; Wang, W.; Cheruiyot, W.K.; Mburu, D.M.; Nyende, A.B.; et al. Effects of increased plastic film residues on soil properties and crop productivity in agro-ecosystem. J. Hazard. Mater. 2021, 414, 125521. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, C.; Gu, Y.; Shi, Y.; Gao, X. Microplastics in plant-soil ecosystems: A meta-analysis. Environ. Pollut. 2022, 308, 119718. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Attinà, E. Early warning indicators of changes in soil ecosystem functioning. Ecol. Indic. 2015, 48, 542–549. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Mallamaci, C.; Sidari, M. Biological indicators to assess short-term soil quality changes in forest ecosystems. Ecol. Indic. 2014, 45, 416–423. [Google Scholar] [CrossRef]
- Meng, F.; Yang, X.; Riksen, M.; Geissen, V. Effect of different polymers of microplastics on soil organic carbon and nitrogen—A mesocosm experiment. Environ. Res. 2022, 204, 111938. [Google Scholar] [CrossRef]
- Gharahi, N.; Zamani-Ahmadmahmoodi, R. Effect of plastic pollution in soil properties and growth of grass species in semi-arid regions: A laboratory experiment. Environ. Sci. Pollut. Res. Int. 2022, 29, 59118–59126. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb–Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic Disguising As Soil Carbon Storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2019, 16, 3–7. [Google Scholar] [CrossRef]
- Schnitzer, M. A Lifetime Perspective on the Chemistry of Soil Organic Matter. In Advances in Agronomy; Academic Press: Oxford, UK, 1999; Volume 68, pp. 1–58. [Google Scholar] [CrossRef]
- DeForest, J.; Zak, D.; Pregitzer, K.; Burton, A. Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol. Biochem. 2004, 36, 965–971. [Google Scholar] [CrossRef]
- Marschner, B.; Kalbitz, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Xiao, M.; Shahbaz, M.; Liang, Y.; Yang, J.; Wang, S.; Chadwicka, D.R.; Jones, D.; Chen, J.; Ge, T. Effect of microplastics on organic matter decomposition in paddy soil amended with crop residues and labile C: A three-source-partitioning study. J. Hazard. Mater. 2021, 416, 126221. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.; Moldrup, P.; Holmstrup, M.; Schjønning, P.; Winding, A.; Mayer, P.; de Jonge, L.W. Soil microbial and physical properties and their relations along a steep copper gradient. Agric. Ecosyst. Environ. 2012, 159, 9–18. [Google Scholar] [CrossRef]
- Naveed, M.; Herath, L.; Moldrup, P.; Arthur, E.; Nicolaisen, M.; Norgaard, T.; Ferré, T.P.; de Jonge, L.W. Spatial variability of microbial richness and diversity and relationships with soil organic carbon, texture and structure across an agricultural field. Appl. Soil Ecol. 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Rillig, M.C.; Muller, L.A.; Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Fitschen, K.; Rillig, M. Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Syst. 2019, 3, 21. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Halley, J.M.; Rillig, M.C. Extinction risk of soil biota. Nat Commun 2015, 6, 8862. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.A.P.; Da Costa, J.; Duarte, A.C.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef]
- Kim, S.W.; An, Y.-J. Soil microplastics inhibit the movement of springtail species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef]
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnol. 2014, 12, 16. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Human health effects of airborne microplastics. In Airborne Microplastics: Analysis, Fate and Human Health Effects; Wang, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 185–223. ISBN 9780443157660. [Google Scholar]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A. Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics. Environ. Sci. Nano 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Bartucci, R.; Paramanandana, A.; Boersma, Y.L.; Olinga, P.; Salvati, A. Comparative study of nanoparticle uptake and impact in murine lung, liver and kidney tissue slices. Nanotoxicology 2020, 14, 847–865. [Google Scholar] [CrossRef]
- Jung, J.-M.; Cho, S.-H.; Jung, S.; Lin, K.-Y.A.; Chen, W.-H.; Tsang, Y.F.; Kwon, E.E. Disposal of plastic mulching film through CO2-assisted catalytic pyrolysis as a strategic means for microplastic mitigation. J. Hazard. Mater. 2022, 430, 128454. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Erratum to: Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2013, 33, 443. [Google Scholar] [CrossRef][Green Version]
- Plastics Europe. Plastics—The Facts 2015: An Analysis of European Plastics Production, Demand and Waste Data. 2015. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2015-Plastics-the-facts.pdf (accessed on 4 September 2022).
- Briassoulis, D. Mechanical behaviour of biodegradable agricultural films under real field conditions. Polym. Degrad. Stab. 2006, 91, 1256–1272. [Google Scholar] [CrossRef]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef]
- Shah, F.; Wu, W. Use of plastic mulch in agriculture and strategies to mitigate the associated environmental concerns. In Advances in Agranomy; Academic Press: Oxford, UK, 2020; Volume 164, pp. 231–287. [Google Scholar] [CrossRef]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.G.; Inglis, D.A.; Marsh, T.L.; Miles, C. Policy considerations for limiting unintended residual plastic in agricultural soils. Environ. Sci. Policy 2017, 69, 81–84. [Google Scholar] [CrossRef]
- World Economic Forum. The New Plastics Economy: Rethinking the Future of Plastics. 2016. Available online: http://coscienzeinrete.net/wp-content/uploads/2017/02/WEF_The_New_Plastics_Economy.pdf (accessed on 31 March 2022).
- Hans, S.; Fletcher, E.; Molteno, S.; Sherrington, C.; Elliott, L.; Kong, M.; Koite, A.; Sastre, S.; Martinez, V. Relevance of Conventional and Biodegradable Plastics in Agriculture. 2021. Available online: https://environment.ec.europa.eu/system/files/2021-09/Agricultural%20Plastics%20Final%20Report.pdf (accessed on 6 March 2023).
- Bonhotal, J. Agricultural Materials Management in New York State; Recycling Agricultural Plastics Project (RAPP), Cornell University: Ithaca, NY, USA, 2015; Available online: https://hdl.handle.net/1813/69518 (accessed on 4 October 2022).
- IFFPG. Irish Farm Film Producers Group. 2001. Available online: https://farmplastics.ie/ (accessed on 4 August 2022).
- González-Sánchez, C.; Martínez-Aguirre, A.; Pérez-García, B.; Martínez-Urreaga, J.; de La Orden, M.U.; Fonseca-Valero, C. Use of residual agricultural plastics and cellulose fibers for obtaining sustainable eco-composites prevents waste generation. J. Clean. Prod. 2014, 83, 228–237. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; Sica, C.; Russo, G. Plastic Materials in European Agriculture: Actual Use and Perspectives. J. Agric. Eng. 2011, 42, 15. [Google Scholar] [CrossRef]
- Geyer, M.; Salama, K. Nachhaltiges Entsorgen von Spargelfolie. Spargel Erdbeerprofi 2023, 25, 32–34. [Google Scholar]
- Clarke, S.P. Recycling Farm Plastic Films. Available online: http://www.omafra.gov.on.ca/english/engineer/facts/95-019.htm (accessed on 21 March 2022).
- ERDE. Sammlung von Spargelfolien—ERDE Bietet Bundesweiten Service: Erntekunststoffe Recycling Deutschland. Available online: https://newsroom.kunststoffverpackungen.de/2021/04/22/sammlung-von-spargelfolien-erde-bietet-bundesweiten-service/ (accessed on 13 July 2021).
- ERDE. IK-Initiative ERDE: Ambitious Collection Goals for 2022. Available online: https://www.erde-recycling.de/en/erde-news/ik-initiative-erde-ambitious-collection-goals-for-2022/ (accessed on 11 April 2022).
- RAFU II. Développement de la Technologie de Dépose des Films Agricoles sur Différentes Culturesprojet RAFU II, CPA. INVENIO, A.D.I.VALOR. 2020. PROJET RAFU II. 52 Pages. Available online: www.ademe.fr/mediatheque (accessed on 16 March 2022).
- Haapala, T.; Palonen, P.; Korpela, A.; Ahokas, J. Feasibility of paper mulches in crop production—A review. Agric. Food Sci. 2014, 23, 60–79. [Google Scholar] [CrossRef]
- Tofanelli, M.B.D.; Wortman, S.E. Benchmarking the Agronomic Performance of Biodegradable Mulches against Polyethylene Mulch Film: A Meta-Analysis. Agronomy 2020, 10, 1618. [Google Scholar] [CrossRef]
- Goldberger, J.R.; DeVetter, L.W.; Dentzman, K.E. Polyethylene and Biodegradable Plastic Mulches for Strawberry Production in the United States: Experiences and Opinions of Growers in Three Regions. HortTechnology 2019, 29, 619–628. [Google Scholar] [CrossRef]
- Goswami, P.; O’Haire, T. Developments in the use of green (biodegradable), recycled and biopolymer materials in technical nonwovens. In Advances in Technical Nonwovens; Woodhead Publishing: Sawston, UK, 2016; pp. 97–114. [Google Scholar] [CrossRef]
- Hartikainen, S. Biodegradability of Nonwoven Fabrics. Bachelor’s Thesis, Tampere University of Applied Sciences, Tampere, Finland, 2015. [Google Scholar]
- Liu, L.; Zou, G.; Zuo, Q.; Li, S.; Bao, Z.; Jin, T.; Liu, D.; Du, L. It is still too early to promote biodegradable mulch film on a large scale: A bibliometric analysis. Environ. Technol. Innov. 2022, 27, 102487. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based plastic mulching films. Polym. Test. 2018, 67, 99–109. [Google Scholar] [CrossRef]
- National Organic Program. Allowed Mulches on Organic Farms and the Future of Biodegradable Mulch. 2015. Available online: https://www.ams.usda.gov/sites/default/files/media/5%20Mulches%20incl%20biodegradable%20FINAL%20RGK%20V2.pdf (accessed on 15 September 2023).
- Sintim, H.Y.; Bandopadhyay, S.; English, M.E.; Bary, A.; Liquet y González, J.E.; DeBruyn, J.M.; Schaeffer, S.M.; Miles, C.A.; Flury, M. Four years of continuous use of soil-biodegradable plastic mulch: Impact on soil and groundwater quality. Geoderma 2021, 381, 114665. [Google Scholar] [CrossRef]
- Global Industry Analysts. Global Biodegradable Mulch Film Market to Reach $83.6 Million by 2023. Available online: https://www.prnewswire.com/news-releases/global-biodegradable-mulch-film-market-to-reach-83-6-million-by-2026–301526421.html (accessed on 15 September 2023).
- ASTM D6400; Standard Specification for Compostable Plastics. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D883; Standard Terminology Relating to Plastics. ASTM International: West Conshohocken, PA, USA, 1946.
- Hayes, D.G.; Flury, M. A New Standard for Biodegradable Plastic Mulch Films EXT-2018-01. 2018. Available online: https://ag.tennessee.edu/biodegradablemulch/Documents/EU%20regs%20factsheet.pdf (accessed on 30 March 2022).
- Mohee, R.; Unmar, G.D.; Mudhoo, A.; Khadoo, P. Biodegradability of biodegradable/degradable plastic materials under aerobic and anaerobic conditions. Waste Manag. 2008, 28, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Kumar Tiwari, A.; Gautam, M.; Maurya, H.K. Recent development of biodegradation techniques of polymer. Int. J. Res. Granthaalayah 2018, 6, 414–452. [Google Scholar] [CrossRef]
- Goldberger, J.R.; Jones, R.E.; Miles, C.A.; Wallace; Russell, W.; Inglis, D.A. Barriers and bridges to the adoption of biodegradable plastic mulches for US specialty crop production. Renew. Agric. Food Syst. 2013, 30, 143–153. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Griffin-LaHue, D.; Ghimire, S.; Yu, Y.; Scheenstra, E.J.; Miles, C.A.; Flury, M. In-field degradation of soil-biodegradable plastic mulch films in a Mediterranean climate. Sci. Total Environ. 2022, 806, 150238. [Google Scholar] [CrossRef]
- Kopitar, D.; Marasovic, P.; Jugov, N.; Schwarz, I. Biodegradable Nonwoven Agrotextile and Films—A Review. Polymers 2022, 14, 2272. [Google Scholar] [CrossRef]
- Restrepo-Osorio, A.; Álvarez-López, C.; Jaramillo-Quiceno, N.; Fernández-Morales, P. Agrotextiles and crop protection textiles. In High Performance Technical Textiles; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Muthu, S.S.; Gardetti, M.Á. Sustainability in the Textile and Apparel Industries: Sourcing Synthetic and Novel Alternative Raw Materials, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9783030380137. [Google Scholar]
- Zhao, Y.; Zhang, F.; Li, L.; Yang, X.; Zhang, F.; Zhao, W.; He, Q. Substitution Experiment of Biodegradable Paper Mulching Film and White Plastic Mulching Film in Hexi Oasis Irrigation Area. Coatings 2022, 12, 1225. [Google Scholar] [CrossRef]
- Güzel, E. Paper Based Mulches as an Alternative to Polyethylene Mulch in Vegetable Production. J. Agric. Mach. Sci. 2014, 1, 73–78. [Google Scholar]
- Gu, X.-B.; Li, Y.-N.; Du, Y.-D. Biodegradable film mulching improves soil temperature, moisture and seed yield of winter oilseed rape (Brassica napus L.). Soil Tillage Res. 2017, 171, 42–50. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Šimůnek, J.; Shi, H.; Hu, Q.; Zhang, Y. Evaluating the effects of biodegradable and plastic film mulching on soil temperature in a drip-irrigated field. Soil Tillage Res. 2021, 213, 105116. [Google Scholar] [CrossRef]
- Ghimire, S.; Flury, M.; Scheenstra, E.J.; Miles, C.A. Sampling and degradation of biodegradable plastic and paper mulches in field after tillage incorporation. Sci. Total Environ. 2020, 703, 135577. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Han, J.; Ren, S.; Zhang, Y.; Zhang, F.; He, Q. Study on anti-aging properties of modified ZnO–SiO2 superhydrophobic coated paper mulch film. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127430. [Google Scholar] [CrossRef]
- Li, A.; Li, K.; Zhang, F.; Ren, S.; Zhang, F.; He, Q. Research on low temperature performance of ZnO/SiO2 composite superhydrophobic paper mulch. J. Mater. Res. Technol. 2021, 14, 851–863. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Ren, S.; Zhang, Y.; Zhang, F. Research progress on preparation and field application of paper mulch. Environ. Technol. Innov. 2021, 24, 101949. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Sanjay; Yogesha, B. Studies on Natural/Glass Fiber Reinforced Polymer Hybrid Composites: An Evolution. Mater. Today Proc. 2017, 4, 2739–2747. [Google Scholar] [CrossRef]
- Bhavani, K.; Mallikarjun, N.; Sunilkumar, N.M. Agro textiles-Their applications in agriculture and scope for utilizing natural fibers in agro tech sector. Int. J. Appl. Home Sci. 2017, 4, 653–662. [Google Scholar]
- Asim, M.; Saba, N.; Jawaid, M.; Nasir, M. Potential of natural fiber/biomass filler-reinforced polymer composites in aerospace applications. In Sustainable Composites for Aerospace Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 253–268. [Google Scholar] [CrossRef]
- Azammi, A.N.; Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.; Asrofi, M.; Atiqah, A. 3—Characterization studies of biopolymeric matrix and cellulose fibres based composites related to functionalized fibre-matrix interface. In Interfaces in Particle and Fibre Reinforced Composites; Goh, K.L., Aswathi, M.K., De Silva, R.T., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 29–93. ISBN 978-0-08-102665-6. [Google Scholar]
- Guerrini, S.; Borreani, G.; Voojis, H. Biodegradable Materials in Agriculture: Case Histories and Perspectives. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–65. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Hosseini, S.B. Natural Fiber Polymer NanocompositesSubrahmanya Bhat. In Fiber-Reinforced Nanocomposites, 1st ed.; Han, B., Sharma, S., Nguyen, T.A., Longbiao, L., Bhat, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–299. ISBN 978-0-12-819904-6. [Google Scholar]
- Hogg, D.; Gibbs, A.; Ettlinger, S.; Hann, S. The Impact of the Use of “Oxo-Degradable” Plastic on the Environment—Final Report; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-61828-4. [Google Scholar]
- Avella, M.; Di Pace, E.; Immirzi, B.; Impallomeni, G.; Malinconico, M.; Santagata, G. Addition of glycerol plasticizer to seaweeds derived alginates: Influence of microstructure on chemical–physical properties. Carbohydr. Polym. 2007, 69, 503–511. [Google Scholar] [CrossRef]
- Mormile, P.; Petti, L.; Rippa, M.; Immirzi, B.; Malinconico, M.; Santagata, G. Monitoring of the degradation dynamics of agricultural films by IR thermography. Polym. Degrad. Stab. 2007, 92, 777–784. [Google Scholar] [CrossRef]
- Malinconico, M.; Immirzi, B.; Santagata; Evelia, G. An Overview on Innovative Biodegradable Materials for Agricultural Applications. In Progress in Polymer Degradation and Stability Research; Nova Science Publishers: New York, NY, USA, 2008; ISBN 978-1-60021-828-6. [Google Scholar]
- Schettini, E.; Vox, G.; de Lucia, B. Effects of the radiometric properties of innovative biodegradable mulching materials on snapdragon cultivation. Sci. Hortic. 2007, 112, 456–461. [Google Scholar] [CrossRef]
- BIO.CO.AGRI. Biodegradable Coverages for Sustainable Agriculture. Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=2357 (accessed on 23 February 2023).
- Vox, G.; Santagata, G.; Malinconico, M.; Immirzi, B.; Scarascia Mugnozza, G.; Schettini, E. Biodegradable films and spray coatings as eco-friendly alternative to petro-chemical derived mulching films. J. Agric. Eng. 2013, 44. [Google Scholar] [CrossRef]
- Sartore, L.; Vox, G.; Schettini, E. Preparation and Performance of Novel Biodegradable Polymeric Materials Based on Hydrolyzed Proteins for Agricultural Application. J. Polym. Environ. 2013, 21, 718–725. [Google Scholar] [CrossRef]
- Sartore, L.; Schettini, E.; Palma, L.; Brunetti, G.; Cocozza, C.; Vox, G. Effect of hydrolyzed protein-based mulching coatings on the soil properties and productivity in a tunnel greenhouse crop system. Sci. Total Environ. 2018, 645, 1221–1229. [Google Scholar] [CrossRef]
- Adhikari, R.; Mingtarja, H.; Freischmidt, G.; Bristow, K.L.; Casey, P.S.; Johnston, P.; Sangwan, P. Effect of viscosity modifiers on soil wicking and physico-mechanical properties of a polyurethane based sprayable biodegradable polymer membrane. Agric. Water Manag. 2019, 222, 346–353. [Google Scholar] [CrossRef]
- Immirzia, B.; Santagataa, G.; Voxb, G.; Schettinib, E. Preparation, characterisation and field-testing of a biodegradable sodium alginate-based spray mulch. Biosyst. Eng. 2009, 102, 461–472. [Google Scholar] [CrossRef]
- Borrowman, C. Degradation, Efficacy, and Environmental Impact of a Sprayable Degradable Plastic Mulch. Master’s Thesis, Monash University, Clayton, Australia, 2020. [Google Scholar]
- Niemann, J.-U.; Menssen, M.; Poehling, H.-M. Reducing initial aphid infestation by use of coloured mulch foils and newly developed biodegradable spray-films. J. Cultiv. Plants 2022, 74, 49–62. [Google Scholar] [CrossRef]
- Giaccone, M.; Cirillo, C.; Scognamiglio, P.; Teobaldelli, M.; Mataffo, A.; Stinca, A.; Pannico, A.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. Biodegradable mulching spray for weed control in the cultivation of containerized ornamental shrubs. Chem. Biol. Technol. Agric. 2018, 5, 21. [Google Scholar] [CrossRef]
- Cline, J.; Neilsen, G.; Hogue, E.; Kuchta, S.; Neilsen, D. Spray-on-mulch Technology for Intensively Grown Irrigated Apple Orchards: Influence on Tree Establishment, Early Yields, and Soil Physical Properties. HortTechnology 2011, 21, 398–411. [Google Scholar] [CrossRef]
- Braunack, M.V.; Zaja, A.; Tam, K.; Filipović, L.; Filipović, V.; Wang, Y.; Bristow, K.L. A Sprayable Biodegradable Polymer Membrane (SBPM) technology: Effect of band width and application rate on water conservation and seedling emergence. Agric. Water Manag. 2020, 230, 105900. [Google Scholar] [CrossRef]
- Remmele, E. Beikrautkontrolle im Gemüsebau mit Einem Spritzbaren Mulchmaterial aus Nachwachsenden Rohstoffen: MuNaRo. Available online: https://www.tfz.bayern.de/stofflichenutzung/projekte/264089/index.php (accessed on 23 February 2023).
- Bhardwaj, R.L.; Kendra, V. Effect of mulching on crop production under rainfed condition—A review. Agric. Rev. 2013, 34, 188. [Google Scholar] [CrossRef]
- Hiltbrunner, J.; Jeanneret, P.; Liedgens, M.; Stamp, P.; Streit, B. Response of Weed Communities to Legume Living Mulches in Winter Wheat. J. Agron. Crop Sci. 2007, 193, 93–102. [Google Scholar] [CrossRef]
- Petersen, J.; Rover, A. Comparison of Sugar Beet Cropping Systems with Dead and Living Mulch using a Glyphosate-resistant Hybrid. J. Agron. Crop Sci. 2005, 191, 55–63. [Google Scholar] [CrossRef]
- Sterrett, S.B.; Hohlt, H.E.; Savage, C.P. Alternative Management Strategies for Tomato Affect Cultural and Economic Sustainability. HortScience 2005, 40, 602–606. [Google Scholar] [CrossRef]
- Dahiya, R.; Ingwersen, J.; Streck, T. The effect of mulching and tillage on the water and temperature regimes of a loess soil: Experimental findings and modeling. Soil Tillage Res. 2007, 96, 52–63. [Google Scholar] [CrossRef]
- Jodaugienė, D.; Pupalienė, R.; Urbonienė, M.; Pranckietis, V.; Pranckietienė, I. The impact of different types of organic mulches on weed emergence. Agron. Res. 2006, 4, 197–201. [Google Scholar]
- Radics, L.; Szné Bognár, E. Comparison of Different Mulching Methods for Weed Control in Organic Green Bean and Tomato. Acta Hortic. 2004, 638, 189–196. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Lenka, N.K.; Dass, A.; Sudhishri, S.; Patnaik, U.S. Soil carbon sequestration and erosion control potential of hedgerows and grass filter strips in sloping agricultural lands of eastern India. Agric. Ecosyst. Environ. 2012, 158, 31–40. [Google Scholar] [CrossRef]
- Tabaglio, V.; Gavazzi, C.; Schulz, M.; Marocco, A. Alternative weed control using the allelopathic effect of natural benzoxazinoids from rye mulch. Agron. Sustain. Dev. 2008, 28, 397–401. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Zhao, X.; Wu, P.; Horwath, W.R.; Li, H.; Jing, Z.; Chen, X. Simulated Study on Effects of Ground Managements on Soil Water and Available Nutrients in Jujube Orchards. Land Degrad. Dev. 2016, 27, 35–42. [Google Scholar] [CrossRef]
- Fernández, C.; Vega, J.A.; Jiménez, E.; Vieira, D.; Merino, A.; Ferreiro, A.; Fonturbel, T. Seeding and mulching + seeding effects on post-fire runoff, soil erosion and species diversity in Galicia (NW Spain). Land Degrad. Dev. 2012, 23, 150–156. [Google Scholar] [CrossRef]
- Abrantes, J.R.; Prats, S.A.; Keizer, J.J.; Lima, J.L. Effectiveness of the application of rice straw mulching strips in reducing runoff and soil loss: Laboratory soil flume experiments under simulated rainfall. Soil Tillage Res. 2018, 180, 238–249. [Google Scholar] [CrossRef]
- Mwango, S.B.; Msanya, B.M.; Mtakwa, P.W.; Kimaro, D.N.; Deckers, J.; Poesen, J. Effectiveness OF Mulching Under Miraba in Controlling Soil Erosion, Fertility Restoration and Crop Yield in the Usambara Mountains, Tanzania. Land Degrad. Dev. 2016, 27, 1266–1275. [Google Scholar] [CrossRef]
- Ruy, S.; Findeling, A.; Chadoeuf, J. Effect of mulching techniques on plot scale runoff: FDTF modeling and sensitivity analysis. J. Hydrol. 2006, 326, 277–294. [Google Scholar] [CrossRef]
- Ji, S.; Unger, P.W. Soil Water Accumulation under Different Precipitation, Potential Evaporation, and Straw Mulch Conditions. Soil Sci. Soc. Am. J. 2001, 65, 442–448. [Google Scholar] [CrossRef]
- Li, R.; Li, Q.; Pan, L. Review of organic mulching effects on soil and water loss. Arch. Agron. Soil Sci. 2021, 67, 136–151. [Google Scholar] [CrossRef]
- Cherr, C.M.; Scholberg, J.M.S.; McSorley, R. Green Manure Approaches to Crop Production: A Synthesis. Agron. J. 2006, 98, 302–319. [Google Scholar] [CrossRef]
- Cline, G.R.; Silvernail, A.F. Residual Nitrogen and Kill Date Effects on Winter Cover Crop Growth and Nitrogen Content in a Vegetable Production System. HortTechnology 2001, 11, 219–225. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, K.; Geyer, M. Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives. Environments 2023, 10, 179. https://doi.org/10.3390/environments10100179
Salama K, Geyer M. Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives. Environments. 2023; 10(10):179. https://doi.org/10.3390/environments10100179
Chicago/Turabian StyleSalama, Kotaiba, and Martin Geyer. 2023. "Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives" Environments 10, no. 10: 179. https://doi.org/10.3390/environments10100179
APA StyleSalama, K., & Geyer, M. (2023). Plastic Mulch Films in Agriculture: Their Use, Environmental Problems, Recycling and Alternatives. Environments, 10(10), 179. https://doi.org/10.3390/environments10100179






