Simple Summary
Shrimp farming is an important source of food and income, but it often requires large amounts of water and can generate waste that negatively affects rivers and lakes. This study explored biofloc technology as a water-saving strategy for rearing juvenile northern river shrimp (Cryphiops caementarius), an endemic freshwater species from Chile that is currently classified as vulnerable. Biofloc systems improve water quality by recycling waste nutrients into microbial aggregates that remain suspended in the water. Two natural sugar-based carbon sources, molasses and chancaca, were used to support biofloc development. Over a 157-day experimental period, shrimp reared in biofloc systems were compared with shrimp maintained under clear-water conditions. Shrimp growth performance was similar among all systems; however, biofloc treatments reduced water exchange by 81.6%. The type of carbon source influenced the structure of the microbial community, and molasses was associated with changes in the stress-related biomarker heat shock protein 70 (Hsp70), linked to physiological responses. These results indicate that biofloc technology can markedly reduce water use while maintaining biological performance, offering a practical and environmentally sustainable approach for the culture of an endemic freshwater shrimp and contributing to the conservation of Chile’s river ecosystems.
Abstract
Biofloc technology (BFT) is based on the reutilization of nitrogenous waste generated by cultured organisms through the biotransformation of these compounds primarily into microbial biomass, allowing a reduction in water exchange. The aim of this study was to evaluate BFT as a water-saving culture strategy, using two carbon sources (chancaca and molasses), and to assess its effects on water-use efficiency, growth performance, digestive enzyme activity, and physiological responses in juvenile northern river shrimp (Cryphiops caementarius). The experiment was conducted in triplicate using 400 L fiberglass tanks, with an initial stocking density of 75 shrimp m−2 and an average individual weight of 0.85 ± 0.65 g, over a 157-day rearing period. Water quality parameters were maintained within suitable ranges throughout the study. Significant differences were observed in the composition of bacterial and plankton communities among the biofloc treatments, whereas no significant differences were detected in growth performance or digestive enzyme activities. Heat shock protein 70 (Hsp70), a stress-related biomarker indicative of physiological responses, exhibited higher levels in the biofloc treatment supplemented with molasses. Overall, BFT treatments reduced water exchange by 81.6% while maintaining comparable biological performance to the control, indicating that biofloc technology represents a water-efficient and environmentally sustainable culture approach for juvenile Cryphiops caementarius, an endemic freshwater shrimp species, particularly in water-limited regions of northern Chile.
1. Introduction
Aquaculture, as an activity aimed at the cultivation and controlled production of hydrobiological organisms, has grown, surpassing capture fisheries as the main producer of aquatic animals. Global aquaculture production reached an unprecedented figure of 130.9 million tons, of which 94.4 million tons correspond to aquatic animals, representing 51% of the total aquatic animal production [1]. The productive growth of aquaculture is directly related to population demographic growth, due to the increased demand for food, such as protein sources, and the stagnation of fisheries. However, this accelerated growth has impacts on the environment, and some of the most questioned are the use and/or consumption of large volumes of water, the generation of effluents, habitat alteration, escapes of cultured species, and the consequent interaction with native species [2,3,4,5,6,7,8]. Among farming systems that operate with zero or minimal water exchange, biofloc technology (BFT) has gained attention due to its capacity to reduce nutrient-rich effluent discharge, minimize risks associated with species escape and disease transmission, and improve water-use efficiency through limited water renewal [9,10,11,12]. In shrimp culture, BFT has been reported to maintain growth performance while modulating microbial communities and physiological responses, and providing additional nutritional contributions through microbial biomass, depending on system configuration and management practices [13,14,15,16]. BFT has been successfully applied in freshwater fish such as tilapia (Oreochromis aureus, O. niloticus, O. mossambicus) [17,18,19]; carp (Cyprinus carpio) [20,21]; tambaqui (Colossoma macropomum) [22,23]; and in marine crustaceans such as Penaeus vannamei [9,11,24], Litopenaeus stylirostris [25], Farfantepenaeus paulensis [26], Fenneropenaeus merguiensis [27], P. monodon [28,29,30], as well as in freshwater prawn Macrobrachium rosenbergii [12,31,32,33,34], Cherax cainii [35], Astacus leptodactylus [36], and Procambarus clarkii [37,38].
BFT is based on the assimilation of inorganic nitrogen by the microbial community present in the culture medium [17,39]. The proportion and predominance of some of these microbial groups are determined by the interaction of various biotic and abiotic factors, presenting an ecological succession in the formation and development of the biofloc over time [40,41,42]. One aspect used to differentiate BFT-based systems is the coloration of the culture medium, which serves as a first practical field tool, although it must be validated with the analysis of physicochemical water quality parameters [43]. In those systems where microalgae predominate, they are known as green water systems, with photoautotrophic characteristics. In contrast, those where the coloration fluctuates from brownish and dark brown tones show a predominance of heterotrophic bacteria over nitrifying bacteria and microalgae [44,45]. In biofloc-based systems, heterotrophic bacteria use organic carbon as their primary energy source and assimilate inorganic nitrogen, particularly ammonia, for the synthesis of cellular proteins, thereby promoting rapid microbial growth and biomass production [46]. This process contributes to mitigating the accumulation of potentially toxic nitrogenous compounds and enhances nutrient recycling by converting waste-derived nitrogen into microbial protein, which may enrich the nutritional environment of the culture system [47]. To stimulate this metabolic pathway, the controlled addition of an external carbon source is required to adjust the carbon-to-nitrogen (C:N) ratio, which is commonly maintained within ranges that favor heterotrophic dominance over autotrophic nitrification [48]. A wide variety of organic carbon substrates have been applied for this purpose and can be classified, according to their carbon release kinetics, as slow- or fast-release sources [49,50,51,52,53]. This release dynamic plays a key role in carbon availability and bacterial activity: slow-release substrates tend to provide a more stable carbon supply, promoting gradual ammonia assimilation and greater system stability, whereas fast-release sources may induce abrupt bacterial proliferation, increasing biofloc formation and leading to greater fluctuations in water quality [54,55]. Among the most widely used carbon sources in different studies related to BFT are molasses, glycerol, wheat flour, starch, among others [56,57,58,59]. It has been described that the application of different external carbon sources influences water quality in culture systems, as well as animal performance and the quality and composition of floc [60,61,62,63,64,65,66,67,68,69].
Considering the importance of carbon source selection for biofloc development and overall system performance, this study evaluated the use of two fast-release carbon sources: molasses, a by-product of sugar production widely applied in biofloc systems, and chancaca, a locally available sugar concentrate traditionally produced and commercialized in Chile. The primary objective of this study was to evaluate biofloc technology as a water-saving culture strategy using different carbon sources, and to assess its effects on water-use efficiency, growth performance, digestive enzyme activity, and stress-related physiological responses in the northern river shrimp Cryphiops caementarius. This species is an endemic freshwater crustacean from Chile and is currently classified as vulnerable according to the official national conservation assessment (Ministerio del Medio Ambiente, Chile, https://clasificacionespecies.mma.gob.cl/) (accessed on 12 July 2025) indicating an elevated risk of population decline under natural conditions. In addition, C. caementarius holds considerable ecological and socio-economic importance in its native range. Despite this relevance, aquaculture research on C. caementarius remains limited, particularly with respect to intensive and water-efficient production systems [50]. Consequently, the evaluation of alternative culture strategies, such as biofloc technology, is especially relevant for this species, as it may contribute to reducing water consumption while maintaining biological performance under controlled conditions.
2. Materials and Methods
The research was performed in the Universidad Católica del Norte (UCN), Guayacán campus, in the city of Coquimbo, Chile, located at coordinates 29°57′56.30″ South and 71°21′11.00″ West, specifically in the Mass Crustacean Culture Laboratory (CMC) of the Faculty of Marine Sciences, Department of Aquaculture. All animal procedures in this study were conducted according to the guidelines for the care and use of the Mass Crustacean Culture Laboratory. Animal studies were reviewed and approved by the Ethics and Biotechnology Committee of the Universidad Católica del Norte (UCN), Coquimbo, Chile (CEC UCN No. 45; Approval Date: 16 November 2021).
2.1. Experimental Design and Conditions
For the initial formation of the biofloc, a 500 L tank was prepared with previously analyzed, filtered, and disinfected freshwater (treated with 15 ppm chlorine and neutralized after 24 h with 5 mg L−1 sodium thiosulfate), and maintained under continuous aeration. Extruded feed was added as the primary organic carbon source, while urea (46%) was supplied as a nitrogen source to promote microalgae proliferation. Sodium bicarbonate was used to provide alkalinity, and the system was inoculated with microalgae—mainly Chlorophyceae—along with the commercial bacterial product EcoPro (EcoMicrobials LLC., Miami, FL, USA), which contains stable spores of Paenibacillus polymyxa, Bacillus megaterium, Bacillus licheniformis, and Bacillus subtilis (minimum guaranteed concentration of 5.5 × 108 CFU g−1). Once the biofloc reached a stable condition, it was divided into two 500 L tanks, each receiving one of the carbon sources evaluated in this study: molasses or chancaca. The carbon sources were diluted in tank water and applied evenly to each treatment to maintain a carbon-to-nitrogen (C:N) ratio of 12:1, following the guidelines proposed by Avnimelech [17] and Ebeling et al. [70]. The required carbon input was calculated based on the target C:N ratio and adjusted according to the estimated carbon content of each source, considering molasses (approximately 30% carbon) and chancaca (approximately 36% carbon) as fast-release substrates. Carbon sources were added daily in dissolved form and in small, incremental doses to minimize abrupt changes in water quality. Total ammonia nitrogen (TAN) concentrations were monitored throughout the experimental period and used as an indirect indicator to fine-tune carbon additions and maintain system stability. The experimental BFT system for each carbon source consisted of three 400 L rectangular units (1.46 m2 each), independently connected to a 500 L reserve tank and a 200 L sedimentation tank. Water circulation was ensured by a submersible pump (Atman AT-105, Guangzhou Ample Technology Co., Ltd., Zhongshan, China, Qmax. 3000 L h−1) to preserve the planktonic community and maintain water quality [9,71]. In contrast, the control treatment (clear water) had no recirculation and received a 50% weekly water exchange. The entire system operated under natural photoperiod, with a constant temperature of 23 °C maintained by 200 W submersible heaters (Whale VK-1000 Zhongshan Enjoyroyal Appliance Co., Ltd., Zhongshan, China). Aeration was provided by microperforated hoses connected to a 2.5 HP blower (Sweetwater, Pentair, Golden Valley, MN, USA). All tanks contained 12 PVC shelters (10–15 cm long, 30–40 mm diameter, joined in groups of 3–4). In the BFT treatments, water addition was limited to compensating for evaporation and solids removal. The different water exchange regimes applied to the control and biofloc treatments were inherent to the operational principles of each culture system. The control treatment followed a conventional management strategy with periodic water renewal, whereas the biofloc treatments were operated under minimal water exchange conditions, consistent with standard BFT practices.
2.2. River Shrimp Stock
A total of 990 juveniles of C. caementarius were used, produced in the UCN laboratory of CMC from ovigerous wild females captured from the natural environment (Limarí River, IV Region, Coquimbo). The organisms were acclimatized to the rearing temperature for three weeks before starting the trial. Each of the rearing tanks was stocked with 110 juveniles with an average body weight of 0.85 ± 0.65 g, with an initial density of 75 shrimp m−2. The animals were fed daily with formulated trout feed at 5% of their biomass. The nutritional composition of the diet was 48.5% crude protein, 18.5% lipids, 1.9% fiber, 12% ash, and 10% moisture. A single pellet size of 3 mm was used throughout the experimental period.
2.3. Water Quality Parameters
Daily during the experiment, dissolved oxygen (DO), temperature, and pH were measured using an HQ40d multiparameter instrument (Hach Company, Loveland, CO, USA). Once a week, total ammonia as nitrogen (TAN), nitrate as nitrogen, nitrite as nitrogen, total suspended solids (TSS), total suspended volatiles (VSS), floc volume (FV), total alkalinity, and hardness were measured. The determination of nitrogen compounds was carried out using the HACH company protocol. The salicylate method was used for Total Ammonia Nitrogen (TAN), the cadmium reduction method for nitrate, and the diazotization method for nitrite. Measurements were performed using a DR 3900 Spectrophotometer (HACH, Loveland, CO, USA). TSS and VSS were measured using 2540D and 2540E method described in American Public Health Association [72]. FV was measured on Imhoff cones (1000-0010 Vitlab, Grossostheim, Germany) following the methodology proposed by Avnimelech and Kochba [73]. Total alkalinity was determined by the Bromophenol blue titration method, with the HI3811alkalinity kit (Hanna Instruments, Smithfield, RI, USA), and total harness was determined by the Titration Method with EDTA 5-B (Hach Company, Loveland, CO, USA).
2.4. Quantification of Total Culturable Bacteria and Vibrio spp.
Biofloc samples were centrifuged at 4200× g for 10 min, after which excess supernatant was removed. The resulting pellet was weighed using an analytical balance and resuspended in 5 mL of sterile saline solution (0.85% NaCl) prior to plating. Both control and biofloc samples were inoculated onto Petri dishes containing Plate Count Agar (PCA) and thiosulfate–citrate–bile salts–sucrose (TCBS) agar using the surface spread technique to quantify total cultivable bacteria (TCB) and Vibrio spp., respectively. PCA plates were incubated at 18 °C for 5 days, whereas TCBS plates were incubated at 18 °C for up to 48 h. Developed colonies were manually counted, and bacterial abundance was expressed as colony-forming units per milliliter (CFU mL−1) [74]. Negative controls consisting of sterile TCBS agar plates were included in each microbiological assay, and no bacterial growth was observed, confirming the absence of cross-contamination during sample processing. A limitation of this study is that bacteriological analysis was restricted to culturable bacteria, which represent only a small fraction of the microbial community in complex environments such as biofloc systems.
2.5. Determination of Phytoplanktonic and Zooplanktonic Communities
At the end of the experiment, 1 L of water was collected from each culture tank, filtered, and the concentrated material preserved in 50 mL bottles with 5% formalin, following the protocol of Azim [75]. Plankton quantification was conducted using a Sedgwick–Rafter counting chamber under a light microscope (Carl Zeiss, Primo Star, Oberkochen, Germany; 10×–40×). Identification of microalgae and planktonic groups was performed using photographs obtained with a Canon EOS Rebel T5 camera (Canon, Tokyo, Japan) and the corresponding taxonomic keys [76,77]. Microalgal density was expressed as cells mL−1. To characterize other organisms (ciliates, rotifers, cladocerans, and nematodes), five 5 mL subsamples from each tank were fixed with 5% formalin and examined directly in the laboratory. Zooplankton were identified to the generic level and quantified as individuals mL−1 [78,79,80].
2.6. Measurement of Growth Performance Parameters
Weight measurements were taken using a Mettler PJ3600 digital scale (Mettler, Columbus, OH, USA) with a precision of 0.01 g. At the end of the experiment, the following performance parameters were evaluated: specific growth rate (SGR) = (lnTW2 − lnTW1) × 100/(T2 − T1); where TW1 and TW2 are total weight at days T1 (start of the experiment) and T2 (after 157 days); survival rate (SR%) = final shrimp number/initial shrimp number) × 100; feed conversion ratio (FCR) = offered feed (g)/(final biomass (g) − initial biomass (g)); productivity (g m−2) = final biomass (g)/Area of the experimental unit (m2).
2.7. Sample Collection
Digestive enzyme activity and physiological responses in shrimp were measured at the end of the experimental period. Twelve shrimp per treatment were removed from the tanks and placed on ice at low temperature (<10 °C) to induce anesthesia prior to sampling. Once sacrificed, each shrimp was dissected into two segments: cephalothorax and abdomen. The cephalothorax was used for the analysis of digestive enzyme activity, while the abdomen was used for physiological response assays. Both segments were placed in sterile 2 mL Eppendorf tubes with RNA Later™ solution (Invitrogen, Thermo Fisher Scientific corporation, Barcelona, Spain) and stored at −80 °C until further analysis.
2.7.1. Digestive Enzyme Analysis
For amylase enzyme activity, Bernfeld’s method [81] was used. A tube with 500 µL of substrate (1% potato starch dissolved in 0.02 M phosphate buffer at pH 6.9 with 0.006 M NaCl) was incubated, and the reaction was initiated by adding 100 µL of enzyme extract, incubating the mixture for 5 min at 25 °C. After the incubation period, 1 mL of the reactive solution (1 g of dinitrosalicylic acid dissolved in 50 mL of distilled water with 30 g of sodium-potassium tartrate tetrahydrate, plus 20 mL of 2 N NaOH, brought to a final volume of 100 mL) was added. To stop the reaction, the mixture was incubated in boiling water for 5 min. It was then cooled for 10 min at room temperature by adding 10 mL of distilled water. A 3 mL aliquot was taken, and absorbance was recorded at 540 nm. Using a standard maltose curve treated in the same way as the samples, a curve was constructed to determine absorbance at various concentrations. Trypsin was performed by the specific methods of Worthington [82]. The trypsin substrate p-Toluene-sulfonyl-l-arginine methyl ester (TAME) was used as the substrate. To 300 µL of TAME, 100 µL of enzyme extract, and 2600 µL of tris-HCl buffer at pH 8.1 were added to the mixture. Absorbance was measured at 247 nm and 25 °C every min for the first 6 min of incubation. Protein concentrations were determined in the enzyme extracts by the method of Lowry et al. [83] with bovine albumin as the standard to establish the specific activities of the enzymes.
2.7.2. Physiological Responses—Heat Shock Protein 70 (Hsp70)
The quantification of Hsp70 was performed using indirect ELISA, with monospecific polyclonal anti-epitope antibodies for Hsp70, developed in mice immunized with the synthetic epitope peptide [84,85]. The ELISA plates were coated with 40 ng µL−1 of total proteins, in a volume of 50 µL in carbonate-bicarbonate buffer (pH 9.6). The plates were then blocked with 5% skim milk dissolved in phosphate-buffered saline (PBS) at 37 °C for 2 h. The plates were washed three times with PBS, and the first antibody, mouse IgG (1:400 dilution) against the invertebrate Hsp70 epitope, was applied and incubated overnight at 4 °C. The plates were washed three times with 1% PBS. The second antibody used was goat Immunoglobulin G (IgG) at 1:2500 dilution, and anti-mouse IgG-HRP (horseradish peroxidase conjugate), which was incubated for 60 min at room temperature. The plates were then washed and developed with the Tetramethylbenzidine (TMB) substrate for 20 min at room temperature in the dark. The reaction was stopped with sulfuric acid, and the absorbance was read at 450 nm using a spectrophotometer [86]. The quantification of Hsp70 was expressed in absorbance values (nm).
2.8. Statistical Analysis
Data analysis was performed using IBM® SPSS® Statistics 20.0 software and Microsoft Office Professional Plus 2013. All data were expressed as mean ± standard deviation (SD). The data was tested for normality and heterogeneity of variance using Kolmogorov–Smirnov test and Levene’s, respectively. The independent sample t test was utilized in testing the significance of differences between the two as carbon source (molasses and chancaca). One-way ANOVA was applied to the water quality parameters. Growth performance, digestive enzyme, and Hsp 70 levels were compared among treatments using the non-parametric Kruskal–Wallis test to detect significant differences among groups. Culture tanks were considered the experimental units (n = 3 per treatment), whereas individual shrimp sampled within each tank were treated as subsamples. For all growth, enzymatic, and physiological variables, individual measurements were averaged at the tank level prior to statistical analysis. Consequently, all statistical comparisons among treatments were performed using tank means, thereby avoiding pseudoreplication and ensuring independence of observations. Differences were considered statistically significant at p < 0.05 [87].
3. Results
3.1. Water Quality
The physical and chemical parameters of water quality monitored throughout the experiment are presented in Table 1. The mean values of temperature (<25 °C), dissolved oxygen (>8 mg L−1), and pH (>7.7) were similar across all treatments, with no present statistically significant differences (p > 0.05). Regarding the nitrogenous compounds, significant differences were observed only in NO3 (p < 0.05). The nitrate, total hardness, and TSS values were lower in the control. The total alkalinity value was higher in the control than in the BFT systems.

Table 1.
Water quality parameters of river shrimp (Cryphiops caementarius) in biofloc system with different carbon sources during the experimental period.
3.2. Total Microorganism Present
The total cultivable bacteria counts are presented in Table 2. The total bacteria count (1.12 and 4.97 × 104 CFU mL−1) was significantly (p < 0.05) higher in the molasses than in the chancaca. The Vibrio spp. was only found in the molasses systems, with a concentration of 4.28 ± 1.51 × 103 CFU mL−1. Overall, the concentrations of phytoplankton and zooplankton in the BFT were similar; all systems contained unicellular algae, bacterial communities, protozoa, rotifers, etc. However, the number of organisms present varied between the two culture systems. In chancaca, a lower number of microalgae and a higher number of zooplankton were found compared to molasses. Zooplankton community presented 12 genera from six different groups (Table 3).

Table 2.
Bacteria composition in biofloc culture with molasses and chancaca as carbon source.

Table 3.
Planktonic abundance in biofloc culture with molasses and chancaca as carbon source.
3.3. Growth Performance
The growth performance results are summarized in Table 4. The final weight, Survival, feed conversion ratio, specific growth rate, and productivity of shrimp did not present significant differences among the treatments (p > 0.05) during all 157 experimental days.

Table 4.
Growth performance of river shrimp (Cryphiops caementarius) in biofloc system with different carbon sources during the experimental period.
3.4. Digestive Enzymes
The results of specific enzymatic activity for trypsin and amylase are presented in Table 5. No significant differences (p > 0.05) were observed among the treatments.

Table 5.
Digestive enzyme activity of river shrimp (Cryphiops caementarius) in a biofloc system with different carbon sources.
3.5. Heat Shock Protein 70 (Hsp70) Response
The Hsp70 response is shown in Figure 1. The values of Hsp70 were significantly higher in molasses (0.109 ± 0.085) compared to chancaca (0.031 ± 0.009) and the control (0.061 ± 0.056) (p < 0.05).
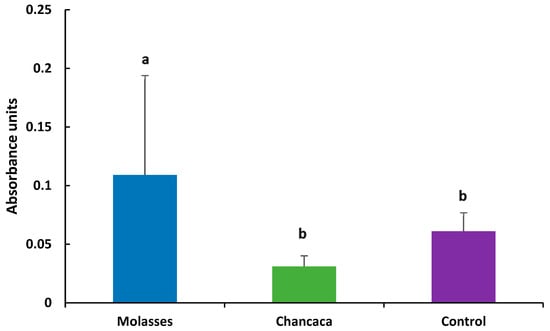
Figure 1.
Detection of Hsp70 by indirect ELISA, expressed as mean absorbance units (450 nm) ± standard deviation (SD) per treatment (n = 12). Statistical differences among treatments were evaluated using the Kruskal–Wallis test. Different letters (a, b) indicate significant differences among treatments (p < 0.05).
4. Discussion
Water quality parameters are critical in aquaculture, as deviations from optimal ranges can negatively affect productivity and lead to economic losses [88]. In BFT systems, these parameters may vary depending on the carbon source used [89,90]. In this study, all measured variables remained within the recommended ranges for the species and for proper biofloc functioning [91,92,93,94]. Among nitrogenous compounds, TAN and nitrite are the most relevant due to their toxicity [95,96]. Both remained within safe limits, with TAN ≤ 1 mg L−1 considered acceptable for fish and crustacean farming [89], and nitrite maintained below the thresholds suggested for BFT systems (<2 mg L−1 [32] and ideally <1 mg L−1 [89]). Although nitrate tends to accumulate in BFT and becomes critical above 400 mg L−1 NO3-N [97,98,99], levels in this study remained non-critical. Overall, both carbon sources effectively supported the control of nitrogen compounds by promoting heterotrophic and nitrifying bacteria or microalgae, thereby converting ammonia into microbial protein, nitrate, or algal biomass [17,70].
In this study, no significant differences were observed in FV and TSS (p > 0.05) among the biofloc treatments, and the values remained within the recommended range for penaeid species [100,101,102,103]. Alkalinity plays an important role in the formation of biofloc by heterotrophic bacteria, in the nitrification process of chemoautotrophic bacteria, and in pH control [70,104]. Therefore, recommended values should range between 100 and 150 mg CaCO3 L−1 [48]. In general, in BFT systems, alkalinity and pH levels tend to decrease, while nitrogenous compounds tend to increase [105]. In the case of M. rosenbergii, recommended alkalinity values exceed 120 mg L−1 as CaCO3 [106]. For C. caementarius, Mendez et al. [107] previously reported experimental alkalinity levels ranging from 208.8 to 255.0 mg CaCO3 L−1. In aquaculture, a moderate degree of water hardness, generally between 25 and 100 mg L−1, is considered beneficial for freshwater crustaceans. In juvenile M. rosenbergii, hardness levels of up to 1000 mg CaCO3 L−1 have been reported to be tolerated without negative effects on survival or growth, highlighting the physiological relevance of mineral-rich waters. Calcium and magnesium play essential roles in freshwater crustaceans, including osmoregulation, exoskeleton mineralization, and molting processes [108]. However, optimal hardness and alkalinity ranges are species-specific and strongly influenced by the natural hydrochemical conditions of the organism’s habitat [109]. According to reports from the Peruvian Marine Institute [110], river systems inhabited by C. caementarius exhibit water hardness values ranging from 154 to 564 mg CaCO3 L−1. Although the hardness levels recorded in the present study were higher than those typically reported, they reflect the mineral-rich freshwater conditions characteristic of river basins in northern Chile. Considering the ecological distribution and physiological adaptation of C. caementarius to hard-water environments, the alkalinity and hardness levels observed in this study can be regarded as environmentally realistic and physiologically relevant for this species. The absence of adverse effects on survival or growth further suggests that these conditions did not impose a significant physiological constraint under the experimental conditions evaluated. The difference in SSV could be due to the composition of the carbon source, as it has been described that molasses contains higher levels of potassium, iron, and manganese compared to other carbon sources such as starch [111,112]. Furthermore, it could be related to the bacterial biomass in the cultivation system, as SSV is considered a measure of bacterial biomass [113].
Bioflocs are complex aggregates composed of bacteria, fungi, algae, protozoa, zooplankton, and particulate organic matter embedded in an extracellular matrix [114,115,116]. Both heterotrophic and autotrophic bacteria play a key role in nutrient recycling and water quality control within BFT systems [54,117]. In biofloc-based culture of Macrobrachium rosenbergii, total bacterial abundances ranging from 0.88 to 4.32 × 108 CFU mL−1 have been reported using different carbon sources, with heterotrophic bacteria levels between 6.33 and 383.33 × 108 CFU mL−1 [57,118]. Similarly, Burford et al. [119] reported bacterial concentrations of 3–5 × 107 CFU mL−1 in Penaeus vannamei culture, while Khanjani et al. [120] documented heterotrophic bacterial levels of approximately 3.4 × 107 CFU mL−1. The bacterial abundances observed in the present study were comparatively lower, which may be attributed to two main factors. First, the carbon-to-nitrogen (C:N) ratio applied (12:1) is known to favor the development of chemoautotrophic nitrifying bacteria rather than heterotrophic populations typically promoted at higher C:N ratios (15–20:1) [121,122]. Second, salinity is a critical driver of heterotrophic bacterial proliferation and nitrification dynamics in BFT systems [118,123]; therefore, comparisons with marine or brackish-water shrimp species should be interpreted cautiously, given the freshwater conditions and species-specific responses of C. caementarius. In BFT systems, Vibrio spp. are commonly detected and may function either as opportunistic pathogens or as organic matter decomposers [124,125]. For example, De Souza et al. [126] reported Vibrio densities below 20 × 102 CFU mL−1 in marine shrimp (F. brasiliensis) cultured with molasses as a carbon source, likely due to the ability of sucrose-fermenting Vibrio species to utilize this substrate [127]. In addition, the minimal or zero water exchange typical of BFT systems may favor the accumulation of organic matter, creating conditions conducive to Vibrio persistence [128]. In the present study, Vibrio spp. densities did not exceed 103 CFU mL−1, a level considered insufficient to induce pathological effects in cultured shrimp [112]. Notably, Vibrio spp. were not detected in the chancaca-based treatments, which may be related to differences in the structure and functional activity of the microbial communities established within the biofloc. Biofloc-associated microorganisms may limit the proliferation of opportunistic bacteria through competition for carbon sources, nutrients, and attachment sites, thereby reducing pathogen establishment [129]. Additionally, some biofloc microorganisms have been reported to produce extracellular compounds capable of interfering with bacterial communication mechanisms, such as quorum sensing, potentially modulating Vibrio growth and virulence [130]. However, the present study does not allow causal relationships to be established, and these mechanisms should therefore be interpreted as plausible hypotheses that warrant further experimental validation.
In studies with freshwater fish such as Ictalurus punctatus and Oreochromis niloticus, as well as with the freshwater shrimp C. caementarius cultivated under BFT conditions, phytoplankton communities were found to be dominated by green algae (Chlorophyta), followed by diatoms (Bacillariophyta) and, finally, cyanobacteria (Cyanophyta) [114,131,132], as observed in this study. Regarding zooplankton organisms, the common organisms described in BFT systems were found [60,133,134,135,136], with ciliates being the most abundant, which play an important role in the nutrition of aquatic microorganisms and in controlling bacterial communities [137]. The second most abundant group were rotifers, which are frequently associated with biofloc [26,138,139]. Other organisms present were copepods, which are beneficial due to their content of long-chain polyunsaturated fatty acids (LC-PUFA), minerals, elements, pigments, and free amino acids [140]. Nematodes were the group that presented the lowest abundance, but they are of great importance, and their abundance is determined by the presence of various ciliates that serve as food for them [141,142,143]. The absence of nematodes in the molasses systems may be attributed to the biological substances and probiotics in the biofloc, which have antiparasitic and antihelminthic activities [144]. A higher concentration of microalgae and bacteria was observed in the molasses systems, but a lower concentration of zooplankton compared to the chancaca systems. This could be due to a process of predation or competition between zooplankton organisms and shrimp [89,116,141]. Additionally, it could be associated with a higher amount of TSS and FV in the molasses, which may serve as substrates for bacteria and microalgae [145,146]. In fact, the volume of settleable solids and the total abundance of bacteria were positively correlated with the abundance of flagellated protozoa, indicating an interaction between these microorganism groups [147]. This variation in the composition of organisms, such as heterotrophic bacteria and/or species of zooplankton, could generate variation in the proximate composition of the bioflocs [115,148].
Under BFT conditions, growth performance indices did not clearly reflect the effect of different carbon sources compared to the control. Comparable findings have been reported in other cultured species. For instance, Emerenciano et al. [71], in a study on F. paulensis reared under BFT versus a clear-water system, found no improvement in growth performance. The authors suggested that this outcome may be related to the feeding habits of shrimp, as the bioflocs may not have been sufficiently attractive to the species. Similarly, Emerenciano et al. [14] reported that growth performance of the marine shrimp F. duorarum did not improve relative to clear-water or water exchange controls. The authors attributed this to several factors, including the species’ lack of domestication, susceptibility to high stocking densities, and lower tolerance to nitrogenous compounds and suspended solids compared with other penaeid shrimp. In the present study, this limitation may also be related to the biological characteristics of C. caementarius, which are comparable to those of the prawn M. rosenbergii. Both species exhibit behaviors such as cannibalism, territoriality, and the dominance of larger males [149,150,151,152]. In this context, Pérez-Fuentes et al. [153] recommended the application of biofloc technology in M. rosenbergii as an alternative strategy, particularly in regions affected by water scarcity where conventional culture systems are not feasible. Similarly, Méndez et al. [152] reported specific growth rate (SGR) values comparable to those obtained in the present study, although associated with lower survival (<38%) under BFT conditions. Survival rates above 60% are generally considered acceptable for freshwater shrimp during juvenile rearing, considering territorial behavior and the well-documented effects of male dominance. According to Valenti et al. [154], survival exceeding 50% between stocking and final harvest is regarded as acceptable in freshwater prawn culture. Regarding feed utilization, the feed conversion ratio (FCR) observed in this study exceeded the range typically reported for shrimp (1.5–2.0) [155,156]. This reduced feed efficiency may be partially attributed to the use of a commercial salmonid diet, selected due to the lack of species-specific formulated feeds for C. caementarius at the time of the experiment. The nutritional mismatch of this diet, together with factors such as feed particle loss, limited feed retention within the system, feeding management constraints, and the influence of biofloc dynamics on feeding behavior, may have collectively contributed to the elevated FCR values. Therefore, these results should be interpreted within the biological and methodological constraints of the experimental design rather than as representative of optimized or commercial-scale production conditions. Overall, the present findings indicate that the application of biofloc technology using molasses and chancaca as carbon sources did not result in improvements in growth performance or feed utilization in juvenile C. caementarius. Comparisons with marine penaeid shrimp species (e.g., P. vannamei and P. monodon) should thus be considered strictly contextual, as substantial differences in physiology, feeding behavior, and culture requirements limit direct extrapolation to freshwater crustaceans. Nevertheless, biofloc systems consistently maintained adequate water quality and achieved substantial reductions in water exchange, highlighting their effectiveness as a water-efficient and environmentally sustainable culture strategy. From this perspective, the principal contribution of biofloc technology for C. caementarius lies not in maximizing productivity, but in enabling culture under water-limited conditions, which is particularly relevant for endemic freshwater species in arid and semi-arid regions.
It has been described that fish and shrimp in BFT systems can consume flocs, and these may have various effects, such as being a potential source of exogenous enzymes and an enhancer of endogenous enzyme synthesis, which could improve feed digestibility [157,158,159,160,161,162]. Additionally, biofloc has the potential to modify the microbiota in the intestines of the host, which improves absorption capacity, growth performance, and overall health [163,164]. Studies by Cardona et al. [165] on L. stylirostris shrimp cultivated in biofloc showed higher enzyme activity levels and greater gene expression for amylase and trypsin, with growth 4.4 times higher than that of shrimp cultivated in clear water. This is in accordance with findings by Anand et al. [29], who measured higher amylase and protease activity in juvenile P. monodon cultivated with a biofloc-supplemented diet, resulting in higher growth compared to control systems. It is not possible to distinguish between enzymatic activities synthesized by the shrimp and those produced by microorganisms. However, it is plausible that extracellular enzymes associated with bioflocs are released into the digestive tract upon ingestion by shrimp [159]. Intestinal tissues, which play a critical role in nutrient metabolism, often exhibit histological alterations that serve as reliable biomarkers of toxic conditions [166]. In our study, no positive effect was observed in the aspects mentioned above; however, no negative effects were noted in the process either, indicating no tissue damage or disruption in the secretion of these enzymes [16].
Among the main advantages attributed to biofloc technology (BFT) are its potential to influence physiological responses and general defense mechanisms, as well as to modulate stress tolerance processes and non-specific responses in fish and shrimp cultured in biofloc-based systems [162,167,168]. Heat shock proteins, particularly Hsp70, are among the most extensively studied stress-related biomarkers and are known to increase in response to environmental stressors and pathogen exposure in a wide range of marine invertebrates [169,170,171]. Importantly, Hsp70 should be interpreted as an indicator of general physiological stress and cellular defense, rather than as a direct or specific marker of immune enhancement. In the present study, when Hsp70 levels were examined in relation to bacteriological findings, it was observed that the treatment in which Vibrio spp. was detected also exhibited the highest Hsp70 levels; both observations corresponded to the molasses-based BFT treatment at the end of the experimental period. This pattern suggests a trade-off associated with the use of fast-release carbon sources such as molasses, which can promote rapid microbial proliferation and higher microbial loads, potentially increasing metabolic demand and cellular stress in the cultured organisms. This association is strictly correlative and does not imply a causal immunological effect of biofloc or carbon source. The elevated Hsp70 levels likely reflect increased cellular stress or microbial challenge rather than an improvement in immune competence. Previous studies have shown that viral and bacterial challenges can induce changes in Hsp70 expression, including increased transcription of LvHsp70 in P. vannamei [172,173]. Similarly, experimental infections of P. monodon with Vibrio harveyi resulted in significant increases in Hsp70 and Hsp90 expression shortly after exposure, reinforcing the role of heat shock proteins as stress-responsive molecules rather than specific immune effectors [174]. Although biofloc-associated microbial components have been suggested to stimulate non-specific defense mechanisms and improve stress tolerance [175], the present study does not provide sufficient evidence to conclude an immunostimulatory effect. Therefore, changes in Hsp70 expression should be interpreted cautiously and limited to their role as indicators of physiological stress and adaptive cellular responses, rather than as proof of enhanced immunity.
It is important to acknowledge that the control and biofloc treatments differed in water exchange regimes, which represents an inherent methodological contrast between conventional and biofloc-based production systems. This difference was intentional and reflects practical management conditions rather than a controlled manipulation of water exchange. Consequently, the results should be interpreted at the system level, focusing on functional performance, water-use efficiency, and physiological responses, rather than as a direct comparison under identical hydraulic conditions. Traditional shrimp farming can require approximately 64,000 L of water per kilogram of shrimp produced, whereas BFT systems typically operate with substantially lower water inputs, often below 100–200 L per kilogram, depending on management practices [176]. In the present study, the total water exchange volume during the experimental period was 350 L per tank in the biofloc treatments. In contrast, control tanks were subjected to an average of two weekly water exchanges equivalent to 50% of the tank volume, resulting in a cumulative water use of approximately 1900 L per tank over the same period. Consequently, water consumption in the biofloc treatments was 5.4-fold lower than in the control, representing a water savings of 81.6%. These results demonstrate that biofloc-based culture markedly reduces water use compared to conventional clear-water systems, thereby contributing to lower environmental impact and improved resource-use efficiency [177]. Consistent with previous reports, BFT systems are characterized by minimal water exchange and reduced effluent discharge, enabling substantial water savings relative to traditional aquaculture practices [32,178,179,180].
5. Conclusions
This study demonstrates that biofloc technology (BFT) can be implemented as an effective water-saving culture strategy for juvenile northern river shrimp (Cryphiops caementarius) under the evaluated experimental conditions. The use of molasses and chancaca as fast-release carbon sources supported biofloc development and allowed system operation with minimal water exchange, resulting in an 81.6% reduction in water use compared to a conventional clear-water system.
Importantly, the application of BFT did not lead to significant improvements in growth performance or digestive enzyme activity relative to the control. Growth-related parameters, including final weight, specific growth rate, survival, feed conversion ratio, and productivity, remained comparable among treatments. These results indicate that, for juvenile C. caementarius, biofloc systems can maintain biological performance while substantially reducing water consumption, rather than enhancing production metrics. Future studies, addressing the limitations of the present work, should incorporate additional immune-related parameters (e.g., phenoloxidase and lysozyme activities) as well as integrative “omics” approaches such as proteomics and metabolomics. These tools may provide deeper mechanistic insight into host–microbe interactions and physiological stress responses under biofloc conditions, aspects that could not be resolved with the experimental scope and biomarkers employed in this study.
Author Contributions
Conceptualization, D.U.W. and M.C.M.; methodology, D.U.W., C.S., C.G. and M.C.M.; formal analysis, C.A.M., D.U.W., C.S. and C.G.; data curation, D.U.W., C.S. and C.G.; writing—original draft preparation, C.A.M., D.U.W.; writing—review and editing, C.A.M., D.U.W., C.S., C.G. and M.C.M.; supervision, M.C.M.; funding acquisition, M.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDEF (Fondo de Fomento al Desarrollo Científico y Tecnológico, Chile), grant number ID15I10353 and ID15I20353.
Institutional Review Board Statement
The authors followed all applicable international, national, and institutional guidelines for the care and use of animals. Studies on animals were reviewed and approved by the ethics and biotechnology committee of the Universidad Católica del Norte (UCN), Coquimbo, Chile, (CEC UCN No. 45; Approval Date: 16 November 2021). In addition, the number of animals was kept to the minimum necessary to obtain scientific results, considering that the gain in knowledge and long-term benefit to the subject species is high. The animals were kept and slaughtered under production conditions; after being immersed in ice, they were subjected to analysis. They were not subjected to any procedures during the experimental period, and all analyses were conducted post mortem.
Informed Consent Statement
Not applicable, as this study did not involve human participants.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We express our sincere gratitude to the research staff of the Laboratorio de Crustaceos from UCN and Laboratorio de Fisiología y Genética Marina (FIGEMA). The authors would like to express our gratitude and appreciation to those who have taken time to critically review this manuscript. This study was part of the second author’s Ph.D. This work and publication were developed with the support of the project FONDEF ID15I20353.
Conflicts of Interest
The authors declare that there are no conflicts of interest or personal relationships that could have influenced the interpretation, analysis, or presentation of the results of this study. One of the authors, David Ulloa, is affiliated with Imenco Aqua Chile; however, this affiliation did not influence the study design, data collection, analysis, or reporting of the results.
References
- FAO. In Brief to The State of World Fisheries and Aquaculture 2024; Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bioflocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Browdy, C.L.; Ray, A.J.; Leffler, J.W.; Avnimelech, Y. Biofloc-based Aquaculture Systems. In Aquaculture Production Systems, 1st ed.; Tidwell, J.H., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 278–307. [Google Scholar] [CrossRef]
- Luo, G.; Avnimelech, Y.; Pan, Y.; Tan, H. Inorganic nitrogen dynamics in sequencing batch reactors using biofloc technology to treat aquaculture sludge. Aquacult. Eng. 2013, 52, 73–79. [Google Scholar] [CrossRef]
- Samocha, T.M.; Braga, A.; Magalhães, V.; Advent, B.; Morris, T. Ongoing studies advance intensive shrimp culture in zero-exchange biofloc raceways. Glob. Aquac. Advocate 2013, 38–40. Available online: https://www.globalseafood.org/advocate/ongoing-studies-advance-intensive-shrimp-culture-in-zero-exchange-biofloc-raceways/?utm_source=copilot.com (accessed on 25 January 2026).
- Lara, G.; Furtado, P.; Hostins, B.; Poersch, L.; Wasielesky, W., Jr. Addition of sodium nitrite and biofilm in a Litopenaeus vannamei biofloc culture system. Lat. Am. J. Aquat. Res. 2026, 44, 760–768. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Ta, V.P.; Tran, N.H.; Chau, T.T.; Le, Q.V.; Nguyen, T.H.V.; Huynh, T.T.; Tran, H.L.; Vo, N.S.; Pham, Q.A.D. Applied biofloc technology for target species in the Mekong Delta in Vietnam: A Review. J. Environ. Sci. Eng. 2017, 6, 165–175. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, K.; Yao, L.; Chen, R.; Liu, S.; Xing, H.; Nie, L.; Wu, Z. Bio-ecological remediation of freshwater aquaculture environments: A systematic review and bibliometric analysis. Water Biol. Secur. 2024, 3, 100229. [Google Scholar] [CrossRef]
- Wasielesky, W., Jr.; Atwood, H.; Stokes, A.; Browdy, C.L. Effect of natural production in a zero-exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 2006, 258, 396–403. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquacult. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Crab, R.; Chielens, B.; Wille, M.; Bossier, P.; Verstraete, W. The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquacult. Res. 2010, 41, 559–567. [Google Scholar] [CrossRef]
- Ekasari, J.; Angela, D.; Waluyo, S.H.; Bachtiar, T.; Surawidjaja, E.H.; Bossier, P.; De Schryver, P. The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 2014, 426–427, 105–111. [Google Scholar] [CrossRef]
- Emerenciano, M.; Cuzon, G.; Paredes, A.; Gaxiola, G. Evaluation of biofloc technology in pink shrimp Farfantepenaeus duorarum culture: Growth performance, water quality, microorganisms profile and proximate analysis of biofloc. Aquacult. Int. 2013, 21, 1381–1394. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, J.; Zhao, C.; Sun, L.; Zhang, X.; Chen, G. Effects of C/N Ratio Control Combined with Probiotics on the Immune Response, Disease Resistance, Intestinal Microbiota and Morphology of Giant Freshwater Prawn (Macrobrachium rosenbergii). Aquaculture 2017, 476, 125–133. [Google Scholar] [CrossRef]
- Popoola, O.M.; Miracle, O.A. Performance of Different Biomaterials as Carbon Sources on the Immunological Response and Oxidative Status of African Catfish Clarias gariepinus in Biofloc Systems. Aquac. Stud. 2022, 2, AQUAST800. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Doan, H.V.; Wannavijit, S.; Tayyamath, K.; Quynh, T.T.D.; Ninyamasiri, P.; Linh, N.V.; Wongmaneeprateep, S.; Rodkhum, C.; Seesuriyachan, P.; Phimolsiripol, Y.; et al. Effects of fermented corn cob on growth performance, digestive enzyme, immune response, and gene expression of Nile tilapia (Oreochromis niloticus) raised in biofloc system. Fish Shellfish Immunol. 2025, 163, 110413. [Google Scholar] [CrossRef]
- Verster, N. Comparison of Growth Rates of Tilapia Species (Oreochromis mossambicus and Oreochromis niloticus) Raised in a Biofloc and a Standard Recirculating Aquaculture (RAS) System. Master’s Thesis, Ghent University Belgium, Ghent, Belgium, 2017. [Google Scholar]
- Adineh, H.; Naderi, M.; Hamidi, M.K.; Harsij, M. Biofloc technology improves growth, innate immune responses, oxidative status, and resistance to acute stress in common carp (Cyprinus carpio) under high stocking density. Fish Shellfish Immunol. 2019, 95, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Adineh, H.; Naderi, M.; Jafaryan, H.; Hamidi, M.K.; Yousefi, M.; Ahmadifar, E. Effect of stocking density and dietary protein level in biofloc system on the growth, digestive and antioxidant enzyme activities, health, and resistance to acute crowding stress in juvenile common carp (Cyprinus carpio). Aquacult. Nutr. 2022, 9344478. [Google Scholar] [CrossRef]
- Dos Santos, R.B.; Izel-Silva, J.; Medeiros, P.A.; Fugimura, M.M.S.; Freitas, T.M.; Ono, E.A.; Claudiano, G.S.; Affonso, E.G. Dietary protein requirement for tambaqui cultivated in biofloc and clear water systems. Aquacult. Int. 2023, 31, 1685–1704. [Google Scholar] [CrossRef]
- Montelo, R.; Dos Santos, R.; Fugimura, M.; Ono, E.; Chaves, F.; Mattioli, C.; Affonso, E. Stocking densities of Colossoma macropomum in the initial grow-out phase using biofloc technology. Aquacult. Int. 2024, 32, 9933–9950. [Google Scholar] [CrossRef]
- Burford, M.; Thompson, P.T.; McIntosh, R.P.; Bauman, R.H.; Pearson, D.C. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture 2004, 232, 525–537. [Google Scholar] [CrossRef]
- Cardona, E.; Lorgeoux, B.; Chim, L.; Goguenheim, J.; Le Delliou, H.; Cahu, C. Biofloc contribution to antioxidant defense status, lipid nutrition and reproductive performance of broodstock of the shrimp Litopenaeus stylirostris: Consequences for the quality of eggs and larvae. Aquaculture 2016, 452, 252–262. [Google Scholar] [CrossRef]
- Ballester, E.L.C.; Abreu, P.C.; Cavalli, R.O.; Emerenciano, M.; De Abreu, L.; Wasielesky, W., Jr. Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero-exchange suspended microbial flocs intensive system. Aquacult. Nutr. 2010, 16, 163–172. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Eslami, J.; Ghaedi, G.; Sourinejad, I. The effects of different stocking densities on nursery performance of Banana shrimp (Fenneropenaeus merguiensis) reared under biofloc condition. Ann. Anim. Sci. 2022, 22, 1291–1299. [Google Scholar] [CrossRef]
- Anand, P.S.S.; Kumar, S.; Panigrahi, A.; Ghoshal, T.; Syama Dayal, J.; Biswas, G.; Sundaray, J.; De, D.; Raja, R.; Deo, A.; et al. Effects of C:N ratio and substrate integration on periphyton biomass, microbial dynamics and growth of Penaeus monodon juveniles. Aquacult. Int. 2013, 21, 511–524. [Google Scholar] [CrossRef]
- Anand, P.S.S.; Kohli, M.P.S.; Kumar, S.; Sundaray, J.K.; Roy, S.D.; Venkateshwarlu, G.; Sinha, A.; Pailan, G.H. Effect of dietary supplementation of biofloc on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture 2014, 418–419, 108–115. [Google Scholar] [CrossRef]
- Anand, P.S.S.; Kumar, S.; Kohli, M.P.S.; Sundaray, J.K.; Sinha, A.; Pailan, G.H.; Roy, D.S. Dietary biofloc supplementation in black tiger shrimp, Penaeus monodon: Effects on immunity, antioxidant and metabolic enzyme activities. Aquacult. Res. 2017, 48, 4512–4523. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Wahab, M.A.; Verdegem, M.C.J.; Huque, S.; Salam, M.A.; Azim, M.E. C/N ratio control and substrate addition for periphyton development jointly enhance freshwater prawn Macrobrachium rosenbergii production in ponds. Aquaculture 2008, 280, 117–123. [Google Scholar] [CrossRef]
- Pérez-Rostro, C.I.; Pérez-Fuentes, J.A.; Hernández-Vergara, M.P. Biofloc, a technical alternative for culturing Malaysian prawn Macrobrachium rosenbergii. In Sustainable Aquaculture Techniques; Hernández-Vergara, M.P., Ed.; IntechOpen: Rijeka, Croatia, 2014; pp. 87–104. [Google Scholar] [CrossRef]
- Miao, S.Y.; Sun, L.S.; Bu, H.Y.; Zhu, J.Y.; Chen, G.H. Effect of molasses addition at C:N ratio of 20:1 on the water quality and growth performance of giant freshwater prawn (Macrobrachium rosenbergii). Aquacult. Int. 2017, 25, 1409–1425. [Google Scholar] [CrossRef]
- Hosain, M.E.; Amin, S.M.N.; Kamarudin, M.S.; Arshad, A.; Romano, N. Effects of C–N ratio on growth, survival and proximate composition of Macrobrachium rosenbergii post larvae reared under a corn starch based zero–exchange brackish water biofloc system. Aquacult. Res. 2021, 52, 3015–3025. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Foysal, M.J.; Gupta, S.K.; Tay, A.; Fotedar, R.; Gagnon, M.M. Effects of carbon source addition in rearing water on sediment characteristics, growth and health of cultured marron (Cherax cainii). Sci. Rep. 2024, 14, 1349. [Google Scholar] [CrossRef]
- Kaya, D.; Genç, E.; Güroy, D.; Dinçer, S.; Yılmaz, B.H.; Yıldız, H.Y. Evaluation of biofloc technology for Astacus leptodactylus: Effect of different stocking densities on production performance and physiological responses. Acta Aquat. Turc. 2021, 17, 569–579. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Li, C.; Zhang, Y.; Wang, Y.; Hou, S.; Cheng, Y.; Li, J. Evaluation of the nutritional quality of edible tissues (muscle and hepatopancreas) of cultivated Procambarus clarkii using biofloc technology. Aquacult. Rep. 2021, 19, 100586. [Google Scholar] [CrossRef]
- Li, J.; Qian, C.; Li, C.; Li, Z.; Xi, Y.; Cheng, Y.; Li, J. Exploration of the optimal stocking density of red swamp crayfish (Procambarus clarkii) larvae by using the biofloc technology. Aquacult. Int. 2023, 31, 1569–1582. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Eslami, J.; Emerenciano, M.G.C. Wheat flour as carbon source on water quality, growth performance, hemolymph biochemical, and immune parameters of Pacific white shrimp (Penaeus vannamei) juveniles in biofloc technology (BFT). Aquacult. Rep. 2025, 40, 102623. [Google Scholar] [CrossRef]
- Yusoff, F.; Zubaidah, M.; Matias, H.; Kwan, T. Phytoplankton succession in intensive marine shrimp culture ponds treated with a commercial bacterial product. Aquacult. Res. 2002, 33, 269–278. [Google Scholar] [CrossRef]
- Martínez-Córdova, L.R.; Emerenciano, M.; Miranda-Baeza, A.; Martínez-Porchas, M. Microbial-based systems for aquaculture of fish and shrimp: An updated review. Rev. Aquac. 2015, 7, 131–148. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Zahedi, S.; Sharifinia, M.; Hajirezaee, S.; Singh, S.K. Biological Removal of Nitrogenous Waste Compounds in the Biofloc Aquaculture System—A Review. Ann. Anim. Sci. 2025, 25, 3–21. [Google Scholar] [CrossRef]
- Pimentel, O.A.L.F.; Amado, A.M.; They, N.H. Biofloc colors as an assessment tool for water quality in shrimp farming with BFT systems. Aquacult. Eng. 2023, 101, 102321. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture; SRAC Publication No. 4503; Biofloc Production Systems for Aquaculture; US Department of Agriculture: Washington, DC, USA, 2013; pp. 1–11. [Google Scholar]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquacult 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Luo, G.; Li, J.; Xu, J.; Liu, W.; Tan, H. Effects of dissolved organic carbon and total ammonia nitrogen concentrations with the same DOC/TAN on biofloc performance. Aquaculture 2023, 574, 739713. [Google Scholar] [CrossRef]
- Zafar, M.A.; Rana, M.M. Biofloc technology: An eco-friendly “green approach” to boost up aquaculture production. Aquacult. Int. 2022, 30, 51–72. [Google Scholar] [CrossRef]
- Samocha, T.M. Sustainable Biofloc Systems for Marine Shrimp; Academic Press: San Diego, CA, USA, 2019; p. 431. [Google Scholar] [CrossRef]
- Serra, F.P.; Gaona, C.A.P.; Furtado, P.S.; Poersch, W.; Wasielesky, W. Use of different carbon sources for the biofloc system adopted during the nursery and grow-out culture of Litopenaeus vannamei. Aquacult. Int. 2015, 23, 1325–1339. [Google Scholar] [CrossRef]
- Ulloa, D.A.; Morales, M.C.; Emerenciano, M.G. Biofloc technology: Principles focused on potential species and the case study of Chilean river shrimp Cryphiops caementarius. Rev. Aquacult. 2020, 12, 1759–1782. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.Z.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquacult. 2021, 13, 1193–1222. [Google Scholar] [CrossRef]
- Hussien, M.H.; Zayd, M.M.; Elshafey, A.E.; Khalafalla, M.M.; Shukry, M.; Abdelmegeid, M.; Okasha, L.A.; Seboussi, R.; Aboraya, M.H.; Elolimy, A.A. Comparing the effect of different carbon sources on growth, blood parameters, immunity, and water quality in Nile Tilapia (Oreochromis niloticus) cultured in a biofloc system. Aquacult. Rep. 2025, 42, 102755. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Chen, Y.; Zhang, S.; Dai, L.; Zhu, W.; Chen, Y. Optimized utilization of organic carbon in aquaculture biofloc systems: A review. Fishes 2023, 8, 465. [Google Scholar] [CrossRef]
- Vinasyiam, A.; Kokou, F.; Ekasari, J.; Schrama, J.W.; Verdegem, M.C. Effects of high wheat bran input on the performance of a biofloc system for Pacific white shrimp (Litopenaeus vannamei). Aquacult. Rep. 2023, 33, 101853. [Google Scholar] [CrossRef]
- Rai, N.; Julka, J.M.; Panigrahi, A.; Das, S.P. Synergistic carbon source utilization in Biofloc aquaculture of common carp (Cyprinus carpio): Impacts on growth, health, and environmental parameters. Front. Mar. Sci. 2025, 12, 1576079. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Yavari, V.; Hoseini, S.J.; Nafisi, M.; Torfi Mozanzadeh, M. Effects of different carbon sources and dietary protein levels in a biofloc system on growth performance, immune response against white spot syndrome virus infection and cathepsin L gene expression of Litopenaeus vannamei. Aquacult. Res. 2019, 50, 1162–1176. [Google Scholar] [CrossRef]
- Hosain, M.E.; Amin, S.M.N.; Arshad, A.; Salleh, M.S.; Karim, M. Effects of carbon sources on the culture of giant river prawn in biofloc system during nursery phase. Aquacult. Rep. 2021, 19, 100607. [Google Scholar] [CrossRef]
- Oliveira, L.K.; Wasielesky, W., Jr.; Tesser, M.B. Fish culture in biofloc technology (BFT): Insights on stocking density carbon sources, C/N ratio, fish nutrition and health. Aquacult. Fish. 2024, 9, 522–533. [Google Scholar] [CrossRef]
- Raza, B.; Zheng, Z.; Yang, W. A Review on Biofloc System Technology, History, Types, and Future Economical Perceptions in Aquaculture. Animals 2024, 14, 1489. [Google Scholar] [CrossRef]
- Monroy-Dosta, M.D.C.; Lara-Andrade, D.; Castro-Mejía, J.; Castro-Mejía, G.; Emerenciano, M. Composición y abundancia de comunidades microbianas asociadas al biofloc en un cultivo de tilapia. Rev. Biol. Mar. Oceanogr. 2013, 48, 511–520. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, S.A.; Wang, A.L. The effect of different carbon sources on the nutritional composition, microbial community and structure of bioflocs. Aquaculture 2016, 465, 88–93. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Li, C.; Deng, Y.; Tadda, M.A.; Lan, L.; Zhu, S.; Liu, D. Effects of different solid carbon sources on water quality, biofloc quality and gut microbiota of Nile tilapia (Oreochromis niloticus) larvae. Aquaculture 2018, 495, 919–931. [Google Scholar] [CrossRef]
- Behera, S.; Das, P.C.; Felix, N.; Ferosekhan, S.; Swain, H.S.; Kumari, R.; Athithan, S.; Padmavathy, P. Effect of different carbon supplements on growth performance and digestive enzyme activities of butter catfish (Ompok bimaculatus Bloch, 1794) in biofloc system. Aquaculture 2025, 603, 742384. [Google Scholar] [CrossRef]
- Luo, G.; Xu, J.; Li, J.; Zheng, H.; Tan, H.; Liu, W. Rapid production bioflocs by inoculation and fertilized with different nitrogen and carbon sources. Aquacult. Eng. 2022, 98, 102262. [Google Scholar] [CrossRef]
- Abiri, S.A.; Chitsaz, H.; Najdegerami, E.H.; Akrami, R.; Jalali, A.S. Influence of wheat and rice bran fermentation on water quality, growth performance, and health status of common carp (Cyprinus carpio L.) juveniles in a biofloc-based system. Aquaculture 2022, 555, 738168. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Abbas, E.M.; Abdelkhalek, N.K.; Ashry, O.A.; Abd El-Fattah, L.S.; El-Sawy, M.A.; Helal, M.F.; El-Haroun, E. Effect of organic carbon source and stocking densities on growth indices, water microflora, and immune-related genes expression of Litopenaeus vannamei larvae in intensive culture. Aquacult. Int. 2022, 546, 737397. [Google Scholar] [CrossRef]
- Ajamhasani, E.; Akrami, R.; Najdegerami, E.H.; Chitsaz, H.; Shamloofar, M. Different carbon sources and probiotics in biofloc-based common carp (Cyprinus carpio) culture: Effects on water quality, growth performance, fish welfare and liver histopathology. J. World Aquac. Soc. 2023, 54, 1427–1717. [Google Scholar] [CrossRef]
- Tasleem, S.; Alotaibi, B.S.; Masud, S.; Habib, S.S.; Acar, Ü.; Gualandi, S.C.; Ullah, M.; Khan, K.; Fazio, F.; Khayyam, K. Biofloc system with different carbon sources improved growth, haematology, nonspecific immunity, and resistivity against the Aeromonas hydrophila in common carp, Cyprinus carpio. Aquac. Res. 2024, 2024, 7652354. [Google Scholar] [CrossRef]
- Ayazo Genes, J.E.; Holanda, M.; Lara, G. Effects of Different Organic Carbon Sources on Water Quality and Growth of Mugil cephalus Cultured in Biofloc Technology Systems. Fishes 2025, 10, 427. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Wasielesky, W., Jr.; Soares, R.B.; Ballester, E.C.; Izeppi, E.M.; Cavalli, R.O. Crescimento e sobrevivência do camarão-rosa (Farfantepenaeus paulensis) na fase de berçário em meio heterotrófico. Acta Sci. Biol. Sci. 2007, 29, 1–7. Available online: https://www.redalyc.org/articulo.oa?id=187115768001 (accessed on 1 January 2025). [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association Inc.: Washington, DC, USA, 1995. [Google Scholar]
- Avnimelech, Y.; Kochba, M. Evaluation of nitrogen uptake and excretion by tilapia in biofloc tanks, using 15N tracing. Aquaculture 2009, 287, 163–168. [Google Scholar] [CrossRef]
- Corry, J.E.L.; Curtis, G.D.W.; Baird, R.M. Handbook of Culture Media for Food and Water Microbiology; Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Azim, M.E. The Potential of Periphyton-Based Aquaculture Production Systems. Ph.D. Thesis, Fish Culture and Fisheries Group, Wageningen University, Wageningen, The Netherlands, 2001. Available online: https://edepot.wur.nl/199010 (accessed on 5 March 2024).
- Parra, O.; Bicudo, C. Algas de Aguas Continentales: Introducción a la Biología y Sistemática; Ediciones Universidad de Concepción: Concepción, Chile, 1996. [Google Scholar]
- Parra, O. Una aproximación sistémica para la evaluación de la biodiversidad algal en ambientes acuáticos continentales de Chile. In Sociedad Ficológica de América Latina y el Caribe; Sociedad Brasileña de Ficología: São Paulo, Brazil, 1998; pp. 167–178. [Google Scholar]
- Streble, H.; Krauter, D. Atlas de los Microorganismos de Agua Dulce; Ediciones Omega, S.A: Barcelona, Spain, 1987; p. 372. [Google Scholar]
- Smirnov, N. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; No. 11; SPB Academic Publishing: Amsterdam, The Netherlands, 1996; p. 197. [Google Scholar]
- Aladro-Lubel, M.A.; Reyes-Santos, M.; Olvera-Bautista, F. Diversidad de los protozoos ciliados. In Biodiversidad del Ecosistema del Pedregal de San Ángel; Lot, A., Cano-Santana, Z., Eds.; Universidad Nacional Autónoma de México: México City, México, 2009; pp. 63–70. [Google Scholar]
- Bernfeld, P. Amylase a and b in Methods in Enzymes; Academic Press: New York, NY, USA, 1951; pp. 144–147. [Google Scholar]
- Worthington, C.; Worthington, V.; Worthington, K.; Decker, L.; Hackler, D.; Worthington, A. Worthington Enzyme Manual Related Biochemical; Worthington Biochemical Corporation: Lakewood, NJ, USA, 2016; p. 78. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bethke, J.; Rojas, V.; Berendsen, J.; Cardenas, C.; Guzman, F.; Gallardo, A.; Mercado, L. Development of a new antibody for detecting natural killer enhancing factor (NKEF)-likeprotein in infected salmonids. J. Fish Dis. 2012, 35, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Morales-Lange, B.; Guzmán, F.; Gallardo, J.A.; Mercado, L. Immunological strategy for detecting the pro-inflammatorycytokine TNF-alpha in salmonids. Electron. J. Biotechnol. 2012, 15, 21. [Google Scholar] [CrossRef]
- Guzmán, F.; Berendsen, J.; González, M.; Arenas, G.; Mercado, L. Epitope design and chemical synthesis to develop antibodies against heat shock protein (Hsp70) in different animal models of stress. New Biotechnol. 2009, 25, S38. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry Principle and Practices of Statistics in Biological Research; W. H. Freeman & Company: San Francisco, CA, USA, 1969; p. 776. [Google Scholar]
- Suneetha, K.; Padmavathi, P.; Chatla, D. Hatchery performance of Pacific white shrimp, Penaeus vannamei in Biofloc technology by using different carbon sources. Blue Biotechnol. 2024, 1, 13. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Martínez-Córdova, L.R.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc technology (BFT): A tool for water quality management in aquaculture. In Water Quality; Tutu, H., Ed.; INTECH: London, UK, 2017; 5th Chapter; pp. 92–109. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Alizadeh, M.; Sharifinia, M. Effects of different carbon sources on water quality, biofloc quality, and growth performance of Nile tilapia (Oreochromis niloticus) fingerlings in a heterotrophic culture system. Aquacult. Int. 2021, 29, 307–321. [Google Scholar] [CrossRef]
- Morales, M.; Mendez, C.; Moreno, J.; Alvarez, C. Biofloc a Nivel Prototipo. Protocolo Para la Producción de Biofloc a Nivel Prototipo, Para Uso en la Acuicultura de Pequeña Escala en Zonas áRidas del Norte de Chile; FONDEF ID15I10353; FONDEF: Coquimbo, Chile, 2018; p. 35. [Google Scholar]
- Moreno-Reyes, J.E.; Morales, M.C.; Meruane, J. A feasible path towards year-round production: Effects of temperature and photoperiod on ovarian maturity of subtropical palaemonid, the river shrimp, Cryphiops caementarius. Aquacult. Rep. 2021, 21, 100809. [Google Scholar] [CrossRef]
- Ferrer-Chujutalli, K.; Sernaqué-Jacinto, J.; Reyes-Avalos, W. Optimal temperature and thermal tolerance of postlarvae of the freshwater prawn Cryphiops (Cryphiops) caementarius acclimated to different temperatures. Heliyon 2024, 10, e25850. [Google Scholar] [CrossRef]
- Mendez, C.A.; Morales, M.C.; Merino, G.E. Effect of different carbon sources on biofloc particle size and settling velocity distribution in biofloc technology aquaculture system for northern river shrimp (Cryphiops caementarius) farming. Aquacult. Int. 2025, 33, 380. [Google Scholar] [CrossRef]
- Chen, J.C.; Lei, S.C. Toxicity of ammonia and nitrite to Penaeus monodon juveniles. J. World Aquacult. Soc. 1990, 21, 300–306. [Google Scholar] [CrossRef]
- Ostrensky, A.; Wasielesky, W. Acute toxicity of ammonia to various life stages of the São Paulo shrimp, Penaeus paulensis Pérez-Farfante, 1967. Aquaculture 1995, 132, 339–347. [Google Scholar] [CrossRef]
- Samocha, T.M.; Wilkenfeld, J.S.; Morris, T.C.; Correia, E.S.; Hanson, T. Intensive raceways without water exchange analyzed for white shrimp culture. Glob. Aquac. Advocate 2010, 13, 22–24. [Google Scholar]
- Krummenauer, D.; Peixoto, S.; Cavalli, R.O.; Poersch, L.H.; Wasielesky, W., Jr. Superintensive Culture of White Shrimp, Litopenaeus vannamei, in a Biofloc Technology System in Southern Brazil at Different Stocking Densities. J. World Aquacult. Soc. 2011, 42, 726–733. [Google Scholar] [CrossRef]
- Souza, J.; Cardozo, A.; Wasielesky, W., Jr.; Abreu, P.C. Does the biofloc size matter to the nitrification process in Biofloc Technology (BFT) Systems. Aquaculture 2019, 500, 443–450. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book, 3rd ed.; The World Aquaculture Society: Baton Rouge, LA, USA, 2009; p. 182. [Google Scholar]
- Gaona, C.A.P.; de Almeida, M.S.; Viau, V.; Poersch, L.H.; Wasielesky, W.J. Effect of different total suspended solids levels on a Litopenaeus vannamei (Boone, 1931) BFT culture system during biofloc formation. Aquacult. Res. 2017, 48, 1070–1079. [Google Scholar] [CrossRef]
- Gaona, C.A.P.; Poersch, L.H.; Krummenauer, D.; Foes, G.K.; Wasielesky, W., Jr. The Effect of Solids Removal on Water Quality, Growth and Survival of Litopenaeus vannamei in a Biofloc Technology Culture System. Int. J. Recirc. Aquacult. 2011, 12, 54–73. [Google Scholar] [CrossRef]
- Schveitzer, R.; Arantes, R.; Custódio, P.F.S.; do Espírito Santo, C.M.; Arana, L.V.; Seiffert, W.Q.; Andreatta, E.R. Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquacult. Eng. 2013, 56, 59–70. [Google Scholar] [CrossRef]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. The effect of different alkalinity levels on Litopenaeus vannamei reared with biofloc technology (BFT). Aquacult. Int. 2015, 23, 345–358. [Google Scholar] [CrossRef]
- Wasielesky, W., Jr.; Atwood, H.; Kegl, R.; Bruce, J.; Stokes, A.; Browdy, C.L. Effect of pH on Growth and Survival of Litopenaeus Vannamei in a Zero Exchange Super-Intensive Culture System. In Proceedings of the World Aquaculture Society Conference, San Antonio, TX, USA, 26 February–2 March 2007; Wiley Blackwell: Hoboken, NJ, USA, 2007; p. 1088. [Google Scholar]
- New, M.B. Farming Freshwater Prawns. A Manual for the Culture of the Giant River Prawn (Macrobrachium rosenbergii); FAO Fisheries Technical Paper 428; FAO: Rome, Italy, 2002; pp. 1–212. [Google Scholar]
- Mendez, C.A.; Morales, M.C.; Merino, G.E. Settling velocity distribution of bioflocules generated with different carbon sources during the rearing of the river shrimp Cryphiops caementarius with biofloc technology. Aquacult. Eng. 2021, 93, 102157. [Google Scholar] [CrossRef]
- González-Vera, C.; Brown, J.H. Effects of alkalinity and total hardness on growth and survival of postlarvae freshwater prawns, Macrobrachium rosenbergii (De Man 1879). Aquaculture 2017, 473, 521–527. [Google Scholar] [CrossRef]
- Romano, N.; Egnew, N.; Quintero, H.; Kelly, A.; Sinha, A.K. The Effects of Water Hardness on the Growth, Metabolic Indicators and Stress Resistance of Largemouth Bass Micropterus salmoides. Aquaculture 2020, 527, 735469. [Google Scholar] [CrossRef]
- Zacarías, S.; Yépez, V. Monitoreo poblacional de camarón de rio estimación de abundancia de adultos en ríos de la costa centro sur. Informe Annu. IMARPE Perú 2007, 44, 34. [Google Scholar]
- Jamir, L.; Kumar, V.; Kaur, J.; Kumar, S.; Singh, H. Composition, valorization and therapeutical potential of molasses: A critical review. Environ. Technol. Rev. 2021, 10, 131–142. [Google Scholar] [CrossRef]
- Tinh, T.H.; Koppenol, T.; Hai, T.N.; Verreth, J.A.J.; Verdegem, M.C.J. Effects of carbohydrate sources on a biofloc nursery system for whiteleg shrimp (Litopenaeus vannamei). Aquaculture 2021, 531, 735795. [Google Scholar] [CrossRef]
- Phuong, T.V.; Hoa, N.V.; Diep, D.X.; Vo, V.; Nhu, M.B. Effects of combined rice flour and molasses use on the growth performance of Pacific white shrimp (Litopenaeus vannamei Boone, 1931) applied biofloc technology. Isr. J. Aquatic. Bamidgeh 2023, 75, 1–18. [Google Scholar] [CrossRef]
- Reis, W.G.; Wasielesky, W.J.; Abreu, P.C.; Brandão, H.; Krummenauer, D. Rearing of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) BFT system with different photoperiods: Effects on the microbial community, water quality and zootechnical performance. Aquaculture 2019, 508, 19–29. [Google Scholar] [CrossRef]
- Maciel de Lima, P.C.; de Andrade, R.J.V.; Elins Moreira da Silva, A.; Campos, C.V.F.d.S.; Oliveira, C.Y.B.; Olivera Gálvez, A.; Brito, L.O. Effects of different molasses application rates on planktonic composition in low salinity biofloc culture of Nile tilapia, Oreochromis niloticus fingerlings. Chem. Ecol. 2022, 38, 913–934. [Google Scholar] [CrossRef]
- Ma, H.; Tian, M.; Zhang, R.; Zhao, J.; Xu, Q.; Duan, M. Effects of soybean oligosaccharides on water quality and microbial community in biofloc systems. Aquacult. Rep. 2025, 42, 102798. [Google Scholar] [CrossRef]
- Vinasyiam, A.; Verdegem, M.C.J.; Ekasari, J.; Schrama, J.W.; Kokou, F. Prokaryotic and eukaryotic microbial community dynamics in biofloc systems supplemented with non-starch polysaccharides. Aquaculture 2025, 594, 741396. [Google Scholar] [CrossRef]
- Hosain, M.E.; Amin, S.N.; Kamarudin, M.S.; Arshad, A.; Karim, M.; Romano, N. Effect of salinity on growth, survival, and proximate composition of Macrobrachium rosenbergii post larvae as well as zooplankton composition reared in a maize starch based biofloc system. Aquaculture 2021, 533, 736235. [Google Scholar] [CrossRef]
- Burford, M.A.; Thompson, P.J.; McIntosh, R.P.; Bauman, R.H.; Pearson, D.C. Nutrient and microbial dynamics in high-intensity, zero-exchange shrimp ponds in Belize. Aquaculture 2003, 219, 393–411. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sajjadi, M.; Alizadeh, M.; Sourinejad, I. Effect of different feeding levels on water quality, growth performance and survival of western white shrimp (Litopenaeus vannamei Boone, 1931) post larvae with application of biofloc technology. Iran. Sci. Fish. J. 2015, 24, 13–28. [Google Scholar]
- Ferreira, G.S.; Santos, D.; Schmachtl, F.; Machado, C.; Fernandes, V.; Bögner, M.; Schleder, D.D.; Seiffert, W.Q.; Vieira, F.N. Heterotrophic, chemoautotrophic and mature approaches in biofloc system for Pacific white shrimp. Aquaculture 2021, 533, 736099. [Google Scholar] [CrossRef]
- Halim, M.A.; Aziz, D.; Arshad, A.; Wong, N.L.W.S.; Karim, M.M.; Syukri Ismail, M.F.; Rahi, M.L. Effects of C/N Ratio for Bi-Culture of Litopenaeus vannamei (Boone, 1931) and Macrobrachium rosenbergii (de Man, 1879) in a Molasses Based Biofloc System. Aquacult. Fish Fish. 2025, 5, e70032. [Google Scholar] [CrossRef]
- Halim, M.A.; Aziz, D.; Arshad, A.; Wong, N.L.W.S.; Karim, M.; Islam, M.A.; Syukri, F.; Rahi, L. Effect of Salinity on Nursery Bi-Culture of Pacific White Leg Shrimp (Litopenaeus vannamei) and Giant Prawn (Macrobrachium rosenbergii) in a Biofloc System. Aquacult. Res. 2025, 5368636. [Google Scholar] [CrossRef]
- Luis-Villaseñor, I.E.; Voltolina, D.; Audelo-Naranjo, J.M.; Pacheco-Marges, M.R.; Herrera-Espericueta, V.E.; Romero-Beltrán, E. Effects of biofloc promotion on water quality, growth, biomass yield and heterotrophic community in Litopenaeus vannamei (Boone, 1931) experimental intensive culture. Ital. J. Anim. Sci. 2016, 14, 332–337. [Google Scholar] [CrossRef]
- Akange, E.T.; Aende, A.A.; Rastegari, H.; Odeyemi, O.A.; Kasan, N.A. Swinging between the beneficial and harmful microbial community in biofloc technology: A paradox. Heliyon 2024, 10, e25228. [Google Scholar] [CrossRef]
- De Souza, D.; Suita, S.; Romano, L.; Wasielesky, W.; Ballester, E. Use of molasses as a carbon source during the nursery rearing of Farfantepenaeus brasiliensis (Latreille, 1817) in a Biofloc technology system. Aquacult. Res. 2014, 45, 270–277. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, S.; Naser, M.; Karim, M. Isolation of Vibrio spp. from Penaeid Shrimp Hatcheries and Coastal Waters of Cox’s Bazar, Bangladesh. Asian J. Exp. Biol. Sci. 2010, 1, 288–293. [Google Scholar]
- Ferreira, C.; Bonetti, C.; Seiffert, Q. Hydrological and water quality indices as management tools in marine shrimp culture. Aquaculture 2011, 318, 425–433. [Google Scholar] [CrossRef]
- Gustilatov, M.; Ekasari, J.; Pande, G.S.J. Protective effects of the biofloc system in Pacific white shrimp (Penaeus vannamei) culture against pathogenic Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2022, 124, 66–73. [Google Scholar] [CrossRef]
- Crab, R.; Lambert, A.; Defoirdt, T.; Bossier, P.; Verstraete, W. The application of bioflocs technology to protect brine shrimp (Artemia franciscana) from pathogenic Vibrio harveyi. J. Appl. Microbiol. 2010, 109, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Schrader, K.K.; Green, B.W.; Perschbacher, P.W. Development of phytoplankton communities and common off-flavors in a biofloc technology system used for the culture of channel catfish (Ictalurus punctatus). Aquacult. Eng. 2011, 45, 118–126. [Google Scholar] [CrossRef]
- Mendez, C.A.; Moreno-Reyes, J.; Galleguillos, C.; Morales, M.C. Effect of different carbon sources on the cultivation of northern river shrimp Cryphiops caementarius in a biofloc system. Ann. Anim. Sci. 2025. ahead of print. [Google Scholar] [CrossRef]
- Loureiro, K.C.; Wasielesky, W., Jr.; Abreu, P.C. The use of protozoan, rotifers and nematodes as live food for shrimp raised in BFT system. Atlântica 2012, 34, 5–12. [Google Scholar] [CrossRef]
- Castro-Mejía, C.; De Lara, A.; Monroy-Dosta, M.; Maya-Gutierrez, S.; Castro-Mejia, J.; Jiménez- Pacheco, F. Presence and abundance of phytoplankton and zooplankton in a Biofloc production system using two carbon sources: 1) Molasses and 2) Molasses + rice powder, culturing Oreochromis niloticus. Rev. Digit. Dep. Hombre Ambiente 2017, 1, 33–42. [Google Scholar]
- Mendoza, L.C.; Pertuz-Buelvas, V.; Espinoza-Araujo, J.; Atencio-García, V.J.; Prieto-Guevara, M.J. Potencialidad del cultivo de bocachico Prochilodus magdalenae con tecnología biofloc. Orinoquia 2021, 25, 25–39. [Google Scholar] [CrossRef]
- Silva, W.A.D.; Silva, J.L.D.; Oliveira, C.Y.B.; de Moraes, A.P.M.; Shinozaki-Mendes, R.A.; Silva, U.L. Effect of stocking density on water quality, plankton community structure, and growth performance of Litopenaeus vannamei (Boone, 1931) post-larvae cultured in low-salinity biofloc system. Int. Aquat. Res. 2022, 14, 107–116. [Google Scholar]
- Becerril-Cortés, D.; Monroy-Dosta, M.; Emerenciano, M.; Castro-Mejía, G.; Sofia, B.; Bermúdez, S.; Vela, G. Effect on nutritional composition of produced bioflocs with different carbon sources (molasses, coffee waste and rice bran) in Biofloc System. Int. J. Fish Aquat. Stud. 2018, 6, 541–547. [Google Scholar]
- Pérez-Fuentes, J.A.; Hernández-Vergara, M.P.; Pérez-Rostro, C.I.; Fogel, I. C:N ratios affect nitrogen removal and production of Nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 2016, 452, 247–251. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mohammadi, A.; Emerenciano, M.G.C. Microorganisms in biofloc aquaculture system. Aquacult. Rep. 2022, 26, 101300. [Google Scholar] [CrossRef]
- Santos, S.M.; Wasielesky, W.; Braga, Í.; Zuñiga, R.; Torres, V.; Christ-Ribeiro, A.; Foes, G. Use of different stocking densities of Litopenaeus vannamei juveniles using “synbiotics”: Effects on water quality, microorganisms, bioflocs composition and zootechnical performance. Aquacult. Int. 2024, 32, 6133–6151. [Google Scholar] [CrossRef]
- Ray, A.J.; Seaborn, G.; Leffler, J.W.; Wilde, S.B.; Lawson, A.; Browdy, C.L. Characterization of microbial communities in minimal-exchange, intensive aquaculture systems and the effects of suspended solids management. Aquaculture 2010, 310, 130–138. [Google Scholar] [CrossRef]
- Chakrapani, S.; Panigrahi, A.; Sundaresan, J.; Mani, S.; Palanichamy, E.; Rameshbabu, V.S.; Krishna, A. Utilization of Complex Carbon Sources on Biofloc System and Its Influence on the Microbial Composition, Growth, Digestive Enzyme Activity of Pacific White Shrimp, Penaeus vannamei Culture. Turk. J. Fish. Aquat. Sci. 2022, 22, TRJFAS18813. [Google Scholar] [CrossRef]
- Al-Souti, A.S.; Zaher, M.M.; Helal, A.M.; Meshhal, D.T.; D.H., M.; Al-Afify, A.D.G.; Rafaey, M.M.; El-Saharty, A.; El-Haroun, E.; Nassif, M.G.; et al. Relation among zootechnical performance, biochemical indicators, water quality, and small invertebrates (zooplankton) abundance reared in biofloc-supplemented systems. Front. Mar. Sci. 2025, 11, 1520765. [Google Scholar] [CrossRef]
- Helal, A.M.; Zaher, M.M.; Meshhal, D.T.; Ashour, M.; Younis, E.M.; Abdelwarith, A.A.; Al-Afify, A.D.G.; Sharawy, Z.Z.; Davies, S.; El-Haroun, E.; et al. Biofloc supplementation improves growth performances, nutrient utilization, and histological status of Nile tilapia (Oreochromis niloticus) while enhancing zooplankton diversity, community, and abundance. Aquaculture 2024, 585, 740711. [Google Scholar] [CrossRef]
- Fróes, C.N.; Fóes, G.; Krummenauer, D.; Ballester, E.; Poersch, L.H.; Wasielesky, W., Jr. Fertilização orgânica com carbono no cultivo intensivo em viveiros com sistema de bioflocos do camarão branco Litopenaeus vannamei. Atlântica 2012, 34, 31–39. Available online: https://periodicos.furg.br/atlantica/article/view/2704/1482 (accessed on 5 March 2024). [CrossRef]
- Soaudy, M.R.; Ghonimy, A.; Greco, L.S.L.; Chen, Z.; Dyzenchauz, A.; Li, J. Total suspended solids and their impact in a biofloc system: Current and potentially new management strategies. Aquaculture 2023, 572, 739524. [Google Scholar] [CrossRef]
- Sena, R.P.O.; Krummenauer, D.; Wasielesky, W., Jr.; Pimentel, O.A.L.F.; Bezerra, A.; dos Santos Junior, J.R.T.; Carvalho, A.; Ravagnan, E.; Bagi, A.; Poersch, L.H.S. The Effects of Predominantly Chemoautotrophic Versus Heterotrophic Biofloc Systems on Nitrifying Bacteria, Planktonic Microorganisms, and Growth of Penaeus vannamei, and Oreochromis niloticus in an Integrated Multitrophic Culture. Fishes 2024, 9, 478. [Google Scholar] [CrossRef]
- Said, M.M.; Zaki, F.M.; Taw, N.; Snyder, G.S. Light limitation alters water quality, biofloc composition, zooplankton community, and performance of the whiteleg shrimp (Litopenaeus vannamei) reared with intensive biofloc. Egypt. J. Aquat. Biol. Fish. 2024, 28, 359–381. [Google Scholar] [CrossRef]
- Boock, M.V.; de Almeida Marques, H.L.; Mallasen, M.; Barros, H.P.; Moraes-Valenti, P.; Valenti, W.C. Effects of prawn stocking density and feeding management on rice-prawn culture. Aquaculture 2016, 451, 480–487. [Google Scholar] [CrossRef]
- Cano, F.; Carrion, S.; Reyes, W. Efecto de altas densidades de siembra en el crecimiento y supervivencia de postlarvas de Cryphiops caementarius (Crustacea: Palaemonidae) en agua salobre. Rev. Citecsa 2014, 5, 62–78. [Google Scholar]
- Rojas, R.; Morales, M.C.; Rivadeneira, M.M.; Thiel, M. Male morphotypes in the Andean River shrimp Cryphiops caementarius (Decapoda: Caridea): Morphology, coloration and injuries. J. Zool. 2012, 288, 21–32. [Google Scholar] [CrossRef]
- Mendez, C.A.; Morales, M.C.; Brokordt, K. Effects of stocking density of the river shrimp Cryphiops caementarius on physiological and performance responses in a biofloc system. Fishes 2024, 9, 377. [Google Scholar] [CrossRef]
- Pérez-Fuentes, J.A.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P. Pond-reared Malaysian prawn Macrobrachium rosenbergii with the biofloc system. Aquaculture 2013, 400–401, 105–110. [Google Scholar] [CrossRef]
- Valenti, W.C.; Daniels, W.H.; New, M.B.; Correia, E.S. Hatchery systems and management. In Freshwater Prawns: Biology and Farming; New, M.B., Valenti, W.C., Tidwell, J.H., D’Abramo, L.R., Kutty, M.N., Eds.; Wiley–Blackwell: Hoboken, NJ, USA, 2010; pp. 55–85. [Google Scholar] [CrossRef]
- Van Wyk, P. Nutrition and Feeding of Litopenaeus vannamei in Intensive Culture Systems. In Farming Marine Shrimp in Recirculating Freshwater Systems; Van Wyk, P., Davis-Hodgkins, M., Laramore, R., Main, J.K., Mountain, L., Scarpa, J., Eds.; Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 1999; pp. 125–139. [Google Scholar]
- Kuhn, D.D.; Lawrence, A.L.; Crockett, J.; Taylor, D. Evaluation of bioflocs derived from confectionary food effluent water as a replacement feed ingredient for fishmeal or soy meal for shrimp. Aquaculture 2016, 454, 66–71. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q.; Zhao, D.H.; Huang, J. Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture 2012, 350–353, 147–153. [Google Scholar] [CrossRef]
- Najdegerami, E.H.; Bakhshi, F.; Lakani, F.B. Effects of biofloc on growth performance, digestive enzyme activities and liver histology of common carp (Cyprinus carpio L.) fingerlings in zero-water exchange system. Fish Physiol. Biochem. 2016, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, L.; Zhang, K.; Xu, W.; Zhao, D.; Mei, L. Effects of different carbon sources addition on nutrition composition and extracellular enzymes activity of bioflocs, and digestive enzymes activity and growth performance of Litopenaeus vannamei in zero-exchange culture tanks. Aquacult. Res. 2016, 47, 3307–3318. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, G.; Tan, H.; Liu, W.; Hou, Z. Growth, digestive enzyme activity and welfare of tilapia (Oreochromis niloticus) reared in a biofloc-based system with poly-β-hydroxybutyric as a carbon source. Aquaculture 2016, 464, 710–717. [Google Scholar] [CrossRef]
- Yu, Z.; Li, L.; Zhu, R.; Li, M.; Duan, J.; Wang, J.Y.; Liu, Y.H.; Wu, L.F. Monitoring of growth, digestive enzyme activity, immune response and water quality parameters of Golden crucian carp (Carassius auratus) in zero-water exchange tanks of biofloc systems. Aquacult. Rep. 2020, 16, 100283. [Google Scholar] [CrossRef]
- Panigrahi, A.; Das, R.R.; Sundaram, M.; Sivakumar, M.R.; Jannathulla, R.; Lalramchhani, C.; Antony, J.; Shyne Anand, P.S.; Vinay Kumar, K.; Jayanthi, M.; et al. Cellular and molecular immune response and production performance of Indian white shrimp Penaeus indicus (H. Milne-Edwards, 1837), reared in a biofloc-based system with different protein levels of feed. Fish Shellfish Immunol. 2021, 119, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Mozanzade, M.T.; Sharifinia, M.; Emerenciano, M.G.C. Biofloc: A sustainable dietary supplement, nutritional value and functional properties. Aquaculture 2023, 562, 738757. [Google Scholar] [CrossRef]
- Torres-Lagos, E.; Henríquez-Castillo, C.; Mendez, C.; Morales, M.C.; Cárcamo, C.B.; Navarrete, P.; Schmitt, P.; Brokordt, K. Biofloc culture system shapes the structure and function of environmental and intestinal bacterial communities in the river prawn (Cryphiops caementarius). Aquacult. Rep. 2024, 39, 102359. [Google Scholar] [CrossRef]
- Cardona, E.; Logeoux, B.; Geffroy, C.; Richard, P.; Saulnier, D.; Gueguen, Y.; Guillou, G.; Chim, L. Relative contribution of natural productivity and compound feed to tissue growth in blue shrimp (Litopenaeus stylirostris) reared in biofloc: Assessment by C and N stable isotope ratios and effect on key digestive enzymes. Aquaculture 2015, 448, 288–297. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tokmechi, A.; Rahmani Farah, K.; Shalizar Jalali, A. Growth performance, haematology, antioxidant status, immune response and histology of common carp (Cyprinus carpio L.) fed biofloc grown on different carbon sources. Aquacult. Res. 2018, 49, 393–403. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and symbiotic improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Biofloc: A sustainable alternative for improving the production of farmed cyprinid species. Aquacult. Rep. 2023, 33, 101748. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.; He, W.; Zheng, Y.; Wang, L.; Xin, Y.; Liu, Y.; Wang, A. Expression of HSP60 and HSP70 in white shrimp, Litopenaeus vannamei in response to bacterial challenge. J. Invertebr. Pathol. 2010, 103, 170–178. [Google Scholar] [CrossRef]
- Hu, B.; Phuoc, L.; Sorgeloos, P.; Bossier, P. Bacterial HSP70 (Dnak) is an efficient immune stimulator in Litopenaeus vannamei. Aquaculture 2014, 418–419, 87–93. [Google Scholar] [CrossRef]
- Brokordt, K.; González, R.; Farías, W.; Winkler, F. Potential Response to Selection of HSP0 as a Component of Innate Immunity in the Abalone Haliotis rufescens. PLoS ONE 2015, 10, e0141959. [Google Scholar] [CrossRef]
- Janewanthanakul, S.; Supungul, P.; Tang, S.; Tassanakajon, A. Heat shock protein 70 from Litopenaeus vannamei (LvHSP70) is involved in the innate immune response against white spot syndrome virus (WSSV) infection. Dev. Comp. Immunol. 2020, 102, 103476. [Google Scholar] [CrossRef]
- Valentim-Neto, P.A.; Moser, J.R.; Fraga, A.P.M.; Marques, M.R.F. Hsp70 expression in shrimp Litopenaeus vannamei in response to IHHNV and WSSV infection. Virusdisease 2014, 25, 437–440. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Leelatanawit, R.; Jiravanichpaisal, P.; Klinbunga, S.; Karoonuthaisiri, N. Expression and distribution of three heat shock protein genes under heat shock stress and under exposure to Vibrio harveyi in Penaeus monodon. Dev. Comp. Immunol. 2010, 34, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Pan, L.; Huang, F.; Wang, C.; Xu, W. Effects of different carbon sources on bioactive compound production of biofloc, immune response, antioxidant level, and growth performance of Litopenaeus vannamei in zero-water exchange culture tanks: Carbon sources affecting biofloc development. J. World Aquacult. Soc. 2016, 47, 566–576. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Martins, M.A.; do Nascimento Vieira, F. Pacific White shrimp and Nile tilapia integrated in a biofloc system under different fish-stocking densities. Aquaculture 2019, 498, 83–89. [Google Scholar] [CrossRef]
- Jing, L.; Ren, H.; Xu, W.; Su, H.; Hu, X.; Wen, G.; Xu, Y.; Tu, L.; Cao, Y. Intensive cultivation of whiteleg shrimp (Litopenaeus vannamei) under biofloc condition in land-based ponds. Aquacult. Rep. 2025, 41, 102690. [Google Scholar] [CrossRef]
- Ridha, M.T.; Hossain, M.A.; Azad, I.S.; Saburova, M. Effects of three carbohydrate sources on water quality, water consumption, bacterial count, growth and muscle quality of Nile tilapia (Oreochromis niloticus) in a biofloc system. Aquac. Rep. 2025, 51, 4225–4237. [Google Scholar] [CrossRef]
- Huang, H.H.; Li, C.Y.; Song, Y.; Lei, Y.J.; Yang, P.H. Growth performance of shrimp and water quality in a freshwater biofloc system with a salinity of 5.0‰: Effects on inputs, costs and wastes discharge during grow-out culture of Litopenaeus vannamei. Aquacult. Eng. 2022, 98, 102265. [Google Scholar] [CrossRef]
- Garcés, S.; Lara, G. Applying Biofloc Technology in the Culture of Mugil cephalus in Subtropical Conditions: Effects on Water Quality and Growth Parameters. Fishes 2023, 8, 420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.