1. Introduction
The pig industry and the formation of its products play an important role in the development of the global economy and the guarantee of food security. With the improvement of the overall scale and productivity of the pig industry, there is growing interest in how to use low-cost unconventional feeds to formulate diets that meet the maintenance and production needs of pigs, so as to increase the output per unit input [
1,
2]. However, due to their high dietary fiber (DF) content, the proportion of these feeds in the complete diets of pigs can only be used at a limited ratio [
3,
4]. Studies have shown that appropriately increasing dietary fiber levels can improve pigs’ feed conversion rate and growth performance [
5], while also regulating the enhancement of animal welfare and intestinal health [
6,
7]. For example, a moderate increase in dietary fiber exerts positive effects on enhancing pigs’ satiety, reducing stereotypic behaviors, promoting chyme emptying in the intestinal lumen, and alleviating intestinal inflammatory responses [
8,
9,
10]. However, when the proportion of fibrous agricultural and sideline by-products in the diet is further increased, that is, when dietary fiber levels are raised further, it often reduces the effective nutrient concentration of the diet and pigs’ production performance [
11,
12]. Therefore, how to improve the utilization rate of unconventional feeds in pig production without additional feed costs is not only an old issue, but also an important problem that needs to be solved urgently at present.
As the main component of the cell wall of plant crops and their by-products, DF is a class of carbohydrate polymers with multiple properties (nutritive, non-nutritive, and anti-nutritive), and its nutritive property is mainly manifested under the action of intestinal microorganisms. It is currently clear that except for a few DFs (some oligosaccharides and resistant starches) which are degraded at the end of the small intestine, most of them are fermented under the action of hindgut microorganisms to produce metabolites such as short-chain fatty acids, thereby providing energy for the animal [
13,
14]. The anti-nutritive property of DF usually appears when its level is too high, which is reflected in reducing the effective nutrients of feed and the production performance of animals [
11]. The non-nutritive effect of DF is mainly determined by its physical properties, such as solubility and apparent viscosity [
15,
16]. Recent studies have shown that the fermentability of DF is related to its solubility. For example, in vitro fermentation experiments using fiber mixtures with different ratios of soluble dietary fiber (SDF) to insoluble dietary fiber (IDF), composed of purified inulin and non-starch polysaccharide mixtures, found that the fermentability of fiber increases with the increase in the proportion of the SDF [
17]. However, this finding is not completely consistent with the results obtained from animal experiments. Studies have found that the concentration of short-chain fatty acids in the feces of growing pigs is positively correlated with the digestibility of IDF, but has no correlation with the digestibility of SDF, suggesting differences between purified DF and natural feed-derived DF [
18]. Compared with many studies on purified DF, our previous studies have confirmed that the chemical structure (β-glucan-to-arabinoxylan ratio, β/AX) and apparent viscosity (AV) of natural feed-derived DF play key roles in regulating its fermentation efficiency and affecting the energy metabolism process of animals [
19]. However, the effects of differences in DF structure and apparent viscosity on the growth performance and product quality of pigs, as well as their mechanisms, remain unclear.
Therefore, this study aims to investigate the effects of differences in DF structure and apparent viscosity on the growth performance, carcass traits, meat quality, and intestinal microbiota of growing pigs under the condition of a relatively high DF supply level. It is expected to provide a reference for the application of unconventional feeds in pig production and the feeding strategy of high DF diets.
2. Materials and Methods
2.1. Ethics Statement
Under the Guidelines for the Care and Use of Experimental Animals from Jilin Agricultural University, we carried out all animal tests. These activities received approval from the Ethics Committee of Jilin Agricultural University (approval number: KT2024001).
2.2. Experimental Design and Animals
We employed a 2 × 3 two-factor factorial treatment arrangement. The primary factors under investigation were two dietary levels of AV and three ratios of dietary β-glucan-to-arabinoxylan (β/AX). We set 36 growing pigs (Duroc × Landrace × Yorkshire) with comparable initial body weights (47.2 ± 1.5 kg) as follows: (1) high AV and high β/AX (HVHβ/AX); (2) high AV and medium β/AX (HVMβ/AX); (3) high AV and low β/AX (HVLβ/AX); (4) low AV and high β/AX (LVHβ/AX); (5) low AV and medium β/AX (LVMβ/AX); and (6) low AV and low β/AX (LVLβ/AX).
2.3. Experimental Diet and Feeding Management
The methodologies for measuring and analyzing the nutritional components and physical characteristics of the feeds and diets followed our earlier works [
19]. The specifics of the nutritional components, DF chemical components, and physical characteristics of the feeds are detailed in
Table S1, while
Table 1 presents the composition and nutritional content of the experimental diets. All experimental diets were formulated to maintain uniform nutrient content, adhering to the standardized ileal digestible crude protein, net energy, and total DF levels recommended by the National Research Council for growing pigs (NRC, 2012) [
20]. The study was conducted over a 35-day period at the Pig Experiment Base of the Jilin Academy of Agricultural Sciences, beginning with a five-day adaptation period followed by 30 days of active experimentation. During the experiment, all pigs were housed in the same feeding trial room, with each pig individually kept in an independent pen of 1.8 m
2. Each pen was equipped with a dedicated feeder and waterer (nipple drinker). The pens featured slatted floors with openings and semi-open partitions, facilitating visual communication and physical contact between adjacent pigs. The ambient temperature was maintained at 22.0 ± 1.0 °C, and the lighting period was approximately from 06:00 to 18:00 daily. Additionally, all pigs remained in good health throughout the experiment and did not receive any medications or vaccines. The feeding regimen involved administering diets at a daily intake that delivered triple the required energy for maintenance (419 kJ/kg body weight 0.75), and body weights were monitored weekly to adjust feed quantities accordingly [
15,
21]. During the experimental period, there were no restrictions on the animals’ water consumption, and they were fed twice daily, once at 08:00 AM and again at 04:00 PM, with each feeding being half of their daily ration.
2.4. Growth Performance
At the initiation and conclusion of the trial, we accurately recorded the pigs’ weight to ascertain their initial weight (IW) and final weight (FW), separately. This data facilitated the calculation of the average daily gain (ADG) throughout the trial. Concurrently, the dietary consumption of each pen was meticulously monitored, allowing for the documentation of the average daily feed intake (ADFI). To compute the feed-to-gain ratio (F/G), the total feed intake per pen was divided by the cumulative weight gain of the pigs housed within that pen.
2.5. Slaughtering Performance and Meat Quality Determination
Following the trial termination, the pigs were transported to the Jilin Zhengda Food Co., Ltd. Slaughterhouse (Changchun, China), where they were processed in alignment with established commercial standards and methodologies. Employing methods delineated in prior research [
22,
23], several key indicators were assessed and calculated, including lean meat percentage, loin-eye area, dressing percentage, carcass weight, fat percentage, back fat thickness, and abdominal fat thickness. These measurements served to evaluate the carcass characteristics comprehensively [
24]. All experimental pigs were weighed 24 h prior to slaughter to ascertain their pre-slaughter live weights. The term “carcass weight” denotes the aggregate weight of the bilateral carcasses following exsanguination and depilation, excluding internal organs, tail, hooves, and head, but retaining the leaf fat and kidneys (while maintaining the leaf fat and kidneys). We measured the dressing percentage as carcass weight to pre-slaughter live weight ratio. The left side of each pig’s carcass was suspended in an inverted position, and the thickness of the fat was measured at three specified points along the dorsal midline: the thickest part, the last rib, and the lumbosacral junction, using a Vernier caliper. An average of these measurements was taken to determine the backfat thickness. In the case of the
Longissimus dorsi muscle (LDM), it was dissected vertically at the position of the last rib on the left side. Sulfuric acid paper was placed over the cross-section, and the perimeter of the eye muscle was outlined with a dark pen to facilitate the measurement of the loin-eye area, which was subsequently quantified using a planimeter (Koizumi Sokki Co., Ltd., Tokyo, Japan). The weights of lean meat and fat were derived from standard formulas. We measured the lean meat weight as −6.9144 + 0.6154 × pre-slaughter live weight −2.6893 × backfat thickness at the 6th–7th ribs; the weight of fat was determined by Fat weight = −26.4 + 0.221 × carcass weight + 1.331. A portable colorimeter (MSEZ1900, Hunter Lab Corp., Reston, VA, USA) was employed to measure the color parameters—lightness (L*), redness (a*), and yellowness (b*)—of the LDM. Furthermore, a pH meter (S20, METTLER TOLEDO, Shanghai, China), calibrated with buffer solutions at pH 4.6 and 7, was utilized to determine the pH levels of each LDM sample at 45 min and 24 h post-mortem, with three replicates per sample. Muscle samples, each measuring 2 × 3 × 4 cm and replicated thrice, were excised from each pig 45 min post-slaughter for analyses of shear force, cooking loss, and drip loss. We determined the drip loss percentage by assessing the weight difference before and after suspension. Cooking loss was quantified by determining the percentage weight loss before and after cooking, relative to the initial weight. We used a texture analyzer (BROOKFIELD CT3 Texture Analyzer, Middleboro, MA, USA) fitted with a Warner-Bratzler shear force attachment for determining shear force. Marbling scores were assigned by three evaluators using the National Pork Producers Council standards for LDM samples refrigerated at 4 °C for 24 h. Finally, the dry matter (DM), crude protein (CP), and intramuscular fat (IMF) contents of the LDM were analyzed according to the protocols established by the Association of Analytical Chemists (AOAC 2007) [
25].
2.6. Blood Biochemistry Analyses
On the morning of the 30th day of the formal experiment, researchers collected blood samples from the anterior vena cava of fasted pigs, which were then aliquoted into regular blood collection tubes (without anticoagulant for serum separation) and blood collection tubes containing the anticoagulant EDTA-K2 (for plasma separation). The collected blood was allowed to stand at ambient temperature for 30 min before being centrifuged at 1500× g for 15 min to separate plasma and serum. The samples were subsequently stored at −80 °C prior to analysis. An automated blood biochemical analyzer (BS-400, Mindray, Shenzhen, China) quantified several serum biochemical parameters, encompassing lactate dehydrogenase (LDH), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glucose (GLU), urea nitrogen (UN), albumin (ALB), and total protein (TP). Concentrations of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) in the plasma were determined using enzyme-linked immunosorbent assay kits (BIM Biosciences, Inc., San Francisco, CA, USA), under the producer’s guidance.
2.7. DNA Extraction, Library Construction, and Sequencing
The gut microbiota analysis included digesta samples from different intestinal segments of all experimental pigs (including the cecum, proximal colon, mid colon, distal colon, and rectum). Specifically, after opening the pigs’ abdominal cavities, the researchers ligated the start and end of the cecum, colon, and rectum before removing the entire large intestine and separating the cecum, colon, and rectum (sufficient ligation was performed to prevent digesta flow within the intestinal lumen). The colon was divided into three segments based on length, including the proximal colon (the side adjacent to the cecum), mid colon, and distal colon (the side adjacent to the rectum). The intestinal digesta samples of all pigs were collected according to the method described by Rattigan et al. and stored at −80 °C for microbiota determination [
26]. We extracted total DNA from various intestinal digesta samples utilizing the E.Z.N.A.
® Soil DNA Kit (Omega Biotechnology, Norcross, GA, USA), following the instructions provided by the manufacturer. After successful quality control (QC) assessments, PCR amplification was conducted using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR amplicons were subsequently purified using a DNA gel extraction and purification kit (PCR Clean-Up Kit, Yuhua, China) and quantified with a Qubit 4.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Libraries were constructed using the NEXTFLEX Rapid DNA-Seq Kit (PerkinElmer, Waltham, MA, USA) and sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA). Following sequencing, QC and assembly of raw sequencing reads were performed using the DADA2 plugin within the Qiime2 pipeline (version 2019.4, Fort Lauderdale, FL, USA) to remove noise and generate amplicon sequence variants (ASVs). Taxonomic classification of the ASVs was conducted employing the Naive Bayes classifier in Qiime2 (version 2019.4, Fort Lauderdale, FL, USA), referencing the Silva 16S rRNA gene database (version 138). Data analysis was facilitated using the MajorBio Cloud Platform (MajorBio, Shanghai, China).
2.8. SCFA Concentration Analyses
The concentration of SCFA in the digesta was determined as per the methodology described by Tong et al. [
27]. We weighed nearly 0.1 g of intestinal digesta, to which PBS solution was added at a 1:9 ratio. The mixture was vortexed for 2 min to ensure thorough mixing. This mixture was then centrifuged at 4500 rpm and 4 °C for 10 min. Subsequently, 300 μL of the resultant supernatant was transferred into a suitable container. To this, 25% metaphosphoric acid was added, maintaining a volume ratio of phosphoric acid to supernatant of 1:5, and the mixture was thoroughly vortexed. Following this, 250 μL of the mixture was withdrawn and co-extracted with an equivalent volume (250 μL) of ethyl acetate by vortexing. The supernatant was carefully aspirated with a syringe, filtered through an organic membrane (0.22 μm), and transferred into a gas-phase vial with an inner cannula. These vials were tightly sealed and stored at −20 °C until analysis. We used a gas chromatography system (Agilent Technologies 7890A-G3440A-GC System; Agilent Technologies, Santa Clara, CA, USA) for determining SCFA concentrations.
2.9. Quantitative Real-Time PCR Analysis
Total RNA was extracted using TRIzol (Takara Bio, Otsu, Japan) from the collected samples. The concentration of total RNA was quantified using a NanoDrop 2000 nucleic acid analyzer (Thermo Fisher, Waltham, MA, USA), and its purity was confirmed (OD260/280 between 1.9 and 2.0, indicating acceptable RNA purity). We used 1% agarose gel electrophoresis for evaluating RNA integrity. For reverse transcription, 1 μg of RNA was processed using a Prime Script
® RT Reagent Kit with gDNase (Takara Bio, Otsu, Japan). Primer sequences were designed based on the conserved regions of gene sequences retrieved from GenBank (National Center for Biotechnology Information, Bethesda, MD, USA) and were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Primer efficiency was verified via standard curve analysis with 10-fold serial dilution of cDNA templates (5 gradients: 10
0, 10
−1, 10
−2, 10
−3, 10
−4), and the efficiency values ranged from 95% to 105% with
R2 > 0.99 for all primers, confirming specific and efficient amplification. A 20 μL reaction mixture, with gene-specific primers and SuperReal PreMix Plus (SYBR Green) Kit (Thermo Fisher, Waltham, MA, USA), was prepared and amplified using the ABI 7300 Real-Time PCR System (Applied Biosystems, Foster, CA, USA). Each sample was analyzed in triplicate. The relative expression levels were quantified using the cycle threshold (Ct) method, with β-actin as the single reference gene. β-actin was selected for normalization due to its stable expression across all experimental groups (Ct value variation < 0.5 among samples). The specific primer sequences are listed in
Table S2.
2.10. Statistical Analysis
The statistical model employed was Yijk = µ + αi + βj + αi × βj + ɛijk, where µ represents the population mean, αi signifies the effect of dietary AV (i = 1, 2), βj denotes the levels of β/AX (j = 1, 2, 3), αi × βj represents the interaction between dietary AV and β/AX, and ɛijk reflects the residual variance. Data were analyzed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA). The assumptions of normality and homogeneity of variance were tested using Levene’s test. A two-way ANOVA was conducted to assess the effects of dietary AV, β/AX, and their interaction. Differences among the six dietary treatments were evaluated using Tukey’s multiple comparison test. Bar charts depicting the results were generated with GraphPad Prism version 10 (GraphPad Software, San Diego, CA, USA). Correlation patterns were analyzed using Pearson’s correlation coefficient and visualized as a heatmap with Origin 2021 software (Origin Lab Corporation, Northampton, MA, USA). Microbial composition and diversity were analyzed on the MajorBio Microbial Cloud platform (MajorBio, Shanghai, China). Statistical significance was noted at p-values below 0.01 (highly significant), below 0.05 (significant), and between 0.05 and 0.1 (indicative of a trend). Results are presented as mean ± standard error.
4. Discussion
Extant research underscores the pivotal role that DF and its microbial fermentation metabolites play in lipid metabolism regulation [
28,
29,
30]. Previous investigations have predominantly concentrated on the quantity or source of DF, or on isolated fiber components and their physicochemical characteristics, to elucidate their beneficial impacts on pigs and other species [
5,
31,
32,
33,
34,
35]. However, the use of synthetic or purified fibers, which constitute single components within the broader spectrum of DF, does not adequately mirror the nutritional characteristics and functional attributes of naturally derived plant fibers. Often, these isolated fibers do not fully capture the comprehensive effects of DF on pig nutrition and metabolism [
19,
36,
37,
38]. In this study, we explore the regulatory effects and potential mechanisms through which the physicochemical characteristics (chemical structure and physical characteristics) of natural DFs influence pig growth performance, carcass traits, meat quality, and gut microbiota composition. Our preliminary findings suggest that dietary β/AX and AV synergistically influence subcutaneous fat deposition and improve certain meat quality characteristics (
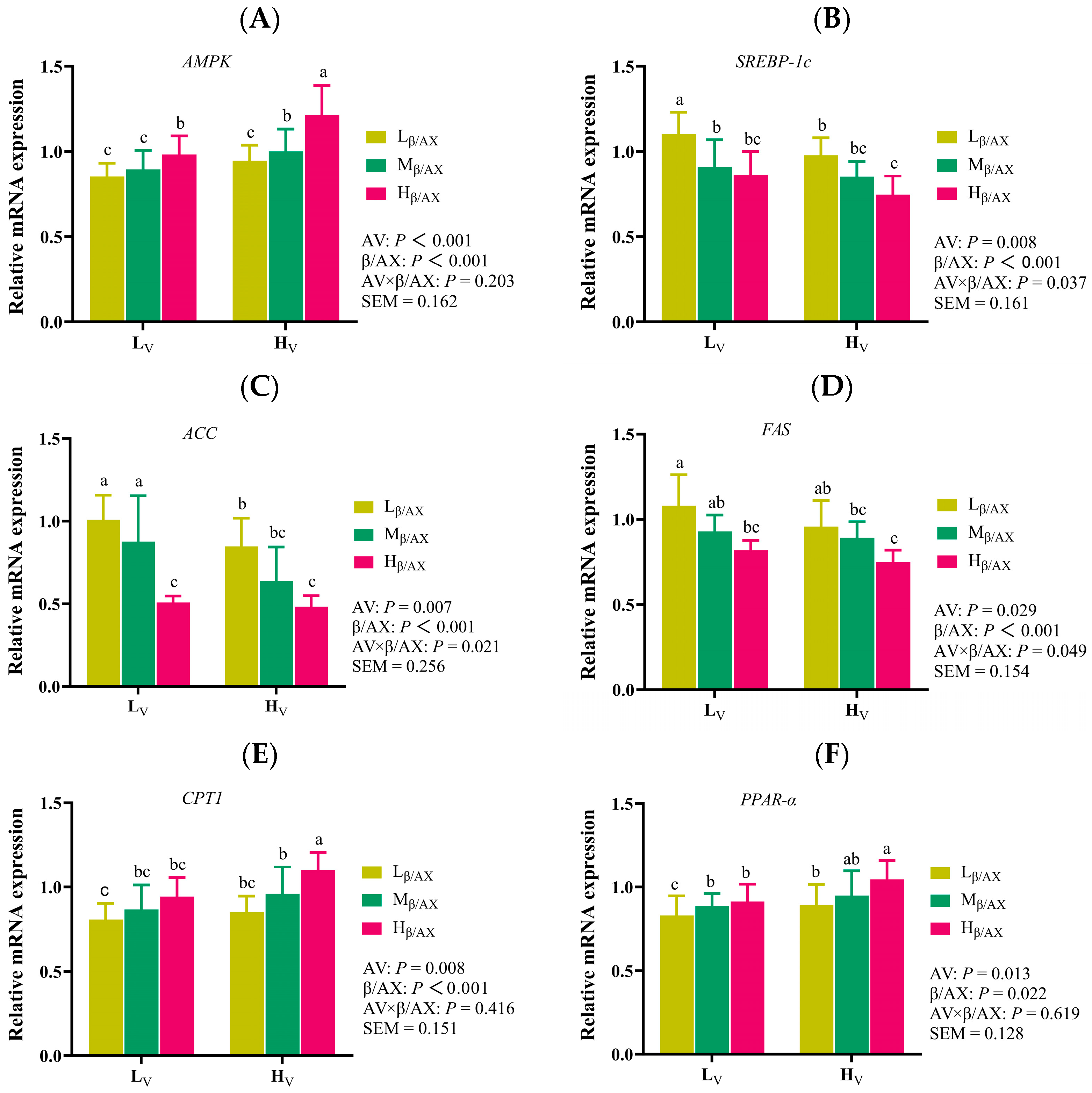
p < 0.05). We observed that modifications in dietary β/AX and AV could regulate serum lipid metabolism indicators and the expression of crucial genes involved in lipid metabolism within the liver. Moreover, microbial sequencing across various intestinal locations indicated that dietary β/AX and AV predominantly affect the microbiome in the middle colon and modulate the abundance of butyrate-producing bacterial genera. These results unveil the potential mechanisms through which the gut-liver axis is influenced by the physicochemical characteristics of DF, thereby regulating subcutaneous fat deposition in pigs.
Numerous studies have demonstrated that excessive lipid accumulation presents significant health risks for both humans and animals [
39,
40,
41]. In the realm of animal production, excessive fat deposition is recognized as a critical factor that adversely affects fattening efficiency, meat quality, reproductive capacity, and immune performance [
42,
43]. DF, a vital component of pig diets, interacts complexly with other nutrients, influencing nutrient digestion and metabolism. Furthermore, it acts as a crucial substrate for gut microbes, undergoing fermentation and degradation to yield metabolites such as SCFA, which contribute to the host’s energy metabolism. In our preceding studies, we elucidated that augmenting dietary β/AX and AV levels diminishes the apparent ileal digestibility of DM and organic matter in pigs, thereby enhancing the excretion of fecal and urinary energy [
19]. Corroborating these findings, the current study reveals that diets enriched with higher β/AX and AV concentrations depress ADG in pigs, subsequently reducing carcass weight and slaughter yield. Intriguingly, while elevated levels of dietary β/AX and AV compromise slaughter performance, they do not detract from the proportion of lean meat. On the contrary, there appears to be an increase in the yield of lean meat per unit body weight. Notably, the augmentation in dietary β/AX and AV markedly curtails subcutaneous fat deposition. These results imply that although modifications in the chemical structure and physical characteristics of DF somewhat impede weight gain efficiency, they concurrently enhance carcass quality and fattening efficiency. Parallel findings were reported by Lu et al. [
29], who observed that raising the dietary neutral detergent fiber content to 16% diminished the pre-slaughter live weight and carcass weight of pigs, as well as the carcass fat content (backfat thickness and fat percentage), while increasing the loin eye area and net lean meat percentage, and ameliorating meat tenderness. These outcomes resonate with those of the present study, which showed that tweaking the chemical structure and physical characteristics of fiber, within a consistent DF level, ameliorates the tenderness and nutritional composition of pork. Additionally, Li et al. [
44] reported analogous effects. Their research indicated that increasing the proportion of ramie fiber in the diet reduced growth performance and slaughter traits such as ADG, carcass weight, and backfat thickness, yet improved meat tenderness and increased protein content in the meat. These collective findings underscore the multifaceted regulatory effects of DF on the growth performance, carcass traits, and meat quality of pigs, influenced not only by its level and source but also by the interaction between its chemical structure and physical characteristics. Additionally, based on the results of this study, reducing dietary β/AX and AV is more beneficial to the weight gain and feed conversion rate of growing pigs (though the differences in dietary β/AX and AV are mainly reflected in subcutaneous fat deposition). Therefore, when growing pigs are fed high-fiber diets or diets supplemented with more agricultural by-products, the effects of dietary fiber structure and apparent viscosity (including interaction effects and independent effects) should be considered. However, appropriately increasing dietary β/AX and AV may be more favorable for the feeding strategy of finishing pigs to improve their finishing efficiency and meat quality.
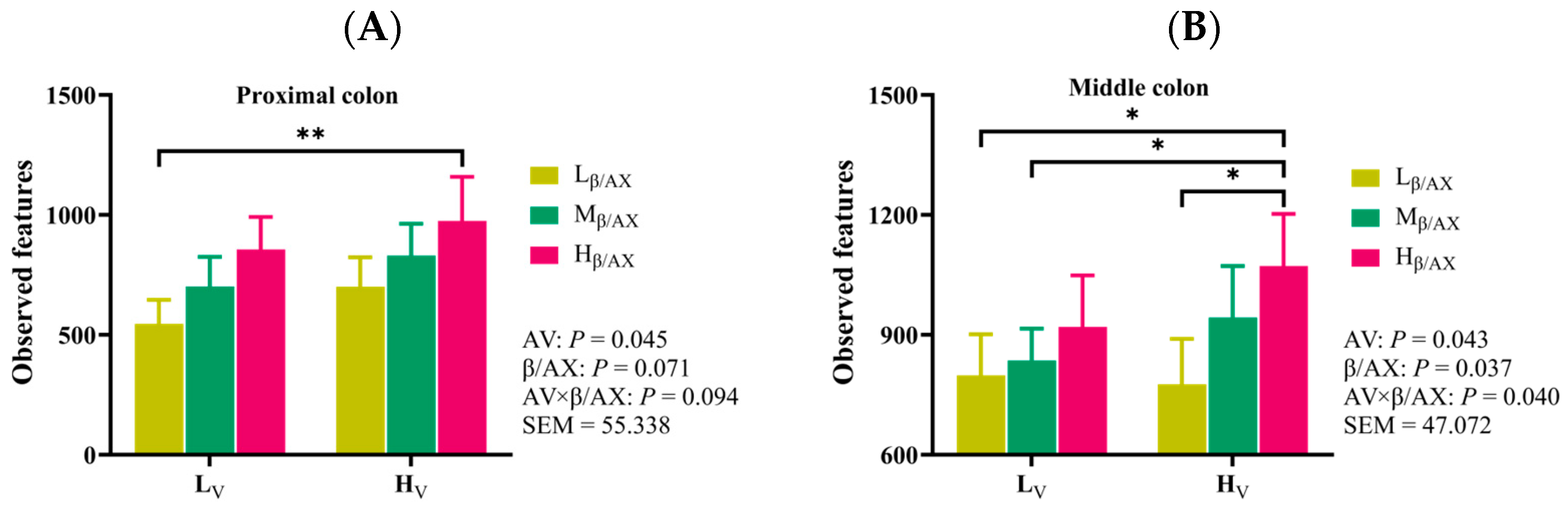
In recent years, a substantial body of research has been devoted to elucidating the intricate relationships between the gut microbiomes of humans and animals and their associations with various diseases, in addition to their significant roles in nutrition, metabolism, physiology, and immune function [
45,
46]. It is now well-established that gut microbes play a pivotal role in regulating the host’s fat deposition phenotype, nutrient utilization efficiency, and production performance [
47,
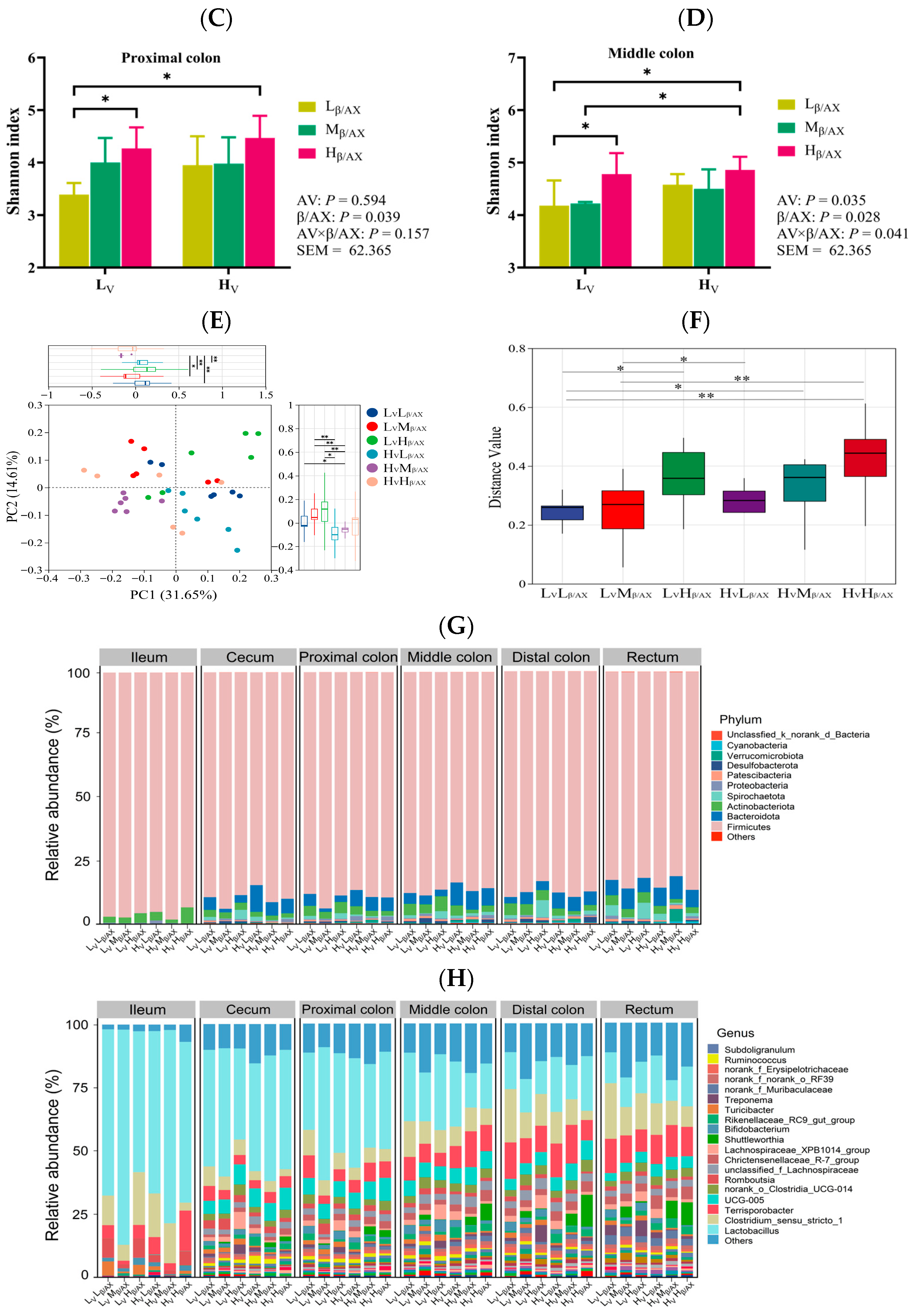
48]. Building on these findings, our study explores the mechanisms by which dietary β/AX and AV influence fat deposition in pigs, mediated by the gut-liver axis effect, which is contingent upon the physicochemical characteristics of DF. Through the analysis of alpha and beta diversities across different groups, our research has identified significant variations in the microbial community structures across various segments of the digestive tract, including the ileum, cecum, proximal colon, middle colon, distal colon, and rectum. Notably, the microbial communities in the middle colon are crucially influenced by the chemical structure and physical characteristics of the DF. Our findings indicate that adjusting the levels of dietary β/AX and AV selectively enhances the relative abundance of acid-producing bacterial genera such as
Terrisporobacter,
Rikenellaceae_RC9,
Shuttleworthia, and
Muribaculaceae in the middle colon. These bacterial genera have been linked to obesity in prior research, suggesting potential interventions and associations [
49,
50,
51]. Furthermore, Jiang et al. [
33] demonstrated that dietary supplementation with β-glucan hydrogels, which are highly water-absorbent, mitigated obesity in mice on a high-fat diet. Their study also revealed a negative correlation between obesity-related traits and the
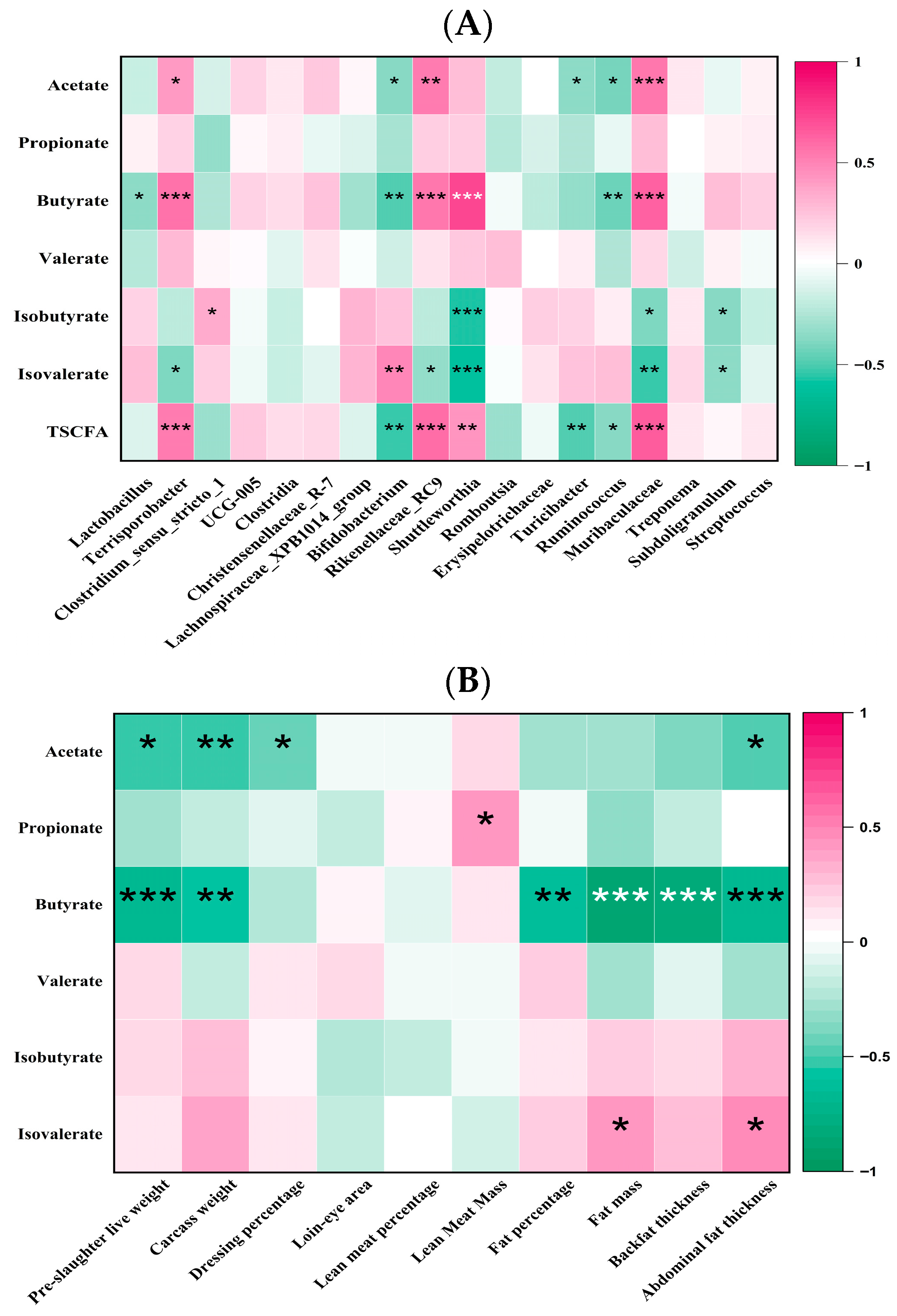
Muribaculaceae genus. Additionally, our correlation analysis indicates that these bacterial genera, which are upregulated by increased levels of dietary β/AX and AV, are positively associated with the production of butyrate in the middle colon, echoing findings from previous studies [
51,
52]. These outcomes suggest that, under consistent dietary nutrient conditions, altering dietary β/AX and AV effectively modulates the proportion of butyrate-producing bacterial genera in the middle colon of pigs and enhances butyrate production.
The significance of SCFA, particularly butyric acid, in linking microbial activities to diverse biological effects and phenotypes has been well-documented [
53,
54]. SCFA serve as vital energy sources for the colonic epithelium. Studies have shown that dietary supplementation with butyric acid enhances energy expenditure and fat oxidation in obese mice, effectively preventing obesity triggered by a high-fat diet [
55]. Furthermore, Zhou et al. [
56] demonstrated that supplementation with a combination of acetic acid, propionic acid, and butyric acid reduces fat deposition in pigs, regardless of the involvement of the gut microbiota. In a recent investigation, the augmentation of β/AX and AV in pig diets was found to synergistically enhance butyrate production, which in turn increased the absorption of butyric acid, as evidenced by a significant elevation in butyrate concentrations in the hepatic portal vein plasma [
19]. Additionally, butyrate production in the middle colon exhibited a strong inverse correlation with several indicators of fat deposition and weight gain, including fat mass, backfat thickness, abdominal fat thickness, fat percentage, pre-slaughter live weight, and carcass weight. These findings suggest that the regulatory effects of dietary β/AX and AV on fat deposition may be mediated through butyrate. Previous research has identified the anti-obesity effects of butyrate as being facilitated by the
AMPK-ACC pathway, which diminishes fat deposition [
56], aligning with the outcomes observed in our study. We noted that an increase in dietary β/AX and AV led to elevated mRNA expression of
AMPK and
PPAR-α in the liver, alongside enhanced activity of
CPT-1. Conversely, mRNA expression of
SREBP-1C in the liver, as well as the activities of
FAS and
ACC, were notably reduced in the group receiving high dietary β/AX and AV. These results corroborate previous findings in pig liver, muscle, and adipose tissues [
57,
58,
59].
AMPK, recognized as a critical energy sensor, governs cellular metabolism and influences lipid metabolic homeostasis [
60]. Research indicates that activation of
AMPK not only promotes energy catabolism, such as fatty acid oxidative catabolism, but also inhibits anabolic processes, including the de novo synthesis of fatty acids [
61]. This dual role establishes
AMPK as a central regulator of lipid synthesis and oxidation.
Additionally,
SREBP-1C and
PPAR-α are pivotal transcription factors that govern the de novo synthesis of fat and the β-oxidation catabolism pathway of fatty acids, separately [
62]. The principal downstream target genes include
FAS,
ACC, and
CPT-1, which play crucial roles in lipid synthesis and metabolism [
63]. Activation of
AMPK typically inhibits the
SREBP-1C pathway while simultaneously enhancing the PPAR-α signaling pathway [
62,
63]. Consequently, the proposed mechanism by which dietary modification with β/AX and AV reduces fat deposition in pigs in this study may involve butyric acid, which, mediated by the chemical structure and physical characteristics of fiber, activates the
AMPK-SREBP-1C pathway and diminishes the activities of
FAS and
ACC, thus curtailing de novo fat synthesis. Conversely, through the
AMPK-PPAR-α pathway, butyric acid decreases ACC activity and augments the expression of
CPT-1, thereby fostering the oxidation of fatty acids [
64]. These effects align with the observed outcomes of serum lipid metabolism indicators. Furthermore, an increase in dietary β/AX and AV was found to decrease serum glucose concentrations, exhibiting an interaction effect. This effect likely stems from alterations in the chemical structure and physical characteristics of DF, which impact the rheological properties and the rate of gastric emptying. Recent studies on the viscosity characteristics of fiber in porcine nutrition have demonstrated that the inclusion of purified β-glucan in the diet elevates the viscosity of intestinal digesta [
15]. Such an increase in viscosity may attenuate the interaction between digestive enzymes and the chyme matrix and engage with the intestinal mucosa to create an absorption barrier, thus reducing the uptake of specific nutrients. Moreover, an enhanced dietary content of β/AX and AV has been associated with increased plasma GLP-1 concentrations, further underscoring the significant role of the chemical and physical characteristics of DF in modulating glucose and lipid metabolism in pigs.
These findings elucidate the intricate interplay between the physicochemical characteristics of DF and host fat deposition mechanisms, including lipid synthesis and metabolism. This evidence offers fresh perspectives on how to optimize fattening efficiency and meat quality in pigs through tailored fiber nutrition and illustrates potential strategies for utilizing DF effectively to manage obesity and prevent the onset of nutritional and metabolic disorders. Further research in this field may reveal the overall effects of DF intervention on lipid metabolism and regulation of animal production performance and welfare, and provide a basis for the feeding strategies of high-fiber diets and the effective utilization of agricultural by-products in pig production. However, this study has limitations such as a relatively short experimental period and a small number of experimental animals. Extending the experimental period will help further understand the overall effects of differences in DF structure and AV on the production performance and product quality of pigs at different physiological stages (growing and finishing phases). In addition, subsequent studies should examine whether the potential mechanisms by which DF structure and AV regulate fat deposition and production performance in pigs are associated with changes in digesta transit kinetics and substrate fermentation kinetics in the large intestine. Therefore, it is necessary to increase the number of experimental animals and extend the experimental period, and combine cutting-edge technologies such as metagenomics, metabolomics, and lipidomics to verify and expand these findings.