Simple Summary
Despite the US Food and Drug Administration’s zero-tolerance policy against Salmonella, several Salmonella-linked outbreaks and recalls linked to pet foods have been reported. Post-processing steps, such as fat and flavor coating, drying, cooling, and packaging are common steps where Salmonella becomes contaminated in dry pet food kibbles. Rendered animal fats and oils are commonly coated on kibbles to enhance palatability and increase energy density. A tiny layer of water in the bottom of bulk fat transport trucks or/and storage tanks could easily harbor Salmonella, leading to its entry into the pet food during coating. In this study, different types of acidulants were applied in the fat and oil system, and their effect against Salmonella in the water or fat phase in different types of fats and oils was evaluated. Our results were promising, wherein all the tested acidulants were effective to mitigate Salmonella from the fat or oil system (both aqueous and fat phase) within 2 h. The findings of this study could be helpful to the rendering and pet food industry in a fight to mitigate Salmonella in pet food.
Abstract
Salmonella-contaminated pet foods could potentially become a source of human salmonellosis. This study evaluated the survival of Salmonella without and with the addition of acidulants in different fat types (chicken fat (CF), canola oil (CO), Menhaden fish oil (FO), lard (La), and tallow (Ta)) commonly used to coat dry pet food kibbles. The minimum inhibitory concentration (MIC) of individual acidulants and the combination were determined using the broth microdilution method. Autoclave-sterilized rendered fats were treated with pre-determined concentrations of antimicrobial acidulants (0.5% sodium bisulfate (SBS), 0.5% phosphoric acid (PA), 0.25% lactic acid (LA), etc.) and incubated overnight at 45 °C. The treated fats were inoculated with approximately eight logs of a Salmonella cocktail. Microbiological analyses were conducted separately for the fat-phase and water-phase at predetermined time intervals (0, 2, 6, 12, and 24 h) by plating them onto TSA plates. After incubating at 37 °C for 24 h, the plate count results were expressed as log CFU/mL. The MIC of SBS was 0.3125%, and of PA and LA were both 0.1953% against cocktail Salmonella serotypes. We observed a possible synergistic effect when SBS and organic acid were combined. All the acidulant tested at targeted concentrations individually as well as in combination with organic acids were highly effective against Salmonella spp. (non-detectable within 2 h) across different fat types. A potent anti-bactericidal effect leading to non-detectable Salmonella immediately (<1 h) at 45 °C was observed in the aqueous phase of the fish oil system, even without the addition of acidulants. These findings are significant for the dry pet food industries, where potential post-processing contamination of Salmonella could be controlled by treating fats and oils with acidulants.
Keywords:
Salmonella spp.; sodium bisulfate; organic acids; chicken fat; canola oil; Menhaden fish oil; lard; tallow 1. Introduction
The use of rendered animal fats as a value-added material in pet food is a common practice. During the rendering process, heat is applied, moisture is removed, and fats are separated. However, food safety concerns arise as animals used in the rendering process are natural microbiological reservoirs, including human pathogens such as Salmonella spp. Clostridium perfringens, Listeria monocytogenes, and Campylobacter jejuni [1].
Continued improvements within the industry have implemented process control monitoring to ensure proven cook times and temperatures have been reached for the inactivation of specific microorganisms deemed to be a food safety hazard [2]. While the rendering industries have an aggressive approach to animal food ingredient quality and safety by using long cook times and high temperatures, contamination with pathogenic microorganisms still occurs. Studies have shown the presence of Salmonella spp. in final rendered products, including protein meals, meat, and bone meal, feather meal, meat meal, and poultry meals [1,3,4,5,6,7]. However, the evaluation of microbial contamination of rendered fats, specifically poultry fat, beef tallow, or other animal fat products, was not included in these surveys. While the rendering process itself is effective in killing pathogens as observed with 0% Salmonella spp. in crax [8], it is a point-in-time mitigation strategy bearing no residual activity. Post-processing contamination from dusts, equipment and humans is considered to be the main source of Salmonella spp. introduction in final rendered products [1,5,8]. Contaminated rendered products have the potential to contaminate animal feed and pet foods. For example, due to the high Salmonella load on chicken offal, viscera, and animal co-products, the incoming raw materials in chicken fat rendering facilities might become a source of Salmonella contamination in the facility and premises if the cleaning and sanitation are not proper. In fact, several outbreaks of human Salmonella infection have been traced back to contaminated animal feed [9,10,11,12]. Application of proper processing temperature and holding time and the use of quality raw ingredients are prime to reduce the risk of pathogen contamination in feed production. Promising research by Cochrane et al. [13] has shown effective mitigation of Salmonella through the addition of chemical additives to rendered animal proteins, including feather meal, avian blood meal, porcine meat, bone meal, and poultry by-product meal.
Dry pet food constitutes the most sold pet food type in the US; USD 5.99 billion worth of dry dog food and USD 2.84 billion worth of dry cat food were sold in 2021 in the USA [14]. Rendered animal fats are commonly incorporated into dry pet food kibbles for added energy, essential fatty acids, and as a palatant. Higher fat content and low water activity in rendered animal fats, both exhibiting bacteriostatic properties, are parameters used in foods to curb microbial growth. However, two major multistate outbreaks of human Salmonella Schwarzengrund and S. Infantis in 2008 and 2012, respectively, were sourced back to contaminated dry pet foods. The pathogens were assumed to have entered during the flavoring and enrobing steps in the coating process. The bulk fats and oils used in pet food industries are transported and stored in bulk trucks and tanks. It is common that these commercial fats hold a small (~3%) moisture, insoluble, and unsaponifiables (MIU).
Acidulants, including sodium bisulfate, phosphoric acids, and organic acids, are known to have an antimicrobial effect against various bacterial pathogens, including Salmonella. Our previous studies have shown that sodium bisulfate (SBS), lactic acid, and phosphoric acid were potent against Salmonella in rendered chicken fat [15]. The fats and oils used to coat dry pet food kibbles differ in the composition of fatty acids. The effect of the composition of fats and oils to mitigate Salmonella has not been evaluated. Therefore, this study aimed to evaluate the survival of outbreak-linked Salmonella serotypes in fats and oils (chicken fat, canola oil, Menhaden fish oil, lard, and tallow) differing in saturation level and treated with food-grade acidulant antimicrobials, such as sodium bisulfate (SBS), lactic acid (LA), phosphoric acid (PA), and a combination of SBS with organic acids (butyric acid (BA), lactic acid (LA), and propionic acid (PrA).
2. Materials and Methods
2.1. Salmonella Serotypes, and Fats/Oil Sources
Salmonella Enteritidis (ATCC 4931; source, gastroenteritis), Salmonella Heidelberg (ATCC 8326), and Salmonella Typhimurium (ATCC 14028; source, poultry) were used in the experiment and were maintained in tryptic soy broth (TSB)-glycerol (7:3) at −80 °C. Prior to use, the frozen cultures were streaked on tryptic soy agar (TSA) plates and incubated at 37 °C for 24 h. A single colony of Salmonella strain was inoculated in 10 mL of TSB and incubated at 37 °C for 18 to 24 h. An equal volume of each serotype, incubated for the same duration, was mixed to make a cocktail of three serotypes.
Chicken fat and beef tallow were provided by Darling Ingredients (Irving, TX, USA). Kroger® Pure Canola Oil was purchased from a local Kroger store. Rendered Menhaden fish oil was provided by an established fish rendering company (Omega Protein®, Inc., Reedville, VA, USA). Morrell snow cap lard was provided by John Morrell & Co. (Cincinnati, OH, USA).
2.2. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) of individual acidulants and combinations was determined using the broth microdilution method [16]. A single colony of each of the three Salmonella serotypes (S. Enteritidis (ATCC 4931), S. Heidelberg (ATCC 8326), and S. Typhimurium (ATCC 14028)) and the cocktail were inoculated in 10 mL of Tryptic Soy Broth (TSB) and incubated at 37 °C for 18 to 24 h. The overnight-grown culture was centrifuged at 5000 rpm for 10 min at room temperature, and the bacterial pellet was re-suspended in fresh TSB.
A volume of 200 µL of an antimicrobial solution consisting of twice the desired final concentration was dispensed in the first well of a 96-well plate (triplicate wells), and 100 µL of sterile water in the rest of the wells. Serial two-fold dilutions of the antimicrobial solutions were performed. One hundred microliters of bacterial culture containing ~5 logs CFU/mL (in 2X TSB) were added to each well to make a final volume of 200 µL. A positive control consisted of Salmonella inoculum only (no antimicrobial), and a negative control consisted of TSB without Salmonella and an antimicrobial agent. The microtiter plate was incubated at 37 °C for 24 h. The MIC was determined to be the lowest concentration of an antimicrobial that inhibited the visible growth of Salmonella after 24 h of incubation at 37 °C.
2.3. Survival of Salmonella in Fats and Oils
For this study, a cocktail of three serotypes was tested in the fat and oil system, both with and without the presence of acidulant antimicrobials. The fat or oil system refers to a mixture of fat or oil with water consisting of a bottom water phase and an upper fat phase for any specific fat/oil type. Based on the MIC of the acidulants and their combination, six different acidulants and their combinations were used to treat the oil and fat system (Table 1). Autoclave-sterilized rendered fats and oils were treated with pre-determined concentrations of antimicrobial acidulants and left overnight at 45 °C. Because we were analyzing the two phases separately, we needed the fat or oil system in molten state, and incubation at 45 °C effectively kept all the fat types in molten state. The acidulants were treated in aqueous form, and ~3% moisture was maintained in all the treatments, thereby creating an aqueous and a fat phase in the fat and oil system. The antimicrobial treated fats were then inoculated with ~8 logs of a Salmonella cocktail. The volume of the acidulants and bacterial suspension were adjusted in a way to achieve ~3% final moisture percentage in the fat system. Microbiological analyses were conducted separately for the fat-phase and water-phase treatments at predetermined time intervals (0, 2, 6, 12, and 24 h). From each subsample, the fat phase and aqueous phases were gently removed by pipetting, diluted in 0.1% peptone water (pre-warmed at 45 °C), and plated onto TSA nutrient plates. For the fat phase, samples were diluted and dispensed onto the agar plates by vigorously vortexing the tubes and removing 100 μL while the suspension was still in the emulsion. A negative control containing fats/oils alone, without antimicrobial or Salmonella spp., was maintained. Fats treated with sterile distilled water instead of acidulant antimicrobials were maintained as controls for each fat type. The plates were incubated at 37 °C for 24 h, and then colonies were counted with results expressed as log CFU/mL. The limit of detection is 1 log CFU/mL in this study. The experiment was a 6 × 5 factorial arrangement of treatments utilizing five fat types and five sampling intervals. The experiment was evaluated in triplicate.

Table 1.
Time to non-detection of Salmonella in acidulants-treated fat or oil system.
3. Results
3.1. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) of acidulant antimicrobials, both individually as well as in combination, against three serotypes of Salmonella, both individually as a cocktail of three serotypes, were determined and summarized in Table 2. Higher minimum concentrations of SBS, LA, and PA were required to inhibit Salmonella Typhimurium, as compared to S. Heidelberg and S. Enteritidis: (0.50% vs. 0.31%), (0.50% vs. 0.20%), and (0.25% vs. 0.10%), respectively. Similarly, for the cocktail of three serotypes, LA and PA were more effective (MIC 0.20%) compared to SBS (MIC 0.31%). When a combination of SBS with lactic acid was used, we observed a potential synergistic effect against individual as well as cocktail serotypes.

Table 2.
Minimum Inhibitory Concentrations (MIC) of acidulants against Salmonella spp.
3.2. Survival of Salmonella in Fats and Oils
The effects of 0.5% SBS treatment on Salmonella-inoculated fats and oils over time are represented in Figure 1. The fat or aqueous phases showing non-detectable Salmonella count at 0 h are not shown in figures (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). In the fat phase of the SBS-treated chicken fat system, the Salmonella count was reduced to 0.77 logs within 0 h and was reduced to a non-detectable level by 2 h, whereas in the aqueous phase, Salmonella was reduced to a detectable level at 0 h. In the case of the control (without SBS) chicken fat system, the Salmonella count was reduced to 2.60 logs and remained at 7.60 logs at 0 h in the fat phase and aqueous phase, respectively. After 2 h, Salmonella was non-detectable in the fat phase but remained >7.13 logs until 24 h in the aqueous phase. The addition of SBS in canola oil also led to Salmonella reduction to a non-detectable level in the aqueous phase and to 1.53 logs in the fat phase within 0 h. In the untreated control canola oil system, the Salmonella count in the fat phase reduced to 0.34 log at 0 h and to a non-detectable level at 2 h, whereas in the aqueous phase, it remained >7.90 logs throughout the incubation. In Menhaden fish oil, in both SBS-treated and control fat systems, Salmonella was reduced to a non-detectable level by 0 h in both fat phases and water phases. Furthermore, SBS-treated lard reduced Salmonella to a non-detectable level in both the fat phase and aqueous phase at 0 h. Similarly, the Salmonella count was non-detectable at 0 h in the fat phase of the control lard system but remained >7.72 logs throughout the 24 h study period. Beef tallow, when treated with SBS, led to Salmonella reduction to a non-detectable level within 0 h in both fat and aqueous phase. Whereas in the control tallow system, though Salmonella lowered to a non-detectable level at 0 h in the fat phase, it remained >7.63 logs throughout the 24 h study period in the aqueous phase.
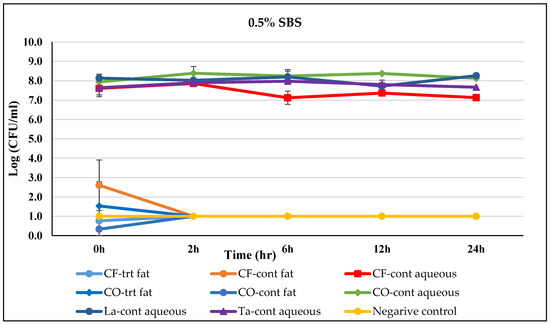
Figure 1.
Mean logarithmic counts (log CFU/mL) of Salmonella spp. in different fat and oil systems with and without the inclusion of 0.5% sodium bisulfate evaluated separately for the aqueous phase and fat phase at 45 °C. Treatments from each phase were plated on TSA at different times. SBS, sodium bisulfate; CF, chicken fat; CO, canola oil; La, Lard; Ta, Tallow; Cont., control. Negative control consisted of fat-oil system without an acidulant and Salmonella inoculation. The limit of detection is 1 log CFU/mL for this study. Because the three replications were averaged, for some of the treatments, the counts on 0 h appeared to be lower-than-detection limit. Error bars are ±1 standard error of the mean.
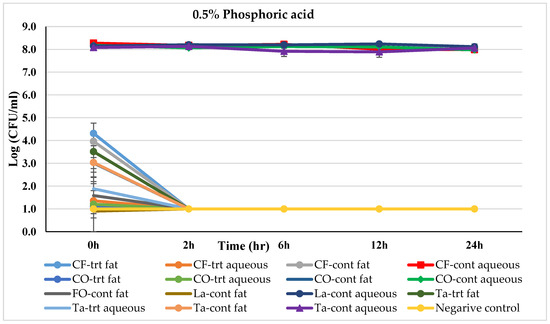
Figure 2.
Mean logarithmic counts (log CFU/mL) of Salmonella spp. in different fat and oil systems with and without the inclusion of 0.5% phosphoric acid evaluated separately for the aqueous phase and fat phase at 45 °C. Treatments from each phase were plated on TSA at different times. SBS, sodium bisulfate; CF, chicken fat; CO, canola oil; FO, fish oil; La, Lard; Ta, Tallow; Cont., control. Negative control consisted of fat-oil system without acidulant and Salmonella inoculation. The limit of detection is 1 log CFU/mL for this study. Because the three replications were averaged, for some of the treatments, the counts on 0 h appeared to be lower-than-detection limit. Error bars are ±1 standard error of the mean.
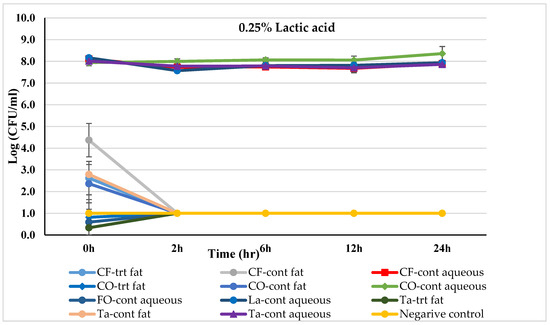
Figure 3.
Mean logarithmic counts (log CFU/mL) of Salmonella spp. in different fat and oil systems with and without the inclusion of 0.25% lactic acid, evaluated separately for the aqueous phase and fat phase at 45 °C. Treatments from each phase were plated on TSA at different times. SBS, sodium bisulfate; CF, chicken fat; CO, canola oil; FO, fish oil; La, Lard; Ta, Tallow; Cont., control. Negative control consisted of fat-oil system without acidulant and Salmonella inoculation. The limit of detection is 1 log CFU/mL for this study. Because the three replications were averaged, for some of the treatments, the counts on 0 h appeared to be lower-than-detection limit. Error bars are ±1 standard error of the mean.
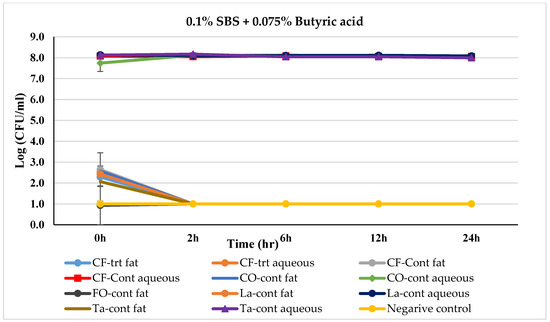
Figure 4.
Mean logarithmic counts (log CFU/mL) of Salmonella spp. in different fat and oil systems with and without the inclusion of 0.1% SBS and 0.075% butyric acid evaluated separately for the aqueous phase and fat phase at 45 °C. Treatments from each phase were plated on TSA at different times. SBS, sodium bisulfate; CF, chicken fat; CO, canola oil; FO, fish oil; La, Lard; Ta, Tallow; Cont., control. Negative control consisted of fat-oil system without acidulant and Salmonella inoculation. The limit of detection is 1 log CFU/mL for this study. Because the three replications were averaged, for some of the treatments, the counts on 0 h appeared to be lower-than-detection limit. Error bars are ±1 standard error of the mean.
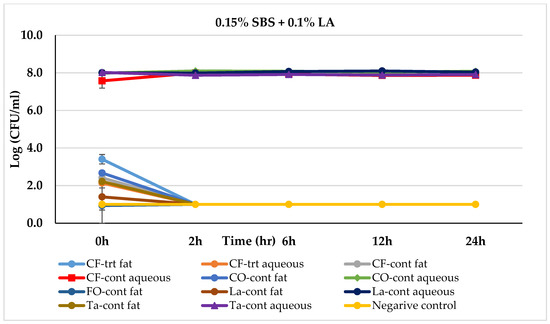
Figure 5.
Mean logarithmic counts (log CFU/mL) of Salmonella spp. in different fat and oil systems with and without the inclusion of 0.15% SBS and 0.1% lactic acid evaluated separately for the aqueous phase and fat phase at 45 °C. Treatments from each phase were plated on TSA at different times. SBS, sodium bisulfate; CF, chicken fat; CO, canola oil; FO, fish oil; La, Lard; Ta, Tallow; Cont., control. Negative control consisted of fat-oil system without acidulant and Salmonella inoculation. The limit of detection is 1 log CFU/mL for this study. Because the three replications were averaged, for some of the treatments, the counts on 0 h appeared to be lower-than-detection limit. Error bars are ±1 standard error of the mean.
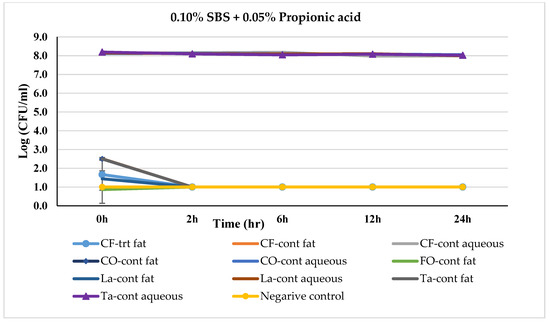
Figure 6.
Mean logarithmic counts (log CFU/mL) of Salmonella spp. in different fat and oil systems with and without the inclusion of 0.10% SBS and 0.05% propionic acid evaluated separately for the aqueous phase and fat phase at 45 °C. Treatments from each phase were plated on TSA at different times. SBS, sodium bisulfate; CF, chicken fat; CO, canola oil; FO, fish oil; La, Lard; Ta, Tallow; Cont., control. Negative control consisted of fat-oil system without acidulant and Salmonella inoculation. The limit of detection is 1 log CFU/mL for this study. Because the three replications were averaged, for some of the treatments, the counts on 0 h appeared to be lower-than-detection limit. Error bars are ±1 standard error of the mean.
The effect of 0.5% phosphoric acid (PA) treatment on Salmonella-inoculated fats and oils over time is represented in Figure 2. The aqueous phase of all the control fat and oil systems, except Menhaden fish oil, was able to contain a Salmonella level between 7.89 logs (in tallow) to 8.27 log CFU/mL (in chicken fat) through the 24 h incubation period. Whereas 1.59 logs of Salmonella were recorded in untreated control Menhaden oil at 0 h, which reduced to a non-detectable level by 2 h. The Salmonella dropped to a non-detectable level in PA-treated Menhaden fish oil system (both aqueous and fat phase) at 0 h.
The effect of 0.25% lactic acid treatment on Salmonella-inoculated fat and oils over time is represented in Figure 3. In the control fat and oil system, Salmonella remained between 7.57 logs (in lard) to 8.36 logs (in canola oil) throughout the incubation period in the aqueous phase, except for Menhaden fish oil, where the Salmonella count dropped to 0.59 log at 0 h and went to a non-detectable level by 2 h. In the case of untreated (control) fat phase, the Salmonella was non-detectable at 0 h in lard and Menhaden fish oil. Whereas Salmonella counts were 4.37, 2.36, and 0.33 logs in chicken fat, canola oil, and tallow, respectively, which declined to a non-detectable level by 2 h. In the lactic acid-treated fat and oil system, Salmonella was non-detectable in the aqueous phase of all fat systems within 0 h. By 2 h, the Salmonella was non-detectable in fat phases of all fats and oil systems.
The effect of a combination of 0.1% SBS + 0.075% BA treatment on Salmonella-inoculated fats and oils over time is shown in Figure 4. In the aqueous phase of control fat and oil systems, Salmonella remained between 7.74 logs (in canola oil) to 8.17 logs (in tallow) throughout the incubation period, except for Menhaden fish oil where the Salmonella count reduced to a non-detectable level within 0 h. Salmonella levels in untreated (control) fat phases in all fats and oils reduced to non-detectable by 2 h. In the case of 0.1% SBS + 0.075% BA treated fat and oil system, except for chicken fats, Salmonella levels were dropped to a non-detectable within 0 h. In chicken fat, both in fat and aqueous phases, Salmonella was non-detectable in 2 h.
Figure 5 shows the effect of a combination of 0.15% SBS + 0.1% LA treatment on Salmonella-inoculated fat and oils over time. In the aqueous phase of the control fat and oil systems, Salmonella levels remained between 7.57 log (in chicken fat) and 8.10 log (in canola oil and Lard) throughout the incubation period, except for the Menhaden fish oil where the Salmonella count was reduced to a non-detectable level within 0 h. In the fat phases of all the untreated (control) oil and fat systems, the Salmonella level was reduced to a non-detectable level in 2 h. In 0.15% SBS + 0.1% LA treated fats and oil systems, a reduction pattern similar to 0.1% SBS + 0.075% BA was observed.
Similarly, the effect of a combination of 0.1% SBS + 0.05% PrA treatment on Salmonella-inoculated fat and oils over time is represented in Figure 6. The Salmonella count was recorded between 7.99 logs (in chicken fat) to 8.19 logs (in tallow) during the 24 h incubation period in the aqueous phase of the control fat and oil systems, except for Menhaden fish oil where the Salmonella level dropped a non-detectable level at 0 h.
Salmonella was non-detectable by 2 h in both the phases of the acidulants-treated fat and oil systems (Table 1) and the fat phase of control fat-oil systems (Table 3). All the treatments, namely 0.5% SBS, 0.5% PA, 0.25% LA, and a combination of SBS with organic acids, were effective in lowering the Salmonella load in both the aqueous phase and fat phase of the fat system to a non-detectable level within 2 h.

Table 3.
Time to non-detection of Salmonella in control (without acidulant) fat or oil system.
4. Discussion
Rendered animal fats and oils vary in the amount of unsaturated fatty acids they contain, and these unsaturated fatty acids are known to exhibit antimicrobial effects [17]. Unsaturated fatty acids are known to have greater antimicrobial activity against Vibrio spp., a gram-negative bacterium than saturated fatty acids [18]. However, saturated fatty acids such as caproic, caprylic, and capric acids also exhibited potent antimicrobial activity against Salmonella [19]. Similarly, it was reported that unsaturated fatty acids such as linolenic and myristoleic acids exerted potent antibacterial activity against H. pylori [20], and linolenic acid against Bacillus cereus and Staphylococcus aureus [21].
In this study, Salmonella was non-detectable by 2 h in the fat phase of both acidulant-treated as well as non-treated (control) fat systems. The reduction in Salmonella from the acidulant-treated fat and oil system could be explained by the antimicrobial effects of acidulants. However, similar findings from the control fat and oil systems indicated the role of the innate antimicrobial effect of fats and oils, especially unsaturated fatty acids content. In addition, the possibility of the presence of Salmonella in a non-detectable form, for example, a viable but non-culturable state (VBNC), could not be negated [22]. However, the reduction in Salmonella from the aqueous phase of the antimicrobial-treated fat system could be credited to the potent action of acidulant antimicrobials which were applied as an aqueous inoculum. A Prior study from our lab validated the effectiveness of acidulants such as SBS in mitigating Salmonella from rendered chicken fat [15].
In the case of canola oil and beef tallow, individual treatment of SBS, phosphoric acid, and lactic acids took 2 h to lower Salmonella to a non-detectable limit (with the exception of 0.5% SBS treatment in tallow), whereas a combination of SBS and organic acids caused an immediate (0 h) reduction in Salmonella to a non-detectable level (Table 2). The latter could potentially be due to the synergistic action of SBS and organic acid. In addition, high levels of polyunsaturated fatty acids (PUFA) in canola oil (31.3%) and tallow (20.3) might also have contributed to the enhanced antimicrobial effect. Similar synergistic effects of SBS and organic acids were also observed against Salmonella in the chicken fat system [15]. The synergistic effect could be supported by the fact that both SBS and organic acids act against bacteria by damaging the cell membrane and causing oxidative damage to the cells [23,24].
Molitor et al. [25] reported that chicken fat contains 36.8% oleic acid (18:1), 21.1% linoleic acid (18:2), and <1% each of alpha-linoleic acid, (18:3:3), eicosenoic acid (20:1), eicosadienoic (20:2), and arachidonic acid (20:4). The level of unsaturated fatty acids in CO was highest, with only less than 8% being saturated fatty acids. However, the majority (~65%) of the unsaturated fatty acids are monounsaturated (oleic acid), and 26.3% are polyunsaturated fatty acids (PUFA), with only 8.6% are linolenic acid, and 17.7% being Linoleic acids [26]. In a study by Deliephan et al. [27], when a mixture of 2-Hydroxy-4-(Methylthio) Butanoic Acid (HMTBa), lactic acid, and phosphoric acid incorporated into canola oil were coated on dry dog food kibbles, Salmonella spp. (Enteritidis, Heidelberg, and Typhimurium) were reduced to a non-detectable level from their initial concentration of approximately eight logs within 72 h. In a related study, the authors also reported that the organic acid mixtures containing HMTBa effectively mitigate Salmonella from food contact surfaces (plastic, rubber, stainless steel, and concrete) commonly used in pet food industry [28]. Phosphoric acid (PA) seemed to be less effective (non-detectable in 2 h) in the aqueous phase as compared to the other acidulants (non-detectable in 0 h) in the canola oil fat system. This is contrary to the effectiveness of phosphoric acid we observed in vitro MIC assay, where a lower concentration of PA was needed to inhibit the visible growth of Salmonella as compared to the SBS and LA. This could partly be explained by the reduced potency of inorganic acid, i.e., phosphoric acid, in the complex organic matrix of the canola oil system.
Menhaden fish oil contains a high amount of unsaturated fatty acids, with 34.8% being PUFA (~29.41% omega 3), and 23.46% monounsaturated fatty acids [29]. Alpha-linoleic acid is the most common omega-3. The reduction in Salmonella to a non-detectable level within 0 h in both fat and aqueous phases of treated FO samples (Table 2) could be explained by the combined effect of the antimicrobial acidulants and the presence of high PUFA [30]. We also observed a very interesting result where Salmonella in the aqueous phase of untreated (control) Menhaden fish oil system was mitigated to a non-detectable level immediately. Out of the six different sets of experiments with different acidulants, Salmonella level in the aqueous phase of non-acidulant-treated Menhaden fish oil dropped to a non-detectable level immediately (0 h) in five of them. Whereas, in one (0.25% lactic acid) treatment, 0.59 log of Salmonella was detected at 0 h, which was reduced to a non-detectable level at 2 h. This minor deviation in only one set could be attributed to sampling or handling variations. Similar results with fish oil were reported in a past study by our lab [22]. As we hypothesized, the absence of detectable Salmonella from the aqueous phase of control Menhaden fish oil samples indicated that the higher the unsaturation level, the more antimicrobial activity an oil/fat possesses, which was similar to the findings by Knapp and Melly [31].
Beef tallow is considered a saturated fat and contains 35–64% unsaturated fatty acids [32,33], with 38.6% oleic acid (18:1), 20.3% linoleic acid (18:2), and <1% each of alpha-linoleic acid, (18:3:3), eicosenoic acid (20:1), eicosadienoic (20:2), arachidonic acid (20:4) [25]. Native Malaysian pork lard contains 45–62% unsaturated fatty acids [32,33], with 17.3% linoleic acid [33]. The high amount of unsaturated fatty acids in lard could have contributed to the reduction in Salmonella to a non-detectable level within 2 h in the control fat phase. Among all the five types of fats and oils tested, pork lard contains the highest level of PUFA, followed by Menhaden fish oil, canola oil, beef tallow, and chicken fat. The immediate reduction in Salmonella to a non-detectable level (0 h) from both aqueous and fat phases of Menhaden oil and lard corresponds to their high level of PUFA. The antimicrobial effect of arachidonic acid (C-20) exhibited a direct relation with the increasing level of unsaturation, with C-20:5 showing the highest reduction and C-20:1 showing the lowest reduction against S. aureus [30] when incubated at room temperature for 1 h. The authors also discovered a similar relation between the reduction in S. aureus and the degree of unsaturation with Oleic acid (C-18). Docosahexaenoic acid (DHA), a PUFA from Sardinell longiceps and Sardinells fimbriata, showed potent antibacterial activity against Salmonella and E. coli [34] in an in vitro assay at 37 °C. A similar efficacy of Menhaden oil and lard was also observed in the case of untreated (control) samples, where the quickest reduction in Salmonella to a non-detectable level was reported as compared to chicken fat, canola oil, and beef tallow (Table 3).
Lamb [32] reported that a cocktail of Salmonella (S. Typhimurium (ATCC 13312), S. choleraesuis subsp. choleraesuis (ATCC 13311), S. Pullorum (ATCC 19945), and S. Choleraesuis subsp. arizonae (ATCC 13314)) survived the 7-day incubation period at 26 °C in duck fat, beef tallow, and pig lard. This finding was contrary to our finding where control fat phase (Salmonella-inoculated fats without antimicrobial addition) reduced the Salmonella cocktail to a non-detectable level in 2 h. The difference in the results could be attributed to the method and enrichment techniques. Our experimental setup simulated the real-life bulk fat storage and transport system by providing ~3% moisture in the bottom aqueous layer and a top fat layer. The author did not incorporate the aqueous layer. Secondly, we inoculated Salmonella as wet inoculum, which could have led to the sedimentation of the pathogen to the aqueous phase. Finally, the enrichment of the inoculated fats before plating could have caused the revival of the stressed cells. In our previous study in chicken fat, we discovered similar findings wherein the fat phase, which was negative for Salmonella on the agar plate, was positive for Salmonella upon enrichment followed by PCR confirmation [22]. In another study by Molitor et al. [25], the authors reported that eight logs CFU/mL of Salmonella cocktail (S. Senftenberg, S. Newport, S. Thompson, and S. Infantis) was reduced to a non-detectable level by day 3 at 48 °C in both choice white grease and beef tallow. The longer survival time in this study could partly be the effect of sampling techniques, where the authors took mixed samples from both the fat phase and water phase. In our study, the separate sampling of the two phases in the fat system yielded a non-detectable Salmonella in 2 h in the fat phase and consistently higher (approximately eight logs) counts throughout the experimental period in the aqueous phase. Therefore, an enrichment followed by a molecular confirmation is necessary to call a fat and oil sample negative [22].
5. Conclusions
In summary, the findings from this study suggest that coating pet foods with rendered animal fats and oils could help in mitigating post-processing Salmonella contamination. The addition of acidulants in the fat and oil system boosts the antimicrobial efficacy of fats and oils. A future study involving the palatability test of these fats and oil-coated dog food is warranted.
Author Contributions
J.D.—conceptualization, data gathering, analysis, writing—original draft preparation; C.G.A.—conceptualization, supervision, funding acquisition, writing—review and editing of original draft. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Fat and Protein Research Foundation and the Pet Food Institute.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided upon reasonable request.
Acknowledgments
We acknowledge the Fat and Protein Research Foundation, Pet Food Institute and the Department of Grain Science and Industry at Kansas State University, Manhattan, KS for their support to complete this study. We are also thankful to Darling Ingredients (Irving, TX, USA), Omega Protein®, Inc., (Reedville, VA, USA), and John Morrell & Co (Cincinnati, OH, USA) for providing the raw materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Denton, J.H.; Coon, C.N.; Pettigrew, J.E.; Parsons, C.M. Historical and Scientific Perspectives of Same Species Feeding of Animal By-Products. J. Appl. Poult. Res. 2005, 14, 352–361. [Google Scholar] [CrossRef]
- Meeker, D.L.; Hamilton, C.R. An Overview of the Rendering Industry. In Essential Rendering: All about the Animal By-Products Industry; Meeker, D.L., Ed.; Kirby Lithographic Company Inc.: Arlington, VA, USA, 2006; pp. 1–17. [Google Scholar]
- Sapkota, A.R.; Lefferts, L.Y.; McKenzie, S.; Walker, P. What Do We Feed to Food-Production Animals? A Review of Animal Feed Ingredients and Their Potential Impacts on Human Health. Environ. Health Perspect. 2007, 115, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.A. A Survey of Salmonella Serovars and Most Probably Numbers in Rendered-Animal-Protein Meals: Inferences for Animal and Human Health. J. Environ. Health 2005, 67, 6. [Google Scholar]
- Kinley, B.; Rieck, J.; Dawson, P.; Jiang, X. Analysis of Salmonella and Enterococci Isolated from Rendered Animal Products. Can. J. Microbiol. 2010, 56, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Laban, S.E.; Moustafa, G.Z.; Anwer, W.; Badawy, E.M. Microbial Load of Poultry By-Products following Rendering Process. Glob. Vet. 2014, 12, 756–759. [Google Scholar]
- Moyle, A.I. Salmonellae in Rendering Plant By-Products. J. Am. Vet. Med. Assoc. 1966, 149, 1172–1176. [Google Scholar]
- Troutt, H.F.; Schaeffer, D.; Kakoma, I.; Pearl, G.G. Prevalence of Selected Foodborne Pathogens in Final Rendered Products: Pilot Study; Director’s Digest 312; Fats and Proteins Research Foundation: Alexandria, VA, USA, 2001. [Google Scholar]
- Clark, C.; Cunningham, J.; Ahmed, R.; Woodward, D.; Fonseca, K.; Isaacs, S.; Ellis, A.; Anand, C.; Ziebell, K.; Muckle, A.; et al. Characterization of Salmonella Associated with Pig Ear Dog Treats in Canada. J. Clin. Microbiol. 2001, 39, 3962–3968. [Google Scholar] [CrossRef]
- Hirsch, W.; Sapiro-Hirsch, R. The Role of Certain Animal Feeding Stuffs, Especially Bone Meal, in the Epidemiology of Salmonellosis. Harefuah 1958, 54, 57–59. [Google Scholar]
- Knox, W.A.; Galbraith, N.S.; Lewis, M.J.; Hickie, G.C.; Johnston, H.H. A Milk-Borne Outbreak of Food Poisoning Due to Salmonella Heidelberg. Epidemiol. Infect. 1963, 61, 175–185. [Google Scholar] [CrossRef]
- Pennington, J.H.; Brooksbank, N.H.; Poole, P.M.; Seymour, F. Salmonella Virchow in a Chicken-Packing Station, and Associated Rearing Units. Br. Med. J. 1968, 4, 804–806. [Google Scholar] [CrossRef]
- Cochrane, R.A. Chemical Mitigation of Microbial Pathogens in Animal Feed, and Ingredients. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2015. [Google Scholar]
- Bedford, E.U.S. Pet Food Industry-Statistics & Facts. 2022. Available online: https://www.statista.com/topics/1369/pet-food/#topicHeaderwrapper (accessed on 9 March 2023).
- Dhakal, J.; Aldrich, C.G.; Knueven, C. Assessing the Efficacy of Sodium Bisulfate and Organic Acid Treatments for Control of Salmonella Typhimurium in Rendered Chicken Fat Applied to Pet Foods. J. Food Prot. 2019, 82, 1864–1869. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. In CLSI document M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free Fatty Acids and Sterols in the Benthic Spawn of Aquatic Molluscs, and Their Associated Antimicrobial Properties. Adv. Exp. Med. Biol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Dhakal, J.; Aldrich, C.G. Use of Medium Chain Fatty Acids to Mitigate Salmonella Typhimurium (ATCC 14028) on Dry Pet Food Kibbles. J. Food Prot. 2020, 83, 1505–1511. [Google Scholar] [CrossRef]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial Actions of Fatty Acids and Monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.S.; Shin, D.H. Antimicrobial Synergistic Effect of Linolenic Acid and Monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002, 50, 2193–2199. [Google Scholar] [CrossRef]
- Dhakal, J.; Aldrich, C.G. A Comparison of Salmonella Survival and Detection Using an Enrichment Technique in Dry- and Wet-Inoculated Rendered Chicken Fat Treated with Sodium Bisulfate. J. Food Prot. 2021, 84, 249–254. [Google Scholar] [CrossRef]
- Bushell, F.M.; Tonner, P.D.; Jabbari, S.; Schmid, A.K.; Lund, P.A. Synergistic Impacts of Organic Acids and pH on Growth of Pseudomonas aeruginosa: A Comparison of Parametric and Bayesian Non-Parametric Methods to Model Growth. Front. Microbiol. 2019, 9, 3196. [Google Scholar] [CrossRef]
- McDaniel, C.; Teng, X.M.; Jaroni, D.; Jadeja, R. Investigation of the Antimicrobial Mode of Action of Sodium Acid Sulfate and Potassium Acid Sulfate. LWT 2021, 148, 111719. [Google Scholar] [CrossRef]
- Molitor, A.; Yucel, U.; Vipham, J.; Jones, C.; Trinetta, V. Effects of Moisture and Temperature on Salmonella Survivability in Beef Tallow, White Grease, and Chicken Rendered Fat. Transl. Anim. Sci. 2021, 5, txab110. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Minor Components in Canola Oil and Effects of Refining on These Constituents: A Review. J. Am. Oil Chem. Soc. 2013, 90, 923–932. [Google Scholar] [CrossRef]
- Deliephan, A.; Dhakal, J.; Subramanyam, B.; Aldrich, C.G. Use of Organic Acid Mixtures Containing 2-Hydroxy-4-(Methylthio) Butanoic Acid (HMTBa) to Mitigate Salmonella enterica, Shiga Toxin-Producing Escherichia coli (STEC) and Aspergillus flavus in Pet Food Kibbles. Animals 2023, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Deliephan, A.; Dhakal, J.; Subramanyam, B.; Aldrich, C.G. Mitigation of Salmonella on food contact surfaces by using organic acid mixtures containing 2-hydroxy-4-(methylthio) butanoic acid (HMTBa). Foods 2023, 12, 874. [Google Scholar] [CrossRef]
- Ossani, G.P.; Denninghoff, V.C.; Uceda, A.M.; Díaz, M.L.; Uicich, R.; Monserrat, A.J. Short-Term Menhaden Oil Rich Diet Changes Renal Lipid Profile in Acute Kidney Injury. J. Oleo Sci. 2015, 64, 497–503. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic Acid and Other Unsaturated Fatty Acids and Some of Their Metabolites Function as Endogenous Antimicrobial Molecules: A Review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Knapp, H.R.; Melly, M.A. Bactericidal Effects of Polyunsaturated Fatty Acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef]
- Lamb, K.E. The Survival of Various Pathogenic Organisms in Fats and Oils. Int. J. Food Microbiol. 2017, 250, 19–24. [Google Scholar]
- Marikkar, J.M.N.; Lim, Y.C.; Ulpathakumbura, B.S.K. Effect of Fractional Crystallization on Fatty Acid and Triacylglycerol Compositions of Selected Native Lipids: An Overview. Molecules 2021, 26, 618. [Google Scholar]
- Chitra Som, R.S.; Radhakrishnan, C.K. Antibacterial activities of polyunsaturated fatty acid extracts from Sardinella longiceps and Sardinella fimbriata. Mar. Drugs 2011, 9, 843–852. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).