Titanian Clinohumite-Bearing Peridotite from the Ulamertoq Ultramafic Body in the 3.0 Ga Akia Terrane of Southern West Greenland
Abstract
1. Introduction
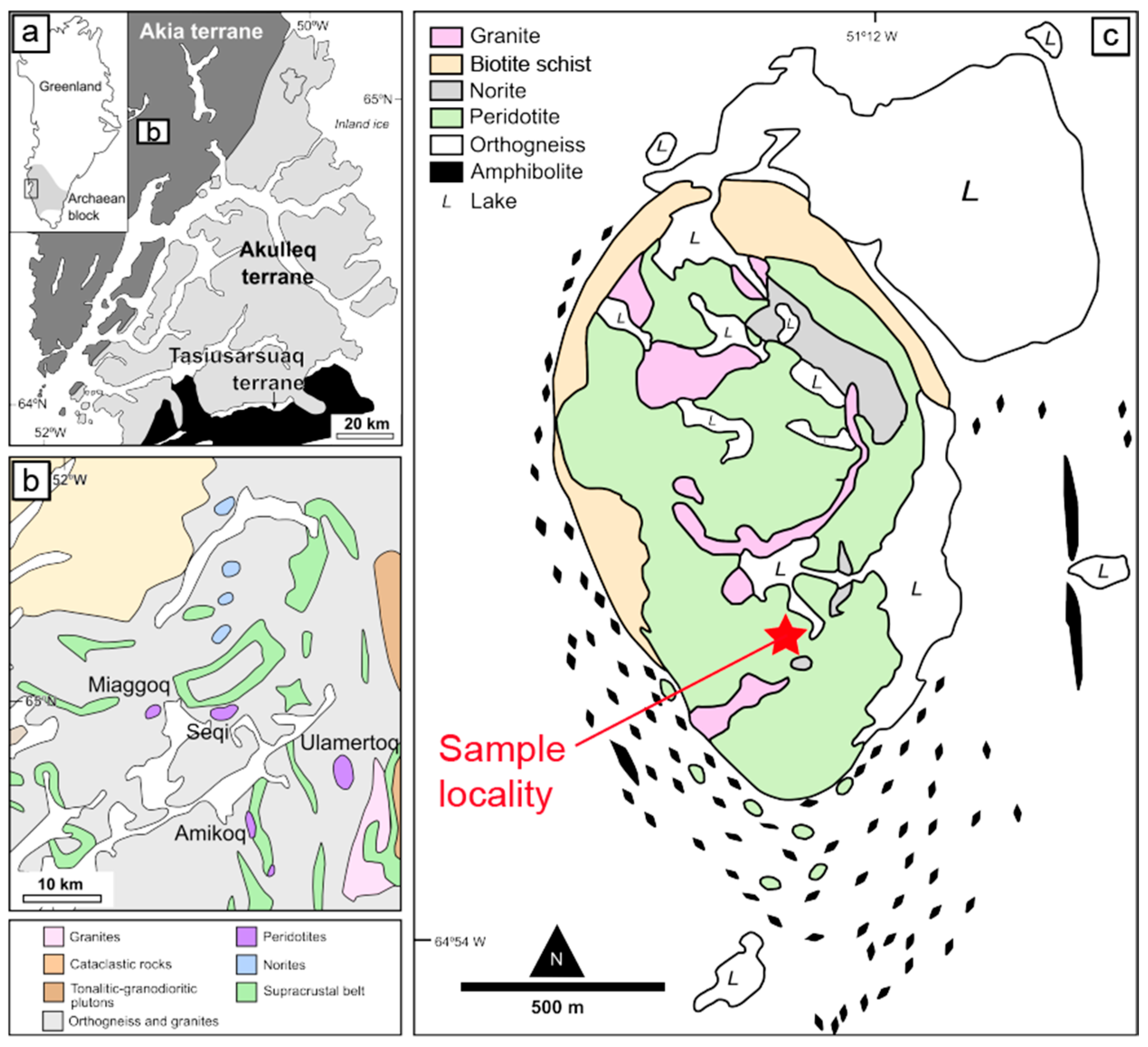
2. Sampling Locality and Methods
2.1. Geological Background
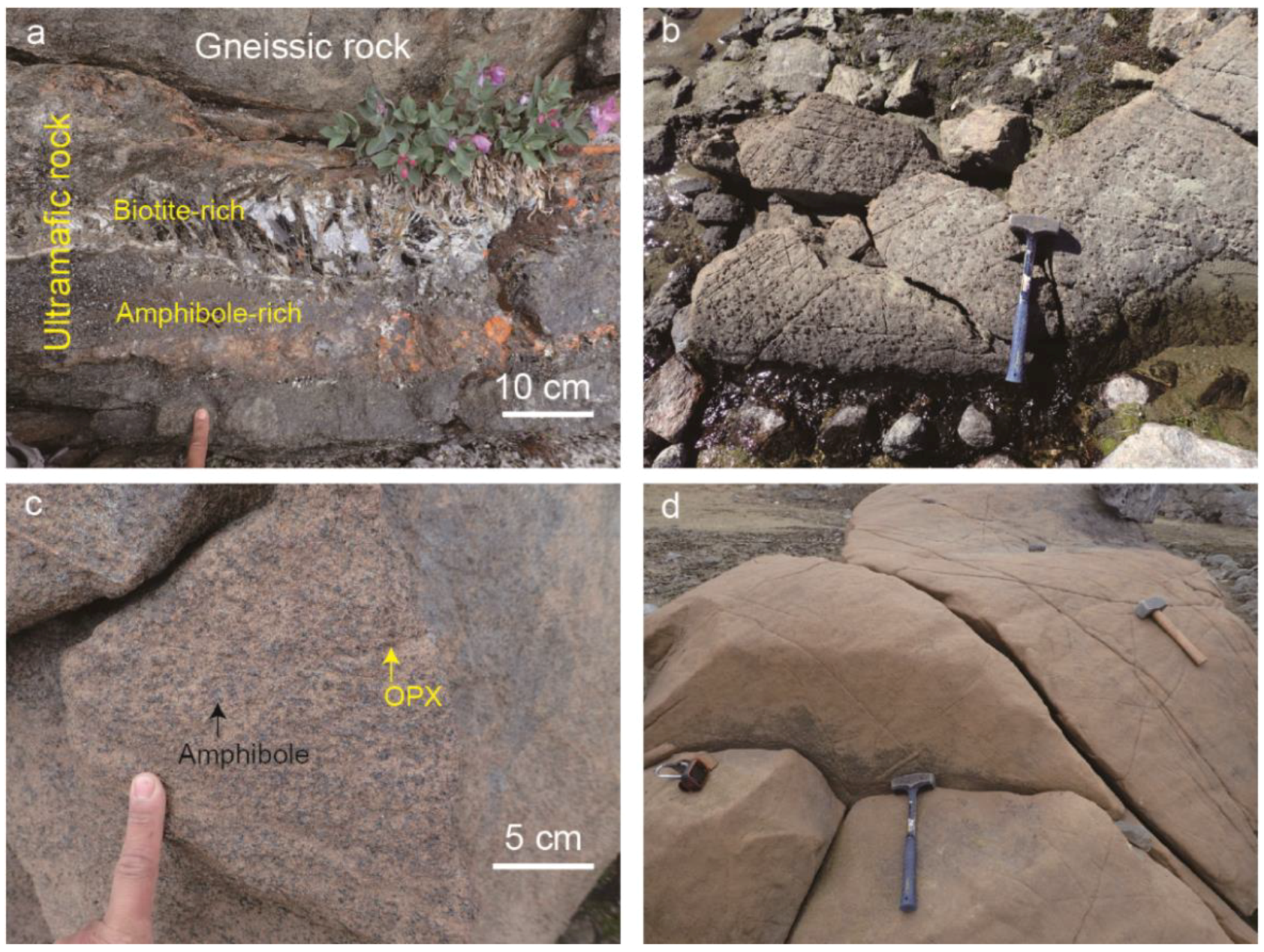
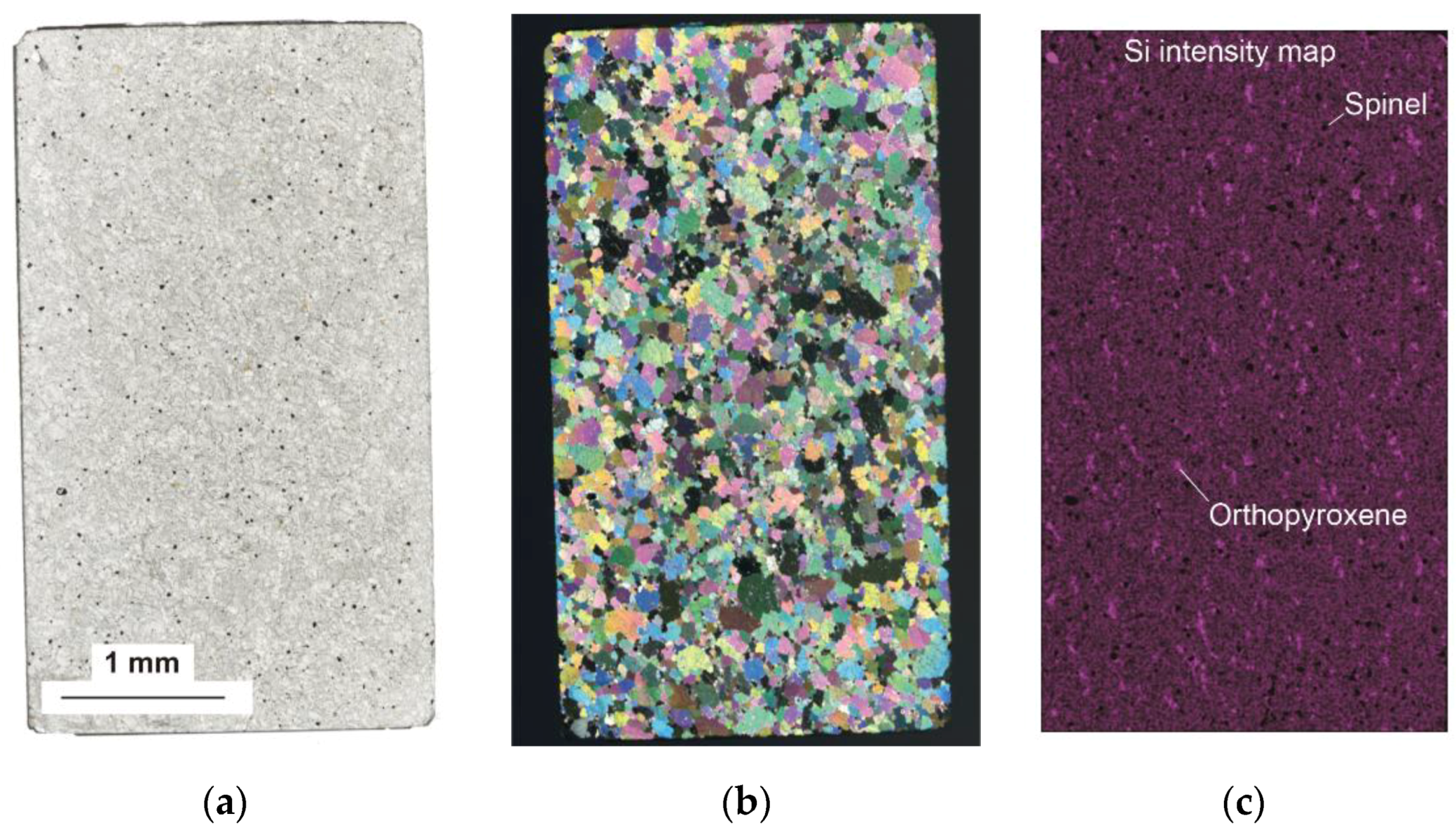
2.2. Sample Description
2.3. Analytical Methods
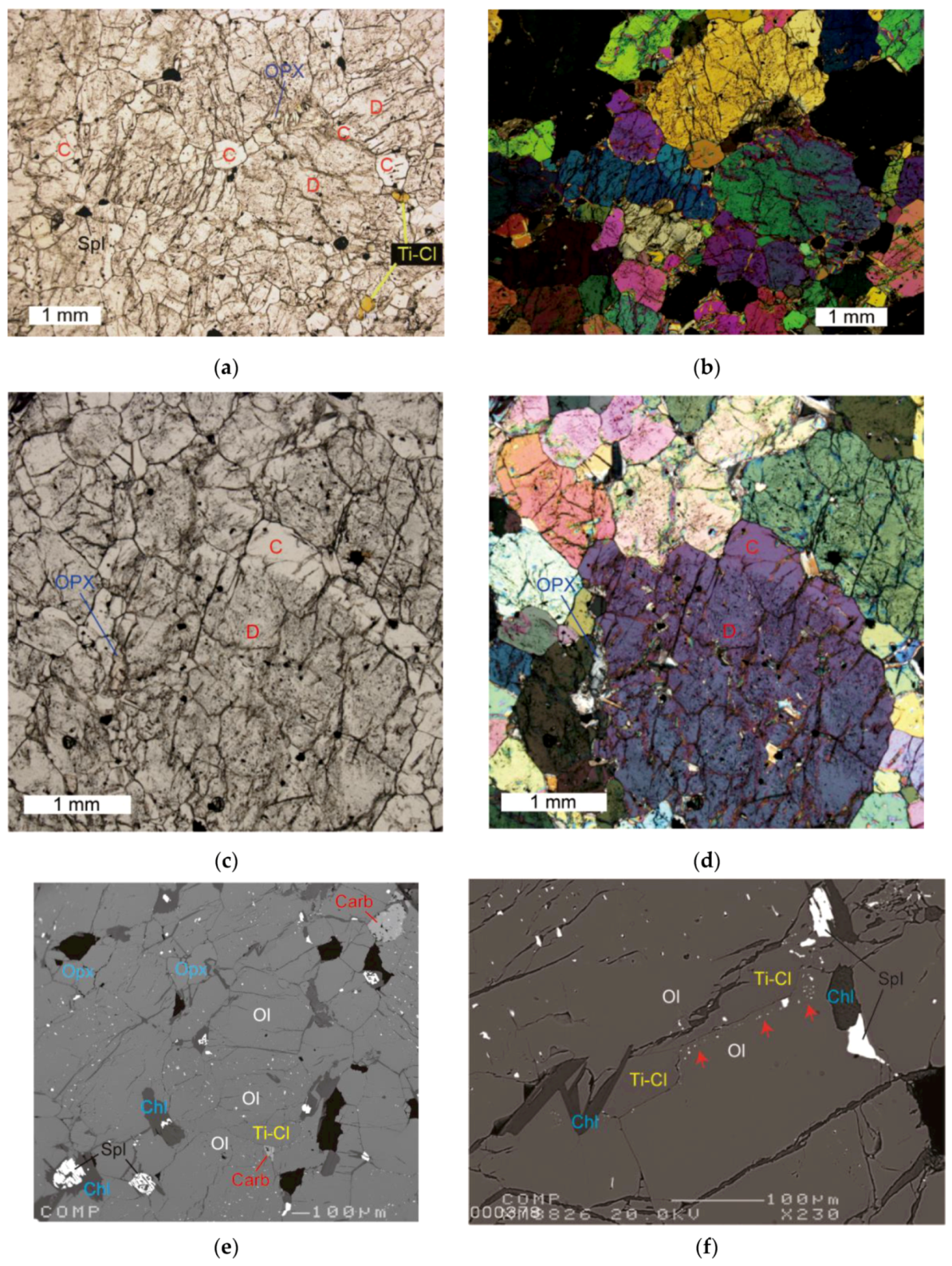
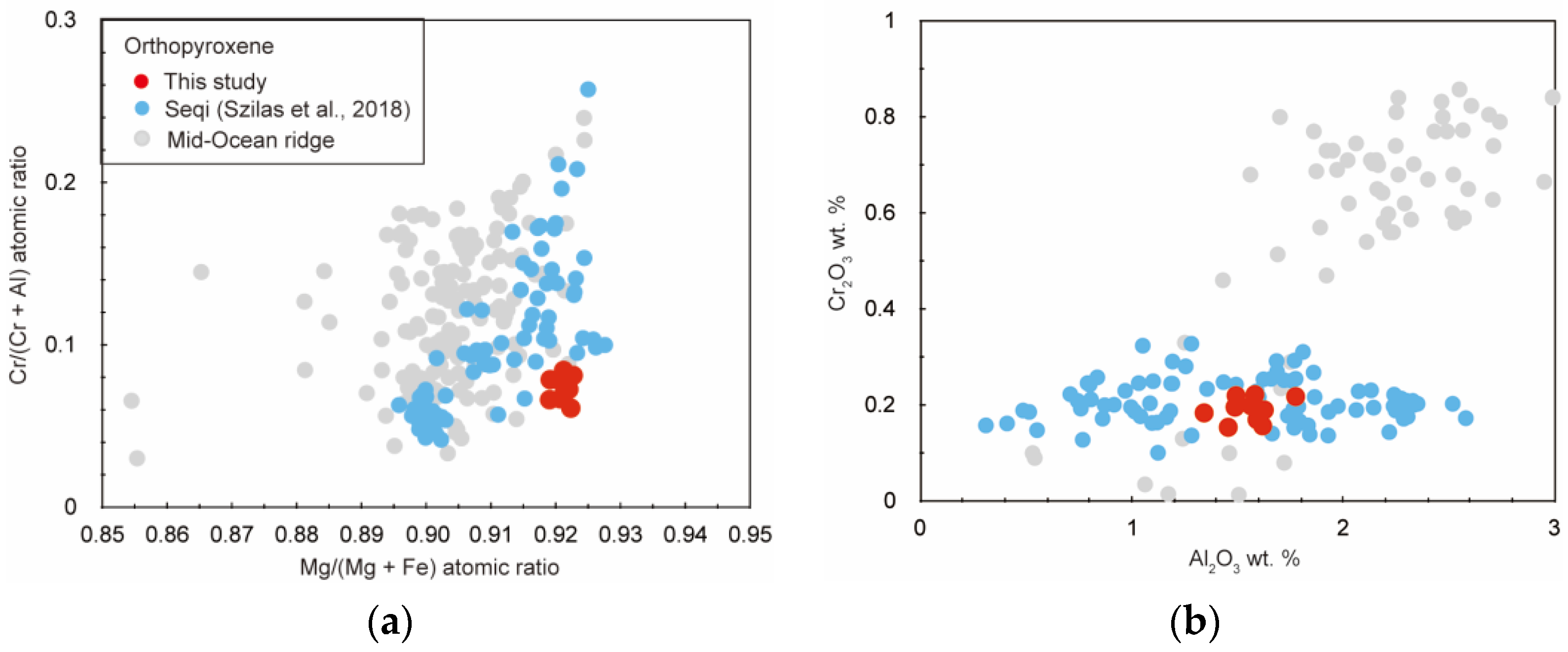
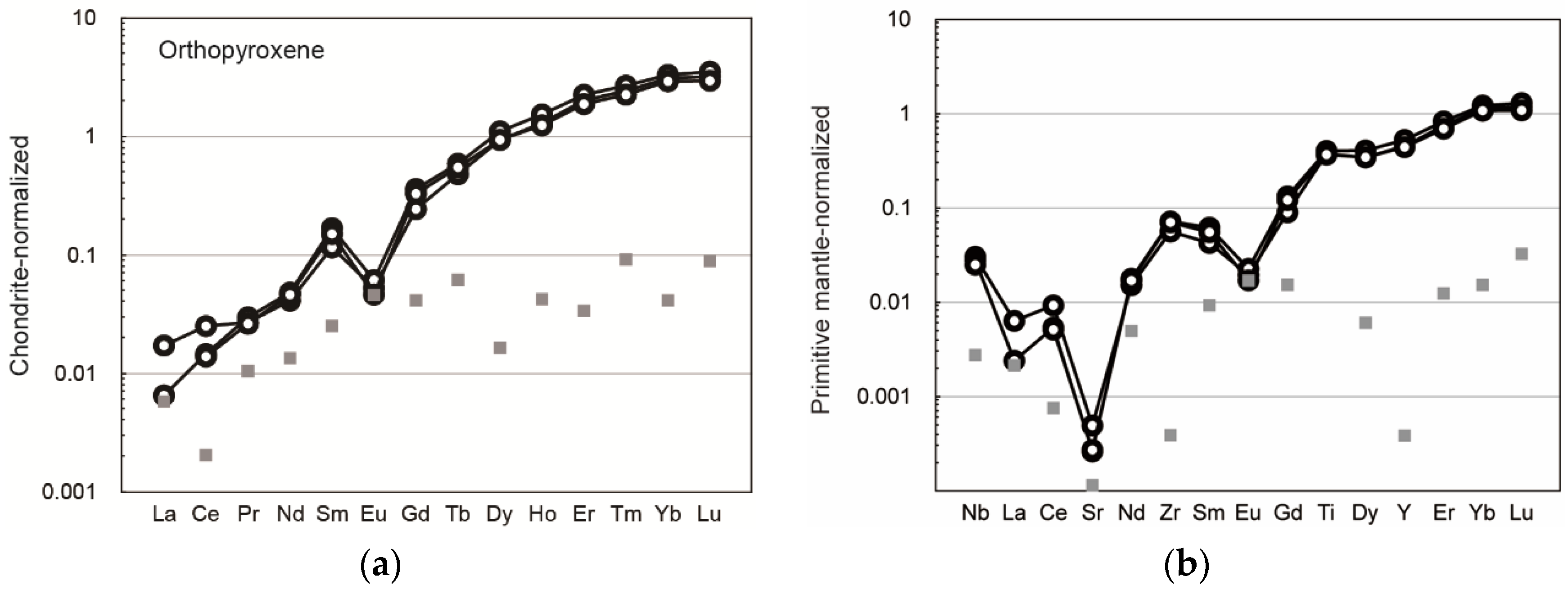
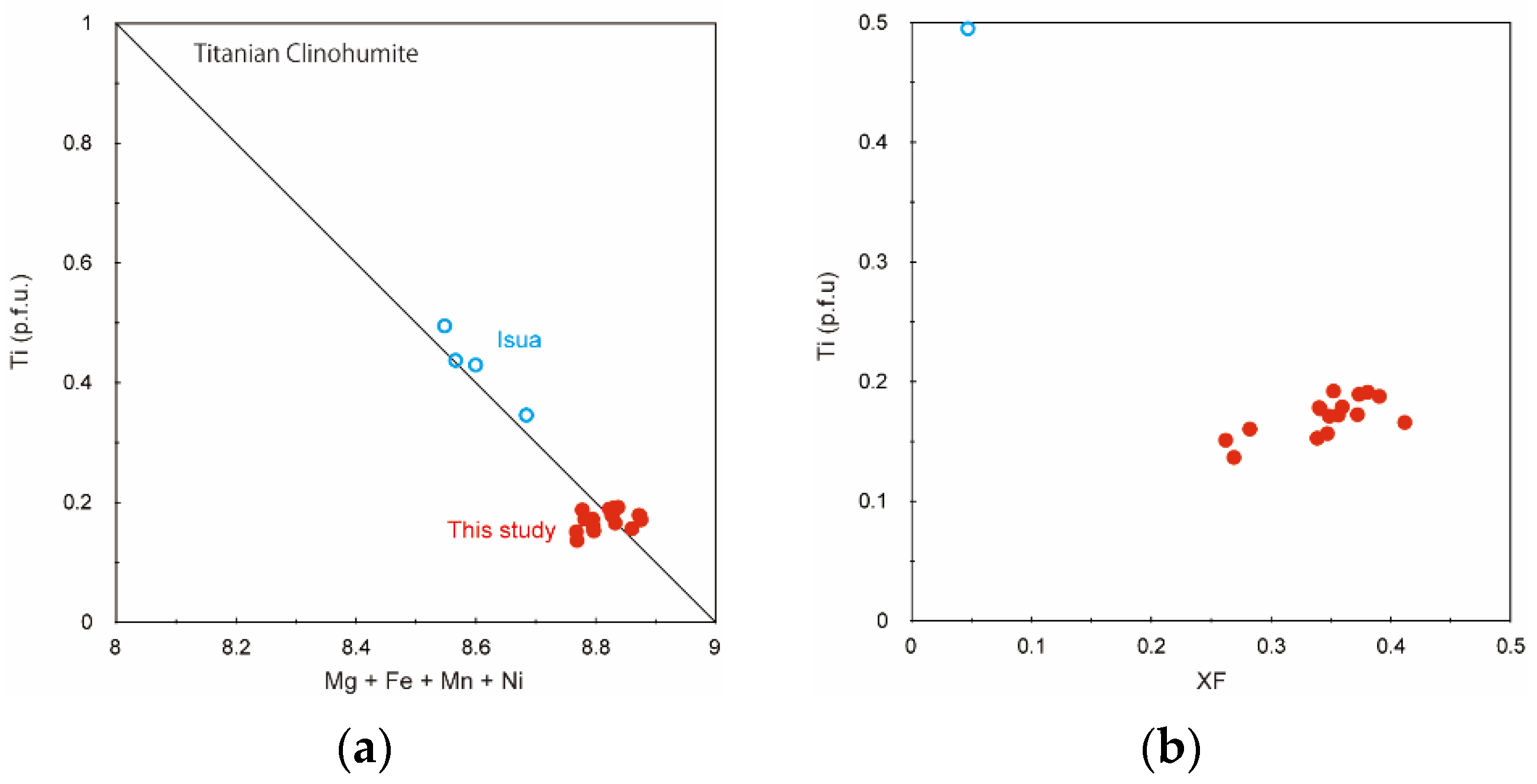
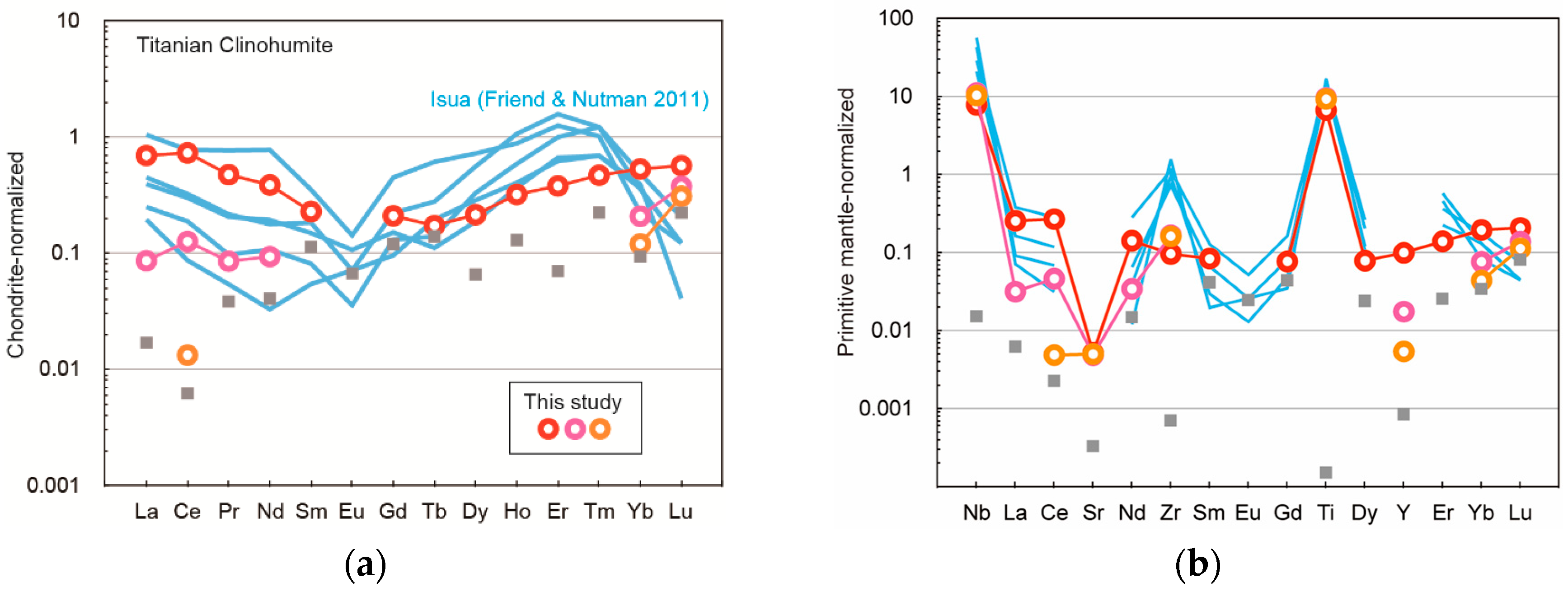
3. Results
4. Discussion
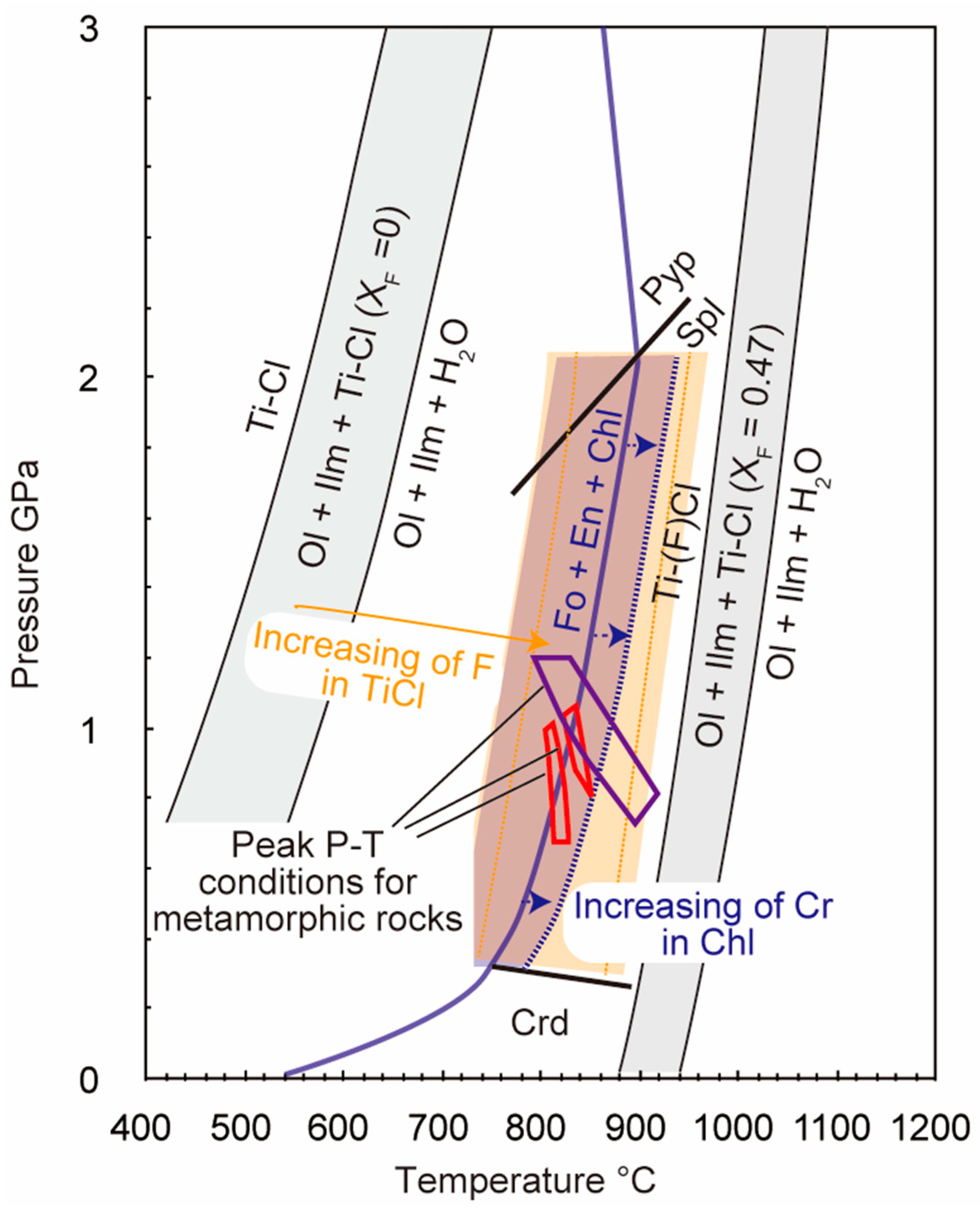
4.1. Pressure-Temperature Conditions for the Titanian Clinohumite-Bearing Peridotite
4.2. Petrogenesis of the Studied Titanian Clinohumite-Bearing Ultramafic Rock
4.3. Comparison with Titanian Clinohumite-Bearing Ultramafic Rock in the Isua Supracrustal Belt and Its Significance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mcgregor, V.R.; Nutman, A.P.; Mcgregor, V.R.; Polytechnic, O. The late Archaean mobile belt through Godthåbsfjord, southern West Greenland: A continent-continent collision zone ? Bull. Geol. Soc. Denmark 1991, 39, 179–197. [Google Scholar]
- Road, S.; Friend, C.R.L.; Nutman, A.P. New pieces to the Archaean terrane jigsaw puzzle in the Nuuk region, southern West Greenland: Steps in transforming a simple insight into a complex regional tectonothermal model. J. Geol. Soc. Lond. 2005, 162, 147–162. [Google Scholar]
- Chadwick, B.; Crewe, M.A. Chromite in the early Archean Akilia association (ca. 3,800 MY), Ivisartoq region, inner Godthabsfjord, southern West Greenland. Econ. Geol. 1986, 81, 184–191. [Google Scholar] [CrossRef]
- Dymek, R.F.; Brothers, S.C.; Craig, M. Petrogenesis of Ultramafic Metamorphic Rocks from. J. Petrol. 1988, 29, 1353–1397. [Google Scholar] [CrossRef]
- Garde, A.A.; Garde, A.A. Accretion and Evolution of an Archaean High-Grade Grey Gneiss—Amphibolite Complex: The Fiskefjord Area, Southern West Greenland; Geological Survey of Denmark and Greenland, Ministry of Environment and Energy: Copenhagen, Denmark, 1997; Volume 177, ISBN 8778710197.
- Friend, C.; Bennett, V.; Nutman, A. Abyssal peridotites >3,800 Ma from southern West Greenland: Field relationships, petrography, geochronology, whole-rock and mineral chemistry of dunite and harzburgite inclusions in the Itsaq Gneiss Complex. Contrib. Mineral. Petrol. 2002, 143, 71–92. [Google Scholar] [CrossRef]
- Nutman, A.P.; Friend, C.R.L. New 1:20,000 scale geological maps, synthesis and history of investigation of the Isua supracrustal belt and adjacent orthogneisses, southern West Greenland: A glimpse of Eoarchaean crust formation and orogeny. Precambrian Res. 2009, 172, 189–211. [Google Scholar] [CrossRef]
- Szilas, K.; Kelemen, P.B.; Rosing, M.T. The petrogenesis of ultramafic rocks in the >3.7Ga Isua supracrustal belt, southern West Greenland: Geochemical evidence for two distinct magmatic cumulate trends. Gondwana Res. 2015, 28, 565–580. [Google Scholar] [CrossRef]
- Szilas, K.; Van Hinsberg, V.; Mcdonald, I.; Næraa, T.; Rollinson, H.; Adetunji, J.; Bird, D. Geoscience Frontiers Highly refractory Archaean peridotite cumulates: Petrology and geochemistry of the Seqi Ultrama fi c Complex, SW Greenland. Geosci. Front. 2018, 9, 689–714. [Google Scholar] [CrossRef]
- van de Löcht, J.; Hoffmann, J.E.; Li, C.; Wang, Z.; Becker, H.; Rosing, M.T.; Kleinschrodt, R.; Münker, C. Earth’s oldest mantle peridotites show entire record of late accretion. Geology 2018, 46, 199–202. [Google Scholar] [CrossRef]
- Szilas, K.; Van Hinsberg, V.J.; Creaser, R.A.; Kisters, A.F.M. The geochemical composition of serpentinites in the Mesoarchaean Tartoq Group, SW Greenland: Harzburgitic cumulates or melt-modified mantle? Lithos 2014, 198–199, 103–116. [Google Scholar] [CrossRef]
- Friend, C.R.L.; Nutman, A.P. Dunites from Isua, Greenland: A ca. 3720 Ma window into subcrustal metasomatism of depleted mantle. Geology 2011, 39, 663–666. [Google Scholar] [CrossRef]
- Rollinson, H. Recognising early Archaean mantle: A reappraisal. Contrib. Mineral. Petrol. 2007, 154, 241–252. [Google Scholar] [CrossRef]
- Pons, M.-L.; Quitte, G.; Fujii, T.; Rosing, M.T.; Reynard, B.; Moynier, F.; Douchet, C.; Albarede, F. Early Archean serpentine mud volcanoes at Isua, Greenland, as a niche for early life. Proc. Natl. Acad. Sci. USA 2011, 108, 17639–17643. [Google Scholar] [CrossRef]
- Szilas, K.; Kelemen, P.B.; Bernstein, S. Peridotite enclaves hosted by Mesoarchaean TTG-suite orthogneisses in the Fiskefjord region of southern West Greenland. GeoResJ 2015, 7, 22–34. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Reddy, S.M.; Nutman, A.P.; Friend, C.R.L.; Bennett, V.C. Earth’s oldest mantle fabrics indicate Eoarchaean subduction. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Rosing, M.T. Metasomatic alteration of ultramafic rocks. In Fluid Movements—Element Transport and the Composition of the Deep Crust; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 187–202. [Google Scholar]
- Rose, N.M.; Rosing, M.T.; Bridgwater, D. The origin of metacarbonate rocks in the Archaean Isua supracrustal belt, West Greenland. Am. J. Sci. 1996, 296, 1004–1044. [Google Scholar] [CrossRef]
- Ribbe, P.H. Titanium, fluorine, and hydroxyl in the humite minerals. Am. Mineral. 1979, 64, 1027–1035. [Google Scholar]
- Fujino, K.; Takeuchi, Y. Crystal chemistry of titanian chondrodite and titanian clinohumite of high-pressure origin. Am. Mineral. 1978, 63, 535–543. [Google Scholar]
- Jones, N.W. Crystallographic nomenclature and twinning in the humite minerals. Am. Mineral. 1969, 54, 309–313. [Google Scholar]
- Aoki, K.; Fujino, K.; Akaogi, M. Mineralogy and Petrology Titanoehondrodite and Titanoelinohumite Derived from the Upper Mantle in the Buell Park Kimberlite, Arizona, USA. Contrib. Mineral. Petrol. 1976, 253, 243–253. [Google Scholar] [CrossRef]
- Mcgetchin, T.R.; Silver, L.T.; Chodos, A.A. Titanoclinohumite’ A possible Mineralogical site for water in the Upper Mantle. J. Geophys. Res. 1970, 75, 255–259. [Google Scholar] [CrossRef]
- Dymek, R.F.; Boak, J.L.; Brothers, S.C. Titanian chondrodite- and titanian clinohumite-bearing metadunite from the 3800 Ma Isua supracrustal belt West Greenland: Chemistry, petrology, and origin. Am. Mineral. 1988, 73, 547–558. [Google Scholar]
- Smith, D. Chlorite-rich ultramafic reaction zones in Colorado Plateau xenoliths: Recorders of sub-Moho hydration. Contrib. Mineral. Petrol. 1995, 121, 185–200. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Kurat, G.; Ntaflos, T. Henrymeyerite in the metasomatized upper mantle of eastern Antarctica. Can. Mineral. 2007, 45, 497–501. [Google Scholar] [CrossRef]
- Möckel, J.R. Structural petrology of the garnet-peridotite of Alpe Arami (Ticino, Switzerland). Leidse Geol. Meded. 1969, 42, 61–130. [Google Scholar]
- Ishibashi, K.; Miyahisa, M.; Sasaki, M. Titanoclinohumite from Fujiwara complex in the Higashi-akaishi Mountainland, Shikoku, Japan. J. Mineral. Petrol. Econ. Geol. 1978, 78, 18–25. [Google Scholar] [CrossRef]
- Trommsdorff, V.; Evans, B.W. Titanian hydroxyl-clinohumite: Formation and breakdown in antigorite rocks (Malenco, Italy). Contrib. Mineral. Petrol. 1980, 72, 229–242. [Google Scholar] [CrossRef]
- Scambrulli, M.; Hoogerduijn Strating, E.H.; Piccardo, G.B.; Vissers, R.L.M.; Rampone, E. Alpine olivine- and titanian clinohumite-bearing assemblages in the Erro-Tobbio peridotite (Voltri Massif, NW Italy). J. Metamorph. Geol. 1991, 9, 79–91. [Google Scholar]
- Shen, T.; Hermann, J.; Zhang, L.; Lü, Z.; Padrón-Navarta, J.A.; Xia, B.; Bader, T. UHP Metamorphism Documented in Ti-chondrodite- and Ti-clinohumite-bearing Serpentinized Ultramafic Rocks from Chinese Southwestern Tianshan. J. Petrol. 2015, 56, 1425–1458. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liou, J.G.; Cong, B.L. Talc-, magnesite- and ti-clinohumite-bearing ultrahigh-pressure meta-mafic and ultramafic complex in the dabie mountains, China. J. Petrol. 1995, 36, 1011–1037. [Google Scholar] [CrossRef]
- Rahn, M.K.; Bucher, K. Petrology Titanian clinohumite formation in the Zermatt-Sl as ophiolites, Central Alps. Mineral. Petrol. 1998, 64, 1–13. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Li, T.; Rumble, D.; Yui, T.F.; Li, L.; Yang, J.S.; Pan, Y.; Liou, J.G. Multiple metasomatism in Sulu ultrahigh-P garnet peridotite constrained by petrological and geochemical investigations. J. Metamorph. Geol. 2007, 25, 149–164. [Google Scholar] [CrossRef]
- Evans, B.W.; Trommsdorff, V. Fluorine hydroxyl titanian clinohumite in Alpine recrystallized garnet peridotite: Compositional controls and petrologic significance. Am. J. Sci. 1983, 283A, 355–369. [Google Scholar]
- López Sánchez-Vizcaíno, V.; Trommsdorff, V.; Gómez-Pugnaire, M.T.; Garrido, C.J.; Müntener, O.; Connolly, J.A.D. Petrology of titanian clinohumite and olivine at the high-pressure breakdown of antigorite serpentinite to chlorite harzburgite (Almirez Massif, S. Spain). Contrib. Mineral. Petrol. 2005, 149, 627–646. [Google Scholar] [CrossRef]
- Garde, A.A.; Friend, C.R.L.; Nutman, A.P.; Marker, M. Rapid maturation and stabilisation of middle Archaean continental crust: Rapid maturation and stabilisation of middle Archaean continental crust: The Akia terrane, southern West Greenland. Bull. Geol. Soc. Denmark 2000, 47, 1–27. [Google Scholar]
- Kirkland, C.L.; Yakymchuk, C.; Hollis, J.; Heide-Jørgensen, H.; Danišík, M. Mesoarchean exhumation of the Akia terrane and a common Neoarchean tectonothermal history for West Greenland. Precambrian Res. 2018, 314, 129–144. [Google Scholar] [CrossRef]
- Gardiner, N.J.; Kirkland, C.L.; Hollis, J.; Szilas, K.; Steenfelt, A.; Yakymchuk, C.; Heide-Jørgensen, H. Building Mesoarchaean crust upon Eoarchaean roots: The Akia Terrane, West Greenland. Contrib. Mineral. Petrol. 2019, 174, 20. [Google Scholar] [CrossRef]
- Guotana, J.M.; Morishita, T.; Yamaguchi, R.; Nishio, I.; Tamura, A.; Tani, K.; Harigane, Y.; Szilas, K.; Pearson, D.; Yamaguchi, R. Contrasting textural and chemical signatures of chromitites in the Mesoarchaean Ulamertoq peridotite body, southern West Greenland. Geosciences 2018, 8, 328. [Google Scholar] [CrossRef]
- Morishita, T.; Arai, S.; Tamura, A. Petrology of an apatite-rich layer in the Finero phlogopite—Peridotite, Italian Western Alps; implications for evolution of a metasomatising agent. Lithos 2003, 69, 37–49. [Google Scholar] [CrossRef]
- Morishita, T.; Tani, K.-I.I.; Soda, Y.; Tamura, A.; Mizukami, T.; Ghosh, B. The uppermost mantle section below a remnant proto-Philippine Sea island arc: Insights from the peridotite fragments from the Daito Ridge. Am. Mineral. 2018, 103, 1151–1160. [Google Scholar] [CrossRef]
- Pearce, N.J.G.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A Compilation of New and Published Major and Trace Element Data for NIST SRM 610 and NIST SRM 612 Glass Reference Materials. Geostand. Geoanalytical Res. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Longerich, H.P.; Gu, D.; Jackson, S.E.; Gü, D. Elemental fractionation in laser ablation inductively coupled plasma mass spectrometry. Fresenius J. Anal. Chem. 1996, 355, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Ishida, Y.; Arai, S.; Shirasaka, M. Determination of multiple trace element compositions in thin (<30 μm) layers of NIST SRM 614 and 616 using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). Geostand. Geoanal. Res. 2005, 29, 107–122. [Google Scholar] [CrossRef]
- Warren, J.M. Global variations in abyssal peridotite compositions. Lithos 2016, 248–251, 193–219. [Google Scholar] [CrossRef]
- Wunder, B. Equilibrium experiments in the system MgO ± SiO2 ± H2O (MSH): Stability fields of clinohumite-OH [Mg9Si4O16(OH)2], chondrodite-OH [Mg5Si2O8(OH)2] and phase A (Mg7Si2O8(OH)6). Contrib. Mineral. Petrol. 1998, 132, 111–120. [Google Scholar] [CrossRef]
- Luoni, P.; Rebay, G.; Spalla, M.I.; Zanoni, D. UHP Ti-chondrodite in the Zermatt-Saas serpentinite: Constraints on a new tectonic scenario. Am. Mineral. 2018, 103, 1002–1005. [Google Scholar] [CrossRef]
- Engi, M.; Lindsley, D.H. Stability of titanian clinohumite: Experiments and thermodynamic analysis. Contrib. Mineral. Petrol. 1980, 72, 415–424. [Google Scholar] [CrossRef]
- Fumagalli, P.; Poli, S.; Fischer, J.; Merlini, M.; Gemmi, M. The high-pressure stability of chlorite and other hydrates in subduction mélanges: Experiments in the system Cr2O3-MgO-Al2O3-SiO2-H2O. Contrib. Mineral. Petrol. 2014, 167, 1–16. [Google Scholar] [CrossRef]
- Weiss, M. Clinohumites: A Field and Experimental Studyitle. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 1997. No. 12202. [Google Scholar]
- Brey, G.; Brice, W.R.; Ellis, D.J.; Green, D.H.; Harris, K.L.; Ryabchikov, I.D. Pyroxene-carbonate reactions in the upper mantle. Earth Planet. Sci. Lett. 1983, 62, 63–74. [Google Scholar] [CrossRef]
- Bucher, K.; Frey, M. Petrogenesis of Metamorphic Rocks; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-662-04916-7. [Google Scholar]
- Trommsdorff, V.; Evans, B.W. Antigorite-ophicarbonates: Phase relations in a portion of the system CaO-MgO-SiO2-H2O-CO2. Contrib. Mineral. Petrol. 1977, 60, 39–56. [Google Scholar] [CrossRef]
- Ulmer, P.; Trommsdorff, V. Phase relations of hydrous mantle subducting to 300 km. Spec. Publ. Geochem. Soc. 1999, 6, 259–282. [Google Scholar]
- Scott, J.M.; Liu, J.; Pearson, D.G.; Waight, T.E. Mantle depletion and metasomatism recorded in orthopyroxene in highly depleted peridotites. Chem. Geol. 2016, 441, 280–291. [Google Scholar] [CrossRef]
- Frost, R. Contact Metamorphism of Serpentinite, Chloritic Blackwall and Rodingite at Paddy-Go-Easy Pass, Central Cascades, Washington. J. Petrol. 1975, 16, 272–313. [Google Scholar] [CrossRef]
- Trommsdorff, V.; Sánchez-Vizcaíno, V.L.; Gomez-Pugnaire, M.T.; Müntener, O. High pressure breakdown of antigorite to spinifex-textured olivine and orthopyroxene, SE Spain. Contrib. Mineral. Petrol. 1998, 132, 139–148. [Google Scholar] [CrossRef]
- Arai, S. “Non-calciferous” orthopyroxene and its bearing on the petrogenesis of ultramafic rocks in Sangun and Joetsu zones. J. Jpn. Assoc. Mineral. Petrol. Econ. Geol. 1974, 69, 343–353. [Google Scholar] [CrossRef]
- Arai, S. Contact metamorphosed dunite-harzburgite complex in the Chugoku district, western Japan. Contrib. Mineral. Petrol. 1975, 52, 1–16. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Shu, J.F.; Mao, H.K.; Liou, J.G. Magnetite lamellae in olivine and clinohumite from Dabie UHP ultramafic rocks, central China. Am. Mineral. 1999, 84, 564–569. [Google Scholar] [CrossRef]
- Connolly, J.A.D. Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet. Sci. Lett. 2005, 236, 524–541. [Google Scholar] [CrossRef]
- Arai, S.; Ishimaru, S.; Okrugin, V.M. Metasomatized harzburgite xenoliths from Avacha volcano as fragments of mantle wedge of the Kamchatka arc: Implication for the metasomatic agent. Isl. Arc 2003, 12, 233–246. [Google Scholar] [CrossRef]
- Arai, S.; Kida, M. Origin of fine-grained peridotite xenoliths from Iraya volcano of Batan Island, Philippines: Deserpentinization or metasomatism at the wedge mantle beneath an incipient arc? Isl. Arc 2008, 9, 458–471. [Google Scholar] [CrossRef]
- McInnes, B.I.; Gregoire, M.; Binns, R.A.; Herzig, P.M.; Hannington, M.D. Hydrous metasomatism of oceanic sub-arc mantle, Lihir, Papua New Guinea: Petrology and geochemistry of fluid-metasomatised mantle wedge xenoliths. Earth Planet. Sci. Lett. 2001, 188, 169–183. [Google Scholar] [CrossRef]
- Iizuka, Y.; Nakamura, E. Experimental Study for of the Slab-mantle Interaction and Implications the Formation of Titanoclinohumite at Deep Subduction Zone subducting oceanic crust ( denoted as slab ) and the overlying wedge mantle in the subduction zone, respectively. The chemi. Proc. Jpn. Acad. 1995, 159–164. [Google Scholar] [CrossRef]
- Vance, J.A.; Dungan, M.A. Formation of peridotites by deserpentinization in the Darrington and Sultan areas, Cascade Mountains, Washington. Geol. Soc. Am. Bull. 1977, 88, 1497. [Google Scholar] [CrossRef]
- Yang, J. Deep subduction of mantle-derived garnet peridotites from the Su-Lu UHP metamorphic terrane in China. J. Metamorph. Geol. 2000, 18, 167–180. [Google Scholar] [CrossRef]
- Polat, A.; Wang, L.; Appel, P.W.U. A review of structural patterns and melting processes in the Archean craton of West Greenland: Evidence for crustal growth at convergent plate margins as opposed to non-uniformitarian models. Tectonophysics 2015, 662, 67–94. [Google Scholar] [CrossRef]

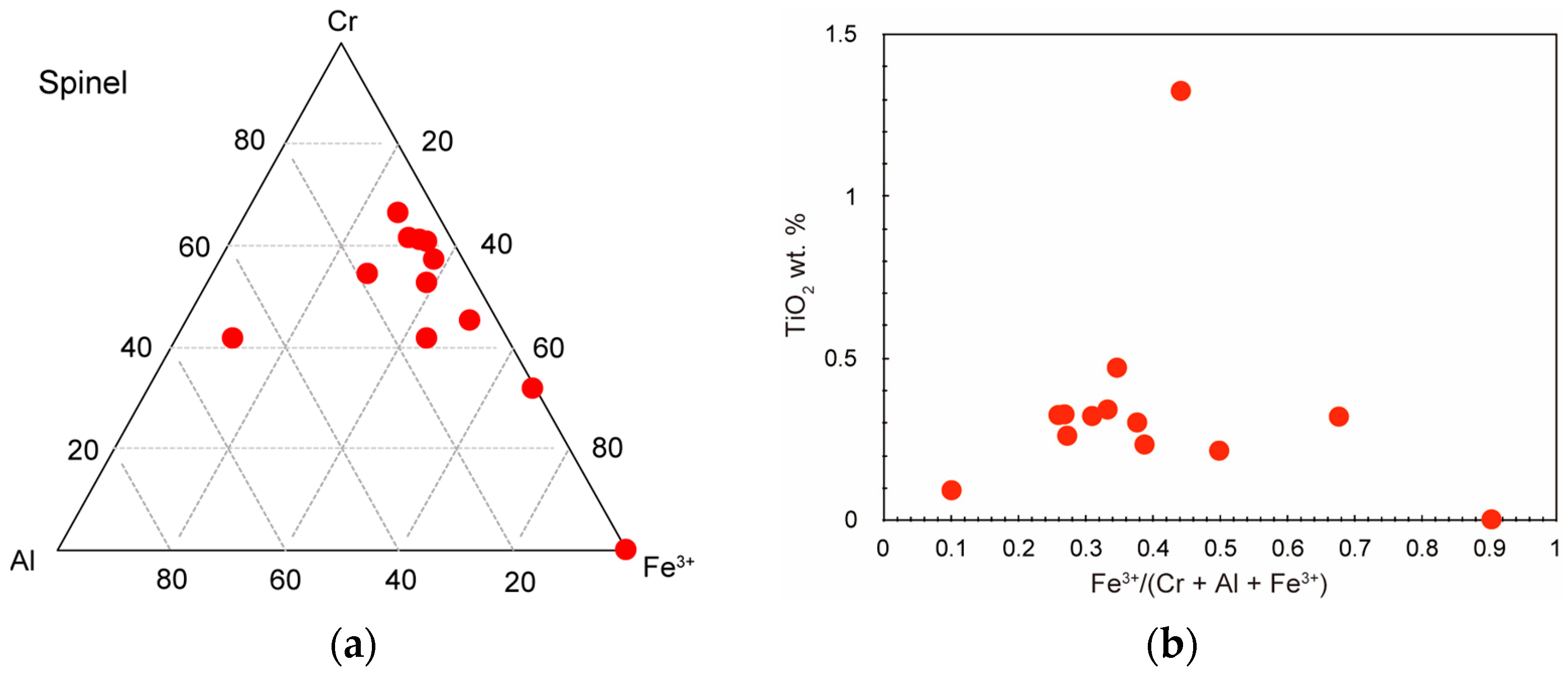
| wt.% | SiO2 | FeO* | MnO | MgO | NiO | Total | Si | Fe* | Mn | Mg | Ni | Total | Fo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dusty | |||||||||||||
| Grain1 | 40.9 | 6.8 | 0.2 | 51.6 | 0.4 | 99.9 | 0.993 | 0.137 | 0.003 | 1.866 | 0.007 | 3.007 | 93.1 |
| Grain1 | 40.9 | 7.1 | 0.1 | 51.8 | 0.4 | 100.3 | 0.990 | 0.144 | 0.003 | 1.866 | 0.007 | 3.010 | 92.9 |
| Grain2 | 41.5 | 6.7 | 0.2 | 52.5 | 0.4 | 101.3 | 0.993 | 0.134 | 0.003 | 1.869 | 0.007 | 3.007 | 93.3 |
| Grain2 | 41.1 | 7.1 | 0.1 | 52.4 | 0.4 | 101.1 | 0.987 | 0.142 | 0.003 | 1.874 | 0.008 | 3.013 | 93.0 |
| Grain3 | 41.1 | 6.9 | 0.1 | 51.7 | 0.4 | 100.3 | 0.994 | 0.139 | 0.003 | 1.862 | 0.008 | 3.006 | 93.1 |
| Grain3 | 41.0 | 6.7 | 0.1 | 51.2 | 0.4 | 99.3 | 0.999 | 0.135 | 0.002 | 1.857 | 0.008 | 3.001 | 93.2 |
| Grain9 | 41.2 | 7.1 | 0.1 | 52.3 | 0.4 | 101.1 | 0.989 | 0.142 | 0.003 | 1.869 | 0.007 | 3.010 | 93.0 |
| Grain9 | 41.5 | 6.8 | 0.2 | 52.5 | 0.4 | 101.4 | 0.992 | 0.135 | 0.003 | 1.869 | 0.008 | 3.008 | 93.3 |
| Grain10/1 | 41.0 | 6.9 | 0.1 | 51.7 | 0.4 | 100.3 | 0.991 | 0.139 | 0.002 | 1.862 | 0.007 | 3.001 | 93.1 |
| Grain10/1 | 40.8 | 6.7 | 0.1 | 51.5 | 0.4 | 99.5 | 0.993 | 0.137 | 0.003 | 1.867 | 0.008 | 3.007 | 93.2 |
| Grain11/1 | 41.0 | 6.6 | 0.1 | 51.2 | 0.4 | 99.3 | 0.998 | 0.135 | 0.003 | 1.858 | 0.008 | 3.001 | 93.2 |
| Grain12/1 | 40.7 | 6.8 | 0.1 | 52.7 | 0.4 | 100.7 | 0.981 | 0.137 | 0.003 | 1.892 | 0.008 | 3.019 | 93.3 |
| Grain13 | 40.8 | 6.8 | 0.1 | 50.2 | 0.4 | 98.3 | 1.004 | 0.140 | 0.003 | 1.841 | 0.008 | 2.996 | 92.9 |
| Grain13 | 41.4 | 6.9 | 0.1 | 51.5 | 0.4 | 100.3 | 1.000 | 0.139 | 0.002 | 1.851 | 0.008 | 3.000 | 93.0 |
| Grain14 | 41.1 | 7.0 | 0.1 | 51.3 | 0.4 | 100.0 | 0.996 | 0.141 | 0.003 | 1.854 | 0.008 | 3.003 | 92.9 |
| Grain20/1 | 41.3 | 6.9 | 0.1 | 51.5 | 0.4 | 100.2 | 0.998 | 0.140 | 0.002 | 1.854 | 0.008 | 3.002 | 93.0 |
| Grain20/1 | 41.0 | 7.2 | 0.1 | 51.6 | 0.4 | 100.3 | 0.993 | 0.145 | 0.002 | 1.859 | 0.007 | 3.007 | 92.8 |
| Dusty Low-NiO | |||||||||||||
| Grain15 | 40.8 | 7.0 | 0.12 | 51.0 | 0.12 | 99.0 | 1.00 | 0.14 | 0.00 | 1.86 | 0.00 | 3.00 | 92.8 |
| Clear | |||||||||||||
| Grain1 | 40.7 | 7.3 | 0.11 | 51.6 | 0.37 | 100.1 | 0.99 | 0.15 | 0.00 | 1.87 | 0.01 | 3.01 | 92.7 |
| Grain1 | 40.7 | 7.1 | 0.13 | 51.7 | 0.39 | 100.0 | 0.99 | 0.14 | 0.00 | 1.87 | 0.01 | 3.01 | 92.9 |
| Grain1 | 40.8 | 7.1 | 0.10 | 51.8 | 0.41 | 100.2 | 0.99 | 0.14 | 0.00 | 1.87 | 0.01 | 3.01 | 92.8 |
| Grain1 | 41.1 | 6.7 | 0.13 | 52.1 | 0.45 | 100.5 | 0.99 | 0.14 | 0.00 | 1.87 | 0.01 | 3.01 | 93.3 |
| Grain5 | 41.3 | 6.8 | 0.07 | 51.7 | 0.42 | 100.3 | 1.00 | 0.14 | 0.00 | 1.86 | 0.01 | 3.00 | 93.1 |
| Grain7 | 41.1 | 7.3 | 0.14 | 52.0 | 0.37 | 100.9 | 0.99 | 0.15 | 0.00 | 1.86 | 0.01 | 3.01 | 92.7 |
| Grain10/2 | 40.2 | 7.3 | 0.14 | 52.4 | 0.39 | 100.5 | 0.98 | 0.15 | 0.00 | 1.89 | 0.01 | 3.02 | 92.8 |
| Grain11/2 | 41.3 | 7.3 | 0.12 | 51.9 | 0.40 | 100.9 | 0.99 | 0.15 | 0.00 | 1.86 | 0.01 | 3.01 | 92.7 |
| Grain11/2 | 40.9 | 7.1 | 0.11 | 50.9 | 0.41 | 99.4 | 1.00 | 0.14 | 0.00 | 1.85 | 0.01 | 3.00 | 92.8 |
| Grain12/1 | 40.8 | 7.2 | 0.10 | 51.8 | 0.38 | 100.3 | 0.99 | 0.15 | 0.00 | 1.87 | 0.01 | 3.01 | 92.7 |
| Grain13/2 | 41.1 | 7.1 | 0.15 | 51.6 | 0.40 | 100.4 | 0.99 | 0.14 | 0.00 | 1.86 | 0.01 | 3.01 | 92.8 |
| Grain13/2 | 40.9 | 7.2 | 0.14 | 50.9 | 0.40 | 99.6 | 1.00 | 0.15 | 0.00 | 1.85 | 0.01 | 3.00 | 92.6 |
| Grain20/2 | 41.2 | 6.9 | 0.09 | 51.8 | 0.42 | 100.4 | 0.99 | 0.14 | 0.00 | 1.86 | 0.01 | 3.00 | 93.0 |
| wt.% | Grain9 | Grain2 | Grain2-2 | Grain10 | Grain11 | Grain3 | Grain20 | Grain20 | Grain7 | Grain7 |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 56.9 | 57.3 | 56.9 | 57.0 | 57.2 | 57.5 | 57.2 | 57.2 | 56.9 | 56.8 |
| TiO2 | 0.10 | 0.08 | 0.07 | 0.09 | 0.08 | 0.10 | 0.08 | 0.12 | 0.08 | 0.09 |
| Al2O3 | 1.6 | 1.5 | 1.3 | 1.6 | 1.5 | 1.6 | 1.6 | 1.8 | 1.5 | 1.6 |
| Cr2O3 | 0.16 | 0.16 | 0.19 | 0.17 | 0.20 | 0.19 | 0.20 | 0.22 | 0.22 | 0.22 |
| FeO* | 5.3 | 5.6 | 5.4 | 5.4 | 5.3 | 5.3 | 5.5 | 5.4 | 5.2 | 5.4 |
| MnO | 0.17 | 0.14 | 0.14 | 0.13 | 0.15 | 0.15 | 0.16 | 0.12 | 0.13 | 0.16 |
| MgO | 35.4 | 35.4 | 35.5 | 35.2 | 35.4 | 35.5 | 35.2 | 35.2 | 35.1 | 35.3 |
| CaO | 0.15 | 0.14 | 0.10 | 0.13 | 0.14 | 0.16 | 0.12 | 0.15 | 0.09 | 0.16 |
| NiO | - | 0.06 | 0.06 | 0.06 | - | - | 0.07 | 0.06 | - | 0.07 |
| Total | 99.9 | 100.3 | 99.7 | 99.7 | 99.9 | 100.6 | 100.1 | 100.2 | 99.2 | 99.8 |
| Si | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 | 1.96 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 0.07 | 0.06 | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.07 |
| Cr | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| Fe* | 0.15 | 0.16 | 0.16 | 0.16 | 0.15 | 0.16 | 0.15 | 0.15 | 0.16 | 0.15 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | 1.81 | 1.81 | 1.82 | 1.80 | 1.81 | 1.80 | 1.81 | 1.80 | 1.80 | 1.80 |
| Ca | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 |
| Ni | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 4.01 | 4.01 | 4.01 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| XMg | 0.922 | 0.919 | 0.921 | 0.921 | 0.923 | 0.921 | 0.923 | 0.922 | 0.919 | 0.921 |
| XCr | 0.061 | 0.067 | 0.085 | 0.067 | 0.082 | 0.067 | 0.082 | 0.073 | 0.079 | 0.076 |
| Grain 1 | Grain 1 | Grain 1 | Grain 1 | Grain 1 | Grain 1 | Grain 2-1 | Grain 2-1 | Grain 2-1 | Grain 2-1 | Grain 2-1 | Grain 2-2 | Grain 2-2 | Grain 2-2 | Grain 3 | Grain 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | margin | margin | margin | margin | margin | rim | margin | core | margin | rim | rim | core | rim | margin | margin | margin |
| SiO2 | 37.6 | 37.3 | 36.8 | 37.0 | 37.5 | 37.9 | 37.4 | 38.2 | 37.2 | 37.6 | 37.9 | 37.2 | 37.4 | 36.5 | 37.0 | 36.8 |
| TiO2 | 2.3 | 2.4 | 2.4 | 2.4 | 2.1 | 2.2 | 2.1 | 1.7 | 2.2 | 2.0 | 1.9 | 2.0 | 1.9 | 2.2 | 2.1 | 2.2 |
| FeO* | 5.4 | 5.4 | 5.2 | 5.3 | 5.4 | 5.1 | 5.3 | 5.6 | 5.2 | 5.4 | 5.3 | 5.4 | 5.3 | 5.5 | 5.4 | 5.3 |
| MnO | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| MgO | 51.7 | 52.1 | 51.9 | 52.0 | 51.5 | 52.4 | 52.2 | 51.6 | 52.1 | 51.7 | 51.6 | 52.4 | 51.4 | 51.9 | 52.5 | 52.3 |
| NiO | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 |
| F | 2.3 | 2.2 | 2.1 | 2.2 | 2.1 | 2.2 | 2.4 | 1.6 | 2.0 | 1.7 | 1.5 | 2.1 | 2.0 | 2.1 | 2.1 | 2.0 |
| H2O | 1.7 | 1.8 | 1.8 | 1.7 | 1.8 | 1.8 | 1.6 | 2.0 | 1.8 | 2.0 | 2.1 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| O = F | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 1.0 | 0.7 | 0.8 | 0.7 | 0.7 | 0.9 | 0.8 | 0.9 | 0.9 | 0.8 |
| Total | 100.3 | 100.5 | 99.7 | 100.1 | 99.9 | 101.0 | 100.4 | 100.4 | 100.1 | 100.0 | 100.0 | 100.4 | 99.4 | 99.5 | 100.3 | 100.0 |
| Si | 4.03 | 3.99 | 3.97 | 3.98 | 4.04 | 4.03 | 4.00 | 4.09 | 3.99 | 4.04 | 3.98 | 4.05 | 3.95 | 3.95 | 3.95 | 4.08 |
| Ti | 0.19 | 0.19 | 0.19 | 0.19 | 0.17 | 0.17 | 0.17 | 0.14 | 0.18 | 0.16 | 0.16 | 0.15 | 0.17 | 0.18 | 0.18 | 0.15 |
| Fe | 0.48 | 0.49 | 0.47 | 0.48 | 0.49 | 0.46 | 0.48 | 0.50 | 0.47 | 0.49 | 0.48 | 0.48 | 0.48 | 0.48 | 0.49 | 0.48 |
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Mg | 8.26 | 8.31 | 8.33 | 8.32 | 8.26 | 8.31 | 8.33 | 8.24 | 8.33 | 8.28 | 8.35 | 8.29 | 8.37 | 8.36 | 8.35 | 8.27 |
| Ni | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Total | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| XMg | 0.941 | 0.942 | 0.943 | 0.942 | 0.941 | 0.945 | 0.943 | 0.940 | 0.944 | 0.941 | 0.943 | 0.942 | 0.942 | 0.941 | 0.943 | 0.942 |
| XF | 0.39 | 0.37 | 0.35 | 0.38 | 0.36 | 0.37 | 0.41 | 0.27 | 0.34 | 0.28 | 0.26 | 0.35 | 0.34 | 0.36 | 0.35 | 0.34 |
| M | 8.78 | 8.82 | 8.84 | 8.83 | 8.78 | 8.80 | 8.83 | 8.77 | 8.83 | 8.80 | 8.77 | 8.86 | 8.80 | 8.87 | 8.88 | 8.87 |
| M/Si | 2.18 | 2.21 | 2.23 | 2.22 | 2.17 | 2.18 | 2.21 | 2.14 | 2.21 | 2.18 | 2.15 | 2.23 | 2.17 | 2.25 | 2.25 | 2.25 |
| Grain9-1 | Grain9-2 | Grain5 | Grain1 | Grain10 | Grain12 | Grain4 | Grain4 | Grain20 | Grain13 | Grain7-1 | Grain7-2 | Grain7-3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | core | core | in ol | core | core | core | core | rim | core | core | core | core | core |
| TiO2 | <0.04 | 0.30 | 0.32 | 0.33 | 0.26 | 1.33 | 0.09 | 0.33 | 0.23 | 0.47 | 0.34 | 0.32 | 0.22 |
| Al2O3 | 0.07 | 2.34 | 0.31 | 3.08 | 8.76 | 6.55 | 26.76 | 9.92 | 4.14 | 2.15 | 2.53 | 3.49 | 2.18 |
| Cr2O3 | 6.2 | 38.9 | 21.2 | 45.2 | 38.9 | 29.1 | 34.4 | 39.6 | 36.8 | 41.0 | 42.0 | 42.6 | 30.5 |
| FeO* | 85.6 | 51.1 | 70.2 | 43.5 | 43.4 | 55.3 | 27.1 | 42.6 | 53.0 | 48.9 | 48.0 | 45.9 | 59.1 |
| MnO | 0.04 | 0.64 | 0.40 | 0.69 | 0.60 | 0.42 | 0.30 | 0.51 | 0.53 | 0.71 | 0.62 | 0.74 | 0.45 |
| MgO | 0.7 | 3.1 | 2.4 | 3.5 | 5.0 | 4.0 | 11.2 | 5.8 | 3.4 | 3.1 | 3.6 | 4.1 | 2.6 |
| NiO | 0.37 | 0.16 | 0.61 | 0.14 | 0.29 | 0.47 | 0.12 | 0.29 | 0.25 | 0.26 | 0.14 | 0.10 | 0.28 |
| Total | 93.1 | 96.6 | 95.4 | 96.5 | 97.2 | 97.2 | 100.0 | 99.1 | 98.3 | 96.7 | 97.1 | 97.4 | 95.4 |
| Ti | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.04 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Al | 0.00 | 0.10 | 0.01 | 0.13 | 0.36 | 0.27 | 0.96 | 0.40 | 0.17 | 0.09 | 0.11 | 0.15 | 0.10 |
| Cr | 0.19 | 1.13 | 0.62 | 1.31 | 1.08 | 0.81 | 0.83 | 1.07 | 1.04 | 1.19 | 1.21 | 1.21 | 0.90 |
| Fe3+ | 1.80 | 0.75 | 1.34 | 0.53 | 0.54 | 0.86 | 0.20 | 0.51 | 0.77 | 0.68 | 0.66 | 0.61 | 0.99 |
| Fe2+ | 0.95 | 0.82 | 0.86 | 0.80 | 0.73 | 0.77 | 0.49 | 0.70 | 0.81 | 0.82 | 0.80 | 0.77 | 0.85 |
| Mn | 0.00 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 |
| Mg | 0.04 | 0.17 | 0.13 | 0.19 | 0.26 | 0.21 | 0.51 | 0.30 | 0.18 | 0.17 | 0.19 | 0.22 | 0.15 |
| Ni | 0.01 | 0.00 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 |
| Total | 3.00 | 3.00 | 3.00 | 3.01 | 3.00 | 2.99 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| XMg | 0.043 | 0.170 | 0.135 | 0.194 | 0.262 | 0.215 | 0.509 | 0.297 | 0.181 | 0.172 | 0.194 | 0.223 | 0.147 |
| XCr | 0.984 | 0.918 | 0.979 | 0.908 | 0.748 | 0.749 | 0.463 | 0.728 | 0.856 | 0.928 | 0.917 | 0.891 | 0.904 |
| YCr | 0.094 | 0.572 | 0.316 | 0.664 | 0.544 | 0.418 | 0.416 | 0.539 | 0.524 | 0.606 | 0.611 | 0.615 | 0.452 |
| YAl | 0.002 | 0.051 | 0.007 | 0.067 | 0.183 | 0.140 | 0.483 | 0.201 | 0.088 | 0.047 | 0.055 | 0.075 | 0.048 |
| YFe3+ | 0.904 | 0.377 | 0.677 | 0.269 | 0.273 | 0.442 | 0.101 | 0.260 | 0.388 | 0.347 | 0.333 | 0.310 | 0.499 |
| wt.% | Grain1/2 | Grain1 | Grain1 | Grain2 | Grain3 |
|---|---|---|---|---|---|
| SiO2 | 32.1 | 31.6 | 31.5 | 32.3 | 30.8 |
| TiO2 | <0.04 | 0.09 | 0.06 | <0.04 | 0.06 |
| Al2O3 | 17.4 | 17.3 | 17.4 | 15.7 | 17.2 |
| Cr2O3 | 1.6 | 1.7 | 1.7 | 1.9 | 1.6 |
| FeO | 2.2 | 2.5 | 2.5 | 2.5 | 2.5 |
| MgO | 32.6 | 32.5 | 32.2 | 33.0 | 32.3 |
| NiO | 0.21 | 0.21 | 0.21 | 0.21 | 0.2 |
| Total | 86.1 | 85.8 | 85.5 | 85.6 | 84.8 |
| F wt.% | 0.12 | 0.15 | 0.35 | 0.18 | 0.36 |
| Si | 2.83 | 2.81 | 2.80 | 2.87 | 2.77 |
| Ti | - | 0.01 | 0.00 | - | 0.00 |
| Al | 1.81 | 1.80 | 1.82 | 1.65 | 1.82 |
| Cr | 0.11 | 0.12 | 0.12 | 0.13 | 0.12 |
| Fe | 0.16 | 0.18 | 0.18 | 0.19 | 0.19 |
| Mg | 4.28 | 4.29 | 4.27 | 4.38 | 4.33 |
| Ni | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 |
| Total | 9.21 | 9.23 | 9.22 | 9.24 | 9.26 |
| XMg | 0.964 | 0.959 | 0.959 | 0.959 | 0.958 |
| XCr | 0.058 | 0.062 | 0.062 | 0.073 | 0.06 |
| ppm | OPX | DL for 100 | Ti Clinohumite | DL for 60 | ||||
|---|---|---|---|---|---|---|---|---|
| Li | 8.4 | 7.6 | 8.7 | 0.1 | 13 | 9 | 7 | 0.6 |
| B | 3.3 | 2.1 | 3.3 | 0.2 | 14 | 8 | 11 | 1.0 |
| Sc | 30.98 | 34.38 | 33.49 | 0.03 | 6 | 8 | 7 | 0.1 |
| Ti | 438 | 478 | 444 | 0.1 | 10,930 | 11,253 | 7866 | 0.2 |
| V | 74 | 73 | 74 | 0.02 | 26 | 32 | 19 | 0.1 |
| Cr | 1771 | 1738 | 1722 | 0.6 | 77 | 81 | 53 | 1.9 |
| Co | 44 | 43 | 43 | 0.006 | 127 | 121 | 129 | 0.0 |
| Ni | 497 | 475 | 497 | 0.3 | 2552 | 2423 | 2733 | 0.7 |
| Rb | < | < | < | 0.008 | < | < | < | 0.029 |
| Sr | 0.005 | 0.005 | 0.010 | 0.002 | 0.097 | 0.095 | 0.101 | 0.007 |
| Y | 1.87 | 2.23 | 1.92 | 0.002 | 0.023 | 0.074 | 0.418 | 0.004 |
| Zr | 0.73 | 0.74 | 0.58 | 0.004 | 1.663 | 1.721 | 0.987 | 0.007 |
| Nb | 0.016 | 0.018 | 0.020 | 0.002 | 6.649 | 7.100 | 5.038 | 0.000 |
| Cs | < | < | < | 0.005 | < | < | < | 0.017 |
| Ba | < | < | < | 0.011 | < | < | 0.053 | 0.033 |
| La | < | 0.002 | 0.004 | 0.001 | < | 0.020 | 0.161 | 0.004 |
| Ce | 0.008 | 0.009 | 0.015 | 0.001 | < | 0.076 | 0.439 | 0.004 |
| Pr | 0.002 | 0.003 | 0.002 | 0.001 | < | 0.008 | 0.043 | 0.004 |
| Nd | 0.021 | 0.022 | 0.019 | 0.006 | < | 0.042 | 0.173 | 0.019 |
| Sm | 0.022 | 0.025 | 0.017 | 0.004 | < | < | 0.033 | 0.017 |
| Eu | 0.003 | 0.003 | 0.003 | 0.003 | < | < | < | 0.004 |
| Gd | 0.065 | 0.071 | 0.048 | 0.008 | < | < | 0.041 | 0.024 |
| Tb | 0.020 | 0.021 | 0.017 | 0.002 | < | < | < | 0.005 |
| Dy | 0.229 | 0.270 | 0.228 | 0.004 | < | < | 0.052 | 0.016 |
| Ho | 0.067 | 0.083 | 0.070 | 0.002 | < | < | 0.017 | 0.007 |
| Er | 0.299 | 0.357 | 0.321 | 0.005 | < | < | 0.060 | 0.011 |
| Tm | 0.055 | 0.066 | 0.060 | 0.002 | < | 0.002 | 0.011 | 0.006 |
| Yb | 0.468 | 0.530 | 0.496 | 0.007 | 0.019 | 0.033 | 0.084 | 0.015 |
| Lu | 0.072 | 0.086 | 0.079 | 0.002 | 0.007 | 0.009 | 0.014 | 0.005 |
| Hf | 0.071 | 0.072 | 0.061 | 0.007 | 0.035 | 0.048 | 0.026 | 0.024 |
| Ta | < | < | < | 0.002 | 0.155 | 0.184 | 0.133 | 0.004 |
| Pb | 0.059 | 0.013 | 0.044 | 0.011 | 0.090 | 0.071 | 0.074 | 0.028 |
| Th | < | < | < | 0.004 | < | < | 0.043 | 0.015 |
| U | < | < | < | 0.004 | 0.041 | 0.017 | 0.071 | 0.008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishio, I.; Morishita, T.; Szilas, K.; Pearson, G.; Tani, K.-I.; Tamura, A.; Harigane, Y.; Guotana, J.M. Titanian Clinohumite-Bearing Peridotite from the Ulamertoq Ultramafic Body in the 3.0 Ga Akia Terrane of Southern West Greenland. Geosciences 2019, 9, 153. https://doi.org/10.3390/geosciences9040153
Nishio I, Morishita T, Szilas K, Pearson G, Tani K-I, Tamura A, Harigane Y, Guotana JM. Titanian Clinohumite-Bearing Peridotite from the Ulamertoq Ultramafic Body in the 3.0 Ga Akia Terrane of Southern West Greenland. Geosciences. 2019; 9(4):153. https://doi.org/10.3390/geosciences9040153
Chicago/Turabian StyleNishio, Ikuya, Tomoaki Morishita, Kristofer Szilas, Graham Pearson, Ken-Ichiro Tani, Akihiro Tamura, Yumiko Harigane, and Juan Miguel Guotana. 2019. "Titanian Clinohumite-Bearing Peridotite from the Ulamertoq Ultramafic Body in the 3.0 Ga Akia Terrane of Southern West Greenland" Geosciences 9, no. 4: 153. https://doi.org/10.3390/geosciences9040153
APA StyleNishio, I., Morishita, T., Szilas, K., Pearson, G., Tani, K.-I., Tamura, A., Harigane, Y., & Guotana, J. M. (2019). Titanian Clinohumite-Bearing Peridotite from the Ulamertoq Ultramafic Body in the 3.0 Ga Akia Terrane of Southern West Greenland. Geosciences, 9(4), 153. https://doi.org/10.3390/geosciences9040153





