Evolutionary Patterns of Intersexual Power
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
2.3. Ancestral State Reconstruction
2.4. Predicting Probability of Exhibiting Male-Biased Power along Phylogeny
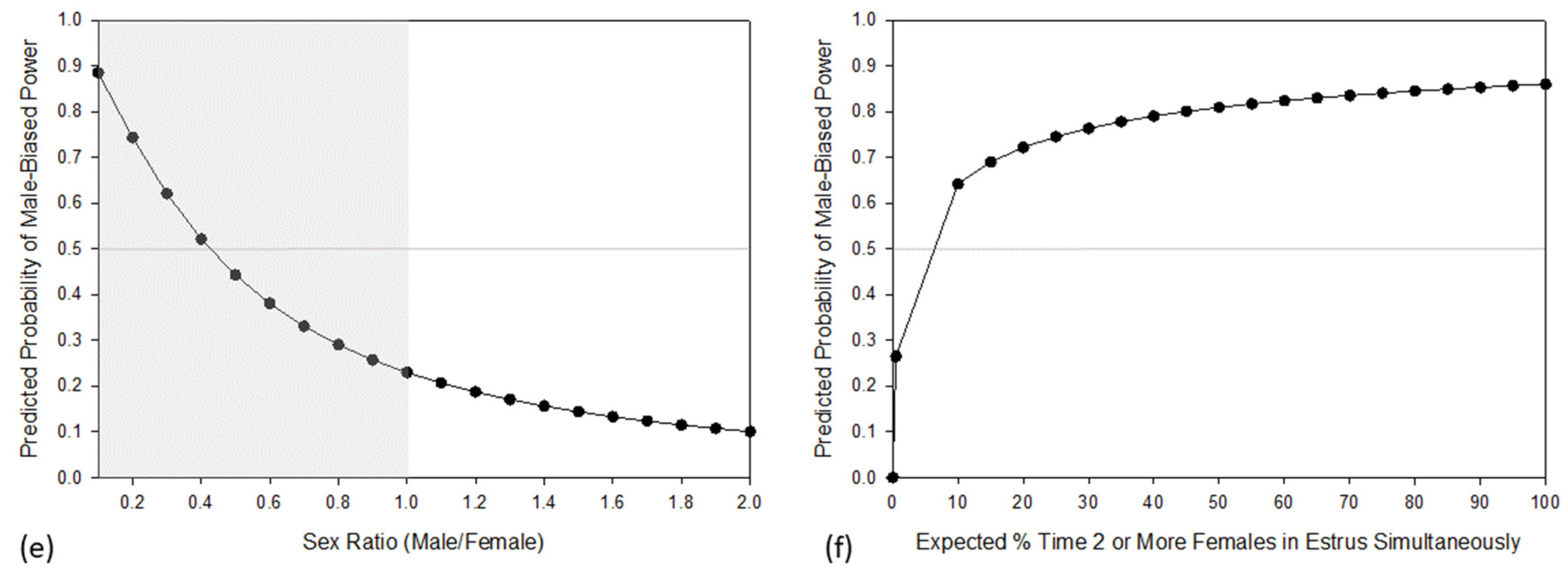
3. Results
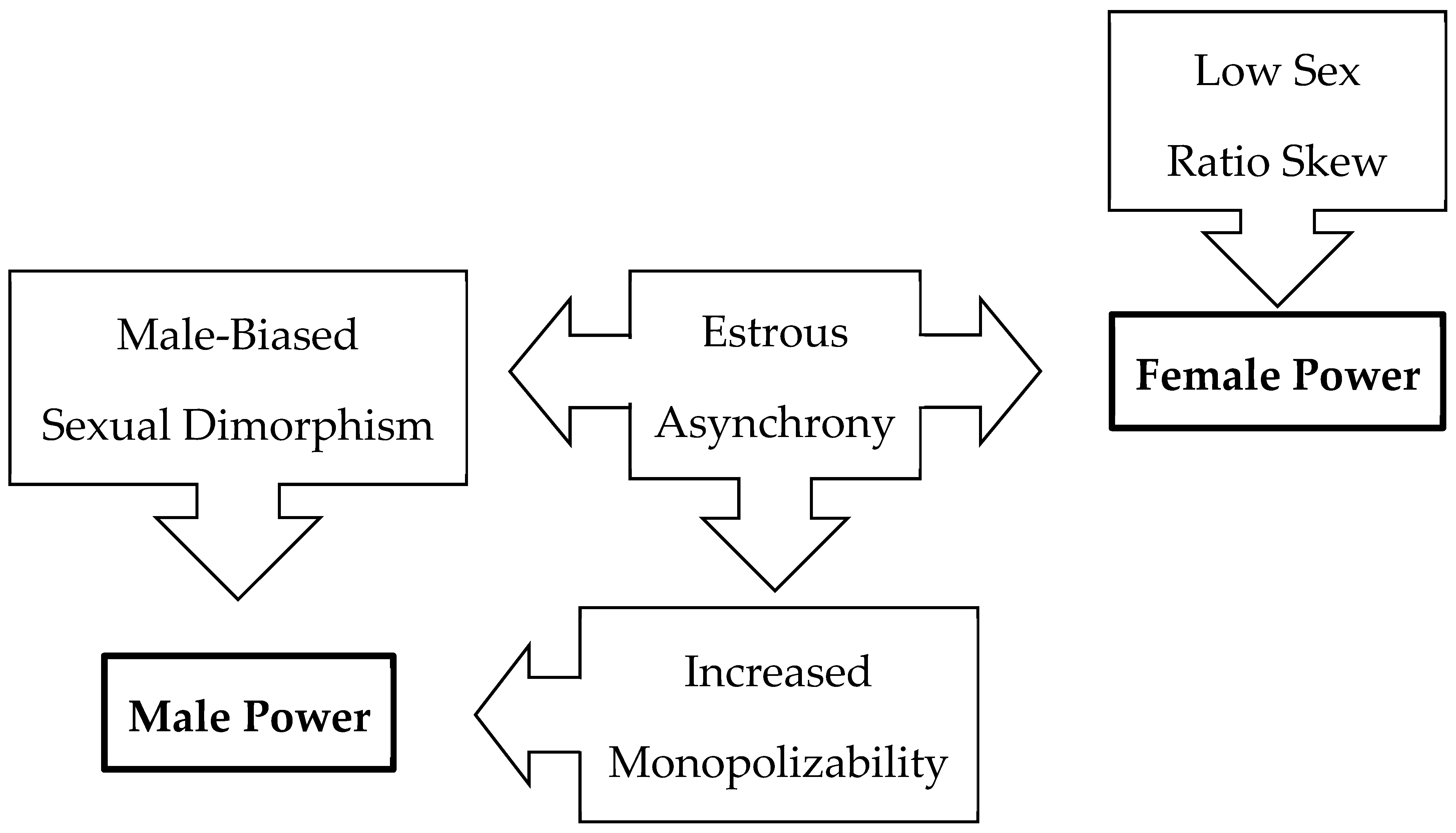
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, H.A. Notes on the Observation and Measurement of Political Power. J. Polit. 1953, 15, 500–516. [Google Scholar] [CrossRef]
- Lewis, R.J. Beyond Dominance: The Importance of Leverage. Q. Rev. Biol. 2002, 77, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. Female Power: A New Framework for Understanding “Female Dominance” in Lemurs. Folia Primatol. 2020, 91, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. Aggression, Rank and Power: Why Hens (and other Animals) Do Not Always Peck According to Their Strength. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200434. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. Female Power in Primates and the Phenomenon of Female Dominance. Annu. Rev. Anthropol. 2018, 47, 533–551. [Google Scholar] [CrossRef]
- Amadon, D. The Significance of Sexual Differences in Size among Birds. Proc. Am. Philos. Soc. 1959, 103, 531–536. [Google Scholar]
- Crook, J. Sexual Selection, Dimorphism, and Social Organization in the Primates. In Sexual Selection and the Descent of Man, 1871–1971; Campbell, B., Ed.; Aldine: Chicago, IL, USA, 1972; pp. 231–281. [Google Scholar]
- Ralls, K. Mammals in Which Females Are Larger than Males. Q. Rev. Biol. 1976, 51, 245–276. [Google Scholar] [CrossRef]
- Jolly, A. The Puzzle of Female Feeding Priority. In Female Primates: Studies by Women Primatologists; Small, M., Ed.; Alan R. Liss: New York, NY, USA, 1984. [Google Scholar]
- Smith, S.M. Raptor “Reverse” Dimorphism Revisited: A New Hypothesis. Oikos 1982, 39, 118–122. [Google Scholar] [CrossRef]
- Hrdy, S. The Woman That Never Evolved; Harvard University Press: Cambridge, UK, 1981. [Google Scholar]
- Wright, P.C. Lemur Traits and Madagascar Ecology: Coping with an Island Environment. Am. J. Phys. Anthropol. 1999, 110 (Suppl. S29), 31–72. [Google Scholar] [CrossRef]
- Richard, A. Aggressive Competition between Males, Female-Controlled Polygyny, and Sexual Monomorphism in a Malagasy Primate, P.v. verreauxi. J. Hum. Evol. 1992, 22, 395–406. [Google Scholar] [CrossRef]
- Lawler, R.R. Causes and Consequences of Differential Reproductive Success in Male White Sifaka (Propithecus verreauxi verreauxi). Ph.D. Dissertation, Yale University, New Haven, CT, USA, 2003. [Google Scholar]
- McCormick, S.K.; Laubach, Z.M.; Strauss, E.D.; Montgomery, T.M.; Holekamp, K.E. Evaluating Drivers of Female Dominance in the Spotted Hyena. Front. Ecol. Evol. 2022, 10, 934659. [Google Scholar] [CrossRef]
- Vullioud, C.; Davidian, E.; Wachter, B.; Rousset, F.; Courtiol, A.; Höner, O.P. Social Support Drives Female Dominance in the Spotted Hyaena. Nat. Ecol. Evol. 2019, 3, 71–76. [Google Scholar] [CrossRef]
- Hemelrijk, C.K.; Seex, L.; Pederboni, M.; Ilany, A.; Geffen, E.; Koren, L. Adult Sex Ratios and Partial Dominance of Females over Males in the Rock Hyrax. Front. Ecol. Evol. 2022, 10, 1004919. [Google Scholar] [CrossRef]
- Hemelrijk, C.; Wantia, J.; Dätwyler, M. Female Co-Dominance in a Virtual World: Ecological, Cognitive, Social and Sexual Causes. Behaviour 2003, 140, 1247–1273. [Google Scholar] [CrossRef]
- Lewis, R.J.; Bueno, G.L.; Di Fiore, A. Variation in Female Leverage: The Influence of Kinship and Market Effects on the Extent of Female Power over Males in Verreaux’s Sifaka. Front. Ecol. Evol. 2022, 10, 851880. [Google Scholar] [CrossRef]
- Voyt, R.A.; Sandel, A.A.; Ortiz, K.M.; Lewis, R.J. Female Power in Verreaux’s Sifaka (Propithecus verreauxi) Is Based on Maturity, Not Body Size. Int. J. Primatol. 2019, 40, 417–434. [Google Scholar] [CrossRef]
- Noë, R.; van Schaik, C.P.; van Hooff, J.A.R.A.M. The Market Effect: An Explanation for Pay-off Asymmetries among Collaborating Animals. Ethology 1991, 87, 97–118. [Google Scholar] [CrossRef]
- Kappeler, P. Female Dominance in Primates and other Mammals. In Perspectives in Ethology; Bateson, P., Klopfer, P., Thompson, N., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 143–158. [Google Scholar]
- French, J.A.; Mustoe, A.C.; Cavanaugh, J.; Birnie, A.K. The Influence of Androgenic Steroid Hormones on Female Aggression in ‘Atypical’ Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130084. [Google Scholar] [CrossRef]
- Kappeler, P.M.; van Schaik, C.P. Evolution of Primate Social Systems. Int. J. Primatol. 2002, 23, 707–740. [Google Scholar] [CrossRef]
- Lindenfors, P.; Gittleman, J.L.; Jones, K.E. Sexual Size Dimorphism in Mammals. In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Oxford University: Oxford, UK, 2007; pp. 16–26. [Google Scholar]
- Kappeler, P.M.; Fichtel, C.; Radespiel, U. The Island of Female Power? Intersexual Dominance Relationships in the Lemurs of Madagascar. Front. Ecol. Evol. 2022, 10, 858859. [Google Scholar] [CrossRef]
- Radespiel, U.; Zimmermann, E. Female Dominance in Captive Gray Mouse Lemurs (Microcebus murinus). Am. J. Primatol. 2001, 54, 181–192. [Google Scholar] [CrossRef]
- Rowell, T.E. The Concept of Social Dominance. Behav. Biol. 1974, 11, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I. Dominance: The Baby and the Bathwater. Behav. Brain Sci. 1981, 4, 419–429. [Google Scholar] [CrossRef]
- Kappeler, P.M.; Schäffler, L. The Lemur Syndrome Unresolved: Extreme Male Reproductive Skew in Sifakas (Propithecus verreauxi), a Sexually Monomorphic Primate with Female Dominance. Behav. Ecol. Sociobiol. 2008, 62, 1007–1015. [Google Scholar] [CrossRef]
- Kappeler, P.M.; Fichtel, C. Eco-Evo-Devo of the Lemur Syndrome: Did Adaptive Behavioral Plasticity Get Canalized in a Large Primate Radiation? Front. Zool. 2015, 12, S15. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, K.E.; Engh, A.L. Reproductive Skew in Female-Dominated Mammalian Societies. In Reproductive Skew in Vertebrates: Proximate and Ultimate Causes; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Mitani, J.C.; Gros-Louis, J.; Richards, A.F. Sexual Dimorphism, the Operational Sex Ratio, and the Intensity of Male Competition in Polygynous Primates. Am. Nat. 1996, 147, 966–980. [Google Scholar] [CrossRef]
- Noë, R.; Hammerstein, P. Biological Markets. Trends. Ecol. Evol. 1995, 10, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Noë, R.; Hammerstein, P. Biological Markets: Supply and Demand Determine the Effect of Partner Choice in Cooperation, Mutualism and Mating. Behav. Ecol. Sociobiol. 1994, 35, 1–11. [Google Scholar] [CrossRef]
- Carnes, L.M.; Nunn, C.L.; Lewis, R.J. Effects of the Distribution of Female Primates on the Number of Males. PLoS ONE 2011, 6, e19853. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.L. The Number of Males in Primate Social Groups: A Comparative Test of the Socioecological Model. Behav. Ecol. Sociobiol. 1999, 46, 1–13. [Google Scholar] [CrossRef]
- Kappeler, P.M. Sex Roles and Adult Sex Ratios: Insights from Mammalian Biology and Consequences for Primate Behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160321. [Google Scholar] [CrossRef] [PubMed]
- Janicke, T.; Fromonteil, S. Sexual Selection and Sexual Size Dimorphism in Animals. Biol. Lett. 2021, 17, 20210251. [Google Scholar] [CrossRef] [PubMed]
- Drews, C. The Concept and Definition of Dominance in Animal Behaviour. Source Behav. 1993, 125, 283–313. [Google Scholar] [CrossRef]
- Watts, D.P. Dominance, Power, and Politics in Nonhuman and Human Primates. In Mind the Gap; Springer: Berlin/Heidelberg, Germany, 2010; pp. 109–138. [Google Scholar]
- Nowak, R.; Walker, E. Walker’s Mammals of the World; Johns Hopkins University Press: Baltimore, MD, USA, 1999; Volume 2. [Google Scholar]
- Pereira, M.E.; Kaufman, R.; Kappeler, P.M.; Overdoff, D.J. Female Dominance Does Not Characterize all of the Lemuridae. Folia Primatol. 1990, 55, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.M.A.; Drea, C.M. Female Rule in Lemurs Is Ancestral and Hormonally Mediated. Sci. Rep. 2015, 5, 9631. [Google Scholar] [CrossRef]
- Smuts, B. Gender, Aggression, and Influence. In Primate Societies; Smuts, B., Cheney, D., Seyfarth, R., Wrangham, R., Struhsaker, T., Eds.; University of Chicago: Chicago, IL, USA, 1987; pp. 400–412. [Google Scholar]
- Wrangham, R.; Peterson, D. Demonic Males: Apes and the Origins of Human Violence; Houghton Mifflin Harcourt: Boston, MA, USA, 1996. [Google Scholar]
- Reichard, U.H.; Barelli, C. Life History and Reproductive Strategies of Khao Yai Hylobates lar: Implications for Social Evolution in Apes. Int. J. Primatol. 2008, 29, 823–844. [Google Scholar] [CrossRef]
- Smith, R.J.; Jungers, W.L. Body Mass in Comparative Primatology. J. Hum. Evol. 1997, 32, 523–559. [Google Scholar] [CrossRef]
- Rowe, R. All the World’s Primates; Pogonias Press: Charlestown, MA, USA, 2011. [Google Scholar]
- Plavcan, J. Sexual Dimorphism in the Dentition of Extant Anthropoid Primates. Ph.D. Dissertation, University of Michigan, Ann Arbor, MI, USA, 1990. [Google Scholar]
- Thorén, S.; Lindenfors, P.; Kappeler, P.M. Phylogenetic Analyses of Dimorphism in Primates: Evidence for Stronger Selection on Canine Size than on Body Size. Am. J. Phys. Anthropol. 2006, 130, 50–59. [Google Scholar] [CrossRef]
- Lemos de Sá, R.M.; Glander, K.E. Capture Techniques and Morphometrics for the Woolly Spider Monkey, or Muriqui (Brachyteles arachnoides, E. Geoffroy 1806). Am. J. Primatol. 1993, 29, 145–153. [Google Scholar] [CrossRef]
- Ives, A.R.; Garland, T. Phylogenetic Logistic Regression for Binary Dependent Variables. Syst. Biol. 2010, 59, 9–26. [Google Scholar] [CrossRef]
- Garland, T.; Dickerman, A.W.; Janis, C.M.; Jones, J.A. Phylogenetic Analysis of Covariance by Computer Simulation. Syst. Biol. 1993, 42, 265–292. [Google Scholar] [CrossRef]
- Springer, M.S.; Meredith, R.W.; Gatesy, J.; Emerling, C.A.; Park, J.; Rabosky, D.L.; Stadler, T.; Steiner, C.; Ryder, O.A.; Janečka, J.E.; et al. Macroevolutionary Dynamics and Historical Biogeography of Primate Diversification Inferred from a Species Supermatrix. PLoS ONE 2012, 7, e49521. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C. The Comparative Approach in Evolutionary Anthropology and Biology; University of Chicago: Chicago, IL, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Detecting Correlated Evolution on Phylogenies: A General Method for the Comparative Analysis of Discrete Characters. Proc. R. Soc. Lond. B Biol. Sci. 1994, 255, 37–45. [Google Scholar] [CrossRef]
- Pennell, M.W.; Eastman, J.M.; Slater, G.J.; Brown, J.W.; Uyeda, J.C.; Fitzjohn, R.G.; Alfaro, M.E.; Harmon, L.J. Geiger v2.0: An Expanded Suite of Methods for Fitting Macroevolutionary Models to Phylogenetic Trees. Bioinformatics 2014, 30, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. Phytools: An R Package for Phylogenetic Comparative Biology (and Other Things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Stafford, B.J.; Szalay, F.S. Craniodental Functional Morphology and Taxonomy of Dermopterans. J. Mammal. 2000, 81, 360–385. [Google Scholar] [CrossRef]
- Kawamichi, T.; Kawamichi, M. Social Organization of Tree Shrews (Tupaia glis). In Primate Behavior and Sociobiology. Proceedings in Life Sciences; Chiarelli, A., Corruccini, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Emmons, L. Tupai: A Field Study of Bornean Treeshrews; University of California Press: Berkeley, CA, USA, 2000; Volume 2. [Google Scholar]
- Janecka, J.E.; Miller, W.; Pringle, T.H.; Wiens, F.; Zitzmann, A.; Helgen, K.M.; Springer, M.S.; Murphy, W.J. Molecular and Genomic Data Identify the Closest Living Relative of Primates. Science 2007, 318, 792–794. [Google Scholar] [CrossRef]
- Kumar, S.; Suleski, M.; Craig, J.M.; Kasprowicz, A.E.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Mol. Biol. Evol. 2022, 39, msac174. [Google Scholar] [CrossRef]
- Roberts, T.E.; Lanier, H.C.; Sargis, E.J.; Olson, L.E. Molecular Phylogeny of Treeshrews (Mammalia: Scandentia) and the Timescale of Diversification in Southeast Asia. Phylogenetics Evol. 2011, 60, 358–372. [Google Scholar] [CrossRef]
- Fleagle, J.G.; Kay, R.F.; Simons, E.L. Sexual Dimorphism in Early Anthropoids. Nature 1980, 287, 328–330. [Google Scholar] [CrossRef]
- Kay, R. Sexual Dimorphism in Ramapithecinae. Proc. Natl. Acad. Sci. USA 1982, 79, 209–212. [Google Scholar] [CrossRef]
- Benefit, B. The Permanent Dentition and Phylogenetic Position of Victoriapithecus from Maboko Island, Kenya. J. Hum. Evol. 1993, 25, 83–172. [Google Scholar] [CrossRef]
- Walker, A.; Teaford, M.F.; Martin, L.; Andrews, P. A New Species of Proconsul from the Early Miocene of Rusinga/Mfangano Islands, Kenya. J. Hum. Evol. 1993, 25, 43–56. [Google Scholar] [CrossRef]
- Simons, E.L.; Rasmussen, D.T.; Gingerich, P.D. New Cercamoniine Adapid from Fayum, Egypt. J. Hum. Evol. 1995, 29, 577–589. [Google Scholar] [CrossRef]
- Simons, E.L.; Seiffert, E.R.; Ryan, T.M.; Attia, Y. A Remarkable Female Cranium of the Early Oligocene Anthropoid Aegyptopithecus zeuxis (Catarrhini, Propliopithecidae). Proc. Natl. Acad. Sci. USA 2007, 104, 8731–8736. [Google Scholar] [CrossRef]
- Schrein, C.M. Metric Variation and Sexual Dimorphism in the Dentition of Ouranopithecus macedoniensis. J. Hum. Evol. 2006, 50, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Gunnell, G.F.; Rose, K.D. Tarsiiformes: Evolutionary History and Adaptation. In The Primate Fossil Record.; Hartwig, W.C., Ed.; Cambridge University: Cambridge, UK, 2002; pp. 45–82. [Google Scholar]
- Coupar, C. Dental Variation in Omomys carteri. Master’s Thesis, California State University, Sacramento, CA, USA, 1996. [Google Scholar]
- Boyer, D.M.; Seiffert, E.R. Patterns of Astragalar Fibular Facet Orientation in Extant and Fossil Primates and Their Evolutionary Implications. Am. J. Phys. Anthropol. 2013, 151, 420–447. [Google Scholar] [CrossRef]
- Boyer, D.M.; Seiffert, E.R.; Gladman, J.T.; Bloch, J.I. Evolution and Allometry of Calcaneal Elongation in Living and Extinct Primates. PLoS ONE 2013, 8, e67792. [Google Scholar] [CrossRef]
- Seiffert, E.; Costeur, L.; Boyer, D. Primate Tarsal Bones from Egerkingen, Switzerland, Attributable to the Middle Eocene Adapiform Caenopithecus lemuroides. PeerJ 2015, 3, e1036. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, P.D.; Smith, B.H.; Rosenberg, K. Allometric Scaling in the Dentition of Primates and Prediction of Body Weight from Tooth Size in Fossils. Am. J. Phys. Anthropol. 1982, 58, 81–100. [Google Scholar] [CrossRef]
- Campione, N.E.; Evans, D.C. A Universal Scaling Relationship between Body Mass and Proximal Limb Bone Dimensions in Quadrupedal Terrestrial Tetrapods. BMC Biol. 2012, 10, 60. [Google Scholar] [CrossRef]
- Harmon, L.J.; Weir, J.T.; Brock, C.D.; Glor, R.E.; Challenger, W. GEIGER: Investigating Evolutionary Radiations. Bioinformatics 2008, 24, 129–131. [Google Scholar] [CrossRef]
- Chase, I.D.; Seitz, K. Self-Structuring Properties of Dominance Hierarchies. A New Perspective. In Advances in Genetics; Academic Press Inc.: New York, NY, USA, 2011; Volume 75, pp. 51–81. [Google Scholar]
- Hand, J. Resolution of Social Conflicts: Dominance, Egalitarianism, Spheres of Dominance, and Game Theory. Q. Rev. Biol. 1986, 61, 201–220. [Google Scholar] [CrossRef]
- de Waal, F.B.M. The Integration of Dominance and Social Bonding in Primates. Source Q. Rev. Biol. 1986, 61, 459–479. [Google Scholar] [CrossRef]
- Lewis, R.J. Subordination Signals Improve the Quality of Social Relationships in Verreaux’s Sifaka: Implications for the Evolution of Power Structures and Social Complexity. Am. J. Phys. Anthropol. 2019, 169, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Leedom, L.J.; Muhtadie, L. The Dominance Behavioral System and Psychopathology: Evidence from Self-Report, Observational, and Biological Studies. Psychol. Bull. 2012, 138, 692–743. [Google Scholar] [CrossRef]
- van Hooff, J.; van Schaik, C. Cooperation in Competition: The Ecology of Primate Bonds. In Coalitions and Alliances in Humans and Other Animals; Harcourt, A., de Waal, F., Eds.; Oxford University: Oxford, UK, 1992; pp. 357–389. [Google Scholar]
- Lewis, R. Male-Female Relationships in Sifaka (Propithecus verreauxi verreauxi): Power, Conflict, and Cooperation. Ph.D. Dissertation, Duke University, Durham, NC, USA, 2004. [Google Scholar]
- Plavcan, J. Sexual Dimorphism in Primate Evolution. Yearb. Phys. Anthropol. 2001, 116, 25–53. [Google Scholar] [CrossRef]
- Gordon, A.D. Scaling of Size and Dimorphism in Primates I: Microevolution. Int. J. Primatol. 2006, 27, 27–61. [Google Scholar] [CrossRef]
- Fairburn, D.J.; Blanckenhorn, W.U.; Székely, T. Sex, Size & Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Oxford University: Oxford, UK, 2007. [Google Scholar]
- Andersson, M. Sexual Selection; Princeton Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Carlson, A.A.; Isbell, L.A. Causes and Consequences of Single-Male and Multimale Mating in Free-Ranging Patas Monkeys, Erythrocebus patas. Anim. Behav. 2001, 62, 1047–1058. [Google Scholar] [CrossRef]
- Di Fiore, A.; Rendall, D. Evolution of Social Organization: A Reappraisal for Primates by Using Phylogenetic Methods. Proc. Natl. Acad. Sci. USA 1994, 91, 9941–9945. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.E.; Thalmann, U. Origin and Evolution of Primate Social Organisation: A Reconstruction. Biol. Rev. 2000, 75, 405–435. [Google Scholar] [CrossRef] [PubMed]
- Hinde, R.; Stevenson-Hinde, J. Towards Understanding Relationships: Dynamic Stability. In Growing Points in Ethology; Bateson, P., Hinde, R., Eds.; Cambridge University: Cambridge, UK, 1976; pp. 451–479. [Google Scholar]
- Rowell, T.E.; Chism, J. Sexual Dimorphism and Mating Systems: Jumping to Conclusions. Hum. Evol. 1986, 1, 215–219. [Google Scholar] [CrossRef]
- Ford, S.M. Evolution of Sexual Dimorphism in Body Weight in Platyrrhines. Am. J. Primatol. 1994, 34, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Dunham, A.E. Battle of the Sexes: Cost Asymmetry Explains Female Dominance in Lemurs. Anim. Behav. 2008, 76, 1435–1439. [Google Scholar] [CrossRef]
- Batista, G.; Zubizarreta, L.; Perrone, R.; Silva, A. Non-Sex-Biased Dominance in a Sexually Monomorphic Electric Fish: Fight Structure and Submissive Electric Signalling. Ethology 2012, 118, 398–410. [Google Scholar] [CrossRef]
- Young, A.; Bennett, N. Intra-Sexual Selection in Cooperative Mammals and Birds: Why Are Females Not Bigger and Better Armed? Philos. Trans. R. Soc. B 2013, 368, 20130075. [Google Scholar] [CrossRef]
- Saccà, T.; Gort, G.; van de Waal, E.; Hemelrijk, C.K. Male Intrasexual Aggression and Partial Dominance of Females over Males in Vervet Monkeys. Front. Ecol. Evol. 2022, 10, 930266. [Google Scholar] [CrossRef]
- Hemelrijk, C.K.; Wubs, M.; Gort, G.; Botting, J.; van de Waal, E. Dynamics of Intersexual Dominance and Adult Sex-Ratio in Wild Vervet Monkeys. Front. Psychol. 2020, 11, 839. [Google Scholar] [CrossRef]
- Szykman, M.; Engh, A.; Van Horn, R.; Funk, S.; Scribner, K.; Holekamp, K. Association Patterns among Male and Female Spotted Hyenas (Crocuta crocuta) Reflect Male Mate Choice. Behav. Ecol. Sociobiol. 2001, 50, 231–238. [Google Scholar] [CrossRef]
- Swanson, E.M.; McElhinny, T.L.; Dworkin, I.; Weldele, M.L.; Glickman, S.E.; Holekamp, K.E. Ontogeny of Sexual Size Dimorphism in the Spotted Hyena (Crocuta crocuta). J. Mammal. 2013, 94, 1298–1310. [Google Scholar] [CrossRef]
- Silk, J.B. Primatological Perspectives on Gender Hierarchies. In Gender Hierarchies; Miller, B., Ed.; Cambridge University Press: Cambridge, UK, 1993; pp. 212–235. [Google Scholar]
- Wasser, S.; Waterhouse, M. The Establishment and Maintenance of Sex Biases. In Social Behavior of Female Vertebrates; Wasser, S., Ed.; Academic Press: New York, NY, USA, 1983; pp. 19–35. [Google Scholar]
- Hare, B.; Wobber, V.; Wrangham, R. The Self-Domestication Hypothesis: Evolution of Bonobo Psychology Is due to Selection against Aggression. Anim. Behav. 2012, 83, 573–585. [Google Scholar] [CrossRef]
- Wang, E.; Milton, K. Intragroup Social Relationships of Male Alouatta palliata on Barro Colorado Island, Republic of Panama. Int. J. Primatol. 2002, 24, 1227–1243. [Google Scholar] [CrossRef]
- Di Fiore, A.; Campbell, C.J. The Atelines: Variation in Ecology, Behavior, and Social Organization. In Primates in Perspective; Campbell, C., Fuentes, A., MacKinnin, K., Panger, M., Bearder, S., Eds.; Oxford University: New York, NY, USA, 2007; pp. 155–185. [Google Scholar]
- Wright, P.C. Variations in Male-Female Dominance and Offspring Care in Non-Human Primates. In Sex and Gender Hierarchies; Miller, B., Ed.; Cambridge University: Cambridge, UK, 1993; pp. 127–145. [Google Scholar]
- Milton, K. Habitat, Diet, and Activity Patterns of Free-Ranging Woolly Spider Monkeys (Brachyteles arachnoides E. Geoffroy 1806). Int. J. Primatol. 1984, 5, 491–514. [Google Scholar] [CrossRef]
- Fontaine, R.; DuMond, F. The Red Uakari in Seminatural Environment: Potentials for Propagation and Studies. In Primate Conservation; Rainer, P., Bourne, G., Eds.; Academic Press: New York, NY, USA, 1977; pp. 167–236. [Google Scholar]
- Wright, P. Biparental Care in Aotus trivirgatus and Callicebus moloch. In Female Primates: Studies by Women Primatologists; Small, M., Ed.; Alan R. Liss: New York, NY, USA, 1984; pp. 59–75. [Google Scholar]
- Kinzey, W. Callicebus. In New World Primates: Ecology, Evolution, and Behavior; Kinzey, W., Ed.; Aldine de Gruyter: New York, NY, USA, 1997; pp. 213–221. [Google Scholar]
- Hartmut, R.; Darns, K. The Social Organization of Marmosets: A Critical Evaluation of Recent Concepts. In Marmosets and Tamarins: Systematics, Behavior, and Ecology; Rylands, A., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 176–199. [Google Scholar]
- Soini, P. The Pygmy Marmoset, Genus Cebuella. In Ecology and Behavior of Neotropical Primates; Mittermeier, R., Coimbra-Filho, A., da Fonseca, G., Eds.; World Wildly Fund: Washington, DC, USA, 1988; Volume 2, pp. 79–129. [Google Scholar]
- Freese, C.; Oppenheimer, J. The Capuchin Monkeys, Genus Cebus. In Ecology and Behavior of Neotropical Primates; Coimbra-Fliho, A., Mittermeier, R., Eds.; Academia Brasileira de Ciencias: Rio de Janeiro, Brazil, 1981; Volume 1, pp. 331–389. [Google Scholar]
- Fragaszy, D.; Visalberghi, E.; Fedigan, F. The Complete Capuchin: The Biology of the Genus Cebus; Cambridge University: Cambridge, UK, 2004. [Google Scholar]
- Perry, S. Male-Female Social Relationships in Wild White-Faced Capuchins (Cebus capucinus). Behaviour 1997, 134, 477–510. [Google Scholar] [CrossRef]
- Gust, T. Moving up the Dominance Hierarchy in Young Sooty Mangabeys. Anim. Behav. 1995, 50, 15–21. [Google Scholar] [CrossRef]
- Hunkler, C.; Bourlière, F.; Bertrand, M. Le Comportement Social de La Mone de Lowe (Cercopithecus campbelli lowei). Folia Primatol. 1972, 17, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Oates, J.F. The Social Life of a Black-and-white Colobus Monkey, Colobus guereza. Z. Tierpsychol. 1977, 45, 1–60. [Google Scholar] [CrossRef]
- Kappeler, P. Lemur Social Structure and Convergence. In Comparative Primate Socioecology; Lee, P., Ed.; Cambridge University: Cambridge, UK, 1999; pp. 271–2999. [Google Scholar]
- Bayart, F.; Simmen, B. Demography, Range Use, and Behavior in Black Lemurs (Eulemur macaco macaco) at Ampasikely, Northwest Madagascar. Am. J. Primatol. 2005, 67, 299–312. [Google Scholar] [CrossRef]
- Curtis, D.; Zaramody, A. Social Structure and Seasonal Variation in the Behaviour of Eulemur mongoz. Folia Primatol. 1999, 70, 79–96. [Google Scholar] [CrossRef]
- Overdorff, D.J.; Merenlender, A.M.; Talata, P.; Telo, A.; Forward, Z.A. Life History of Eulemur fulvus rufus From 1988–1998 in Southeastern Madagascar. Yearb. Phys. Anthropol. 1999, 108, 295–310. [Google Scholar] [CrossRef]
- Mitchell, G. Behavioral Sex Differences in Nonhuman Primates; Van Nostrand Rheinhold: New York, NY, USA, 1979. [Google Scholar]
- Robbins, M. Gorillas: Diversity in Ecology and Behavior. In Primates in Perspective; Campbell, C., Fuentes, A., MacKinnon, K., Panger, M., Bearder, S., Eds.; Oxford University: Oxford, UK, 2007; pp. 305–320. [Google Scholar]
- Grassi, C. The Behavioral Ecology of Hapalemur griseus griseus: The Influences of Microhabitat and Population Density on This Small-Bodied Folivore (Madagascar). Ph.D. Dissertation, University of Texas-Austin, Austin, TX, USA, 2002. [Google Scholar]
- Leighton, D.R. Gibbons: Territoriality and Monogamy. In Primate Societies; Smuts, B., Cheney, D., Seyfarth, R., Wrangham, R., Struhsaker, T., Eds.; University of Chicago: Chicago, IL, USA, 1987; pp. 135–145. [Google Scholar]
- Gittins, S.P.; Raemaekers, J.J. Siamang, Lar, and Agile Gibbons. In Malayan Forest Primates: Ten Years’ Study in Tropical Rainforest; Chivers, D., Ed.; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Pollock, J. Female Dominance in Indri indri. Folia Primatol. 1979, 31, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Jolly, A. Lemur Social Behavior and Primate Intelligence: The Step from Prosimian to Monkey Intelligence Probably Took Place in a Social Context. Science 1966, 153, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Bales, K.; Dietz, J. A New “Founder Effect”—Establishment of Dominance in Wild Golden Lion Tarmarins Groups. Am. J. Primatol. 1999, 49, 31. [Google Scholar]
- Schulze, H.; Meier, B. Behavior of Captive Loris tardigradus nordicus: A Qualitative Description, Including Some Information about Morphological Bases of Behavior. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Gerald, A., Doyle, G., Izard, M., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 221–249. [Google Scholar]
- Sterck, E.H.M.; Steenbeek, R. Female Dominance Relationships and Food Competition in the Sympatric Thomas Langur and Long-Tailed Macaque. Behaviour 1997, 134, 749–774. [Google Scholar] [CrossRef]
- Chaffin, C.; Friedlen, K.; de Waal, F. Dominance Style of Japanese Macaques Compared with Rhesus and Stumptail Macaques. Am. J. Primatol. 1995, 35, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Chapais, B. Reproductive Activity in Relation to Male Dominance and the Likelihood of Ovulation in Rhesus Monkeys. Behav. Ecol. Sociobiol. 1983, 12, 215–228. [Google Scholar] [CrossRef]
- Oi, T. Patterns of Dominance and Affiliation in Wild Pig-Tailed Macaques (Macaca nemestrina nemestrina) in West Sumatra. Int. J. Primatol. 1990, 11, 339–356. [Google Scholar] [CrossRef]
- Koyama, N. Dominance, Grooming, and Clasped-Sleeping Relationships among Bonnet Monkeys in India. Primates 1973, 14, 225–244. [Google Scholar] [CrossRef]
- Berman, C.M.; Ionica, C.S.; Li, J. Dominance Style among Macaca thibetana on Mt. Huangshan, China. Int. J. Primatol. 2004, 25, 1283–1312. [Google Scholar] [CrossRef]
- Setchell, J.M.; Vaglio, S.; Moggi-Cecchi, J.; Boscaro, F.; Calamai, L.; Knapp, L.A. Chemical Composition of Scent-Gland Secretions in an Old World Monkey (Mandrillus sphinx): Influence of Sex, Male Status, and Individual Identity. Chem. Senses 2010, 35, 205–220. [Google Scholar] [CrossRef]
- White, F.J.; Wood, K.D. Female Feeding Priority in Bonobos, Pan paniscus, and the Question of Female Dominance. Am J Primatol. 2007, 69, 837–850. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Hsu, M. Occurrence of Infanticide among Wild Proboscis Monkeys (Nasalis larvatus) in Sabah, Northern Borneo. Folia Primatol. 2005, 76, 177–179. [Google Scholar] [CrossRef]
- Hager, R.; Welker, C. Female Dominance in African Lorises (Otolemur garnettii). Folia Primatol. 2001, 72, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Surbeck, M.; Hohmann, G. Intersexual Dominance Relationships and the Influence of Leverage on the Outcome of Conflicts in Wild Bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 2013, 67, 1767–1780. [Google Scholar] [CrossRef]
- Stumpf, R. Chimpanzees and Bonobos: Diversity within and between Species. In Primates in Perspective; Campbell, C., Fuentes, A., MacKinnon, K., Panger, M., Bearder, S., Eds.; Oxford University: Oxford, UK, 2007; pp. 321–344. [Google Scholar]
- Strum, S. Almost Human: A Journey into the World of Baboons; W. W. Norton: New York, UK, USA, 1987. [Google Scholar]
- Hausfater, G. Dominance and Reproduction in Baboons (Papio cynocephalus): A Quantitative Analysis; S. Karger: Basel, Switzerland, 1975; ISBN 3805521391. [Google Scholar]
- Crook, J. Primate Societies and Individual Behaviour. J. Psychosom. Res. 1968, 12, 11–19. [Google Scholar] [CrossRef]
- Maestripieri, D.; Mayhew, J.; Carlson, C.L.; Hoffman, C.L.; Radtke, J.M. One-Male Harems and Female Social Dynamics in Guinea Baboons. Folia Primatol. 2006, 78, 56–68. [Google Scholar] [CrossRef]
- Kitchen, D.M.; Beehner, J.C.; Bergman, T.J.; Cheney, D.L.; Crockford, C.; Engh, A.L.; Fischer, J.; Seyfarth, R.M.; Wittig, R.M. The Causes and Consequences of Male Aggression Directed at Female Chacma Baboons. In Sexual Coercion in Primates and Humans: An Evolutionary Perspective on Male Aggression against Females; Muller Mnwrangham, R.W., Ed.; Harvard University Press: Cambridge, MA, USA, 2009; pp. 128–156. [Google Scholar]
- Struhsaker, T. The Red Colobus Monkey; University of Chicago: Chicago, IL, USA, 1975. [Google Scholar]
- Pochron, S.T.; Fitzgerald, J.; Gilbert, C.C.; Lawrence, D.; Grgas, M.; Rakotonirina, G.; Ratsimbazafy, R.; Rakotosoa, R.; Wright, P.C. Patterns of Female Dominance in Propithecus diadema edwardsi of Ranomafana National Park, Madagascar. Am. J. Primatol. 2003, 61, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.F.; Nicoll, M.E. Female Social Dominance and Basal Metabolism in a Malagasy Primate, Propithecus verreauxi. Am. J. Primatol. 1987, 12, 309–314. [Google Scholar] [CrossRef]
- Lippold, L.K. The Douc Langur: A Time for Conservation. In Primate Conservation; Prince Rainier, H., Bourne, G., Eds.; Academic Press: New York, NY, USA, 1977; pp. 513–538. [Google Scholar]
- Epple, G. Notes of the Establishment and Maintenance of the Pair Bond in Saguinus fuscicollis. In The Biology and Conservation of the Callitrichidae; Kleiman, K., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1977; pp. 23–38. [Google Scholar]
- Mitchell, C. The Ecological Basis for Female Social Dominance: A Behavioral Study of the Squirrel Monkey (Saimiri sciureus) in the Wild. Ph.D. Dissertation, Princeton University, Princeton, NJ, USA, 1990. [Google Scholar]
- Boinski, S.; Sughrue, K.; Selvaggi, L.; Quatrone, R.; Henry, M.; Cropp, S. An Expanded Test of the Ecological Model of Primate Social Evolution: Competitive Regimes and Female Bonding in Three Species of Squirrel Monkeys (Saimiri oerstedii, S. boliviensis and S.sciureus). Behaviour 2002, 139, 227–261. [Google Scholar] [CrossRef]
- Mendoza, S.; Lowe, E.; Levine, S. Social Organization and Social Behavior in Two Subspecies of Squirrel Monkeys (Saimiri sciureus). Folia Primatol. 1978, 30, 126–144. [Google Scholar] [CrossRef]
- Hrdy, S. The Langurs of Abu; Harvard University Press: Cambridge, UK, 1977. [Google Scholar]
- Bernstein, I. Activity Patterns in a Gelada Monkey Group. Folia Primatol. 1975, 23, 50–71. [Google Scholar] [CrossRef]
- Fedigan, L. Primate Paradigms: Sex Roles and Social Bonds; University of Chicago: Chicago, IL, USA, 1992. [Google Scholar]
- Raps, S.; White, F.J. Female Social Dominance in Semi-Free-Ranging Ruffed Lemurs (Varecia variegata). Folia Primatol. 1995, 65, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, P.D. New Species of Eocene Primates and Phylogeny of European Adapidae. Folia Primatol. 1977, 28, 60–80. [Google Scholar] [CrossRef]
- International Commission on Stratigraphy. 2017. Available online: https://Stratigraphy.org (accessed on 21 September 2023).
- Gingerich, P.D. Early Eocene Cantius torresi—Oldest Primate of Modern Aspect from North America. Nature 1986, 319, 319–321. [Google Scholar] [CrossRef]
- Gradstein, F.M.; Ogg, J.G.; Smith, A.G.; Agterberg, F.; Bleeker, W.; Cooper, R.; Davydov, V.; Gibbard, P.; Hinnov, L.; House, M.R.; et al. A Geologic Time Scale; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Gunnell, G.F. Notharctine Primates (Adapiformes) from the Early to Middle Eocene (Wasatchian-Bridgerian) of Wyoming: Transitional Species and the Origins of Notharctus and Smilodectes. J. Hum. Evol. 2002, 43, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, E.R.; Simons, E.L.; Boyer, D.M.; Perry, J.M.G.; Ryan, T.M.; Sallam, H.M. A Fossil Primate of Uncertain Affinities from the Earliest Late Eocene of Egypt. Proc. Natl. Acad. Sci. USA 2010, 107, 9712–9717. [Google Scholar] [CrossRef]
- Vizcaíno, S.F.; Bargo, M.S.; Kay, R.F.; Fariña, R.A.; Di Giacomo, M.; Perry, J.M.G.; Prevosti, F.J.; Toledo, N.; Cassini, G.H.; Fernicola, J.C. A Baseline Paleoecological Study for the Santa Cruz Formation (Late-Early Miocene) at the Atlantic Coast of Patagonia, Argentina. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 292, 507–519. [Google Scholar] [CrossRef]
- Smith, T.; Rose, K.D.; Gingerich, P.D.; Sabloff, J.A. Rapid Asia-Europe-North America Geographic Dispersal of Earliest Eocene Primate Teilhardina during the Paleocene-Eocene Thermal Maximum. Proc. Natl. Acad. Sci. USA 2006, 103, 11223–11227. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, P.D. Cranial Morphology and Adaptations in Eocene Adapidae. I. Sexual Dimorphism in Adapis magnus and Adapis parisiensis. Am. J. Phys. Anthropol. 1981, 56, 217–234. [Google Scholar] [CrossRef]
- Gingerich, P. Sexual Dimorphism in Earliest Eocene Cantius torresi (Mammalia, Primates, Adapoidea). In Contributions from the Museum of Paleontology; University of Michigan: Ann Arbor, MI, USA, 1995; Volume 29, pp. 185–199. [Google Scholar]
- Krishtalka, L.; Stucky, R.K.; Christopher Beard, K. The Earliest Fossil Evidence for Sexual Dimorphism in Primates. Proc. Natl. Acad. Sci. USA 1990, 87, 5223–5226. [Google Scholar] [CrossRef] [PubMed]
- Simons, E.L.; Plavcan, P.J.; Fleagle, J.G. Canine Sexual Dimorphism in Egyptian Eocene Anthropoid Primates: Catopithecus and Proteopithecus. Proc. Natl. Acad. Sci. USA 1999, 96, 2559–2562. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.; Perry, J.; Malinzak, M.; Allen, K.; Kirk, E.; Plavcan, J.; Fleagle, J. The Paleobiology of Santacrucian Primates. In Early Miocene Paleobiology in Patagonia: High-Latitude Paleocommunities of the Santa Cruz Formation; Vizcaino, S., Kay, R., Bargo, M., Eds.; Cambridge University: Cambridge, UK, 2012; pp. 306–330. [Google Scholar]



| Power Category * | Body Mass Ratio (BMR) | Canine Ratio (CR) | Sex Ratio (SR) | ||||
|---|---|---|---|---|---|---|---|
| Node (LCA) | Scaled Likelihood of Male Power | Predicted Probability of Male Power | MLE of Reconstructed Body Mass Ratio | Predicted Probability of Male Power | MLE of Reconstructed Canine Ratio | Predicted Probability of Male Power | MLE of Reconstructed Sex Ratio |
| Primates | 0.535 | 0.58 (0.55–0.60) | 1.14 (1.00–1.30) | 0.73 (0.67–0.78) | 1.18 (0.92–1.51) | 0.33 (0.44–0.23) | 0.67 (0.48–0.94) |
| Strepsirrhini | 0.447 | 0.58 (0.55–0.60) | 1.14 (1.00–1.30) | 0.71 (0.66–0.76) | 1.09 (0.89–1.34) | 0.31 (0.42–0.22) | 0.70 (0.50–0.98) |
| Haplorhini # | 0.579 | 0.58 (0.55–0.60) | 1.14 (1.00–1.30) | ||||
| Lemuriformes | 0.018 | 0.57 (0.54–0.59) | 1.10 (0.95–1.27) | 0.71 (0.66–0.75) | 1.05 (0.87–1.28) | 0.28 (0.37–0.20) | 0.79 (0.58–1.09) |
| Lorisiformes | 0.569 | 0.57 (0.55–0.60) | 1.14 (0.98–1.32) | 0.70 (0.66–0.75) | 1.05 (0.88–1.25) | 0.32 (0.45–0.21) | 0.69 (0.46–1.04) |
| Anthropoidea | 0.844 | 0.58 (0.56–0.60) | 1.16 (1.02–1.32) | 0.75 (0.70–0.79) | 1.27 (1.03–1.57) | 0.36 (0.46–0.28) | 0.60 (0.45–0.79) |
| Platyrrhini | 0.876 | 0.58 (0.55–0.60) | 1.15 (1.01–1.31) | 0.74 (0.70–0.78) | 1.23 (1.03–1.45) | 0.35 (0.43–0.27) | 0.63 (0.49–0.81) |
| Catarrhini | 0.950 | 0.59 (0.57–0.62) | 1.27 (1.09–1.48) | 0.78 (0.73–0.81) | 1.44 (1.18–1.77) | 0.40 (0.49–0.31) | 0.54 (0.41–0.70) |
| Cercopithecoidea | 0.998 | 0.61 (0.58–0.63) | 1.38 (1.18–1.61) | 0.81 (0.77–0.84) | 1.68 (1.38–2.06) | 0.44 (0.53–0.35) | 0.48 (0.37–0.61) |
| Hominoidea | 0.927 | 0.61 (0.58–0.63) | 1.36 (1.15–1.61) | 0.77 (0.73–0.81) | 1.40 (1.15–1.70) | 0.39 (0.50–0.30) | 0.54 (0.40–0.73) |
| Dataset | Independent Variable | Estimate | SE | t | Bootstrapped p-Value | Bootstrapped Mean of the Intercept (b0) | Bootstrapped Mean of Independent Variable (b1) |
|---|---|---|---|---|---|---|---|
| All primates | Body mass dimorphism a | 1.573 | 0.436 | 3.612 | *** | 0.2134 | 1.6228 |
| Canine length dimorphism a | 2.678 | 0.636 | 4.214 | *** | 0.8117 | 2.7104 | |
| Expected estrous overlap b | 0.773 | 0.339 | 2.277 | * | −0.5840 | 0.9596 | |
| Sex ratio a | −3.210 | 0.865 | 3.711 | *** | −1.2963 | −3.2791 | |
| Excluding extremely | Expected estrous overlap b | 1.221 | 0.499 | 2.445 | ** | −0.6490 | 1.2333 |
| dimorphic taxa c | Sex ratio a | −2.946 | 0.954 | 3.087 | *** | −1.2069 | −3.2538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, R.J.; Kirk, E.C.; Gosselin-Ildari, A.D. Evolutionary Patterns of Intersexual Power. Animals 2023, 13, 3695. https://doi.org/10.3390/ani13233695
Lewis RJ, Kirk EC, Gosselin-Ildari AD. Evolutionary Patterns of Intersexual Power. Animals. 2023; 13(23):3695. https://doi.org/10.3390/ani13233695
Chicago/Turabian StyleLewis, Rebecca J., E. Christopher Kirk, and Ashley D. Gosselin-Ildari. 2023. "Evolutionary Patterns of Intersexual Power" Animals 13, no. 23: 3695. https://doi.org/10.3390/ani13233695
APA StyleLewis, R. J., Kirk, E. C., & Gosselin-Ildari, A. D. (2023). Evolutionary Patterns of Intersexual Power. Animals, 13(23), 3695. https://doi.org/10.3390/ani13233695







