Impact of Biosecurity on Production Performance and Antimicrobial Usage in Broiler Farms in Cameroon
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Design
2.2. Data Collection
2.2.1. Biosecurity Assessment
2.2.2. Evaluation of Antibiotic Usage
- -
- The UDD describes the amount of active substance actually administered to the animals in mg/kg. It was calculated by dividing the amount of antimicrobial compound administered (mg) by the number of broilers multiplied by the average weight at the time of treatment (treatment duration) [30];
- -
- The ADD, which is the assumed average dose per day per kilogram of chicken of a specific drug, was collected from the drug’s instruction leaflet;
- -
- The amount used to produce one kilogram of meat was obtained by dividing the total amount of active substance (AS) used by the total weight of the subjects during treatment;
- -
- -
- Treatment incidence for chickens is defined as the number of chickens per 1000 that were treated daily with a UDD or ADD [32]. TI was calculated using the following formula:
- -
- The number of days at risk corresponds to the duration in days during which the broiler may have been exposed to one or more treatments.
- -
- The weight of the subjects was obtained by multiplying the number of chickens by their average weight.
2.2.3. Evaluation of Production Performance of Broiler Farms
- Mortality rate
- Feed Conversion Ratio
- Average daily gain
- Performance Index
2.3. Statistical Analysis
3. Results
3.1. Profiles of Respondents and Characteristics of Farms
3.2. Biosecurity in Broiler Farms
3.3. Antibiotic Usage
3.3.1. Quantity of Antibiotics Used
3.3.2. Association Between Biosecurity and Antibiotic Usage
3.4. Production Performances
3.4.1. Performance of Broiler Farms
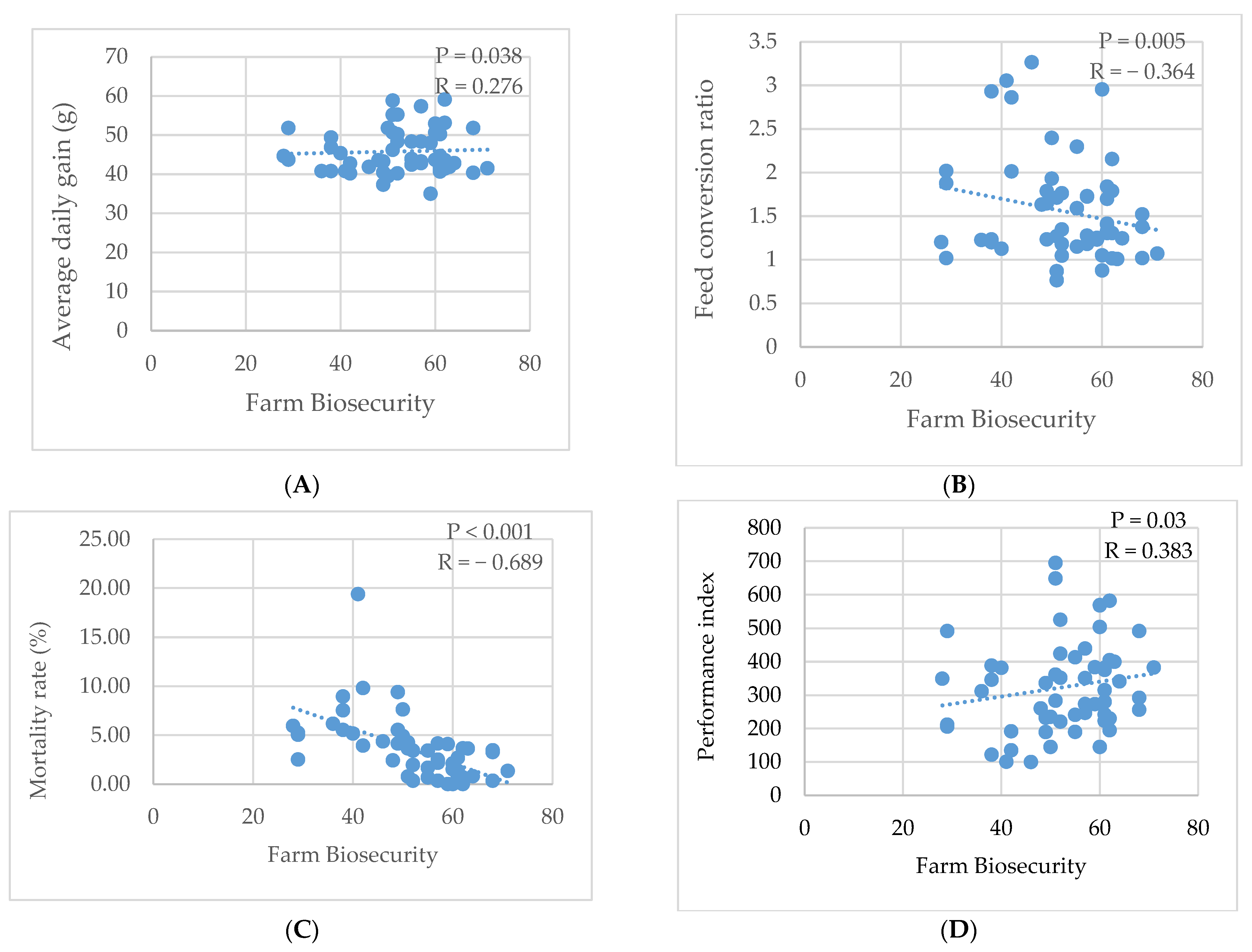
3.4.2. Relation Between Biosecurity and Production Performances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADG | Average daily gain |
| FCR | Feed conversion ratio |
| PI | Performance index |
| AMU | Antimicrobial usage |
| AMR | Antimicrobial resistance |
| TI | Treatment incidence |
| UDD | Daily dose used |
| ADD | Animal daily dose |
| AS | Active substance |
| HPCIA | Highest priority critically important antimicrobials |
| CIA | Critically important antimicrobials |
| HIA | Highly important antimicrobials |
References
- Monamele, G.C.; Phalla, Y.; Albert, E.; Vernet, M.; Wade, A. Evidence of exposure and human seroconversion during an outbreak of avian influenza A (H5N1) among poultry in Cameroon. Emerg. Microbes Infect. 2019, 8, 186–196. [Google Scholar] [CrossRef]
- MINEPIA. Ministry of Livestock, Fisheries and Animal Industry. In Statistical Yearbook of the Ministry of Livestock, Fisheries and Animal Industries of Cameroon; MINEPIA: Yaoundé, Cameroon, 2019. [Google Scholar]
- Mpouam, S.E.; Ikoum, D.; Hadja, L.; Kilekoung Mingoas, J.P.; Saegerman, C. Parallel multicriteria decision analysis for sub-national prioritization of zoonoses and animal diseases in Africa: The case of Cameroon. PLoS ONE 2024, 19, e0295742. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Ahmed Laloui, H.; Kara, R.; Dems, M.A.; Cherb, N.; Klikha, A.; Blake, D.P. The financial cost of coccidiosis in Algerian chicken production: A major challenge for the poultry sector. Avian Pathol. 2024, 53, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.K.; Tchouankui, H.N.; Ngapagna, A.N. Epidemiological features of highly pathogenic avian influenza in Cameroon. Vet. Med. Int. 2019, 1, 3796369. [Google Scholar] [CrossRef]
- Paudel, S.; Apostolakos, I.; Vougat Ngom, R.; Tilli, G.; de Carvalho Ferreira, H.C.; Piccirillo, A. A systematic review and meta-analysis on the efficacy of vaccination against colibacillosis in broiler production. PLoS ONE 2024, 19, e0301029. [Google Scholar] [CrossRef] [PubMed]
- Tilli, G.; Ngom, R.V.; de Carvalho Ferreira, H.C.; Apostolakos, I.; Paudel, S.; Piccirillo, A. A systematic review on the role of biosecurity to prevent or control colibacillosis in broiler production. Poult. Sci. 2024, 103, 103955. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Ofori, L.A.; Akenten, C.W.; Fosu, D.; Ofori, S.; Lamshöft, M.; May, J.; Danso, K.O.; Krumkamp, R.; Dekker, D. Antimicrobial usage in commercial and domestic poultry farming in two communities in the Ashanti region of Ghana. Antibiotics 2021, 10, 800. [Google Scholar] [CrossRef]
- Vougat Ngom, R.; Laconi, A.; Tolosi, R.; Akoussa, A.M.; Ziebe, S.D.; Kouyabe, V.M.; Piccirillo, A. Resistance to medically important antimicrobials in broiler and layer farms in Cameroon and its relation with biosecurity and antimicrobial use. Front. microbiol. 2025, 15, 1517159. [Google Scholar] [CrossRef]
- Hassan, M.M.; Kalam, M.A.; Alim, M.A.; Shano, S.; Nayem, M.R.K.; Badsha, M.R.; Al Mamun, M.A.; Hoque, A.; Tanzin, A.Z.; Nath, C.H.; et al. Knowledge, attitude, and practices on antimicrobial use and antimicrobial resistance among commercial poultry farmers in Bangladesh. Antibiotics 2021, 10, 784. [Google Scholar] [CrossRef]
- Vougat Ngom, R.R.B.; Tomdieu, T.; Ziébé, R.; Foyet, H.S.; Moritz, M.; Vondou, L.; Schrunk, D.E.; Imerman, P.M.; Rumbeiha, W.K.; Garabed, R.B. Quality of veterinary pharmaceuticals and their use by pastoralists in the Far North Region of Cameroon. Pastoralism 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Mouiche, M.M.M.; Mazra, M.; Moffo, F.; Mpouam, S.E.; Ngoujigne, A.I.; Feussom, K.J.M.; Akoda, G.K.; Awah-Ndukum, J. Veterinary drugs market in Cameroon: Regulations, organization and distribution channels. Inter-Afr. Bur. Anim. Resour. 2018, 66, 591–605. [Google Scholar]
- Muloi, D.M.; Kasudi, M.R.; Murungi, M.K.; Ibayil, E.L.; Kahariri, S.; Karimi, C.; Korir, M.; Muasa, B.; Mwololo, D.; Ndanyi, R.; et al. Analysis of antibiotic use and access to drugs among poultry farmers in Kenya. One Health 2025, 20, 100987. [Google Scholar] [CrossRef] [PubMed]
- Teko-Agbo, A.; Akoda, K.; Assoumy, A.M.; Kadja, M.C.; Niang, E.M.M.; Messomo, N.F.; Walbadet, L.; Abiola, F.A. Qualité des médicaments vétérinaires en circulation au Cameroun et au Sénégal. Dakar Méd. 2009, 54, 226–234. [Google Scholar]
- Vidhamaly, V.; Bellingham, K.; Newton, P.N.; Caillet, C. The quality of veterinary medicines and their implications for One Health. BMJ Glob. Health 2022, 7, e008564. [Google Scholar] [CrossRef]
- Cavany, S.; Nanyonga, S.; Hauk, C.; Lim, C.; Tarning, J.; Sartorius, B.; Dolecek, C.; Caillet, C.; Newton, P.N.; Cooper, B.S. The uncertain role of substandard and falsified medicines in the emergence and spread of antimicrobial resistance. Nat. Commun. 2023, 14, 6153. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Bose, P.; Rahman, M.Z.; Muktaruzzaman, M.; Sultana, P.; Ahamed, T.; Khatun, M.M. A review of antimicrobial usage practice in livestock and poultry production and its consequences on human and animal health. J. Adv. Vet. Res. 2024, 11, 675–685. [Google Scholar] [CrossRef]
- Sartorius, B.; Gray, A.P.; Weaver, N.D.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Mestrovic, T.; Chung, E.; Wool, E.E.; Han, C.; et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: A cross-country systematic analysis. Lancet Glob. Health 2024, 12, e201–e216. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Huber, N.; Andraud, M.; Sassu, E.L.; Prigge, C.; Zoche-Golob, V.; Käsbohrer, A.; D’Angelantonio, D.; Viltrop, A.; Żmudzki, J.; Jones, H.; et al. What is a biosecurity measure? A definition proposal for animal production and linked processing operations. One Health 2022, 15, 100433. [Google Scholar] [CrossRef]
- Vougat Ngom, R.; Laconi, A.; Mouiche, M.M.M.; Ayissi, G.J.; Akoussa, A.M.; Ziebe, D.S.; Tilli, G.; Zangue, A.H.; Piccirillo, A. Methods and Tools Used for Biosecurity Assessment in Livestock Farms in Africa: A Scoping Review. Transbound. Emerg. Dis. 2024, 2024, 5524022. [Google Scholar] [CrossRef]
- Vougat Ngom, R.; Ayissi, G.J.; Akoussa, A.M.; Laconi, A.; Jajere, S.M.; Zangue, H.A.; Piccirillo, A. A Systematic Review and Meta-Analysis of the Efficacy of Biosecurity in Disease Prevention and Control in Livestock Farms in Africa. Transbound. Emerg. Dis. 2024, 2024, 8683715. [Google Scholar] [CrossRef] [PubMed]
- Gelaude, P.; Schlepers, M.; Verlinden, M.; Laanen, M.; Dewulf, J. Biocheck.UGent: A quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult. Sci. 2014, 93, 2740–2751. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.; Koolman, L.; Whyte, P.; Lynch, H.; Coffey, A.; Lucey, B.; Egan, J.; O’Connor, L.; Bolton, D. Maximising productivity and eliminating campylobacter in broilers by manipulating stocking density and population structure using ‘biosecurity cubes’. Pathogens 2021, 10, 492. [Google Scholar] [CrossRef]
- Dhaka, P.; Chantziaras, I.; Vijay, D.; Bedi, J.S.; Makovska, I.; Biebaut, E.; Dewulf, J. Can improved farm biosecurity reduce the need for antimicrobials in food animals? A scoping review. Antibiotics 2023, 12, 893. [Google Scholar] [CrossRef]
- Moffo, F.; Mouiche, M.M.M.; Djomgang, H.K.; Tombe, P.; Wade, A.; Kochivi, F.L.; Dongmo, J.B.; Mbah, C.K.; Mapiefou, N.P.; Mingoas, J.P.K.; et al. Associations between antimicrobial use and antimicrobial resistance of Escherichia coli isolated from poultry litter under field conditions in Cameroon. Prev. Vet. Med. 2022, 204, 105668. [Google Scholar] [CrossRef]
- Djamen, C.T.; Nyembo, C.K.; Ngouana, T.R.; Tchouan, G.D.; Donfack, M.; Fokam, A.B.T.; Kana, J.R. The Effects of Allium sativum on Growth Performance, Kidney and Liver Function Markers, Microbial Flora and Feed Digestibility in Broiler Chickens. J. World’s Poult. Sci. 2024, 3, 1–11. [Google Scholar] [CrossRef]
- Tossou, L.M.; Houndonougbo, M.F.; Abiola, F.A.; Chrysostome, C.A.A.M. Etude comparée des performances de production et de la qualité organoleptique de la viande de trois souches de poulets chair (Hubbard, Cobb et Ross) élevés au Bénin. Sci. Vie Terre Agron. 2014, 2, 1–35. [Google Scholar]
- Timmerman, T.; Dewulf, J.; Catry, B.; Feyen, B.; Opsomer, G.; de Kruif, A.; Maes, D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev. Vet. Med. 2006, 74, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Rahmatallah, N.; el Rhaffouli, H.; Lahlou Amine, I.; Sekhsokh, Y.; Fassi Fihri, O.; el Houadfi, M. Consumption of antibacterial molecules in broiler production in Morocco. Vet. Med. Sci. 2018, 4, 80–90. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Carrique-Mas, J.J.; Ngo, T.H.; Ho, H.M.; Ha, T.T.; Campbell, J.I.; Nguyen, T.N.; Hoang, N.N.; Pham, V.M.; Wagenaar, J.A. Prevalence and risk factors for carriage of antimicrobial-resistant Escherichia coli on household and small- scale chicken farms in the Mekong Delta of Vietnam. J. Antimicrob. Chemother. 2015, 70, 2144–2152. [Google Scholar] [CrossRef]
- Torrey, S.; Mohammadigheisar, M.; Nascimento dos Santos, M.; Rothschild, D.; Dawson, L.C.; Liu, Z.; Kiarie, E.G.; Edwards, A.M.; Mandell, I.; Karrow, N.; et al. In pursuit of a better broiler: Growth, efficiency, and mortality of 16 strains of broiler chickens. Poult. Sci. 2021, 100, 100955. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). WHO List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/ (accessed on 20 April 2024).
- Nyokabi, N.S.; Phelan, L.; Lindahl, J.F.; Berg, S.; Muunda, E.; Mihret, A.; Wood, J.L.N.; Moore, H.L. Exploring veterinary students’ awareness and perception of zoonoses risks, infection control practices, and biosecurity measures in Ethiopia. Front. Vet. Sci. 2024, 11, 1385849. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.; El-Sayed, H.S.; Samir, A.; Abdelaty, M.F.; Hamed, E.A.; Roshdy, H. A cross-sectional survey for the assessment of biosecurity measures in small-scale duck farms in Qalyoubia, Egypt: Comprehensive evaluation and procedural recommendations. Vet. World. 2023, 16, 607–617. [Google Scholar] [CrossRef]
- Zaki, M.S.; Fahmy, H.A.; Khedr, M.H.A.; Goha, M.A.A.; Attia, A.S.A. Relationship between poultry biosecurity assessments and Escherichia coli prevalence in poultry farms. J. Adv. Vet. Res. 2024, 14, 362–367. [Google Scholar]
- Dhakal, A.; Devkota, S.; Jethara, S.B.; Yadav, R.K.; Phuyal, P. Assessment of Biosecurity in Poultry Farms in Chitwan, Nepal. Vet. Med. Sci. 2025, 11, e70232. [Google Scholar] [CrossRef]
- Elhassan, M.M.O.; Lamyia, M.A.; Enan, S.A.; Salman, A.M.A.; Mustafa, E.A. Evaluation of Internal and External Biosecurity Measures and Their Association with Mycoplasma gallisepticum Infection in Broiler Farms in Khartoum State, Sudan. J. Appl. Vet. Sci. 2024, 9, 64–73. [Google Scholar] [CrossRef]
- Ibrahim, N.; Chantziaras, I.; Mohsin, M.A.S.; Boyen, F.; Fournié, G.; Islam, S.S.; Berge, A.C.; Caekebeke, N.; Joosten, P.; Dewulf, J. Quantitative and qualitative analysis of antimicrobial usage and biosecurity on broiler and Sonali farms in Bangladesh. Prev. Vet. Med. 2023, 217, 105968. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Holistic Strategies to Control Salmonella Infantis: An Emerging Challenge in the European Broiler Sector. Microorganisms 2023, 11, 1765. [Google Scholar] [CrossRef]
- Moffo, F.; Mouiche, M.M.M.; Kochivi, F.L.; Dongmo, J.B.; Djomgang, H.K.; Tombe, P.; Mbah, C.K.; Mapiefou, N.P.; Mingoas, J.-P.K.; Awah-Ndkum, J. Knowledge, attitudes, practices and risk perception of rural poultry farmers in Cameroon to antimicrobial use and resistance. Prev. Vet. Med. 2020, 182, 105087. [Google Scholar] [CrossRef]
- Azabo, R.; Mshana, S.; Matee, M.; Kimera, S.I. Antimicrobial usage in cattle and poultry production in Dar es Salaam, Tanzania: Pattern and quantity. BMC Vet. Res. 2022, 18, 1–12. [Google Scholar] [CrossRef]
- Yévenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martín, B.S.; Maddaleno, A.; Hidalgo, H.; Cornejo, J. Detection of antimicrobial residues in poultry litter: Monitoring a risk through a selective and sensitive HPLC–MS/MS method. Animals 2021, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Vougat Ngom, R.; Jajere, S.M.; Ayissi, G.J.; Tanyienow, A.; Moffo, F.; Watsop, H.M.; Mimboe, L.; Mouiche, M.M.M.; Schüpbach-Regula, G.; Carmo, L.P. Unveiling the landscape of resistance against high priority critically important antimicrobials in food-producing animals across Africa: A scoping review. Prev. Vet. Med. 2024, 226, 106173. [Google Scholar] [CrossRef]
- Bamidele, O.; Amole, T.A.; Oyewale, O.A.; Bamidele, O.O.; Yakubu, A.; Ogundu, U.E.; Ajayi, F.O.; Hassan, W.A. Antimicrobial usage in smallholder poultry production in Nigeria. Vet. Med. Int. 2022, 2022, 7746144. [Google Scholar] [CrossRef]
- Mahmood, Q.; Chantziaras, I.; Yasir, A.; Dewulf, J. Establishing defined daily and course doses for antimicrobials used in Pakistani broilers to enable farm-level quantification and comparison of antimicrobial use. Prev. Vet. Med. 2024, 233, 106348. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Chaisowwong, W.; Ong-Artborirak, P.; Thongprachum, A. The use of critically important antimicrobials for human use in poultry farming in Pakistan: A cross-sectional study among different types of farmers. Vet. Integr. Sci. 2025, 23, e2025026-1-12. [Google Scholar] [CrossRef]
- Hosain, M.Z.; Lutful Kabir, S.M.; Kamal, M.M. Antimicrobial uses for livestock production in developing countries. Vet. World 2021, 14, 210–221. [Google Scholar] [CrossRef]
- Jarrige, N.; Cazeau, G.; Morignat, E.; Chanteperdrix, M.; Gay, E. Quantitative and qualitative analysis of antimicrobial usage in white veal calves in France. Prev. Vet. Med. 2017, 144, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, G.F.; Rodriguez, C. Tetracyclines in food and feeding stuffs: From regulation to analytical methods, bacterial resistance, and environmental and health implications. J. Anal. Methods Chem. 2017, 2017, 1315497. [Google Scholar] [CrossRef]
- Ayebare, D.; Mbatidde, I.; Kemunto, N.P.; Muloi, D.M.; Ibayi, E.L.; Nielsen, S.S.; Ndoboli, D.; Roesel, K.; Tenhagen, B.-A.; Moodley, A. Biosecurity measures and effects on health performance and antibiotic use in semi-intensive broiler farms in Uganda. One Health 2025, 20, 101039. [Google Scholar] [CrossRef]
- Umair, M.; Tahir, M.F.; Ullah, R.W.; Ali, J.; Siddique, N.; Rasheed, A.; Akram, M.; Zaheer, M.U.; Mohsin, M. Quantification and trends of antimicrobial use in commercial broiler chicken production in Pakistan. Antibiotics 2021, 10, 598. [Google Scholar] [CrossRef]
- Messomo, F.N. Etude de la Distribution et de la Qualité des Médicaments Vétérinaires au Cameroun. Ph.D. Thesis, Inter-State School of Veterinary Sciences and Medicine, Dakar, Senegal, 2006. Available online: http://www.memoireonline.com/06/07/478/m_etude-distribution-qualite-medicaments-veterinaires-cameroun20.html (accessed on 1 March 2017).
- Waktole, H.; Ayele, Y.; Ayalkibet, Y.; Teshome, T.; Muluneh, T.; Ayane, S.; Borena, B.M.; Abayneh, T.; Deresse, G.; Asefa, Z.; et al. Prevalence, molecular detection, and antimicrobial resistance of Salmonella isolates from poultry farms across central Ethiopia: A cross-sectional study in Urban and Peri-Urban Areas. Microorganisms 2024, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Witeska, M. Review: Antimicrobial resistance of Klebsiella pneumoniae isolated from poultry, cattle and pigs. Animal 2024, 18, 101345. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, O.; Makanjuola, M.; Bankole, N.; Olanike, A.; Awoseyi, A.; Awoyomi, O.J. Biosecurity and Antimicrobial Use Practices in Live Bird Markets within Abeokuta Metropolis, Southwest, Nigeria: A Preliminary Survey. Maced. Vet. Rev. 2021, 44, 187–202. [Google Scholar] [CrossRef]
- Ramukhithi, T.F.; Nephawe, K.A.; Mpofu, T.J.; Raphulu, T.; Munhuweyi, K.; Ramukhithi, F.V.; Mtileni, B. An Assessment of Economic Sustainability and Efficiency in Small-Scale Broiler Farms in Limpopo Province: A Review. Sustainability 2023, 15, 2030. [Google Scholar] [CrossRef]
- Khan, S.U.; Fouzder, S.K.; Sarkar, P.K. Productivity and profitability of commercial broiler chickens under various farming conditions. J. Exp. Biol. Agric. Sci. 2023, 11, 209–215. [Google Scholar] [CrossRef]
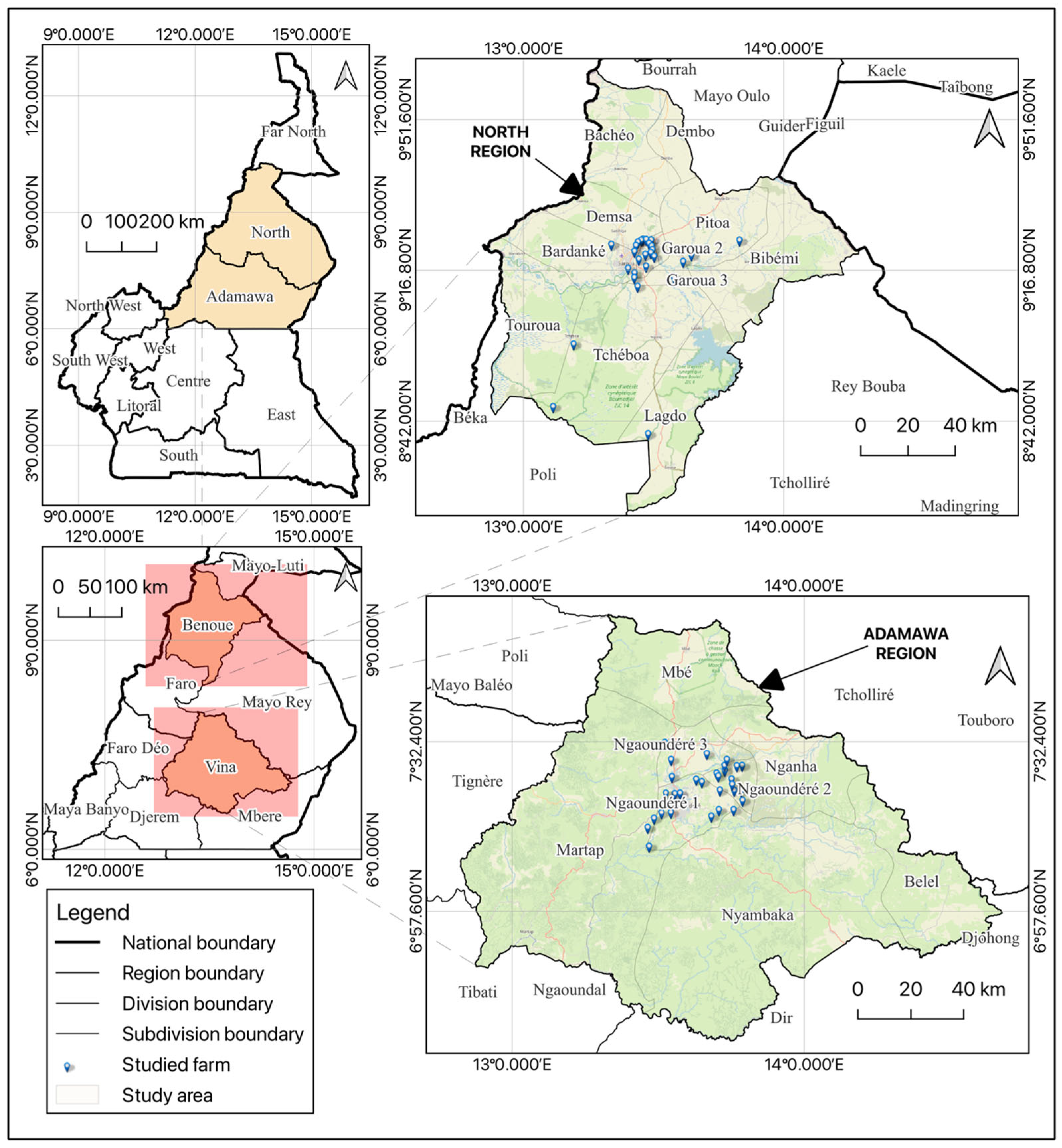

| Variables | Number (n) | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 45 | 78.9 |
| Female | 12 | 21.1 |
| Age (years) | ||
| 20–30 | 13 | 22.9 |
| 31–40 | 34 | 60.1 |
| 41–50 | 6 | 9.9 |
| 51–60 | 4 | 7.1 |
| Educational level | ||
| No education | 5 | 9.0 |
| Primary | 8 | 14.5 |
| Secondary | 26 | 44.7 |
| Higher | 18 | 31.8 |
| Training in poultry farming | ||
| Yes | 26 | 45.4 |
| No | 31 | 54.6 |
| Years of experience in poultry farming | ||
| 2–10 | 47 | 82.6 |
| 11–20 | 7 | 11.9 |
| 21–30 | 3 | 5.5 |
| Poultry farming as main activity | ||
| Yes | 13 | 24.1 |
| No | 44 | 75.9 |
| Variables | Average | SD | Min | Median | Max |
|---|---|---|---|---|---|
| External biosecurity | 50 | 12 | 23 | 53 | 72 |
| Purchase of one-day chicks | 48 | 23 | 9 | 51 | 100 |
| Depopulation of broilers | 38 | 18 | 17 | 34 | 72 |
| Feed and water | 30 | 19 | 5 | 32 | 77 |
| Removal of manure and carcasses | 35 | 25 | 0 | 45 | 78 |
| Visitors and farmworkers | 55 | 17 | 19 | 60 | 80 |
| Material supply | 74 | 29 | 0 | 100 | 100 |
| Infrastructure and biological vectors | 63 | 16 | 28 | 65 | 90 |
| Location of the farm | 54 | 20 | 19 | 61 | 100 |
| Internal biosecurity | 56 | 9 | 31 | 59 | 73 |
| Disease management | 66 | 17 | 23 | 70 | 100 |
| Cleaning and disinfection | 37 | 910 | 19 | 37 | 67 |
| Materials and measurements between compartments | 78 | 22 | 0 | 82 | 100 |
| Overall biosecurity score | 52 | 10 | 28 | 52 | 71 |
| Variables | Active Substance | ITUDD (Min–Max) | TIADD (Min–Max) |
|---|---|---|---|
| HPCIA | |||
| Quinolones | Enrofloxacin | 78.3 (66.7–111.1) | 23.40 (0.1–98.8) |
| Norfloxacin | 90.9 (66.7–111.1) | 57.7 (13.8–137.2) | |
| Macrolides | Erythromycin | 88.9 (66.7–133.3) | 3.51 (1.4–5.6) |
| Thiacynate | |||
| Polymyxins | Colistin | 82.4 (66.7–111.1) | 51.6 (0.3–920.9) |
| HIA | |||
| Beta–lactams | Amoxicillin | 66.7 (66.7) | 22.5 (14.4–31.0) |
| Tetracyclines | Oxytetracycline | 87.9 (44.4–155.6) | 34.4 (0.1–233.3) |
| Doxycycline | 91.4 (66.7–111.1) | 178.8 (19.0–397.0) | |
| Sulfonamides | Sulfadimethoxine | 88.9 (66.7–111.1) | 272.8 (90.0–698.1) |
| Sulfadiazine | 88.9 (66.7–111.1) | 13.9 (6.1–25.8) | |
| Trimethoprim | 86.5 (45.3–111.1) | 18.8 (1.2–93.1) | |
| Amphenicols | Florfenicol | 44.4 (44.4) | 16.3 (16.3) |
| CIA | |||
| Aminoglycosides | Streptomycin | 100.0 (66.7–133.3) | 4.6 (3.5–5.6) |
| Neomycin | 90.9 (66.7–155.6) | 14.2 (0.4–129.8) | |
| Macrolides | Tylosin | 90.4 (66.7–111.1) | 61.8 (5.9–198.5) |
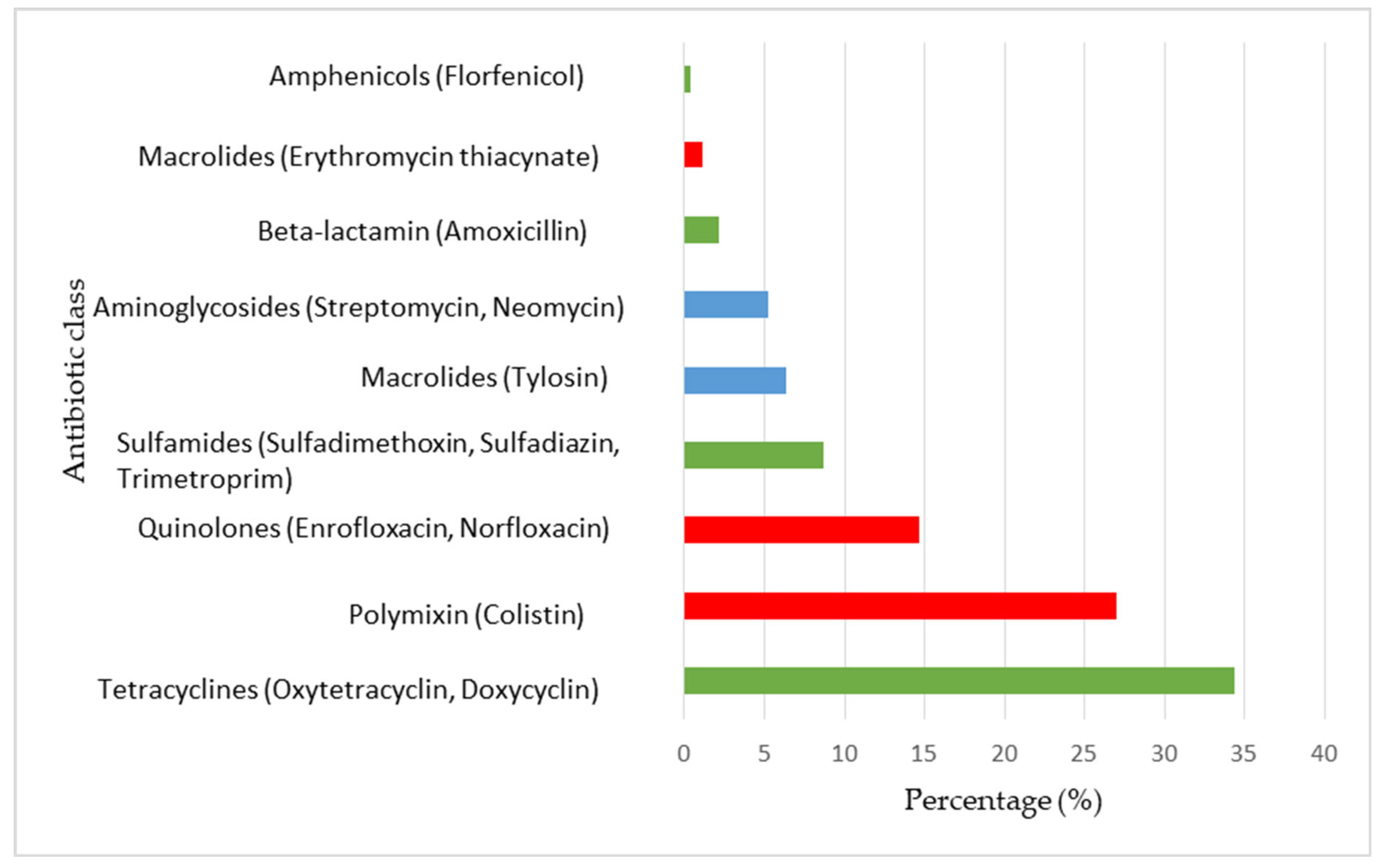
| Variables | Active Substance | Quantity (mg) | Percentage (%) |
|---|---|---|---|
| HPCIA | 113.8 | 14.5 | |
| Quinolones | Enrofloxacin | 7.9 | 1.0 |
| Norfloxacin | 27.7 | 3.5 | |
| Macrolides | Erythromycin thiacynate | 6.8 | 0.9 |
| Polymyxins | Colistin | 71.4 | 9.1 |
| HIA | 509.5 | 64.9 | |
| Beta-lactams | Amoxicillin | 105.0 | 13.4 |
| Tetracyclines | Oxytetracycline | 36.8 | 4.7 |
| Doxycycline | 198.0 | 25.2 | |
| Sulfonamides | Sulfadimethoxine | 49.1 | 6.3 |
| Sulfadiazine | 98.6 | 12.6 | |
| Trimethoprim | 14.7 | 1.9 | |
| Amphenicols | Florfenicol | 7.3 | 0.9 |
| CIA | 161.1 | 20.5 | |
| Aminoglycosides | Streptomycin | 2.0 | 0.3 |
| Neomycin | 61.4 | 7.8 | |
| Macrolides | Tylosin | 97.7 | 12.5 |
| Active Substance | Underdosing (%) | Correctly Dosing (%) | Overdosing (%) | Total Number of Treatments |
|---|---|---|---|---|
| Amoxicilline | 5 (100) | 0 | 0 | 5 |
| Colistine | 43 (69.3) | 6 (9.7) | 13 (21.0) | 62 |
| Doxycycline | 2 (22.2) | 0 | 7 (77.8) | 9 |
| Enrofloxacine | 22 (95.7) | 1 (4.3) | 0 | 23 |
| Erythromycin thiacynate | 3 (100) | 0 | 0 | 3 |
| Florfénicol | 1 (100) | 0 | 0 | 1 |
| Neomycine | 10 (80.9) | 0 | 1 (9.1) | 11 |
| Norfloxacine | 9 (81.8) | 0 | 2 (18.2) | 11 |
| Oxytetracycline | 56 (82.4) | 6 (8.8) | 6 (8.8) | 68 |
| Streptomycine | 2 (100) | 0 | 0 | 2 |
| Sulfadiazine | 6 (100) | 0 | 0 | 6 |
| Sulfadiméthoxine | 0 | 0 | 4 (100) | 4 |
| Trimethoprime | 9 (90.0) | 1 (1.0) | 0 | 10 |
| Tylosine | 11 (73.3) | 0 | 4 (26.7) | 15 |
| Total | 179 (77.8) | 14 (6.1) | 37 (16.1) | 230 |
| Variable | Good Biosecurity | Poor Biosecurity |
|---|---|---|
| TIUDD | 68.41 ± 38.73 a | 75.23 ± 40.13 a |
| TIADD | 40.89 ± 24.74 a | 39.58 ± 25.82 a |
| Qkg | 35.66 ± 15.10 a | 47.75 ± 26.20 a |
| Variable | R2 | p-Value |
|---|---|---|
| TIUDD | −0.099 | 0.100 |
| TIADD | −0.180 | 0.771 |
| Qkg | −0.008 | 0.889 |
| Variables | Average ± Standard Deviation |
|---|---|
| Average daily gain (g) | |
| Starter | 25.35 ± 3.51 b |
| Grower | 59.56 ±13.45 a |
| Finisher | 53.34 ±13.12 a |
| Average | 45.71 ± 5.14 |
| Feed conversion ratio | |
| Starter | 2.39 ± 0.82 a |
| Grower | 1.69 ± 0.87 b |
| Finisher | 1.60 ± 0.51 b |
| Average | 1.59 ± 0.48 |
| Mortality rate (%) | |
| Starter | 1.77 ± 1.28 a |
| Grower | 1.13 ± 0.87 ab |
| Finisher | 0.76 ± 0.52 b |
| Total | 3.55 ± 3.30 |
| Performance index | 317.76 ± 115.56 |
| Production Performances | Good Biosecurity | Poor Biosecurity | p-Value |
|---|---|---|---|
| Average daily gain (g) | |||
| Startup | 26.22 ± 3.20 a | 25.30 ± 4.49 a | 0.200 |
| Growth | 62,08 ± 11,40 a | 54.03 ± 15.03 a | 0.103 |
| Finishing | 53,07 ± 14.71 a | 53.77 ± 11.34 a | 0.517 |
| Average | 46.54 ± 5.18 a | 43.80 ± 4.16 b | 0.034 |
| Feed conversion ratio | |||
| Startup | 2.13 ± 0.39 a | 2.57 ± 1.08 b | 0.007 |
| Growth | 1.49 ± 0.52 a | 1.99 ± 1.16 a | 0.096 |
| Finishing | 1.59 ± 0.61 a | 1.75 ± 0.58 b | 0.029 |
| Average | 1.50 ± 0.35 a | 1.72 ± 0.57 b | 0.026 |
| Mortality rate (%) | |||
| Startup | 1.10 ± 0.92 a | 3.16 ± 2.22 b | <0.001 |
| Growth | 0.47 ± 0.16 a | 2.22 ± 1.85 b | <0.001 |
| Finishing | 0.41 ± 0.19 a | 2.09 ± 1.49 b | 0.008 |
| Total | 2.47 ± 1.42 a | 6.65 ± 3.12 b | <0.001 |
| Performance Index | 339.21 ± 105.79 a | 268.22 ± 101.09 b | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziebe, S.D.; Vougat Ngom, R.; Akoussa, A.M.M.; Bogning, H.P.; Zangue, H.A. Impact of Biosecurity on Production Performance and Antimicrobial Usage in Broiler Farms in Cameroon. Animals 2025, 15, 1771. https://doi.org/10.3390/ani15121771
Ziebe SD, Vougat Ngom R, Akoussa AMM, Bogning HP, Zangue HA. Impact of Biosecurity on Production Performance and Antimicrobial Usage in Broiler Farms in Cameroon. Animals. 2025; 15(12):1771. https://doi.org/10.3390/ani15121771
Chicago/Turabian StyleZiebe, Stephane D., Ronald Vougat Ngom, Adonis M. M. Akoussa, Henry P. Bogning, and Henriette A. Zangue. 2025. "Impact of Biosecurity on Production Performance and Antimicrobial Usage in Broiler Farms in Cameroon" Animals 15, no. 12: 1771. https://doi.org/10.3390/ani15121771
APA StyleZiebe, S. D., Vougat Ngom, R., Akoussa, A. M. M., Bogning, H. P., & Zangue, H. A. (2025). Impact of Biosecurity on Production Performance and Antimicrobial Usage in Broiler Farms in Cameroon. Animals, 15(12), 1771. https://doi.org/10.3390/ani15121771






