Sex Differences in Sexual Motivation in Humans and Other Mammals: The Role of Conscious and Unconscious Processes
Abstract
1. Introduction
2. Approaches to Sexual Motivation
2.1. Instinct Theories
2.2. Sexual Economics Theory
2.3. Self-Determination Theory
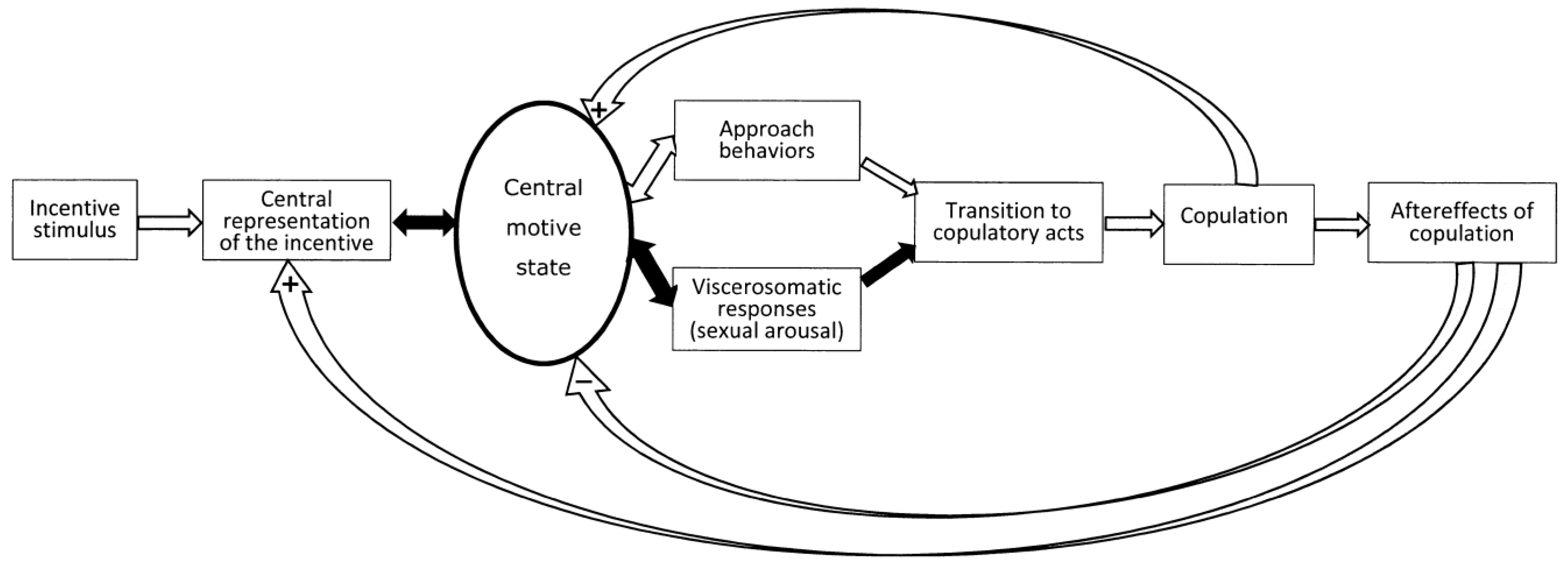
2.4. Incentive Motivation Theories
2.5. Other Models
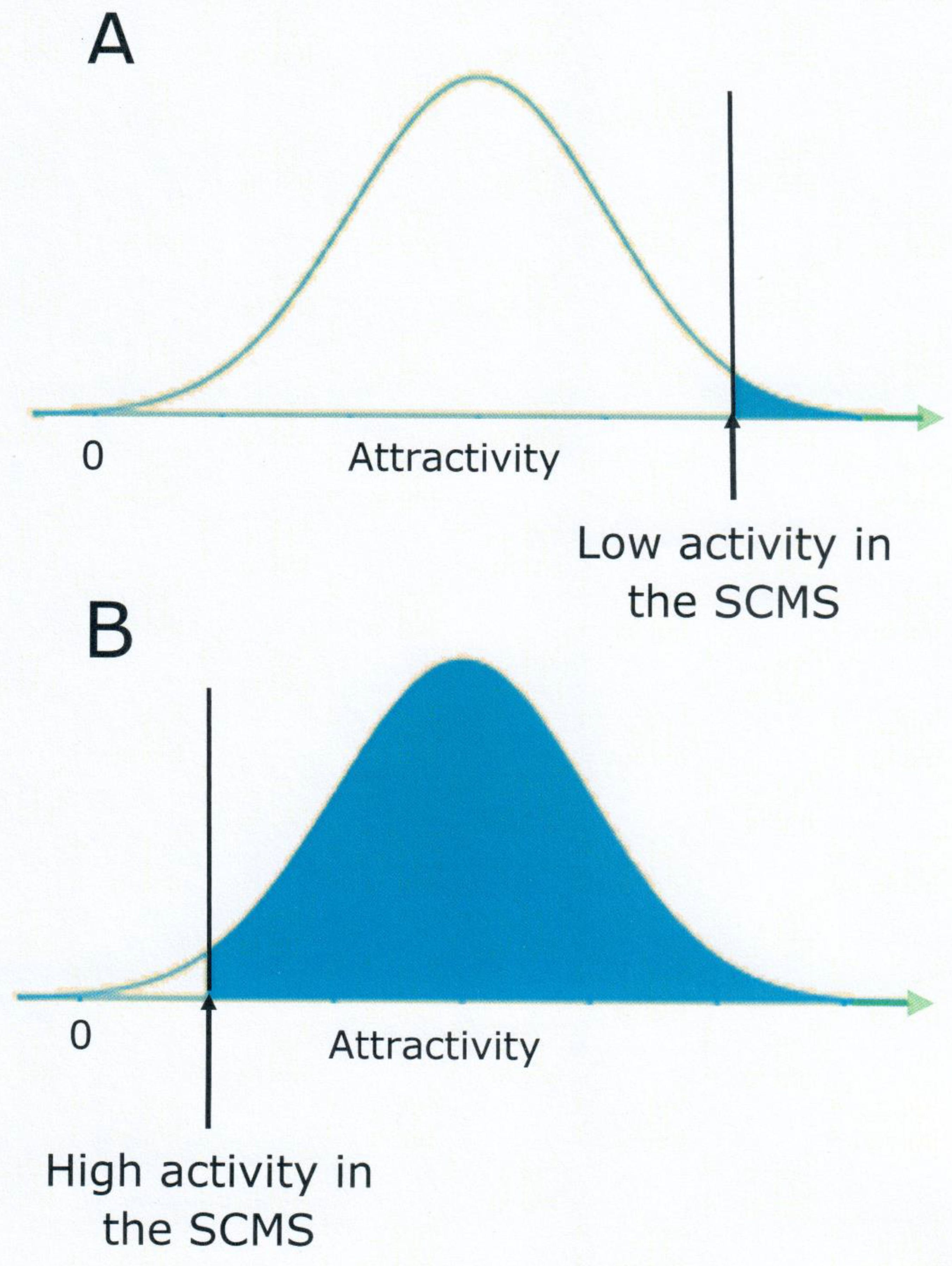
3. Is Sexual Motivation a Trait or a State?
4. Quantification of Sexual Motivation in Non-Human Animals and in Humans
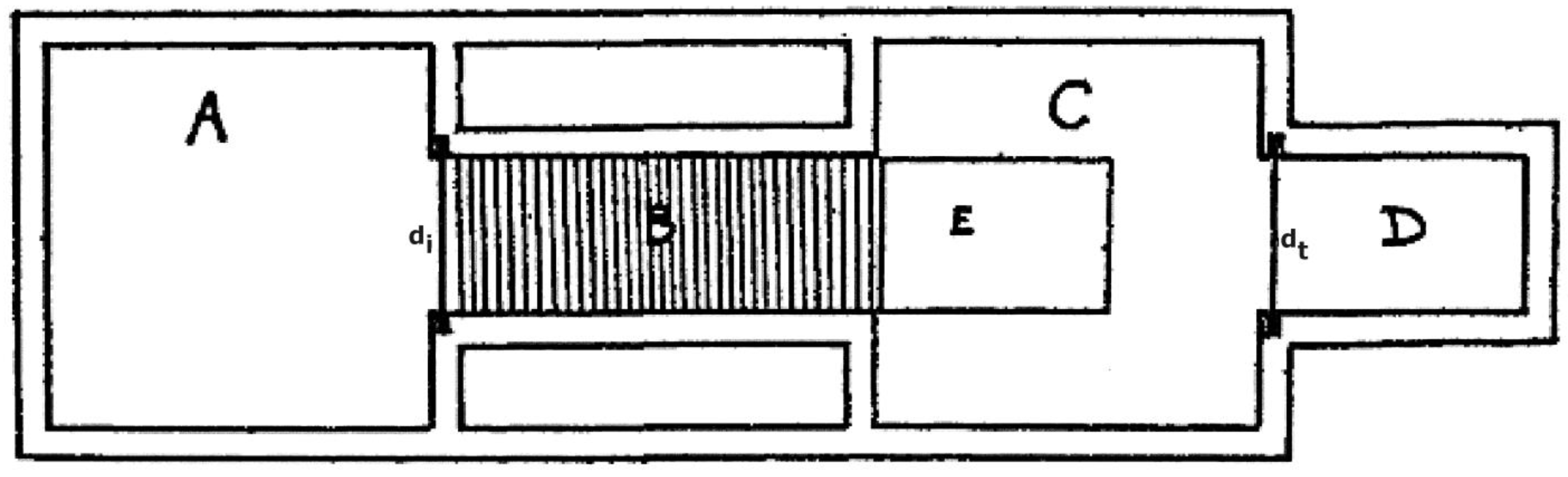
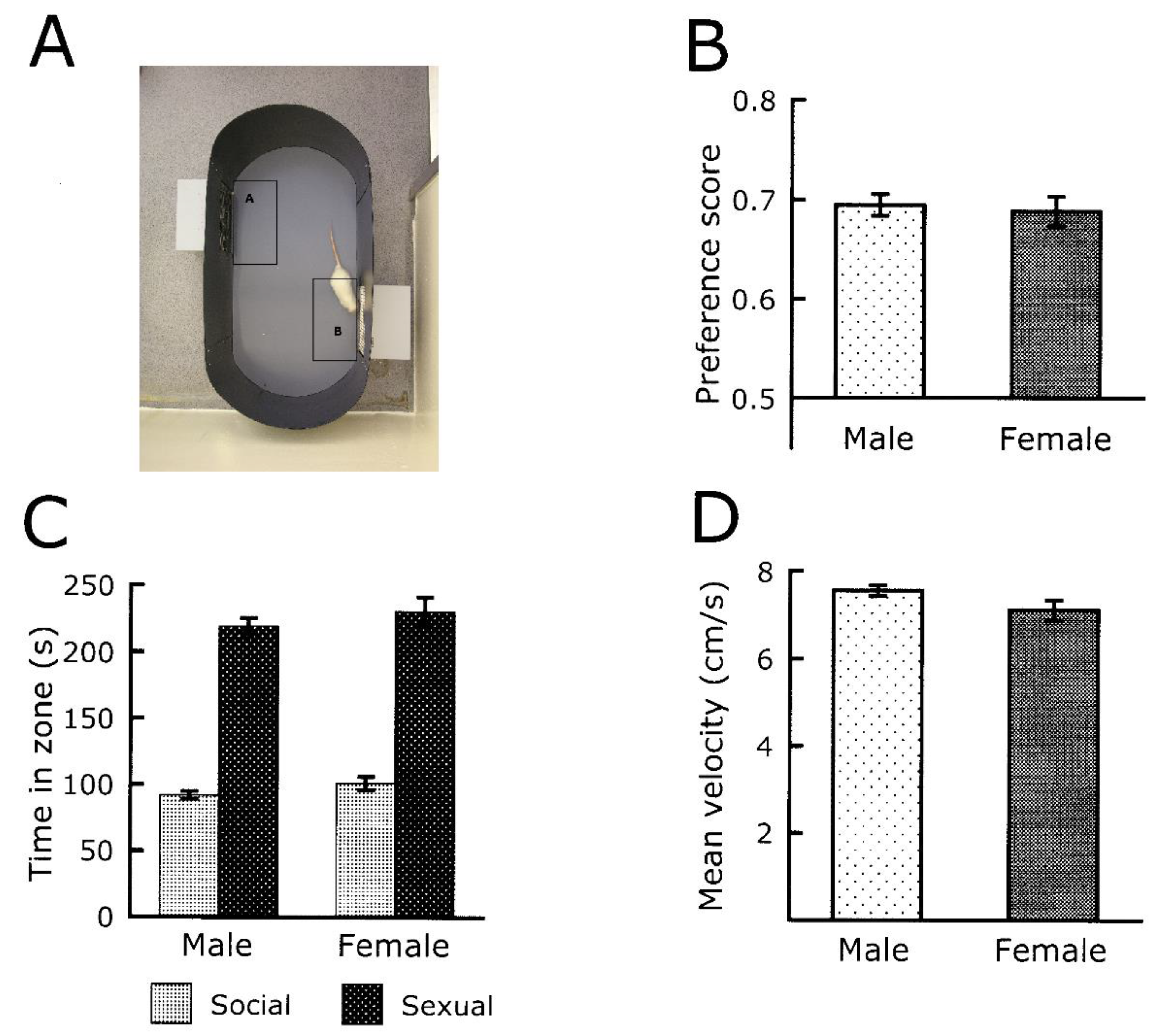
4.1. Non-Human Animals
4.2. Humans
4.2.1. Conscious vs. Unconscious Sexual Motivation
4.2.2. Self-Report Questionnaires
4.2.3. Tests for Implicit (Unconscious) Sexual Motivation
4.2.4. Subliminal Priming
4.2.5. Attention to Sexual Incentives and Cognitive Processing
4.2.6. Genital Responses
4.2.7. Anal Responses
4.2.8. Spinal Reflexes
4.2.9. Frequency of Masturbation in Infants
5. Sex Comparisons
5.1. Sexual Motivation in Male and Female Rodents
5.2. Sexual Motivation in Non-Human Primates
5.3. Sexual Motivation in Humans
5.3.1. Implicit Motivation
5.3.2. Effects of Subliminal Priming
5.3.3. Attention to Sexual Incentives and Cognitive Processing
5.3.4. Genital Responses
5.3.5. Spinal Reflex Responses
5.3.6. Masturbation in Infants
5.4. Summary of Sex Differences
6. Why Are Men and Women Responding Differently to Questionnaires?
6.1. Classical Explanations
6.2. The Role of Sexual Experiences
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lancaster, R.N. The Trouble with Nature. Sex in Science and Popular Culture; University of Caifornia Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Baumeister, R.F.; Catanese, K.R.; Vohs, K.D. Is there a gender difference in strength of sexual drive? Theoretical views, conceptual distinctions, and a review of relevant evidence. Pers. Soc. Psychol. Rev. 2001, 5, 242–273. [Google Scholar] [CrossRef]
- Lippa, R.A. Sex differences in sex drive, sociosexuality, and height across 53 nations: Testing evolutionary and social structural theories. Arch. Sex. Behav. 2009, 38, 631–651. [Google Scholar] [CrossRef]
- Frankenbach, J.; Weber, M.; Loschelder, D.D.; Kilger, H.; Friese, M. Sex drive: Theoretical conceptualization and meta-analytic review of gender differences. Psychol. Bull. 2022, 148, 621–661. [Google Scholar] [CrossRef]
- Levin, R.J.; Wagner, G. Orgasm in women in the laboratory—Quantitative studies on duration, intensity, latency, and vaginal blood flow. Arch. Sex. Behav. 1985, 14, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.G.; Fisher, T.D. Truth and consequences: Using the bogus pipeline to examine sex differences in self-reported sexuality. J. Sex Res. 2003, 40, 27–35. [Google Scholar] [CrossRef]
- Milani, S.; Brotto, L.A.; Kingstone, A. “I can see you”: The impact of implied social presence on visual attention to erotic and neutral stimuli in men and women. Can. J. Hum. Sex. 2019, 28, 105–119. [Google Scholar] [CrossRef]
- Beaussart, M.L.; Kaufman, J.C. Gender differences and the effects of perceived internet privacy on self-reports of sexual behavior and sociosexuality. Comput. Human Behav. 2013, 29, 2524–2529. [Google Scholar] [CrossRef]
- Suschinsky, K.D.; Fisher, T.D.; Maunder, L.; Hollenstein, T.; Chivers, M.L. Use of the bogus pipeline increases sexual concordance in women but not men. Arch. Sex. Behav. 2020, 49, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Kellie, D.J.; Dixson, B.J.W.; Brooks, R.C. Papa don’t preach? Using lies to expose the truth about who suppresses female sexuality. Hum. Nat.-Interdiscip. Biosoc. Perspect. 2020, 31, 222–248. [Google Scholar] [CrossRef]
- Krumpal, I. Determinants of social desirability bias in sensitive surveys: A literature review. Qual. Quant. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- Ågmo, A.; Laan, E. Sexual incentive motivation, sexual behavior, and general arousal: Do rats and humans tell the same story? Neurosci. Biobehav. Rev. 2022, 135, 104595. [Google Scholar] [CrossRef] [PubMed]
- Ågmo, A.; Laan, E. The sexual incentive motivation model and its clinical applications. J. Sex Res. 2023, 60, 969–988. [Google Scholar] [CrossRef]
- Laan, E.; Everaerd, W. Determinants of female sexual arousal: Psychophysiological theory and data. Annu. Rev. Sex Res. 1995, 6, 32–76. [Google Scholar]
- Schultheiss, O.C.; Köllner, M.G. Implicit motives. In Handbook of Personality: Theory and Research, 4th ed.; John, O.P., Robbins, R.W., Eds.; The Guilford Press: New York, NY, USA, 2021; pp. 385–410. [Google Scholar]
- DiClemente, R.J. Validity of self-reported sexual behavior among adolescents: Where do we go from here? AIDS Behav. 2016, 20, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Moll, A. Untersuchungen Über Die Libido Sexualis; Buchhandlung H. Kornfeld: Berlin, Germany, 1897. [Google Scholar]
- McDougall, W. An Introduction to Social Psychology (Revised Edition). Supplementary Chapter 5: The Sex Instinct; John W. Luce & Co.: Boston, MA, USA, 1926. [Google Scholar]
- McDougall, W. The definition of the sexual instinct. Proc. R. Soc. Med. 1914, 7, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Freud, S. Drei Abhandlungen Zur Sexualtheorie; F. Deuticke: Leipzig, Germany, 1905. [Google Scholar]
- Freud, S. Triebe und Triebschicksale. Int. Z. Arztl. Psychoanal. 1915, 3, 84–100. [Google Scholar]
- Baumeister, R.F.; Vohs, K.D. Sexual economics: Sex as female resource for social exchange in heterosexual interactions. Pers. Soc. Psychol. Rev. 2004, 8, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.F.; Reynolds, T.; Winegard, B.; Vohs, K.D. Competing for love: Applying sexual economics theory to mating contests. J. Econ. Psychol. 2017, 63, 230–241. [Google Scholar] [CrossRef]
- Van Valkenburgh, S.P. Digesting the Red Pill: Masculinity and neoliberalism in the manosphere. Men Masc. 2021, 24, 84–103. [Google Scholar] [CrossRef]
- Deci, E.L.; Ryan, R.M. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychol. Inq. 2000, 11, 227–268. [Google Scholar] [CrossRef]
- Gravel, E.E.; Pelletier, L.G.; Reissing, E.D. “Doing it” for the right reasons: Validation of a measurement of intrinsic motivation, extrinsic motivation, and amotivation for sexual relationships. Pers. Individ. Dif. 2016, 92, 164–173. [Google Scholar] [CrossRef]
- Gravel, E.E.; Reissing, E.D.; Pelletier, L.G. The ebb and flow of sexual well-being: The contributions of basic psychological needs and autonomous and controlled sexual motivation to daily variations in sexual well-being. J. Soc. Pers. Relatsh. 2020, 37, 2286–2306. [Google Scholar] [CrossRef]
- Shoikhedbrod, A.; Rosen, N.O.; Corsini-Munt, S.; Harasymchuk, C.; Impett, E.A.; Muise, A. Being responsive and self-determined when it comes to sex: How and why sexual motivation is associated with satisfaction and desire in romantic relationships. J. Sex Res. 2023, 60, 1113–1125. [Google Scholar] [CrossRef]
- Smith, C.V. In pursuit of ‘good’ sex: Self-determination and the sexual experience. J. Soc. Pers. Relatsh. 2007, 24, 69–85. [Google Scholar] [CrossRef]
- Lauderdale, M.; Yli-Piipari, S.; Irwin, C.; Layne, T. Gender differences regarding motivation for physical activity among college students: A self-determination approach. Phys. Educat. 2015, 72, 153–172. [Google Scholar] [CrossRef]
- Guérin, E.; Bales, E.; Sweet, S.; Fortier, M. A meta-analysis of the influence of gender on self-determination theory’s motivational regulations for physical activity. Can. Psychol.-Psychol. Can. 2012, 53, 291–300. [Google Scholar] [CrossRef]
- Leblanc, V.; Bégin, C.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in dietary intakes: What is the contribution of motivational variables? J. Hum. Nutr. Diet. 2015, 28, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bindra, D. How adaptive behaviour is produced: A perceptual-motivational alternative to response reinforcement. Behav. Brain Sci. 1978, 1, 41–52. [Google Scholar] [CrossRef]
- Bindra, D. A Theory of Intelligent Behavior; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Toates, F. An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. J. Sex Res. 2009, 46, 168–193. [Google Scholar] [CrossRef]
- Singer, B.; Toates, F.M. Sexual motivation. J. Sex Res. 1987, 23, 481–501. [Google Scholar] [CrossRef]
- Ågmo, A. Sexual motivation. An inquiry into events determining the occurrence of sexual behavior. Behav. Brain Res. 1999, 105, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, O.C. An incentive motivation account of motives. In Implicit Motives, 2nd ed.; Schultheiss, O.C., Pang, J.S., Eds.; Oxford University Press: Oxford, UK, 2024. [Google Scholar]
- Ågmo, A.; Laan, E. Sexual incentive motivation and sexual behavior: The role of consent. Annu. Rev. Psychol. 2024, 75, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Toates, F. A motivation model of sex addiction—Relevance to the controversy over the concept. Neurosci. Biobehav. Rev. 2022, 142, 104872. [Google Scholar] [CrossRef] [PubMed]
- Wolchik, S.A.; Beggs, V.E.; Wincze, J.P.; Sakheim, D.K.; Barlow, D.H.; Mavissakalian, M. The effect of emotional arousal on subsequent sexual arousal in men. J. Abnorm. Psychol. 1980, 89, 595–598. [Google Scholar] [CrossRef]
- Laan, E.; Everaerd, W.; Evers, A. Assessment of female sexual arousal: Response specificity and construct validity. Psychophysiology 1995, 32, 476–485. [Google Scholar] [CrossRef]
- Gaither, G.A.; Plaud, J.J. The effects of secondary stimulus characteristics on men’s sexual arousal. J. Sex Res. 1997, 34, 231–236. [Google Scholar] [CrossRef]
- McConaghy, N. Penile volume responses to moving and still pictures of male and female nudes. Arch. Sex. Behav. 1974, 3, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, K.N.; Dawson, S.J.; Shelley, A.J.; Pukall, C.F. Concurrent measurement of genital lubrication and blood flow during sexual arousal. Biol. Psychol. 2019, 145, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Everaerd, W.; Spiering, M.; Janssen, J. Automatic processes and the appraisal of sexual stimuli: Toward an information processing model of sexual arousal. J. Sex Res. 2000, 37, 8–23. [Google Scholar] [CrossRef]
- Ventura-Aquino, E.; Ågmo, A. The elusive concept of sexual motivation: Can it be anchored in the nervous system? Front. Neurosci. 2023, 17, 1285810. [Google Scholar] [CrossRef]
- Both, S.; Everaerd, W.; Laan, E. Desire emerges from excitement. A psychophysiological perspective on sexual motivation. In Psychophysiology of Sex; Janssen, E., Ed.; Indiana University Press: Bloomington, IN, USA, 2007; pp. 327–339. [Google Scholar]
- Smid, W.J.; Wever, E.C. Incentive theory of sexual motivation. In The Wiley Handbook on the Theories, Assessment and Treatment of Sexual Offending; Boer, D.P., Ed.; Wiley: Chichester, UK, 2016; pp. 141–164. [Google Scholar]
- Ventura-Aquino, E.; Portillo, W.; Paredes, R.G. Sexual motivation: A comparative approach in vertebrate species. Curr. Sex. Health Rep. 2018, 10, 114–123. [Google Scholar] [CrossRef]
- Basson, R. The female sexual response: A different model. J. Sex Marital Ther. 2000, 26, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Basson, R. A model of women’s sexual arousal. J. Sex Marital Ther. 2002, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Janssen, E. The dual control model of male sexual response: A theoretical approach to centrally mediated erectile dysfunction. Neurosci. Biobehav. Rev. 2000, 24, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Bancroft, J. The dual control model of sexual response: A scoping review, 2009–2022. J. Sex Res. 2023, 60, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Kagerer, S.; Walter, B.; Vaitl, D.; Klucken, T.; Wehrum-Osinsky, S. Trait sexual motivation questionnaire: Concept and validation. J. Sex. Med. 2015, 12, 1080–1091. [Google Scholar] [CrossRef]
- Velten, J.; Dawson, S.J.; Suschinsky, K.; Brotto, L.A.; Chivers, M.L. Development and validation of a measure of responsive sexual desire. J. Sex Marital Ther. 2020, 46, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Timmers, A.D.; Dawson, S.J.; Chivers, M.L. The effects of gender and relationship context cues on responsive sexual desire in exclusively and predominantly androphilic women and gynephilic men. J. Sex Res. 2018, 55, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Prause, N.; Janssen, E.; Hetrick, W.P. Attention and emotional responses to sexual stimuli and their relationship to sexual desire. Arch. Sex. Behav. 2008, 37, 934–949. [Google Scholar] [CrossRef]
- Giargiari, T.D.; Mahaffey, A.L.; Craighead, W.E.; Hutchison, K.E. Appetitive responses to sexual stimuli are attenuated in individuals with low levels of sexual desire. Arch. Sex. Behav. 2005, 34, 547–556. [Google Scholar] [CrossRef]
- Stark, R.; Klein, S.; Kruse, O.; Weygandt, M.; Leufgens, L.K.; Schweckendiek, J.; Strahler, J. No sex difference found: Cues of sexual stimuli activate the reward system in both sexes. Neuroscience 2019, 416, 63–73. [Google Scholar] [CrossRef]
- Steyer, R.; Mayer, A.; Geiser, C.; Cole, D.A. A theory of states and traits—Revised. Annu. Rev. Clin. Psychol. 2015, 11, 71–98. [Google Scholar] [CrossRef]
- Fleeson, W. Toward a structure- and process-integrated view of personality: Traits as density distributions of states. J. Pers. Soc. Psychol. 2001, 80, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, K.M.; Vazire, S. The incremental validity of average state self-reports over global self-reports of personality. J. Pers. Soc. Psychol. 2018, 115, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Breil, S.M.; Schweppe, P.C.; Geukes, K.; Biesanz, J.C.; Quintus, M.; Wagner, J.; Wrzus, C.; Nestler, S.; Back, M.D. The incremental validity of average states: A replication and extension of Finnigan and Vazire (2018). J. Pers. Soc. Psychol. 2022, 123, e23–e37. [Google Scholar] [CrossRef] [PubMed]
- Rauthmann, J.F.; Horstmann, K.T.; Sherman, R.A. Do self-reported traits and aggregated states capture the same thing? A nomological perspective on trait-state homomorphy. Soc. Psychol. Personal. Sci. 2019, 10, 596–611. [Google Scholar] [CrossRef]
- Lin, C.J.; Thornton, M. Evidence for bidirectional causation between trait and mental state inferences. J. Exp. Soc. Psychol. 2023, 108, 104495. [Google Scholar] [CrossRef]
- Warren, M.T.; Galla, B.M.; Grund, A. Using Whole Trait Theory to unite trait and state mindfulness. J. Res. Pers. 2023, 104, 104372. [Google Scholar] [CrossRef]
- Spiteri, T.; Ågmo, A. Modèles precliniques du désir sexuel. Sexologies 2006, 15, 241–249. [Google Scholar] [CrossRef]
- Ågmo, A.; Turi, A.L.; Ellingsen, E.; Kaspersen, H. Preclinical models of sexual desire: Conceptual and behavioral analyses. Pharmacol. Biochem. Behav. 2004, 78, 379–404. [Google Scholar] [CrossRef]
- Ågmo, A. Unconditioned sexual incentive motivation in the male Norway rat (Rattus norvegicus). J. Comp. Psychol. 2003, 117, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, B.J.; Lindström, L.H. Sexual motivation in the female rat. A methodological study applied to the investigation of the effect of estradiol benzoate. Acta Physiol. Scand. Suppl. 1973, 389, 1–80. [Google Scholar] [PubMed]
- Jenkins, M. The effect of segregation on the sex behavior of the white rat as measured by the obstruction method. Genet. Psychol. Monogr. 1928, 3, 457–571. [Google Scholar]
- Hetta, J.; Meyerson, B.J. Sexual motivation in the male rat. A methodological study of sex-specific orientation and the effects of gonadal hormones. Acta Physiol. Scand. 1978, (Suppl. S453), 1–67. [Google Scholar]
- Ågmo, A. Male rat sexual behavior. Brain Res. Protoc. 1997, 1, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Massen, C.; Bredenkamp, J. Die Wundt-Bühler-Kontroverse aus der Sicht der heutigen kognitiven Psychologie. Z. Psychol. 2005, 213, 109–114. [Google Scholar] [CrossRef]
- Weinberger, J. William James and the unconscious: Redressing a century-old misunderstanding. Psychol. Sci. 2000, 11, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.A.; Morsella, E. The unconscious mind. Perspect. Psychol. Sci. 2008, 3, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Galison, P. Blacked-out spaces: Freud, censorship and the re-territorialization of mind. Br. J. Hist. Sci. 2012, 45, 235–266. [Google Scholar] [CrossRef]
- Freud, S. The unconscious (1915). In The Standard Edition of the Complete Psychological Works of Sigmund Freud; Freud, S., Ed.; Hogarth Press and the Institute of Psychoanalysis: London, UK, 1957; Volume 14, pp. 159–204. [Google Scholar]
- Arrington, R.; Cofrancesco, J.; Wu, A.W. Questionnaires to measure sexual quality of life. Qual. Life Res. 2004, 13, 1643–1658. [Google Scholar] [CrossRef]
- Grover, S.; Shouan, A. Assessment scales for sexual disorders—A review. J. Psychosexual Health 2020, 2, 121–138. [Google Scholar] [CrossRef]
- Spector, I.P.; Carey, M.P.; Steinberg, L. The sexual desire inventory: Development, factor structure, and evidence of reliability. J. Sex Marital Ther. 1996, 22, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Ostovich, J.M.; Sabini, J. How are sociosexuality, sex drive, and lifetime number of sexual partners related? Pers. Soc. Psychol. Bull. 2004, 30, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Reis, D.; Friese, M. Development and validation of the trait sexual motivation scale (TSMS). J. Pers. Assess. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Nisbett, R.E.; Wilson, T.D. Telling more than we can know: Verbal reports on mental processes. Psychol. Rev. 1977, 84, 231–259. [Google Scholar] [CrossRef]
- Haeffel, G.J.; Howard, G.S. Self-report: Psychology’s four-letter word. Am. J. Psychol. 2010, 123, 181–188. [Google Scholar] [CrossRef]
- Borgmann, M.; Brandner, L.M.; D’Urso, D.; Gonin-Spahni, S.; Znoj, H.J.; Werner, M.A. A psychometric study of a trait and state assessment of sexual pleasure—The Amsterdam sexual pleasure inventory. J. Sex Res. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.D.; Murray, H.A. A method for investigating fantasies: The thematic apperception test. Arch. Neurol. Psychiatry 1935, 34, 289–306. [Google Scholar] [CrossRef]
- Gruber, N.; Kreuzpointner, L. Measuring the reliability of picture story exercises like the TAT. PLoS ONE 2013, 8, e79450. [Google Scholar] [CrossRef]
- McClelland, D.C.; Koestner, R.; Weinberger, J. How do self-attributed and implicit motives differ? Psychol. Rev. 1989, 96, 690–702. [Google Scholar] [CrossRef]
- Hinzmann, J.; Khalaidovski, K.; Kullmann, J.S.; Brummer, J.; Braun, S.; Kurz, P.; Wagner, K.M.; Schultheiss, O.C. Developing a causally valid picture-story measure of sexual motivation: I. Effects of priming. Motiv. Sci. 2023, 9, 157–174. [Google Scholar] [CrossRef]
- Schultheiss, O.C.; Hinzmann, J.; Bergmann, S.; Matthes, M.; Beyer, B.; Janson, K.T. Developing a causally valid picture-story measure of sexual motivation: II. Effects of film clips. Motiv. Sci. 2023, 9, 272–287. [Google Scholar] [CrossRef]
- Schultheiss, O.C.; Hinzmann, J.C. Developing a measure for the need for sex. In Implicit Motives; Schultheiss, O.C., Pang, J.S., Eds.; Oxford University Press: Oxford, UK, 2024. [Google Scholar]
- Hill, C.A. Implicit and explicit sexual motives as related, but distinct characteristics. Basic Appl. Soc. Psych. 2016, 38, 59–88. [Google Scholar] [CrossRef]
- Hill, C.A.; Preston, L.K. Individual differences in the experience of sexual motivation: Theory and measurement of dispositional sexual motives. J. Sex Res. 1996, 33, 27–45. [Google Scholar] [CrossRef]
- Greenwald, A.G.; McGhee, D.E.; Schwartz, J.L. Measuring individual differences in implicit cognition: The implicit association test. J. Pers. Soc. Psychol. 1998, 74, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, A.; Steinman, R.B. The Single Category Implicit Association Test as a measure of implicit social cognition. J. Pers. Soc. Psychol. 2006, 91, 16–32. [Google Scholar] [CrossRef] [PubMed]
- de Jong, D.C.; Reis, H.T.; Peters, B.J.; DeHaan, C.; Birnbaum, G.E. The role of implicit sexual desire in romantic relationships. Pers. Individ. Dif. 2019, 149, 46–56. [Google Scholar] [CrossRef]
- Elgendi, M.; Kumar, P.; Barbic, S.; Howard, N.; Abbott, D.; Cichocki, A. Subliminal priming-state of the art and future perspectives. Behav. Sci. 2018, 8, 54. [Google Scholar] [CrossRef]
- Moore, T.; Zirnsak, M. Neural mechanisms of selective visual attention. Annu. Rev. Psychol. 2017, 68, 47–72. [Google Scholar] [CrossRef]
- Watson, P.; Pearson, D.; Wiers, R.W.; Le Pelley, M.E. Prioritizing pleasure and pain: Attentional capture by reward-related and punishment-related stimuli. Curr. Opin. Behav. Sci. 2019, 26, 107–113. [Google Scholar] [CrossRef]
- Spiering, M.; Everaerd, W. The sexual unconscious. In Psychophysiology of Sex; Janssen, E., Ed.; Indiana University Press: Bloomington, IN, USA, 2007; pp. 166–184. [Google Scholar]
- Mennemeier, M. Arousal, attention, and perception. In The Roots of Cognitive Neuroscience: Behavioral Neurology and Neuropsychology; Chatterjee, A., Coslett, H.B., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 131–170. [Google Scholar]
- Legrand, L.B.; Del Zotto, M.; Tyrand, R.; Pegna, A.J. Basic instinct undressed: Early spatiotemporal processing for primary sexual characteristics. PLoS ONE 2013, 8, e69726. [Google Scholar] [CrossRef]
- Ziogas, A.; Habermeyer, E.; Santtila, P.; Poeppl, T.B.; Mokros, A. Neuroelectric correlates of human sexuality: A review and meta-analysis. Arch. Sex. Behav. 2023, 52, 497–596. [Google Scholar] [CrossRef]
- Giuliano, F.; Bernabé, J.; Rampin, O.; Courtois, F.; Benoit, G.; Rosseau, J.P. Telemetric monitoring of intracavernous pressure in freely moving rats during copulation. J. Urol. 1994, 152, 1271–1274. [Google Scholar] [CrossRef]
- d’Isa, R.; Gerlai, R. Designing animal-friendly behavioral tests for neuroscience research: The importance of an ethological approach. Front. Behav. Neurosci. 2023, 16, 1090248. [Google Scholar] [CrossRef] [PubMed]
- Kuban, M.; Barbaree, H.E.; Blanchard, R. A comparison of volume and circumference phallometry: Response magnitude and method agreement. Arch. Sex. Behav. 1999, 28, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, T.M. Devices and methods to measure female sexual arousal. Sex. Med. Rev. 2015, 3, 225–244. [Google Scholar] [CrossRef]
- Handy, A.B.; McMahon, L.N.; Meston, C.M. Local responses to genital arousal—Mechanisms of lubrication. Curr. Sex. Health Rep. 2022, 14, 247–253. [Google Scholar] [CrossRef]
- Tajkarimi, K.; Burnett, A.L. The role of genital nerve afferents in the physiology of the sexual response and pelvic floor function. J Sex Med. 2011, 8, 1299–1312. [Google Scholar] [CrossRef]
- Alkatout, I.; Wedel, T.; Pape, J.; Possover, M.; Dhanawat, J. Pelvic nerves - from anatomy and physiology to clinical applications. Transl. Neurosci. 2021, 12, 362–378. [Google Scholar] [CrossRef]
- Bohlen, J.G.; Held, J.P.; Sanderson, M.O.; Ahlgren, A. The female orgasm: Pelvic contractions. Arch. Sex. Behav. 1982, 11, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, J.G.; Held, J.P.; Sanderson, M.O. The male orgasm: Pelvic contractions measured by anal probe. Arch. Sex. Behav. 1980, 9, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, J.G.; Held, J.P. Anal probe for monitoring vascular and muscular events during sexual response. Psychophysiology 1979, 16, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Sawatsky, M.L.; Suschinsky, K.D.; Lavrinsek, S.; Chivers, M.L.; Lalumiere, M.L. Can the vaginal photoplethysmograph and its associated methodology be used to assess anal vasocongestion in women and men? Arch. Sex. Behav. 2021, 50, 3865–3888. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Bradley, M.M.; Lang, P.J.; Requin, J. Modulation of spinal reflexes: Arousal, pleasure, action. Psychophysiology 1995, 32, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Brunia, C.H.M.; Boelhouwer, A.J.W. Reflexes as a tool: A window in the central nervous system. In Advances in Psychophysiology; JAI Press: Stamford, CT, USA, 1988; Volume 3, pp. 1–67. [Google Scholar]
- Moss, F.A. Study of animal drives. J. Exp. Psychol. 1924, 7, 165–185. [Google Scholar] [CrossRef]
- Warner, L.H. A study of sex drive in the white albino rat by means of the obstruction method. In Comparative Psychology Monographs; University of California Press: Berkely, CA, USA, 1927; Volume 4, pp. 1–68. [Google Scholar]
- Diserens, C.M.; Vaughn, J. The experimental psychology of motivation. Psychol. Bull. 1931, 28, 15–65. [Google Scholar] [CrossRef]
- Warden, C.J.; Nissen, H.W. An experimental analysis of the obstruction method for measuring animal drives. J. Comp. Psychol. 1928, 8, 325–342. [Google Scholar] [CrossRef]
- Dixson, A.F. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Human Beings; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Wallen, K. Sex and context: Hormones and primate sexual motivation. Horm. Behav. 2001, 40, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.D.; Bartlett, E.S. Penile erection: A reflection of sexual arousal and arousability in male chimpanzees. Physiol. Behav. 1997, 61, 425–432. [Google Scholar] [CrossRef]
- Eberle, M.; Kappeler, P.M. Selected polyandry: Female choice and inter-sexual conflict in a small nocturnal solitary primate (Microcebus murinus). Behav. Ecol. Sociobiol. 2004, 57, 91–100. [Google Scholar] [CrossRef]
- Eberle, M.; Kappeler, P.M. Sex in the dark: Determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav. Ecol. Sociobiol. 2004, 57, 77–90. [Google Scholar] [CrossRef]
- Stumpf, R.M.; Boesch, C. Male aggression and sexual coercion in wild West African chimpanzees, Pan troglodytes verus. Anim. Behav. 2010, 79, 333–342. [Google Scholar] [CrossRef]
- Tutin, C.E.G. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 1979, 6, 29–38. [Google Scholar] [CrossRef]
- Watts, D.P. Effects of male group size, parity, and cycle stage on female chimpanzee copulation rates at Ngogo, Kibale National Park, Uganda. Primates 2007, 48, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T. Sexual behavior of adult male chimpanzees of the Mahale Mountains national park, Tanzania. Primates 1997, 38, 379–398. [Google Scholar] [CrossRef]
- Stanford, C.B. The social behavior of chimpanzees and bonobos: Empirical evidence and shifting assumptions. Curr. Anthropol. 1998, 39, 399–420. [Google Scholar] [CrossRef]
- Deschner, T.; Heistermann, M.; Hodges, K.; Boesch, C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm. Behav. 2004, 46, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Heldstab, S.A.; van Schaik, C.l.P.; Müller, D.W.H.; Rensch, E.; Lackey, L.B.; Zerbe, P.; Hatt, J.M.; Clauss, M.; Matsuda, I. Reproductive seasonality in primates: Patterns, concepts and unsolved questions. Biol. Rev. 2021, 96, 66–88. [Google Scholar] [CrossRef]
- Rooker, K.; Gavrilets, S. On the evolution of sexual receptivity in female primates. Sci. Rep. 2020, 10, 11945. [Google Scholar] [CrossRef]
- Dewitte, M. Gender differences in liking and wanting sex: Examining the role of motivational context and implicit versus explicit processing. Arch. Sex. Behav. 2015, 44, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Roath, O.K.; Chen, X.; Kolacz, J. Predictors of participation for sexuality items in a U.S. population-based online survey. Arch. Sex. Behav. 2023, 52, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.; Janssen, E.; Turner, S.L. Classical conditioning of sexual arousal in women and men: Effects of varying awareness and biological relevance of the conditioned stimulus. Arch. Sex. Behav. 2004, 33, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gillath, O.; Collins, T. Unconscious desire: The affective and motivational aspects of subliminal sexual priming. Arch. Sex. Behav. 2016, 45, 5–20. [Google Scholar] [CrossRef]
- Novák, O.; Bártová, K.; Vagenknecht, V.; Klapilová, K. Attention bias and recognition of sexual images. Front. Psychol. 2020, 11, 556071. [Google Scholar] [CrossRef]
- Henderson, R.R.; Bradley, M.M.; Lang, P.J. Modulation of the initial light reflex during affective picture viewing. Psychophysiology 2014, 51, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.B.; Deuter, C.E.; Hengesch, X.; Schachinger, H. The time course of pupil dilation evoked by visual sexual stimuli: Exploring the underlying ANS mechanisms. Psychophysiology 2017, 54, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Morandini, J.S.; Veldre, A.; Hsu, K.J.; Dar-Nimrod, I. Ethnic differences in visual attention to sexual stimuli among Asian and White heterosexual women and men. Pers. Individ. Dif. 2020, 155, 109630. [Google Scholar] [CrossRef]
- Carvalho, J.; Rosa, P.J. Gender differences in the emotional response and subjective sexual arousal toward non-consensual sexual intercourse: A pupillometric study. J. Sex. Med. 2020, 17, 1865–1874. [Google Scholar] [CrossRef]
- Wiemer, J.; Kurstak, S.; Sellmann, F.; Lindner, K. Sexual stimuli cause behavioral disinhibition in both men and women, but even more so in men. Arch. Sex. Behav. 2023, 52, 1445–1460. [Google Scholar] [CrossRef]
- O’Kane, K.M.K.; Milani, S.; Chivers, M.L.; Dawson, S.J. Gynephilic men’s and androphilic women’s visual attention patterns: The effects of gender and sexual activity cues. J. Sex Res. 2023, 60, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Chivers, M.L.; Bailey, J.M. A sex difference in features that elicit genital response. Biol. Psychol. 2005, 70, 115–120. [Google Scholar] [CrossRef]
- Heiman, J.R. A psychophysiological exploration of sexual arousal patterns in females and males. Psychophysiology 1977, 14, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Gomes, A.Q.; Laja, P.; Oliveira, C.; Vilarinho, S.; Janssen, E.; Nobre, P. Gender differences in sexual arousal and affective responses to erotica: The effects of type of film and fantasy instructions. Arch. Sex. Behav. 2013, 42, 1011–1019. [Google Scholar] [CrossRef]
- Both, S.; Spiering, M.; Everaerd, W.; Laan, E. Sexual behavior and responsiveness to sexual stimuli following laboratory-induced sexual arousal. J. Sex Res. 2004, 41, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Peterson, Z.D.; Janssen, E.; Laan, E. Women’s sexual responses to heterosexual and lesbian erotica: The role of stimulus intensity, affective reaction, and sexual history. Arch. Sex. Behav. 2010, 39, 880–897. [Google Scholar] [CrossRef] [PubMed]
- Suschinsky, K.D.; Dawson, S.J.; Chivers, M.L. Assessing gender-specificity of clitoral responses. Can. J. Hum. Sex. 2020, 29, 57–64. [Google Scholar] [CrossRef]
- Bouchard, K.N.; Timmers, A.D.; Chivers, M.L. Gender-specificity of genital response and self-reported sexual arousal in women endorsing facets of bisexuality. J. Bisexuality 2015, 15, 180–203. [Google Scholar] [CrossRef]
- Timmers, A.D.; Bouchard, K.N.; Chivers, M.L. Effects of gender and sexual activity cues on the sexual responses of women with multidimensionally defined bisexuality. J. Bisexuality 2015, 15, 154–179. [Google Scholar] [CrossRef]
- Mavissakalian, M.; Blanchard, E.B.; Abel, G.C.; Barlow, D.H. Responses to complex erotic stimuli in homosexual and heterosexual males. Br. J. Psychiatry 1975, 126, 252–257. [Google Scholar] [CrossRef]
- Sakheim, D.K.; Barlow, D.H.; Beck, J.G.; Abrahamson, D.J. A comparison of male heterosexual and male homosexual patterns of sexual arousal. J. Sex Res. 1985, 21, 183–198. [Google Scholar] [CrossRef]
- Cerny, J.A.; Janssen, E. Patterns of sexual arousal in homosexual, bisexual, and heterosexual men. Arch. Sex. Behav. 2011, 40, 687–697. [Google Scholar] [CrossRef]
- Chivers, M.L. The specificity of women’s sexual response and its relationship with sexual orientations: A review and ten hypotheses. Arch. Sex. Behav. 2017, 46, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Sawatsky, M.L.; Dawson, S.J.; Lalumière, M.L. Genital lubrication: A cue-specific sexual response? Biol. Psychol. 2018, 134, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Both, S.; Everaerd, W.; Laan, E. Modulation of spinal reflexes by aversive and sexually appetitive stimuli. Psychophysiology 2003, 40, 174–183. [Google Scholar] [CrossRef]
- Both, S.; Van Boxtel, G.; Stekelenburg, J.; Everaerd, W.; Laan, E. Modulation of spinal reflexes by sexual films of increasing intensity. Psychophysiology 2005, 42, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Ajlouni, H.K.; Daoud, A.S.; Ajlouni, S.F.; Ajlouni, K.M. Infantile and early childhood masturbation: Sex hormones and clinical profile. Ann. Saudi Med. 2010, 30, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Unal, F. Predisposing factors in childhood masturbation in Turkey. Eur. J. Pediatr. 2000, 159, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bakwin, H. Masturbation in infants. J. Pediatr. 1952, 40, 675–678. [Google Scholar] [CrossRef]
- Giorgi, G.; Siccardi, M. Ultrasonographic observation of a female fetus’ sexual behavior in utero. Am. J. Obstet. Gynecol. 1996, 175, 753. [Google Scholar] [CrossRef]
- Rodríguez Fernández, V.; López Ramón y Cajal, C. In utero gratification behaviour in male fetus. Prenat. Diagn. 2016, 36, 985–986. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S. Childhood and Sexuality; Blackwell: Oxford, UK, 1982. [Google Scholar]
- Friedrich, W.N.; Grambsch, P.; Broughton, D.; Kuiper, J.; Beilke, R.L. Normative sexual behavior in children. Pediatrics 1991, 88, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, W.N.; Fisher, J.; Broughton, D.; Houston, M.; Shafran, C.R. Normative sexual behavior in children: A contemporary sample. Pediatrics 1998, 101, e9. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.L.; Schick, V.; Reece, M.; Herbenick, D.; Sanders, S.A.; Dodge, B.; Fortenberry, J.D. Prevalence, frequency, and associations of masturbation with partnered sexual behaviors among us adolescents. Arch. Pediatr. Adolesc. Med. 2011, 165, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.D.; Hawkes, G. Theorizing the Sexual Child in Modernity; Palgrave McMillan: New York, NY, USA, 2010. [Google Scholar]
- Emmerink, P.M.J.; van den Eijnden, R.J.J.M.; Vanwesenbeeck, I.; ter Bogt, T.F.M. The relationship between endorsement of the sexual double standard and sexual cognitions and emotions. Sex Roles 2016, 75, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Leitenberg, H.; Detzer, M.J.; Srebnik, D. Gender differences in masturbation and the relation of masturbation experience in preadoloscence and/or early adolescence to sexual behavior and sexual adjustment in young adulthood. Arch. Sex. Behav. 1993, 22, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.H.; Simon, W. Sexual Conduct: The Social Sources of Human Sexuality, 2nd ed.; AldineTransaction: New Brunswick, NJ, USA, 2002. [Google Scholar]
- Foucault, M. Histoire de la Sexualité; Gallimard: Paris, France, 1976. [Google Scholar]
- Gagnon, J.H. The explicit and implicit use of the scripting perspective in sex research. Annu. Rev. Sex Res. 1990, 1, 1–43. [Google Scholar]
- Ward, L.M.; Rosenscruggs, D.; Aguinaldo, E.R. A scripted sexuality: Media, gendered sexual scripts, and their impact on our lives. Curr. Dir. Psychol. Sci. 2022, 31, 369–374. [Google Scholar] [CrossRef]
- Prentice, D.A.; Carranza, E. What women and men should be, shouldn’t be, are allowed to be, and don’t have to be: The contents of prescriptive gender stereotypes. Psychol. Women Q. 2002, 26, 269–281. [Google Scholar] [CrossRef]
- Endendijk, J.J.; van Baar, A.L.; Deković, M. He is a stud, she is a slut! A meta-analysis on the continued existence of sexual double standards. Pers. Soc. Psychol. Rev. 2020, 24, 163–190. [Google Scholar] [CrossRef]
- Mischel, W. A social learning view of sex differences in behavior. In The Development of Sex Differences; Maccoby, E.E., Ed.; Stanford Universsity Press: Stanford, CA, USA, 1966; pp. 56–81. [Google Scholar]
- Tolman, D.L. Dilemmas of Desire: Teenage Girls Talk about Sexuality; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Chodorow, N. The Reproduction of Mothering: Psychoanalysis and the Sociology of Gender; University of California Press: Berkeley, CA, USA, 1978. [Google Scholar]
- Chodorow, N. Individualizing Gender and Sexuality: Theory and Practice; Routledge: New York, NY, USA, 2012. [Google Scholar]
- Trivers, R.L. Parental investment amnd sexual selection. In Sexual Selection and the Decent of Man, 1871–1971; Campbell, B., Ed.; Heinemann Educational: London, UK, 1972; pp. 136–179. [Google Scholar]
- Oliver, M.B.; Hyde, J.S. Gender differences in sexuality: A meta-analysis. Psychol. Bull. 1993, 114, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Conley, T.D.; Klein, V. Women get worse sex: A confound in the explanation of gender differences in sexuality. Perspect. Psychol. Sci. 2022, 17, 960–978. [Google Scholar] [CrossRef] [PubMed]
- Klein, V.; Laan, E.; Brunner, F.; Briken, P. Sexual pleasure matters (especially for women)—Data from the German sexuality and health survey (GeSiD). Sex. Res. Soc. Policy 2022, 19, 1879–1887. [Google Scholar] [CrossRef]
- Döring, N.; Mohseni, M.R. Der Gender Orgasm Gap. Ein kritischer Forschungsüberblick zu Geschlechterdifferenzen in der Orgasmus-Häufigkeit beim Heterosex. Z. Sex. Forsch. 2022, 35, 73–87. [Google Scholar] [CrossRef]
- Frederick, D.A.; St John, H.K.; Garcia, J.R.; Lloyd, E.A. Differences in orgasm frequency among gay, lesbian, bisexual, and heterosexual men and women in a US national sample. Arch. Sex. Behav. 2018, 47, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Herbenick, D.; Schick, V.; Sanders, S.A.; Reece, M.; Fortenberry, J.D. Pain experienced during vaginal and anal intercourse with other-sex partners: Findings from a nationally representative probability study in the United States. J. Sex. Med. 2015, 12, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Elmerstig, E.; Wijma, B.; Swahnberg, K. Prioritizing the partner’s enjoyment: A population-based study on young Swedish women with experience of pain during vaginal intercourse. J. Psychosomat. Obstet. Gynecol. 2013, 34, 82–89. [Google Scholar] [CrossRef]
- Pastor-Moreno, G.; Ruiz-Pérez, I.; Sordo, L.; Henares-Montiel, J. Frequency, types, and manifestations of partner sexual violence, non-partner sexual violence and sexual harassment: A population study in Spain. Int. J. Environ. Res. Public Health 2022, 19, 8108. [Google Scholar] [CrossRef] [PubMed]
- Panel on Measuring, Rape, and Sexual Assault. Estimating the Incidence of Rape and Sexual Assault; National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Rudman, L.A.; Glick, P.; Marquardt, T.; Fetterolf, J.C. When women are urged to have casual sex more than men are: Perceived risk moderates the sexual advice double standard. Sex Roles 2017, 77, 409–418. [Google Scholar] [CrossRef]
- Baranowski, A.M.; Hecht, H. Gender differences and similarities in receptivity to sexual invitations: Effects of location and risk perception. Arch. Sex. Behav. 2015, 44, 2257–2265. [Google Scholar] [CrossRef]
- Sierra, J.C.; Alvarez-Muelas, A.; Sánchez-Fuentes, M.D. Sexual function and relationship satisfaction among women who have experienced intimate partner violence. Curr. Sex. Health Rep. 2023, 15, 280–290. [Google Scholar] [CrossRef]
- Eilers, M.A. What a (young) woman wants: Concurrent effects of desire to avoid pregnancy and desire for sex on sexual intercourse and contraceptive use. Demography 2022, 59, 2271–2293. [Google Scholar] [CrossRef]
- Laan, E.T.M.; Klein, V.; Werner, M.A.; van Lunsen, R.H.W.; Janssen, E. In pursuit of pleasure: A biopsychosocial perspective on sexual pleasure and gender. Int. J. Sex. Health 2021, 33, 516–536. [Google Scholar] [CrossRef]
- Hawkins, S.E.; DeLuca, H.K.; Claxton, S.E.; Baker, E.A. Sexual behaviors, satisfaction, and intentions to engage in casual sexual relationships and experiences in emerging adulthood. Arch. Sex. Behav. 2023, 52, 1575–1591. [Google Scholar] [CrossRef]
- Uecker, J.E.; Martinez, B.C. When and why women regret sex in hookups more than men do: An analysis of the online college social life survey. Sociol. Q. 2017, 58, 470–494. [Google Scholar] [CrossRef]
- Brotto, L.A.; Yule, M.A. Physiological and subjective sexual arousal in self-identified asexual women. Arch. Sex. Behav. 2011, 40, 699–712. [Google Scholar] [CrossRef]
- Heiman, J.R.; Rupp, H.; Janssen, E.; Newhouse, S.K.; Brauer, M.; Laan, E. Sexual desire, sexual arousal and hormonal differences in premenopausal US and Dutch women with and without low sexual desire. Horm. Behav. 2011, 59, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Laan, E.; van Driel, E.M.; van Lunsen, R.H.W. Genital responsiveness in healthy women with and without sexual arousal disorder. J. Sex. Med. 2008, 5, 1424–1435. [Google Scholar] [CrossRef]
- Sarin, S.; Amsel, R.; Binik, Y.M. A streetcar named “derousal”? A psychophysiological examination of the desire-arousal distinction in sexually functional and dysfunctional women. J. Sex Res. 2016, 53, 711–729. [Google Scholar] [CrossRef]
- Dunn, K.M.; Croft, P.R.; Hackett, G.I. Satisfaction in the sex life of a general population sample. J. Sex Marital Ther. 2000, 26, 141–151. [Google Scholar] [CrossRef]
- Colson, M.H.; Lemaire, A.; Pinton, P.; Hamidi, K.; Klein, P. Sexual behaviors and mental perception, satisfaction and expectations of sex life in men and women in France. J Sex Med. 2006, 3, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Freihart, B.K.; Stephenson, K.R.; Williams, P.B.; Meston, C.M. Sexual satisfaction and gender differences. In Encyclopedia of Quality of Life and Well-Being Research; Maggino, F., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–3. [Google Scholar]
- Peragine, D.E.; Skorska, M.N.; Maxwell, J.A.; Impett, E.A.; VanderLaan, D.P. A learning experience? Enjoyment at sexual debut and the gender gap in sexual desire among emerging adults. J. Sex Res. 2022, 59, 1092–1109. [Google Scholar] [CrossRef] [PubMed]
- Maunder, L.; Micanovic, N.; Huberman, J.S.; Chivers, M.L. Orgasm consistency and its relationship to women’s self-reported and genital sexual response. Can. J. Hum. Sex. 2022, 31, 32–45. [Google Scholar] [CrossRef]
| Parameter | Species | Result | References |
|---|---|---|---|
| Proportion of animals crossing grid in the Columbia Obstruction Apparatus | Rat | F > M | [120] |
| Number of grid crossings in the Columbia Obstruction Apparatus | Rat | F > M | [72,121] |
| Number of grid crossings in the Columbia Obstruction Apparatus | Rat | F = M | [123] |
| Number of partners per unit time | Mouse lemur | F = M | [127,128] |
| Number of partners per unit time | Chimpanzee | F = M | [129,130,131,132] |
| Number of partners per unit time | Bonobo | F = M | [133] |
| Identification of sexual pictures | Human | F = M | [141] |
| Fixation on genitals while observing sex | Human | F = M | [144] |
| Pupil diameter while watching non-consensual sex | Human | F = M | [145] |
| Implicit motivation as evaluated with the implicit AMORE test | Human | F = M | [95] |
| Implicit motivation as evaluated with the Implicit Association Test | Human | F = M | [137] |
| Implicit motivation as evaluated with the modified Implicit Association Test | Human | F = M | [99] |
| Enhanced motivation after subliminal priming with sexual incentives | Human | F = M | [139,140] |
| Identification of sexual pictures | Human | F = M | [141] |
| Pupil dilation in response to sexual stimuli | Human | F = M | [143,145,146] |
| Gaze fixation on genital area | Human | F = M | [7,144] |
| Genital responses to sexual incentives | Human | F = M | [14,42,150,151] |
| Spinal reflex facilitation by sexual incentives | Human | F = M | [161] |
| Frequency of masturbation in infants | Human | F > M | [163,164] |
| Frequency of masturbation in adolescents | Human | F < M | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touraille, P.; Ågmo, A. Sex Differences in Sexual Motivation in Humans and Other Mammals: The Role of Conscious and Unconscious Processes. Behav. Sci. 2024, 14, 277. https://doi.org/10.3390/bs14040277
Touraille P, Ågmo A. Sex Differences in Sexual Motivation in Humans and Other Mammals: The Role of Conscious and Unconscious Processes. Behavioral Sciences. 2024; 14(4):277. https://doi.org/10.3390/bs14040277
Chicago/Turabian StyleTouraille, Priscille, and Anders Ågmo. 2024. "Sex Differences in Sexual Motivation in Humans and Other Mammals: The Role of Conscious and Unconscious Processes" Behavioral Sciences 14, no. 4: 277. https://doi.org/10.3390/bs14040277
APA StyleTouraille, P., & Ågmo, A. (2024). Sex Differences in Sexual Motivation in Humans and Other Mammals: The Role of Conscious and Unconscious Processes. Behavioral Sciences, 14(4), 277. https://doi.org/10.3390/bs14040277





