Simple Summary
Fish, like all animals, need enzymes that can break down and rebuild the material surrounding their cells. These enzymes, called matrix metalloproteases, are essential for growth, tissue repair, and defense against disease. Despite their importance, we know little about how the genes behind these enzymes evolved in fish, which make up the largest and most diverse group of vertebrates. In this study, we traced the history of the genes that produce collagen-degrading metalloproteases across different animals, with a focus on fish. We discovered that while some of these genes remain single copies, others were duplicated during fish evolution and kept their function. By studying two important farmed fish, Sparus aurata (gilthead sea bream) and Dicentrarchus labrax (European sea bass), we found that the duplicated genes are regulated differently at different developmental stages or in different tissues. Our findings provide new insights into the biology and evolution of fish and may help improve aquaculture practices by linking gene function to fish development and health.
Abstract
Matrix metalloproteases (MMPs) are zinc-dependent endopeptidases that are critical for extracellular matrix (ECM) remodeling, with key roles in tissue development and repair, and immune responses. Despite their evolutionary and functional importance, the diversification and regulatory dynamics of MMPs genes in teleosts remain poorly understood. This study investigates the evolutionary history of MMPs with collagenolytic activity in the vertebrates with an emphasis on teleosts. Using comparative genomics and phylogenetics we identified conserved single-copy mmp2 and mmp9 genes and duplicated mmp11 and mmp13 paralogs in all non-salmonid teleosts. Phylogenetic and synteny analyses suggest that mmp11 paralogs originated from the teleost-specific genome duplication (TSGD), whereas the origin of mmp13 paralogs originated from a more complex evolutionary scenario. Protein domain analysis confirmed conserved catalytic motifs across species, supporting functional retention. The expression patterns of paralog genes were studied in two model marine teleosts, the Sparus aurata (gilthead sea bream) and Dicentrarchus labrax (European sea bass). Developmental and adult tissue transcriptome analyses revealed three major expression patterns among the paralogs: similar expression, stage-specific or tissue-specific expression. The overall data point to varied evolutionary dynamics of MMP genes in the teleosts, tracing their origin to different whole-genome duplication events. Expression profiles on paralog genes in model teleosts suggest regulatory sub-functionalization as the most possible fate of retained MMPs paralogs in teleosts following whole-genome duplication.
1. Introduction
Extracellular matrix (ECM) consists of a complex network of macromolecules, providing structural stability and support to the cells [1]. ECM acts essentially as a “scaffold”, facilitating cell adhesion and migration [2]. Proteins such as collagen provide structural integrity, and glycoproteins, such as fibronectin and laminin, promote cell adhesion. In addition, proteoglycans play a vital role in stability by contributing to viscosity and compressive resistance. Collagen is the most abundant protein in ECM and it is characterized by a triple-helix conformation. Key biological processes, namely embryogenesis, tissue remodeling, osteogenesis, and angiogenesis require collagen degradation performed by collagenolytic enzymes such as matrix metalloproteases (MMPs) [3,4,5].
Matrix metalloproteases (MMPs) are a family of zinc-dependent endopeptidases, playing a key role in the regulation of ECM remodeling, tissue growth and homeostasis in general. They belong to the metzincin superfamily characterized by a conserved zinc-binding motif (metzincin motif-HEXXHXXGXXH) where the three histidine residues are coordinated with a zinc ion, essential for MMPs catalytic activity [6]. These proteases are present in all kingdoms of life, and they are found in both vertebrates and invertebrates [7,8]. Phylogenetic studies suggest that MMP vertebrate gene family was expanded from a common protostome-deuterostome ancestor through multiple rounds of genome duplications. However, prior to the emergence of vertebrates the family included a low number of gene members (2 MMPs in protostomes and 5 MMPs in urochordates). Interestingly, although teleosts have undergone an extra round of genome duplication compared to other vertebrates, they possess a similar number of genes to the other vertebrates, with some of the family members missing [6].
Whole-genome duplication (WGD) events have been a recurring phenomenon throughout the evolutionary history of vertebrates and other eukaryotes contributing to the long-term evolutionary success of species-rich lineages [9,10]. Unlike duplication of individual genes or small gene clusters, WGD events stand out to facilitate the divergence of entire networks of ohnologues, driving molecular and phenotypic complexity [11]. Ohnologs or paralogs are gene duplicates that originate from whole-genome duplication events and are named after Susumu Ohno, who first proposed their evolutionary importance. Vertebrates have undergone two shared whole-genome duplication events (1R and 2R) [12] and teleosts, after their diversification as a lineage, have undergone a third WGD event (3R or TS-WGD) 320–350 Mya [13,14]. After a WGD event, the trajectory of duplicated genes is directly dependent on evolutionary pressures [15]. Because both gene copies can initially perform the same function, one copy can accumulate mutations without compromising organismal fitness. This functional redundancy temporarily relaxes purifying selection, making ohnologs less subject to evolutionary constraints and allowing sequence and regulatory divergence to occur. Duplicated genes are free of evolutionary constraints, creating fertile ground for the accumulation and establishment of mutations [16]. This can lead to a functional loss of one of the two copies, creating pseudogenes, provided the gene is not under evolutionary pressure. On the other hand, the maintenance of paralogs genes, either by acquiring a new function or by diverging the function of one, is driven by the advantages of duplicating a genetic locus under selection [17]. These mechanisms can act sequentially or simultaneously during gene evolution, contributing to the differential fate of paralogs genes. Ultimately, the establishment of duplicated gene loci depends on several factors, such as the presence of neutral or deleterious mutations, selection factors, and, in some cases, population size [18].
Progress in unraveling the evolutionary history of various gene families has been achieved through the sequencing of complete teleost genomes. However, there is a noticeable lack of literature on matrix metalloproteinases (MMPs), a gene family whose evolution and transcriptional regulation remain poorly understood despite their significant relevance in many eukaryotic species. While insights into the evolution and transcriptional regulation of various gene families have been gained, the influence of these processes on the MMP gene family is still largely unknown [19].
Teleost fish species occupy a critical position in the vertebrate lineage, allowing us to explore the evolutionary dynamics of matrix metalloproteinases (MMPs) with collagenolytic activity, in the context of whole-genome duplications (2R and 3R events). Sparus aurata (gilthead seabream) and Dicentrarchus labrax (European seabass) serve as valuable models due to their phylogenetic relevance, conservation, and accessibility [20,21]. Studying MMPs in these species provides insights into the conservation and diversification of MMP functions within teleosts. Additionally, their accessibility and economic importance make gilthead sea bream (S. aurata) and European sea bass (D. labrax) feasible choices for experimental research, facilitating the collection of samples and the undertaking of analyses across various tissues and developmental stages. This unique combination of phylogenetic significance, conservation relevance, and experimental feasibility make these fish species ideal candidates for advancing our understanding of MMP biology in aquatic environments. In this study, we tested the hypothesis that the collagenolytic matrix metalloproteases (MMPs) paralogs in teleosts have undergone regulatory sub-functionalization following gene duplication, resulting in distinct expression patterns in development and tissue remodeling. Using gilthead sea bream (S. aurata) and European sea bass (D. labrax) as model species, we integrated comparative genomic and transcriptomic analyses to (i) trace the evolutionary divergence of MMPs paralogs, and (ii) evaluate their tissue- and stage-specific expression.
2. Materials and Methods
2.1. Phylogenetic Analysis
2.1.1. Database Mining and Sequence Retrieval
A comprehensive database mining was performed to identify genes belonging to the Matrix Metalloproteases family, as described in the HGNC database for humans. Subsequently, for members of the gene family with collagenolytic activity, we utilized the BLASTP algorithm of the ENSEMBL database (ENSEMBL 105) [22] to retrieve homologous genes from twenty species. Specifically, sequences belonging to representatives of teleosts (13), a representative of Actinopterygii, representatives of Sarcopterygii (5), and a representative of Chondrichthyes were selected to elucidate the evolutionary path of the MMP2, MMP9, MMP11, and MMP13 genes (Table 1). The taxonomic sampling strategy was designed to span key vertebrate lineages and to provide phylogenetic resolution across major evolutionary transitions.

Table 1.
Species selected for the phylogenetic analysis and the accession numbers of each species genome. *: Stickleback and Tetraodon hadn’t any accession number and we report the Ensembl Assembly ID.
Representatives of Sarcopterygii were included as outgroups that have only undergone the two early vertebrate whole-genome duplications (1R and 2R), allowing for comparisons against lineages that experienced additional genome duplication. The holostei species (Lepisosteus oculatus), a non-teleost actinopterygii, was selected because it diverged just prior to the teleost-specific genome duplication (TSGD) and can act as a closely related outgroup for identifying the duplication origin of teleost paralogs. Teleost species were sampled across major clades to ensure broad coverage of the TSGD-derived lineage, while the Chondrichthyes species (Callorhinchus milii) served as a more distant outgroup to root the phylogeny and distinguish ancient from lineage-specific duplication events. This sampling enabled inference of ohnolog retention patterns and duplication timing.
In addition, to examine exon–intron organization, the gene structures of all mmps of gilthead sea bream (S. aurata) and European sea bass (D. labrax) were visualized using the Gene Structure Display Server (GSDS 2.0) [23]. For each gene, the coding sequence (CDS) and the corresponding genomic DNA sequence were retrieved from Ensembl and aligned in GSDS to infer intron positions relative to exon boundaries (Supplementary Figures S1–S4).
2.1.2. Sequence Alignments and Phylogenetic Analysis
The retrieved sequences were used for the reconstruction of the phylogenetic tree and sequences of low quality were removed. Multiple sequence alignment was performed using the MAFFT algorithm (version 7) [24] along with the GUIDANCE2 algorithm [25] to gain statistical confidence for each aligned site. The multiple alignment data were uploaded to MEGAX [26] and the best-fitting substitution model was identified for each gene based on the Bayesian Information Criterion (BIC). The selected models used for tree reconstruction are reported in the corresponding figure legends. Lastly, Maximum Likelihood (ML) algorithm was used, and 100 bootstrap repetitions were performed to statistically analyze the sequence branches. In addition to the ML algorithm, a tree using MrBayes (version 3.2.7) and Bayesian inference was used to gain statistical confidence for each branch of the tree, using the default parameters (two independent runs of 1 million generations, sampling every 100 generations, and applying a burn-in of 25%) [27].
2.1.3. Collinearity and Synteny Analysis and Duplicate Gene Origin Classification
Phylogenetic analysis helps elucidate the evolutionary relationships among genes or species, providing insights into their common ancestry, divergence, and adaptation. But this type of analysis cannot answer questions about genomic regions with conserved architecture, which synteny can. This is pivotal for identifying orthologs and paralogs, helping to understand the evolutionary forces that shaped the genomic landscape.
Two different approaches were used to evaluate the conservation level of the genes of interest among the species studied. Firstly, collinearity analysis was performed using MCscanX (Multiple Collinearity Scan X, version 1.0.0), to examine the collinearity in the genomes of gilthead sea bream (S. aurata) and European sea bass (D. labrax). The detailed pipeline was according to Tsipourlianos et al., 2024 [28]. Secondly, to evaluate the conservation level of the adjacent gene environment for the genes of interest, the upstream and the downstream localization was examined for the absence or presence of a conservation level in the surrounding genomic area using BioMart tool of the ENSEMBL database [29]. Lastly, the MCScanX tool called duplicate gene classifier was used in order to classify duplicated genes according to their evolutionary origin [30].
2.1.4. Functional Conservation Estimate
The Interpro database [31] was used to identify conserved regions in proteins of gilthead sea bream (S. aurata) and European sea bass (D. labrax) and compare with human homologous proteins. Then, the IBS (Illlustrator for Biological Sequences) Software (version 1.0) was used to visualize the protein domains and their functions [32].
2.2. Gene Expression Profiling
2.2.1. Larvae Rearing and Sampling
Egg hatching and larval rearing for both European sea bass and gilthead sea bream were performed by using the methodology of Papandroulakis et al. at the Institute of Marine Biology, Biotechnology and Aquaculture (HCMR) [33]. Fish larvae were kept at 20 °C for gilthead sea bream (S. aurata) and 17 °C for European sea bass (D. labrax) from egg (epiboly) to metamorphosis stage. The same sampling protocol was followed for both species. Briefly, larvae samples were collected at three developmental stages, first feeding (FF); notochord flexion (FL); and mid-metamorphosis (MM), anesthetized using 2-Phenoxyethanol, rinsed with clean sea water and fixed in RNA later solution. Samples were stored at −20 °C until extraction [34].
2.2.2. RNA Extraction and Transcriptome Sequencing
Larvae homogenization was performed through glass beads beating homogenization at 6000 rpm for 30 s (tissue homogenizer, Precellys, Bertin Technologies, Montigny-le-Bretonneux, France). To obtain sufficient total RNA, 3–5 individuals were pooled per sample at the FF stage, whereas individual extractions were performed at the FL and MM stages. Total RNA was extracted using E.Z.N.A.® Total RNA Kit I (OMEGA bio-tek, Omega Bio-tek, Inc., Atlanta, GA, USA) with slight refinements. To totally remove traces of genomic DNA an extra step of DNAse treatment was performed with DNA-free™ DNA Removal Kit (Invitrogen, Waltham, MA, USA). Total RNA was evaluated via gel electrophoresis and was quantified using Qubit™ RNA BR Assay Kit (Invitrogen). Subsequently, twelve RNA samples were pooled, equimolarly for each developmental stage and sent to Novogene Inc. (Europe, Cambridge, UK), for transcriptome sequencing, using Novaseq 6000 Illumina’s platform (Illumina, San Diego, CA, USA) and 150 bp paired-end reads were generated producing approximately 30 million reads per sample, with 97% passing Q20. A pair of fastq files for each sample (R1: forward and R2: reverse) were acquired after the transcriptome sequencing and the bioinformatics analysis was performed according to Stamperna et al. and Robinson et al. [35,36]. The detailed pipeline can be found in the public repository Github (https://github.com/RafaelAngelakopoulos/Bioz_lab/tree/0f040a4aee3536952a6df587f25a02ddb74fa61b/RNAseq, accessed on 9 November 2025) and the transcriptome data are submitted in SRA (European sea bass (D. labrax): PRJNA1050410 and gilthead sea bream (S. aurata): PRJNA1050571).
In addition, publicly available transcriptome data (FASTQ files) from various adult tissues were obtained for both gilthead sea bream (S. aurata) and European sea bass (D. labrax) and processed with the same pipeline. Moreover, to account for potential batch effects in the transcriptomic data, we applied the ComBat-Seq model [37] from the sva R package (v3.56.0) [38]. The accession numbers of the data are shown in Supplementary Table S1.
3. Results
3.1. Phylogenetic Analysis
Using HGNC database, we identified MMP1, MMP2, MMP9, MMP11 and MMP13 genes as MMPs with collagenolytic activity. Gene names of fish paralogs and human homologs were inconsistent within Ensembl annotation, with some being marked as novel genes. Standardizing gene names was imperative to improve clarity throughout the manuscript. In cases Ensembl annotation lacked consistency in gene names, a nomenclature based on the human homolog (best BLAST hit) was adopted, appending an ascending number to each paralog. The correspondence between the masked gene names and the according Ensembl gene IDs is shown in Table 2.

Table 2.
Correspondence between the masked gene names and the according Ensembl gene IDs in both gilthead sea bream and European sea bass.
Among the five genes initially identified within the human genome, we discovered an alternative repertoire in both gilthead sea bream (S. aurata) and European sea bass (D. labrax), each harboring a total of six genes. Interestingly, mmp1 was not identified in teleost genomes. Duplications of mmp13 and mmp11 genes were recorded, while mmp2 and mmp9 harbored one copy each. The detailed number of genes in each species is summarized in Figure 1.
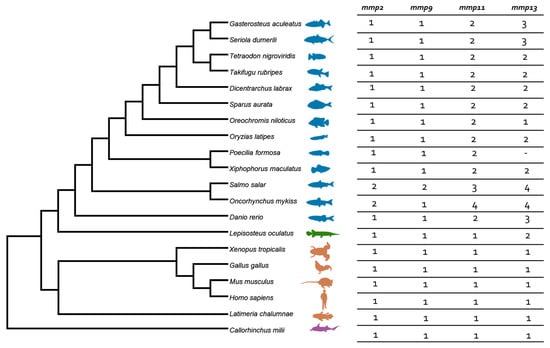
Figure 1.
Species tree and summarized number of genes of mmps per species. Teleosts are presented in blue, Lepisosteus oculatus (spotted gar) in green, sarcopterygii in orange, and C. milli in purple. Colors are consistent in all gene trees.
We used both Maximum Likelihood (ML) and Bayesian inference methodologies for tree reconstruction. Topologies of both methodologies were similar, so we integrated the results from both statistical models on the tree in a way of ML bootstraps/Bayesian probability.
The phylogenetic analysis of the mmp2 gene (Figure 2) revealed a conserved single-copy presence across all species, except within the Salmonidae branch, where a duplication event is present, resulting in the retention of two gene copies. This observation aligns with the established fourth round of genome duplication specific to the Salmonidae lineage (4R). A similar topology was observed in the mmp9 tree (Figure 3), wherein the gene maintained a single-copy status across diverse species, except for the Salmonidae branch, where gene duplication was evident. Notably, within this branch, only Salmo salar (salmon) exhibited the retention of paralogs derived from the 4R event, whereas Oncorhynchus mykiss (rainbow trout) retained a single copy of the mmp9 gene, suggesting a probable loss of the second copy under evolutionary pressures.
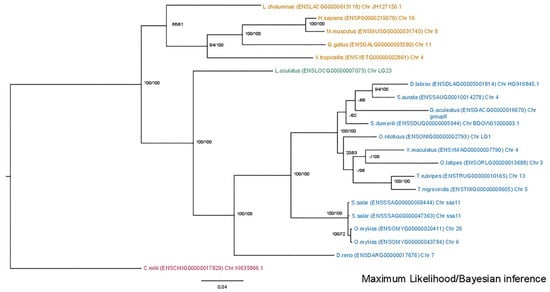
Figure 2.
Phylogenetic tree of the mmp2 protein is depicted, with teleosts represented in blue, Lepisosteus oculatus (spotted gar) in green, sarcopterygii in orange, and C. milli in purple. Bootstraps from the Maximum Likelihood method are denoted on the right of “/”, while those from Bayesian inference are on the left. The LG + G replacement model was employed for reconstructing the ML tree.
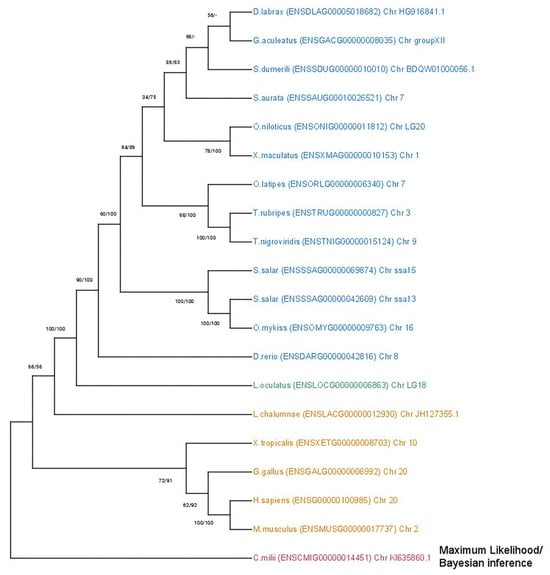
Figure 3.
Phylogenetic tree of the mmp9 protein is depicted, with teleosts represented in blue, Lepisosteus oculatus (spotted gar) in green, sarcopterygii in orange, and C. milli in purple. Bootstraps from the Maximum Likelihood method are denoted on the right of “/”, while those from Bayesian inference are on the left. The LG + G replacement model was employed for reconstructing the ML tree.
Regarding the duplicated genes mmp11 and mmp13 two topologies were discerned. The mmp11 topology suggests that both paralogs originated from a whole-genome duplication event, most likely the TSGD, occurring in the common ancestor of teleosts (Figure 4). Most teleost representatives possess two copies of this gene, with the exception of the Salmonidae lineage, where four copies were identified. This inference is strongly supported by both the bootstrapping and Bayesian probabilities in the base of teleosts, splitting from spotted gar (Lepisosteus oculatus), a species that predated the TSGD. The tree is outgrouped by Sarcopterygii, although Latimeria chalumnae (Coelacanth) aligned with Actinopterygii albeit in a less supported branch. Lastly, the tree is rooted from Callorhinchus milii (Elephant shark) following the species tree topology.
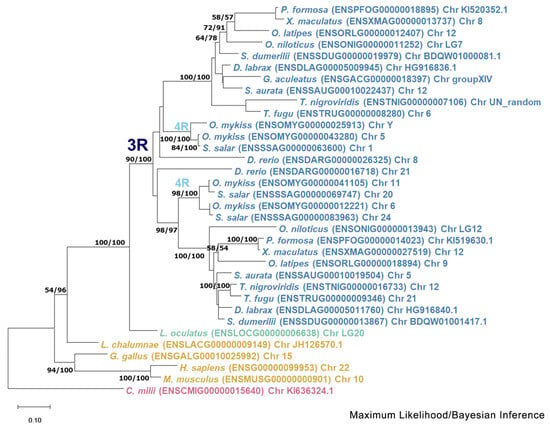
Figure 4.
Phylogenetic tree of the mmp11 protein is depicted, with teleosts represented in blue, Lepisosteus oculatus (spotted gar) in green, sarcopterygii in orange, and Callorhinchus milii (Elephant shark) in purple. Bootstraps from the Maximum Likelihood method are denoted in the right of “/”, while those from Bayesian inference are on the left. The JTT + G + I replacement model was employed for reconstructing the ML tree. The main duplication events are annotated on the tree.
Phylogenetic analysis of the mmp13 gene (Figure 5) reveals a discernible divergence of Actinopterygii from Sarcopterygii. The inferred topology does not provide enough clarity to confidently hypothesize the evolutionary scenario leading to the paralogs. Specifically, the tree configuration places two copies of the spotted gar gene across both teleost clades, with Sarcopterygii forming an outgroup. The placement of Danio rerio (zebrafish) outside of the spotted gar (Lepisosteus oculatus) suggests that the branching order of these species is not well-resolved, further complicating the interpretation of the tree.
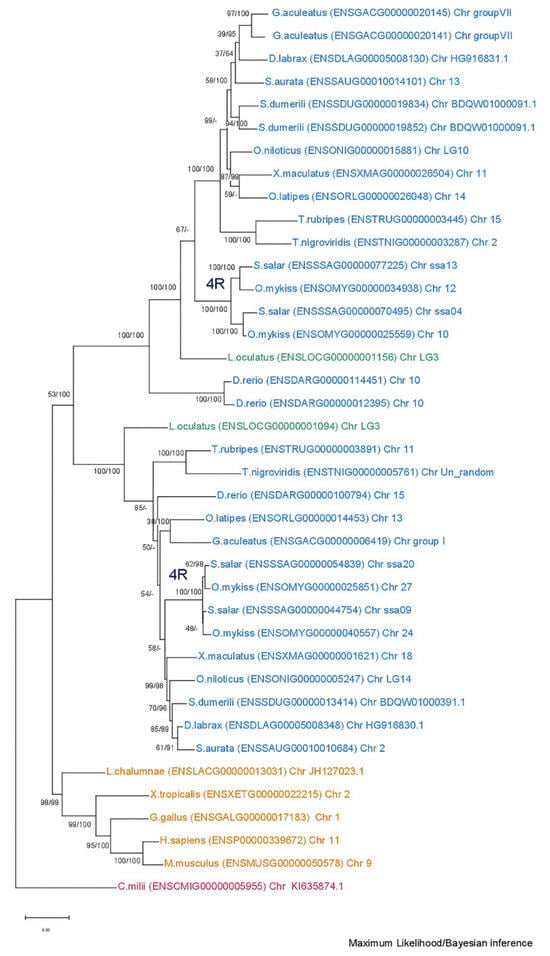
Figure 5.
Phylogenetic tree of the mmp13 protein is depicted, with teleosts represented in blue, Lepisosteus oculatus (spotted gar) in green, sarcopterygii in orange, and Callorhinchus milii (Elephant shark) in purple. Bootstraps from the Maximum Likelihood method are denoted on the right of “/”, while those from Bayesian inference are on the left. The JTT + G + I replacement model was employed for reconstructing the ML tree. Main duplication events are annotated on the tree.
Finaly, for better understanding the evolutionary relationship between the genes in study, a family unrooted tree was reconstructed using the ML algorithm. The topology revealed suggests that mmp2 and mmp9, the two gelatinases, are more closely related than mmp13 and mmp11, with the latter being the most diverged (Figure 6).
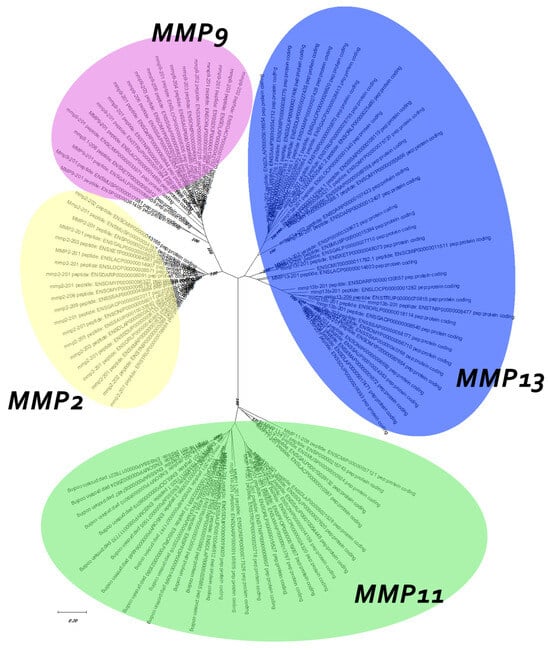
Figure 6.
Unrooted family tree of MMPs under study. Model used for the ML tree: WAG + G + I.
3.2. Synteny Analysis
A macro- and micro-synteny approach was employed to investigate whether the gene paralogs of mmp11 and mmp13 originated from genome duplications. Genome duplications typically exhibit characteristic synteny, as blocks of genes within a genome may be found on two distinct chromosomes. The macro-synteny analysis unveiled shared syntenic regions within the genomes of both gilthead sea bream and European seabass MMP paralogs (Supplementary Figures S5 and S6). Furthermore, employing McScan-X tools for collinearity block construction and the “duplicate gene classifier” led to the categorization of paralogs into four categories, as delineated by Wang et al. (2012) [30] (Table 3). In addition, micro-synteny analysis was conducted using Biomart data for all species used for tree reconstructions. The comparisons, extending six (6) genes upstream and downstream of the gene of interest, revealed distinct instances of genes that appear to be duplicated across various teleosts. The micro-synteny analysis revealed high conservation of synteny across species for the mmp13 gene (Figure 7). In contrast, for the mmp11 gene, although the majority of the upstream and downstream genes were present across all species, they were not arranged in conserved blocks (Figure 8).

Table 3.
Duplicate genes classification in gilthead sea bream and European sea bass using McScan-X tools.
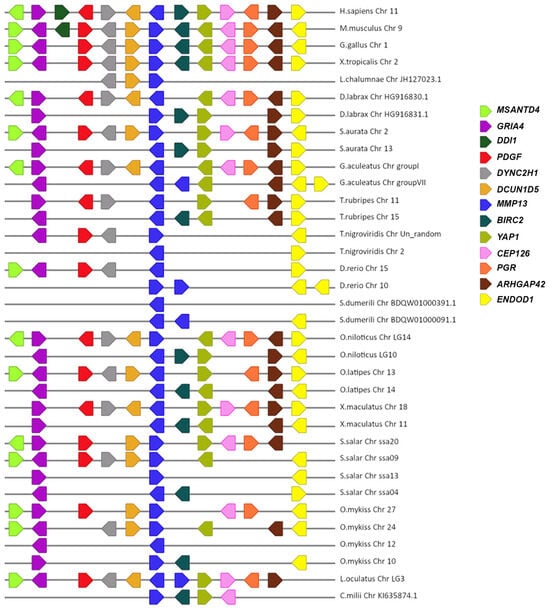
Figure 7.
The micro-synteny of mmp13 is visually depicted in the figure, where colored arrows denote homologous genes, and the arrowhead signifies the direction of predicted gene transcription. Horizontal lines represent chromosome fragments from the included species. Distances between genes or gene length are not drawn to scale.
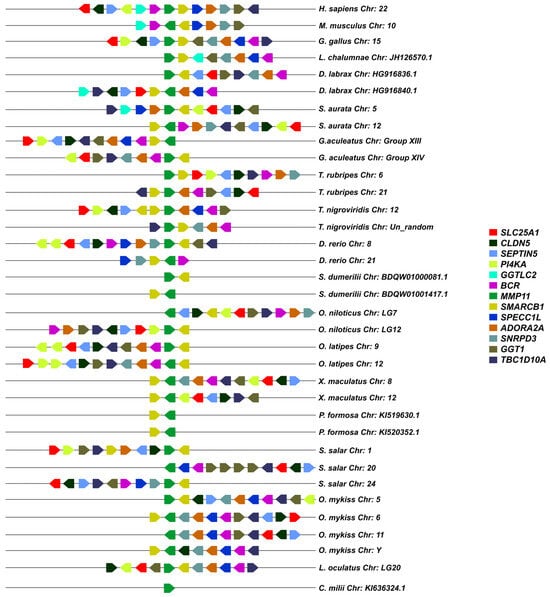
Figure 8.
The micro-synteny of mmp11 is visually depicted in the figure, where colored arrows denote homologous genes, and the arrowhead signifies the direction of predicted gene transcription. Horizontal lines represent chromosome fragments from the included species. Distances between genes or gene length are not drawn to scale.
3.3. Comparative Protein Structure Analysis
Protein reconstruction was performed using the IBS illustrator, revealing the structural domains of MMPs in humans (H. sapiens), gilthead sea bream (S. aurata) and European sea bass (D. labrax). Notably, all metalloproteases exhibited several structural similarities, including the presence of a signal peptide, facilitating their secretion as proenzymes. In addition, the copies of the duplicated genes mmp11 and mmp13 displayed a high degree of conservation, featuring comparable-sized catalytic and regulatory domains. Moreover, both copies retained intact active sites and metal ion-binding sites, affirming their catalytic activity (Figure 9, Figure 10, Figure 11 and Figure 12). It is important to highlight the highly conserved sequence (VAAHEXGHXXXXGXXH) observed in the active site of the catalytic domain of all metalloproteases under study in both gilthead sea bream (S. aurata) and European sea bass (D. labrax). Generalizing this finding, the same sequence was aligned and retrieved from all species and all metalloproteases under study, including hagfish (Eptatretus burgeri) and lamprey (Petromyzon marinus). The percentage of conservation remains high throughout vertebrate evolution (Figure 13).
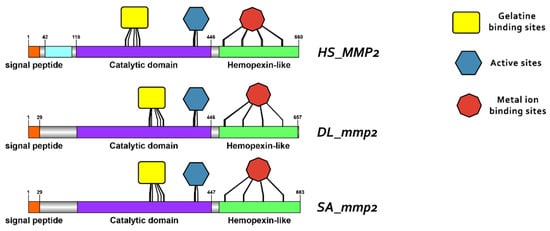
Figure 9.
Domain architecture of MMP2 proteins in human (H. sapiens), gilthead sea bream (S. aurata) and European sea bass (D. labrax), including the signal peptide (orange), proteoglycan binding site (PGBD) (light blue), catalytic domain (purple), hemopexin-like domain (light green), gelatine binding sites (yellow), metal ion-binding site (red), and the active sites (dark blue) of the enzyme. The PGBD domain could not be identified in gilthead sea bream (S. aurata) and European sea bass (D. labrax).
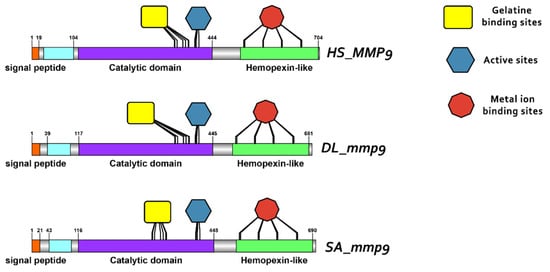
Figure 10.
Domain architecture of MMP9 proteins in human, gilthead sea bream and European seabass, including the signal peptide (orange), proteoglycan binding site (PGBD) (light blue), catalytic domain (purple), hemopexin-like domain (light green), gelatine binding sites (yellow), metal ion-binding site (red), and the active sites (dark blue) of the enzyme.
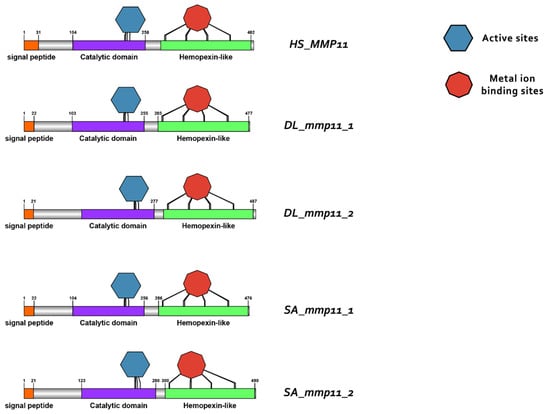
Figure 11.
Domain architecture of MMP11 proteins in human (H. sapiens), gilthead sea bream (S. aurata) and European sea bass (D. labrax), including the signal peptide (orange), catalytic domain (purple), hemopexin-like domain (light green), metal ion-binding site (red), and the active sites (dark blue) of the enzyme.
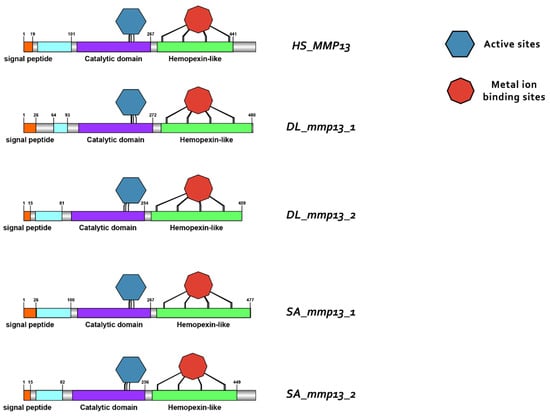
Figure 12.
Domain architecture of MMP13 proteins in human (H. sapiens), gilthead sea bream (S. aurata) and European sea bass (D. labrax), including the signal peptide (orange), proteoglycan binding site (PGBD) (light blue), catalytic domain (purple), hemopexin-like domain (light green), metal ion-binding site (red), and the active sites (dark blue) of the enzyme.

Figure 13.
Percentage of conservation in each position of the highly conserved sequence VAAHEXGHXXXXGXXH in vertebrates.
3.4. Gene Expression Analysis
3.4.1. Developmental Expression Patterns
Transcriptome analysis revealed three distinct expression patterns of mmp11 and mmp13 paralogs, both across different developmental stages and within individual stages in both species: (i) paralogs exhibited comparable expression levels, (ii) one paralog was overexpressed in comparison with its copy, indicative of differential regulatory mechanisms influencing individual expression patterns, and (iii) paralogs exhibited stage-specific expression, implying a nuanced regulation during ontogeny. Genes with fewer than 20 reads were characterized as not detectable.
While mmp11 exhibited a similar expression pattern in both species across and within developmental stages, the expression patterns of mmp13 varied subtly. Specifically, mmp13.2 demonstrated expression not detectable above threshold in the FF stage in gilthead sea bream, whereas in European sea bass, the mmp13.2 paralog exhibited expression not detectable above threshold in the FL stage. This nuanced divergence mmp13 expression underlines the intricacies of regulatory mechanisms governing specific paralogs and highlights subtle variations in their stage specific/temporal expression profiles between the two species. Single-copy genes, i.e., mmp2 and mmp9, exhibited a similar ontogenetic expression pattern in both species. This concurrence implies a conserved regulatory mechanism for these genes, elucidating a shared ontogenetic regulation in both species (Figure 14 and Figure 15).
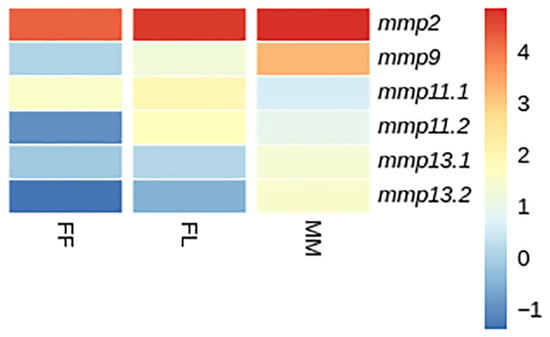
Figure 14.
Heatmap of gene expression of mmps genes in gilthead sea bream (S. aurata) between developmental stages. FF: first feeding; FL: flexion of notochord; MM: mid-metamorphosis. Expression levels are expressed as log2CPM (counts per million).
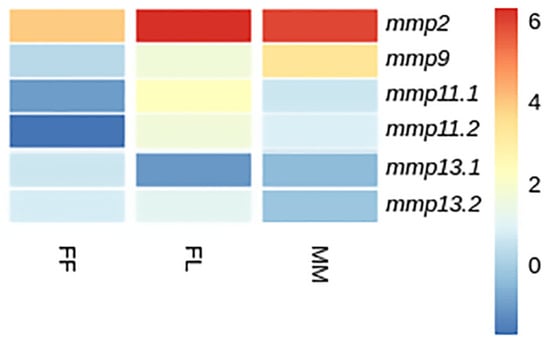
Figure 15.
Heatmap of gene expression of mmps genes in European sea bass (D. labrax) between developmental stages. FF: first feeding; FL: flexion of notochord; MM: mid-metamorphosis. Expression levels are expressed as log2CPM (counts per million).
3.4.2. Tissue Expression Patterns
Our investigation to elucidate the expression patterns of MMPs paralogs extended to the analysis of publicly available transcriptome sequencing data, from adult tissues for both species. Within the constraints of the available data, we acknowledge that not all tissues were included in the study to allow for a more comprehensive analysis. As with developmental expression patterns, the same three patterns of expression were observed (Figure 16 and Figure 17).
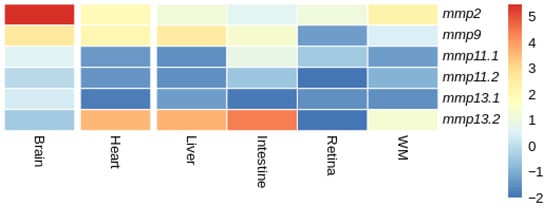
Figure 16.
Heatmap of gene expression of mmps genes in gilthead sea bream (S. aurata) across tissues. Expression levels are expressed as log2CPM (counts per million).
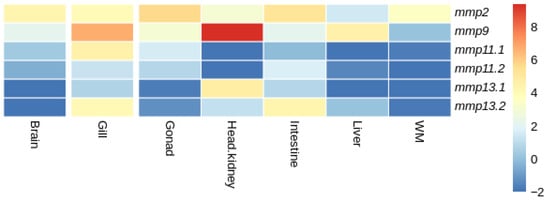
Figure 17.
Heatmap of gene expression of mmps genes in European sea bass (D. labrax) bream across tissues. Expression levels are expressed as log2CPM (counts per million).
In gilthead sea bream (S. aurata), both mmp11 paralogs were expressed at similarly low levels in the heart, liver and white muscle. Their expression was low in all tissues, yet it differentiated in the intestine, retina and brain with mmp11.1 overexpressed in comparison with mmp11.2 (Figure 16). Similarly, mmp13.2 was overexpressed in comparison with mmp13.1 in the liver, intestine, heart and white muscle, while it exhibited expression not detectable above threshold in the retina.
In European sea bass (D. labrax), expression not detectable above threshold was observed for both mmp11 paralogs in head kidney, while expression not detectable above threshold was observed for mmp11.1 and mmp11.2 in the liver and white muscle, respectively. In the gills, gonads, intestine, and liver the two paralogues were differentially expressed; the expression of mmp11.1 was higher in the gills and gonad, while mmp11.2 was expressed more in the intestine and liver. Expression not detectable above threshold was observed for both mmp13 paralogs in the brain, while expression not detectable above threshold was observed for the mmp13.1 paralog in the liver and white muscle (Figure 17).
When comparing the expression patterns of MMP paralogs between gilthead sea bream (S. aurata) and European sea bass (D. labrax), distinct differences were observed within common tissues. In particular, mmp11.1 in European sea bass (D. labrax) showed expression not detectable above threshold in the liver, in contrast to the uniform expression observed in gilthead sea bream (S. aurata). Similarly, mmp11.2 was not expressed in the white muscle of European sea bass (D. labrax) while both paralogs were expressed in gilthead sea bream (S. aurata). In European sea bass (D. labrax), neither of the two mmp13 paralogs were expressed in the brain, while in gilthead sea bream (S. aurata), both paralogs were expressed, albeit at relatively low levels. Similarly, in European sea bass (D. labrax)only one of the two paralogs of mmp13 was expressed with expression not detectable above threshold being observed for mmp13.1, whereas both mmp13 paralogs were expressed in gilthead sea bream (S. aurata). A ubiquitous expression of mmp2 and mmp9 that have no paralogs was observed in both species.
4. Discussion
4.1. Evolutionary Path of Matrix Metalloproteases in Teleosts
Matrix metalloproteases are present across all domains of life, showing remarkable complexity in vertebrates. Phylogenetic studies reveal orthologous genes in Ciona intestinalis (Sea vase), an invertebrate of the subphylum Urochordata and a close relative of vertebrates, suggesting that MMP evolution predates the vertebrate-urochordate divergence [6]. The intricate diversity within vertebrate MMPs, however, originates from evolutionary events during early vertebrate lineage development, involving extensive duplication of primordial genes. Successive duplications gave rise to the MMP gene family in vertebrates. While teleosts underwent a lineage-specific whole-genome duplication (TSGD), a similar number of genes are found in both humans and teleosts, implying a low retention of gene duplicates in teleosts [39]. The absence of certain human orthologs in teleosts, such as MMP1 and MMP8 genes, suggests the specific loss of these genes in the teleost lineage. This could be attributed to various factors, including functional redundancy, as other collagenolytic metalloproteases like MMP13 are found in two copies [40]. This redundancy may enable teleosts to achieve collagenolytic functions without relying on MMP1 and MMP8. Additionally, distinct biomechanical and physiological demands on connective tissues, such as teleosts lacking the tendon system and having more complex forms of collagen, might have influenced the collagenolytic machinery in teleosts, rendering these metalloproteases redundant [41].
In this study, we systematically identified and characterized matrix metalloproteinase (MMP) genes with collagenolytic activity. Through phylogenetic analysis, we traced the presence of paralogs within the genomes of teleost species, notably mmp11 and mmp13, back to events such as the Teleost-specific genome duplication (TSGD) and the second round of vertebrate whole-genome duplication (2R), unlike mmp2 and mmp9 that were found in one copy, with the exception of the Salmonidae that underwent a lineage-specific genome duplication after the TSGD (SsWGD). The findings for mmp2 and mmp9, retaining one copy in teleosts is in agreement with analogous phylogenetic investigations in Ctenopharyngodon idella (grass carp) and Pelteobagrus fulvidraco (yellow catfish) [42,43]. Also, these genes share a structural similarity across species, with similar domains, suggesting the conservation of the protein across all species [43].
Two paralogs of mmp11 were identified in all teleosts but the salmonids. Phylogenetic analysis suggests that these paralogs most probably derived from the TSGD, as one copy of the gene is found in non-teleost vertebrates, and the spotted gar (L. oculatus) serves as an outgroup of a well-supported duplication node at the base of teleosts. Macro-synteny analysis provided syntenic regions in both chromosomes where mmp11 resides (Supplementary Figure S1). Micro-synteny analysis for the mmp11 gene revealed a less conserved landscape across species. The genes were not arranged in conserved blocks and classified from MCScanX tool as “dispersed”, which is not expected from paralogs derived from genome duplications [44,45]. Additionally, the analysis indicated chromosome rearrangements, consistent with the process of rediploidization, after duplication events [9,46,47] (Figure 7). This discrepancy likely reflects post-WGD genome rearrangements, gene relocations, or annotation inconsistencies that disrupt conserved collinearity, leading to misclassification in computational frameworks. Such limitations are well-documented in comparative genomic analyses, particularly for older duplication events where synteny erosion may obscure true duplication origins. Unlike mmp11, which showed a lack of conserved synteny, mmp13 exhibited high synteny conservation, with a conserved gene block across species (Figure 8).
Similarly, in previous studies on the genomes of zebrafish (D. rerio) [7], fugu (T. rubripes) [48,49], and medaka (O. latipes) [50], two copies of mmp13 were identified, while in Atlantic salmon (S. salar) and rainbow trout (O. mykiss), four paralogs were identified [51,52]. Although the zebrafish (D. rerio) paralog (mmp13.2) does not seem to follow the species tree and serves as an outgroup for both spotted gar (L. oculatus) and the rest of the teleosts, this could result from poor sequence quality or divergent evolution. Other phylogenetic analyses suggests that divergent evolution is quite common after whole-genome duplication events [53,54]. The presence of two copies of mmp13 could be interpreted as a balancing counteract to the lack of mmp1 and mmp8 through sub-functionalization or gene dosage effect of the two paralogs. Although teleosts underwent the TSGD, the origins of the paralogs of mmp13 seems more vague since two copies of mmp13 can also be found in spotted gar, which did not undergo the TSGD [55]. Two possible scenarios may explain this pattern. In the first scenario, a duplication event occurred in the common ancestor of teleosts and spotted gar, followed by the pseudogenization of one or more TSGD-derived mmp13 copies in teleosts. In the second scenario, the duplication in spotted gar arose independently, while teleosts retained both mmp13 paralogs that originated from the TSGD. These alternative evolutionary pathways are illustrated in Figure 18. Interestingly, mmp1 and mmp8 genes are missing from the spotted gar (L. oculatus) genome too, emphasizing the importance of the retention of mmp13 paralogs. This scenario is further supported by the finding of high sequence identity between the mammals’ genes MMP1 and MMP13 (86% amino acid identity) [56].
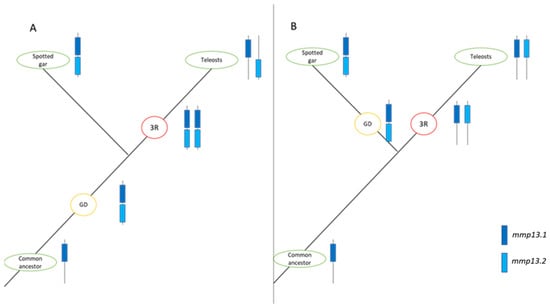
Figure 18.
Possible scenarios for the evolutionary pathway of mmp13 in spotted gar (L. oculatus) and Teleosts. mmp13.1 is represented in dark blue, and mmp13.2 in light blue. Red circles denote Teleost-Specific Whole-Genome Duplication (TS-WGD or 3R), while orange circles indicate gene or genome duplication (GD). (A). In this scenario, duplication occurred in a common ancestor, followed by the conversion of genes resulting from TS-WGD into pseudogenes. (B). This scenario suggests an independent gene duplication in the spotted gar (L. oculatus) lineage, with the conservation of TS-WGD products in teleosts.
4.2. Conservation of Structure
Protein domain and motifs analysis using the Interpro database highlighted structural similarities among MMPs, emphasizing the conserved nature of key domains for protein function. The catalytic residues of an enzyme comprise the amino acids located in the active center responsible for accelerating the enzyme-catalyzed reaction by lowering the activation energy of the reaction [57]. Evolution tends to strictly conserve the site of the catalytic metal ion. Even small variations that could affect the metal site of a metalloenzyme as MMPs are under strong selection [58]. Functional traits that provide adaptive advantages in each environment are preserved by natural selection. Preserving critical functional domains and residues across species reflect the evolutionary importance of maintaining enzymatic activity and substrate specificity [57,58]. The loss of catalytic residues in enzymes may have been the reason behind lost paralogs of MMPs. The highly conserved sequence (VAAHEXGHXXXXGXXH) in the active site of the catalytic domain of all MMPs under study across vertebrates accentuates the functional significance of this region and underscores the functionality of the proteins.
4.3. Expression Analysis
Expression analysis in both tissues and developmental stages of gilthead sea bream (S. aurata) and European sea bass (D. labrax) unveiled regulatory patterns among the paralogs, with three expression patterns being emerged, indicating potential divergences in regulatory mechanisms. Since these genes are crucial in bone [59,60,61] and tissue remodeling [62] and participate in immune responses [63], they hold great significance in the development of fish larvae. Larvae undergo dramatic changes with tissue growth, bone expansion and the need to survive in a somewhat hostile environment. Through strictly controlled degradation of the extracellular matrix (ECM) by MMPs, tissue is rearranged, facilitating the making of normal structures and ensuring physiological functions [64]. MMPs are also involved in the degradation of bone matrix during bone resorption, a process mediated by osteoclasts; in addition, MMPs help in wound healing and tissue repair, facilitating the breakdown of damaged ECM [65,66,67,68]. In the context of fish development, MMPs play a role in shaping the initial skeletal structures and modulating the ECM, ensuring proper tissue turnover [4]. Furthermore, in scenarios like fin regeneration, MMPs facilitate the remodeling of tissues, showcasing their importance in regenerative processes [69,70].
Studies in zebrafish (D. rerio) showed that one of the two paralogs is not detectable during embryogenesis, while the other appears to play a crucial role in hatching [48]. Regarding the role of mmp13 in bone remodeling in zebrafish (D. rerio), there are studies that conclude that mmp13.1 may be restricted to the endoskeletal part of the fin and the axial skeleton of zebrafish (D. rerio), and that mmp13.2 plays an important role in fin bone formation [71,72,73].
In tissues, mmp11 and mmp13 paralogs showed tissue-specific expression in both species, with inter-species differences observed. Drawing data from expression atlas (https://www.ebi.ac.uk/gxa/home, accessed on 27 January 2024) for mammals such as human (H. sapiens) (Illumina body map) and mouse (M. musculus) (Fantom5 project) for MMP11 expression across tissues, we can see a ubiquitous expression. On the contrary, in gilthead sea bream (S. aurata) and European sea bass (D. labrax) the expression of the paralogs of mmp11 is tissue-specific (Figure 16 and Figure 17).
The expression of MMP13 is tissue-specific in humans, with only a few tissues expressing this gene. On the contrary, in rodents like mice, and rabbits [74], the expression is extended to more tissues. MMP1, absent in teleosts, shares high homology with MMP13 in mammals and is ubiquitous in humans. Interestingly, there is also a rodent-specific duplication of MMP1, though no relevant expression data is available [56].
On the other hand, at least one mmp13 paralog was expressed in almost all tissues studied in gilthead sea bream (S. aurata) and European sea bass (D. labrax) except of the brain tissue in European sea bass (D. labrax). A study conducted by Castillo-Briceno and colleagues in different tissues of adult samples of gilthead sea bream (S. aurata) confirmed the expression of mmp13.2 across all tissues studied, although it should be noted that only that paralog was taken into consideration, probably due to shortcomings of the databases at the time [75].
4.4. Fate of Paralogs
The complex genetic interaction network of dispensable WGD paralogs provides insight into the long-standing question of why paralogs with overlapping functions are retained on an evolutionary time scale [76]. Duplicated genes are initially redundant in function, meaning duplicates have the same function, with none or minor phenotypic consequences in the case that one copy is eliminated [18]. The remaining paralogous gene is sufficient to compensate for the loss of the second paralog. Therefore, redundant duplicates provide an ideal source of genetic material that can be used as the origin of new genes [17]. Paralogs can follow different fates after the initial duplication. Paralogs can be maintained or eliminated through selective pressure during evolution. In general, the loss of an extra copy is more frequent than the maintenance of a functionally redundant copy [9,18,49,77] and that appears to be the case of mmp2 and mmp9 paralogs where the subsequent rediploidization process after a WGD led the paralogs from the TSGD being lost [78]. Phylogenetic analysis of the mmp2 and mmp9 genes revealed a conserved single-copy presence across all teleosts, except within the Salmonidae branch, where two copies were observed, consistent with the fourth round of genome duplication specific to this lineage.
In circumstances where the duplicated gene is retained, mutations may arise in the coding or regulatory sequence at different positions of each duplicate, leading to changes in the coding sequence and the protein structure, or the spatiotemporal regulation of the gene expression [18,49,79,80,81,82,83]. While transcriptome analysis provides insight into the expression patterns of the retained paralogs of mmp11 and mmp13 in gilthead sea bream (S. aurata) and European sea bass (D. labrax), determining their exact evolutionary fate requires more direct functional assays.
Considering conserved catalytic domain sequences between paralogs, we hypothesize that the catalytic function of both paralogs is retained. The identification of distinct expression levels and patterns either between developmental stages or across tissues suggests a degree of regulatory sub-functionalization. The nuanced variations in expression may be ascribed to mutations in regulatory elements or the acquisition of specific cis-regulatory motifs, handing a differential response of the paralogs. However, since mmp11 is expressed across tissues in mammals and in teleosts pose a tissue-specific expression and mmp13 seems to have broader function in teleosts, compensating probably for the loss of MMP1, the regulatory neo-functionalization cannot be excluded.
4.5. Limitations of the Study
While this study provides biologically meaningful insights into the evolution and expression of collagenolytic MMPs in teleosts and advances our current knowledge, certain methodological limitations should be acknowledged.
RNA-seq analyses for developmental stages were conducted on pooled samples without biological replicates, limiting the statistical power for differential expression. Similarly, publicly available transcriptomic datasets used for tissue expression analysis also lacked replicates, constraining the robustness of statistical comparisons. Although all analyses followed best practices for non-replicated datasets (edgeR), and expression patterns were consistent across stages and species and biologically meaningful, the findings should be interpreted with caution and validated experimentally.
Additionally, functional predictions of protein domains are relied on in silico tools, which are subject to limitations such as annotation quality and algorithmic assumptions. Some undetected domains may reflect technical artifacts rather than true biological absence. As such, domain-based inferences should be considered preliminary until supported by experimental validation.
5. Conclusions
This study tested the hypothesis that matrix metalloprotease (MMP) paralogs in teleosts, specifically mmp11 and mmp13, have undergone regulatory divergence following major duplication events such as the second round of vertebrate whole-genome duplication (2R) and the teleost-specific genome duplication (TSGD). Phylogenetic and synteny analyses confirmed a teleost-specific expansion of collagenolytic MMPs and revealed the absence of mmp1, a major interstitial collagenase present in mammals. Our data suggests that this loss may have driven compensatory functional specialization of mmp13a and mmp13b, which display differential and tissue-specific transcriptional regulation in gilthead sea bream (S. aurata) and European sea bass (D. labrax). Through comparative phylogenetic analysis and transcriptomic profiling, we demonstrate that these paralogs are not only retained but have acquired distinct expression patterns across tissues and developmental stages. This divergence, despite conservation of key catalytic residues, supports a model of regulatory sub-functionalization and potentially regulatory neo-functionalization. These findings provide mechanistic insight into how gene duplicates evolve under selective pressures to perform complementary roles in processes such as tissue remodeling, immune modulation, and skeletal development. Future functional studies, including gene knockout or knockdown experiments, are essential to validate the physiological relevance of these paralogs and to further elucidate the consequences of their differential regulation. Overall, this work offers a refined perspective on how genome duplication events contribute to the diversification and specialization of gene functions, enhancing our understanding of the molecular evolution of vertebrate genomes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani15223270/s1, Figure S1. Exon–intron structure of mmp2 genes in Dicentrarchus labrax (DL), Sparus aurata (SA), and Homo sapiens (HS). Schematic representation of the gene architecture of mmp2 orthologs based on genomic and coding sequences. Coding sequences (CDS) are shown as purple boxes, introns as black lines, and untranslated regions (UTRs or upstream/downstream regions) as yellow boxes. The direction of transcription proceeds from 5′ to 3′. Scale bars indicate relative distances; Figure S2. Exon–intron structure of mmp9 genes in Dicentrarchus labrax (DL), Sparus aurata (SA), and Homo sapiens (HS). Schematic representation of the gene architecture of mmp9 orthologs based on genomic and coding sequences. Coding sequences (CDS) are shown as purple boxes, introns as black lines, and untranslated regions (UTRs or upstream/downstream regions) as yellow boxes. The direction of transcription proceeds from 5′ to 3′. Scale bars indicate relative distances; Figure S3. Exon–intron structure of mmp11 genes in Dicentrarchus labrax (DL), Sparus aurata (SA), and Homo sapiens (HS). Schematic representation of the gene architecture of mmp11 orthologs based on genomic and coding sequences. Coding sequences (CDS) are shown as purple boxes, introns as black lines, and untranslated regions (UTRs or upstream/downstream regions) as yellow boxes. The direction of transcription proceeds from 5′ to 3′. Scale bars indicate relative distances; Figure S4. Exon–intron structure of mmp13 genes in Dicentrarchus labrax (DL), Sparus aurata (SA), and Homo sapiens (HS). Schematic representation of the gene architecture of mmp13 orthologs based on genomic and coding sequences. Coding sequences (CDS) are shown as purple boxes, introns as black lines, and untranslated regions (UTRs or upstream/downstream regions) as yellow boxes. The direction of transcription proceeds from 5′ to 3′. Scale bars indicate relative distances; Figure S5. Circos plot depicting the paralogue relationships between gilthead sea bream genes. Duplicated gene relationships between gilthead sea bream chromosomes are represented by inner lines; Figure S6. Circos plot depicting the paralogue relationships between European sea bass genes. Duplicated gene relationships between European sea bass chromosomes are represented by inner lines. Table S1. Public RNAseq data from various tissues in both S. aurata and D. labrax. Missing tissue for each species is reported with “-”.
Author Contributions
Conceptualization, K.A.M. and R.A.; methodology, R.A., A.T. and T.G.; sampling, A.T.; formal analysis, R.A., A.T. and I.D.M.; investigation, R.A., A.T. and I.D.M.; data curation, R.A., A.T. and I.D.M.; writing—original draft preparation, R.A. and T.G.; writing—review and editing, T.G., Z.M. and K.A.M.; visualization, R.A. and I.D.M.; funding acquisition, K.A.M.; supervision, K.A.M.; project administration, Z.M. and K.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work funded by the European Union through project H2020-SFS2016-2-727610 “PerformFISH: Consumer-driven production: Integrating Innovative Approaches for Competitive and Sustainable Performance across the Mediterranean Aquaculture Value Chain”. This output reflects only the authors; views, and the European Union cannot be held responsible for any use that may be made of the information contained herein.
Institutional Review Board Statement
Fish were reared at the Hellenic Center of Marine Research (HCMR) in Crete, Greece. The HCMR facilities are certified from the national veterinary authority (code GR94FISH0001) and are licensed for operations of breeding and experimentation with fish issued by Region of Crete, General Directorate of Agricultural & Veterinary no. 3989/01.03.2017 (approval codes EL91-BIObr-03 and EL91-BIOexp-04). The experimental protocol was approved by the Veterinarian Authority of the Region of Crete with the 255,332/29-11-2017 document. Animal experiments were carried out in accordance with the EU Directive 2010/63/EU.
Informed Consent Statement
Not applicable.
Data Availability Statement
All consensus sequences were retrieved from the Short Read archive (SRA) under Accession No (D. labrax: PRJNA1050410 and S. aurata: PRJNA1050571).
Acknowledgments
The authors acknowledge HCMR Hellenic Centre for Marine Research for supplying the samples. The authors also acknowledge the Aqua-FAANG project for generating the transcriptome sequencing data that is publicly available in the SRA.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sherman, V.R.; Yang, W.; Meyers, M.A. The Materials Science of Collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50. [Google Scholar] [CrossRef]
- Moorehead, C.; Prudnikova, K.; Marcolongo, M. The Regulatory Effects of Proteoglycans on Collagen Fibrillogenesis and Morphology Investigated Using Biomimetic Proteoglycans. J. Struct. Biol. 2019, 206, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.E.; Vuong, T.T.; Rønning, S.B.; Kolset, S.O. Matrix Metalloproteinases in Fish Biology and Matrix Turnover. Matrix Biol. 2015, 44–46, 86–93. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (MMPs): Chemical–Biological Functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Huxley-Jones, J.; Clarke, T.K.; Beck, C.; Toubaris, G.; Robertson, D.L.; Boot-Handford, R.P. The Evolution of the Vertebrate Metzincins; Insights from Ciona Intestinalis and Danio Rerio. BMC Evol. Biol. 2007, 7, 63. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix Metalloproteinases: Evolution, Gene Regulation and Functional Analysis in Mouse Models. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef]
- Marino-Puertas, L.; Goulas, T.; Gomis-Rüth, F.X. Matrix Metalloproteinases Outside Vertebrates. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2026–2035. [Google Scholar] [CrossRef]
- Robertson, F.M.; Gundappa, M.K.; Grammes, F.; Hvidsten, T.R.; Redmond, A.K.; Lien, S.; Martin, S.A.M.; Holland, P.W.H.; Sandve, S.R.; Macqueen, D.J. Lineage-Specific Rediploidization Is a Mechanism to Explain Time-Lags between Genome Duplication and Evolutionary Diversification. Genome Biol. 2017, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.S.; Van de Peer, Y.; Braasch, I.; Meyer, A. Comparative Genomics Provides Evidence for an Ancient Genome Duplication Event in Fish. Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 1661–1679. [Google Scholar] [CrossRef]
- Conant, G.C.; Wolfe, K.H. Turning a Hobby into a Job: How Duplicated Genes Find New Functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S. Evolution by Gene Duplication; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Taylor, J.S.; Braasch, I.; Frickey, T.; Meyer, A.; Van de Peer, Y. Genome Duplication, a Trait Shared by 22,000 Species of Ray-Finned Fish. Genome Res. 2003, 13, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Van De Peer, Y. From 2R to 3R: Evidence for a Fish-Specific Genome Duplication (FSGD). BioEssays 2005, 27, 937–945. [Google Scholar] [CrossRef]
- Szklarczyk, R.; Huynen, M.A.; Snel, B. Complex Fate of Paralogs. BMC Evol. Biol. 2008, 8, 337. [Google Scholar] [CrossRef]
- Edger, P.P.; Pires, J.C. Gene and Genome Duplications: The Impact of Dosage-Sensitivity on the Fate of Nuclear Genes. Chromosom. Res. 2009, 17, 699–717. [Google Scholar] [CrossRef]
- Glasauer, S.M.K.K.; Neuhauss, S.C.F.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Drobek, M. Paralogous Genes Involved in Embryonic Development: Lessons from the Eye and Other Tissues. Genes 2022, 13, 2082. [Google Scholar] [CrossRef]
- Young, D.; Das, N.; Anowai, A.; Dufour, A. Matrix Metalloproteases as Influencers of the Cells’ Social Media. Int. J. Mol. Sci. 2019, 20, 3847. [Google Scholar] [CrossRef]
- Pauletto, M.; Manousaki, T.; Ferraresso, S.; Babbucci, M.; Tsakogiannis, A.; Louro, B.; Vitulo, N.; Quoc, V.H.; Carraro, R.; Bertotto, D.; et al. Genomic Analysis of Sparus Aurata Reveals the Evolutionary Dynamics of Sex-Biased Genes in a Sequential Hermaphrodite Fish. Commun. Biol. 2018, 1, 119. [Google Scholar] [CrossRef] [PubMed]
- Tine, M.; Kuhl, H.; Gagnaire, P.A.; Louro, B.; Desmarais, E.; Martins, R.S.T.; Hecht, J.; Knaust, F.; Belkhir, K.; Klages, S.; et al. European Sea Bass Genome and Its Variation Provide Insights into Adaptation to Euryhalinity and Speciation. Nat. Commun. 2014, 5, 5770. [Google Scholar] [CrossRef]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Ridwan Amode, M.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Ashkenazy, H.; Katoh, K.; Pupko, T. GUIDANCE2: Accurate Detection of Unreliable Alignment Regions Accounting for the Uncertainty of Multiple Parameters. Nucleic Acids Res. 2015, 43, W7–W14. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Tsipourlianos, A.; Cardoso, J.C.R.; Angelakopoulos, R.; Kotoula, A.; Power, D.M.; Mamuris, Z.; Moutou, K.A. Regulatory Subfunctionalization Drives OXPHOS Evolution in Teleosts. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kinsella, R.J.; Kähäri, A.; Haider, S.; Zamora, J.; Proctor, G.; Spudich, G.; Almeida-King, J.; Staines, D.; Derwent, P.; Kerhornou, A.; et al. Ensembl BioMarts: A Hub for Data Retrieval across Taxonomic Space. Database 2011, 2011, bar030. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Ma, J.; Luo, X.; Nie, P.; Zuo, Z.; Lahrmann, U.; Zhao, Q.; Zheng, Y.; Zhao, Y.; et al. IBS: An Illustrator for the Presentation and Visualization of Biological Sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef]
- Papandroulakis, N.; Divanach, P.; Anastasiadis, P.; Kentouri, M. The Pseudo-Green Water Technique for Intensive Rearing of Sea Bream (Sparus aurata) Larvae. Aquac. Int. 2001, 9, 205–216. [Google Scholar] [CrossRef]
- Kourkouta, C.; Tsipourlianos, A.; Power, D.M.; Moutou, K.A.; Koumoundouros, G. Variability of Key-Performance-Indicators in Commercial Gilthead Seabream Hatcheries. Sci. Rep. 2022, 12, 17896. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Cañon-Beltrán, K.; Dovolou, E.; Kalemkeridou, M.; Nanas, I.; Rizos, D.; Moutou, K.A.; Mamuris, Z.; Amiridis, G.S. Oviductal Epithelial Cells Transcriptome and Extracellular Vesicles Characterization during Thermoneutral and Heat Stress Conditions in Dairy Cows. Theriogenology 2022, 187, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting Batch Effects in Microarray Expression Data Using Empirical Bayes Methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Amălinei, C.; Căruntu, I.; Anca Bălan, R. Biology of Metalloproteinases. Rom. J. Morphol. Embryol. 2007, 48, 323–334. [Google Scholar]
- Gout, J.F.; Hao, Y.; Johri, P.; Arnaiz, O.; Doak, T.G.; Bhullar, S.; Couloux, A.; Guérin, F.; Malinsky, S.; Potekhin, A.; et al. Dynamics of Gene Loss Following Ancient Whole-Genome Duplication in the Cryptic Paramecium Complex. Mol. Biol. Evol. 2023, 40, msad107. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ma, L.; Wang, G.; Li, M.; Zhang, Z. Old Genes Experience Stronger Translational Selection than Young Genes. Gene 2016, 590, 29–34. [Google Scholar] [CrossRef]
- Xu, X.Y.; Shen, Y.B.; Fu, J.J.; Liu, F.; Guo, S.Z.; Yang, X.M.; Li, J. Le Matrix Metalloproteinase 2 of Grass Carp Ctenopharyngodon Idella (CiMMP2) Is Involved in the Immune Response against Bacterial Infection. Fish Shellfish Immunol. 2012, 33, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Wang, Y.; Hong, J.; Xu, C.; Chen, H.; Zhou, S.B. Characterization of MMP-9 Gene from a Normalized CDNA Library of Kidney Tissue of Yellow Catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2015, 45, 260–267. [Google Scholar] [CrossRef]
- Aase-Remedios, M.E.; Ferrier, D.E.K. Improved Understanding of the Role of Gene and Genome Duplications in Chordate Evolution With New Genome and Transcriptome Sequences. Front. Ecol. Evol. 2021, 9, 703163. [Google Scholar] [CrossRef]
- Catchen, J.M.; Conery, J.S.; Postlethwait, J.H. Automated Identification of Conserved Synteny after Whole-Genome Duplication. Genome Res. 2009, 19, 1497–1505. [Google Scholar] [CrossRef]
- Luo, J.; Chai, J.; Chai, J.; Wen, Y.; Wen, Y.; Wen, Y.; Tao, M.; Lin, G.; Liu, X.; Ren, L.; et al. From Asymmetrical to Balanced Genomic Diversification during Rediploidization: Subgenomic Evolution in Allotetraploid Fish. Sci. Adv. 2020, 6, 7677–7704. [Google Scholar] [CrossRef]
- Woods, I.G.; Kelly, P.D.; Chu, F.; Ngo-Hazelett, P.; Yan, Y.-L.; Huang, H.; Postlethwait, J.H.; Talbot, W.S. A Comparative Map of the Zebrafish Genome. Genome Res. 2000, 10, 1903–1914. [Google Scholar] [CrossRef]
- Small, C.D.; El-Khoury, M.; Deslongchamps, G.; Benfey, T.J.; Crawford, B.D. Matrix Metalloproteinase 13 Activity Is Required for Normal and Hypoxia-Induced Precocious Hatching in Zebrafish Embryos. J. Dev. Biol. 2020, 8, 3. [Google Scholar] [CrossRef]
- Woolfe, A.; Elgar, G. Comparative Genomics Using Fugu Reveals Insights into Regulatory Subfunctionalization. Genome Biol. 2007, 8, R53. [Google Scholar] [CrossRef]
- Wyatt, R.A.; Keow, J.Y.; Harris, N.D.; Haché, C.A.; Li, D.H.; Crawford, B.D. The Zebrafish Embryo: A Powerful Model System for Investigating Matrix Remodeling. Zebrafish 2009, 6, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, D.J.; Johnston, I.A. A Well-Constrained Estimate for the Timing of the Salmonid Whole Genome Duplication Reveals Major Decoupling from Species Diversification. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132881. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Thorgaard, G.H. Tetraploidy and the Evolution of Salmonid Fishes. In Evolutionary Genetics of Fishes; Springer: Boston, MA, USA, 1984; pp. 1–53. [Google Scholar] [CrossRef]
- Grone, B.P.; Maruska, K.P. Divergent Evolution of Two Corticotropin-Releasing Hormone (CRH) Genes in Teleost Fishes. Front. Neurosci. 2015, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, J.; Qu, C.; Wang, L.; Huang, G.; Niu, P.; Zhong, Z.; Hong, F.; Wang, G.; Postlethwait, J.H.; et al. Molecular Evolution and Functional Divergence of Zebrafish (Danio rerio) Cryptochrome Genes. Sci. Rep. 2015, 5, 8113. [Google Scholar] [CrossRef]
- Braasch, I.; Gehrke, A.R.; Smith, J.J.; Kawasaki, K.; Manousaki, T.; Pasquier, J.; Amores, A.; Desvignes, T.; Batzel, P.; Catchen, J.; et al. The Spotted Gar Genome Illuminates Vertebrate Evolution and Facilitates Human-Teleost Comparisons. Nat. Genet. 2016, 48, 427–437. [Google Scholar] [CrossRef]
- Foley, C.J.; Kuliopulos, A. Mouse Matrix Metalloprotease-1a (Mmp1a) Gives New Insight Into MMP Function. J. Cell. Physiol. 2014, 229, 1875. [Google Scholar] [CrossRef]
- Ribeiro, A.J.M.; Tyzack, J.D.; Borkakoti, N.; Holliday, G.L.; Thornton, J.M. A Global Analysis of Function and Conservation of Catalytic Residues in Enzymes. J. Biol. Chem. 2020, 295, 314. [Google Scholar] [CrossRef] [PubMed]
- Valasatava, Y.; Rosato, A.; Furnham, N.; Thornton, J.M.; Andreini, C. To What Extent Do Structural Changes in Catalytic Metal Sites Affect Enzyme Function? J. Inorg. Biochem. 2018, 179, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Wargelius, A.; Fjelldal, P.G.; Grini, A.; Gil-Martens, L.; Kvamme, B.O.; Hansen, T. MMP-13 (Matrix MetalloProteinase 13) Expression Might Be an Indicator for Increased ECM Remodeling and Early Signs of Vertebral Compression in Farmed Atlantic Salmon (Salmo salar L.). J. Appl. Ichthyol. 2010, 26, 366–371. [Google Scholar] [CrossRef]
- de Vrieze, E.; Sharif, F.; Metz, J.R.; Flik, G.; Richardson, M.K. Matrix Metalloproteinases in Osteoclasts of Ontogenetic and Regenerating Zebrafish Scales. Bone 2011, 48, 704–712. [Google Scholar] [CrossRef]
- Liu, R.; Imangali, N.; Ethiraj, L.P.; Carney, T.J.; Winkler, C. Transcriptome Profiling of Osteoblasts in a Medaka (Oryzias latipes) Osteoporosis Model Identifies Mmp13b as Crucial for Osteoclast Activation. Front. Cell Dev. Biol. 2022, 10, 775512. [Google Scholar] [CrossRef]
- Mazzoni, T.S.; Nostro, F.L.L.; Antoneli, F.N.; Quagio-Grassiotto, I. Action of the Metalloproteinases in Gonadal Remodeling during Sex Reversal in the Sequential Hermaphroditism of the Teleostei Fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae). Cells 2018, 7, 34. [Google Scholar] [CrossRef]
- Castillo-Briceño, P.; Sepulcre, M.P.; Chaves-Pozo, E.; Meseguer, J.; García-Ayala, A.; Mulero, V. Collagen Regulates the Activation of Professional Phagocytes of the Teleost Fish Gilthead Seabream. Mol. Immunol. 2009, 46, 1409–1415. [Google Scholar] [CrossRef]
- Mundell, N.A.; Jessen, J.R.; Mundell, N.A.; Jessen, J.R.; Desimone, D.W. Extracellular Matrix Remodeling in Zebrafish Development. In Extracellular Matrix in Development; Springer: Berlin/Heidelberg, Germany, 2013; pp. 187–218. [Google Scholar] [CrossRef]
- Bai, S.; Thummel, R.; Godwin, A.R.; Nagase, H.; Itoh, Y.; Li, L.; Evans, R.; McDermott, J.; Seiki, M.; Sarras, M.P. Matrix Metalloproteinase Expression and Function during Fin Regeneration in Zebrafish: Analysis of MT1-MMP, MMP2 and TIMP2. Matrix Biol. 2005, 24, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ceballos-Francisco, D.; Guardiola, F.A.; Huang, D.; Esteban, M.Á. Skin Wound Healing in Gilthead Seabream (Sparus aurata L.) Fed Diets Supplemented with Arginine. Fish Shellfish Immunol. 2020, 104, 347–358. [Google Scholar] [CrossRef]
- Schmidt, J.G.; Andersen, E.W.; Ersbøll, B.K.; Nielsen, M.E. Muscle Wound Healing in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 48, 273–284. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of Matrix Metalloproteinase in Wound Healing. Am. J. Transl. Res. 2022, 14, 4391. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y. Role of Matrix Metalloproteinases in Skeletal Muscle. Cell Adhes. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Murawala, H.; Patel, S.; Ranadive, I.; Desai, I.; Balakrishnan, S. Variation in Expression and Activity Pattern of Mmp2 and Mmp9 on Different Time Scales in the Regenerating Caudal Fin of Poecilia latipinna. J. Fish Biol. 2018, 92, 1604–1619. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Akimenko, M.A. Inhibition of Mmp13a during Zebrafish Fin Regeneration Disrupts Fin Growth, Osteoblasts Differentiation, and Laminin Organization. Dev. Dyn. 2020, 249, 187–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Zhao, G.; Yokoyama, T.; Vega, H.; Huang, Y.; Sood, R.; Bishop, K.; Maduro, V.; Accardi, J.; et al. ATP6V1H Deficiency Impairs Bone Development through Activation of MMP9 and MMP13. PLoS Genet. 2017, 13, e1006481. [Google Scholar] [CrossRef]
- Kessels, M.Y.; Huitema, L.A.F.; Boeren, S.; Kranenbarg, S.; Schulte-Merker, S.; Van Leeuwen, J.L.; De Vries, S.C. Proteomics Analysis of the Zebrafish Skeletal Extracellular Matrix. PLoS ONE 2014, 9, e90568. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional Regulation of Collagenase (MMP-1, MMP-13) Genes in Arthritis: Integration of Complex Signaling Pathways for the Recruitment of Gene-Specific Transcription Factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Castillo-Briceño, P.; Arizcun-Arizcun, M.; Meseguer, J.; Mulero, V.; García-Ayala, A. Correlated Expression Profile of Extracellular Matrix-Related Molecules during the Inflammatory Response of the Teleost Fish Gilthead Seabream. Dev. Comp. Immunol. 2010, 34, 1051–1058. [Google Scholar] [CrossRef]
- Kuzmin, E.; Vandersluis, B.; Ba, A.N.N.; Wang, W.; Koch, E.N.; Usaj, M.; Khmelinskii, A.; Usaj, M.M.; van Leeuwen, J.; Kraus, O.; et al. Exploring Whole-Genome Duplicate Gene Retention with Complex Genetic Interaction Analysis. Science 2020, 368, eaaz5667. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.; Koop, B.F.; Sandve, S.R.; Miller, J.R.; Kent, M.P.; Nome, T.; Hvidsten, T.R.; Leong, J.S.; Minkley, D.R.; Zimin, A.; et al. The Atlantic Salmon Genome Provides Insights into Rediploidization. Nature 2016, 533, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Hou, G.Y.; Kong, X.F.; Li, C.Y.; Zeng, J.M.; Li, H.D.; Xiao, G.B.; Li, X.M.; Sun, X.W. The Fate of Recent Duplicated Genes Following a Fourth-Round Whole Genome Duplication in a Tetraploid Fish, Common Carp (Cyprinus carpio). Sci. Rep. 2015, 5, srep08199. [Google Scholar] [CrossRef]
- Duarte, J.M.; Cui, L.; Wall, P.K.; Zhang, Q.; Zhang, X.; Leebens-Mack, J.; Ma, H.; Altman, N.; DePamphilis, C.W. Expression Pattern Shifts Following Duplication Indicative of Subfunctionalization and Neofunctionalization in Regulatory Genes of Arabidopsis. Mol. Biol. Evol. 2006, 23, 469–478. [Google Scholar] [CrossRef]
- Maouche, A.; Curran, E.; Goupil, A.S.; Sambroni, E.; Bellaiche, J.; Le Gac, F.; Lareyre, J.J. New Insights into the Evolution, Hormonal Regulation, and Spatiotemporal Expression Profiles of Genes Involved in the Gfra1/Gdnf and Kit/Kitlg Regulatory Pathways in Rainbow Trout Testis. Fish Physiol. Biochem. 2018, 44, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.R.; Berg, J.J.; Blinka, S.; Rausher, M.D.; Baum, D.A. Precise Spatio-Temporal Regulation of the Anthocyanin Biosynthetic Pathway Leads to Petal Spot Formation in Clarkia gracilis (Onagraceae). New Phytol. 2013, 197, 958–969. [Google Scholar] [CrossRef]
- Shew, C.J.; Carmona-Mora, P.; Soto, D.C.; Mastoras, M.; Roberts, E.; Rosas, J.; Jagannathan, D.; Kaya, G.; O’Geen, H.; Dennis, M.Y. Diverse Molecular Mechanisms Contribute to Differential Expression of Human Duplicated Genes. Mol. Biol. Evol. 2021, 38, 3060–3077. [Google Scholar] [CrossRef]
- MacCarthy, T.; Bergman, A. The Limits of Subfunctionalization. BMC Evol. Biol. 2007, 7, 213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).