How Much Does Stress Cost? A Case–Control Study on Vagally Mediated Heart Rate Variability Responses in Anxious and Non-Anxious Individuals During a Cognitive Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Mertgen, A. Vagal Tank Theory: The Three Rs of Cardiac Vagal Control Functioning—Resting, Reactivity, and Recovery. Front. Neurosci. 2018, 12, 257628. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Studinger, P. Counterpoint: Cardiovascular Variability Is Not an Index of Autonomic Control of the Circulation. J. Appl. Physiol. (1985) 2006, 101, 676–682. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 108292. [Google Scholar] [CrossRef] [PubMed]
- Massaro, S.; Pecchia, L. Heart Rate Variability (HRV) Analysis: A Methodology for Organizational Neuroscience. Organ. Res. Methods 2019, 22, 354–393. [Google Scholar] [CrossRef]
- Lo Turco, G.; Grimaldi Di Terresena, L. Analisi spettrale dell’Heart Rate Variability in pazienti con disturbi psichici: Valutazione del sistema nervoso autonomo nei disturbi psicotici, d’ansia e dell’umore [Spectral analysis of Heart Rate Variability in psychiatric patients: Autonomic nervous system evaluation in psychotic, anxiety and depressive disorders]. Riv. Psichiatr. 2012, 47, 139–148. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Abhishekh, H.A.; Nisarga, P.; Kisan, R.; Meghana, A.; Chandran, S.; Raju, T.; Sathyaprabha, T.N. Influence of Age and Gender on Autonomic Regulation of Heart. J. Clin. Monit. Comput. 2013, 27, 259–264. [Google Scholar] [CrossRef]
- Fagard, R.H. A Population-Based Study on the Determinants of Heart Rate and Heart Rate Variability in the Frequency Domain. Verh. K. Acad. Geneeskd. Belg. 2001, 63, 57–91. [Google Scholar]
- Fraley, M.A.; Birchem, J.A.; Senkottaiyan, N.; Alpert, M.A. Obesity and the Electrocardiogram. Obes. Rev. 2005, 6, 275–281. [Google Scholar] [CrossRef]
- Pousset, F.; Copie, X.; Lechat, P.; Jaillon, P.; Boissel, J.P.; Hetzel, M.; Fillette, F.; Remme, W.; Guize, L.; Le Heuzey, J.Y. Effects of Bisoprolol on Heart Rate Variability in Heart Failure. Am. J. Cardiol. 1996, 77, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Wilhelm, M.; Salzmann, S.; Rief, W.; Euteneuer, F. A Meta-Analysis of Heart Rate Variability in Major Depression. Psychol. Med. 2019, 49, 1948–1957. [Google Scholar] [CrossRef]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.A.; Kemp, A.H. Anxiety Disorders Are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef]
- Nayak, S.K.; Pradhan, B.; Mohanty, B.; Sivaraman, J.; Ray, S.S.; Wawrzyniak, J.; Jarzębski, M.; Pal, K. A Review of Methods and Applications for a Heart Rate Variability Analysis. Algorithms 2023, 16, 433. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235. [Google Scholar] [CrossRef]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association; DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Pedrabissi, L.; Santinello, M. Verifica della validità dello STAI forma Y di Spielberger [Verification of the validity of the STAI, Form Y, by Spielberger]. Giunti Organ. Spec. 1989, 191–192, 11–14. [Google Scholar]
- Friedman, B.H. An Autonomic Flexibility-Neurovisceral Integration Model of Anxiety and Cardiac Vagal Tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The Relation of Strength of Stimulus to Rapidity of Habit-Formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and Cognitive Performance: Attentional Control Theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 436204. [Google Scholar] [CrossRef]
- Williams, D.W.P.; Thayer, J.F.; Koenig, J. Resting Cardiac Vagal Tone Predicts Intraindividual Reaction Time Variability during an Attention Task in a Sample of Young and Healthy Adults. Psychophysiology 2016, 53, 1843–1851. [Google Scholar] [CrossRef]
- Williams, P.G.; Cribbet, M.R.; Tinajero, R.; Rau, H.K.; Thayer, J.F.; Suchy, Y. The Association between Individual Differences in Executive Functioning and Resting High-Frequency Heart Rate Variability. Biol. Psychol. 2019, 148, 107772. [Google Scholar] [CrossRef]
- Kim, M.S.; Yoon, J.H.; Hong, J.M. Early Differentiation of Dementia with Lewy Bodies and Alzheimer’s Disease: Heart Rate Variability at Mild Cognitive Impairment Stage. Clin. Neurophysiol. 2018, 129, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.A.; Heathers, J.A.; Abbott, M.J.; Kemp, A.H.; Quintana, D.S. Worry is associated with robust reductions in heart rate variability: A transdiagnostic study of anxiety psychopathology. BMC Psychol. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.D. Biofeedback: Methods and Procedures in Clinical Practice; Biofeedback Press: San Francisco, CA, USA, 1977. [Google Scholar]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and Backward Span for Verbal and Visuo-Spatial Data: Standardization and Normative Data from an Italian Adult Population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Liu, T.-H.; Wang, Z.; Xie, F.; Liu, Y.-Q.; Lin, Q. Contributions of Aversive Environmental Stress to Migraine Chronification: Research Update of Migraine Pathophysiology. World J. Clin. Cases 2021, 9, 2136–2145. [Google Scholar] [CrossRef]
- Kessler, R.C.; Greenberg, P.E. The economic burden of anxiety and stress disorders. In Neuropsychopharmacology: The Fifth Generation of Progress; American College of Neuropsychopharmacology: Brentwood, TN, USA, 2002; pp. 981–992. [Google Scholar]
- Zahn, D.; Adams, J.; Krohn, J.; Wenzel, M.; Mann, C.G.; Gomille, L.K.; Jacobi-Scherbening, V.; Kubiak, T. Heart Rate Variability and Self-Control—A Meta-Analysis. Biol. Psychol. 2016, 115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Williams, D.P.; Kemp, A.H.; Thayer, J.F. Vagally Mediated Heart Rate Variability in Headache Patients—A Systematic Review and Meta-Analysis. Cephalalgia 2016, 36, 265–278. [Google Scholar] [CrossRef]
- Pruneti, C.; Ferrari, S.; Guidotti, S. A Narrative Review of Heart Rate Variability as a Good Index of Psychophysical Health in Athletes and in Biofeedback Training. J. Clin. Sport Psychol. 2023, 18, 422–449. [Google Scholar] [CrossRef]
- Carnevali, L.; Bignami, E.; Gambetta, S.; Barbetti, M.; Procopio, M.; Freyrie, A.; Carbognani, P.; Ampollini, L.; Sgoifo, A. Cardiac Autonomic and Cortisol Stress Responses to Real Operations in Surgeons: Relationship with Individual Psychobiological Characteristics and Experience. Biopsychosoc. Med. 2023, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Alloy, L.B. Atypical Reactivity of Heart Rate Variability to Stress and Depression across Development: Systematic Review of the Literature and Directions for Future Research. Clin. Psychol. Rev. 2016, 50, 67–79. [Google Scholar] [CrossRef]
- Wekenborg, M.K.; Schwerdtfeger, A.; Rothe, N.; Penz, M.; Walther, A.; Kirschbaum, C.; Thayer, J.F.; Wittling, R.A.; Hill, L.B.K. Determining the Direction of Prediction of the Association between Parasympathetic Dysregulation and Exhaustion Symptoms. Sci. Rep. 2022, 12, 10648. [Google Scholar] [CrossRef]
- Schiweck, C.; Piette, D.; Berckmans, D.; Claes, S.; Vrieze, E. Heart Rate and High Frequency Heart Rate Variability during Stress as Biomarker for Clinical Depression. A Systematic Review. Psychol. Med. 2019, 49, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.S.; Lee, J.F.; Chen, C.C.; Chang, Y.C. Reactive heart rate variability in male patients with first-episode major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Lombardi, F. Physiology and Clinical Implications of Variability of Cardiovascular Parameters with Focus on Heart Rate and Blood Pressure. Am. J. Cardiol. 1994, 73, C3–C9. [Google Scholar] [CrossRef]
- Alhalabi, L.; Singleton, M.J.; Oseni, A.O.; Shah, A.J.; Zhang, Z.M.; Soliman, E.Z. Relation of Higher Resting Heart Rate to Risk of Cardiovascular Versus Noncardiovascular Death. Am. J. Cardiol. 2017, 119, 1003–1007. [Google Scholar] [CrossRef]
- Sirigatti, S.; Stefanile, C. 16PF-5 Adattamento Italiano; OS Organizzazioni Speciali: Florence, Italy, 2001. [Google Scholar]
| Variable | Anxiety Group (n = 17) | Control Group (n = 24) | t or χ2 | p | |
|---|---|---|---|---|---|
| Age, M (SD) | 24.21 (3.69) | 25.5 (2.95) | 1.23 | 0.11 | |
| Sex, N (%) | 0.21 | 0.65 | |||
| Male | 9 (22%) | 11 (27%) | |||
| Female | 8 (20%) | 13 (31%) | |||
| Marital status, N (%) | 0.73 | 0.39 | |||
| Unmarried | 17 (100%) | 23 (96%) | |||
| Married/cohabitant | 0 (0%) | 1 (4%) | |||
| Education level, N (%) | 1.47 | 0.69 | |||
| High school graduation | 7 (17%) | 12 (29.3%) | |||
| University degree | 10 (24.4%) | 12 (29.3%) | |||
| Variable | Anxiety Group (n = 19) | Control Group (n = 22) | t or χ2 | p | |
|---|---|---|---|---|---|
| Age, M (SD) | 24.10 (3.75) | 25.64 (2.77) | 1.52 | 0.06 | |
| Sex, N (%) | 0.63 | 0.42 | |||
| Male | 8 (20%) | 12 (29%) | |||
| Female | 11 (27%) | 10 (24%) | |||
| Marital status, N (%) | 0.88 | 0.34 | |||
| Unmarried | 19 (100%) | 21 (96%) | |||
| Married/cohabitant | 0 (0%) | 1 (4%) | |||
| Education level, N (%) | 0.56 | 0.75 | |||
| High school graduation | 10 (24.4%) | 9 (22%) | |||
| University degree | 9 (22%) | 13 (31.6%) | |||
| Variable | Anxiety Group (n = 17) | Control Group (n = 24) | t (40) | p | Cohen’s D | |||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| Heart rate (bpm) | ||||||||
| Baseline | 67.55 | 9.10 | 65.40 | 9.44 | −0.73 | 0.23 | −0.23 | |
| Reactivity | 9.29 | 6.80 | 8.63 | 7.10 | −0.30 | 0.38 | −0.09 | |
| Recovery | −9.83 | 7.06 | −8.94 | 8.30 | 0.36 | 0.36 | 0.11 | |
| Log-HF | ||||||||
| Baseline | 6.35 | 1.07 | 7.21 | 0.97 | 2.68 | 0.05 | 0.85 | |
| Reactivity | −0.31 | 0.55 | −0.61 | 0.80 | −1.32 | 0.10 | −0.42 | |
| Recovery | 0.24 | 0.63 | 0.45 | 0.74 | 0.97 | 0.17 | 0.30 | |
| Log-RMSSD | ||||||||
| Baseline | 3.71 | 0.54 | 4.08 | 0.46 | 2.34 | 0.01 | 0.74 | |
| Reactivity | −0.19 | 0.30 | −0.27 | 0.35 | −0.81 | 0.21 | −0.25 | |
| Recovery | −0.17 | 0.29 | 0.22 | 0.33 | −0.43 | 0.33 | −0.14 | |
| Variable | Anxiety Group (n = 19) | Control Group (n = 22) | t (40) | p | Cohen’s D | |||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| Heart rate (bpm) | ||||||||
| Baseline | 68.50 | 9.03 | 64.38 | 9.21 | −1.44 | 0.08 | −0.45 | |
| Reactivity | 6.98 | 4.53 | 10.57 | 8.17 | −1.70 | 0.10 | 0.53 | |
| Recovery | −7.56 | 6.44 | −10.83 | 9.37 | −1.36 | 0.18 | −0.42 | |
| Log-HF | ||||||||
| Baseline | 6.59 | 1.10 | 7.08 | 1.07 | 1.45 | 0.15 | 0.45 | |
| Reactivity | −0.23 | 0.44 | −0.71 | 0.83 | −2.26 | 0.03 | −0.70 | |
| Recovery | 0.13 | 0.49 | 0.57 | 0.79 | 2.11 | 0.04 | 0.66 | |
| Log-RMSSD | ||||||||
| Baseline | 3.82 | 0.53 | 4.02 | 0.50 | 1.20 | 0.24 | 0.38 | |
| Reactivity | −0.16 | 0.29 | −0.30 | 0.35 | −1.35 | 0.18 | −0.41 | |
| Recovery | 0.12 | 0.26 | 0.27 | 0.35 | −1.57 | 0.12 | 0.49 | |
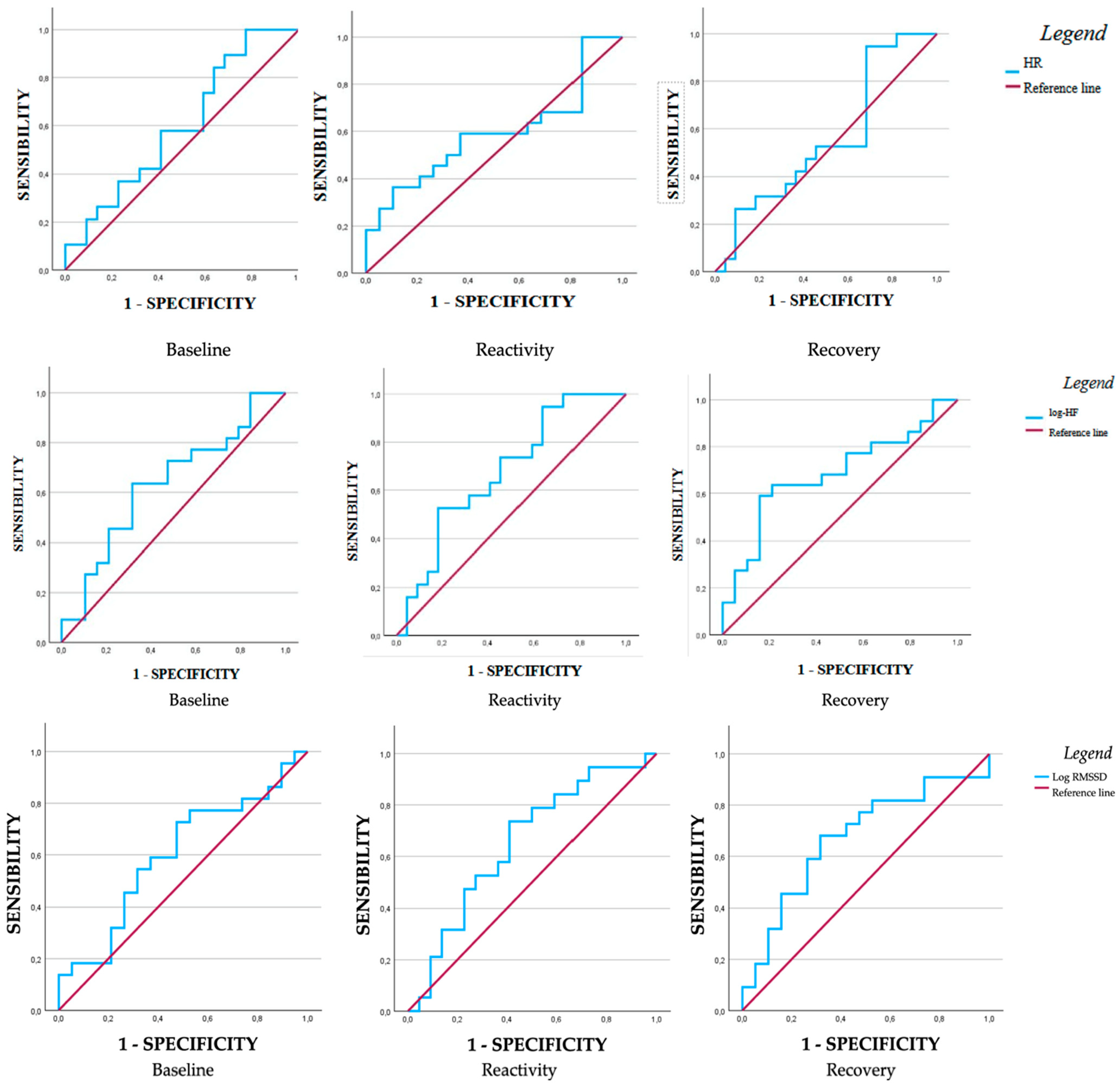
| AUC | SE | CI 95% (LL-UL) | p | Sensibility | Specificity | Youden Index | ||
|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | ||||||||
| Baseline | 0.60 | 0.09 | 0.43–0.77 | 0.26 | 0.89 | 0.78 | 0.21 | |
| Reactivity | 0.59 | 0.09 | 0.41–0.76 | 0.33 | 0.59 | 0.36 | 0.22 | |
| Recovery | 0.56 | 0.09 | 0.37–0.73 | 0.53 | 0.94 | 0.72 | 0.22 | |
| Log-HF | ||||||||
| Baseline | 0.63 | 0.09 | 0.46–0.87 | 0.80 | 0.63 | 0.32 | 0.32 | |
| Reactivity | 0.68 | 0.08 | 0.51–0.84 | 0.03 | 0.52 | 0.18 | 0.34 | |
| Recovery | 0.32 | 0.08 | 0.15–0.48 | 0.03 | 0.59 | 0.16 | 0.43 | |
| Log-RMSSD | ||||||||
| Baseline | 0.60 | 0.09 | 0.43–0.78 | 0.25 | 0.72 | 0.47 | 0.25 | |
| Reactivity | 0.65 | 0.09 | 0.48–0.82 | 0.08 | 0.73 | 0.40 | 0.33 | |
| Recovery | 0.67 | 0.09 | 0.50–0.84 | 0.05 | 0.68 | 0.32 | 0.36 | |
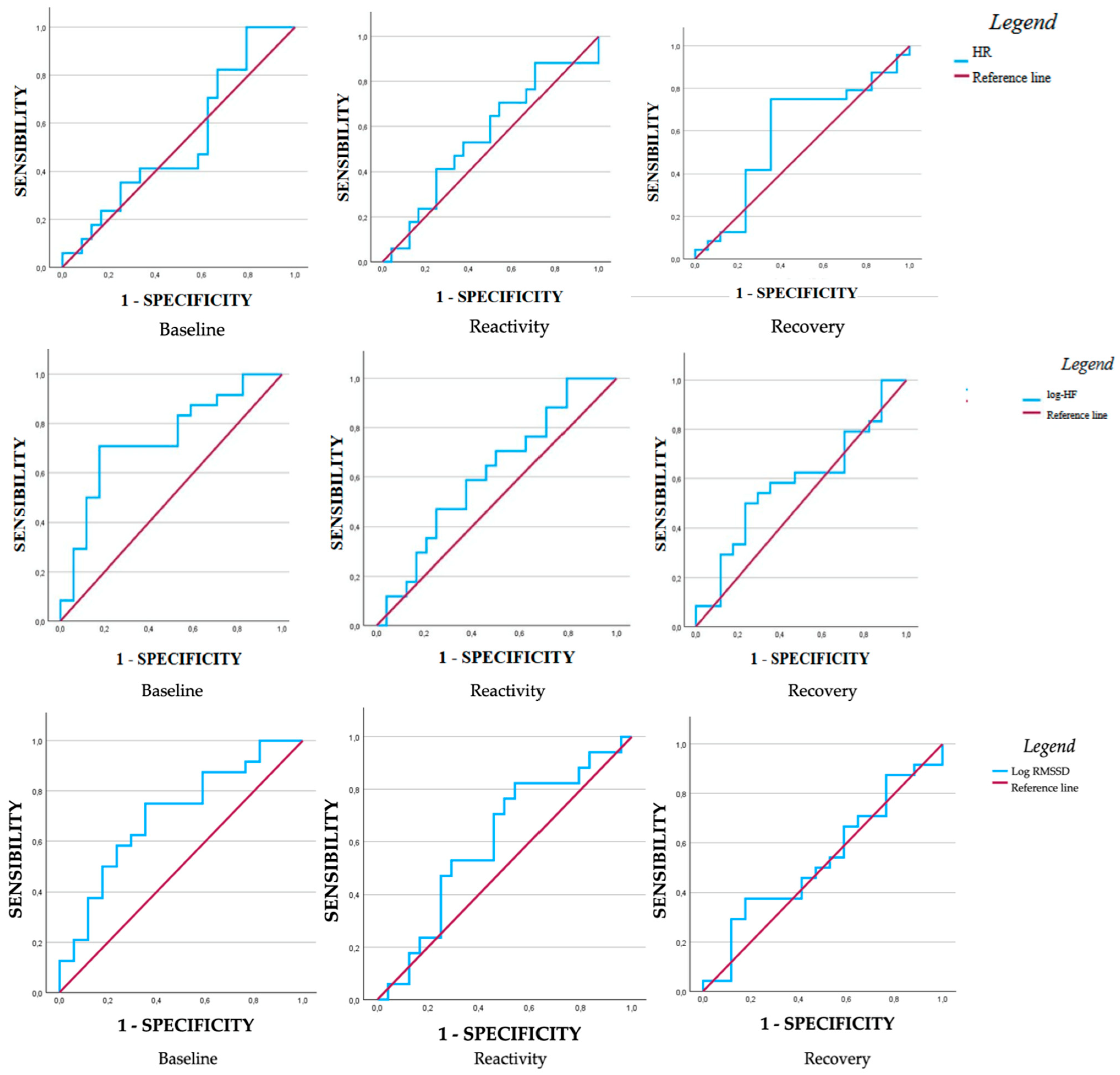
| AUC | SE | CI 95% (LL-UL) | p | Sensibility | Specificity | Youden Index | ||
|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | ||||||||
| Baseline | 0.60 | 0.09 | 0.34–0.71 | 0.75 | 0.82 | 0.67 | 0.16 | |
| Reactivity | 0.56 | 0.09 | 0.37–0.74 | 0.54 | 0.88 | 0.77 | 0.17 | |
| Recovery | 0.59 | 0.09 | 0.40–0.77 | 0.35 | 0.75 | 0.35 | 0.40 | |
| Log-HF | ||||||||
| Baseline | 0.73 | 0.08 | 0.58–0.89 | 0.003 | 0.71 | 0.18 | 0.53 | |
| Reactivity | 0.61 | 0.09 | 0.44–0.79 | 0.20 | 0.59 | 0.37 | 0.21 | |
| Recovery | 0.58 | 0.09 | 0.40–0.76 | 0.36 | 0.54 | 0.29 | 0.25 | |
| Log-RMSSD | ||||||||
| Baseline | 0.70 | 0.08 | 0.54–0.87 | 0.01 | 0.75 | 0.35 | 0.40 | |
| Reactivity | 0.60 | 0.09 | 0.43–0.78 | 0.25 | 0.76 | 0.50 | 0.26 | |
| Recovery | 0.52 | 0.09 | 0.35–0.71 | 0.73 | 0.37 | 0.18 | 0.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirco, D.; Guidotti, S.; Pruneti, C. How Much Does Stress Cost? A Case–Control Study on Vagally Mediated Heart Rate Variability Responses in Anxious and Non-Anxious Individuals During a Cognitive Task. Med. Sci. 2025, 13, 205. https://doi.org/10.3390/medsci13030205
Chirco D, Guidotti S, Pruneti C. How Much Does Stress Cost? A Case–Control Study on Vagally Mediated Heart Rate Variability Responses in Anxious and Non-Anxious Individuals During a Cognitive Task. Medical Sciences. 2025; 13(3):205. https://doi.org/10.3390/medsci13030205
Chicago/Turabian StyleChirco, Daniele, Sara Guidotti, and Carlo Pruneti. 2025. "How Much Does Stress Cost? A Case–Control Study on Vagally Mediated Heart Rate Variability Responses in Anxious and Non-Anxious Individuals During a Cognitive Task" Medical Sciences 13, no. 3: 205. https://doi.org/10.3390/medsci13030205
APA StyleChirco, D., Guidotti, S., & Pruneti, C. (2025). How Much Does Stress Cost? A Case–Control Study on Vagally Mediated Heart Rate Variability Responses in Anxious and Non-Anxious Individuals During a Cognitive Task. Medical Sciences, 13(3), 205. https://doi.org/10.3390/medsci13030205







