Multi-Omics Deciphers Divergent Mechanisms in Differentially Cardiac-Remodeled Yili Horses Under Conditions of Equivalent Power Output
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design and Horse Grouping
2.3. Echocardiographic Detection of Cardiac Structure and Function
2.4. Blood Sample Collection
2.5. Metabolomic Analysis: Experimental Design and Methods
2.5.1. Plasma Lipidomic Detection (UPLC-MS/MS)
2.5.2. Data Preprocessing
2.5.3. PCA and OPLS-DA for Differential Lipid Screening
2.5.4. KEGG Enrichment Analysis of Differentially Expressed Metabolites
2.6. Transcriptomics Analysis
2.6.1. RNA Extraction and Library Construction
2.6.2. Library Quality Control and Sequencing
2.6.3. Bioinformatics Analysis
2.6.4. Differential Gene Screening
2.6.5. Functional Annotation and Pathway Analysis of DE mRNAs
2.6.6. Isolation, Library Construction, Sequencing, and Identification of miRNAs
2.6.7. Target Gene Prediction of DEmiRNAs
2.7. Integration of Transcriptomic and Metabolomic Data
2.8. miRNA Isolation, cDNA Library Construction, and Sequencing Identification
2.9. RT-qPCR Validation of mRNA and miRNA
2.10. Statistical Analysis
3. Results
3.1. Echocardiographic Parameters of Horses
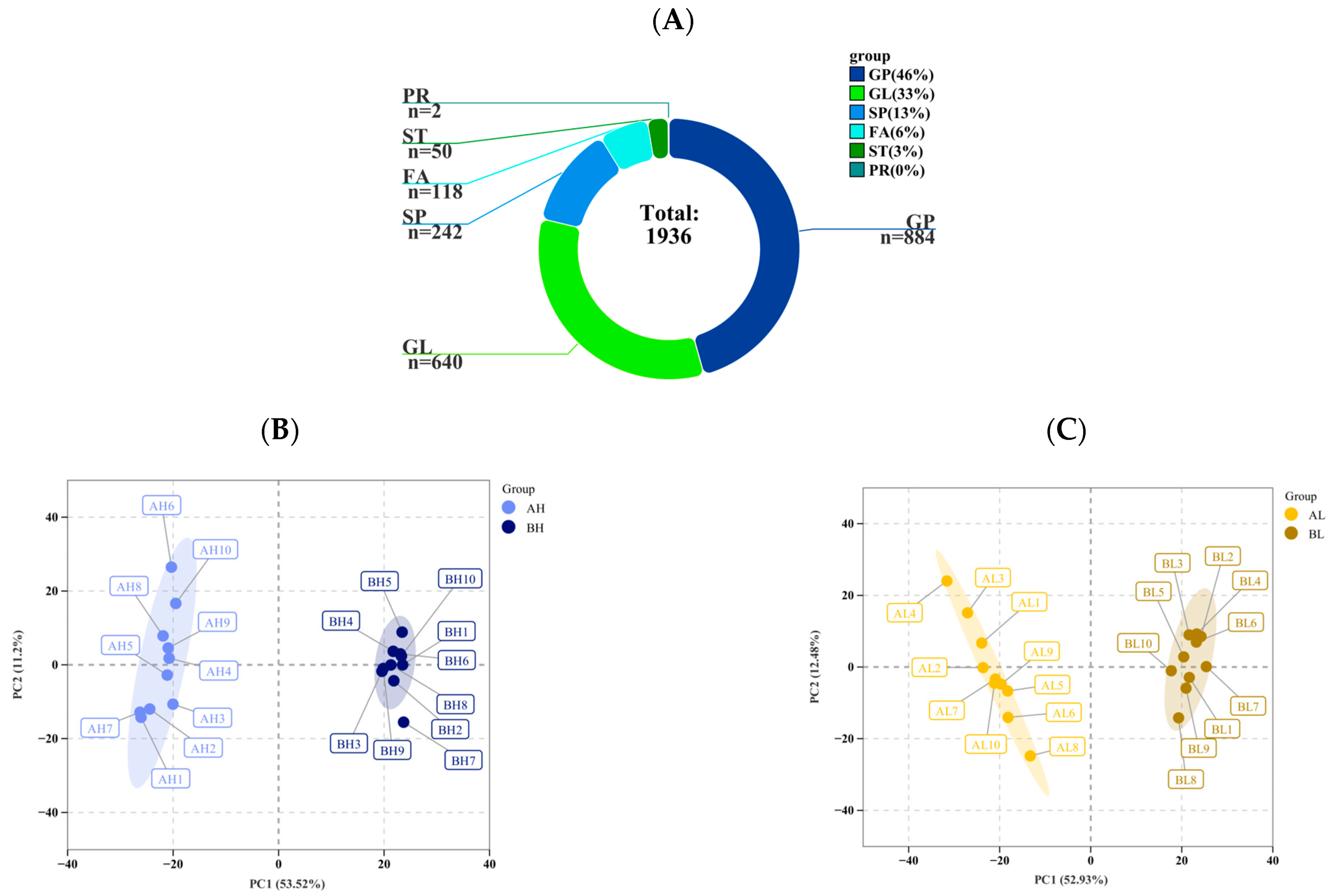
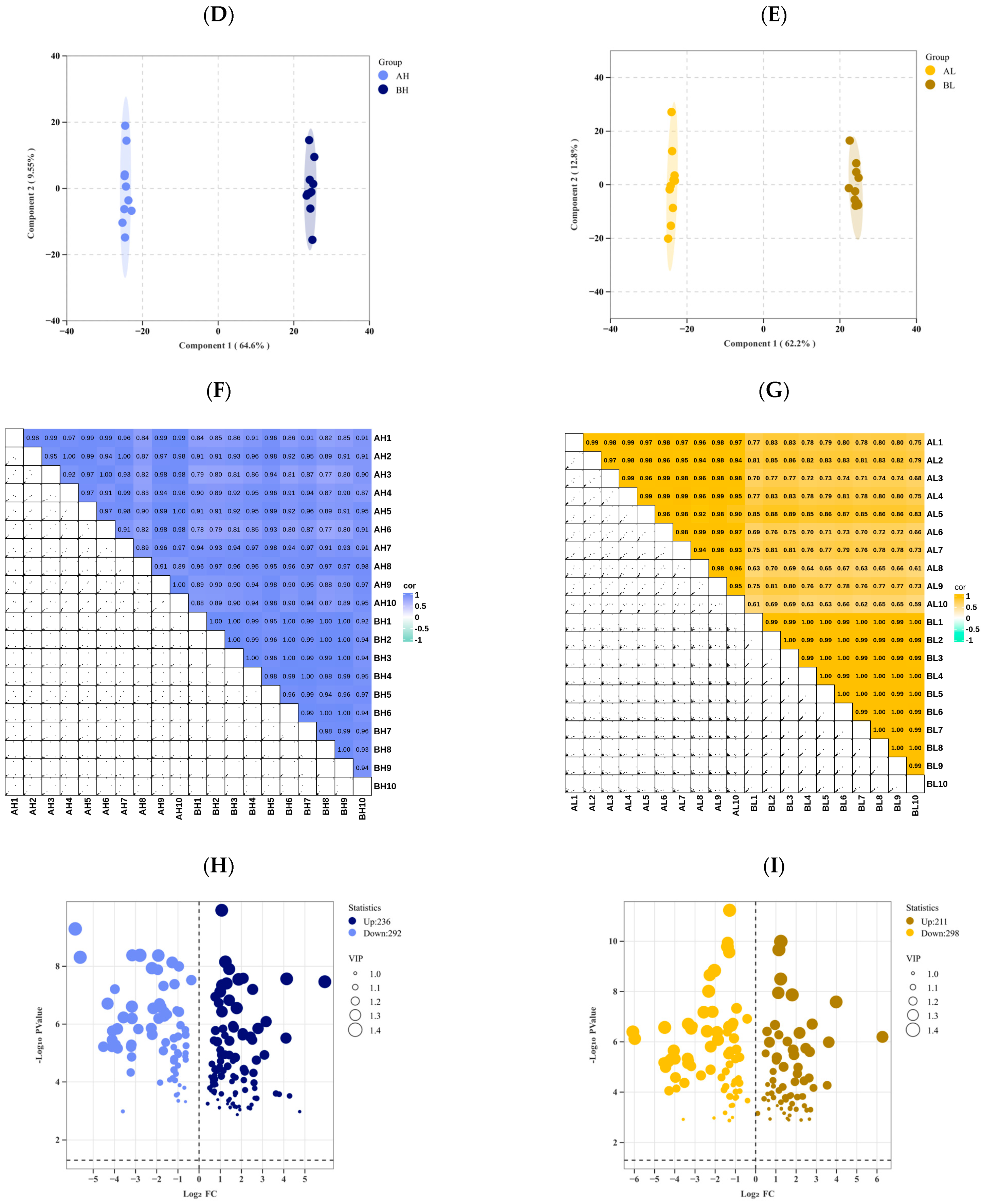
3.2. Metabolomic Analysis
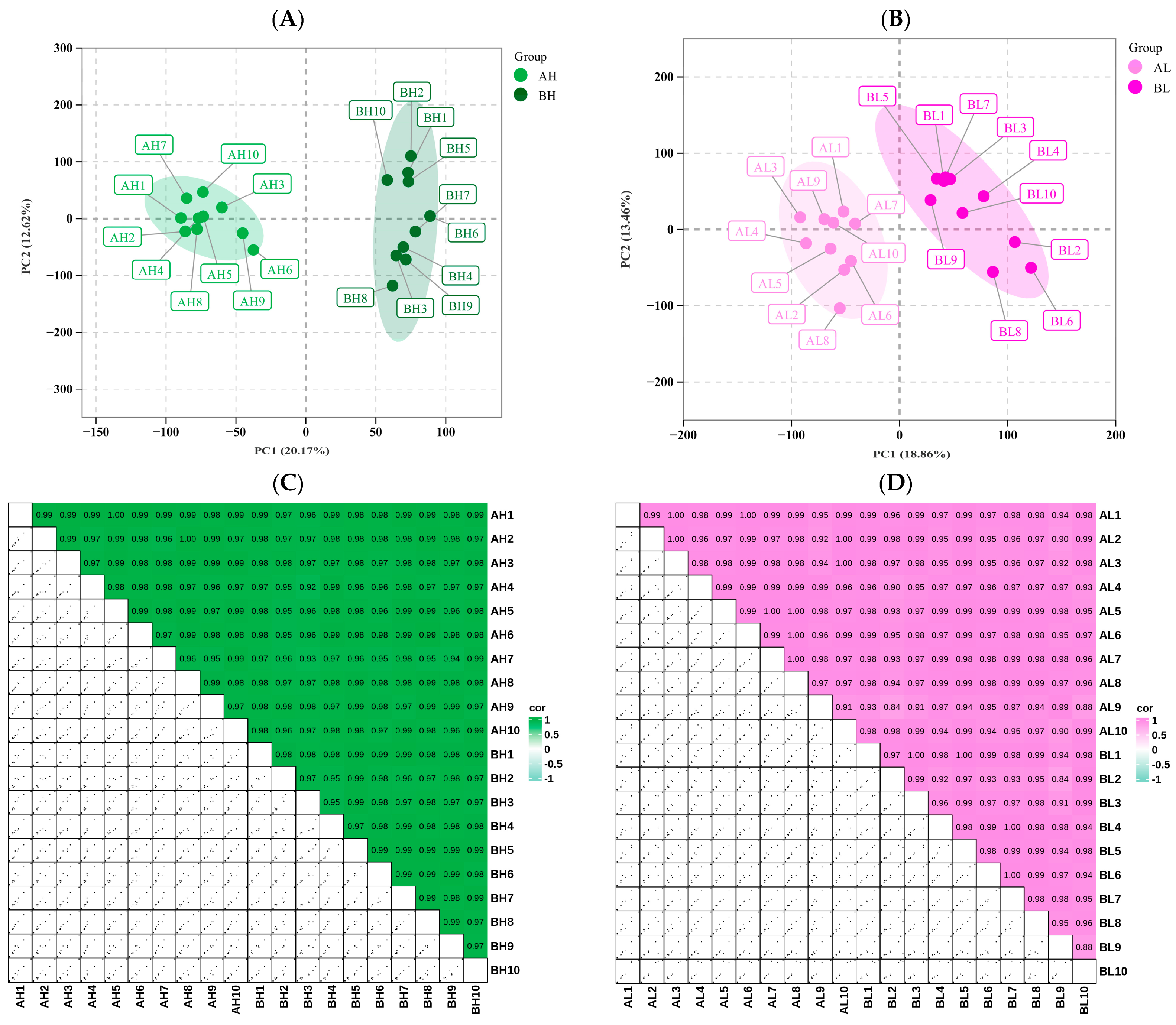
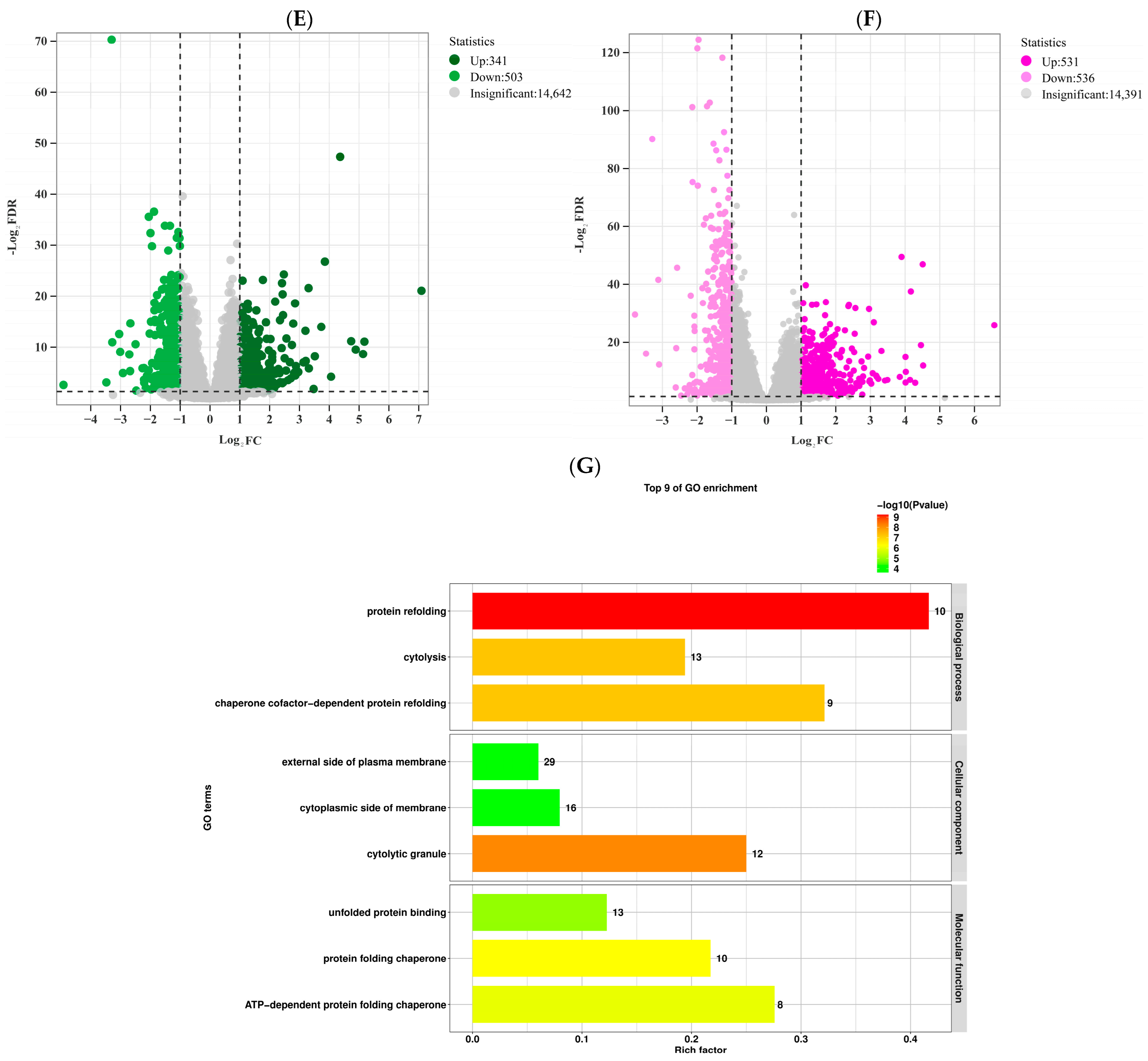
3.3. Transcriptomic Analysis
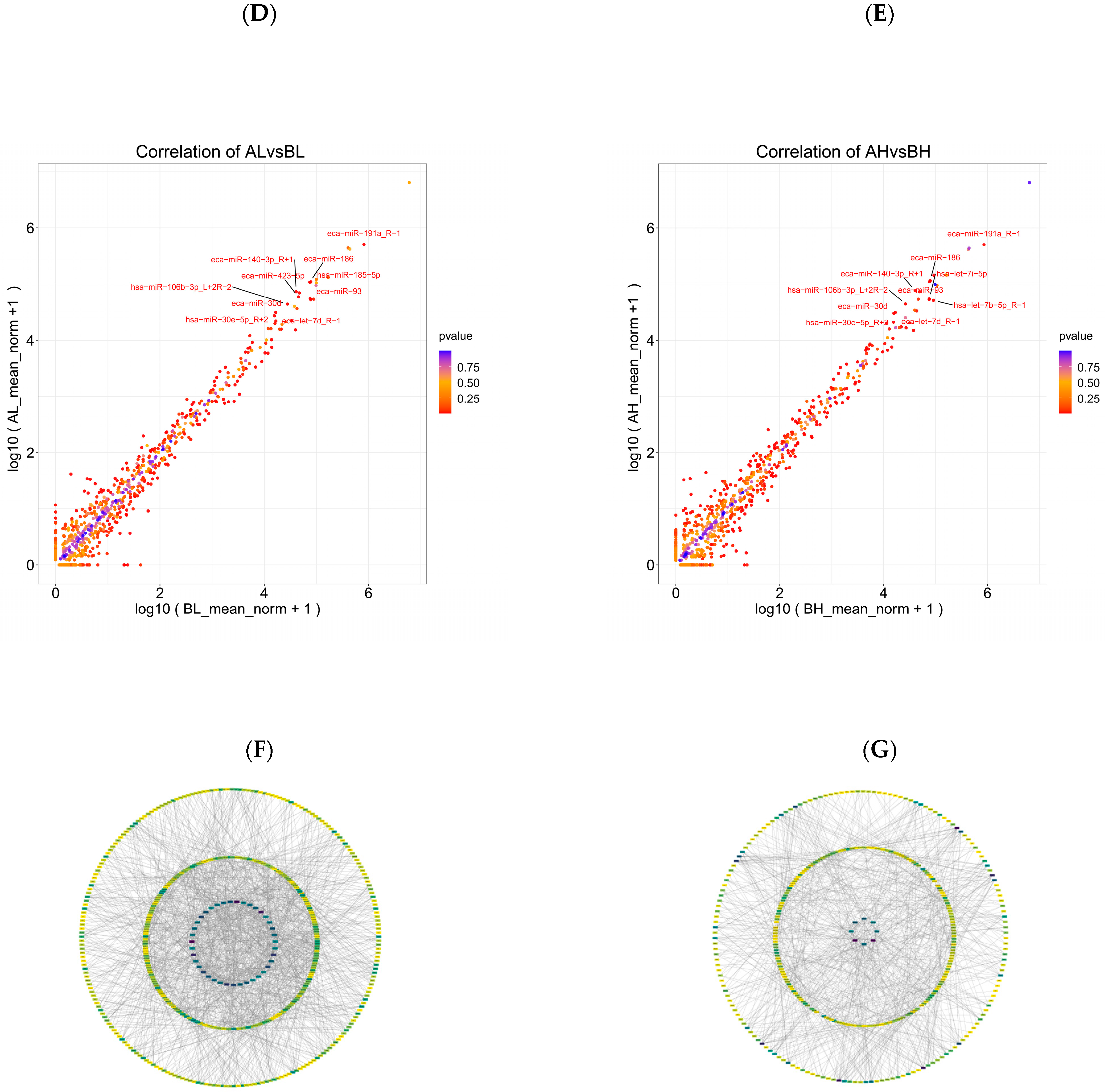
3.4. miRNA Analysis
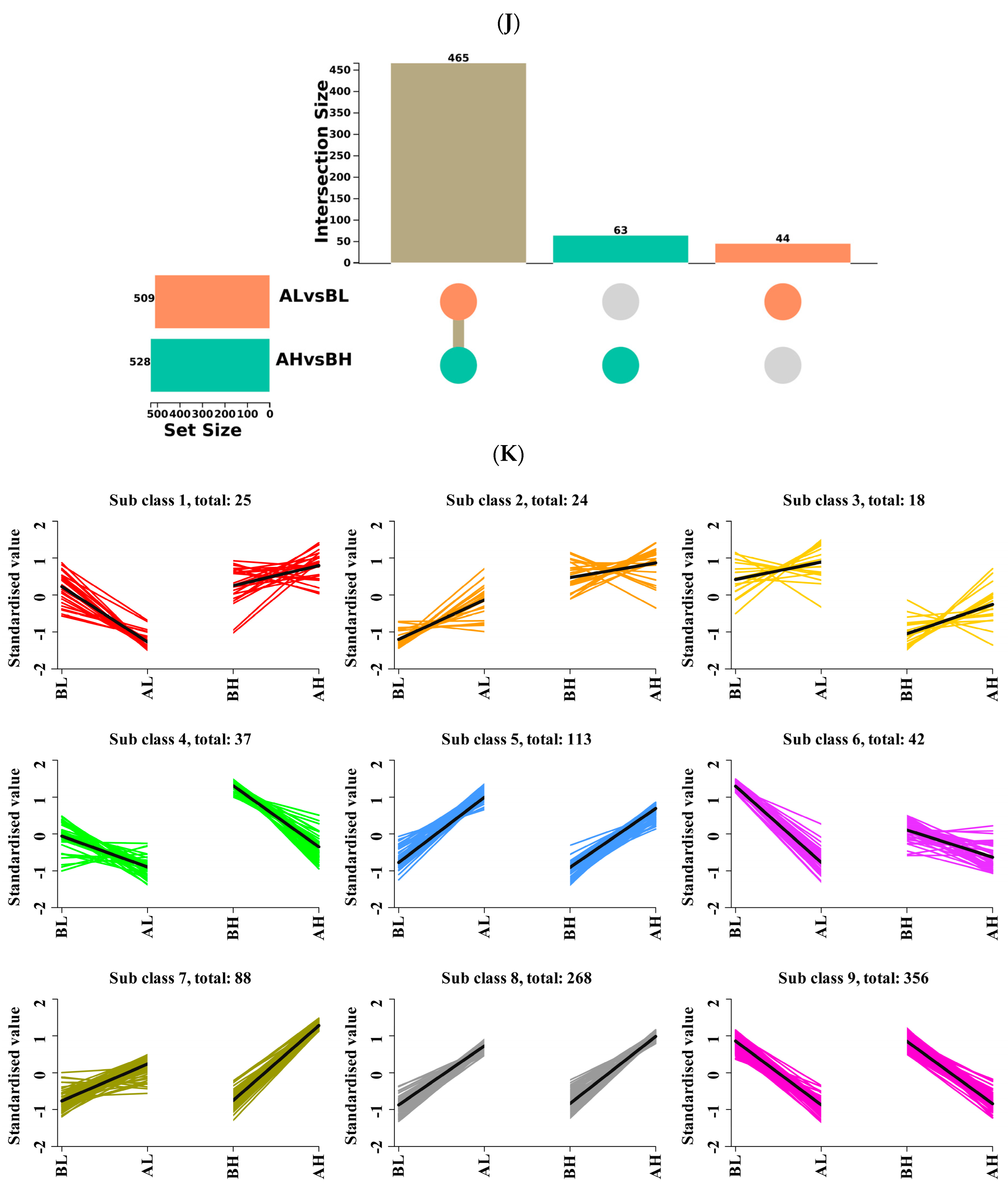
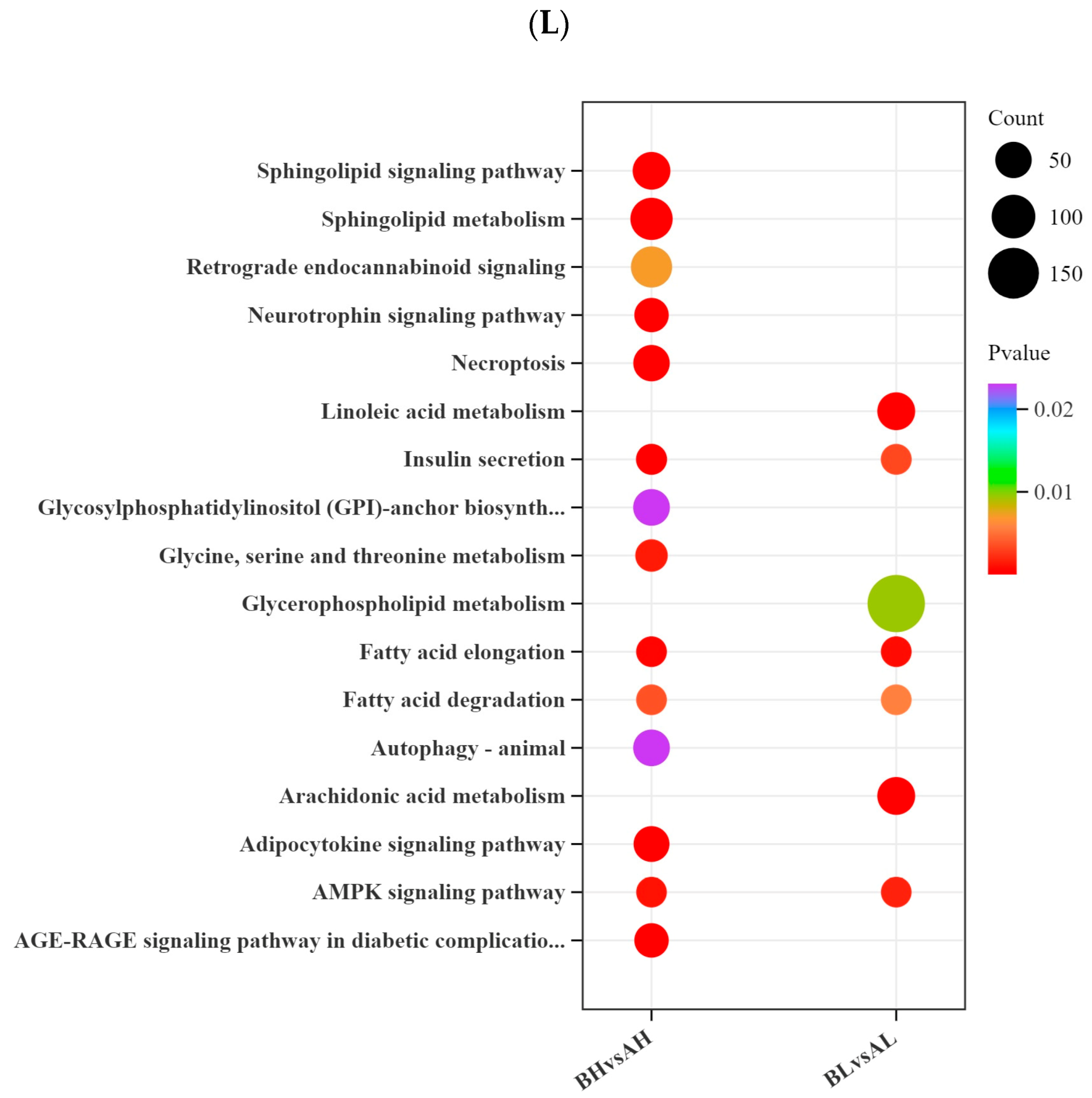
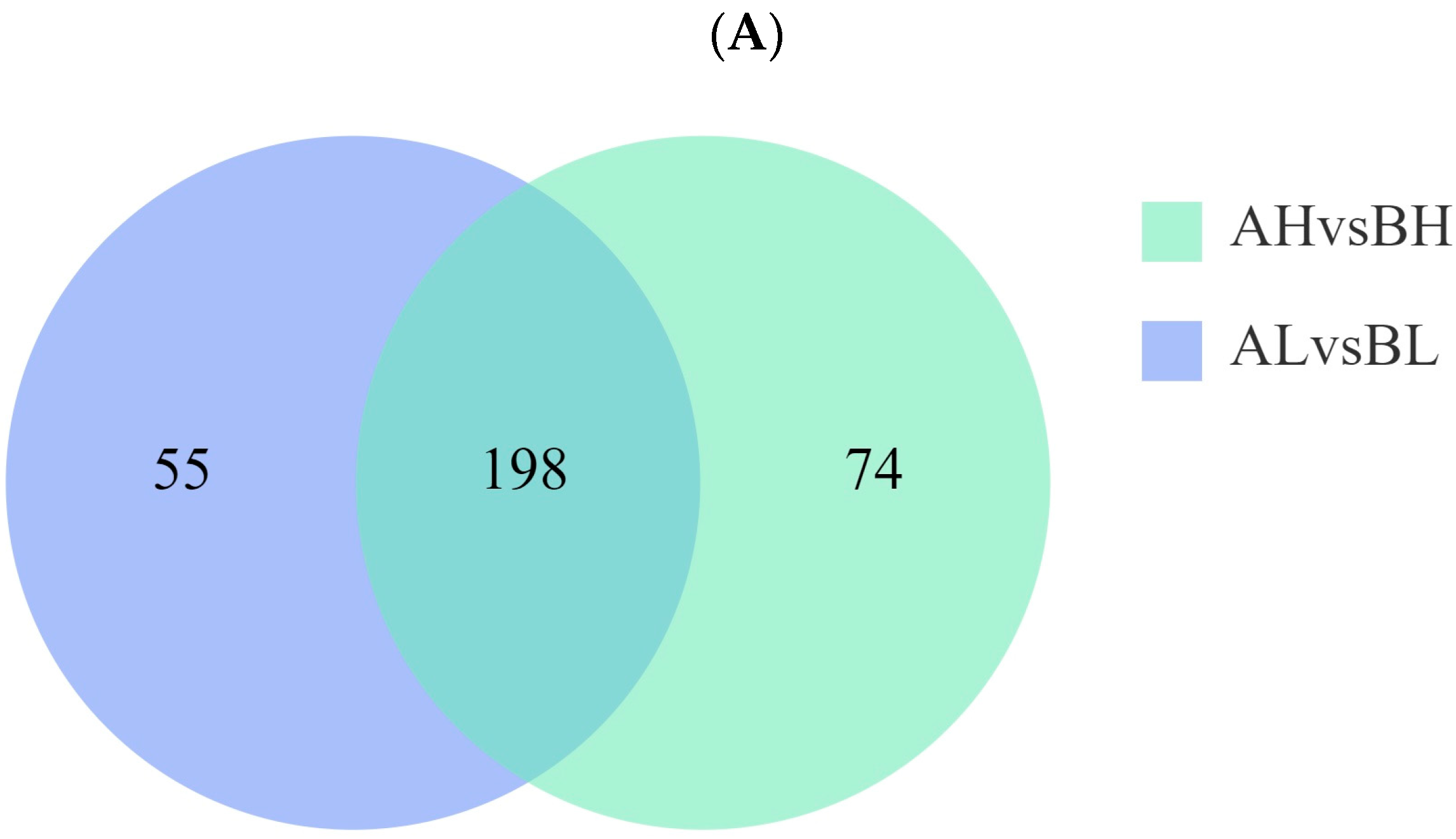
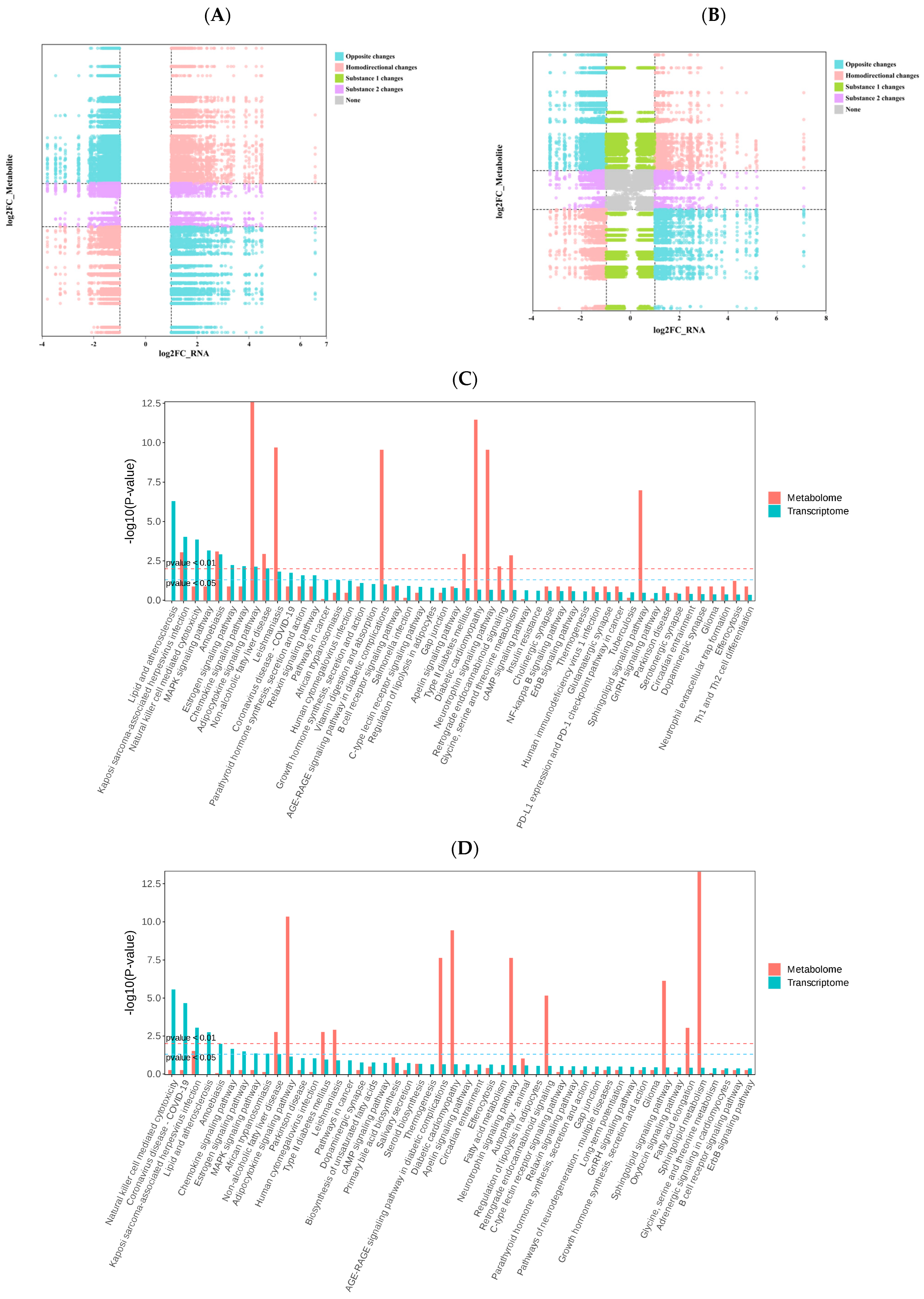
3.5. Integrated Transcriptomic and Metabolomic Analysis
4. Discussion
4.1. Differences in Cardiac Structure and Function of Yili Horses with Varying Degrees of Physiological Cardiac Remodeling
4.2. Differences in Metabolomic Responses to Exercise in Yili Horses with Varying Degrees of Physiological Cardiac Remodeling
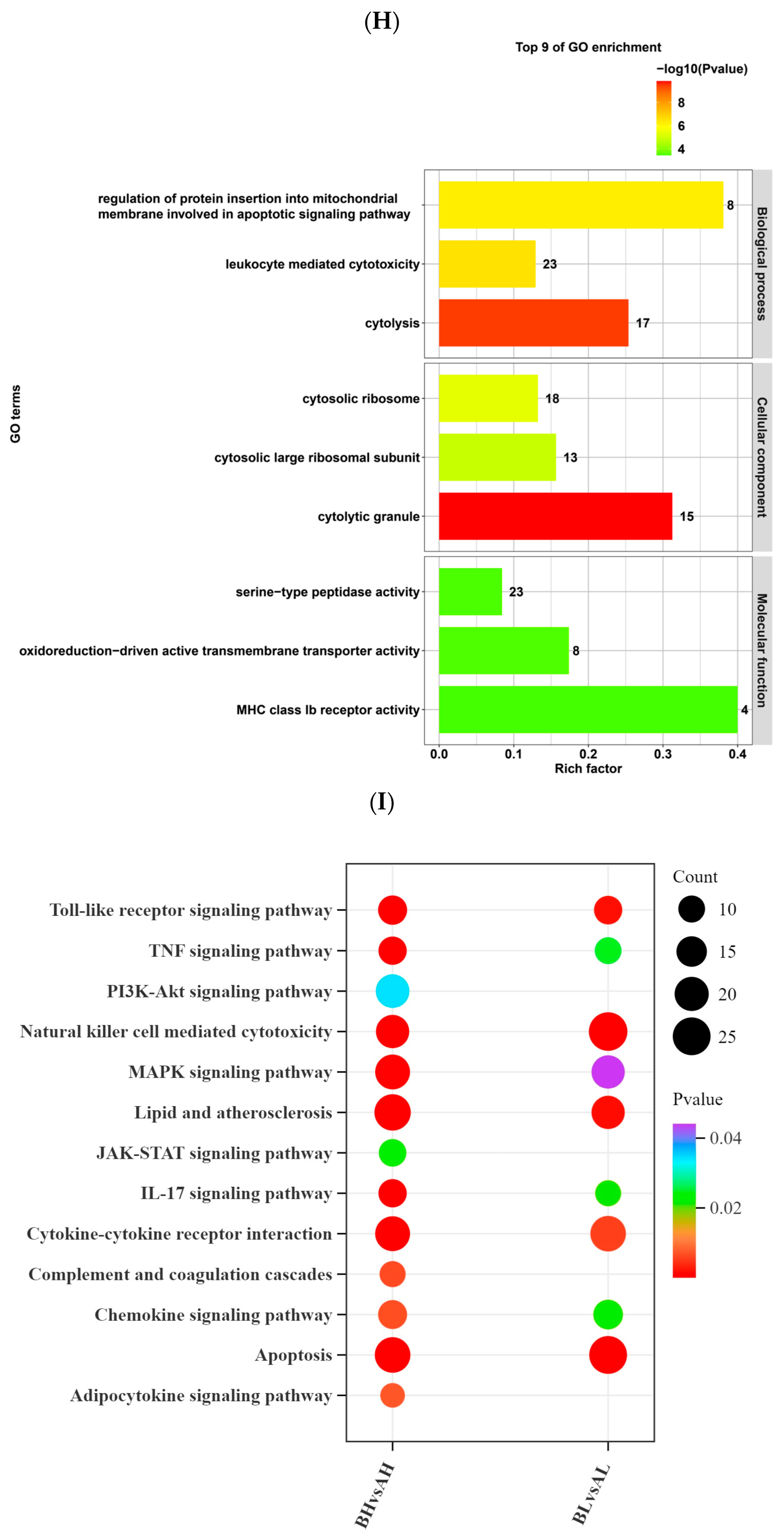
4.3. Transcriptomic Differences in Yili Horses with Varying Degrees of Physiological Cardiac Remodeling Before and After Exercise
4.4. Integrated Transcriptomic and Metabolomic Analysis of Yili Horses with Varying Degrees of Physiological Cardiac Remodeling
4.4.1. Sphingolipid Metabolism and Sphingolipid Signaling Pathway
4.4.2. Adipocytokine Signaling Pathway
4.4.3. AMPK Signaling Pathway
4.4.4. Fatty Acid Elongation
4.4.5. Alpha-Linolenic Acid, Arachidonic Acid, and Linoleic Acid Metabolism
4.4.6. Glycine, Serine, and Threonine Metabolism
4.4.7. Limitations Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Innocentiis, C.; Ricci, F.; Khanji, M.Y.; Aung, N.; Tana, C.; Verrengia, E.; Petersen, S.E.; Gallina, S. Athlete’s heart: Diagnostic challenges and future perspectives. Sports Med. 2018, 48, 2463–2477. [Google Scholar] [CrossRef]
- Klein, D.J.; Anthony, T.G.; McKeever, K.H. Metabolomics in equine sport and exercise. J. Anim. Physiol. Anim. Nutr. 2021, 105, 140–148. [Google Scholar] [CrossRef]
- Heaney, L.M.; Deighton, K.; Suzuki, T. Non-targeted metabolomics in sport and exercise science. J. Sports Sci. 2017, 37, 959–967. [Google Scholar] [CrossRef]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Meaney, M.P.; Dew, D.A.; Pappan, K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R68–R74. [Google Scholar] [CrossRef]
- Pohjanen, E.; Thysell, E.; Jonsson, P.; Eklund, C.; Silfver, A.; Carlsson, I.B.; Lundgren, K.; Moritz, T.; Svensson, M.B.; Antti, H. A multivariate screening strategy for investigating metabolic effects of strenuous physical exercise in human serum. J. Proteome Res. 2007, 6, 2113–2120. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; Fitas, A.; Marszalek, M.; Simon, J.E.; De Rosa, S.; Wiecha, S.; Palatini, J.; Postula, M.; Malek, L.; et al. Altered circulating microRNA profiles after endurance training: A cohort study of ultramarathon runners. Front. Physiol. 2022, 12, 792931. [Google Scholar] [CrossRef]
- Liu, X.; Platt, C.; Rosenzweig, A. The role of MicroRNAs in the cardiac response to exercise. Cold Spring Harb. Perspect. Med. 2017, 7, a029850. [Google Scholar] [CrossRef]
- Drown, M.; Crawford, D.; Oleksiak, M. mRNA expression explains metabolic and thermal physiology. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y. mRNA metabolism in cardiac development and disease: Life after transcription. Physiol. Rev. 2020, 100, 673–694. [Google Scholar] [CrossRef]
- Yuan, T.; Wu, M.; Zhu, C.; Yu, H.; Pham, M.D.; Bottermann, K.; Mao, Y.; Langner, M.; Peitzsch, M.; Das, A.P.; et al. Combinatorial miRNA1a/15b interference drives adult cardiac regeneration. medRxiv 2024. [Google Scholar] [CrossRef]
- Fulghum, K. Metabolic Foundations of Exercise-Induced Cardiac Growth. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 2022. [Google Scholar]
- Wang, T.; Yang, X.; Zeng, Y.; Wang, J.; Li, X.; Shen, Z.; Liu, H.; Zhang, Y.; Chen, W.; Xu, L.; et al. Integrating miRNA, mRNA, and Targeted Metabolomics Analyses to Explore the Regulatory Mechanism of Cardiac Remodeling in Yili Horses. Biology 2025, 14, 1535. [Google Scholar] [CrossRef]
- Ringmark, S.; Lindholm, A.; Hedenström, U.; Lindinger, M.; Dahlborn, K.; Kvart, C.; Jansson, A. Reduced high intensity training distance had no effect on VLa4 but attenuated heart rate response in 2–3-year-old Standardbred horses. Acta Vet. Scand. 2015, 57, 17. [Google Scholar] [CrossRef]
- Wang, T.; Meng, J.; Peng, X.; Huang, J.; Huang, Y.; Yuan, X.; Li, K.; Chen, W.; Zhang, L.; Liu, M.; et al. Metabolomics analysis and mRNA/miRNA profiling reveal potential cardiac regulatory mechanisms in Yili racehorses under different training regimens. PLoS ONE 2025, 20, e0322468. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; De Luca, R.; Di Paolo, F.M.; Spataro, A.; Culasso, F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef]
- Baggish, A.L.; Wang, F.; Weiner, R.B.; Elinoff, J.M.; Tournoux, F.; Boland, A.; Picard, M.H.; Hutter, A.M., Jr.; Wood, M.J. Training-specific changes in cardiac structure and function: A prospective and longitudinal assessment of competitive athletes. J. Appl. Physiol. 2008, 104, 1121–1128. [Google Scholar] [CrossRef]
- Choi, N.S.; Jung, I.W.; Kang, H.S.; Cho, C.W.; Kim, K.S.; Kim, M.S.; Song, J.S.; Bae, J.H. Echocardiographic Evaluation of Left Ventricle before and after Maximum Exercise in Track Athletes. J. Korean Soc. Echocardiogr. 1996, 4, 72–79. [Google Scholar] [CrossRef][Green Version]
- Stöhr, E.J.; González-Alonso, J.; Shave, R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H478–H487. [Google Scholar] [CrossRef]
- Treibert, J.; Friederich, J.; Fischer, S.; Küchenhoff, H.; Wess, G. Reference intervals for various measurements of canine left atrial size and function obtained using two-dimensional and three-dimensional echocardiography. J. Vet. Cardiol. 2024, 52, 43–60. [Google Scholar] [CrossRef]
- Wang, T.; Meng, J.; Yang, X.; Zeng, Y.; Yao, X.; Ren, W. Differential Metabolomics and Cardiac Function in Trained vs. Untrained Yili Performance Horses. Animals 2025, 15, 2444. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Meng, J.; Wang, J.; Ren, W.; Yang, X.; Adina, W.; Bao, Y.; Zeng, Y.; Yao, X. Absolute Quantitative Lipidomics Reveals Differences in Lipid Compounds in the Blood of Trained and Untrained Yili Horses. Vet. Sci. 2025, 12, 255. [Google Scholar] [CrossRef]
- Tsougos, E.; Angelidis, G.; Gialafos, E.; Tzavara, C.; Tzifos, V.; Tsougos, I.; Georgoulias, P. Myocardial strain may predict exercise tolerance in patients with reduced and mid-range ejection fraction. Hell. J. Cardiol. 2018, 59, 331–335. [Google Scholar] [CrossRef]
- Grenacher, P.A.; Schwarzwald, C.C. Assessment of left ventricular size and function in horses using anatomical M-mode echocardiography. J. Vet. Cardiol. 2010, 12, 111–121. [Google Scholar] [CrossRef]
- Cockcroft, S. Mammalian lipids: Structure, synthesis and function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef]
- Cui, S.F.; Li, W.; Niu, J.; Zhang, C.Y.; Chen, X.; Ma, J.Z. Acute responses of circulating microRNAs to low-volume sprint interval cycling. Front. Physiol. 2015, 6, 311. [Google Scholar] [CrossRef]
- Da Dalt, L.; Cabodevilla, A.G.; Goldberg, I.J.; Norata, G.D.; Giorgino, T.; Quagliarini, F. Cardiac lipid metabolism, mitochondrial function, and heart failure. Cardiovasc. Res. 2023, 119, 1905–1914. [Google Scholar] [CrossRef]
- Foran, D.; Antoniades, C.; Akoumianakis, I. Emerging roles for sphingolipids in cardiometabolic disease: A rational therapeutic target? Nutrients 2024, 16, 3296. [Google Scholar] [CrossRef]
- Li, Q.; Wu, M.; Fang, G.; Li, K.; Cui, W.; Li, L.; Li, X.; Wang, J.; Cang, J. MicroRNA-186-5p downregulation inhibits osteoarthritis development by targeting MAPK1. Mol. Med. Rep. 2021, 23, 253. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Venkatesan, C.; Abdelhalim, H.; Zeeshan, S.; Arima, Y.; Linna-Kuosmanen, S.; Ahmed, Z. Genomic approaches to identify and investigate genes associated with atrial fibrillation and heart failure susceptibility. Hum. Genom. 2023, 17, 47. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Liu, X.; Qin, M. Absence of Rgs5 influences the spatial and temporal fluctuation of cardiac repolarization in mice. Front. Physiol. 2021, 12, 622084. [Google Scholar] [CrossRef]
- Yang, Q.H.; Yang, M.; Zhang, L.L.; Xiao, M.C.; Zhao, Y.; Yan, D.X. The mechanism of miR-23a in regulating myocardial cell apoptosis through targeting FoxO3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5789–5797. [Google Scholar]
- Smith, A.S.T.; Macadangdang, J.; Leung, W.; Laflamme, M.A.; Kim, D.-H. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol. Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef]
- Gan, J.; Yuan, J.; Liu, Y.; Lu, Z.; Xue, Y.; Shi, L.; Zeng, H. Circular RNA_101237 mediates anoxia/reoxygenation injury by targeting let-7a-5p/IGF2BP3 in cardiomyocytes. Int. J. Mol. Med. 2020, 45, 451–460. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, D.; Zhu, Q.; Xiang, Y.; He, Y.; Zhang, H. ALAS2 overexpression alleviates oxidative stress-induced ferroptosis in aortic aneurysms via GATA1 activation. J. Thorac. Dis. 2024, 16, 2510. [Google Scholar] [CrossRef]
- Bei, Y.; Lu, D.; Meng, X.; Zhu, Y.; Liang, X.; Xiao, J. P5399 microRNA-486 mediates exercise-induced cardiac growth and prevents cardiac ischemia-reperfusion injury. Eur. Heart J. 2019, 40 (Suppl. S1), ehz746.0359. [Google Scholar] [CrossRef]
- de Oliveira, G.P., Jr.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Reis, A.M.M.; Marçola, T.G.; Teixeira-Neto, A.R.; Franco, O.L.; Pereira, R.W. Effects of endurance racing on horse plasma extracellular particle miRNA. Equine Vet. J. 2021, 53, 618–627. [Google Scholar] [CrossRef]
- Ke, H.; Chen, Z.; Zhao, X.; Yang, C.; Luo, T.; Ou, W.; Wang, L.; Liu, H. Research progress on activation transcription factor 3: A promising cardioprotective molecule. Life Sci. 2023, 328, 121869. [Google Scholar] [CrossRef]
- Webb, R.; Hughes, M.G.; Thomas, A.W.; Morris, K. The ability of exercise-associated oxidative stress to trigger redox-sensitive signalling responses. Antioxidants 2017, 6, 63. [Google Scholar] [CrossRef]
- Behera, S.; Ghosh Roy, A. Exercise-induced differential transcriptional output of AMPK signalling improves axon regeneration and functional recovery. bioRxiv 2024. [Google Scholar] [CrossRef]
- An, H.; Jang, Y.; Choi, J.; Hur, J.; Kim, S.; Kwon, Y. New insights into AMPK, as a potential therapeutic target in metabolic dysfunction-associated steatotic liver disease and hepatic fibrosis. Biomol. Ther. 2024, 33, 18. [Google Scholar] [CrossRef]
- Coven, D.L.; Hu, X.; Cong, L.; Bergeron, R.; Shulman, G.I.; Hardie, D.G.; Young, L.H. Physiological role of AMP-activated protein kinase in the heart: Graded activation during exercise. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E629–E636. [Google Scholar] [CrossRef]
- Fang, C.; Pan, J.; Qu, N.; Lei, Y.; Han, J.; Zhang, J.; Han, D. The AMPK pathway in fatty liver disease. Front. Physiol. 2022, 13, 970292. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shangguan, R.; Chen, S.; Lai, X.; Han, H.; Sun, J. Mechanism of Fatty Acid Metabolism and Regulation by Lactate During Exercise in White Adipose and Skeletal Muscle Tissue: A Review. Sports Med.-Open 2025, 11, 76. [Google Scholar] [CrossRef]
- Pranzini, E.; Muccillo, L.; Nesi, I.; Santi, A.; Mancini, C.; Lori, G.; Genovese, M.; Lottini, T.; Comito, G.; Caselli, A.; et al. Limiting serine availability during tumor progression promotes muscle wasting in cancer cachexia. Cell Death Discov. 2024, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zha, C.; Shao, F.; Wang, L.; Tan, B. Amino acids regulate energy utilization through mammalian target of rapamycin complex 1 and adenosine monophosphate activated protein kinase pathway in porcine enterocytes. Anim. Nutr. 2020, 6, 98–106. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yang, X.; Ren, W.; Meng, J.; Yao, X.; Chu, H.; Yao, R.; Zhai, M.; Zeng, Y. Multi-Omics Deciphers Divergent Mechanisms in Differentially Cardiac-Remodeled Yili Horses Under Conditions of Equivalent Power Output. Animals 2025, 15, 3251. https://doi.org/10.3390/ani15223251
Wang T, Yang X, Ren W, Meng J, Yao X, Chu H, Yao R, Zhai M, Zeng Y. Multi-Omics Deciphers Divergent Mechanisms in Differentially Cardiac-Remodeled Yili Horses Under Conditions of Equivalent Power Output. Animals. 2025; 15(22):3251. https://doi.org/10.3390/ani15223251
Chicago/Turabian StyleWang, Tongliang, Xixi Yang, Wanlu Ren, Jun Meng, Xinkui Yao, Hongzhong Chu, Runchen Yao, Manjun Zhai, and Yaqi Zeng. 2025. "Multi-Omics Deciphers Divergent Mechanisms in Differentially Cardiac-Remodeled Yili Horses Under Conditions of Equivalent Power Output" Animals 15, no. 22: 3251. https://doi.org/10.3390/ani15223251
APA StyleWang, T., Yang, X., Ren, W., Meng, J., Yao, X., Chu, H., Yao, R., Zhai, M., & Zeng, Y. (2025). Multi-Omics Deciphers Divergent Mechanisms in Differentially Cardiac-Remodeled Yili Horses Under Conditions of Equivalent Power Output. Animals, 15(22), 3251. https://doi.org/10.3390/ani15223251





