Breeding and Ontogeny of the Aquarium-Traded Scissortail Rasbora (Rasbora trilineata)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of the Broodstock
2.2. Breeding and Larval-Rearing Experiments
2.3. Data Analyses
2.4. Observation of Embryonic and Larval Development
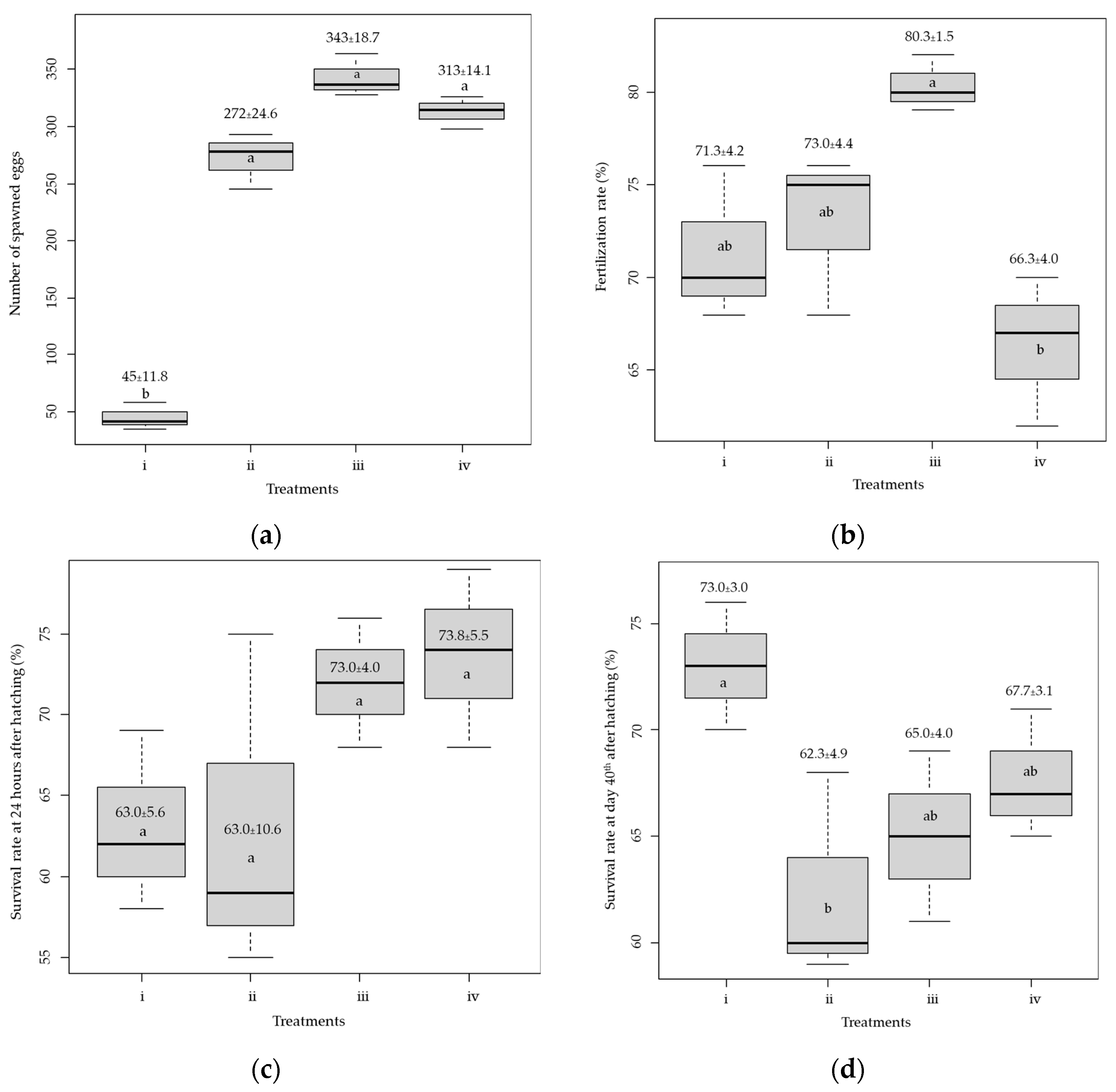
3. Results
3.1. Spawning Success and Nursing of Larvae
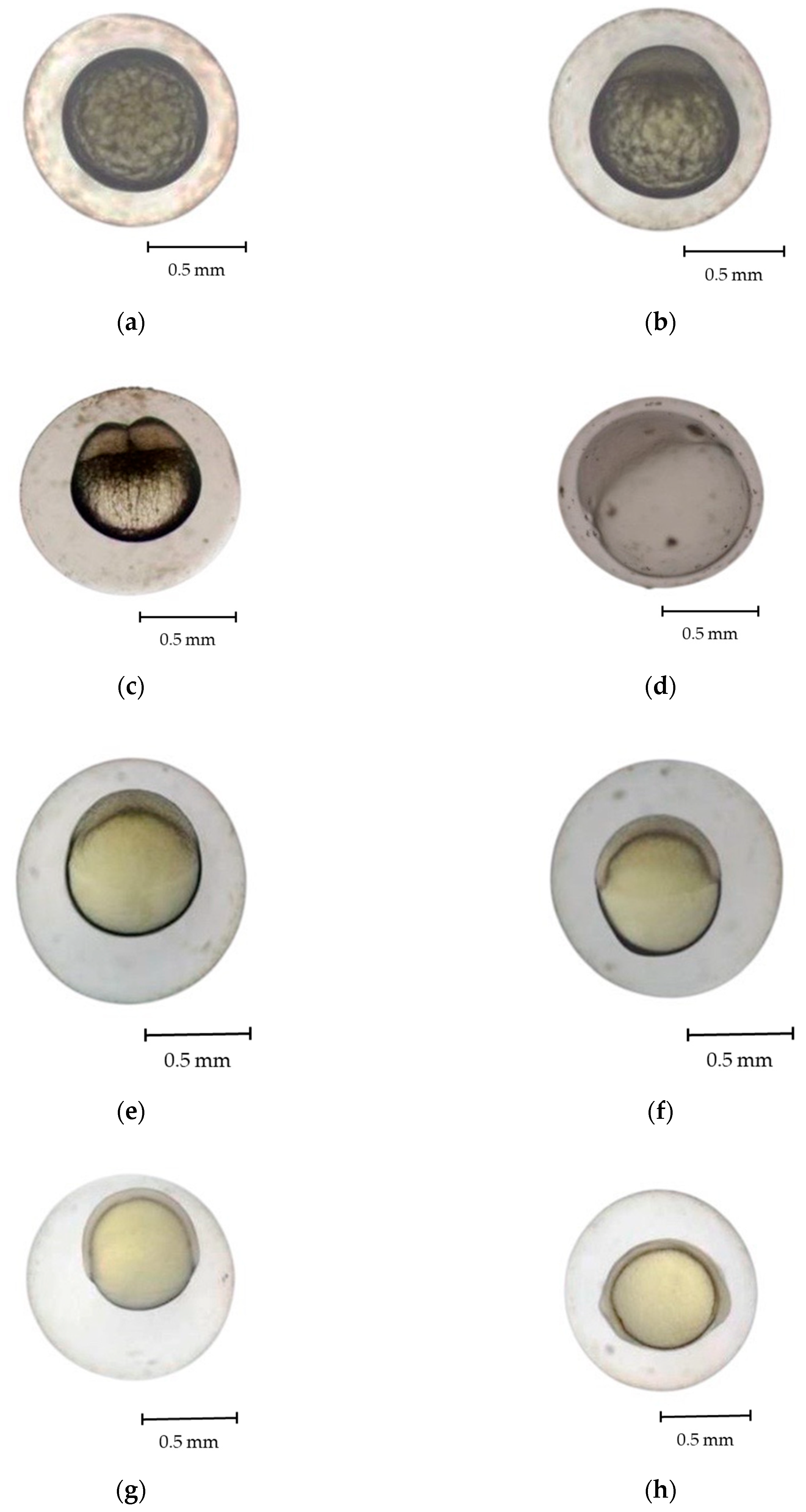
3.2. Development of Eggs and Larvae
- Zygote stage (Figure 3b)
- Cleavage stage (Figure 3c)
- Morula stage (Figure 3d)
- Blastula stage (Figure 3e,f)
- Gastrula stage (Figure 3g,h)
- Segmentation stage (Figure 3i,j)
- Pharyngeal stage
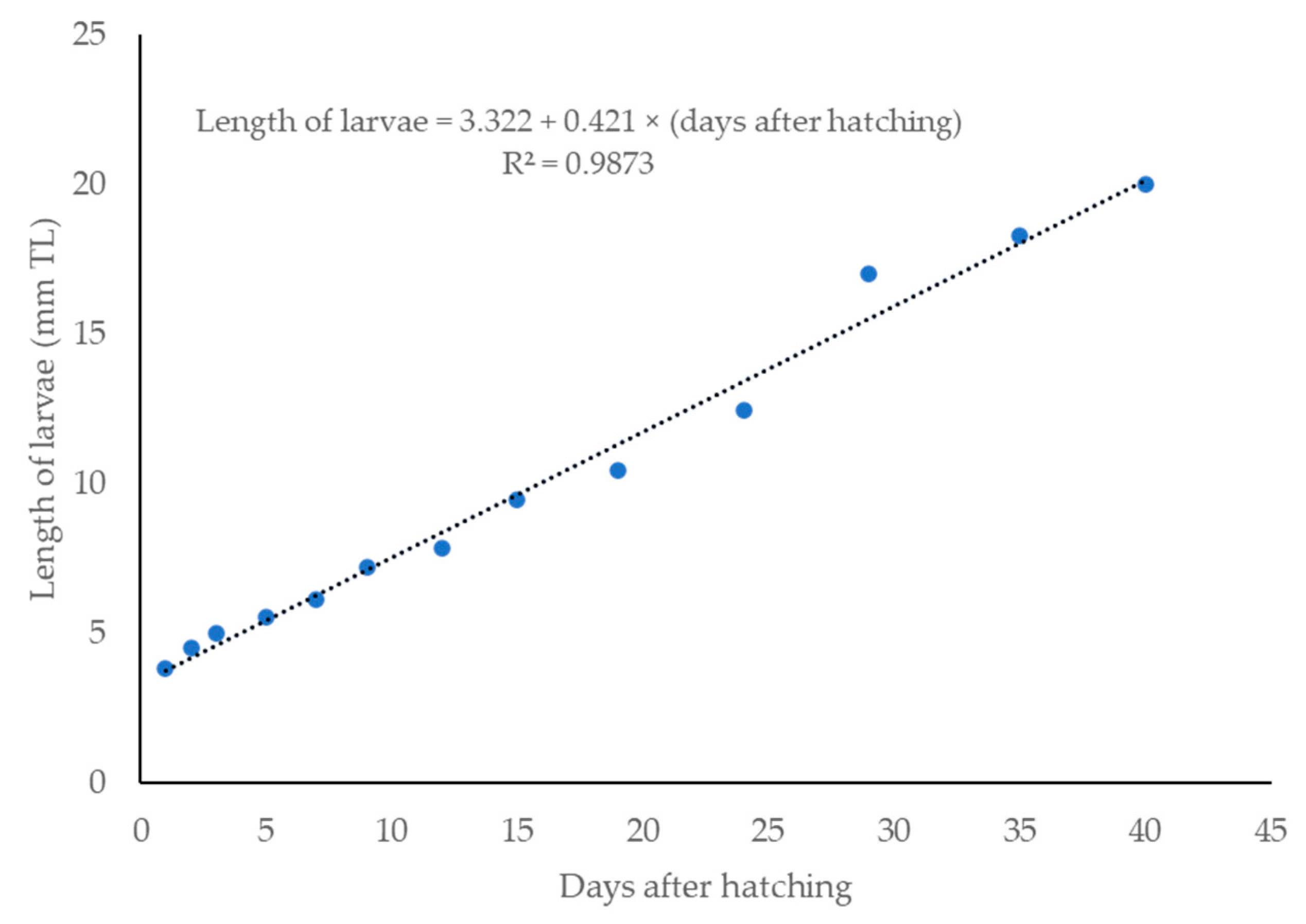
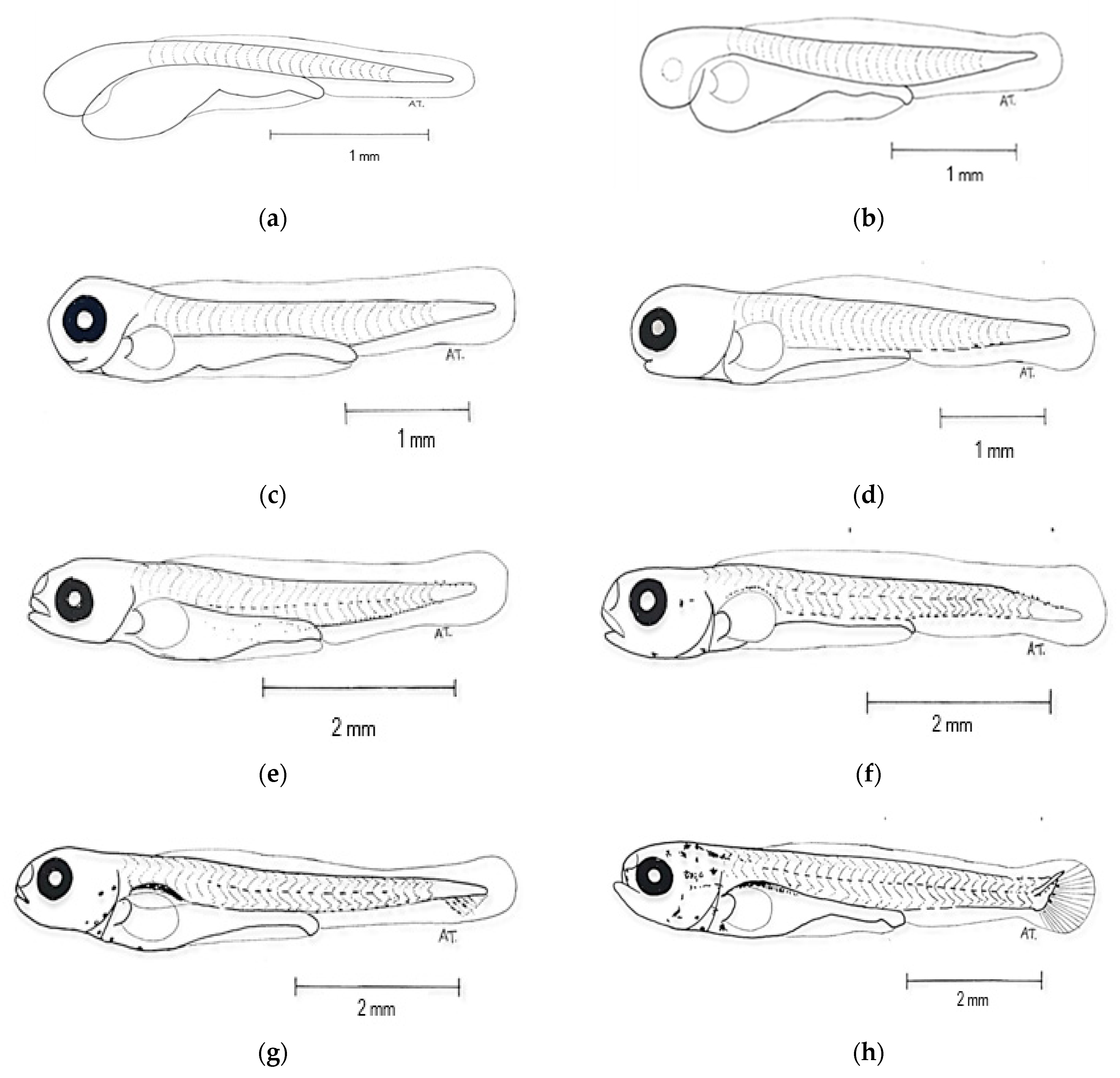
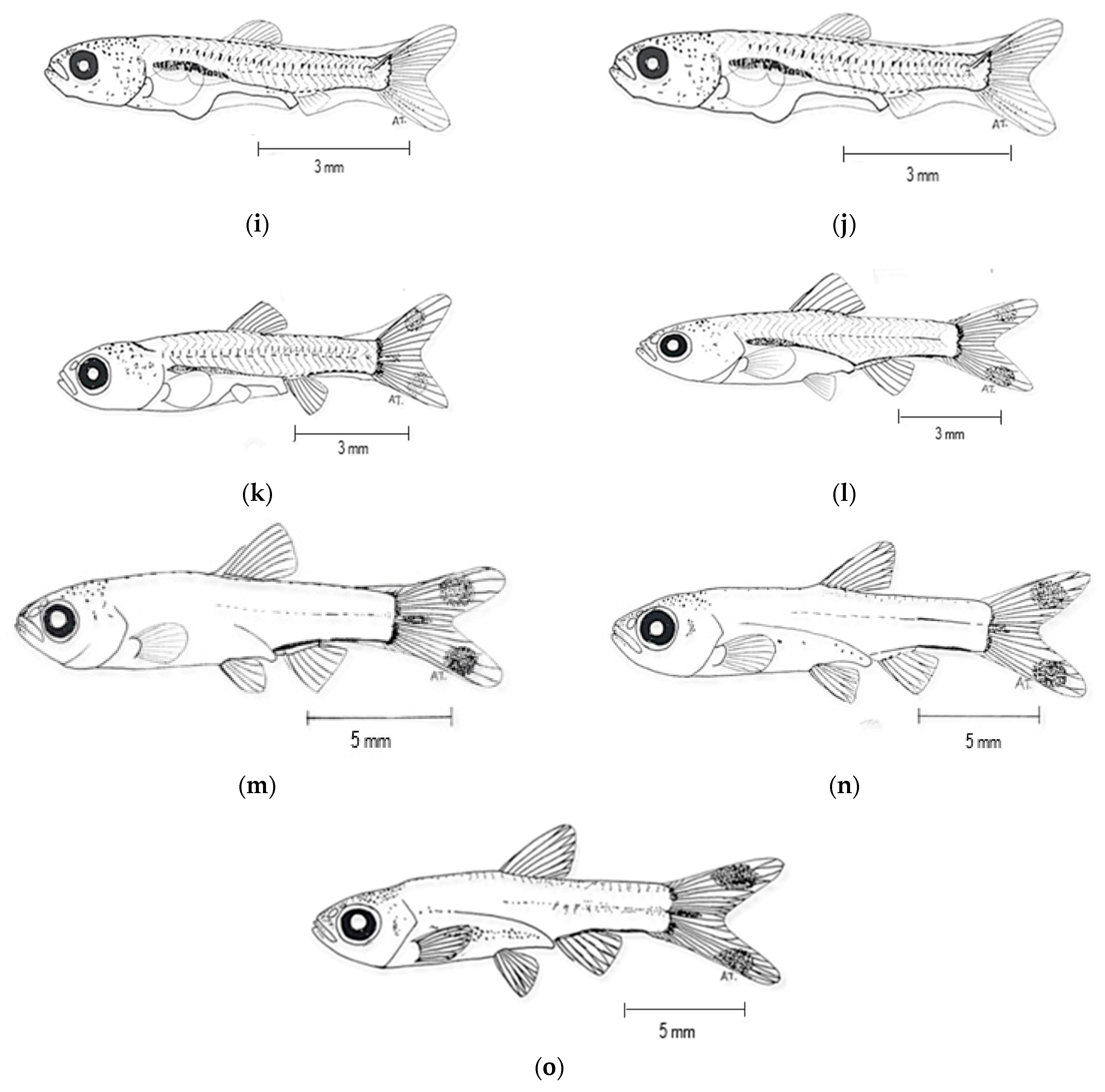
3.3. Larval Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAH | Day after hatching |
| hpf | Hours post fertilization |
| TL | Total length |
References
- Liao, T.Y.; Kullanfer, S.O.; Fang, F. Phylogenetic analysis of the genus Rasbora (Teleostei: Cyprinidae). Zool. Scr. 2010, 39, 155–176. [Google Scholar] [CrossRef]
- Ho, C.W.; Liu, M.Y.; Chen, M.H. Complete mitochondrial genome of Rasbora trilineata (Cypriniformes, Cyprinidae). Mitochondrial DNA Part A 2016, 27, 1755–1757. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase; Version (10/2024); Quantitative Aquatics, Inc.: Los Banos, Philippines, 2024; Available online: www.fishbase.org (accessed on 14 April 2025).
- Vidthayanon, C.; Kannasut, J.; Nabhitabhata, J. The Diversity of Freshwater Fish Species in Thailand; Office of Environmental Policy and Planning (ONEP): Bangkok, Thailand, 1997; 102p. [Google Scholar]
- Rainboth, W.J. Fishes of the Cambodian Mekong. In FAO Species Identification Field Guide for Fishery Purposes; FAO: Rome, Italy, 1996; 265p. [Google Scholar]
- Saowakoon, S.; Saowakoon, H.; Chandam, S.; Athainsee, A.; Pitsamayarom, K. Study on Biology, Breeding and Nursing for Commercial Culture of Scissortail Rasbora; Rajamangala University of Technology Isan, Ministry of Education: Surin, Thailand, 2008; 78p. (In Thai) [Google Scholar]
- Poling, K.R.; Brunjes, P.C. Sensory deafferentation and olfactory bulb morphology in the zebrafish and related species. Brain Res. 2000, 856, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sholihah, A.; Delrieu-Trottin, E.; Sukmono, T.; Dahruddin, H.; Risdawati, R.; Elvyra, R.; Wibowo, A.; Kustiati, K.; Busson, F.; Sauri, S.; et al. Disentangling the taxonomy of the subfamily Rasborinae (Cy priniformes, Danionidae) in Sundaland using DNA barcodes. Sci. Rep. 2020, 10, 2818. [Google Scholar] [CrossRef] [PubMed]
- Aiumsumang, S.; Phimphan, S.; Tanomtong, A.; Supiwong, W. Chromosome study of Rasbora trilineata and Rasbora borapetensis (Cyprinidae, Cypriniformes): Reveal by conventional staining technique. Food Agric. Sci. Technol. 2022, 8, 29–39. [Google Scholar]
- Miao, W.; Silva, S.D.; Davy, B. (Eds.) Inland Fisheries Enhancement and Conservation in Asia; RAP Publication 2010/22; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; 189p. [Google Scholar]
- Jutagate, T.; Rattanachai, A. Inland fisheries resource enhancement and conservation in Thailand. In Inland Fisheries Resource Enhancement and Conservation in Asia-Pacific; De Silva, S.S., Davy, B., Wieman, M., Eds.; FAO/RAP Publication 2010/22; FAO/RAP: Bangkok, Thailand, 2010; pp. 133–146. [Google Scholar]
- Permana, A.; Priyadi, A.; Musa, A.; Nur, B.; Cindelaras, S.; Rohmy, S.; Budi, D.S. Induced spawning of harlequin rasbora (Trigonostigma heteromorpha) using tiger barb (Puntius tetrazona) stimuli: Techniques and outcomes. Int. J. Fish. Aquat. Stud. 2024, 12, 140–144. [Google Scholar] [CrossRef]
- Anshori, K.; Hananya, A.; Pratama, S.F.; Raharjeng, A.R.P.; Suci, D.A.; Retnoaji, B. Artificial breeding technology for mass field cultivation of Indonesian wader fish (Rasbora lateristriata Bleeker, 1854). AIP Conf. Proc. 2024, 3132, 060001. [Google Scholar]
- Rey, J.; Widiyanto, S.; Retnoaji, B. Maturation of female yellow rasbora (Rasbora lateristriata Bleeker, 1854) using Oodev at different doses in feed. J. Trop. Biodivers. Biotechnol. 2023, 8, 75916. [Google Scholar] [CrossRef]
- Adawiyah, L.A.; Sulmartiwi, L.; Bodur, T.; Budi, D.S. Induction of spermiation using Ovaprim™ with topical gill method in the silver rasbora (Rasbora argyrotaenia). Theriogenology 2019, 126, 172–176. [Google Scholar] [CrossRef]
- Budi, D.S.; Ulkhaq, M.F.; Kenconojati, H. Increasing of milt production in male silver rasbora (Rasbora argyrotaenia) administered Ovaprim. Indian Vet. J. 2020, 97, 15–17. [Google Scholar]
- Budi, D.S.; Puspitasari, S.; Febrianti, R.P.N.; Bodur, T.; Mukti, A.T. A novel technique for mass induction of propagation in small fish species: Hormone immersion. Anim. Reprod. Sci. 2023, 255, 107280. [Google Scholar] [CrossRef] [PubMed]
- Retnoaji, B.; Nurhidayat, L.; Pratama, S.F.; Anshori, K.; Hananya, A.; Sofyantoro, F.; Bessho, Y. Embryonic development of Indonesian native fish yellow rasbora (Rasbora lateristriata). J. King Saud Univ.-Sci. 2023, 35, 102810. [Google Scholar] [CrossRef]
- Paul, S.; Sarmah, S.K. Study of embryonic and larval development of Rasbora daniconius. Biol. Forum 2019, 11, 72–76. [Google Scholar] [CrossRef]
- Prastika, A.B.; Sulmartiwi, L.; Lutfiyah, L. Effect of sublethal dose organophosphate pesticides on embryogenesis and hatching rate of silver rasbora eggs (Rasbora argyrotaenia). IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012065. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 23 December 2023).
- Langeland, J.; Kimmel, C.B. The embryology of fish. In Embryology: Constructing the Organism; Gilbert, S.F., Raunio, A.M., Eds.; Sinauer Associates: Sunderland, MA, USA, 1997; pp. 383–407. [Google Scholar]
- Aral, F.; Şahınöz, E.; Doğu, Z. Embryonic and larval development of freshwater fish. In Recent Advances in Fish Farms; Aral, F., Doğu, Z., Eds.; IntechOpen Limited: London, UK, 2011; pp. 83–94. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Kahar, R.; Ahmad, N.; Arai, T. Year-round spawning of three tropical Cypriniformes fishes in Southeast Asia. Sci. Rep. 2023, 13, 8971. [Google Scholar] [CrossRef] [PubMed]
- Ningrum, D.R.K.; Budi, D.S.; Sulmartiwi, L. Spawning induction of silver rasbora (Rasbora argyrotaenia) using Ovaprim different doses. J. Ilmu-Ilmu Perair. Pesisir Dan Perikanan 2019, 8, 117–124. [Google Scholar] [CrossRef]
- Petchrit, N. Breeding and Nursing of Brilliant Rasbora, Rasbora Einthovenii (Bleeker, 1851); Extension Paper No. 1/2021; Satun Inland Aquaculture Research and Development Center, Inland Aquaculture Research and Development Bureau, Department of Fisheries: Satun, Thailand, 2021; 31p. (In Thai) [Google Scholar]
- Wang, Y.; Hu, M.; Cao, L.; Yang, Y.; Wang, W. Effects of daphnia (Moina micrura) plus chlorella (Chlorella pyrenoidosa) or microparticle diets on growth and survival of larval loach (Misgurnus anguillicaudatus). Aquacult. Int. 2008, 16, 361–368. [Google Scholar] [CrossRef]
- Aly, S.M.; ElBanna, N.I.; Fathi, M. Chlorella in aquaculture: Challenges, opportunities, and disease prevention for sustain able development. Aquacult. Int. 2024, 32, 1559–1586. [Google Scholar] [CrossRef]
- Joshua, W.J.; Kamarudin, M.S.; Ikhsan, N.; Yusoff, F.M.; Zulperi, Z. Development of enriched Artemia and Moina in larvi culture of fish and crustaceans: A review. Latin Am. J. Aquat. Res. 2022, 50, 144–157. [Google Scholar] [CrossRef]
- Rizzo, E.; Sato, Y.; Barreto, B.P.; Godinho, H.P. Adhesiveness and surface patterns of eggs in neotropical freshwater teleosts. J. Fish Biol. 2002, 61, 615–632. [Google Scholar] [CrossRef]
- Sado, T.; Kimura, S. Descriptive morphology of the eggs, larvae, and juveniles of two cyprinid fishes belonging to the Zacco temminckii species’ group. Ichthyol. Res. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Park, J.M.; Mun, S.J.; Yim, H.S.; Han, K.H. Egg development and larvae and juveniles morphology of carp, Cyprinus carpio in Korean. Dev. Reprod. 2017, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Kupren, K.; Nowosad, J.; Kucharczyk, D. Growth priorities of the four riverine cyprinids during early ontogeny. Animals 2023, 13, 2345. [Google Scholar] [CrossRef]
- Khan, S.A.; Sherzada, S.; Ahmad, Q.A.; Hussain, A.; Hussain, N.; Nowosad, J. Captive breeding and early developmental dynamics of Cirrhinus mrigala: Implications for sustainable seed production. Animals 2024, 14, 2799. [Google Scholar] [CrossRef]
- Ribeiro, L.; Hubert, F.N.; Rodrigues, V.; Rojas-Garcia, C.; Dinis, M.T. Understanding fish larvae’s feeding biology to improve aquaculture feeding protocols. Oceans 2022, 3, 94–113. [Google Scholar] [CrossRef]
- Ng, P.K.; Tan, H.H. Freshwater fishes of Southeast Asia: Potential for the aquarium fish trade and conservation issues. Aquar. Sci. Conserv. 1997, 1, 79–90. [Google Scholar] [CrossRef]



| Embryonic Stages | Approximate Time * | Explicit Remarks During Development |
|---|---|---|
| Zygote | 10 min | Cell division begins. |
| Cleavage | ||
| • 2-cell | 14 min | Division of 1 cell into 2 cells. |
| • Second cleavage | 17 min | Division of 2 cells into 4 cells. |
| • Third cleavage | 20 min | Division of 4 cells into 8 cells. |
| • Fourth cleavage | 30 min | Continued cell division, entering the morula stage. |
| Morula | 2 h, 30 min | Cells divide and arrange into multiple layers, resembling a mulberry-like structure. |
| Blastula | 3 h, 30 min | Increase in the number of cells. |
| • Dome stage | 4 h, 30 min | Cells arrange into a dome shape, transitioning towards a horizontal alignment. |
| Gastrula | 5 h | Cells organize into a germ ring (germ layer formation). |
| Segmentation | ||
| • Head bud stage | 8 h | The head bud and tail bud become visible. |
| • Primordium | 8 h, 30 min | The optic bud appears in the head region. |
| • Somite stage | 9 h | Smites begin to form along the body axis. |
| 11 h, 30 min | Somite count increases. | |
| 12 h, 55 min | Formation of myotomes and initial muscle movements. | |
| 15 h, 30 min | Movement of the body begins with the embryo wriggling inside the egg. | |
| 11 h, 30 min | Somite count increases. | |
| Pharyngula | 17 h | Formation of the chorion membrane. |
| 18 h | Hatchling emerges from the egg as a free-swimming larva. |
| Days After Hatching (DAH) | Development Description |
|---|---|
| Early hatched | The mouth has not developed, the yolk sac is fully present, there is a fin fold, and the body axis is straight. |
| 12 h AH | The larval length increased while the yolk sac became smaller and slimmer at early hatching. Rudimentary pectoral fins begin to develop on each side of the body. |
| 1 DAH | Spines begin to appear at the anterior portion of the larval. |
| 2 DAH | The body is attached to the horizontal spinal cord. |
| 3 DAH | The indentation of the mouth appeared, forming a gap. The yolk sac became thinner and crenulated below the stomach. |
| 5 DAH | The larvae began to show more swimming activities at the bottom and the edge of the aquarium. |
| 7 DAH | The rudimentary caudal fins started to develop. |
| 9 DAH | Melanophores were spotted at the operculum near the head of the larvae. The lateral line was visible from the head to the tail. |
| 12 DAH | The operculum became thicker and fully covered the gills. Caudal and dorsal fins started to develop, and the caudal fin became forked in the middle. |
| 15 DAH | The mouth had fully developed, and feeding began at 15 DAH with Moina. Melanophores were spotted at the caudal fin. |
| 19 DAH | Pectoral fins, anal fins, dorsal fin, and caudal fin had fully developed. |
| 24 DAH | Pelvic fins started to develop, and their size increased. |
| 29 DAH | The fish reached the juvenile stage as early as 28–30 DAH. Scales fully covered its body. Fin shapes and colors were similar to adults. |
| 35 DAH | The fish behavior was similar to that of adult R. trilineata, showing a uniform swimming pattern, swimming in schools. |
| 40 DAH | The fish could be fed with artificial feed, and they had reached the minimum size for export. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasamawut, K.; Udduang, S.; Kattakdad, S.; Danwandee, K.; Jutagate, A.; Saowakoon, S.; Jutagate, T. Breeding and Ontogeny of the Aquarium-Traded Scissortail Rasbora (Rasbora trilineata). Animals 2025, 15, 1823. https://doi.org/10.3390/ani15131823
Kasamawut K, Udduang S, Kattakdad S, Danwandee K, Jutagate A, Saowakoon S, Jutagate T. Breeding and Ontogeny of the Aquarium-Traded Scissortail Rasbora (Rasbora trilineata). Animals. 2025; 15(13):1823. https://doi.org/10.3390/ani15131823
Chicago/Turabian StyleKasamawut, Krittima, Suriya Udduang, Supalug Kattakdad, Kasama Danwandee, Achara Jutagate, Samnao Saowakoon, and Tuantong Jutagate. 2025. "Breeding and Ontogeny of the Aquarium-Traded Scissortail Rasbora (Rasbora trilineata)" Animals 15, no. 13: 1823. https://doi.org/10.3390/ani15131823
APA StyleKasamawut, K., Udduang, S., Kattakdad, S., Danwandee, K., Jutagate, A., Saowakoon, S., & Jutagate, T. (2025). Breeding and Ontogeny of the Aquarium-Traded Scissortail Rasbora (Rasbora trilineata). Animals, 15(13), 1823. https://doi.org/10.3390/ani15131823





