Simple Summary
Symbiotic copepods usually live in the gills, mantle cavity and visceral mass of the marine mollusks, with hosts including polyplacophorans, bivalves, scaphopods, gastropods, and cephalopods. However, our understanding is limited regarding these copepods that are symbiotic with mollusks in the global oceans. To close this gap, we compiled a detailed list of copepods associated with mollusks found in the global oceans based on a review of the existing literature. This list includes information on species diversity, host associations, and geographical distributions. We report that 342 species of symbiotic copepods are associated with more than 435 species of mollusks, primarily bivalves and gastropods, with a few found in other molluscan classes. The most common order of the symbiotic copepods is Cyclopoida. However, many species have not been properly studied or collected, emphasizing the requirement for more research to completely understand the diversity of copepods symbiotic with mollusks in the global oceans.
Abstract
Symbiotic copepods have a wide host group, including not only invertebrates but also vertebrates, with variable symbiotic sites and morphological characteristics. Even though symbiotic copepods exhibit remarkable diversity, our knowledge of them is still very limited, causing significant lacunae in our understanding of their taxonomic characteristics, host associations, and geographical distributions. To fill these knowledge gaps, we have compiled a comprehensive list of symbiotic copepods and their molluscan hosts in the global oceans based on an extensive literature review. The inventory provides a comprehensive synthesis of the diversity, hosts, and geographical distributions of the symbiotic copepods. This review summarizes information on copepods symbiotic with mollusks from 1863 to 2025. Our compilation records a total of 342 symbiotic copepod species associated with more than 435 species of mollusks. This total includes some copepod species for which no specific host has been identified. For each copepod species, we provide details on its hosts, geographical distributions and the original references.
1. Introduction
Copepods are one of the most species-rich animal groups on the earth and can be found in seawater or freshwater, where they lead planktonic, benthic, or parasitic lives [1,2]. To date, approximately 15,045 species have been described, about one-third of which are commensal or parasitic [3]. As the most abundant group of marine zooplankton, copepods are an indispensable component of the food chain. Within marine food webs, they play a crucial role: on one hand, they drive the operation of biogeochemical cycles, and on the other hand, they facilitate the smooth transfer of energy from primary producers to higher trophic levels. Due to the high sensitivity of copepod populations to the impacts of climate change and human activities, they have become highly valuable model organisms in the fields of ecology and ecotoxicology [4,5]. However, there are still significant gaps in our knowledge of these crucial organisms. Firstly, their taxonomic system remains unstable. Based on morphological characteristics, the classification system of Huys and Boxshall divides copepods into 10 orders [2]. However, the phylogenetic relationships among the entire subclass Copepoda and its various groups are still controversial [2]. For instance, Khodami et al. reclassified the order Poecilostomatoida into Cyclopoida, while some families formerly in Poecilostomatoida were assigned to the suborder Ergasilida within Cyclopoida [6]. Secondly, the research focus is heavily skewed. The majority of research on marine symbiotic copepods has focused on the parasitic copepods of fish, particularly genera Caligus Müller O.F., 1785 and Lepeophtheirus von Nordmann, 1832, commonly known as sea lice, which pose significant threats to aquaculture [7,8]. In contrast, relatively scant studies have been carried out regarding the symbiotic biology of copepods that associate with invertebrates [9]. This narrow focus substantially limits a comprehensive understanding of the diversity and ecological functions of these invertebrate-symbiotic groups.
Mollusks are important hosts for symbiotic copepods, with hosts spanning polyplacophorans, bivalves, scaphopods, gastropods, and cephalopods. It has been known for more than a century that mollusks are appropriate hosts for copepods [10]. Over 430 mollusk species serve as hosts, with the majority belonging to bivalves [11]. According to Boxshall and O’Reilly, around 280 copepod species are known to act as parasites or associates with mollusks, and an overwhelming proportion of these are marine species within the order Cyclopoida [12]. As a form of symbiosis, parasitism in molluscan copepods manifests in two primary types: ectoparasitism and endoparasitism [13]. Most of these copepods have cyclopiform or only slightly modified bodies, which applies particularly to ectoparasitic forms found on the gills and in the mantle cavity of the mollusks. In contrast, the more modified forms have elongate bodies, often with reduced segmentation and appendages, and in many cases, they inhabit the intestinal tracts of their hosts [14]. With the development of semi-intensive, intensive brackish water and marine aquaculture, the importance of parasitic copepods as disease-causing agents is becoming more and more obvious [15]. Consequently, understanding the biology of parasites is not only critical for advancing ecological knowledge, but also essential as a prerequisite for the conservation of host populations [16]. However, there are still many gaps in the taxonomic research on symbiotic copepods of mollusks. So far, the copepods of mollusks have only been summarized in specific sea areas of some countries, such as Korea and Japan, but a global review of these symbiotic copepods is currently lacking. In order to understand the species compositions and host distributions of copepod species, and enrich the data of marine biodiversity, we review the literature and summarize the copepod species of mollusks in a table.
2. Methods
A scoping review was conducted to synthesize existing knowledge on symbiotic copepods of mollusks in the global oceans. Databases were searched using a combination of the terms “mollus*”, “Bivalv*”, “Cephalopod*”, “Gastropod*”, “Polyplacophora*”, “Monoplacophora*”, “Aplacophora*”, “Scaphopod*”, “parasit*”, “symbio*”, “associ*” and “copepod*”. For each search result obtained, the abstract and introduction were reviewed to determine relevance. In addition to the databases, gray literature from university theses and conference presentations was searched.
The data are presented as a table compiled from the literature. The symbiotic copepods are presented in alphabetical order within the categories of order, family, genus, and species, with records of their hosts, geographical locations, and references.
3. Results
The symbiotic copepods with mollusks recorded in this study comprise 342 species. These species are classified into five orders, 28 families, and 86 genera, including one unidentified order and one unidentified family. Six additional copepod specimens remained unidentified at the species level (Table 1; Supplementary Materials).

Table 1.
Taxon of copepods symbiotic with mollusks in the global oceans.
Cyclopoida includes 17 families and 65 genera; Siphostomatoida includes five families and 11 genera; Harpacticoida includes four families and seven genera; and Monstrilloida includes one family and two genera. These copepods have a symbiotic relationship with most groups of mollusks, including polyplacophorans, bivalves, scaphopods, gastropods, and cephalopods. Among these hosts, bivalves constitute the most common host group, with over 205 species identified, followed by approximately 165 species of gastropods and about 65 species from other molluscan classes. Among these symbiotic copepods, Cyclopoida and Siphostomatoida are primarily associated with bivalves; Harpacticoida is found predominantly in cephalopods; and Monstrilloida occurs mainly in gastropods.
Based on the geographical distributions of copepods symbiotic with mollusks, a collection area map (as depicted in Figure 1) was constructed. Through this map, the specific geographical locations of the symbiotic copepods can be directly discerned. Following the classification scheme by Spalding et al., the marine ecoregions are divided into 12 realms [183,184]. Copepods symbiotic with mollusks are widely distributed across diverse ecosystems and exhibit a global geographic range (Figure 2). The majority of documented observations are concentrated in the Temperate Northern Pacific (114 species) and the Temperate Northern Atlantic (79 species), while research data from regions such as the Temperate Southern Africa (one species) and the Arctic (two species) remain relatively scarce. In this paper, the Cyclopoida is the dominant order (295 species), followed by the Harpacticoida (19 species), Siphonostomatoida (17 species), Monstrilloida (four species), and one unidentified order (one species). In addition, six specimens remained unidentified at the species level (Figure 3). Among the symbiotic copepods, the order Cyclopoida accounts for the largest proportion and the order Monstrilloida has the smallest proportion.
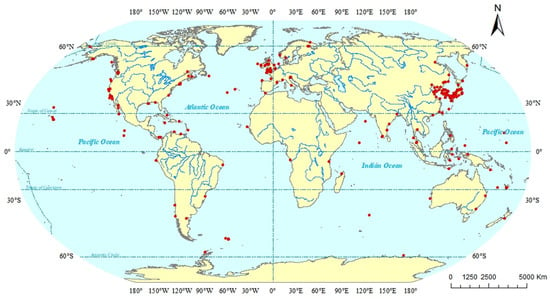
Figure 1.
Distribution map of sampling sites. Note: Records without coordinates (data not available) not marked on the map.
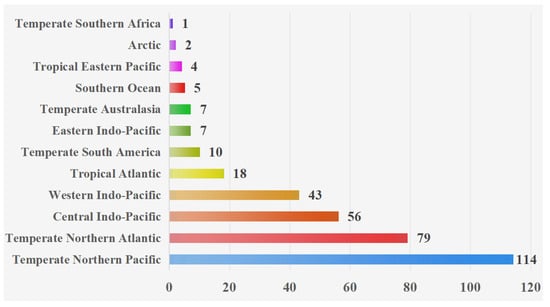
Figure 2.
The number of symbiotic copepods of mollusks in various realms. Note: Species without clearly defined realms are not represented in the diagram.
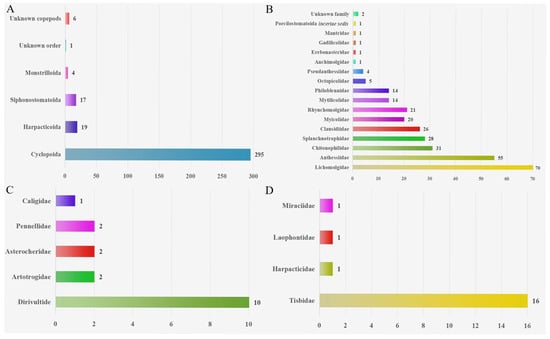
Figure 3.
(A): Order-level distribution of symbiotic copepods. (B): Composition of families in Cyclopoida. (C): Composition of families in Siphonostomatoida. (D): Composition of families in Harpacticoida.
4. Discussion
Copepods are usually small and inconspicuous aquatic crustaceans, but they are extremely numerous. Due to the economic value of hosts, most studies on marine symbiotic copepods in the world have mainly focused on copepods of fish, while relatively few studies have been conducted on the copepods symbiotic with invertebrates [9].
In this review, symbiotic copepods of mollusks are found in 42 countries (as shown in Table 1). Among them, the copepods of mollusks from Japan account for the largest proportion, which may be related to its geographical location and economic condition. Japan is bordered by the Pacific Ocean to the east and the Sea of Japan to the west, which provides this country with abundant fishery resources and important sea transportation routes. Consequently, it has certain advantages in the research on copepods symbiotic with mollusks compared with other countries. Symbiotic copepods are distributed in Pacific Ocean, Atlantic Ocean, Indian Ocean and Arctic Ocean (as shown in Table 1 and Figure 1). Among them, the largest number of known symbiotic copepod species has been found in the Pacific Ocean, while the fewest have been found in the Arctic Ocean. This low diversity in the Arctic Ocean is likely related to its harsh climatic conditions. Due to the cold climate in the Arctic Ocean, scientific research work is rarely carried out.
Copepods symbiotic with mollusks are predominantly found in the Temperate Northern Pacific and the Temperate Northern Atlantic. This distribution pattern may be attributed to the complex coastlines of these regions, which feature diverse habitat types. The high habitat heterogeneity provides a wider range of ecological niches and host options for different symbiotic copepods. Moreover, these areas are home to many developed nations (e.g., Japan and the United States) and developing nations (e.g., China). These countries possess a long-standing tradition in oceanographic research, substantial funding, and well-established scientific institutions. These factors collectively contribute to the particularly rich species records in these regions. In contrast, the scarcity of research data from the Temperate Southern Africa and the Arctic may be due to limited research resources and marine science infrastructure in coastal countries of the southern temperate zone, leading to a lack of systematic marine biological surveys. The extreme environmental conditions in the Arctic, characterized by high sampling difficulty, prohibitive costs, and extensive sea ice cover for most of the year, further restrict fieldwork opportunities.
The four orders of Copepoda (Cyclopoida, Harpacticoida, Siphonostomatoida and Monstrilloida) have been reported [14,176,177]. Among the symbiotic copepods, the order Cyclopoida accounts for the largest proportion (86.09%). The remaining groups are Harpacticoida (5.63%), Siphonostomatoida (5.02%) and Monstrilloida (1.19%) (Figure 3). The reasons may be that the biological characteristics of cyclopoid copepods make them more suitable for living in molluscan hosts, and may also be that copepods of other orders have not been thoroughly discovered. In addition, compared with the more than 100,000 species of marine mollusks worldwide [185], a large number of molluscan hosts remain to be examined, which will facilitate the discovery of more unknown species. Several symbiotic copepod taxa exhibit distinct host specificity. For example, Cholidya polypi Farran, 1914 is currently known to be associated with cephalopods, with no records from hosts of other classes [171], thus demonstrating strict host specificity. In contrast, Pseudomyicola spinosus (Raffaele and Monticelli, 1885) is a symbiont associated with over 50 species of bivalves [114], exhibiting a broad host range. It is possible that the few symbionts found in some molluscan groups are due to inadequate research, thereby underestimating their symbiotic diversity. Some copepod groups that associate with only a few molluscan hosts may have evolved highly specialized attachment structures or life cycles, restricting them to utilizing specific host types.
There are also relevant reports on the impact of symbiotic copepods, particularly those that are parasitic on mollusks. Ho and Zheng described Ostrincola koe Tanaka, 1961 as the primary cause of mass mortalities of the cultured hard clam Meretrix meretrix (Linnaeus), which occurred in 1988 and 1989 in China [186]; Pectinophilus ornatus Nagasawa et al., 1988, a pathogen in northern Japan, parasitizes the gills of the Japanese scallop Mizuhopecten yessoensis (Jay), reaching infection rates of up to 100% in young scallops [136,187]; Mytilicola intestinalis Stuer, 1902, a copepod parasite of the gut of mussels, is endemic along European coasts and has been responsible for heavy mortalities [188]; Mytilicola orientalis Mori, 1935 can damage the inner wall of the intestine of Magallana gigas (Thunberg), leading to the death of oysters and a consequent reduction in production [189]. Currently, the marine biological diseases resulting from parasitic copepods are becoming increasingly severe and meriting greater attention. To undertake this task effectively, researchers must be well-versed in the latest developments in parasitology-related disciplines, including biology, ecology, phylogeny, and zoogeography. It is essential to promote international collaboration among experts from various countries to conduct more in-depth research on these significant symbiotic copepods and effectively prevent the diseases they cause.
5. Conclusions
In summary, this review offers a comprehensive survey of the species of symbiotic copepods of mollusks in the global oceans. To date, 342 species of these symbiotic copepods have been identified within more than 435 molluscan hosts. Bivalves are the most common host group. Among the symbiotic copepods, the majority (86.25%) belong to Cyclopoida, while the remaining portions consist of Harpacticoida (5.55%), Siphonostomatoida (4.97%) and Monstrilloida (1.19%). The Temperate Northern Pacific (especially the waters around Japan) contains the most known symbiotic copepod species, while the Temperate Southern Africa contains the fewest. This pattern likely reflects disparities in research effort, not actual biodiversity. The review also details the host species, geographical locations and compiles a bibliography of symbiotic copepods, thereby extending our comprehension of these organisms. It is highly probable that more species of symbiotic copepods as well as a richer diversity of species will be discovered in the future. Nevertheless, our understanding of the impacts of these symbiotic copepods on their molluscan hosts remains limited. The majority of species have been reported to do harm to the economically significant mollusks. It is imperative to highlight that further research and exploration are essential to enhance our understanding and to devise strategies for the prevention and control of the threats posed by these symbiotic associations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani16020212/s1, Description of the dataset with specific information relative to copepod symbiotic with mollusks, order, suborder, family, subfamily, genus, hosts, site of infection, geographical locations [dates] and references.
Author Contributions
J.S.: conceptualization, data curation, formal analysis, investigation, writing—original draft. H.J.: conceptualization, data curation, formal analysis, investigation, writing—review and editing. X.D.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing. C.X.: data curation, investigation, methodology, supervision, writing—review and editing. M.S.C.: data curation, investigation, methodology, writing—review and editing. Z.L.: data curation, investigation, methodology, writing—review and editing. X.L.: conceptualization, data curation, formal analysis, investigation, methodology, software, supervision, validation, visualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Hebei Province (No. D2025204008); the Science Research Project of Hebei Education Department (No. KY2025010); the Scientific Research Project for Talented Scholars of Hebei Agricultural University (No. YJ2020020); the Innovation and Entrepreneurship Project of the Ocean College, Hebei Agricultural University (No. 2021KY15); and the National Natural Science Foundation of China (No. 32300422).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
Thanks are expressed for the researchers who obtained and published the data adopted in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mauchline, J. The Biology of Calanoid Copepods; Academic Press: San Diego, CA, USA, 1998; pp. 1–721. [Google Scholar]
- Huys, R.; Boxshall, G.A. Copepod Evolution; Ray Society: London, UK, 1991; pp. 1–468. [Google Scholar]
- World Copepods Database. Available online: http://www.marinespecies.org/copepoda/ (accessed on 1 July 2013).
- Feng, J.; Mazzei, M.; Di Gregorio, S.; Niccolini, L.; Vitiello, V.; Ye, Y.; Guo, B.; Yan, X.; Buttino, I. Marine copepods as a microbiome hotspot: Revealing their interactions and biotechnological applications. Water 2023, 15, 4203. [Google Scholar] [CrossRef]
- Vakati, V.; Fuentes-Reinés, J.M.; Wang, P.; Wang, J.; Dodsworth, S. A summary of Copepoda: Synthesis, trends, and ecological impacts. J. Oceanol. Limnol. 2023, 41, 1050–1072. [Google Scholar] [CrossRef]
- Khodami, S.; Mercado-Salas, N.F.; Tang, D.; Martinez Arbizu, P. Molecular evidence for the retention of the Thaumatopsyllidae in the order Cyclopoida (Copepoda) and establishment of four suborders and two families within the Cyclopoida. Mol. Phylogenet. Evol. 2019, 138, 43–52. [Google Scholar] [CrossRef]
- Costello, M.J. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 2006, 22, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, J.; Gonzalez, L.; George-Nascimento, M. Native sea lice (Copepoda: Caligidae) infestation of salmonids reared innetpen systems in southern Chile. Aquaculture 1998, 166, 241–246. [Google Scholar] [CrossRef]
- O’Reilly, M.G. A guide to polychaete-infesting copepods from British waters. Porc. Newsl. 1991, 5, 63–70. [Google Scholar]
- Stock, J.H. On Copepoda associated with Dutch molluscs. Basteria 1965, 29, 65–71. [Google Scholar]
- Humes, A.G. How many copepods? Hydrobiologia 1994, 292, 1–7. [Google Scholar] [CrossRef]
- Boxshall, G.A.; O’Reilly, M. The first parasitic copepod from a scaphopod mollusc host. System. Parasitol. 2015, 90, 113–124. [Google Scholar] [CrossRef]
- Roberts, L.S.; Janovy, J. Foundations of Parasitology, 6th ed.; The McGraw-Hill Companies: New York, NY, USA, 2000; 670p. [Google Scholar]
- Boxshall, G. Cructacean parasites. In Marine Parasitology; Rohde, K., Ed.; CSIRO Publishing: Melbourne, VIC, Australia, 2005; pp. 129–130. [Google Scholar]
- Johnson, S.C.; Treasurer, J.W.; Bravo, S.; Nagasawa, K.; Kabata, Z. A review of the impact of parasitic copepods on marine aquaculture. Zool. Stud. 2004, 43, 229–243. [Google Scholar]
- Nichols, E.; Gómez, A. Conservation education needs more parasites. Biol. Conserv. 2011, 144, 937–941. [Google Scholar] [CrossRef]
- Humes, A.G.; Boxshall, G.A. Poecilostome copepods associated with bivalve molluscs and a brachiopod at Hong Kong. J. Nat. Hist. 1988, 22, 537–544. [Google Scholar] [CrossRef]
- Humes, A.G. Cyclopoid copepods associated with Tridacnidae (Mollusca, Bivalvia) at Eniwetok Atoll. Proc. Biol. Soc. Wash. 1971, 84, 345–358. [Google Scholar]
- Humes, A.G. Cyclopoid copepods associated with marine bivalve mollusks in New Caledonia. Cah. L’office Rech. Sci. Tech. Outre-Mer (ORSTOM) Série Océanographie 1973, 6, 3–25. [Google Scholar]
- Ho, J.S. Cladistics of the Anthessius Della Valle, 1880: A major genus of poecilostome copepods parasitic in Mollusca. Phuket Mar. Biol. Cent. Spec. Publ. 1997, 17, 483–493. [Google Scholar]
- Humes, A.G.; Stock, J.H. Three new species of Anthessius (Copepoda, Cyclopoida, Myicolidae) associated with Tridacna from the Red Sea and Madagascar. Bull. Sea Fish. Res. Stat. Israel. 1965, 40, 49–74. [Google Scholar]
- Moles, J.; Avila, C.; Kim, I.H. Anthessius antarcticus n. sp. (Copepoda: Poecilostomatoida: Anthessiidae) from Antarctic waters living in association with Charcotia granulosa (Mollusca: Nudibranchia: Charcotiidae). J. Crustac. Biol. 2015, 35, 97–104. [Google Scholar] [CrossRef]
- López-González, P.J.; Conradi, M.; Naranjo, S.; García-Gómez, J.C. A new species of Anthessius (Copepoda: Poecilostomatoida) associated with Berthella stellata (Risso, 1826) (Gastropoda: Opisthobranchia). Proc. Biol. Soc. Wash 1992, 105, 240–248. [Google Scholar]
- Monod, T.; Dollfus, R.P. Les Copépodes parasites de mollusques. Ann. Parasitol. 1932, 10, 129–204. [Google Scholar] [CrossRef]
- Kim, I.H. Poecilostomatoid copepods associated with bivalves in Korea and their distribution. Zool. Stud. 2004, 43, 187–192. [Google Scholar]
- Hashimoto, R.; Uyeno, D. First specimen based record of Anthessius cucullatus (Copepoda: Cyclopoida: Anthessiidae) associated with Aplysia spp. (Gastropoda: Aplysiida: Aplysiidae) from coastal waters of Kagoshima, Southern Japan. Species Divers. 2023, 28, 189–197. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.H. Three new species of Anthessius (Copepoda, Cyclopoida, Anthessiidae) associated with mollusks. Anim. Syst. Evol. Divers. 2021, 37, 187–204. [Google Scholar]
- Humes, A.G. Cyclopoid copeopods associated with Tridacnidae (Mollusca, Bivalvia) in the Moluccas Indonesia. Proc. Biol. Soc. Wash. 1976, 89, 491–508. [Google Scholar]
- Humes, A.G.; Ho, J.S. New species of the genus Anthessius (Copepoda, Cyclopoida) associated with mollusks in Madagascar. Cah. L’office Rech. Sci. Tech. Outre-Mer (ORSTOM) Série Océanographie 1965, 3, 79–113. [Google Scholar]
- Illg, P.L. Marine copepods of the genus Anthessius from the Northeastern Pacific Ocean. Pac. Sci. 1960, 14, 337–372. [Google Scholar]
- Ueda, H.; Nagai, H.; Hibino, M.; Tanaka, M. Redescription of a symbiotic poecilostomatoid copepod Anthessius graciliunguis Do and Kajihara from plankton: The second record of the species and first record of the male. Plankton Benthos Res. 2006, 1, 102–108. [Google Scholar] [CrossRef]
- Kim, I.H.; Sato, S. A review of copepods associated with bivalves in Japan, with description of two new species (Crustacea, Copepoda, Cyclopoida). Bull. Tohoku Univ. Mus. 2010, 9, 1–22. [Google Scholar]
- Do, T.T.; Kajihara, T. Two poecilostomatoid copepods, Anthessius graciliunguis n. sp. and Modiolicola bifidus Tanaka, 1961 from the blue mussel, Mytilus edulis galloprovincialis Lamarck, in Japan. Fish Pathol. 1984, 19, 5–15. [Google Scholar] [CrossRef]
- Uyeno, D.; Nagasawa, K. New species of the copepod genus Anthessius Della Valle, 1880 (Poecilostomatoida: Anthessiidae) from Turbo marmoratus Linnaeus (Gastropoda: Turbinidae) collected during the KUMEJIMA 2009 Expedition. Zootaxa 2012, 3367, 60–68. [Google Scholar] [CrossRef]
- Suh, H.L. Anthessius kimjensis, a new species of Anthessiidae (Copepoda; Poecilostomatoida) associated with the pelecypod Solen grandis Dunker in Korea. Hydrobiologia 1993, 259, 187–193. [Google Scholar] [CrossRef]
- Humes, A.G. New poecilostomatoid copepod (Anthessiidae) associated with the opisthobranch Discodoris heathi off California. Beaufortia 1997, 47, 163–169. [Google Scholar]
- Ho, J.S.; Kim, I.H. Copepod parasites of Gastropoda from Korea. Korean J. Zool. 1992, 35, 240–255. [Google Scholar]
- Stock, J.H. Copepoda associated with Neapolitan Mollusca. Publ. Staz. Zool. Napoli 1959, 31, 43–58. [Google Scholar]
- Reddiah, K. Copepods associated with Indian mollusca. Anthessius mytilicolus n. sp. from Mytilus viridis at Ennore. J. Mar. Biol. Ass. India 1966, 8, 290–294. [Google Scholar]
- Wilson, C.B. Parasitic copepods from the Pacific Coast. Am. Midl. Nat. 1935, 16, 776–797. [Google Scholar] [CrossRef]
- Kim, I.H. Poecilostome copepods (Crustacea: Cyclopoida) associated with marine invertebrates from tropical waters. Korean J. Syst. Zool. Spec. Iss. 2009, 7, 1–90. [Google Scholar]
- Ho, J.S. A new species of copepod associated with Pleurobranchaea californica (Gastropoda: Opisthobranchia) with discussion on Anthessius associated with notaspidean sea slugs. Veliger 1983, 25, 393–398. [Google Scholar]
- Tanaka, O. On copepods associated with marine pelecypods in Kyushu. J. Fac. Agric. Kyushu Univ. 1961, 11, 249–273. [Google Scholar] [CrossRef]
- Devi, S.L. A new species of copepod Anthessius placunae from the gills of window-pane oyster Pracenta pracenta (Linneaus). J. Mar. Biol. Assoc. India 1979, 21, 143–146. [Google Scholar]
- Della, V.A. Sui Coriceidi parassiti, e sull’anatomia del gen. Lichomolgus. Mitt. Zool. Stat. Neapel 1881, 2, 83–106. [Google Scholar]
- Claus, C. Ueber neue oder wenig bekannte halbparasitische Copepoden insbesondere der Lichomolgiden-und Ascomyzontiden-Gruppe. Arb. Zool. Inst. Univ. Wien 1889, 8, 327–370. [Google Scholar]
- Stock, J.H. Copepoda Poecilostomatoida associated with Bivalvia from New Guinea. Hydrobiologia 1995, 312, 37–45. [Google Scholar] [CrossRef]
- Stock, J.H.; Humes, A.G.; Gooding, R.U. Copepoda associated with west Indian invertebrates-III. The genus Anthessius (Cyclopoida, Myicolidae). Stud. Fauna Cur. 1963, 17, 1–37. [Google Scholar]
- Stock, J.H. Sur quelques copépodes associés aux invertébrés des côtes du Roussillon. Crustaceana 1960, 1, 218–257. [Google Scholar] [CrossRef]
- Izawa, K. Two new parasitic copepods (Cyclopoida: Myicolidae) from Japanese gastropod molluscs. Publ. Seto Mar. Biol. Lab. 1976, 23, 213–227. [Google Scholar] [CrossRef]
- Uyeno, D. Two new species of Panaietis (Copepoda: Cyclopoida: Anthessiidae) associated with vetigastropods (Gastropoda) in coastal waters of southern Japan. Zootaxa 2019, 4652, 135–144. [Google Scholar] [CrossRef]
- Uyeno, D. Copepods (Cyclopoida) associated with top shells (Vestigastropoda: Trochoidea: Tegulidae) from coastal waters in southern Japan, with descriptions of three new species. Zootaxa 2016, 4200, 109–130. [Google Scholar] [CrossRef]
- Ho, J.S. Parasitic Copepoda of gastropods from the Sea of Japan. Annu. Rep. Sado Mar. Biol. Stn. Niigata Univ. 1981, 11, 23–42. [Google Scholar]
- Lalitha, D.S.; Bhavanarayana, P.V. Conchyliurus bhimilensis n. sp. (Copepoda) from a bivalve mollusc Meretrix casta (Cherm) off Bhimynipatnam. Curr. Sci. 1976, 45, 675–676. [Google Scholar]
- Reddiah, K. Copepods associated with Indian mollusks (B). Description of two new Conchyliurus species from Meretrix meretrix (L.). Crustaceana 1961, 2, 300–312. [Google Scholar] [CrossRef]
- Reddiah, K. Copepods associated with Indian mollusks (A). Description of Conchyliurus maximus sp. nov., (Cyclopoida-Clausidiidae) from Sanguinolaria (Soletellina) diphos (Gmelin) (Lamellibranchiata-Psammobiidae). J. Zool. Soc. India 1961, 12, 137–146. [Google Scholar]
- Pillai, N.K. Copepods associated with South Indian invertebrates. Proc. Indian Acad. Sci. 1963, 58, 235–247. [Google Scholar] [CrossRef]
- Ho, J.S.; Kim, I.H. Copepod parasites of a commercial clam (Meretrix meretrix) from Phuket, Thailand. Hydrobiologia 1995, 308, 13–21. [Google Scholar] [CrossRef]
- Holmes, J.M.C. Records of some interesting copepods belonging to the Clausidiidae, a family new to Ireland. Irish Nat. J. 1986, 22, 30–32. [Google Scholar]
- Gooding, R.U. On some Copepoda from Plymouth, mainly associated with invertebrates, including three new species. J. Mar. Biol. Assoc. UK 1957, 36, 195–221. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kim, I.H. Description of Conchyliurus dispar n. sp. (Copepoda, Cyclopoida, Clausidiidae) associated with the bivalve Barnea manilensis (Philippi) from the Yellow Sea with a discussion of the male morphotypes in the genus. Zootaxa 2014, 3760, 471–478. [Google Scholar] [CrossRef]
- Kim, I.H. Two new species of poecilostomatoid copepods associated with the bivalve Dosinella penicillata in the Yellow Sea. Korean J. Biol. Sci. 1997, 1, 15–23. [Google Scholar] [CrossRef]
- Humes, A.G.; Cressey, R.F. Copepod parasites of mollusks in West Africa. Bull. Inst. Fr. Afr. Noire A 1958, 20, 921–942. [Google Scholar]
- Ho, J.S.; Kim, I.H. Copepod parasites of commercial bivalves in Korea II. Copepods from cultured bivalves. Bull. Korean Fish. Soc. 1991, 24, 369–396. [Google Scholar]
- Kim, I.H. Copepodid stages of Conchyliurus quintus Tanaka, 1961 (Poecilostomatoida, Clausidiidae) associated with bivalve mollusks. Hydrobiologia 1994, 292, 161–169. [Google Scholar] [CrossRef]
- Du, X.; Sun, S.C. Studies on cyclopoid copepods parasitic in bivalvian mollusks from the Yellow Sea and the Bohai Sea. Period. Ocean Univ. China 2022, 52, 42–55, (In Chinese with English Abstract). [Google Scholar]
- Bocquet, C.; Stock, J.H. Copépodes parasites d’invertébrés des côtes de France. I. Sur deux genres de la famille des Clausidiidae, commensaux de mollusques: Hersiliodes Canu et Conchyliurus nov. gen. Proc. Kon. Ned. Akad. Wet. Ser. C 1957, 60, 212–222. [Google Scholar]
- Bocquet, C.; Stock, J.H. Copépodes parasites d’invértébres des côtes de France. II. Notes taxonomiques et écologiques sur la famille des Mytilicolidae. Proc. Kon. Ned. Akad. Wet. Ser. C 1957, 60, 223–232. [Google Scholar]
- Bocquet, C.; Stock, J.H. Copépodes parasites d’invértébres des côtes de France. III. Notes taxonomiques et écologiques sur la famille des Mytilicolidae. Proc. Kon. Ned. Akad. Wet. Ser. C 1957, 60, 233–239. [Google Scholar]
- Pelseneer, P. Copépodes parasites de mollusques. Soc. R. Zool. Belg. 1929, 59, 33–49. [Google Scholar]
- Kim, I.H.; Stock, J.H. A new species of Clausididae (Copepoda, Poecilostomatoida) associated with the bivalve Ruditapes philippinarum in Korea. Cah. Biol. Mar. 1996, 37, 1–6. [Google Scholar]
- Defaye, D.; Toda, T. A new species of Hyphalion (Copepoda, Poecilostomatoidea, Clausidiidae) from off North Peru, in the Eastern Pacific. Bull. Mus. Natl. Hist. Nat. Paris 4e Sér. 1994, 16, 87–94. [Google Scholar]
- Kim, I.H.; Ho, J.S. Copepod parasites of commercial bivalves from Korea I. Two new poecilostomatoid species from Solen grandis Dunker in the Yellow Sea. Anim. Syst. Evol. Divers. 1991, 7, 1–11. [Google Scholar]
- Allen, J.A. Myocheres inflata a new species of parasitic copepod from the Bahamas. J. Parasitol. 1956, 42, 60–67. [Google Scholar] [CrossRef]
- Pearse, A.S. A second report on parastitic copepods collected at Beaufort, N.C.J. Elisha Mitchell Sci. Soc. 1948, 64, 127–131. [Google Scholar]
- Humes, A.G. Copepodids and adults of Leptinogaster major (Williams, 1907), a poecilostomatoid copepod living in Mya arenaria L. and other marine bivalve mollusks. Fish. Bull. 1986, 84, 227–245. [Google Scholar]
- Williams, L.W. List of the Rhode Island Copepoda, Phyllopoda, and Ostracoda, with new species of Copepoda. Annu. Rep. Comm. Inland Fish. Rhode Isl. 1907, 37, 69–79. [Google Scholar]
- Ho, J.S.; Wardle, W.J. Pholadicola intestinalis, new genus and species, a clausidiid copepod parasitic in a deep-burrowing clam from Texas. Bull. Mar. Sci. 1992, 51, 37–44. [Google Scholar]
- Humes, A.G. A new poecilostomatoid copepod (Erebonasteridae) from deep-sea cold seeps at the West Florida Escarpment. Hydrobiologia 1989, 175, 175–182. [Google Scholar] [CrossRef]
- Humes, A.G.; Segonzac, M. Copepoda from deep-sea hydrothermal sites and cold seeps: Description of a new species of Aphotopontius from the East Pacific Rise and general distribution. Cah. Biol. Mar. 1998, 39, 51–62. [Google Scholar]
- Humes, A.G.; Stock, J.H. A Revision of the Family Lichomolgidae Kossmann, 1877, Cyclopoid Copepods Mainly Associated with Marine Invertebrates; Smithsonian Institution Press: Washington, DC, USA, 1973; pp. 1–365. [Google Scholar]
- Clarke, C.L.; Klussmann-Kolb, A. Parasitic copepods in the sea hare Dolabrifera brazieri (Gastropoda: Opisthobranchia: Anaspidea). J. Mar. Biol. Assoc. UK 2003, 83, 793–796. [Google Scholar] [CrossRef]
- Holmes, J.M.C.; Minchin, D. A new species of Herrmannella (Copepoda, Poecilostomatoida, Sabelliphilidae) associated with the oyster Ostrea edulis L. Crustaceana 1991, 60, 258–269. [Google Scholar] [CrossRef]
- Humes, A.G. Cyclopoid copepods of the genus Paranthessius associated with marine pelecypods in the West Indies. Bull. Mar. Sci. 1970, 20, 605–625. [Google Scholar]
- Herdman, W.A.; Thompson, I.C.; Scott, A. On the plankton collected continuously during two traverses of the North Atlantic in the summer of 1897; with descriptions of new species of copepoda; and an appendix on dredging in Puget Sound. Proc. Trans. Liverp. Biol. Soc. 1897, 12, 33–90. [Google Scholar]
- Lynch, S.A.; Rowley, A.F.; Longshaw, M.; Malham, S.K.; Culloty, S.C. Diseases of molluscs. In Invertebrate Pathology; Rowley, A.F., Coates, C.J., Whitten, M.M., Eds.; Oxford University Press: Oxford, UK, 2022; pp. 171–215. [Google Scholar]
- Lee, J.; Chang, C.Y.; Kim, I.H. Symbiotic copepods (Cyclopoida and Siphonostomatoida) collected by light trap from Korea. ZooKeys 2022, 1115, 1–71. [Google Scholar] [CrossRef]
- Bocquet, C.; Stock, J.H. Copépodes parasites d’invertébrés des côtes de France. X. Sur les espèces de Paranthessius (Cyclopoida, Lichomolgidae) du groupe des Herrmannella, associées à pélécypodes. Proc. Kon. Ned. Akad. Wet. Ser. C 1959, 62, 238–249. [Google Scholar]
- Bocquet, C.; Stock, J.H. Sur la présence de Lichomolgus leptodermatus Gooding, copépode associé au pélécypode Cardium crassum Gmelin, sur les côtes françaises de la Manche. Bull. Soc. Linn. Normandie 1959, 9, 119–120. [Google Scholar]
- Kim, I.H. Herrmannella hoonsooi, a new species of Copepoda (Poecilostomatoida, Sabelliphilidae) associated with a bivalve from Korea. Korean J. Syst. Zool. 1992, 3, 77–84. [Google Scholar]
- Humes, A.G. The copepod genus Herrmannella (Poecilostomatoida) associated with marine bivalve mollusks at Kodiak Island, Alaska. Pac. Sci. 1995, 49, 289–295. [Google Scholar]
- Humes, A.G. Cyclopoid copepods of the genus Paranthessius associated with marine pelecypods in Chile. Proc. United States Natl. Mus. 1967, 124, 1–18. [Google Scholar] [CrossRef]
- Illg, P.L. A review of the copepod genus Paranthessius Claus. Proc. United States Natl. Mus. 1949, 99, 391–428. [Google Scholar] [CrossRef]
- Atkins, D. Two parasites of the common cockle Cardium edule; a rhabdocoele Paravortex cardii Hallez and a copepod Paranthessius rostratus (Canu). J. Mar. Biol. Assoc. UK 1934, 19, 669–676. [Google Scholar] [CrossRef]
- Ho, J.S. Anchicaligus nautili (Willey), a caligid copepod parasitic on Nautilus in Palau, with discussion of Caligulina Heegaard, 1972. Publ. Seto Mar. Biol. Lab. 1980, 25, 157–165. [Google Scholar] [CrossRef]
- Humes, A.G. Two new copepods (Cyclopoida, Lichomolgidae) from marine pelecypods in Madagascar. Crustac. Suppl. 1968, 1, 65–81. [Google Scholar]
- Humes, A.G. The cyclopoid copepod Pseudomyicola spinosus (Raffaele and Monticelli) from marine pelecypods, chiefly in Bermuda and the West Indies. Beaufortia 1968, 14, 203–226. [Google Scholar]
- Kim, I.H. Redescription of two species of Copepoda (Poecilostomatoida, Lichomolgidae) associated with the bivalve Dosinorbis japonicus from the Korea Strait. Korean J. Syst. Zool. 2002, 18, 23–33. [Google Scholar]
- Ko, Y.; Murakami, Y.; Daika, K. The biology of the commensal copepods in Japanese marine bivalves. Rec. Oceanogr. Works Jpn. Spec. 1962, 6, 113–119. [Google Scholar]
- Do, T.T.; Kajihara, T. Studies of parasitic copepod fauna and biology of Pseudomyicola spinosus, associated with blue mussel, Mytilus edulis galloprovincialis in Japan. Bull. Ocean Res. Inst. Univ. Tokyo 1986, 23, 1–63. [Google Scholar]
- Yamaguti, S. Parasitic copepods from mollusks of Japan, I. Jpn. J. Zool. 1936, 7, 113–127. [Google Scholar]
- Jones, J.B. Lichomolgus uncus n. sp. (Copepoda: Cyclopoida) an associate of the mussel Perna canaliculus Gmelin. J. Roy. Soc. New. Zeal. 1976, 6, 301–305. [Google Scholar] [CrossRef]
- Kim, I.H. Modiolicola avdeevi, a new sabelliphilid copepod (Poecilostomatoida) from a bivalve in the Sea of Japan. Anim. Syst. Evol. Divers. 1995, 11, 315–321. [Google Scholar]
- Costanzo, G.; Calafiore, N. Seasonal fluctuation of Modiolicola insignis Aurivillius, 1882 (Copepoda: Poecilostomatoida: Sabelliphilidae), associated with Mytilus galloprovincialis in Lake Faro (Messina). J. Crustac. Biol. 1987, 7, 77–86. [Google Scholar] [CrossRef]
- Kim, I.H. Copepods (Crustacea) associated with marine invertebrates from Great Barier Reef, Australia. Korean J. Syst. Zool. 2004, 20, 109–140. [Google Scholar]
- Reddiah, K.; Williamson, D.I. On Modiolicola inermis Canu and Modiolicola maxima (Thompson), lichomolgid copepods associated with Pectinid lamellibranchs. Ann. Mag. Nat. Hist. 1958, 1, 689–701. [Google Scholar] [CrossRef]
- Aurivillius, C.W.S. Bidrag Till Kännedomen Om Krustaceer, Som Lefva Hos Mollusker Och Tunikater; Öfvers. Kongl. Vet.—Akad. Forh: Stockholm, Sweden, 1882; pp. 41–117. [Google Scholar]
- Thompson, I.C. Revised report on the Copepoda of Liverpool Bay. Proc. Trans. Liverp. Biol. Soc. 1893, 7, 175–230. [Google Scholar]
- Avdeev, G.V. Dva novykh vida kopepod (Sabellipilidae, Poecilostomatoida) ot dvustborchatikh molluskov Zaliva Petra Belikogo Yaponskogo Morya. Zool. Zhurnal 1987, 66, 608–613, (In Russian with English Summary). [Google Scholar]
- Uyeno, D. A checklist of symbiotic copepods from marine mollusks in Japan. Taxa Proc. Jpn. Soc. Syst. Zool. 2020, 49, 23–44. [Google Scholar]
- Hoshina, T.; Kuwabara, R. On a new parasitic copepod, Parapanaietis turbo n. sp. obtained from marine gastropod. Turbo stenogyrus Fischer. J. Tokyo Univ. Fish. 1958, 44, 69–72. [Google Scholar]
- Kim, I.H.; Ohtsuka, S.; Yokosaka, K.; Ito, K. Redescription and taxonomic remarks of the lichomolgid copepod Paraphiloconcha meretricis (Crustacea: Copepoda: Poecilostomatoida) parasitic on the bivalve Meretrix lamarckii from Japan. Species Divers. 2004, 9, 331–341. [Google Scholar] [CrossRef]
- López-González, P.J.; Pascual, S. A new species of Stellicola Kossmann, 1877 (Copepoda, Lichomolgidae) off the Atlantic coast of the Iberian Peninsula. Hydrobiologia 1996, 339, 1–6. [Google Scholar] [CrossRef]
- Ho, J.S. Phylogenetic analysis of the Myicolidae, a family of poecilostome copepods chiefly parasitic in marine bivalve mollusks. Acta Zool. Taiwan. 1992, 3, 67–77. [Google Scholar]
- Humes, A.G. Copépodes parasites de mollusques à Madagascar. Mém. Inst. Sci. Madagascar. 1959, 2, 285–342. [Google Scholar]
- Ho, J.S. Cladistics of Ostrincola (Copepoda), a genus of pests in clam culture. PMBC Spec. Publ. 1998, 18, 237–242. [Google Scholar]
- Ho, J.S.; Liu, W.C.; Lin, C.L. New record of Ostrincola koe Tanaka, 1961 (Copepoda: Poecilostomatoida: Myicolidae) from hard clam (Meretrix lusoria) of Taiwan, with key to species of genus Ostrincola Wilson, 1944. J. Fish. Soc. Taiwan 2012, 39, 137–148. [Google Scholar]
- Lin, C.L.; Ho, J.S. Poecilostomatoid copepods parasitic in bivalve mollusks of Taiwan. Publ. Seto Mar. Biol. Lab. 1999, 38, 201–218. [Google Scholar] [CrossRef]
- Ho, J.S.; Kim, I.H. Ostrincola breviseti n. sp., a copepod parasite of an oyster from Penang, Malaysia. Beaufortia 1990, 41, 91–95. [Google Scholar]
- Humes, A.G. Ostrincola and Pseudomyicola (Crustacea, Copepoda, Poecilostomatoida) associated with marine bivalve mollusks on the Pacifc coast of Panama. Proc. Biol. Soc. Wash. 1984, 97, 589–600. [Google Scholar]
- Wilson, C.B. Parasitic copepods in the United States National Museum. Proc. United States Natl. Mus. 1944, 94, 529–582. [Google Scholar] [CrossRef]
- Ho, J.S.; Yoosukh, W. Ostrincola humesi sp. nov., a myicolid copepod (Poecilostomatoida) parasitic in rock oysters cultured in the Gulf of Thailand. Hydrobiologia 1994, 288, 151–155. [Google Scholar] [CrossRef]
- Reddiah, K. Copepods associated with Indian mollusks (C). Ostrincola portonoviensis n. sp. from commercial bivalves at Portonovo, S. India. Crustaceana 1962, 4, 1–6. [Google Scholar] [CrossRef]
- Ho, J.S.; Kim, I.H. A new genus of poecilostome copepod of the family Myicolidae parasitic in a commercial clam from Malaysia. J. Nat. Hist. 1992, 26, 303–309. [Google Scholar] [CrossRef]
- Cáceres-Martínez, C.; Chávez-Villalba, J.; Garduño-Méndez, L. First record of Pseudomyicola spinosus in Argopecten ventricosus in Baja California, Mexico. J. Invertebr. Pathol. 2005, 89, 95–100. [Google Scholar] [CrossRef]
- Raffaele, F.; Monticelli, F.S. Descrizione di un nuvov Lichomogus parasita del Mytilus galloprovincialis Lk. Atti. Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1885, 1, 302–307. [Google Scholar]
- Stock, J.H. The names of certain cyclopoid copepods associated with invertebrates in the Black Sea. Crustaceana 1969, 17, 220–222. [Google Scholar] [CrossRef]
- Yamaguti, S. A new commensal copepod from Brachiodontes senhausi (Reeve). Trans. Am. Microsc. Soc. 1939, 58, 371–373. [Google Scholar] [CrossRef]
- Humes, A.G.; Ho, J.S. Mytilicola fimbriatus sp. n., a cyclopoid copepod parasitic in the marine pelecypod, Arca decussata, in Madagascar. J. Parasitol. 1970, 56, 584–587. [Google Scholar] [CrossRef]
- Steele, S.; Mulcahy, M.F. Impact of the copepod Mytilicola orientalis on the Pacific oyster Crassostrea gigas in Ireland. Dis. Aquat. Organ. 2001, 47, 145–149. [Google Scholar] [CrossRef]
- Humes, A.G. Mytilicola porrecta n. sp. (Copepoda: Cyclopoida) from the intestine of marine pelecypods. J. Parasitol. 1954, 40, 186–194. [Google Scholar] [CrossRef]
- Mori, T. Mytilicola orientalis, a new species of parasitic Copepoda. Zool. Soc. Jpn. 1935, 47, 687–689. [Google Scholar]
- Nagasawa, K.; Nitta, M. Rediscovery of Mytilicola orientalis (Copepoda: Mytilicolidae) from wild Pacific oysters Crassostrea gigas in Japan. Biogeography 2014, 16, 49–51. [Google Scholar]
- Chew, K.K.; Sparks, A.K.; Katkansky, S.C. First record of Mytilicola orientalis Mori in the California mussel Mytilus californianus Conrad. J. Fish. Board Can. 1964, 21, 205–207. [Google Scholar] [CrossRef]
- Goater, C.P.; Weber, A.E. Factors affecting the distribution and abundance of Mytilicola orientalis (Copepoda) in the mussel, Mytilus trossulus, in Barkley Sound, B.C.J. Shellfish Res. 1997, 15, 681–684. [Google Scholar]
- Nagasawa, K.; Bresciani, J.; Lützen, J. Morphology of Pectenophilus ornatus, new genus, new species, a copepod parasite of the Japanese scallop Patinopecten yessoensis. J. Crustac. Biol. 1988, 8, 31–42. [Google Scholar] [CrossRef]
- Nagasawa, K. The biology of the parasitic copepod, Pectenophilus ornatus, of pectinid bivalves in Japan: An overview. Biogeography 1999, 1, 3–18. [Google Scholar]
- Shimura, S.; Kuwabara, R. Trochicola japonicus sp. nov., a mytilicolid copepod parasitic in the short neck clam (Tapes philippinarum) from Lake Hamana, Japan. Fish Pathol. 1984, 18, 191–197. [Google Scholar] [CrossRef]
- Du, X.; Dong, C.; Sun, S.C. Octopicola huanghaiensis n. sp. (Copepoda: Cyclopoida: Octopicolidae), a new parasitic copepod of the octopuses Amphioctopus fangsiao (d’Orbigny) and Octopus minor (Sasaki) (Octopoda: Octopodidae) in the Yellow Sea. System. Parasitol. 2018, 95, 905–912. [Google Scholar] [CrossRef]
- Humes, A.G. Octopicola stocki n. sp. (Copepoda, Cyclopoida), associated with an Octopus in Madagascar. Crustaceana 1963, 5, 271–280. [Google Scholar] [CrossRef]
- Cavaleiro, F.I.; Ho, J.S.; Iglesias, R.; García-Estévez, J.M.; Santos, M.J. Revisiting the octopicolid copepods (Octopicolidae: Octopicola Humes, 1957): Comparative morphology and an updated key to species. System. Parasitol. 2013, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.H.; Humes, A.G.; Gooding, R.U. Copepoda associated with West Indian invertebrates-IV. The genera Octopicola, Pseudanthessius and Meomicola (Cyclopoida, Lichomolgidae). Stud. Fauna Curaçao Other Caribb. Isl. 1963, 18, 1–74. [Google Scholar]
- Guillén-Hernández, S.; López-Struck, A.; González-Salas, C.; Aguirre-Macedo, M.L. Octopus maya parasites off the yucatán peninsula, Mexico I Faunal assemblages. Dis. Aquat. Org. 2018, 130, 37–43. [Google Scholar] [CrossRef]
- Schwabe, E. First record of a potential philoblennid-polyplacophoran association with the description of Acanthopleuricola sirenkoi, new genus and new species. Spixiana 2021, 44, 145–158. [Google Scholar]
- Uyeno, D.; Ogasaka, R.; Nagasawa, K. Nippoparasitus unoashicola, a new genus and species of philoblennid copepod (Cyclopoida) parasitic on the Pacific sugar limpet, Patelloida saccharina (Patellogastropoda: Lottiidae) from the intertidal zone of eastern Japan. Zootaxa 2016, 4174, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Huys, R. Splanchnotrophid systematics: A case of polyphyly and taxonomic myopia. J. Crustac. Biol. 2001, 21, 106–156. [Google Scholar] [CrossRef]
- Ho, J.S.; Kim, I.H. New species of Doridicola (Copepoda, Rhynchomolgidae) from Thailand, with a cladistic analysis of the genus. J. Crustac. Biol. 2001, 21, 78–89. [Google Scholar] [CrossRef]
- Humes, A.G.; Stock, J.H. Redefinition of the genus Doridicola Leydig, 1853, synonymy of Metaxymolgus Humes and Stock, 1972, and establishment of a new genus, Critomolgus (Copepoda, Poecilostomatoida, Lichomolgidae). Bull. Zool. Mus. 1983, 9, 93–96. [Google Scholar]
- Brunckhorst, D.J. Decription of a new species of Doridocola (Copepoda: Cyclopoida) from a nudibranch host, with notes on copepod-nudibranch associations. Proc. R. Soc. Qld. 1985, 96, 49–59. [Google Scholar]
- Kim, I.H. Two new species of Doridicola (Copepoda: Cyclopoida: Rhynchomolgidae) associated with opisthobranch mollusks from Korea. Korean J. Syst. Zool. 2007, 23, 117–126. [Google Scholar] [CrossRef]
- Anton, R.F.; Schories, D.; Jörger, K.M.; Kaligis, F.; Schrödl, M. Description of four new endoparasitic species of the family Splanchnotrophidae (Copepoda, Poecilostomatoida) from nudibranch and sacoglossan gastropod hosts. Mar. Biodiv. 2016, 46, 183–195. [Google Scholar] [CrossRef]
- Salmen, A.; Wilson, N.G.; Schrödl, M. Scanning electron microscopical description and biology of three new endoparasitic Ceratosomicola species from tropical Indo-Pacific nudibranch hosts (Crustacea, Copepoda, Poecilostomatoida, Splanchnotrophidae). Spixiana 2008, 31, 47–69. [Google Scholar]
- Uyeno, D.; Nagasawa, K. Four new species of splanchnotrophid copepods (Poecilostomatoida) parasitic on doridacean nudibranchs (Gastropoda, Opistobranchia) from Japan, with proposition of one new genus. ZooKeys 2012, 247, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, D.; Kohtsuka, H.; Maeno, A. Ceratosomicola oki n. sp., a new species of Copepod (Cyclopoida: Splanchnotrophidae) parasitic on the chromodoridid nudibranch Glossodoris misakinosibogae Baba, 1988 off the Oki Islands, Japan, with microanatomical observation using micro-CT. Zool. Sci. 2021, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Anton, R.F.; Schrödl, M. The “inner values” of an endoparasitic copepod-computer-based 3D-reconstruction of Ismaila aliena (Copepoda; Poecilostomatoida; Splanchnotrophidae). Spixiana 2013, 36, 183–199. [Google Scholar]
- Belcik, F.P. The male of Ismaila monstrosa Bergh, 1867 (Copepoda, Splanchnotrophidae). Crustaceana 1981, 40, 16–25. [Google Scholar] [CrossRef]
- Uyeno, D.; Hirose, E. Lomanoticola nishiharai n. sp., a new species of copepod parasitic on the facelinid nudibranch, Sakuraeolis enosimensis (Baba, 1930), from the Seto Inland Sea, Western Japan, including histological observations of the female lateral body process. Zool. Sci. 2018, 35, 382–387. [Google Scholar] [CrossRef]
- Abad, M.; Díaz-Agras, G.; Urgorri, V. Anatomical description and biology of the splanchontrophid Splanchnotrophus gracilis Hancock & Norman, 1863 found parasitizing the doridacean nudibranch Trapania tartanella Ihering, 1886 at the Ría de Ferrol (Galicia, NW Iberian Peninsula). Thalassas 2011, 27, 49–60. [Google Scholar]
- Marshall, H.C.; Hayward, P.J. The effects of Splanchnotrophus willemi infecting Ancula gibbosa (Gastropoda: Opisthobranchia: Nudibranchia). J. Mar. Biol. Assoc. UK 2006, 86, 1437–1441. [Google Scholar] [CrossRef]
- Schwabe, E.; Holtheuer, J.; Schories, D. First record of a mesoparasite (Crustacea, Copepoda) infesting a polyplacophoran (Mollusca, Polyplacophora) in Chilean waters, with an overview of the family Chitonophilidae (Crustacea & Mollusca). Spixiana 2014, 37, 165–182. [Google Scholar]
- Avdeev, G.V.; Sirenko, B.I. New and recognized species of copepods (Chitonophilidae)-parasites of chitons of Northern Pacific. Parazitologiia 2005, 39, 516–543. [Google Scholar] [PubMed]
- Nagasawa, K.; Bresciani, J.; Lützen, J. Ischnochitonika japonica, new species (Copepoda), a parasite on Ischnochiton (Ischnoradsia) hakodadensis (Pilsbry) (Polyplacophora: Ischnochitonidae) from the Sea of Japan. J. Crustac. Biol. 1991, 11, 315–321. [Google Scholar] [CrossRef]
- Huys, R.; López-González, P.J.; Roldán, E.; Luque, Á.A. Brooding in Cocculiniform limpets (Gastropoda) and familial distinctiveness of the Nucellicolidae (Copepoda): Misconceptions reviewed from a chitonophilid perspective. Biol. J. Linn. Soc. 2002, 75, 187–217. [Google Scholar] [CrossRef]
- Lamb, E.J.; Boxshall, G.A.; Mill, P.J.; Grahame, J. Postembryonic stages of Nucellicola holmanae Lamb et al., 1996 (Copepoda, Poecilostomatoida), an endoparasite of the dog whelk Nucella lapillus (Gastropoda). J. Mar. Syst. 1998, 15, 261–267. [Google Scholar] [CrossRef]
- Enshina, I.; Krupenko, D.; Kremnev, G.; Miroliubov, A.; Savchenko, A.; Huys, R. New records of Nucellicola Lamb, Boxshall, Mill & Grahame, 1996 (Copepoda: Chitonophilidae) from true whelks (Gastropoda: Buccinidae) in the Russian Arctic. Plankton Benthos Res. 2025, 20, 242–245. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Boxshall, G.A.; Torigoe, K. A new genus and species of the family Mantridae (Copepoda: Cyclopoida) infesting the bivalve Pseudochama retroversa from the Seto Inland Sea, western Japan. J. Nat. Hist. 2000, 34, 1967–1976. [Google Scholar] [CrossRef]
- Avdeev, G.V.; Kurochkin, I.V. Teredoika aspectabilis sp. n., a new endoparasitic copepod from the gastropod mollusc, Clinopegma unicum, in the sea of Okhotsk. Parazitologiia 1977, 11, 544–546. [Google Scholar] [PubMed]
- Huys, R. Harpacticoid copepods—Their symbiotic associations and biogenic substrata: A review. Zootaxa 2016, 4174, 448–729. [Google Scholar] [CrossRef]
- Avdeev, G.V. Amplipedicola pectinatus gen. et sp. n. (Copepoda, Harpacticoida, Tisbidae), a parasite of octopuses in the Bering Sea. Crustaceana 2010, 83, 1363–1370. [Google Scholar] [CrossRef]
- Avdeev, G.V. New harpacticoid copepods associated with Pacific cephalopods. Crustaceana 1986, 51, 49–65. [Google Scholar] [CrossRef]
- Humes, A.G.; Voight, J.R. Cholidya polypi (Copepoda: Harpacticoida: Tisbidae), a parasite of deep-sea octopuses in the North Atlantic and Northeastern Pacific. Ophelia 1997, 46, 65–81. [Google Scholar] [CrossRef]
- López-González, P.J.; Bresciani, J.; Huys, R.; González, Á.F.; Guerra, Á.; Pascual, S. Description of Genesis vulcanoctopusi gen. et sp. nov. (Copepoda: Tisbidae) parasitic on a hydrothermal vent octopod and a reinterpretation of the life cycle of cholidyinid harpacticoids. Cah. Biol. Mar. 2000, 41, 241–253. [Google Scholar]
- Komeda, S.; Ohtsuka, S.; Huys, R. A new genus and species of oceanic planktonic Tisbidae (Crustacea, Copepoda, Harpacticoida) with enlarged modified eyes. ZooKeys 2024, 1191, 307–338. [Google Scholar] [CrossRef] [PubMed]
- McAlice, B.J. On the male of Monstrilla helgolandica Claus (Copepoda, Monstrilloida). J. Crustac. Biol. 1985, 5, 627–634. [Google Scholar] [CrossRef]
- Jeon, D.; Lee, W.; Soh, H.Y. A new genus and two new species of monstrilloid copepods (Copepoda: Monstrillidae): Integrating morphological, molecular phylogenetic, and ecological evidence. J. Crustac. Biol. 2018, 38, 45–65. [Google Scholar] [CrossRef]
- Gejima, K.; Ohtsuka, S.; Konishi, K.; Nagasawa, K. Research on parasitic organisms of mollusks I. The parasitic situation of Pinnotheres sinensis and monstrilloid copepods occurring in bivalves. In Proceedings of the 37th Meeting of the Carcinological Society of Japan, Toba, Japan, 6–7 November 1999. (In Japanese). [Google Scholar]
- Suárez-Morales, E.; Scardua, M.P.; Da Silva, P.M. Occurrence and histopathological effects of Monstrilla sp. (Copepoda: Monstrilloida) and other parasites in the brown mussel Perna perna from Brazil. J. Mar. Biol. Assoc. UK 2010, 90, 953–958. [Google Scholar] [CrossRef]
- Kim, I.H. Copepoda of Artotrogidae (Siphonostomatoida) from the Sea of Japan. Anim. Syst. Evol. Divers. 1996, 12, 397–466. [Google Scholar]
- Ridewood, W.G. On Obesiella lyonsiellae, a new genus of copepod crustacean. Zool. J. Linn. Soc. 1903, 28, 463–465. [Google Scholar] [CrossRef]
- Humes, A.G.; Lutz, R.A. Aphotopontius acanthinus, new species (Copepoda: Siphonostomatoida), from deep-sea hydrothermal vents on the East Pacific Rise. J. Crustac. Biol. 1994, 14, 337–345. [Google Scholar] [CrossRef]
- Perkins, P.S. The life history of Cardiodectes medusaeus (Wilson), a copepod parasite of lanternfishes (Myctophidae). J. Crustac. Biol. 1983, 3, 70–87. [Google Scholar] [CrossRef]
- Wilson, C.B. Description of a new genus and species of copepod parasitic in a shipworm. J. Wash. Acad. Sci. 1942, 32, 60–62. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Korzhavina, O.A.; Gubareva, N.V.; Kitashov, A.V.; Britayev, T.A.; Ivanenko, V.N. From microscale interactions to macroscale patterns in copepod-crinoid symbiosis. Animals 2024, 14, 877. [Google Scholar] [CrossRef]
- Khan, B.M.; Liu, Y. Marine mollusks: Food with benefits. Compr. Rev. Food Sci. Food Saf. 2019, 18, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Zheng, G.X. Ostrincola koe (Copepoda, Myicolidae) and mass mortality of cultured hard clam (Meretrix meretrix) in China. Hydrobiologia 1994, 284, 169–173. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsutani, T. Infection of the parasitic copepod, Pectenophilus ornatus on juvenile Japanese scallop, Patinopecten yessoensis. Aquac. Sci. 2009, 57, 513–514. [Google Scholar]
- Blateau, D.; Le Coguic, Y.; Mialhe, E.; Grizel, H. Mussel (Mytilus edulis) treatment against the red copepod Mytilicola intestinalis. Aquaculture 1992, 107, 165–169. [Google Scholar] [CrossRef]
- Holmes, J.M.C.; Minchin, D. Two exotic copepods imported into Ireland with the Pacific oyster Crassostrea gigas (Thunberg). Irish Nat. J. 1995, 25, 17–20. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.