A New Species of the Genus Gekko (Squamata: Sauria: Gekkonidae) from the Dabie Mountains, China †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Molecular Data and Phylogenetic Analyses
2.3. Morphological Analyses
3. Results
3.1. Phylogenetic Analyses
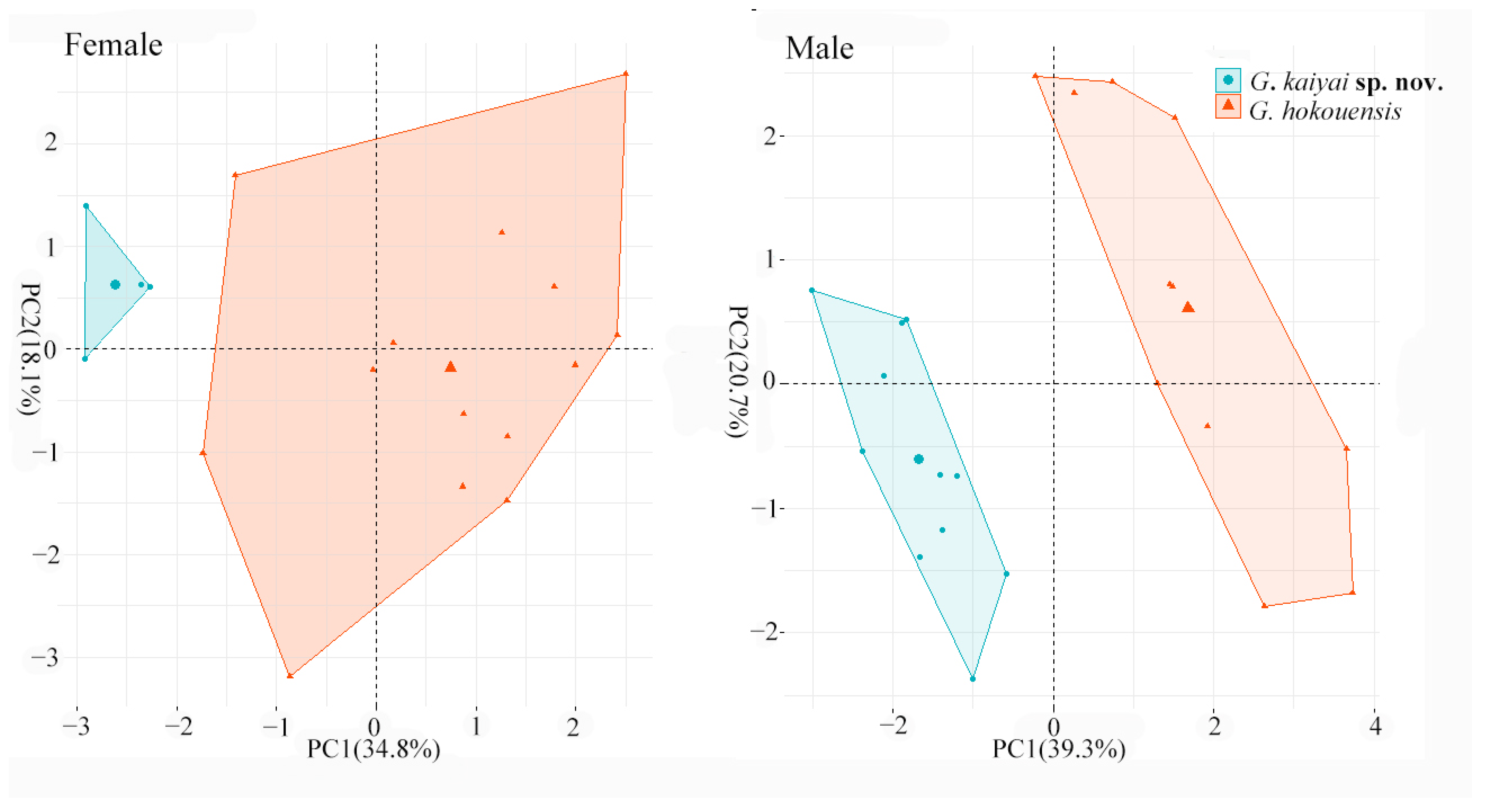
3.2. Morphological Analyses
3.2.1. Taxonomic Accounts
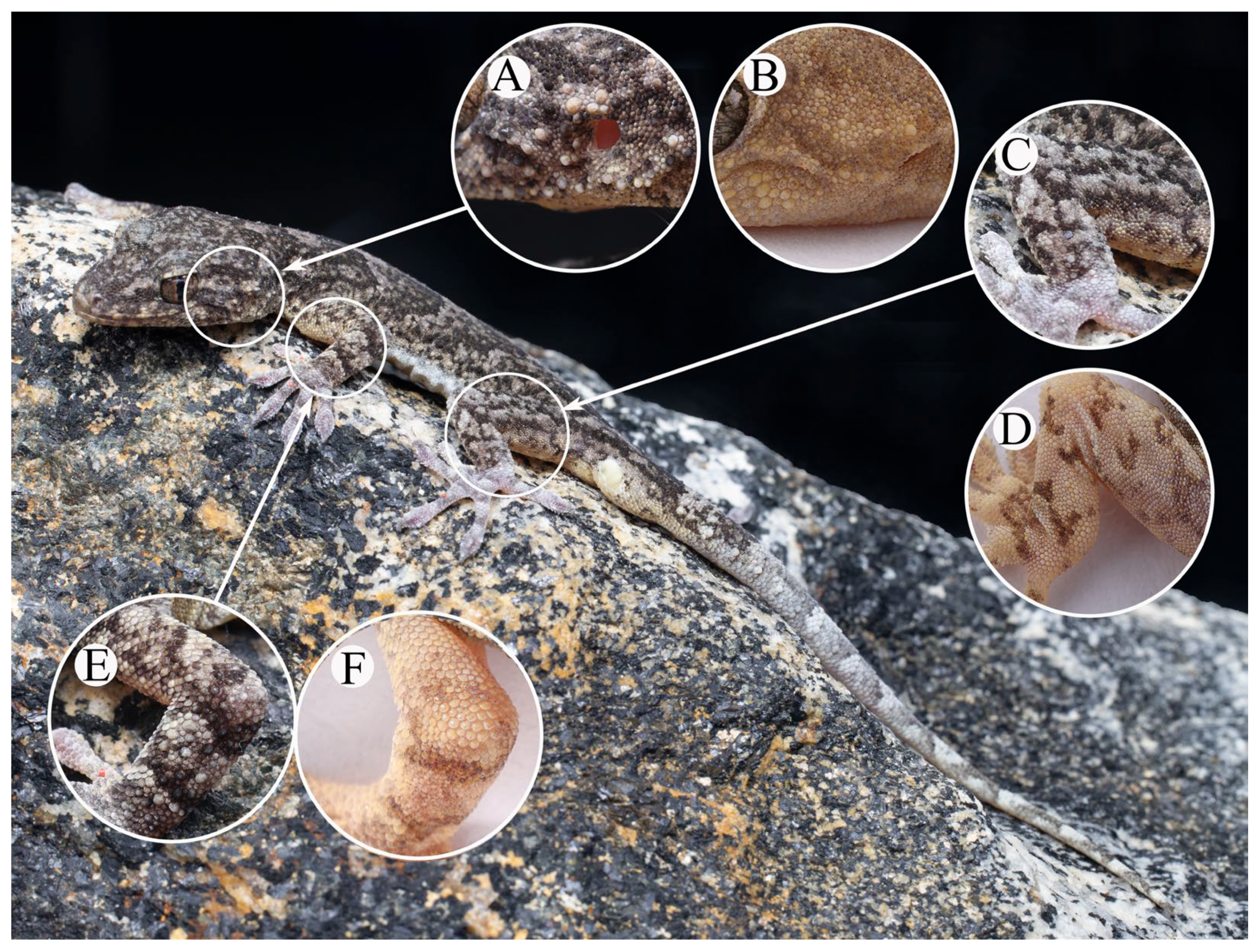
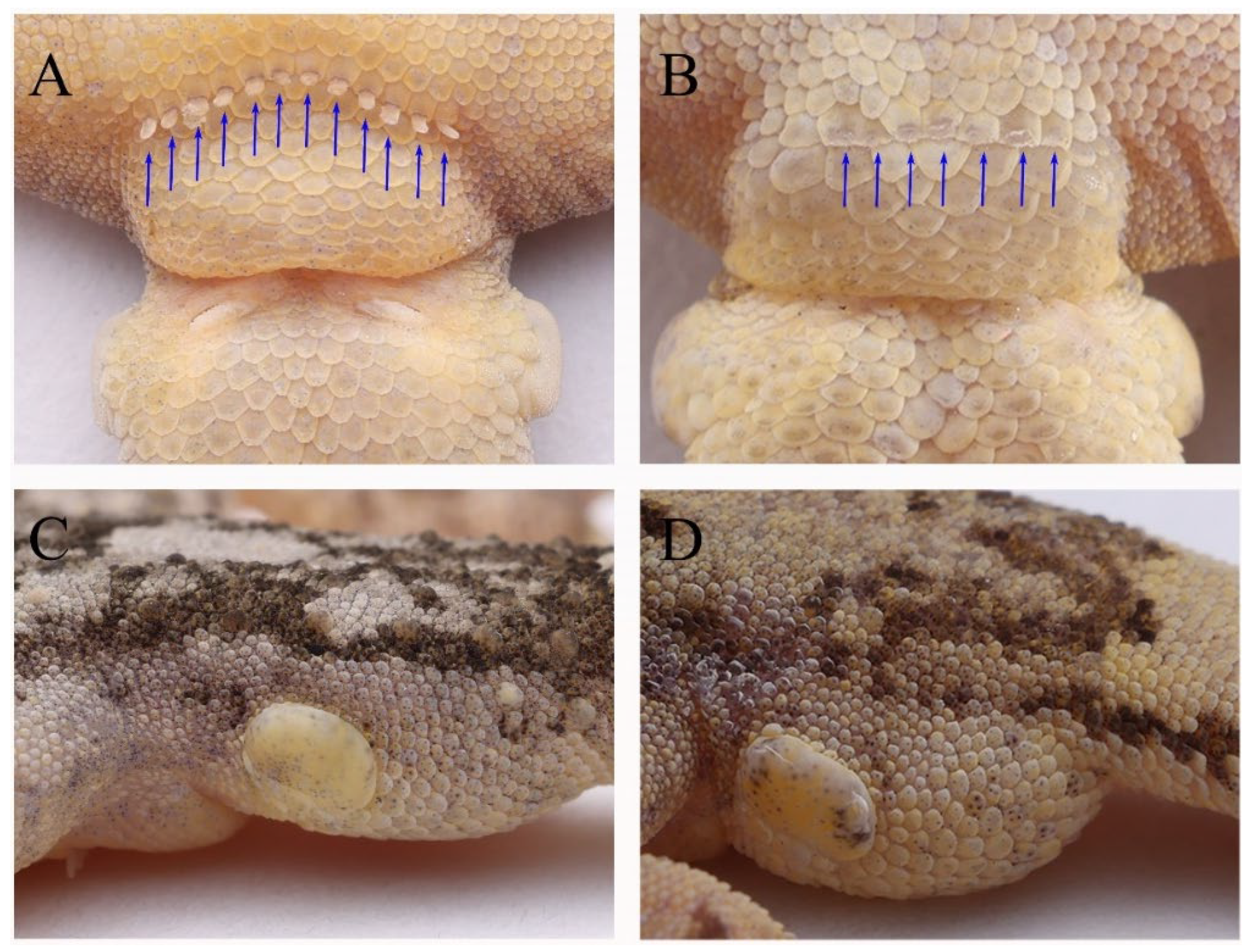
3.2.2. Gekko (Japonigekko) kaiyai sp. nov. Zhang, Wu, and Zhang
3.2.3. Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, P.L., Jr.; Guo, X.G.; Travers, S.L.; Su, Y.C.; Olson, K.V.; Bauer, A.M.; Grismer, L.L.; Siler, C.D.; Moyle, R.G.; Andersen, M.J.; et al. Parachute geckos free fall into synonymy: Gekko phylogeny, and a new subgeneric classification, inferred from thousands of ultraconserved elements. Mol. Phylogenet. Evol. 2020, 146, 106731. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Q. New species of Gekko (Squamata: Sauria: Gekkonidae) from China: Morphological and molecular evidence. Zootaxa 2008, 1778, 59–68. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptiledatabase.org (accessed on 12 February 2023).
- Cai, B.; Ji, X.; Wang, Y.Y.; Rao, D.Q.; Huang, S.; Wang, Y.Z.; Song, Z.B.; Guo, X.G.; Jiang, J.P. An Annotated List of Lizards (Sauria: Squamata) Recorded from the People’s Republic of China. Asian Herpetol. Res. 2022, 13, 64–74. [Google Scholar]
- Hou, Y.M.; Shi, S.C.; Wang, G.; Shu, G.C.; Zheng, P.Y.; Qi, Y.; Liu, G.H.; Jiang, J.P.; Xie, F. A New Species of the Gekko japonicus Group (Squamata: Gekkonidae) from Southwest China. Asian Herpetol. Res. 2021, 12, 36–48. [Google Scholar]
- Lyu, Z.T.; Lin, C.Y.; Ren, J.; Jiang, K.; Zhang, Y.P.; Qi, S.; Wang, J. Review of the Gekko (Japonigekko) subpalmatus complex (Squamata, Sauria, Gekkonidae), with description of a new species from China. Zootaxa 2021, 4951, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Rösler, H.; Bauer, A.M.; Heinicke, M.P.; Greenbaum, E.; Jackman, T.; Nguyen, T.Q.; Ziegler, T. Phylogeny, taxonomy, and zoogeography of the genus Gekko Laurenti, 1768 with the revalidation of G. reevesii Gray, 1831 (Sauria: Gekkonidae). Zootaxa 2011, 2989, 1–50. [Google Scholar] [CrossRef]
- Pan, T.; Zhou, W.L.; Shi, W.B.; Zhao, K.; Chen, J.Y.; Wang, W.G.; Chu, J.; Pu, H.G.; Gu, C.M.; Zhang, B.W. Species richness of amphibians and reptiles in Dabie Mountains, China. Chin. J. Zool. 2014, 49, 195–206. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 545. [Google Scholar]
- Yan, J.; Ma, X.Y.; Du, J.; Zhou, K.Y. DNA barcoding of nine species of Gekko from China. J. Nan Jing Norm. Univ. Nat. Sci. Ed. 2010, 33, 84–90. [Google Scholar]
- Kumazawa, Y.; Endo, H. Mitochondrial genome of the Komodo dragon: Efficient sequencing method with reptile-oriented primers and novel gene rearrangements. DNA Res. 2004, 11, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.Y.; Li, H.D.; Han, D.M.; Bauer, A.M.; Feng, J.Y. The complete mitochondrial genome of Gekko gecko (Reptilia: Gekkonidae) and support for the monophyly of Sauria including Amphisbaenia. Mol. Phylogenet. Evol. 2006, 40, 887–892. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.; Drummond, A.J. Tracer 1.6. Available online: http://tree.bio.ed.ac.uk/software/tracer/ (accessed on 2 October 2022).
- Thorpe, R.S. Quantitative handling of characters useful in snake systematics with particular reference to interspecific variation in the Ringed Snake, Natrix natrix (L.). Biol. J. Linn. Soc. 1975, 7, 27–43. [Google Scholar] [CrossRef]
- Thorpe, R.S. A review of the numerical methods for recognizing and analyzing racial differentiation. In Numerical Taxonomy; Felsenstein, J., Ed.; NATO ASI Series (Series G: Ecological Sciences); Springer: Berlin, Germany, 1983; Volume 1, pp. 404–423. [Google Scholar]
- Turan, C. A note on the examination of morphometric differentiation among fish populations: The Truss System. Turk. Zool. Derg. 1999, 23, 259–263. [Google Scholar]
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef]
- Reist, J.D. A empirical evaluation of coefficients used in residualand allometric adjustment of size covariation. Can. J. Zool. 1986, 64, 1363–1368. [Google Scholar] [CrossRef]
- McCoy, M.W.; Bolker, B.M.; Osenberg, C.W.; Miner, B.G.; Vonesh, J.R. Size correction: Comparing morphological traits among populations and environments. Oecologia 2006, 148, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K. Three new species of Gekko and remarks on Gekko hokouensis (Lacertiformis, Gekkonidae). Acta Zootaxon. Sin. 1982, 7, 438–446. [Google Scholar]
- Zhao, E.; Zhao, K.; Zhou, K. Reptilia, Fauna Sinica, Reptilia Vol. 2, Squamata (Lacertilia); Science Press: Beijing, China, 1999; pp. 1–394. [Google Scholar]
- Boulenger, G.A. LV—Descriptions of new lizards in the British Museum. Nat. Hist. 1907, 19, 486–489. [Google Scholar] [CrossRef][Green Version]
- Goris, R.C.; Maeda, N. Guide to the Amphibians and Reptiles of Japan; Krieger Publishing Company: Malabar, FL, USA, 2004; pp. 176–178. [Google Scholar]
- Luu, V.Q.; Calame, T.; Nguyen, T.Q.; Ducle, M.; Bonkowski, M.; Ziegler, T. A new species of the Gekko japonicus group (Squamata: Gekkonidae) from central Laos. Zootaxa 2014, 3895, 73–88. [Google Scholar] [CrossRef]
- Luu, V.Q.; Calame, T.; Nguyen, T.Q.; Ducle, M.; Ziegler, T. Morphological and molecular review of the Gekko diversity of Laos with descriptions of three new species. Zootaxa 2015, 3986, 279–306. [Google Scholar] [CrossRef]
- Luu, V.Q.; Nguyen, T.Q.; Le, M.D.; Bonkowski, M.; Ziegler, T. A new karst dwelling species of the Gekko japonicus group (Squamata: Gekkonidae) from central Laos. Zootaxa 2017, 4263, 179–193. [Google Scholar] [CrossRef]
- Ngo, V.T.; Bauer, A.M.; Wood, P.L.; Grismer, J.L. A new species of Gekko Laurenti, 1768 (Squamata: Gekkonidae) from Dong Nai Province, Southeastern Vietnam. Zootaxa 2009, 2238, 33–42. [Google Scholar]
- Ngo, V.T.; Gamble, T. A new species of Gekko (Squamata: Gekkonidae) from Tà Kóu Nature Reserve, Binh Thuan Province, Southern Vietnam. Zootaxa 2010, 2346, 17–28. [Google Scholar]
- Nguyen, T.Q.; Wang, Y.Y.; Yang, J.H.; Lehmann, T.; Le, M.D.; Ziegler, T.; Bonkowski, M. A new species of the Gekko japonicus group (Squamata: Sauria: Gekkonidae) from the border region between China and Vietnam. Zootaxa 2013, 3652, 501–518. [Google Scholar] [CrossRef]
- Ota, H.; Lau, M.W.; Weidenhöfer, T.; Yasukawa, Y.; Bogadek, A. Taxonomic review of the geckos allied to Gekko chinensis Gray 1842 (Gekkonidae, Reptilia) from China and Vietnam. Trop. Zool. 1995, 8, 181–196. [Google Scholar] [CrossRef]
- Phung, T.M.; Ziegler, T. Another new Gekko species (Squamata: Gekkonidae) from southern Vietnam. Zootaxa 2011, 3129, 51–61. [Google Scholar] [CrossRef]
- Rösler, H.; Ziegler, T.; Thanh, V.N.; Herrmann, H.W.; Boehme, W. A New Lizard of the Genus Gekko Laurenti, 1768 (Squamata: Sauria: Gekkonidae) from the Phong Nha-Ke Bang National Park, Quang Binh Province, Vietnam 1. Bonn. Zool. Beitr. 2005, 53, 135–148. [Google Scholar]
- Rösler, H.; Truong, Q.N.; Kien, V.D.; Cuc, T.H.; Tao, T.N.; Ziegler, T. A new species of the genus Gekko Laurenti (Squamata: Sauria: Gekkonidae) from Vietnam with remarks on G. japonicus (Schlegel). Zootaxa 2010, 2329, 56–68. [Google Scholar] [CrossRef]
- Rösler, H.; Tiedemann, F. Gekko melli Vogt, 1922 and its types (Reptilia, Sauria, Gekkonidae). Zoosyst. Evol. 2007, 83, 105–108. [Google Scholar] [CrossRef]
- Song, M. A new species of Gekko from Shaanxi. Acta Herpetol. Sin. 1985, 4, 329–330. [Google Scholar]
- Stejneger, L. Herpetology of Japan and Adjacent Territory; US Government Printing Office: Washington, DC, USA, 1907; pp. 507–509. [Google Scholar]
- Toda, M.; Sengoku, S.; Hikida, T.; Ota, H. Description of two new species of the genus Gekko (Squamata: Gekkonidae) from the Tokara and Amami Island Groups in the Ryukyu Archipelago, Japan. Copeia 2008, 2008, 452–466. [Google Scholar] [CrossRef]
- Yang, J.H. A new species of the genus Gekko Laurenti (Squamata: Sauria: Gekkonidae) from Guangxi, China. Zootaxa 2015, 3936, 287–295. [Google Scholar] [CrossRef]
- Grismer, L.L.; del Pinto, L.; Quah, E.S.H.; Anuar, S.; Cota, M.; McGuire, J.A.; Iskandar, D.T.; Wood Jr, P.L.; Grismer, J.L. Phylogenetic and multivariate analyses of Gekko smithii Gray, 1842 recover a new species from Peninsular Malaysia and support the resurrection of G. albomaculatus (Giebel, 1861) from Sumatra. Vertebr. Zool. 2022, 72, 47–80. [Google Scholar] [CrossRef]
- Sites, J.J.W.; Marshall, J.C. Delimiting species: A Renaissance issue in systematic biology. Trends Ecol. Evol. 2003, 18, 462–470. [Google Scholar] [CrossRef]
- Padial, J.M.; Miralles, A.; De la Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]

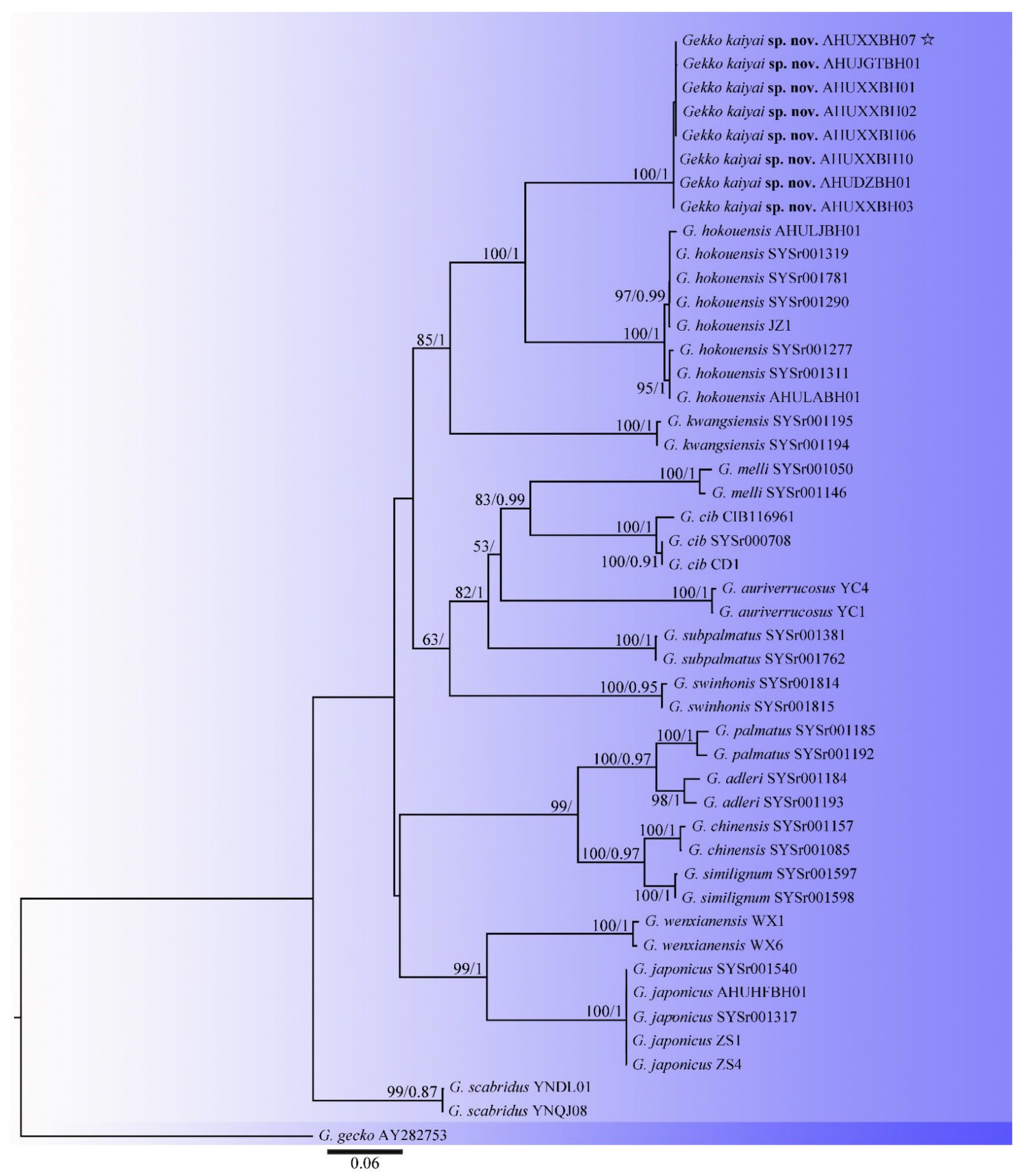




| ID | Species | Locality | Voucher ID | 16S | CYTB | COI | Renference |
|---|---|---|---|---|---|---|---|
| 1 | G. kaiyai sp. nov. | China: Henan: Xinyang: Xinxian | AHUXXBH01 | OQ780318 | OQ743839 | OQ788612 | This study |
| 2 | G. kaiyai sp. nov. | China: Henan: Xinyang: Xinxian | AHUXXBH02 | OQ780319 | OQ743840 | OQ788613 | This study |
| 3 | G. kaiyai sp. nov. | China: Henan: Xinyang: Xinxian | AHUXXBH03 | OQ780320 | OQ743841 | OQ788614 | This study |
| 4 | G. kaiyai sp. nov. | China: Henan: Xinyang: Luoxian | AHULXBH01 | OQ780321 | OQ743842 | OQ788615 | This study |
| 5 | G. kaiyai sp. nov. | China: Henan: Xinyang: shangcheng | AHUJGTBH01 | OQ780322 | OQ743843 | OQ788616 | This study |
| 6 | G. kaiyai sp. nov. | China: Henan: Xinyang: Xinxian | AHUXXBH06 | OR381680 | OR394955 | OR394958 | This study |
| 7 | G. kaiyai sp. nov. | China: Henan: Xinyang: Xinxian | AHUXXBH07 | OR381681 | OR394956 | OR394959 | This study |
| 8 | G. kaiyai sp. nov. | China: Henan: Xinyang: Xinxian | AHUXXBH10 | OR381682 | OR394957 | OR394960 | This study |
| 9 | G. adleri | China: Guangxi: Daxin County | SYSr001184 | MW451636 | MW448266 | N/A | Lyu et al., 2021 [6] |
| 10 | G. adleri | China: Guangxi: Ningming County | SYSr001193 | MW451640 | MW448270 | N/A | Lyu et al., 2021 [6] |
| 11 | G. auriverrucosus | China: Shanxi: Yuncheng | YC1 | N/A | EU417692 | EU417716 | Zhou et al., 2008 [2] |
| 12 | G. auriverrucosus | China: Shanxi: Yuncheng | YC4 | N/A | EU417695 | EU417719 | Zhou et al., 2008 [2] |
| 13 | G. cib | China: Sichuan: Chengdu | CD1 | N/A | EU417696 | EU417713 | Zhou et al., 2008 [2] |
| 14 | G. cib | China: Sichuan: Chengdu City | CIB116961 | MW451623 | MW448256 | N/A | Lyu et al., 2021 [6] |
| 15 | G. cib | China: Sichuan: Chengdu City | SYSr000708 | MW451629 | MW448260 | N/A | Lyu et al., 2021 [6] |
| 16 | G. chinensis | China: Guangdong: Neilingding Island | SYSr001157 | MW451634 | MW448264 | N/A | Lyu et al., 2021 [6] |
| 17 | G. chinensis | China: Guangdong: Shenzhen City | SYSr001085 | MW451632 | MW448262 | N/A | Lyu et al., 2021 [6] |
| 18 | G. hokouensis | China: Anhui: Hefei: Lujiang | AHUHFBH01 | OQ780323 | OQ743844 | N/A | This study |
| 19 | G. hokouensis | China: Anhui: Liuan: Shucheng | AHUSCBH01 | OQ780324 | OQ743845 | OQ788617 | This study |
| 20 | G. hokouensis | China: Fujian: Mt. Wuyi | SYSr001290 | MW451647 | MW448277 | N/A | Lyu et al., 2021 [6] |
| 21 | G. hokouensis | China: Fujian: Shaowu City | SYSr001277 | MW451646 | MW448276 | N/A | Lyu et al., 2021 [6] |
| 22 | G. hokouensis | China: Hunan: Hengdong County | SYSr001781 | MW451665 | MW448295 | N/A | Lyu et al., 2021 [6] |
| 23 | G. hokouensis | China: Hunan: Mt. Hengshan | SYSr001319 | MW451650 | MW448280 | N/A | Lyu et al., 2021 [6] |
| 24 | G. hokouensis | China: Jiangxi: Mt. Meiling | SYSr001311 | MW451648 | MW448278 | N/A | Lyu et al., 2021 [6] |
| 25 | G. hokouensis | China: Anhui: Jinzhai | JZ1 | N/A | EU417689 | EU417720 | Zhou et al., 2008 [2] |
| 26 | G. japonicus | China: Anhui: Liuan: Huoshan | AHULABH11 | N/A | OQ743846 | OQ788618 | This study |
| 27 | G. japonicus | China: Guangxi: Guanyang County | SYSr001540 | MW451656 | MW448286 | N/A | Lyu et al., 2021 [6] |
| 28 | G. japonicus | China: jiangxi: Lushan | SYSr001317 | MW451649 | MW448279 | N/A | Lyu et al., 2021 [6] |
| 29 | G. japonicus | China: Zhejiang: Zhoushan | ZS1 | N/A | EU417683 | EU417723 | Zhou et al., 2008 [2] |
| 30 | G. japonicus | China: Zhejiang: Zhoushan | ZS4 | N/A | EU417686 | EU417726 | Zhou et al., 2008 [2] |
| 31 | G. kwangsiensis | China: Guangxi: Wuming County | SYSr001194 | MW451641 | MW448271 | N/A | Lyu et al., 2021 [6] |
| 32 | G. kwangsiensis | China: Guangxi: Wuming County | SYSr001195 | MW451642 | MW448272 | N/A | Lyu et al., 2021 [6] |
| 33 | G. melli | China: Guangdong: Mt. Yinping | SYSr001146 | MW451633 | MW448263 | N/A | Lyu et al., 2021 [6] |
| 34 | G. melli | China: Guangdong: Mt. Yinping | SYSr001050 | MW451631 | MW448261 | N/A | Lyu et al., 2021 [6] |
| 35 | G. palmatus | China: Guangxi: Napo County | SYSr001185 | MW451637 | MW448267 | N/A | Lyu et al., 2021 [6] |
| 36 | G. palmatus | China: Guangxi: Nonggang Nature Reserve | SYSr001192 | MW451639 | MW448269 | N/A | Lyu et al., 2021 [6] |
| 37 | G. scabridus | China: Yunnan: Dali | YNDL01 | N/A | N/A | HM802949 | Yan et al., 2010 [10] |
| 38 | G. scabridus | China: Yunnan: Dali | YNDL08 | N/A | N/A | HM802950 | Yan et al., 2010 [10] |
| 39 | G. similignum | China: Haihan: Mt. Wuzhi | SYSr001597 | MW451658 | MW448288 | N/A | Lyu et al., 2021 [6] |
| 40 | G. similignum | China: Haihan: Mt. Wuzhi | SYSr001598 | MW451659 | MW448289 | N/A | Lyu et al., 2021 [6] |
| 41 | G. subpalmatus | China: Zhejiang: Zhoushan Island | SYSr001381 | MW451653 | MW448283 | N/A | Lyu et al., 2021 [6] |
| 42 | G. subpalmatus | China: Zhejiang: Fenghua County | SYSr001762 | MW451662 | MW448292 | N/A | Lyu et al., 2021 [6] |
| 43 | G. swinhonis | China: Hebei: Zunhua County | SYS r001814 | MW451666 | MW448296 | N/A | Lyu et al., 2021 [6] |
| 44 | G. swinhonis | China: Hebei: Zunhua County | SYS r001815 | MW451667 | MW448297 | N/A | Lyu et al., 2021 [6] |
| 45 | G. wenxianensis | China: Gansu: Wenxian | WX1 | N/A | EU417677 | EU417703 | Zhou et al., 2008 [2] |
| 46 | G. wenxianensis | China: Gansu: Wenxian | WX6 | N/A | EU417682 | EU417708 | Zhou et al., 2008 [2] |
| outgoup | |||||||
| 47 | G. (G.) gecko | China: Guangxi: Nanning City | N/A | AY282753 | AY282753 | AY282753 | Zhou et al., 2006 [12] |
| Measurements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||
| G. kaiyai sp. nov. (n = 11) | G. hokouensis (n = 11) | G. kaiyai sp. nov. (n = 4) | G. hokouensis (n = 14) | G. kaiyai sp. nov. vs. G. hokouensis | G. kaiyai sp. nov. vs. G. hokouensis | |||||
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | |||
| SVL | 50.03–61.56 | 57.97 ± 3.91 | 48.17–62.6 | 56 ± 497 | 56.98–64.99 | 62.3 ± 3.13 | 50.26–63.11 | 55.77 ± 4.83 | 0.326 | 0.0239 * |
| AG | 22.96–30.95 | 26.91 ± 2.32 | 17.89–27.17 | 23.28 ± 2.36 | 27.68–34.24 | 30.57 ± 2.63 | 27.68–34.24 | 25.01 ± 2.98 | 0.0006 *** | 0.0136 * |
| HL | 13.34–15.73 | 14.83 ± 0.73 | 13.01–16.33 | 14.55 ± 0.93 | 15.71–16.14 | 15.94 ± 0.86 | 15.71–16.14 | 14.44 ± 1.03 | 0.94 | 0.00194 ** |
| HW | 11.39–13.48 | 12.63 ± 0.60 | 10.38–12.53 | 11.52 ± 0.71 | 12.96–13.77 | 13.26 ± 0.84 | 12.96–13.77 | 11.26 ± 1.22 | 0.0000 *** | 0.0006 *** |
| HH | 4.30–6.71 | 5.34 ± 0.56 | 4.48–8.05 | 6.31 ± 0.87 | 5.05–5.46 | 5.3 ± 0.61 | 5.05–5.46 | 6.14 ± 0.69 | 0.0005 *** | 0.0164 * |
| SE | 4.05–4.98 | 4.59 ± 0.25 | 4.06–5.81 | 4.93 ± 0.59 | 4.49–5.15 | 4.79 ± 0.44 | 4.49–5.15 | 4.63 ± 0.42 | 0.0552 | 0.783 |
| ED | 2.9–3.9 | 3.41 ± 0.25 | 2.47–3.18 | 2.87 ± 0.22 | 3.11–3.27 | 3.21 ± 0.40 | 3.11–3.27 | 2.95 ± 0.28 | 0.0000 *** | 0.352 |
| TD | 0.97–1.56 | 1.22 ± 0.19 | 0.51–0.97 | 0.79 ± 0.15 | 1.31–1.68 | 1.53 ± 0.29 | 1.31–1.68 | 0.99 ± 0.34 | 0.0001 *** | 0.0074 ** |
| EE | 3.57–4.88 | 4.37 ± 0.37 | 3.91–5.05 | 4.45 ± 0.36 | 4.61–4.98 | 4.78 ± 0.37 | 4.61–4.98 | 4.5 ± 0.57 | 0.204 | 0.882 |
| RW | 1.95–2.97 | 2.59 ± 0.32 | 1.57–2.23 | 1.95 ± 0.67 | 2.64–2.94 | 2.84 ± 0.43 | 2.64–2.94 | 1.98 ± 0.37 | 0.244 | 0.0000 *** |
| RH | 0.93–1.4 | 1.13 ± 0.13 | 0.61–5.35 | 2.36 ± 0.67 | 0.97–1.35 | 1.18 ± 0.20 | 0.97–1.35 | 1.07 ± 0.22 | 0.1 | 0.361 |
| ML | 1.52–2.13 | 1.74 ± 0.17 | 1.53–1.92 | 1.78 ± 0.12 | 1.69–1.89 | 1.78 ± 0.15 | 1.69–1.89 | 1.85 ± 0.24 | 0.295 | 0.401 |
| MW | 0.66–0.99 | 0.85 ± 0.08 | 0.78–1.24 | 1.02 ± 0.13 | 0.93–1.1 | 1.03 ± 0.07 | 0.93–1.1 | 0.95 ± 0.19 | 0.0011 ** | 0.26 |
| Morphometric Characteristics | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | |
| SVL | −0.2752 | 0.1692 | 0.3835 | −0.0212 | −0.1268 | −0.1214 | 0.0492 | 0.9830 |
| AG | −0.2496 | 0.3650 | 0.1457 | −0.4916 | −0.3759 | −0.0181 | 0.2899 | −0.0633 |
| HL | −0.4581 | −0.2206 | 0.0884 | 0.1963 | 0.1405 | −0.3404 | −0.7896 | 0.0224 |
| HW | −0.0341 | −0.1749 | 0.5379 | −0.1723 | −0.3297 | −0.3630 | −0.1878 | −0.0832 |
| HH | 0.1627 | −0.5465 | −0.1819 | −0.3927 | 0.4034 | −0.0260 | 0.1856 | 0.0264 |
| ED | −0.2986 | 0.2448 | −0.5670 | 0.2064 | −0.3050 | −0.4042 | −0.0188 | −0.0976 |
| TD | −0.4409 | 0.1503 | −0.1211 | 0.2323 | −0.2462 | −0.5201 | 0.3002 | −0.1048 |
| EE | −0.1381 | −0.5411 | 0.1123 | 0.4397 | 0.3066 | −0.3896 | 0.1596 | −0.0149 |
| RH | −0.2892 | −0.2212 | −0.2958 | −0.4689 | 0.3413 | −0.3216 | 0.3247 | −0.0037 |
| MW | −0.3667 | −0.1897 | −0.2554 | −0.1599 | 0.4329 | −0.2105 | −0.0054 | 0.0214 |
| Eigenvalues | 3.3832 | 1.8118 | 1.4060 | 0.9876 | 3.9305 | 2.0704 | 0.9878 | 0.9353 |
| Percentage of total variance | 34.8323 | 18.1183 | 14.0603 | 9.8761 | 39.3049 | 20.7039 | 9.8781 | 9.3625 |
| Cumulative percentage | 34.8323 | 52.9506 | 67.0109 | 76.8870 | 39.3049 | 60.0088 | 69.8869 | 79.2395 |
| Characters | MaxSVL | SPL | IFL | N to R | I | IO | PM | DTR | SMC | SR | V | LT1 | LT4 | Web | Fore Tubercles | Hind Tubercles | Tail Tubercles | PP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G. kaiyai sp. nov. | 64.99 | 9–12 | 9–13 | 1 | 1–1 | 22–33 | 1 | 11–18 | 157–209 | 99–121 | 30–43 | 8–9 | 7–11 | 0 | 1 | 1 | 1 | 9–12 |
| G. aaronbaueri | 80 | 13–14 | 10–11 | 1 | 0–1 | 34–37 | 1 | 0–0 | – | 98–104 | 39–43 | 14–17 | 14–16 | – | 0 | 0 | 0 | 3–4 |
| G. adleri | 75.3 | 10–15 | 9–13 | 1 | 1–1 | 27–36 | 1 | 7–11 | 168–190 | 123–144 | 35–44 | 11–14 | 11–15 | 1 | 0 | 1 | 1 | 17–21 |
| G. auriverrucosus | 69 | 9–11 | 9–11 | 0 | 0–1 | 25–25 | 0 | 16–20 | – | – | – | 6–8 | 6–8 | 0 | 1 | 1 | 1 | 8–11 |
| G. bonkowskii | 69.2 | 12–14 | 10–11 | 1 | 0 | 26–27 | 1 | 0–0 | 154–169 | 117–117 | 37–40 | 11–13 | 15–15 | 1 | 0 | 0 | 0 | 6–6 |
| G. canhi | 99.2 | 14–14 | 10–12 | 1 | 1–1 | 49–50 | 1 | 11–12 | 168–170 | 205–227 | 49–51 | 13–16 | 14–17 | 0 | 0 | 1 | 0 | 5–5 |
| G. chinensis | 72 | 10–14 | 9–13 | 1 | 1–1 | 35–48 | 1 | 10–10 | 156–167 | 118–140 | 37–39 | 8–10 | 9–12 | 1 | 0 | 1 | 1 | 17–27 |
| G. cib | 66.4 | 10–12 | 10–14 | 1 | 1–2 | 28–36 | 1 | 0–0 | 171–196 | 128–149 | 37–45 | 9–13 | 9–17 | 1 | 0 | 0 | 0 | 7–9 |
| G. hokouensi | 70 | 10–14 | 8–11 | 1 | 1–1 | 30–33 | 1 | 12–18 | 153–174 | 119–130 | 36–43 | 8–11 | 15–18 | 0 | 0 | 0 | 1 | 5–9 |
| G. japonicus | 74 | 9–13 | 8–13 | 1 | 0–1 | 32–35 | 1 | 9–14 | 169–188 | 130–144 | 39–44 | 10–12 | 14–16 | 0 | 1 | 1 | 1 | 6–9/4–8 |
| G. jinjiangensis | 61.6 | 7–10 | 6–9 | 1 | 0–1 | 20–24 | 1 | 12–16 | 146–169 | 111–149 | 31–47 | 8–11 | 11–15 | 0 | 1 | 1 | 1 | 4–5 |
| G. (J.)khunkhamensis | 75.2 | 10 | 9 | – | 0 | 31–32 | – | – | 181–185 | 127–138 | 42–45 | 13–14 | 14–15 | 1 | 0 | 0 | 0 | 0–0 |
| G. kwangsiensis | 69.7 | 10–12 | 11–13 | 1 | 0–1 | 29–31 | 1 | 9–11 | 185–208 | 143–156 | 41–45 | 11–13 | 13–18 | 1 | 0 | 0 | 1 | 9–11 |
| G. lauhachindai | 98 | 11–12 | 11 | 0 | 0 | 24 | 1 | 14 | – | 112–121 | 32 | 12–14 | 13–15 | 1 | 0 | 0 | 0 | 12–14 |
| G. liboensis | 85 | 12–12 | 11–11 | 1 | 0 | 40–40 | 0 | 10–10 | – | – | – | 8–8 | 9–9 | 0 | 0 | 0 | – | – |
| G. melli | 84.6 | 10–13 | 9–12 | 1 | 1–1 | 34–40 | 0 | 0–0 | 181–200 | 147–160 | 43–49 | 10–12 | 11–14 | 1 | 0 | 0 | 0 | 9–11 |
| G. nadenensis | 77.1 | 12–14 | 10–12 | 1 | 0 | 28–30 | 1 | 0–0 | 175–185 | 123–140 | 43–49 | 13–15 | 14–16 | 1 | 0 | 0 | 0 | 6–6 |
| G. palmatus | 79.7 | 11–15 | 9–13 | 1 | 0–3 | 27–36 | 1 | 4–12 | 160–191 | 116–147 | 36–47 | 10–13 | 10–16 | 1 | 0 | 0 | 1 | 23–30 |
| G. scabridus | 64 | 9–11 | 9–11 | 1 | 1–2 | 30–30 | 1 | 17–21 | – | – | – | 6–9 | 7–9 | 0 | 1 | 1 | 1 | 10–15 |
| G. scientiadventura | 73 | 12–14 | 9–13 | 1 | 0 | 41–51 | 1 | 0–0 | 118–140 | 139–143 | 38–48 | 12–15 | 14–17 | 1 | 0 | 0 | 0 | 5–8 |
| G. sengchanthavongi | 77.3 | 8–10 | 6–7 | 1 | 0 | 28–32 | 1 | 0–0 | 175–184 | 120–135 | 35–43 | 11–14 | 13–17 | 1 | 0 | 0 | 0 | 4–5 |
| G. shibatai | 70.9 | 10–13 | 10–14 | 1 | 0–1 | 37–52 | 0 | 5–14 | – | 114–134 | – | – | 9–16 | 0 | 0 | 0 | 1 | 0–3 |
| G. similignum | 58.9 | 12–14 | 11–11 | 1 | 1–1 | 46–48 | 0 | 11–11 | – | 144–153 | – | 11–13 | 12–14 | 1 | 0 | 0 | 1 | 17–17 |
| G. subpalmatus | 72 | 8–12 | 7–12 | 1 | 1–1 | 32–32 | 0 | 0–0 | – | – | 48–48 | 7–9 | 7–10 | 1 | 0 | 0 | 0 | 5–11 |
| G. swinhonis | 66 | 7–12 | 7–11 | 1 | – | 23–24 | 0 | 6–8 | – | – | 40–40 | 6–9 | 6–9 | 0 | 1 | 1 | – | 7–9 |
| G. taibaiensis | 69 | 9–10 | 8–10 | 1 | – | 28–28 | – | – | – | – | – | 6–7 | 7–8 | – | – | – | – | 4–6 |
| G. tawaensis | 71 | 15–15 | 13–13 | 1 | 2–2 | – | 0 | 0–0 | – | – | – | 10–10 | 12–12 | 0 | 0 | 0 | 0 | 0–0 |
| G. thakhekensis | 79.2 | 12–14 | 10–11 | 1 | 0 | 22–26 | 1 | 0–0 | 165–174 | 110–116 | 32–40 | 11–13 | 14–15 | 1 | 0 | 0 | 0 | 1–5 |
| G. truongi | 95.9 | 13–15 | 11–13 | 1 | 0–1 | 45–48 | 1 | 0–0 | 160–172 | 131–143 | 35–36 | 11–13 | 15–17 | 0 | 0 | 0 | 0 | 10–11 |
| G. vertebralis | 69.2 | 10–15 | 10–15 | 1 | 0–2 | 35–50 | 0 | 2–12 | – | 112–139 | – | – | 9–17 | 0 | 0 | 0 | 0 | 0–1 |
| G. vietnamensis | 91 | 11–12 | 10–11 | – | – | 38–46 | – | – | – | – | – | – | – | 0 | – | – | – | 0–0 |
| G. wenxianensis | 59 | 12–12 | 11–11 | 1 | 1–1 | – | 1 | 10–10 | – | – | 42–44 | 6–6 | 9–9 | 0 | 0 | 1 | – | 6–8 |
| G. yakuensis | 72 | 12–13 | 9–13 | 1 | 1–1 | – | 0 | – | – | – | – | 10–10 | 15–15 | 0 | 0 | 0 | 1 | 6–8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wu, A.; Cai, B.; Wang, L.; Pang, D.; Ma, H.; Yu, L.; Li, X.; Huang, H.; Zeng, L.; et al. A New Species of the Genus Gekko (Squamata: Sauria: Gekkonidae) from the Dabie Mountains, China. Animals 2023, 13, 3796. https://doi.org/10.3390/ani13243796
Zhang C, Wu A, Cai B, Wang L, Pang D, Ma H, Yu L, Li X, Huang H, Zeng L, et al. A New Species of the Genus Gekko (Squamata: Sauria: Gekkonidae) from the Dabie Mountains, China. Animals. 2023; 13(24):3796. https://doi.org/10.3390/ani13243796
Chicago/Turabian StyleZhang, Caiwen, Afang Wu, Bo Cai, Lanrong Wang, Dapeng Pang, Haohao Ma, Lei Yu, Xiangyang Li, Hua Huang, Lin Zeng, and et al. 2023. "A New Species of the Genus Gekko (Squamata: Sauria: Gekkonidae) from the Dabie Mountains, China" Animals 13, no. 24: 3796. https://doi.org/10.3390/ani13243796
APA StyleZhang, C., Wu, A., Cai, B., Wang, L., Pang, D., Ma, H., Yu, L., Li, X., Huang, H., Zeng, L., Li, L., Yan, J., Li, P., & Zhang, B. (2023). A New Species of the Genus Gekko (Squamata: Sauria: Gekkonidae) from the Dabie Mountains, China. Animals, 13(24), 3796. https://doi.org/10.3390/ani13243796







