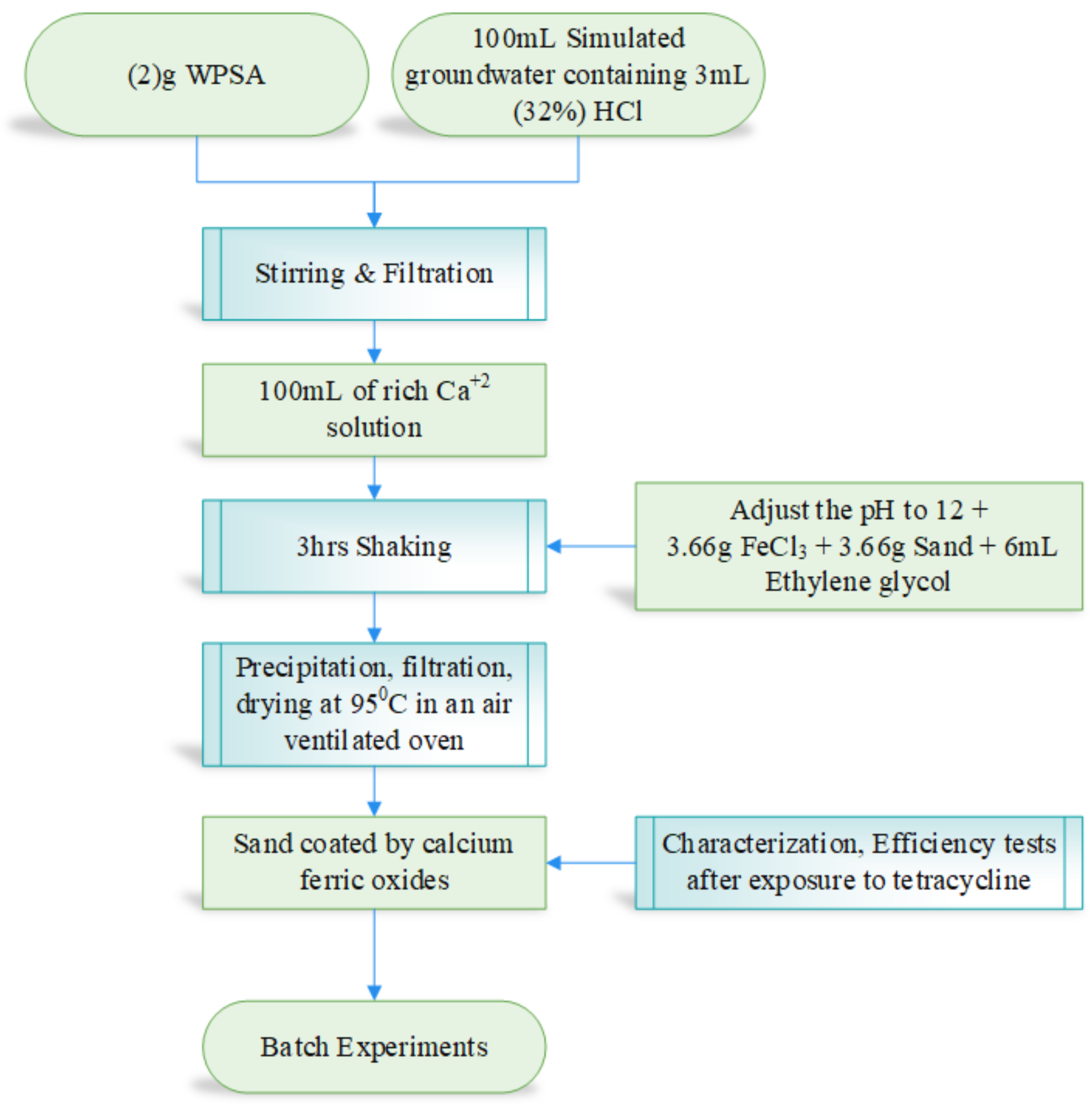
1. Introduction
Tetracycline (TC) is a broad-spectrum antibiotic which consists of 59.4540% of carbon (C), 5.4430% hydrogen (H), 6.3032% nitrogen (N), and 28.800% of oxygen (O) [
1], with amphoteric, phenolic, and alcoholic properties [
2]. Owing to the presence of a dimethylammonium group, a phenolic diketone moiety, and a tricarbonyl system, TC exists primarily as cationic (
) at pH less than 3.3, zwitterionic (
) at pH between 3.3 and 7.7, and anionic (
) and (
) at pH greater than 7.7 [
3,
4]. TC is massively produced due to its extensive use in treating a wide range of bacterial infections [
5]. Additionally, it is widely used for veterinary and agricultural purposes [
6,
7]. Unfortunately, the excessive use of antibiotics threatens the environment and public health. In fact, Germany, China, the EU, and Switzerland are among the countries with relatively high TC usage. For instance, in 2013, China used 6950 tonnes of TC-based antibiotics [
8]. The consumption of TC-based antibiotics in Germany in 2016 reached 542 tonnes. In 2017, the United Kingdom used 104.9 tonnes of TC-based antibiotics for animal treatment and 48.2 tonnes for human treatment [
9]. This vast global usage of TC-based antibiotics is the main reason for the presence of TC in surface waters [
10].
The presence of TC in water is a problematic issue for the water industry due to the complex composition of the TC, which prohibits the efficient removal of TC in conventional wastewater treatment facilities [
11]. As with any other organic chemical compound, TC-based antibiotics undergo various physical, chemical, and biological processes in the aquatic environment that lead to the partial or complete degradation of the TC-based antibiotics. The level and pace of the degradation process depend on the physicochemical characteristics of the TC-based antibiotics, the characteristics of the bed soil, and the ambient conditions, such as the temperature and presence of other chemicals [
12]. During the degradation journey in the aquatic environment, the TC-based antibiotics may migrate from one phase to another (from water to soil and vice versa) due to the adsorption and desorption, leaching, drainage, absorption by crops, dispersion, and volatilisation. Furthermore, the structure of TC-based antibiotics may alter to another structure during this journey through transformation processes, such as hydrolysis, photocatalytic degradation, bioremediation, oxidisation, and reduction. TC may have a partitioning, distribution, and adsorption coefficient of 15,278 L/kg [
10] and, in some other forms, may reach 312,447 L/kg [
13]. Bao et al. [
14] confirmed tetracycline may have a partitioning, distribution, and adsorption coefficient of 15,278 L/kg, and may reach 312,447 L/kg in some forms [
13]. This demonstrates that TCs are very durable and non-degradable substances because any compound with a sorption coefficient of 4000 L/kg is considered very resistant and non-degradable [
15].
Although many methods are used to remove TC-based antibiotics from wastewater and water, such as biodegradation, they do not enjoy high removal efficiency. Currently, adsorption is the predominant method for removing TC-based antibiotics from water and wastewater [
16,
17]. Several materials have been used as adsorbents to remove TC from water, including chitosan [
18], montmorillonite [
19], activated carbon (AC) [
20], and nanotubes of carbon [
21]. However, the wide use of these materials is restricted by their high fabrication costs, dumping, and regenerating costs [
22,
23]. Therefore, several attempts have been made to develop cost-effective and eco-friendly adsorbents. For example, many studies focused on coating sand particles with different waste materials, such as cement kiln dust, and use these particles as a promising adsorbent for the removal of organics and heavy metals from water and wastewater [
7,
24].
In this context, the current study aims to develop a new adsorbent made from sand coated with calcium ferric oxides; the latter was fabricated using wastepaper sludge ash (WPSA). It is noteworthy that the WPSA was used in this study as a source of calcium for many reasons; firstly, its chemical composition of the WPSA is rich in calcium [
25]. Secondly, huge amounts of WPSA are produced annually worldwide [
26]. Finally, local authorities in the UK and Europe encourage recycling paper waste [
27,
28]. Paper mill sludge is the semi-solid slurry gathered in effluent treatment units during deinking and repulping.
3. Previous Uses of Calcium Ferric Oxides
The literature shows many trials to synthesise different types of calcium ferric oxides. For instance, Chatterjee and Chakraborty [
30] synthesised Ca
4Fe
9O
17 using a mixture of potassium ferrocyanide trihydrate and calcium acetate hydrate. The dry mixture was thermally decomposed at a temperature of 700 °C and then used as a photocatalyst for the degradation of organic dyes. Sadrolhosseini et al. [
31] prepared CaFe
2O
4 using Fe(NO
3)
3·H
2O, Ca(NO
3)
2·H
2O and polyvinyl alcohol by the thermal treatment method (at temperatures of 362 and 860 K). The produced calcium ferric oxides have been used to remove the Methylene blue and Methylene Orange dyes from water. Vanags et al. [
32] made another trial to synthesise Ca
2Fe
2O
5 using Fe(NO
3)·9H
2O and Ca(NO
3)
2·4H
2O by the sol-gel auto-combustion method at temperatures ranging between 300 and 800 °C. The produced adsorbent exhibited high efficiency in the degradation of methylene blue from water. Additionally, some studies focused on the development of nano-particles of the CaFe
2O
4; for example, Sulaimana [
33] synthesised CaFe
2O
4 nano-particles by mixing a 1:1 molar ratio of nitrate Ca(NO
3)
2 and ferric nitrate Fe(NO
3)
3, then calcinated at a temperature of 550 °C. However, these nano-particles were applied in drug delivery.
The above short literature review shows the application of calcium ferric oxide as a coating layer for inert materials, such as sand, though its use as an adsorbent for water treatment has not yet been investigated. Additionally, the novel difference, to the knowledge of the authors, between the current study and the literature is that the previous studies used elevated temperatures up to 800 °C to fabricate the calcium ferric oxide, which is not an eco-friendly method, while the present study used a temperature of 95 °C (for drying purposes). Finally, the present study utilised a by-product (wastepaper sludge ash (WPSA)) as a source of calcium, which could make the new adsorbent an eco-friendly alternative to the traditional adsorbents. These differences between the current and previous studies could be enough of a reason to make the new adsorbent an eco-friendly alternative to traditional adsorbents. At the same time, there is still a need for further studies on the ability of this new adsorbent to remove other pollutants and investigate the effects of competitive ions on its efficiency in the removal of the targeted pollutants.
In conclusion, the authors believe this work represents the initial step for this new approach. Still, there are many steps that other researchers should take to make the new adsorbent applicable for industrial purposes.
6. Characterisation
Several characterisation analyses were performed to investigate the properties of sorbents. A Shimadzu XRD-6000 X-ray diffractometer, made by Shimadzu Corporation (Kyoto, Japan) was used to conduct X-ray diffraction (XRD) investigations on both uncoated and calcium ferric oxide-coated sand to determine the crystal structure. The Scanning electron microscopy (SEM) tests were carried out using scanning electron microscopy, FEI Inspect-S (SEM) variable vacuum (0.1–30KV range). Particle size distribution has been performed using Beckman Coulter (LS 3 320), and the total carbon has been performed using Primacs SERIES Carbon/Nitrogen Analysers.
The content of the quantitative oxide was measured using X-ray fluorescence analysis (XRF) type Shimadzu EDX 720 analyser, made by Shimadzu Corporation (Kyoto, Japan). Furthermore, the specific gravity has been measured using a Quantachrome helium pycnometer. Finally, the concentrations of calcium and tetracycline were measured using a Benchtop Visible Spectrophotometer UV-Spectrophotometer (HACH DR-3900) made by Hach Lange GmbH Headquarter, D-40549 Düsseldorf, Germany.
7. Results and Discussion
7.1. Extraction of Calcium Ions (Ca2+) from WPSA
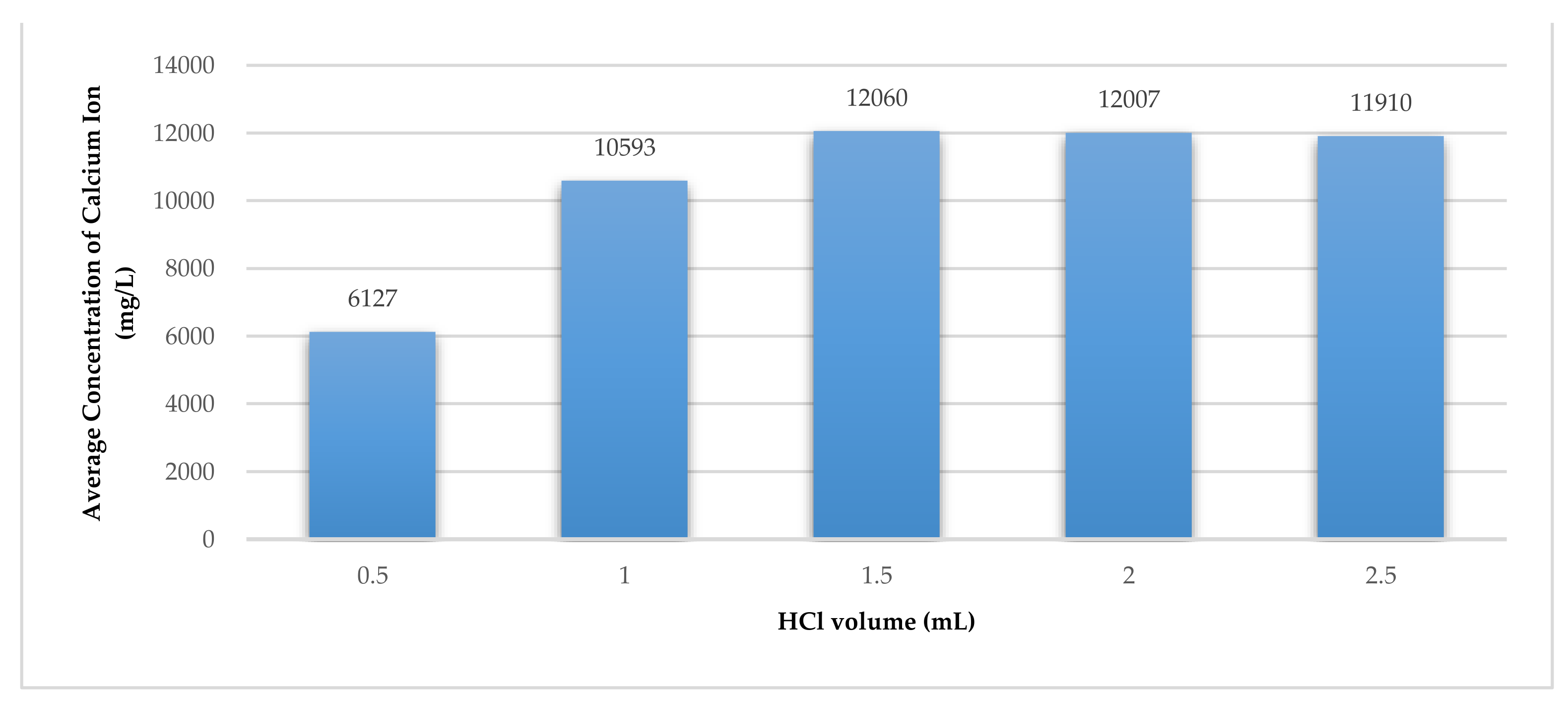
As mentioned above, the extraction of the Ca2+ ions from the WPSA was done by mixing 1.0 g of WPSA with 100 mL of deionised water containing 0.5, 1, 1.5, 2, and 2.5 mL of 32% HCl; then, the mixture was stirred at 200 rpm for 3 h. The mixture was then filtered using Whatman No.1 filter paper manufactured by Merck Life Science UK Ltd., Gillingham, United Kingdom.
The concentration of Ca
2+ ion in the filtrate was measured using a Hach Lang Spectrophotometer (DR3900) and LCK 327 cuvettes. The results showed the WPSA could produce 12,060 mg/L of Ca
2+ ion, as shown in
Figure 5.
7.2. Synthesise of the Novel Coated Sand Adsorbent (QS/CFO)
The co-precipitation method stated by Sulaiman et al. [
38] has been adopted to synthesise the new adsorbent. The performance of prepared coated sand by calcium ferric oxides has been evaluated by measuring the removal efficiency of TC by applying 0.5 g of sorbent to 50 mL of contaminated water with an initial TC concentration (
C0) of 50 mg/L and initial pH of 7. The mixture was shaken for 3 h at a speed of 200 rpm.
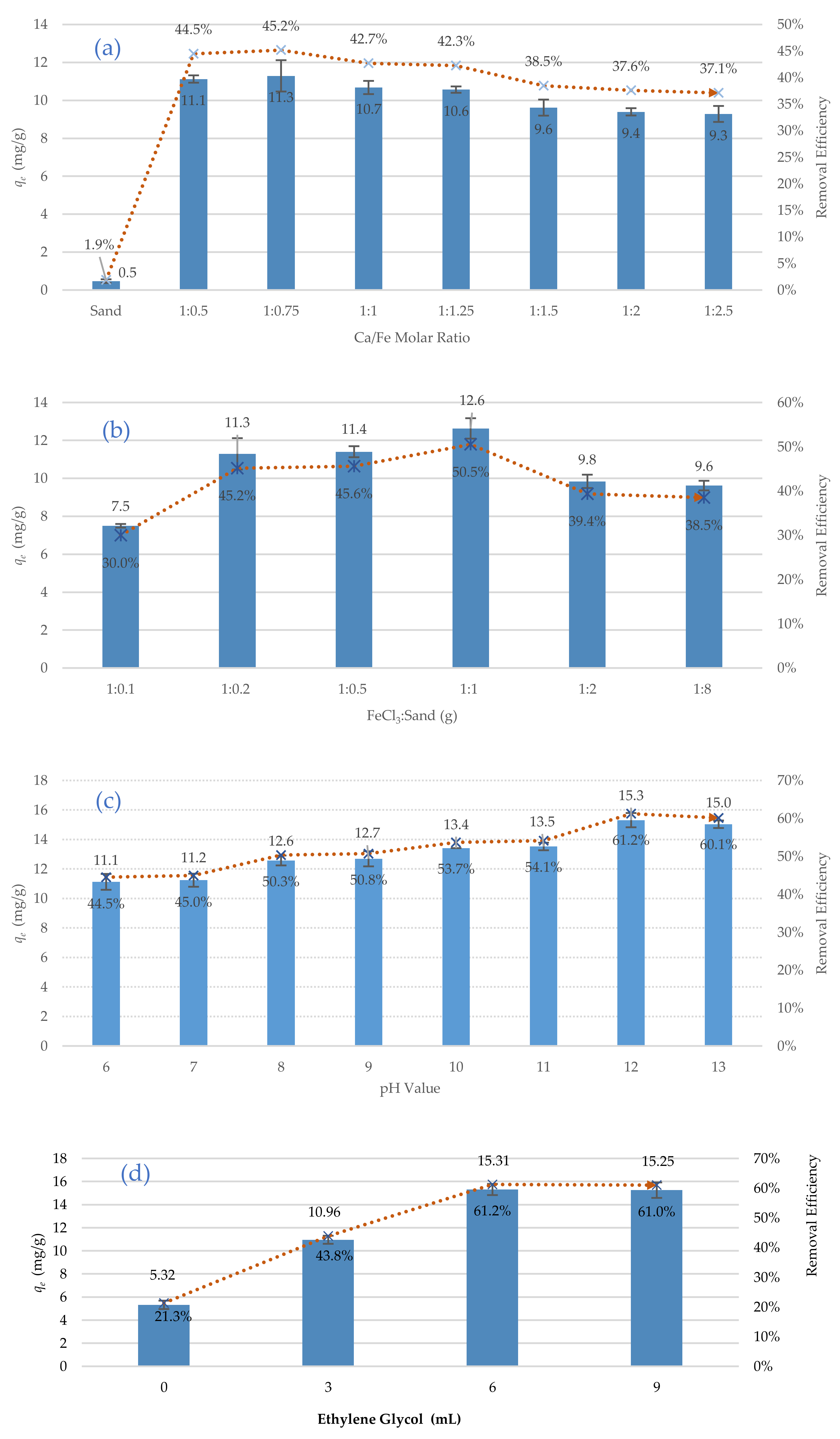
In this stage of work, the effects of six key synthesising parameters on the performance of the new adsorbent were investigated in batch experiments. The studied parameters were the Fe/Ca molar ratio, FeCl3/Sand ratio, pH of the synthesising aqueous environment, Ethylene glycol dose, drying temperatures, and recoating cycles.
Initially, a set of experiments were carried out to determine the best Fe/Ca molar ratio that provides the best adsorption of TC. The optioned results showed a significant effect of the Fe/Ca molar ratio on the removal efficiency, see
Figure 6a. A Fe/Ca molar ratio of 1/0.75 was selected as the best ratio in this work, as it provides the maximum removal efficiency (45.2%). Additionally, it was noticed that the use of uncoated sand achieved the lowest removal (1.9%). Another set of batch experiments was carried out under the same operational conditions mentioned previously to specify the FeCl
3/Sand ratio. The results of these experiments,
Figure 6b, indicated that a FeCl
3/Sand ratio of 1/1 provided the best removal efficiency (50.5%). Therefore, this ratio was adopted in the synthesis of the new adsorbent. The effect of the pH of the synthesising aqueous environment on the removal efficiency was also investigated in batch experiments. The best removal (61.2%) was noticed at a pH of 12, as illustrated in
Figure 6c. The effects of four different doses of Ethylene glycol (0 to 9 mL) on the removal efficiency of TC were studied, and it was noticed that the best removal (61.2%) was achieved at the Ethylene glycol dose of 6 mL, as illustrated in
Figure 6d. In terms of drying temperatures, the coated sand was dried at four different temperatures (75, 85, 95, and 105 °C), and then the coated sand was used to remove TC. The results of
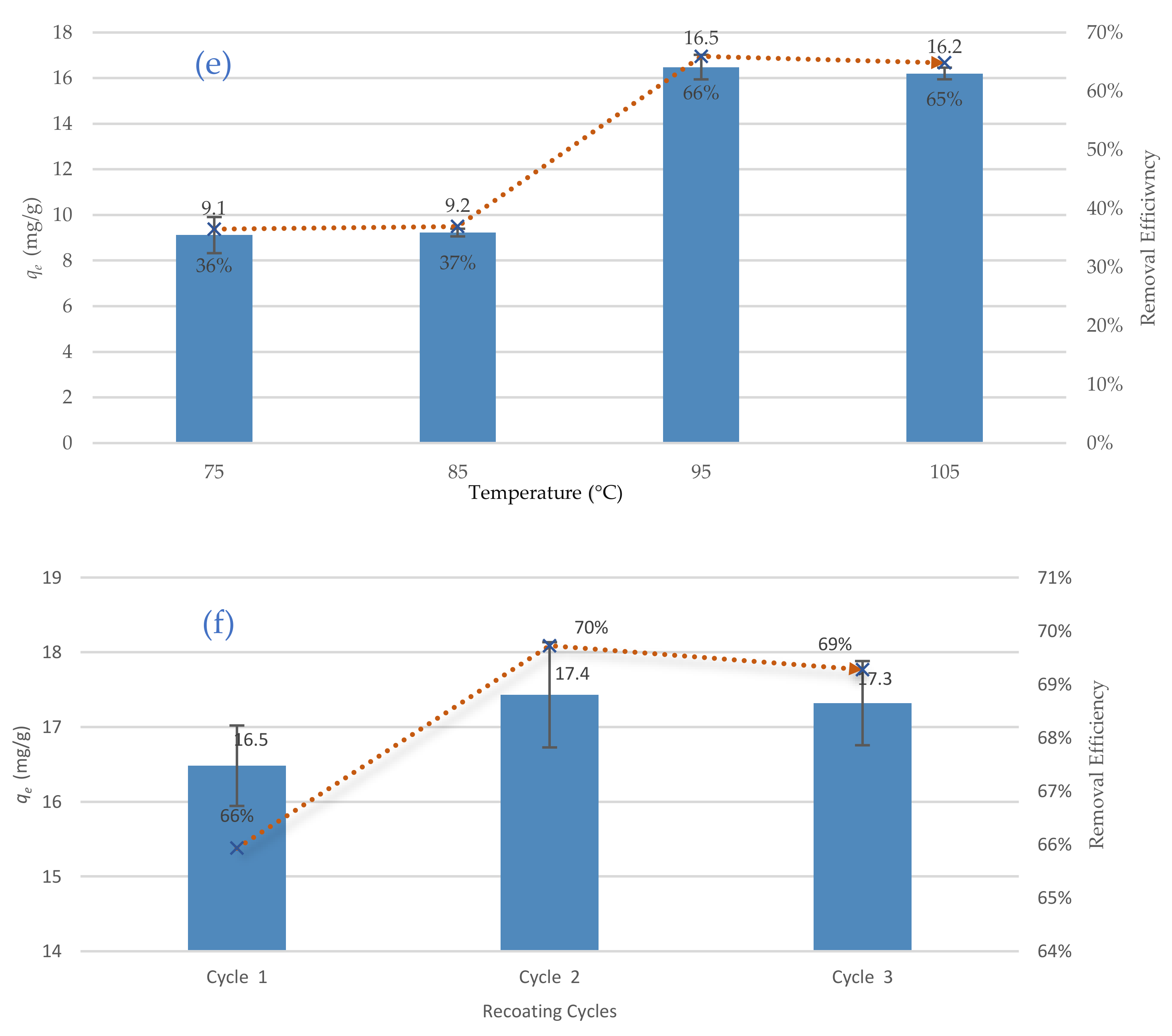
Figure 6e show the best adsorption of TC happened at a temperature of 95 °C. Lee and Lee [
39] explain this behaviour by changing the specific surface area; the specific surface area of the dried coated sand at a temperature of 95 °C was to be greater than the specific surface area of that dried at a temperature of 105 °C. It is noteworthy the majority of the synthesising processes in the literature were conducted at drying temperatures between 100 and 105 °C. Therefore, the present work could be a cost-effective and eco-friendly alternative to the traditional synthesising processes as it minimises the drying temperature by 5 °C. Finally, the effects of the recoating cycles on the removal efficiency of TC were investigated by repeating the coating process three times. The removal efficiency was increased from 66% (one coating cycle) to 70% (at three recoating cycles), see
Figure 6f. This increase of the removal efficiency could be explained by filling uncovered spaces on the surface of the sand with calcium ferric oxide, which improves the removal efficiency. In this study, however, one coating cycle was adopted due to the relatively low increase of the removal efficiency (only 4%). In summary, the best synthesising conditions for the new adsorbent are Fe/Ca molar ratio, FeCl
3/Sand ratio, pH of the synthesising aqueous environment, Ethylene glycol dose, drying temperatures, and recoating cycles of 1/0.75, 1:1, 12, 6 mL, 95 °C and one coating cycle, respectively.
7.3. Characterisation of The New Adsorbent
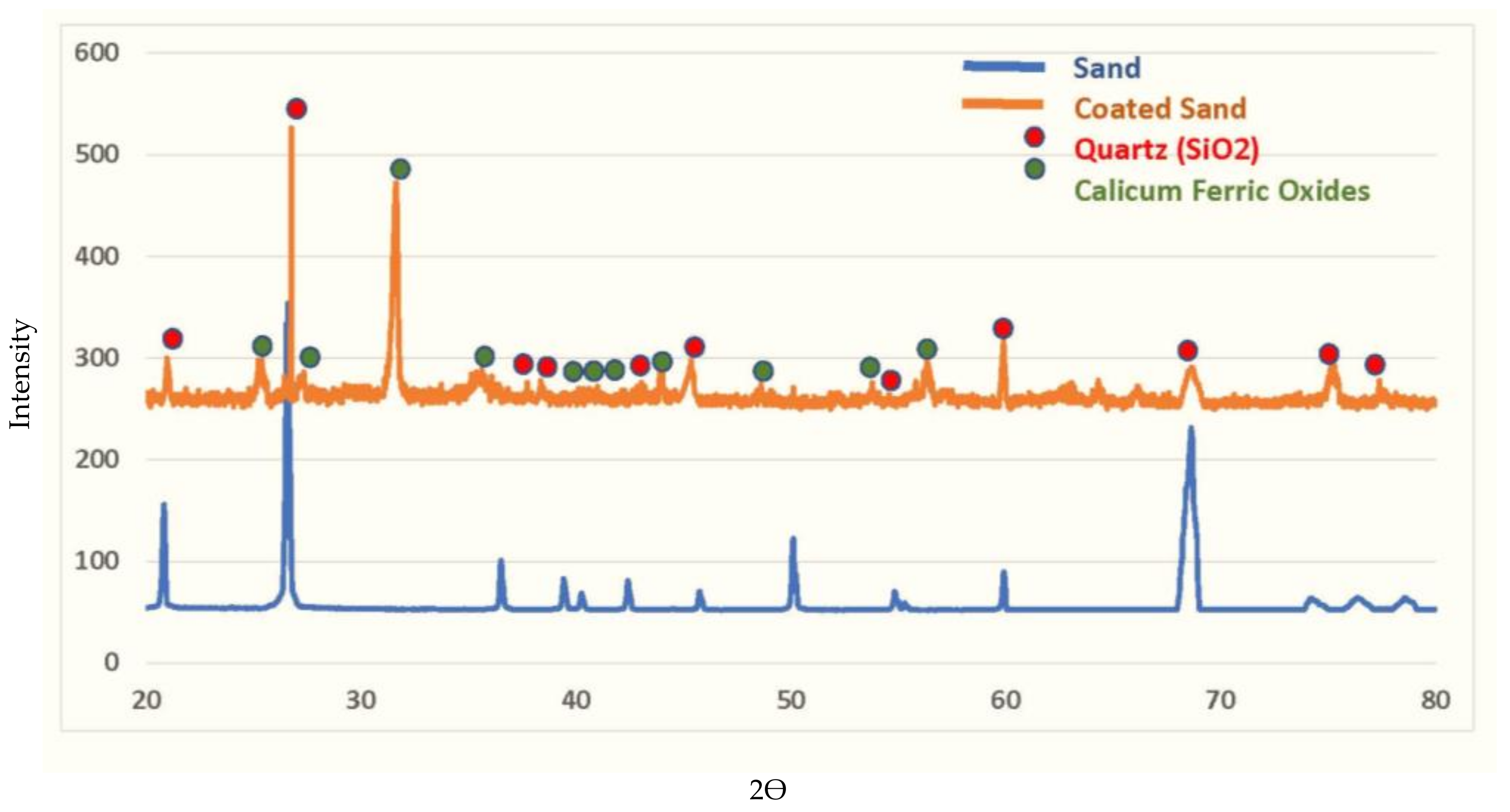
A series of characterisation tests were carried out to understand the mechanism of TC adsorption by the new adsorbent. Initially, the chemical composition of the sand before and after coating was characterised using XRD analysis, as seen in
Figure 7. The XRD results revealed the presence of diffraction reflections at degree 2theta (25.28, 26.28, 32.45, 36.28, 39.42, 40.25, 42.41, 44.28, 50.09, 54.10, and 56.44) that correspond to the presence of calcium ferric oxides pattern. The confirmation of the presence of calcium ferric oxides has been achieved by the PANalytical/X’Pert HighScore Plus software, version (3.0.0) for XRD powder diffraction measurements. The X’Pert HighScore Plus programme validated the synthesis of calcium iron oxides (CaFe
2O
4, Ca
4Fe
9O
17, Ca
0.15Fe
2.85O
4, Ca
3Fe
15O
25, CaFe
4O
7) on the surface of the raw sand. The XRD reflections describe the new sites formed on the sand’s surface, which turned the inert sand into reactive material. As a result, the new sites will oversee tetracycline removal from aqueous solutions. Based on a comparison of the “Joint Committee on Powder Diffraction Standards (JCPDSs)” and the quartz pattern obtained by this method, it seems that the silica component is the primary ingredient that is responsible for the appearance of reflections. The presence of silica oxide in the sand structure is indicated by the peaks at 21.29, 27.65, 38.26, 38.27, 43.27, 45.28, 54.26, 60.31, 64.32, 69.28, 74.63, and 77.58. The development of new reflections on the sand profile proves the formation of a new layer covering the sand; this layer will be responsible for the attraction and hunting of TC.
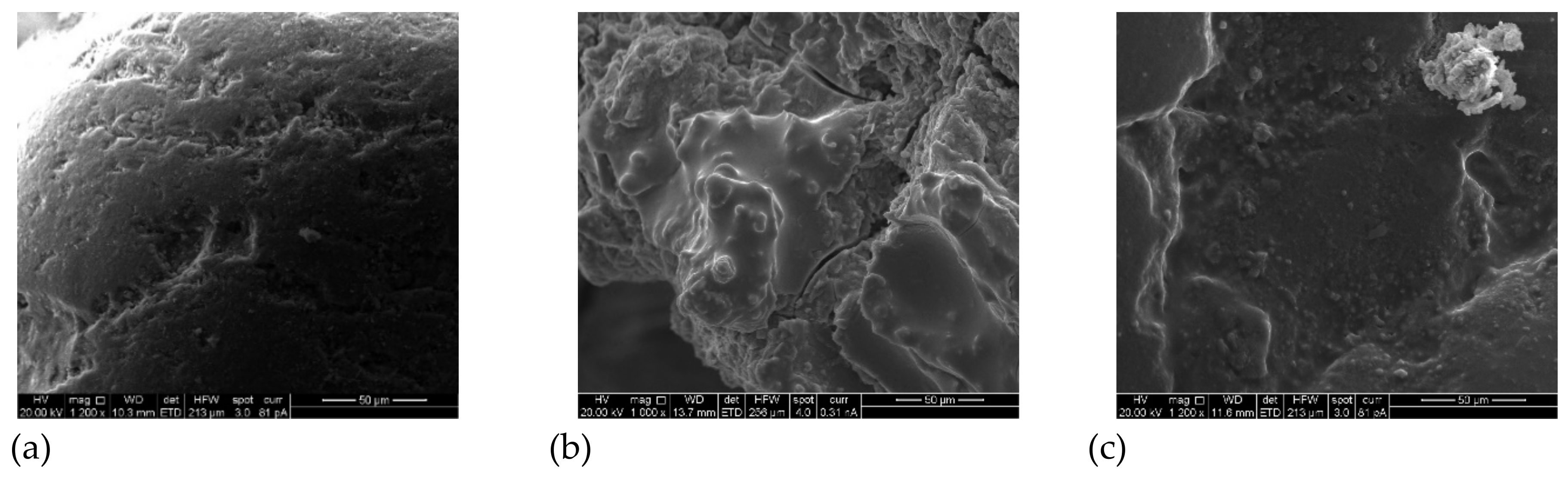
As shown in
Figure 8, SEM images illustrate the morphological properties of the new adsorbent before and after the adsorption of TC.
Figure 8a shows a nonhomogeneous morphology for the porous surface of the sand, yet the surface structure is relatively compact and chaotic.
Figure 8b shows the surface roughness and fractures of sand generated by the coating of the inert sand that resulted in the production of a rough layer in the coated sand consisting of coarsely aggregated surfaces (high surface-to-volume ratio) with inconsistent orientations and sizes. The latter increases the surface area of the new adsorbent, which in turn enhances the adsorption efficiency. There is also an increase of the size of cracks on the surfaces of the coated sand particles due to the influence of heating and drying temperatures.
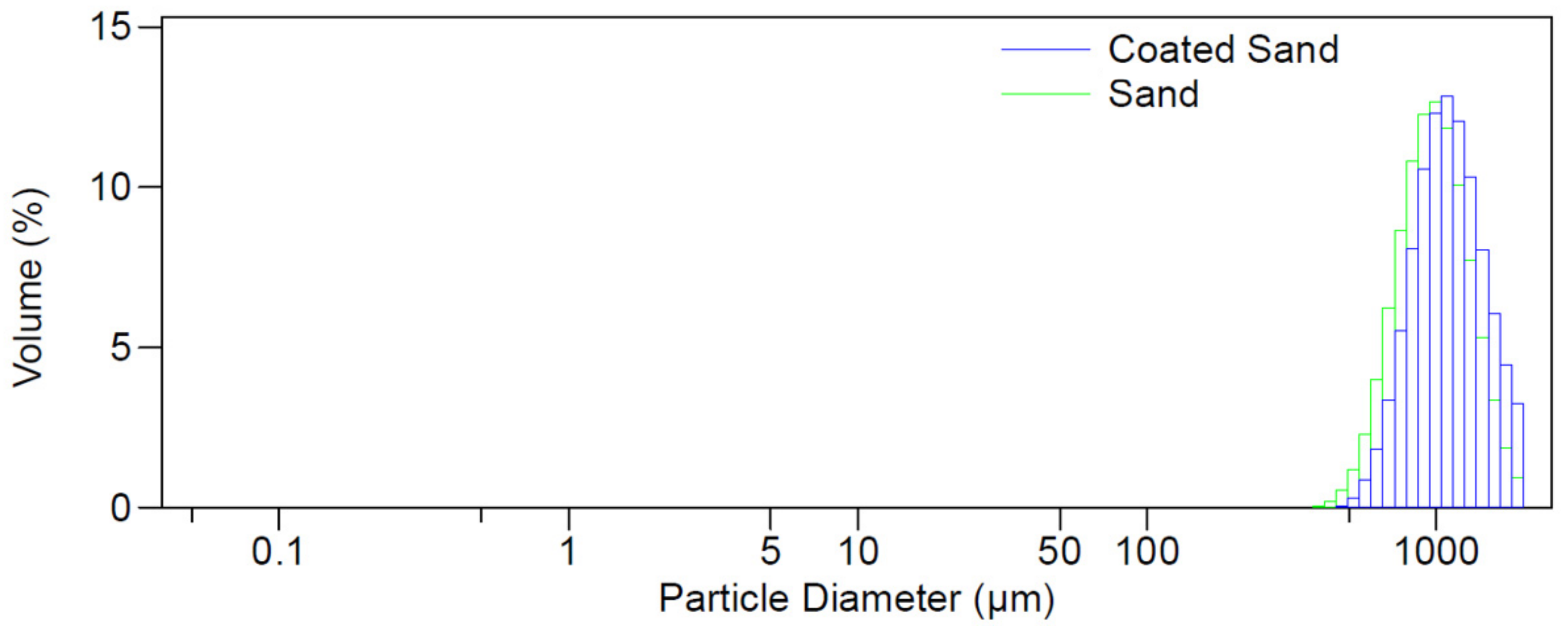
Figure 8c reveals a noticeable difference in the shape of the new adsorbent before and after TC adsorption, which is related to the interaction with contaminant molecules. This finding agrees with particle size analyser data, where the mean particle size of sand increased from 1013 micrometres to 1140 micrometres before and after coating with calcium ferric oxide, respectively, as shown in
Figure 9.
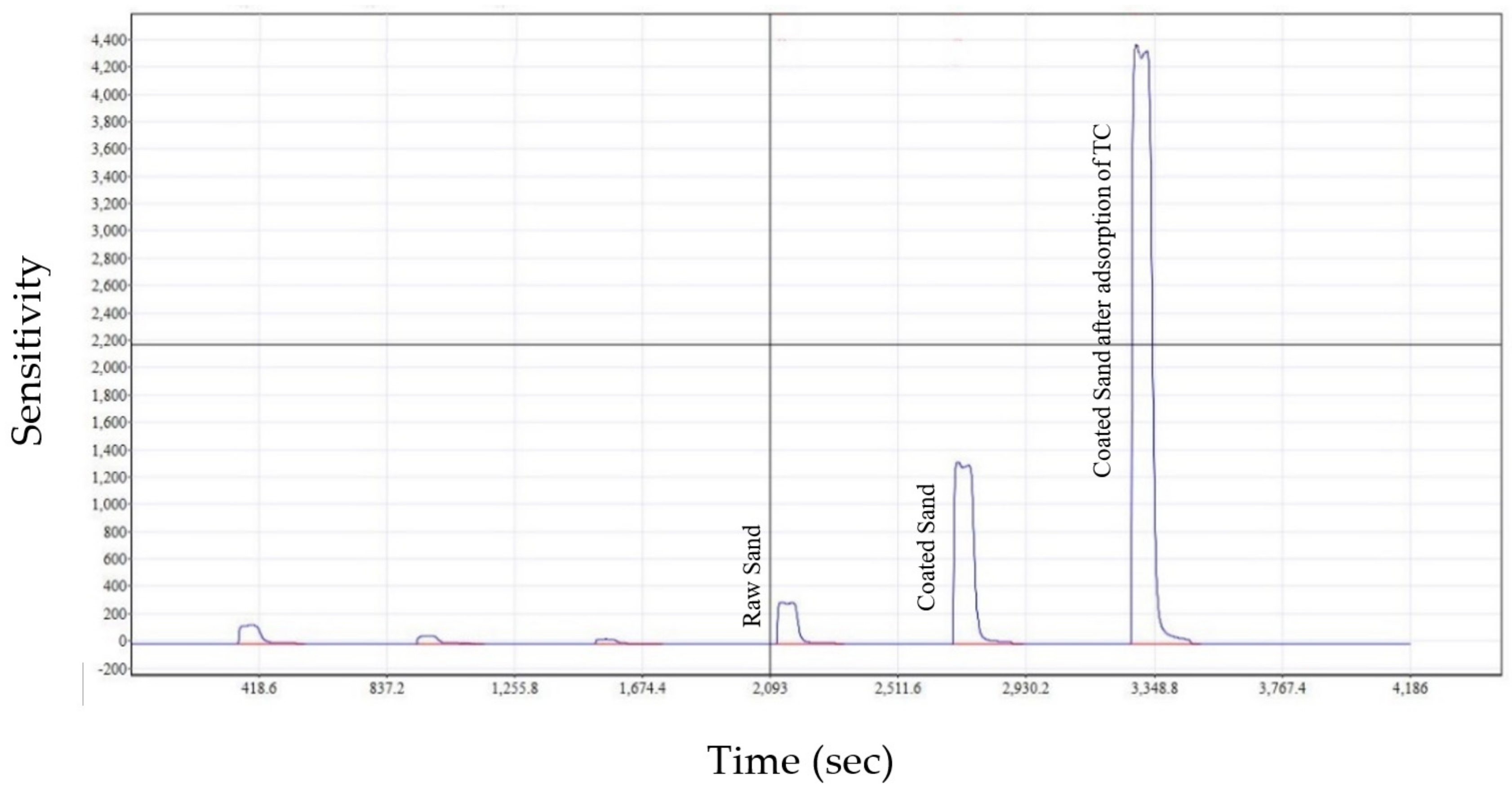
The Primacs SERIES Carbon/Nitrogen Analyser, manufactured by Skalar Analytical B.V (Breda, The Netherlands) was used to compare the total carbon content of virgin and coated sand before and after the sorption process. This test revealed that the raw sand contains a minimal quantity of carbon (at a sensitivity of 250), as shown in
Figure 10. However, the sensitivity increased from 250 to 1300 after the coating process due to the carbon added by ethylene glycol. The sensitivity then stepped up to 4300 following the TC adsorption by the coated sand. This increase of sensitivity demonstrates the efficacy of the adsorbing layer plantation and the high efficiency of TC adsorption. It is noteworthy to mention that the first three peaks in
Figure 10 are blanks.
7.4. Batch Tests
Batch tests are used to determine the best conditions for achieving the maximum removal of TC by the developed adsorbent. These parameters included in the batch experiments were the solution’s initial pH, contact time, adsorbent mass, initial concentration (
C0) of the TC, and agitation speed. The experiments were initiated by adding 50 mL of contaminated water (
V) with different
C0 of TC (10, 50, 100, 150, and 200 mg/L) into 250 mL flasks containing 0.1 g of the new adsorbent. The flasks were shaken at a speed of 200 rpm for 3 hrs. The effects of the initial pH of the aqueous solution were examined at a range of 3 to 13, the effects of the adsorbent dose were examined at a range of 0.05 to 0.5 g/ 100 mL, and the contact time was tested up to 3 h. The solution was filtered using grade 5 Whatman filter papers to separate the solid particles. Then, a set of Hach-Lang LCK 327 cuvettes and a Hach-Lang spectrophotometer (DR3900), made by Hach Lange GmbH Headquarter (Düsseldorf, Germany) were used to determine the final concentration (
Ce) of TC in the solution after filtration. The amount of TC loaded on the adsorbent (
qe, mg/g) was calculated using Equation (4), which is based on the mass balance principle.
where
qe is the amount of adsorbate loaded on the adsorbent (mg/g),
C0 and
Ce are the initial and final concentration of TC (mg/L), respectively,
V is the volume of aqueous solution (L), and
m is the mass of adsorbent (g). The removal efficiency (
) of TC was calculated using the following Equation (5):
The results of the effects of the studied parameters are explained below.
7.4.1. Effects of Operating Parameters on TC Removal
Effects of contact time
The time required to reach equilibrium in the batch study is a critical factor in defining the diffusion of contaminants between aqueous and solid phases. Therefore, the effects of contact time on the adsorption of TC onto the new adsorbent were investigated at different contact times (0 to 250 min). The experiments were repeated for different initial concentrations of TC (10 to 200 mg/L) to understand the effects of TC concentration on the performance of the new adsorbent. The initial pH, the dose of adsorbent, and agitating speed were kept constant at 7, 0.1 g/50 mL, and 200 rpm, respectively.
Figure 11a shows the results of these experiments; it can be seen from this figure that the TC elimination percentages increased quickly during the first hour of experiments, then slowed down during the rest of the experiments. Slower adsorption might be due to the increase of the number of occupied sites on the adsorbent’s surface. It can be noticed that 180 min is sufficient to reach the maximum adsorption of TC; however, most of the TC was adsorbed within the first 60 min. In terms of TC concentration, it was noticed that increasing TC concentration from 10 to 200 mg/L dropped the removal efficiency from 90% to 21% respectively. The limited availability of empty sites on the surface of the adsorbent could explain this decrease of the removal efficiency with the increase of TC concentration.
Therefore, a contact time of 180 min will be used in the rest of the experiments.
Effects of initial pH
At room temperature, the sorption of TC onto the new adsorbent were examined at different pH levels (3 to 12) of the aqueous solution at a constant
C0 and sorbent dose of 50 mg/L and 0.1 g, respectively. The findings demonstrated that raising the pH value increases the removal efficacy until the pH reaches 10, and then the removal efficiency becomes stable (see
Figure 11b). The ∆pH/solid addition method was utilised to evaluate the zeta potential of the new adsorbent. It was found that the point of zero charges (pHpzc) value was 5, as shown in
Figure 11c, and the sorbent’s surface becomes positively charged after this pH, which tends to generate an electrostatic interaction with the negatively charged pollutant. The adsorption of TC is most likely due to the π-π electron donor–acceptor interactions. The increment in TC adsorption is also related to the pore filling and the effect of H-bonding [
40].
Effects of adsorbent dose
The effects of the adsorbent dose on the removal of TC were studied using masses of the new adsorbent (between 0.05 to 0.5 g/50 mL), keeping the values of
C0, initial pH, contact time, and agitation speed constant at 50 mg/L, 7, 3 h, and 200 rpm, respectively.
Figure 11d shows that increasing the new adsorbent dose from 0.05 to 0.3 g/50 mL significantly increases the removal of TC from 53.4% to 91.8%, respectively. However, increasing the dose to more than 0.3 g/50 mL did not increase the removal efficiency. Therefore, a dose of 0.3 g/50 mL will be used in the rest of the experiments.
Effects of agitation speed
The change in TC removal with the agitation speed was studied at different speeds (0 to 250 rpm), keeping the rest of the parameters constant at the optimum values obtained from the previous experiments. The obtained results are shown in
Figure 11e, which shows that the lowest removal of TC (35%) was obtained at 0 rpm (no agitation), while the maximum removal (91.8%) was obtained at a speed of 200 rpm. The increase of the TC removal with the agitation speed could be explained by the fact that increasing the agitation speed resulted in appropriate contact between the TC and the adsorbent.
7.4.2. Desorption Kinetics
Calculating the desorption properties is crucial in evaluating the adsorbent’s potential and efficacy. Therefore, the desorption of the TC that adsorbed on the new adsorbent was measured by soaking 0.3 g of saturated new adsorbent (used in the removal of TC of the solution in the previous experiments) in 50 mL of deionised water for an extended contact time of 2880 min (48 h). The concentration of the TC in the deionised water was measured using a Hach-Lang spectrophotometer and LCK 327 cuvvets. The results of the desorption tests, as shown in
Figure 11f, proved that the maximum released amount of TC into the deionised water after 2880 minutes did not exceed 0.17mg/L, which demonstrates that the forces between composite sorbent and TC are quite strong and that this sorbent is effective and suitable for removing TC from water.
7.4.3. Adsorbent Regeneration
The regeneration of the synthesised new adsorbent must be assessed to determine whether the used adsorbent might be reused again or not. Therefore, 0.5 M HCl was used to wash the used adsorbent to extract the adsorbed TC, and deionised water was then used to wash the adsorbent. The washed adsorbent was then used to remove TC from water under the optimum operating conditions obtained from the batch experiments. This process was repeated four times (four regeneration cycles). The results of TC removal efficiency after each regeneration cycle are shown in
Figure 11g, which shows that the TC removal efficiency declines after each regeneration cycle, where it declined from 65% to 30% after one and four regeneration cycles. According to the results, the produced adsorbent could be regenerated a few times before the final disposal.
7.5. Kinetic of Adsorption
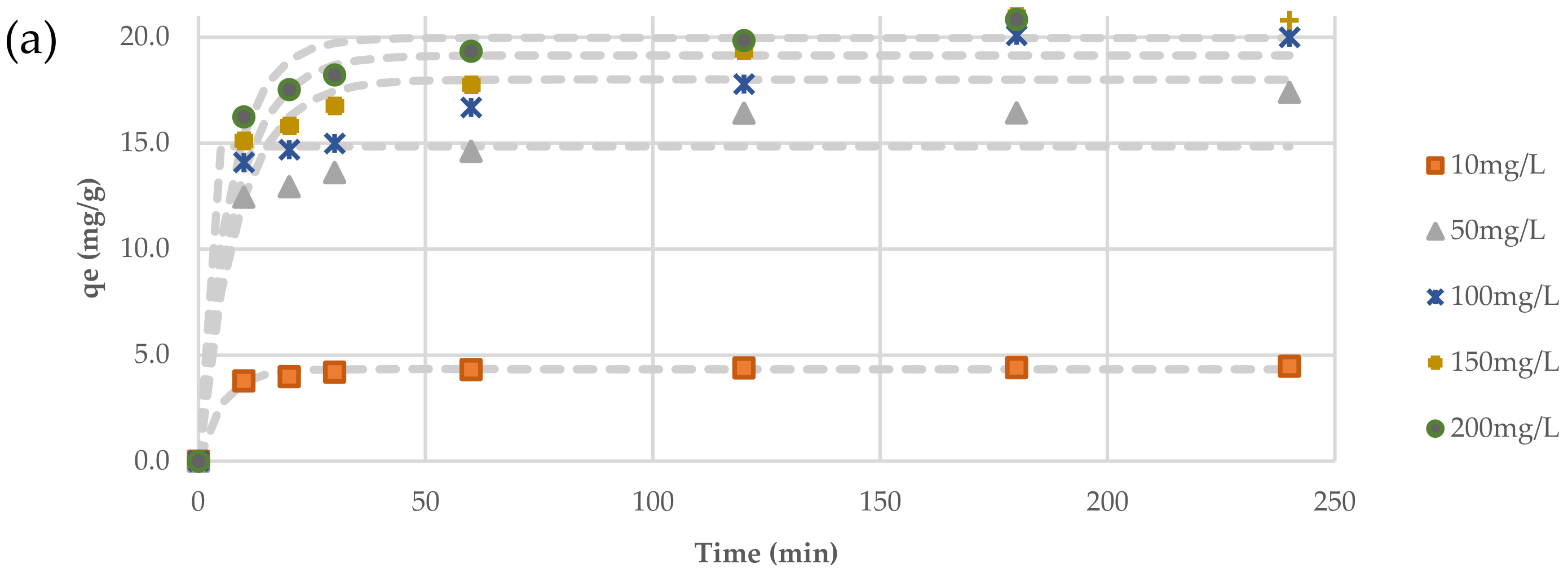
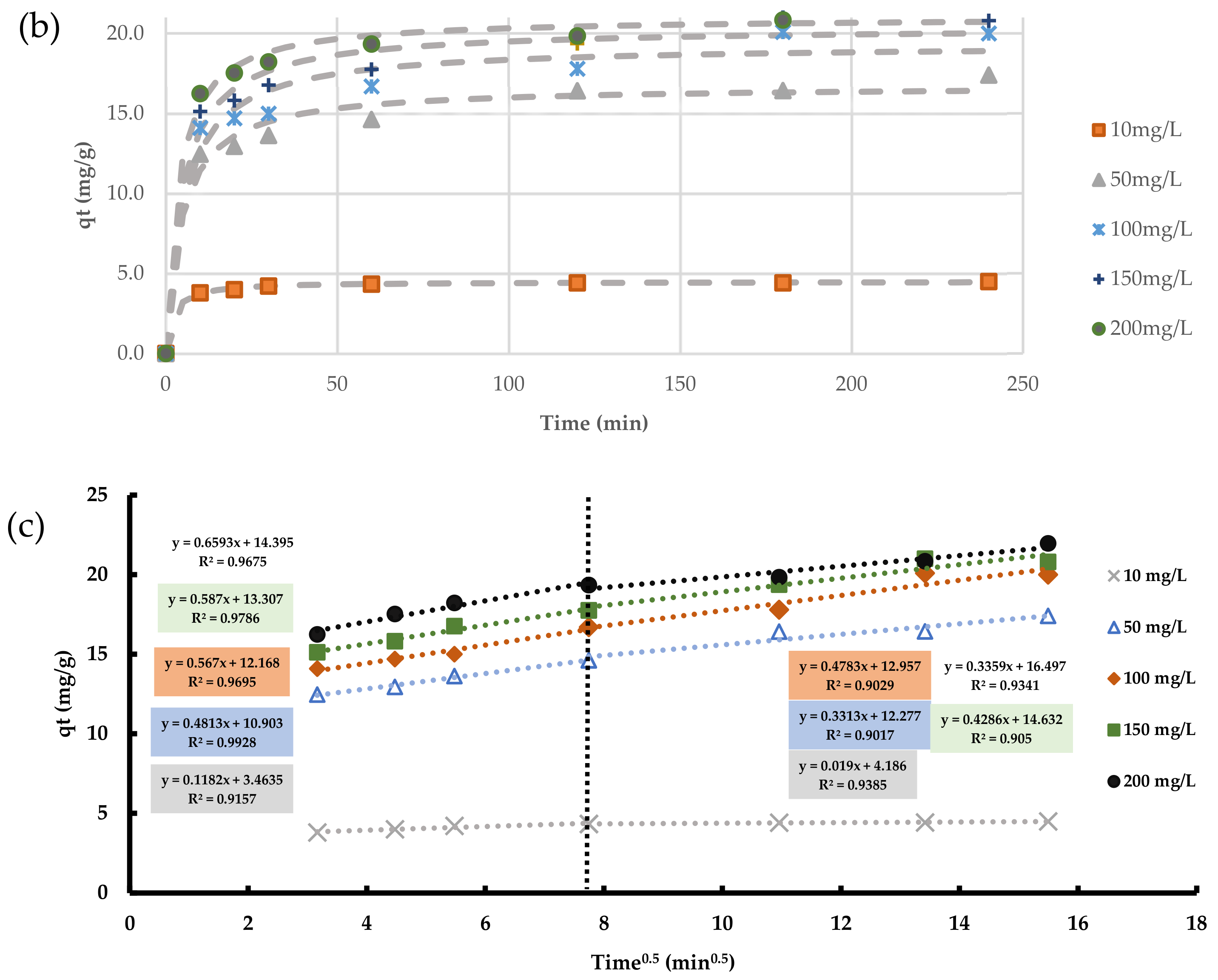
Kinetic studies of the variation of TC adsorption with contact time onto the new adsorbent at various initial concentrations of TC were fitted with non-linear analysis using the “Solver” tool in Microsoft Excel 365. The kinetic experiments were carried out under the same optimal conditions obtained from the previous batch experiments.
Table 3 shows the constants of the kinetic models according to Equations (1)–(3) derived from the fitting process. Statistical metrics (sum of squared error (SSE) and coefficient of determination (R
2)) are also derived to describe the convergence between the theoretical models and the experimental data, as shown in
Figure 12a,b. Kinetic fitting in this figure and
Table 3 revealed that the Pseudo-Second-Order model was the best in expressing the adsorption process of TC because it has the largest R
2 and the smallest SSE. Furthermore, the measured adsorbed quantity of TC (
qe) was close to the predicted values, which is evidence of the model’s applicability. These results confirmed that the TC removal is owing to chemical bonds (i.e., chemisorption). However, the kinetics models alone are insufficient for defining the processes involved in the sorption process; hence, the intra-particle diffusion model was employed in this study.
The intra particle diffusion model (Equation (3)) is fitted with experimental observations to specify the contribution of intra-particle diffusion and film diffusion to the contaminant adsorption. The results are presented in
Figure 12c. Since the relationship between
qt and
t0.5 is linear, intra-particle diffusion should occur, and this diffusion is the rate-controlling process. However,
Figure 12c shows the intercept for this plot is not zero, which means another process other than intra-particle diffusion has led to the adsorption of TC.
Tetracycline (TC) is a group of broad-spectrum antibiotics that are amphoteric due to the presence of the dimethylammonium group, phenolic diketone moiety, and the tricarbonyl system. It exists primarily as a cationic () at pH less than 3.3, zwitterionic () between pH 3.3–7.7, and anionic () and () at pH greater than 7.7, The Zeta potential test proves that the surface charge of the (CFO-SS) is negative under (5) and positive over (5); this will enable the QS/CFO to interact with (H+) and (O−) ions on the TC, which leads to an electrostatic attraction between the TC and the (QS/CFO) surface. Furthermore, the SEM images proved the increment of surface roughness of the coated sand due to the deposition of calcium ferric oxides and the drying process. The latter improves the interlock of the TC molecules within the surface of the coated sand through intraparticle diffusion; this phenomenon was also proved by the intraparticle diffusion kinetics. Additionally, the iron and calcium ions on the surface of the coated sand react with a pair of electrons and π-bonding electrons in TC functional groups (hydroxyl, carbonyl, and amino groups), forming TC-Fe, TC-Ca complexes to strengthen the cation-π bonding.
8. Conclusions
This article investigates the development of a sustainable and eco-friendly adsorbent via the reaction of calcium derived from wastepaper sludge ash with FeCl3. Based on the results of the batch experiments, the new adsorbent showed a good ability to remove TC from water, and the best performance of the new adsorbent (achieved TC removal of 92%) can be obtained at pH 10, agitation speed of 200 rpm, and contact time of 3 hrs. Furthermore, the outputs of the kinetic study indicated that the adsorption of TC on the new adsorbent is via chemical forces, and the Pseudo-Second-Order model is suitable for expressing the adsorption of TC on the new adsorbent.
Finally, future studies are still needed to investigate the applicability of the new adsorbent for the removal of other pollutants. Additionally, it is recommended to investigate the possibility of using other by-products as sources of calcium or other useful ions to remove antibiotics from the aquatic environment.