Simple Summary
Excellent quality of early embryonic development contributes to a successful pregnancy. At present, most in vitro cultured embryos can only develop to the blastocyst stage at most, because an in vitro culture (IVC) system cannot replace the physiological environment in vivo. During IVC, excessive accumulated reactive oxygen species in embryos cannot be easily metabolized, which will cause oxidative stress and suppress embryo development. In this study, we found that anti-oxidation capacity of early embryo was improved by adding 6-gingerol to IVC. Moreover, 6-gingerol can also improve blastocyst rate, cell proliferation, mitochondrial function, inhibit cell apoptosis, autophagy, and regulate functional genes expression in blastocyst. These results are helpful to optimize the early embryo culture system, and thus provide a theoretical basis for improving the early embryo quality and the efficiency of subsequent pregnancy.
Abstract
6-Gingerol, the main active ingredient in ginger, exhibits a variety of biological activities, such as antioxidant, anti-inflammatory, and anticancer activities, and can affect cell development. However, the effects of 6-gingerol on mammalian reproductive processes, especially early embryonic development, are unclear. This study explored whether 6-gingerol can be used to improve the quality of in vitro-cultured porcine embryos. The results showed that 5 μM 6-gingerol significantly increased the blastocyst formation rates of porcine early embryos. 6-Gingerol attenuated intracellular reactive oxygen species accumulation and autophagy, increased intracellular glutathione levels, and increased mitochondrial activity. In addition, 6-gingerol upregulated NANOG, SRY-box transcription factor 2, cytochrome c oxidase subunit II, mechanistic target of rapamycin kinase, and RPTOR independent companion of MTOR complex 2 while downregulating Caspase 3, baculoviral IAP repeat containing 5, autophagy related 12, and Beclin 1. Most importantly, 6-gingerol significantly increased the levels of p-extracellular regulated protein kinase 1/2 while reducing the levels of p-c-Jun N-terminal kinase 1/2/3 and p-p38. These results indicate that 6-gingerol can promote the development of porcine early embryos in vitro.
Keywords:
6-gingerol; porcine embryo; reactive oxidative stress; proliferation; apoptosis; autophagy 1. Introduction
In vitro embryo culture systems, including procedures for in vitro maturation (IVM), in vitro fertilization (IVF) and in vitro embryo culture (IVC), are currently widely used for embryonic developmental mechanism research, in vitro embryo production, and human assisted reproduction [1]. However, it is difficult to simulate the optimal embryonic growth environment in vitro, and embryo quality and subsequent development ability are still low, especially in porcine in vitro embryo production systems [2]. Therefore, continuous development and optimization of these culture systems are important for improving porcine early embryo quality [3].
The addition of hormones [4,5] and growth factors [6] can improve the quality of early embryo development in vitro. However, the overall efficiency of embryo production in vitro is still low [1,7]. One of the most important potential reasons is that reactive oxygen species (ROS) can inhibit the development of porcine embryos [8]. The oxygen concentration in the normal reproductive tract is lower than the oxygen concentration in air, and oxygen levels higher than those in the body can cause ROS accumulation [9]. Oxidative stress caused by ROS accumulation can easily cause DNA damage in cells and oxidative modification of proteins in cells, which further cause mitochondrial destruction and lead to cell death [10]. Therefore, it is critical to use antioxidants to inhibit oxidation during porcine embryonic development. Previous studies showed that antioxidants, such as Vitamin C and coenzyme Q10, are effective in reducing ROS accumulation during both in vivo and in vitro embryo development [11,12]. Exploring additional antioxidants and clarifying their mechanisms is meaningful for improving embryo quality.
Ginger is commonly used in functional dietary supplements, beverages, and foods (sugar products, jams, and pickled products) [13]. In Asia and some non-Asian countries, ginger has been used as a medicine to treat diseases such as arthritis, indigestion, diabetes, and irregular menstruation [14]. 6-Gingerol (6-G) extracted from ginger has the greatest abundance of biologically active compounds of any ginger component and exerts various pharmacological effects, including antioxidant, apoptosis- and autophagy-regulating, cell proliferation-promoting, and mitochondrial function-maintaining effects [15]. Importantly, 6-G exerts pharmacological effects by regulating the mitogen-activated protein kinase (MAPK) signaling pathway [16] and inhibits apoptosis to attenuate myocardial ischemia/reperfusion injury by activating the phosphatidylinositol 3-kinase/Akt and high mobility group box 2-c-Jun N-terminal kinase 1/2/3 (JNK1/2/3)-nuclear factor kappa B pathways [17]. However, few studies on the effects and mechanisms of 6-G on animal reproduction have been carried out, especially with regard to early embryos. Whether 6-G can be used as an antioxidant in in vitro embryo production and the possible related mechanisms are not clear.
Porcine parthenogenetically activated (PA) embryos can be acquired in a relatively short amount of time, they are also frequently used in early embryo development-related studies. Studies indicated that parthenogenetic embryos could retain the female’s genetic characteristics, which were used in studies of mitochondrial functions, production of autologous stem cells, and cytoplasmic activity [18,19]. In this study, the potential effects of 6-G on oxidative stress, cell proliferation, apoptosis, autophagy, mitochondrial function, and embryo quality were explored in PA embryos. These results are helpful to optimize the embryo IVC system, and thus provide a theoretical basis for improving the early embryo quality.
2. Materials and Methods
All chemicals and reagents were purchased from Sigma-Aldrich/Merck unless expressly stated otherwise. According to the Institutional Animal Care and Use Committee of Jilin University (IACUC-ID-201802070), all experiments were carried out in the Experimental Animal Center of the Jilin University.
2.1. Oocyte Collection and IVM
Cumulus-oocyte complexes were collected from 3–6 mm follicles of prepubertal gilt ovaries and placed in a 4-well culture plate with 500 μL of IVM medium for 44 h. The details are indicated in the Supplementary Materials.
2.2. Parthenogenetic Activation and In Vitro Embryo Culture
Mature oocytes were parthenogenetically activated. Next, the oocytes were cultured in IVC medium with cytochalasin B (#C6762) for 3 h. Then, the oocytes were transferred into IVC medium with/without 0 μM, 5 μM, 10 μM, and 20 μM 6-G (#S3836, Selleck Chemicals, Shanghai, China). The rate of blastocyst formation was calculated as the ratio of the number of blastocysts to the number of cleavages. The details are indicated in the Supplementary Materials.
2.3. Cell Proliferation Analysis
Briefly, embryonic cell proliferation capacity was analyzed by a 5-ethynyl-2′-deoxyuridine (EdU) assay with a BeyoClick™ EdU Cell Proliferation Kit (#C0075; Beyotime, Shanghai, China). The cell proliferation rate was calculated as the number of EdU-positive cells to the total number of cells in blastocysts. The details are indicated in the Supplementary Materials.
2.4. Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End-Labeling (TUNEL) Assays
Briefly, the cell apoptosis level was analyzed by an In Situ Cell Death Detection Kit (#11684795910; Roche, Mannheim, Germany). The ratio of the number of TUNEL-positive nuclei to the total number of nuclei was calculated as the apoptosis rate. The details are indicated in the Supplementary Materials.
2.5. ROS and Glutathione (GSH) Assays
Briefly, 4-cell-stage porcine embryos were incubated in 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH; #C2938; Invitrogen, Rochester, NY, USA) and 10 μM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC; #C12881; Invitrogen), respectively. The details are indicated in the Supplementary Materials.
2.6. Mitochondrial Membrane Potential (MMP, ΔΨm) Assay
Briefly, 4-cell-stage embryos were incubated in 2 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine-iodide dye (JC-1; #M34152; Invitrogen). The details are indicated in the Supplementary Materials.
2.7. Determination of ATP Levels
Briefly, the ATP levels in 4-cell-stage embryos were measured using an ATP Determination Kit (#A22066; Invitrogen). The details are indicated in the Supplementary Materials.
2.8. Immunofluorescence
Briefly, embryos were fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. Then, the embryos were blocked in 5% BSA and incubated with a primary antibody against microtubule-associated protein 1 light chain 3 beta (LC3B; #ab63817; Abcam, Cambridge, MA, USA). Then, the embryos were incubated with a secondary antibody (#ab150073; Abcam) for 1 h. The nuclei were stained with Hoechst 33342. The details are indicated in the Supplementary Materials.
2.9. RNA Extraction and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
Briefly, mRNA was extracted using the Dynabeads mRNA DIRECT Purification Kit (#61011; Invitrogen). A TIANScript First Strand cDNA Synthesis Kit (#KR118; Tiangen Biotech Co., Beijing, China) was used to synthesize cDNA. Gene expression was quantified using the 2−ΔΔCt method with 18S rRNA as the standard. The details are indicated in the Supplementary Materials and all the primers are listed in Supplementary Table S1.
2.10. Western Blot Analysis
Briefly, a RIPA lysis buffer (#R0010; Solarbio, Beijing, China) was used for total protein extraction. Proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (0.45 μm; #IPVH00010; Millipore, Bedford, MA, USA). The membranes were blocked with 5% BSA and incubated with a primary antibody. Then, the membranes were incubated with secondary antibodies. The details are indicated in the Supplementary Materials and the antibody information is shown in Supplementary Table S2.
2.11. Statistical Analysis
All calculations were performed using SPSS software v.22.0 (SPSS, Inc., Chicago, IL, USA). Data from two groups were compared using the Student’s t-test. Tests with three or more means were analyzed using a one-way ANOVA (Tukey-Kramer). All data are presented as mean ± SD. The total numbers of embryos used (N) in each group and replicates (R) in each experiment are shown in the results and figure legends. p < 0.05 and p < 0.01 were considered to indicate significant differences.
3. Results
3.1. 6-G Improved the Blastocyst Formation Rate
To explore the effects of 6-G on early embryo development, we incubated parthenogenetic porcine early embryos with different 6-G concentrations (0, 5, 10, and 20 μM). As shown in Figure 1, the blastocyst formation rates were higher in the 5 μM 6-G-supplied group than in the other groups at day 5, day 6, and day 7. On day 5, the blastocyst formation rates were 32.46% ± 2.06% (N = 173), 44.67% ± 1.77% (N = 177, p < 0.01), 33.56% ± 2.54% (N = 176, p = 0.903), and 25.45% ± 1.42% (N = 181, p = 0.011) with 0 μM, 5 μM, 10 μM, and 20 μM 6-G, respectively. On day 6, these rates were 39.23% ± 2.42% (N = 173), 48.04% ± 2.54% (N = 177, p = 0.027), 40.88% ± 3.99% (N = 176, p = 0.899), and 33.62% ± 2.57% (N = 181, p = 0.171) with the aforementioned 6-G concentrations, respectively, and on day 7, these rates were 40.90% ± 2.55% (N = 173), 54.78% ± 5.46% (N = 177, p < 0.01), 44.30% ± 3.96% (N = 176, p = 0.711), and 37.57% ± 2.77% (N = 181, p = 0.724), respectively. Therefore, a concentration of 5 μM was selected for further research.
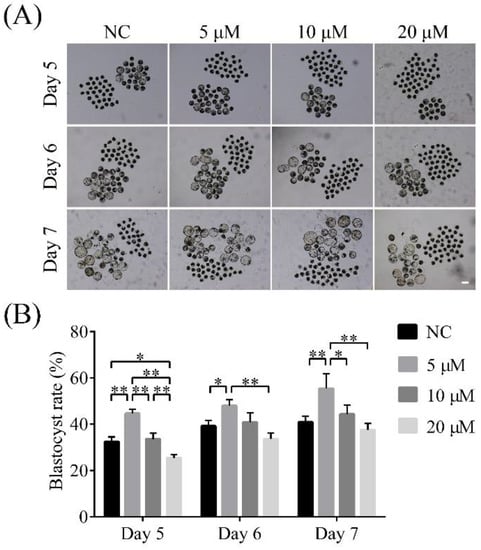
Figure 1.
Effects of 6-gingerol (6-G) on blastocyst formation rate of porcine early embryos. (A) Representative images of embryos on day 5, day 6, and day 7 treated with 0 μM, 5 μM, 10 μM, or 20 μM 6-G, respectively. Scale bar = 200 μm. (B) Blastocyst formation rates on day 5, day 6, and day 7 treated with 0 μM, 5 μM, 10 μM, or 20 μM 6-G, respectively. R = 3. * p < 0.05; ** p < 0.01.
3.2. 6-G Enhanced Cell Proliferation
Subsequently, we examined the effect of 6-G on proliferation in early embryos by the EdU assay. As shown in Figure 2, compared with the NC group, the 6-G treatment group exhibited a significantly higher proportion of proliferating cells among the total number of cells (48.87% ± 12.46% (N = 70) versus 40.66% ± 12.57% (N = 71), p < 0.01). Based on the above results, 6-G can enhance cell proliferation during early embryo development.
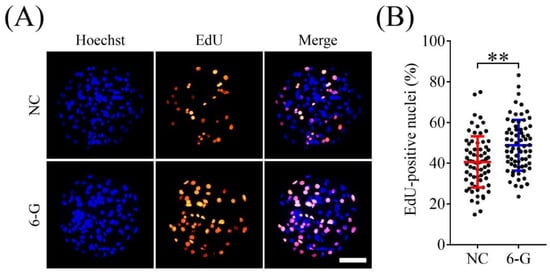
Figure 2.
Effects of 6-gingerol (6-G) on cell proliferation in blastocysts. (A) Representative 5-ethynyl-2′-deoxyuridine (EdU) staining images of embryos with or without 6-G at day 7. (B) Proportions of proliferating cells to total numbers of cells in embryos with or without 6-G treatment. R = 5. Bar = 100 μm. ** p < 0.01.
3.3. 6-G Reduced Apoptosis of Porcine Embryos
As shown in Figure 3, the proportion of TUNEL-positive nuclei in the 6-G-treated group was significantly lower than that in the NC group (3.94 ± 1.87% (N = 61) versus 5.70 ± 2.36% (N = 62), p < 0.01). This suggests that treatment with 6-G reduces apoptosis in blastomeres of porcine embryos.
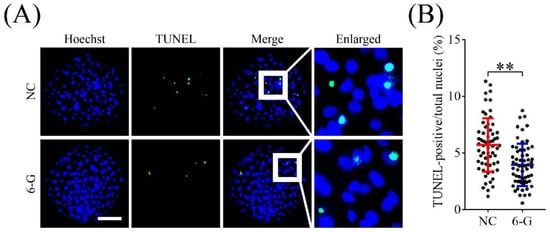
Figure 3.
Effects of 6-gingerol (6-G) on cell apoptosis in blastocysts. (A) Representative staining images of Hoechst and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) of blastocysts. Bar = 100 μm. (B) Apoptotic rates in blastocysts with or without 6-G treatment. R = 5. ** p < 0.01.
3.4. 6-G Enhanced Antioxidant Capacity in Porcine Embryos
To investigate whether 6-G can inhibit ROS accumulation, we used DCFH fluorescent probes to detect ROS levels in 4-cell-stage embryos. As shown in Figure 4, the fluorescence intensities of DCFH in blastomeres were significantly lower (0.79 ± 0.13-fold; NNC = 163, N6-G = 168; p < 0.01) in the 6-G treatment group than in the NC group (Figure 4A). In addition, we analyzed the relative CMF2HC level to detect GSH levels in 4-cell-stage embryos and found that they were significantly higher (1.26 ± 0.12-fold; NNC = 140, N6-G = 155; p < 0.01) in the 6-G treatment group than in the NC group (Figure 4B).
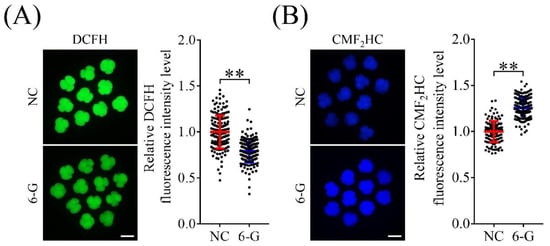
Figure 4.
Effects of 6-gingerol (6-G) on reactive oxygen species (ROS) and glutathione (GSH) levels. (A) Representative 2′,7′-dichlorodihydrofluorescein diacetate (DCFH) staining images of 4-cell-stage embryos with or without 6-G. The relative DCFH fluorescence intensity decreased significantly in 4-cell-stage embryos with 6-G. R = 5. Bar = 100 μm. (B) Representative 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC) staining images of 4-cell-stage embryos with or without 6-G. The relative CMF2HC fluorescence intensity increased significantly in 4-cell-stage embryos with 6-G. R = 5. Bar = 100 μm. ** p < 0.01.
3.5. 6-G Improved Mitochondrial Function
Mitochondrial activity directly affects embryonic cell proliferation and developmental potential. To study whether 6-G can improve mitochondrial function in porcine early embryos, we measured MMP in 4-cell-stage embryos. As shown in Figure 5A, the JC-1Red/Green fluorescence intensity ratio in 4-cell-stage embryos was significantly higher (1.40 ± 0.14-fold; NNC = 81, N6-G = 70; p < 0.01) in the 6-G-treated group than in the NC group (Figure 5B). In addition, the ATP level in 6-G treated embryos was also higher (1.30 ± 0.03-fold; NNC, N6-G = 270; p < 0.01) than that in non-6-G-treated embryos (Figure 5C).
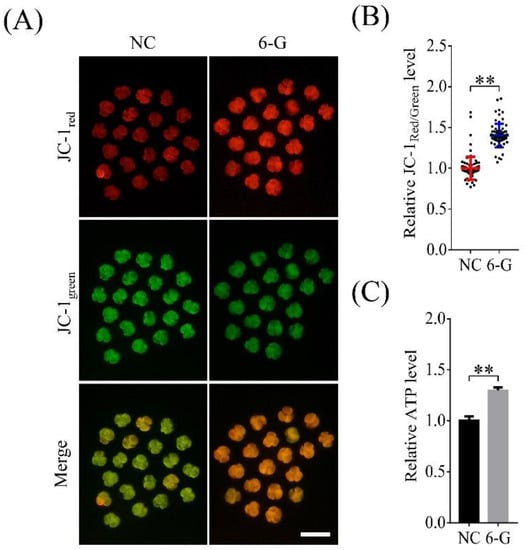
Figure 5.
Effects of 6-gingerol (6-G) on mitochondrial function in 4-cell-stage embryos. (A) Representative images of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine-iodide dye (JC-1) in the NC and 6-G treatment groups. Bar = 200 μm. (B) Relative JC-1Red/Green fluorescence intensity ratios with or without 6-G treatment. R = 4. (C) Relative ATP level change in 4-cell-stage embryos after 6-G treatment. R = 3. ** p < 0.01.
3.6. 6-G Inhibited Autophagy in Blastocysts
Excessive ROS production can induce autophagy in cells. Therefore, we analyzed the autophagy levels of embryos after 6-G treatment. As shown in Figure 6A, compared with the NC group, the 6-G group exhibited a significantly lower number of intracellular LC3B-positive puncta (0.76 ± 0.33-fold; NNC = 57, N6-G = 64; p < 0.01). This finding indicates that 6-G may reduce intracellular autophagy levels.
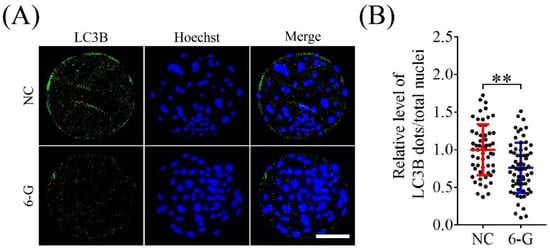
Figure 6.
6-Gingerol (6-G) treatment affects the autophagy levels in porcine early embryos. (A) Representative immunofluorescence images of microtubule-associated protein 1 light chain 3 beta (LC3B) in blastocysts. Bar = 100 μm. (B) Proportions of LC3B-positive puncta in blastocysts with or without 6-G treatment. R = 5. ** p < 0.01.
3.7. 6-G Regulated Embryo Pluripotency, Apoptosis, Autophagy, and Proliferation-Related Gene Expression in Blastocysts
To study the potential mechanisms by which 6-G promotes early embryo development, we examined the expression of related genes (shown in Figure 7). Compared with NC embryos, 6-G-treated embryos exhibited significant upregulation of embryo pluripotency- (NANOG, 1.31 ± 0.10-fold, p = 0.039; SRY-box transcription factor 2, SOX2, 1.39 ± 0.16-fold, p = 0.047; NNC, N6-G = 90), proliferation- (mechanistic target of rapamycin kinase, mTOR, 1.43 ± 0.17-fold, p = 0.039; NNC, N6-G = 90), hatching- (cytochrome c oxidase subunit II, COX2, 1.42 ± 0.16-fold, p = 0.032; NNC, N6-G = 90) related genes, and the apoptosis-related gene RPTOR independent companion of MTOR complex 2 (RICTOR, 1.46 ± 0.11-fold, p = 0.018; NNC, N6-G = 90) and significant downregulation of the apoptosis- (caspase 3, CASP3, 0.53 ± 0.16-fold, p < 0.01; baculoviral IAP repeat containing 5, BIRC5, 0.61 ± 0.22-fold, p = 0.023; NNC, N6-G = 90) and the autophagy- (autophagy related 12, ATG12, 0.60 ± 0.16-fold, p = 0.028; beclin 1, BECN1, 0.68 ± 0.16-fold, p = 0.030; NNC, N6-G = 90) related genes. However, 6-G did not significantly increase the expression level of OCT4 (1.10 ± 0.09-fold, p = 0.256; NNC, N6-G = 90).
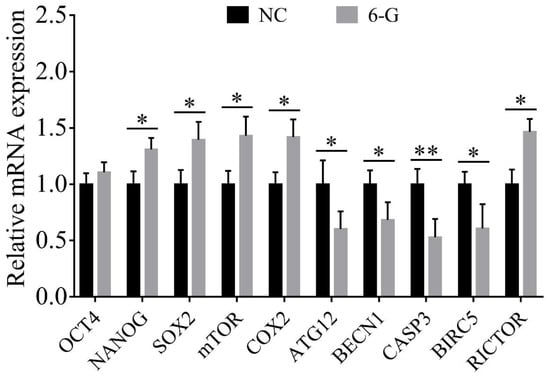
Figure 7.
Results of quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis of functional gene expression. Expression of embryo pluripotency- (OCT4; NANOG; and SRY-box transcription factor 2, SOX2), proliferation- (mechanistic target of rapamycin kinase, mTOR), hatching (cytochrome c oxidase subunit II, COX2), apoptosis- (RPTOR independent companion of MTOR complex 2, RICTOR; caspase 3, CASP3; and baculoviral IAP repeat containing 5, BIRC5), and autophagy- (autophagy related 12, ATG12; beclin 1, BECN1) related genes were all detected in blastocysts at day 7. R = 3. * p < 0.05; ** p < 0.01.
3.8. 6-G Regulated MAPKs Activations in Blastocysts
The MAPK signaling pathway can regulate cell proliferation, differentiation, apoptosis, and autophagy. Therefore, we used Western blotting to detect the effects of 6-G treatment on the extracellular regulated protein kinase 1/2 (ERK1/2), JNK1/2/3, and p38 signaling pathways. As shown in Figure 8, the addition of 6-G significantly increased the level of p-ERK1/2 (1.52 ± 0.14-fold, p < 0.01; NNC, N6-G = 90; Figure 8A), while reducing the levels of p-p38 (0.51 ± 0.13-fold, p < 0.01; NNC, N6-G = 90; Figure 8B), and p-JNK1/2/3 (0.81 ± 0.05-fold, p < 0.01; NNC, N6-G = 90; Figure 8C).
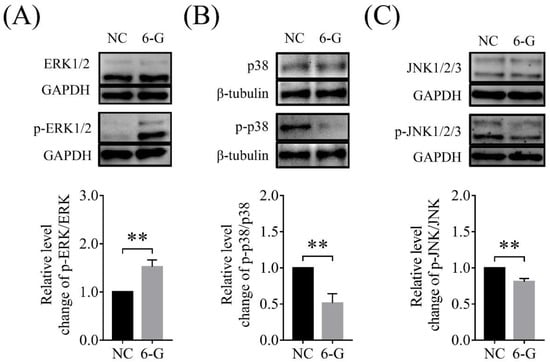
Figure 8.
Effects of 6-gingerol (6-G) on the extracellular regulated protein kinase 1/2 (ERK1/2), c-Jun N-terminal kinase 1/2/3 (JNK1/2/3), and p38 pathways. (A) The level of p-ERK1/2 in the 6-G treatment group was higher than that of the NC group. R = 3. (B) The level of p-p38 in the 6-G treatment group was lower than that of the NC group. R = 3. (C) The level of p-JNK1/2/3 in the 6-G treatment group was lower than that of the NC group. R = 3. ** p < 0.01.
4. Discussion
Studies have shown that 6-G alleviates oxidative damage and inflammation in the ovaries [20,21]. Porcine early embryos are more sensitive to oxidative stress during in vitro embryo production than in vivo due to their unique structures [22]. The results of this study indicate that the addition of 6-G can effectively improve the developmental potential of early porcine embryos.
In this study, 6-G treatment effectively reduced the accumulation of ROS in blastomeres at the 4-cell stage, likely because 6-G can effectively prevent -OH-induced DNA damage, in particular by scavenging various free radicals [23]. This effect was likely mediated by 6-G-induced high levels of GSH. Intracellular ROS levels are highly correlated with GSH levels. The findings show that when ROS levels in the body are increased, 6-G-mediated enhancement of the GSH defense system can increase the removal of excess oxygen free radicals and maintain dynamic redox balance. These results are consistent with previous suggestions that 6-G can alleviate ROS-induced diseases and conditions by increasing GSH levels [24]; such conditions include impaired ovarian follicle development and abnormal fertilization [25].
At the 4-cell stage, zygotic genome activation is essential for porcine early embryo development and pregnancy [26,27]. During IVC of embryos, activation of the zygotic genome generally occurs 24–48 h after parthenogenetic activation or IVF. This stage is more sensitive than other stages to negative conditions in the external environment, especially the accumulation of ROS, which leads to the failure of zygotic genome activation and leads to the suppression of embryo development [28]. The results indicate that 6-G may exert biological effects at least as early as at this stage.
Mitochondria play essential roles during embryo development both before and after zygotic genome activation [29]. Excessive accumulation of ROS causes oxidative damage, MMP depolarization [30], aging, and apoptosis [31]. In this study, we found that 6-G supplementation significantly increased the MMP and ATP level in blastomeres at the 4-cell stage, improved the ability of embryos to proliferate and effectively reduced the number of apoptotic cells in the embryos. This result is consistent with previous findings that 6-G inhibits disruption of MMP [32] and is probably related to the roles of 6-G in increasing the activity of the mitochondrial enzymes NADH oxidase, succinate dehydrogenase, and Sirtuin 3 [33]. Previous studies have also shown that 6-G can reduce intracellular ROS levels by regulating nuclear factor kappa B translocation [34]. In addition, 6-G upregulated antioxidant enzymes, such as glyoxylate carboligase and heme oxygenase 1, and further protects against cytotoxicity and apoptotic cell death resulting from processes such as ROS-induced DNA fragmentation, disruption of MMP, and autophagy. These effects seem to be mediated by regulation of nuclear factor 2 [35], p38 MAPK, JNK [36], and phosphatidylinositol 3-kinase/Akt [37]. In this study, we found that 6-G increased p-ERK levels and decreased p-JNK and p-p38 levels. This indicates that 6-G exerts a regulatory effect on cell survival or apoptosis by affecting the dynamic balance between the growth factor-activated ERK pathway and the stress-activated JNK-p38 pathway [38]. On the one hand, this regulation depends on ERK pathway-mediated direct targeting and regulation of the cell cycle and indirect regulation of RNA metabolism and transport [39]. On the other hand, it also depends on the regulation of the JNK pathway by various cellular stress and growth factors [40]. 6-G may enable the effects mediated by these pathways to regulate biological processes, such as cell morphology changes, immune responses, and apoptosis [41,42].
To explore the potential mechanism by which 6-G promotes embryo development, we also examined functional gene expression changes. The results showed that the addition of 6-G significantly upregulated the anti-apoptotic gene RICTOR and downregulated the pro-apoptotic gene CASP3. These factors help stabilize the intracellular environments of blastomeres. The results are consistent with the findings that 6-G can improve embryo development and reduce apoptosis. Early embryos with stable cell states and enhanced mitochondrial function show high proliferation ability and reduced nuclear apoptosis rates [43,44]. Notably, the quality of blastomeres is an important basis for embryo development before implantation [45]. Combined with the result that 6-G improved the proliferation-related gene mTOR [46], these findings suggest that 6-G can stabilize or even optimize the intracellular environment of blastomeres, creating a relatively stable internal environment and promoting cell proliferation and development. This study also revealed that the addition of 6-G can significantly increase the cell proliferation capacity in blastocysts. Interestingly, 6-G significantly decreased the expression level of the apoptosis-related gene BIRC5. BIRC5 is widely known as an anti-apoptosis gene. However, a previous study has found that overexpression of BIRC5 significantly inhibits cell survival [47]. In addition, compared with those in unfrozen bovine blastocysts, the levels of apoptosis and the expression of apoptosis-related genes BIRC5 and CASP3 were also significantly increased in frozen embryos [48], which was similar to our results that 6-G reduced the level of apoptosis and downregulated the expression levels of BIRC5 and CASP3 in porcine blastocysts. We speculate that this may be because the addition of 6-G improves the embryonic development environment while the high level of anti-apoptotic factors is not needed. In addition, embryo pluripotency-related genes (NANOG and SOX2) were significantly upregulated in this study, which indicates that 6-G has the potential to promote establishment of the epiblast and hypoblast [49], improve the quality of inner cell masses [50,51], and stabilize the basic functions of embryonic stem cells [52,53]. However, there are some reports showing that there are differences in the expression of NANOG and SOX2 during embryo development not only between embryos produced in vivo and in vitro, but also between parthenogenetic and IVF embryos [54]. Additional research about the roles of 6-G in IVF and somatic cell nuclear transfer embryos is still needed. The upregulation of COX2 observed in this study suggests that 6-G has potential effects on early embryo implantation and decidualization [55,56]. Our results showed that the expression levels of autophagy-related genes ATG12 and BECN1 with 6-G treatment. ATG12 can target the elongation of the autophagosome membrane. A previous study showed that inhibition of ATG12 significantly decreased the autophagy level [57]. BECN1 also plays important roles in autophagy [58]. However, mTOR plays opposite roles in the regulation of autophagy [59]. The early stages of the autophagic process are inhibited by mTOR, and mTOR can also regulate the lysosomal degradative capacity by preventing the transactivation of genes encoding catalytic, regulatory, and structural factors [60]. These results were related to the inhibition of autophagosome formation with 6-G treatment, indicating that 6-G can significantly inhibit the autophagy level in porcine early embryos. Moreover, autophagy is one of the most important factors in mediating apoptosis [61], which indicates that the decreased level of autophagy with 6-G treatment may also be one of the reasons for the inhibition of apoptosis.
Conversely, some studies have shown that 6-G can play roles in inducing ROS production, reducing mitochondrial function, and promoting autophagy and apoptosis. However, such studies have mostly focused on cancer research [62]. In addition, we believe that different effects are caused by different concentrations of 6-G. 6-G (20 µM) inhibited blastocyst formation. This is similar to the results showing that an appropriate concentration of antioxidant supplements could improve early embryo development by reducing oxidative stress, while they would inhibit early embryo development at a high concentration [63,64,65]. This may be because oxidative stress signaling is also required during early embryonic development. A large amount of 6-G will reduce the ROS level too much. Reductive stress is just as dangerous as oxidative stress [66]. At the same time, the concentration of 6-G in the culture medium may decrease with time. However, during the 7 days of in vitro culture, the corresponding concentration of 6-G still affected the embryonic development. Furthermore, many studies have shown that biological substances can induce ROS production and promote apoptosis in cancer cells while exerting beneficial effects on normal cells, promoting cell proliferation and enhancing embryo development [67]. These different effects are related mainly to the abnormal physiological statuses of cancer cells, such as their abnormal gene expression (including that of insulin-like growth factor-1, DNA methyltransferase 1, and histone deacetylases) [68], enzyme activity [69], and activation of signaling pathways including the AKT, MAPK, and nuclear factor kappa B pathways [70]. In this study, 6-G was found to regulate the p38, JNK, and ERK pathways. However, how 6-G regulates key downstream genes through these pathways during early embryo development still needs further research.
In summary, our results indicate that the addition of 6-G to IVC systems increases GSH and regulates functional gene expression and the ERK, JNK, and p38 signaling pathways, thereby reducing intracellular ROS accumulation, autophagy, and apoptosis, and enhancing mitochondrial activity and cell proliferation to improve porcine preimplantation embryo development and competence. Our findings will suggest new methods and provide a theoretical basis for improving the quality of embryo development in vivo and in vitro.
5. Conclusions
6-G improved blastocyst rate, cell proliferation, mitochondrial function, and inhibited cell apoptosis and autophagy by reducing oxidative stress. Moreover, 6-G can regulate related gene expressions in blastocyst.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13081315/s1, Table S1: Primer sequence for qRT-PCR; Table S2: Antibodies used for Western blotting; Supplementary Materials and Methods.
Author Contributions
Conceptualization, C.C. and J.Z.; methodology, C.C., B.Y. and H.J.; software, W.Y. and Y.P.; validation, C.C., Y.G. and S.L.; formal analysis, W.Y., Y.P. and X.P.; investigation, W.Y., Y.P., X.P., Z.L., C.L. and L.Y.; resources, Y.G. and S.L.; data curation, W.Y., Y.P., X.P., Z.L. and C.L.; writing—original draft, Y.P.; writing—review and editing, H.J. and W.Y.; visualization, H.J.; supervision, H.J.; project administration, N.-h.K.; funding acquisition, C.C. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Science and Technology Project of Jilin Province (SXGJSF2017-6, Jiabao Zhang; and 20200703013ZP, Hao Jiang).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article or supplementary material.
Acknowledgments
We thank Fushi Quan and Wei Gao from Jilin Provincial Key Laboratory of Animal Model for their assistance with experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fowler, K.E.; Mandawala, A.A.; Griffin, D.K.; Walling, G.A.; Harvey, S.C. The production of pig preimplantation embryos in vitro: Current progress and future prospects. Reprod. Biol. 2018, 18, 203–211. [Google Scholar] [CrossRef]
- Abeydeera, L.R. In vitro fertilization and embryo development in pigs. Reprod. (Camb. Engl.) Suppl. 2001, 58, 159–173. [Google Scholar] [CrossRef]
- Gil, M.A.; Cuello, C.; Parrilla, I.; Vazquez, J.M.; Roca, J.; Martinez, E.A. Advances in swine in vitro embryo production technologies. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, F.A.; Semple, E.; Schmidt, C.H.; St John, E.; Bartlewski, P.M.; King, W.A. Thyroid hormone supplementation improves bovine embryo development in vitro. Hum. Reprod. 2010, 25, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Yamanaka, T.; Terada, Y.; Murakami, T.; Yajima, A. Growth hormone improves mouse embryo development in vitro, and the effect is neutralized by growth hormone receptor antibody. Tohoku J. Exp. Med. 1998, 184, 113–122. [Google Scholar] [CrossRef]
- Kawamura, K.; Chen, Y.; Shu, Y.; Cheng, Y.; Qiao, J.; Behr, B.; Pera, R.A.; Hsueh, A.J. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS ONE 2012, 7, e49328. [Google Scholar] [CrossRef]
- Grupen, C.G. The evolution of porcine embryo in vitro production. Theriogenology 2014, 81, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Booth, P.J.; Holm, P.; Callesen, H. The effect of oxygen tension on porcine embryonic development is dependent on embryo type. Theriogenology 2005, 63, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Im, G.S.; Lai, L.; Liu, Z.; Hao, Y.; Wax, D.; Bonk, A.; Prather, R.S. In vitro development of preimplantation porcine nuclear transfer embryos cultured in different media and gas atmospheres. Theriogenology 2004, 61, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cheng, D.; Gao, X.; Bao, J.; Ma, X.; Wang, H. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reprod. Domest. Anim. 2012, 47, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Singletary, K. Ginger: An overview of health benefits. Nutr. Today 2010, 45, 171–183. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.J.B.C.; Medicine, A. Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement. Altern. Med. 2015, 15, 258. [Google Scholar] [CrossRef]
- Radhakrishnan, E.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.T.; Soniya, E.V.; Anto, R.J. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Jiang, Y.; Wang, N.; Li, F.; Xin, H. 6-Gingerol Attenuates Ischemia-Reperfusion-Induced Cell Apoptosis in Human AC16 Cardiomyocytes through HMGB2-JNK1/2-NF-kappaB Pathway. Evid.-Based Complement. Altern. Med. 2019, 2019, 8798653. [Google Scholar] [CrossRef]
- Bing, Y.; Che, L.; Hirao, Y.; Takenouchi, N.; Rodríguez-Martínez, H.; Nagai, T. Parthenogenetic activation and subsequent development of porcine oocytes activated by a combined electric pulse and butyrolactone I treatment. J. Reprod. Dev. 2003, 49, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Kondo, T.; Ikeda, S.; Imai, H.; Yamada, M. Effects of EDTA saturated with Ca2+ (Ca-EDTA) on pig, bovine and mouse oocytes at the germinal vesicle stage during maturation culture and the involvement of chelation of Zn2+ in pronuclear formation induction by Ca-EDTA. Reproduction 2002, 124, 235–240. [Google Scholar] [CrossRef]
- Pournaderi, P.S.; Yaghmaei, P.; Khodaei, H.; Noormohammadi, Z.; Hejazi, S.H. The effects of 6-Gingerol on reproductive improvement, liver functioning and Cyclooxygenase-2 gene expression in estradiol valerate—Induced polycystic ovary syndrome in Wistar rats. Biochem. Biophys. Res. Commun. 2017, 484, 461–466. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Ojo, M.; Afolabi, T.T.; Arowoogun, M.D.; Nwawolor, D.; Farombi, E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017, 270, 15–23. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Suzuki, K.; Yoneda, A.; Watanabe, T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004, 62, 1186–1197. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Chen, L.; Lu, W.; Chen, X.; Han, L.; Chen, D.J. Protective Effect Against Hydroxyl Radical-induced DNA Damage and Antioxidant Mechanism of [6]-gingerol: A Chemical Study. Bull. Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Mohd Sahardi, N.F.N.; Makpol, S. Ginger (Zingiber officinale Roscoe) in the Prevention of Ageing and Degenerative Diseases: Review of Current Evidence. Evid.-Based Complement. Altern. Med. 2019, 2019, 5054395. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, P.; Laurincik, J.; Viuff, D.; Fair, T.; Zakhartchenko, V.; Rosenkranz, C.; Avery, B.; Rath, D.; Niemann, H.; Thomsen, P.D.; et al. Activation of ribosomal RNA genes in preimplantation cattle and swine embryos. Anim. Reprod. Sci. 2000, 60–61, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, B.; Wrenzycki, C.; Strejcek, F.; Laurincik, J.; Holm, P.; Ochs, R.L.; Rosenkranz, C.; Callesen, H.; Rath, D.; Niemann, H.; et al. Expression of Nucleolar-Related Proteins in Porcine Preimplantation Embryos Produced In Vivo and In Vitro1. Biol. Reprod. 2004, 70, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Herrler, A. Regulation of proliferation and apoptosis during development of the preimplantation embryo and the placenta. Birth Defects Res. Part C 2005, 75, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Chakraborty, D.; Ghosh, S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Lycopodine triggers apoptosis by modulating 5-lipoxygenase, and depolarizing mitochondrial membrane potential in androgen sensitive and refractory prostate cancer cells without modulating p53 activity: Signaling cascade and drug-DNA interaction. Eur. J. Pharmacol. 2013, 698, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Lee, K.C.; Chen, S.Y.; Chang, H.H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem. Biophys. Res. Commun. 2009, 382, 134–139. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Yao, L.; Ma, P.; Chen, Z.; Han, T.-L.; Yuan, C.; Zhang, J.; Jiang, L.; Liu, L.J.T.; et al. 6-gingerol ameliorates age-related hepatic steatosis: Association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2019, 362, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kim, Y.; Na, K.-M.; Surh, Y.-J.; Kim, T.-Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, G.H.; Kim, C.-Y.; Jang, J.-H. [6]-Gingerol attenuates β-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2011, 49, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Kang, M.; Han, Y.; Zhang, T.; Quan, W.; Gao, J. 6-Gingerols (6G) reduces hypoxia-induced PC-12 cells apoptosis and autophagy through regulation of miR-103/BNIP3. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Zhuang, X.-D.; Xu, Z.-W.; Lu, L.-H.; Guo, H.-L.; Wu, W.-K.; Liao, X.-X. Higenamine combined with [6]-gingerol suppresses doxorubicin-triggered oxidative stress and apoptosis in cardiomyocytes via upregulation of PI3K/Akt pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 970490. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.B.; Uhlitz, F.; Bluthgen, N. A compendium of ERK targets. FEBS Lett. 2017, 591, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.; Liu, T.; Wang, J.; Dai, W.; Wang, F.; Zheng, Y.; Chen, K.; Li, S.; Abudumijiti, H.; et al. Protective effects of astaxanthin on ConA-induced autoimmune hepatitis by the JNK/p-JNK pathway-mediated inhibition of autophagy and apoptosis. PLoS ONE 2015, 10, e0120440. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.-G.; Qu, J.; Zhang, Q.; Prasad, C.; Wei, Z.-J. Assessment of anti-cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells. Food Chem. Toxicol. 2017, 109, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.-F.; Zong, S.-H.; Zhang, Z.-Y.; Fu, S.-W.; Li, K.-K.; Fang, Y.; Lu, L.; Xiao, D.-Q. The role of 6-gingerol on inhibiting amyloid β protein-induced apoptosis in PC12 cells. Rejuvenation Res. 2015, 18, 413–421. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [CrossRef]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.T.; Guo, J.; Niu, Y.J.; Cui, X.S. The toxic effect of aflatoxin B1 on early porcine embryonic development. Theriogenology 2018, 118, 157–163. [Google Scholar] [CrossRef]
- Murakami, M.; Ichisaka, T.; Maeda, M.; Oshiro, N.; Hara, K.; Edenhofer, F.; Kiyama, H.; Yonezawa, K.; Yamanaka, S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 2004, 24, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Tsuji, N.; Endoh, T.; Yagihashi, A.; Watanabe, N. Survivin enhances Fas ligand expression via up-regulation of specificity protein 1-mediated gene transcription in colon cancer cells. J. Immunol. 2004, 172, 3922–3929. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, E.Y.; Cui, X.S.; Tae, J.C.; Lee, W.D.; Kim, N.H.; Park, S.P.; Lim, J.H. Increase in DNA fragmentation and apoptosis-related gene expression in frozen-thawed bovine blastocysts. Zygote 2006, 14, 125–131. [Google Scholar] [CrossRef]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Negron-Perez, V.M.; Zhang, Y.; Hansen, P.J. Single-cell gene expression of the bovine blastocyst. Reproduction 2017, 154, 627–644. [Google Scholar] [CrossRef]
- Ortega, M.S.; Kelleher, A.M.; O’Neil, E.; Benne, J.; Cecil, R.; Spencer, T.E. NANOG is required to form the epiblast and maintain pluripotency in the bovine embryo. Mol. Reprod. Dev. 2019, 87, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Tada, M.; Kubota, H.; Kimura, H.; Hatano, S.Y.; Suemori, H.; Nakatsuji, N.; Tada, T. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol. Cell. Biol. 2005, 25, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Rodda, D.J.; Chew, J.L.; Lim, L.H.; Loh, Y.H.; Wang, B.; Ng, H.H.; Robson, P. Transcriptional regulation of nanog by OCT4 and SOX2. J. Biol. Chem. 2005, 280, 24731–24737. [Google Scholar] [CrossRef] [PubMed]
- Magnani, L.; Cabot, R.A. In vitro and in vivo derived porcine embryos possess similar, but not identical, patterns of Oct4, Nanog, and Sox2 mRNA expression during cleavage development. Mol. Reprod. Dev. 2008, 75, 1726–1735. [Google Scholar] [CrossRef]
- Shah, B.H.; Catt, K.J. Metabolism. Roles of LPA3 and COX-2 in implantation. Trends Endocrinol. Metab. 2005, 16, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, K.; Bell, R.; Lisowski, A.; English, M.; Saldeen, S.; Khan, K.N.M. Expression of cyclooxygenase-2 in embryonic and fetal tissues during organogenesis and late pregnancy. Birth Defects Res. Part A 2003, 67, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharm. 2019, 119, 109415. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell. Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological properties of 6-gingerol: A brief review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef]
- Liu, R.P.; Wang, X.Q.; Wang, J.; Dan, L.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Oroxin A reduces oxidative stress, apoptosis, and autophagy and improves the developmental competence of porcine embryos in vitro. Reprod. Domest. Anim. 2022, 57, 1255–1266. [Google Scholar] [CrossRef]
- Yu, W.J.; Chen, C.Z.; Peng, Y.X.; Li, Z.; Gao, Y.; Liang, S.; Yuan, B.; Kim, N.H.; Jiang, H.; Zhang, J.B. Schisanhenol improves early porcine embryo development by regulating the phosphorylation level of MAPK. Theriogenology 2021, 175, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Ji, H.W.; He, S.Y.; Liu, R.P.; Wang, X.Q.; Wang, J.; Huang, C.M.; Xu, Y.N.; Li, Y.H.; Kim, N.H. Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy. Vet. Sci. 2023, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Kim, J.; Lim, J.; Lee, E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010, 74, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.S.; Gupta, K.; Gupta, S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis 2012, 33, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in ApcMin mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem. Pharmacol. 2013, 85, 667–672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).