Simple Summary
The flat mite family Tenuipalpidae includes 41 genera and more than 1100 species worldwide, and is considered one of the most important families of phytophagous mites. The Ultratenuipalpus is a small genus with 25 known species present in almost all zoogeographic regions. Here, a new species Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. is described from specimens collected on ferns from Brazil. It represents the first species of the genus described from the country. The type species of the genus Ultratenuipalpus meekeri (De Leon) is redescribed based on types and newly collected material from Mexico. Highly detailed low-temperature scanning electron image (LT-SEM) micrographs and DNA barcodes are provided for both species. The taxonomy of the genus Ultratenuipalpus and the ontogenetic additions of leg setae are discussed.
Abstract
Species of the genus Ultratenuipalpus bear a broad subquadrate propodosoma with many large, flattened, lanceolate to ovate dorsal setae. They also bear some plesiomorphic character states, such as the presence of three pairs of ventral ps setae. Here, we describe Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. based on adult females, males, and immatures, collected on ferns from Brazil. We also re-describe Ultratenuipalpus meekeri (De Leon), the type species of the genus, based on types and newly collected material from Mexico, and include additional novel data (e.g., dorsal and ventral ornamentation, leg chaetotaxy, and setal measurements) in a standardized form. We include highly detailed images obtained using LT-SEM, accompanied by DNA barcodes, for both species. The ontogenetic additions of leg chaetotaxy are presented and discussed.
1. Introduction
The Ultratenuipalpus Mitrofanov is a small genus of the family Tenuipalpidae (Trombidiformes: Tetranychoidea), with 25 known species to date [1,2,3]. Most species are described from three countries: New Zealand (10 species), Australia (3), and Chile (3) [3,4]. Species of the genus bear a broad subquadrate propodosoma with many large, flattened, lanceolate, and/or obovate to ovate dorsal setae [2]. They also bear some potentially plesiomorphic character states, such as the presence of three pairs of pseudanal setae (ps1–ps3) and the absence of genital plates [1,2], which are both shared across the Tetranychoidea [5].
The Ultratenuipalpus shares many character states with the genera Extenuipalpus and Tenuipalpus, such as having the prodorsum broader than the opisthosoma [2,6]. The Ultratenuipalpus and Extenuipalpus also share the character of dorsal opisthosomal setae h2 not being flagellate; the Ultratenuipalpus and Tenuipalpus sensu stricto share the presence of lateral body projections associated with prodorsal setae sc2 [2,6].
The genus Extenuipalpus was recently reinstated and includes only three species described from South Africa [2,3], while Tenuipalpus is the largest genus in the flat mite family, with over 300 described species worldwide [3]. According to Beard et al. [2], the Ultratenuipalpus and these two genera are closely allied, and the Extenuipalpus may occupy a position intermediate between the Ultratenuipalpus and Tenuipalpus.
Species of the Ultratenuipalpus occur on different families of ferns (e.g., Dennstaedtiaceae, Thelypteridaceae), conifers (e.g., Araucariaceae, Podocarpaceae), monocots (e.g., Arecaceae, Asteliaceae), and dicots plants (e.g., Proteaceae, Rubiaceae) [3,4]. While most species have only ever been recorded from the original host plant, there are some species (e.g., U. aberrans, U. coprosmae) that have been found on multiple plants of different families.
Here, we describe a new species of the Ultratenuipalpus collected on ferns from Brazil and re-describe the type species of the genus, U. meekeri (De Leon), in a standardized form. As these two species are morphologically similar and share several character states, including the shape of dorsal setae and chaetotaxy of the legs, molecular analyses were undertaken to confirm their separation using material freshly collected from Brazil and Mexico.
2. Materials and Methods
A portion of the samples collected of each species was maintained in 70% ethanol for subsequent use in low temperature SEM (LT-SEM) studies. Mites for LT-SEM were studied using the previously described methodology [7]. Another portion of the samples of each species was maintained in 100% alcohol for a subsequent molecular analysis.
DNA was extracted from individual mites using the QIAGEN DNeasy Blood & Tissue kits following standard protocols with the following exceptions: (1) mite specimens were carefully pierced with a sterilized minutin pin and then incubated overnight in a solution of buffer ATL and Proteinase K as per instructions and (2) a final elution was performed with 100 μL to increase the total DNA concentration. A portion of cytochrome oxidase I was amplified by PCR with previously published primers [8,9]. The amplification reactions were performed in 25 μL volumes containing 2.5 μL of manufacturer supplied buffer, 0.2 μL (5 units) of Platinum Taq polymerase (Invitrogen), 2.5 μL dNTP (0.25 mM of each base), 1 μL of each primer (10 mM), 2.5 μL of MgCl2 (25 mM), 11.3 μL of ddH2O, and 4 μL of the template DNA. The samples were denatured at 94 °C for 2 min, followed by 30 cycles of 1 min denaturation at 92 °C, 1 min annealing at 50 °C, and 1.5 min extension at 72 °C, with a final elongation of 10 min after the completion of all cycles. PCR products were visualized on a 1% agarose gel saturated with GelRed (Biotium, Hayward, CA, USA). DNA was then purified with a QIAquick PCR Purification kit (Qiagen, Germantown, MD, USA). The amplified fragments were sent to Macrogen USA for sequencing. No additional primers were used for sequencing. COI sequences have been deposited in GenBank (https://www.ncbi.nlm.nih.gov/, accessed on 15 January 2023).
All measurements are given in micrometers (μm). Measurements are presented for the holotype followed by the range for all types in parentheses. The number of leg setae is written as the total number of setae followed by the number of solenidia in parentheses. Terminology of leg and body setation is adapted from [10,11,12]. Photographs of slide-mounted specimens were obtained using a Zeiss Axioscope™ microscope (Carl Zeiss Inc., Thornwood, NY, USA) with a differential interference contrast (DIC) 100× Plan Apochromatic objective with an NA 1.4.
Type specimens and vouchers of non-type specimens are deposited in the Collection of Acari, Departamento de Zoologia e Botânica, UNESP, São José do Rio Preto, State of São Paulo, Brazil (DZSJRP, http://www.splink.cria.org.br, accessed on 10 December 2022) and in the National Insect and Mite Collection, National Museum of Natural History, Smithsonian Institution, located at the Systematic Entomology Laboratory (SEL), USDA, Beltsville, MD, USA (NMNH). The holotype of U. meekeri is deposited in the Museum of Comparative Zoology (MCZ), Harvard University, Cambridge, MA, USA.
3. Results
3.1. Description of Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov.
- Family Tenuipalpidae Berlese, 1913
- Genus Ultratenuipalpus Mitrofanov, 1973
- Type species: Ultratenuipalpus meekeri (De Leon), 1957
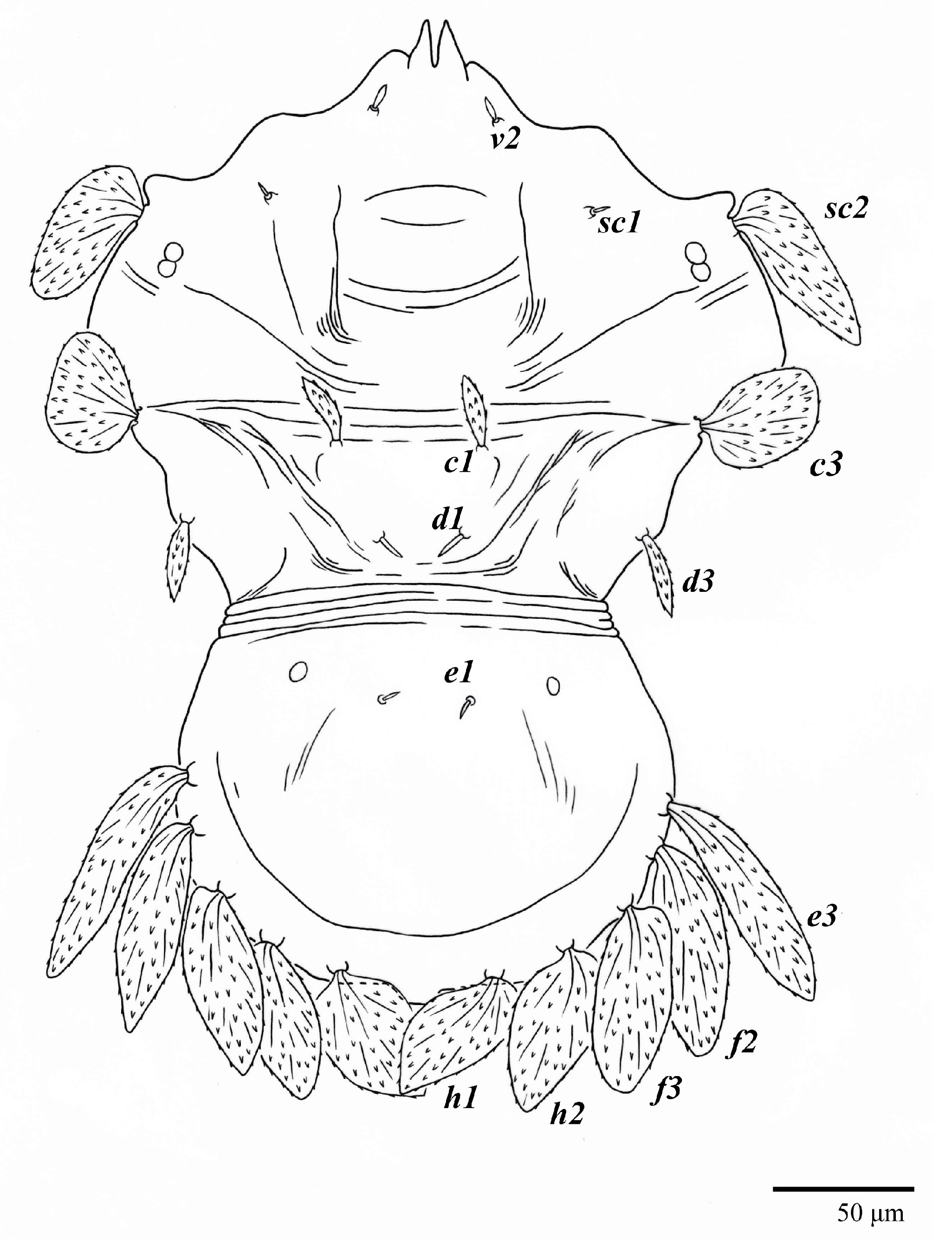
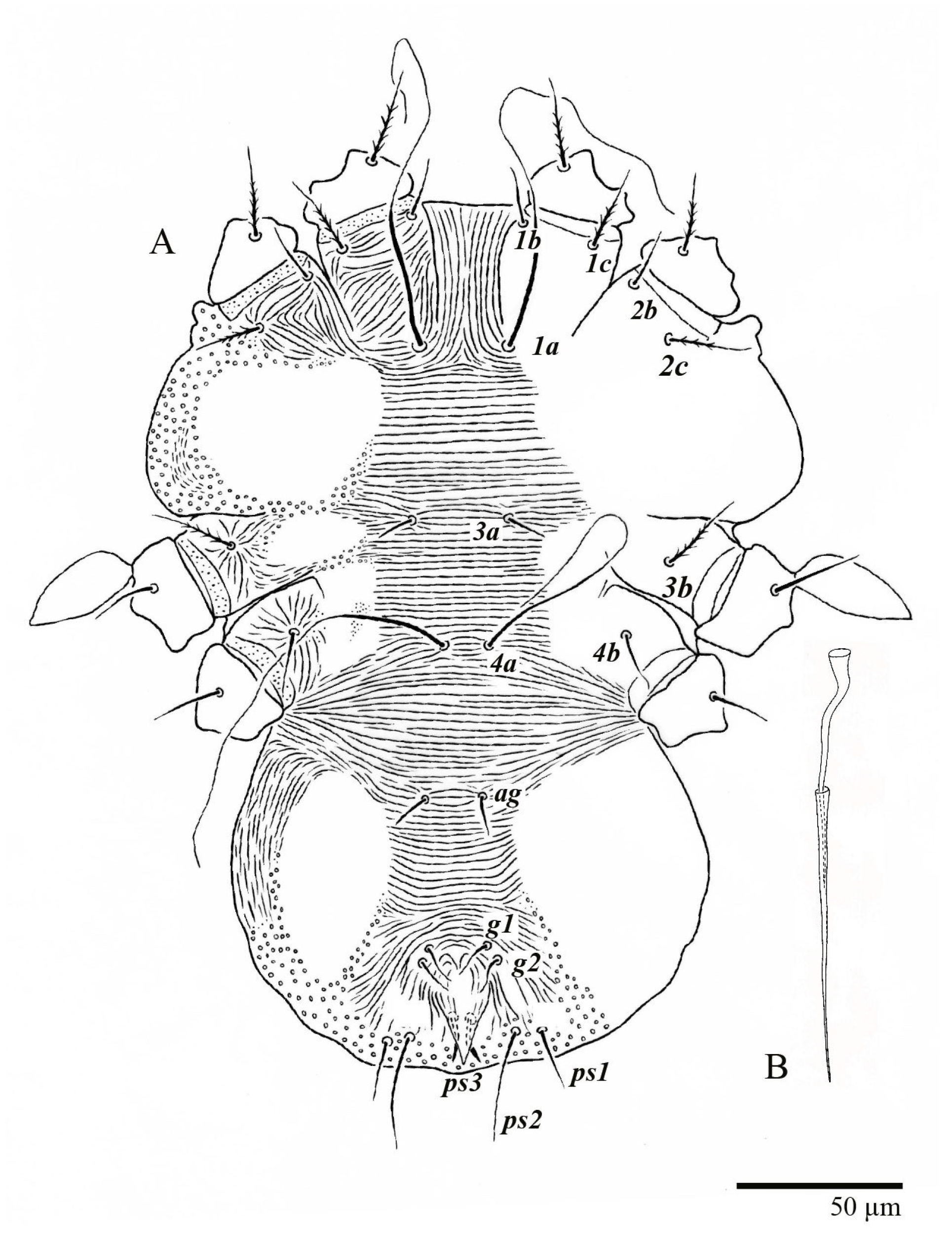
Diagnosis of the genus (Based on [2]). “Body shape from elongate-ovate to broadly rounded; broad propodosoma differentiated from narrower opisthosoma (although anterior opisthosoma is broad at junction with propodosoma). Anterior margin of prodorsum usually with median forked projection forming a short notch.” Prodorsum with one pair of lateral body projections anterior to setae sc2 present or absent. Posterior margin of opisthosoma with a broad rounded projection between setae h1 usually present. “Prodorsal shield divided by two oblique folds running from vicinity of the eyes angled medially to posterior margin of shield, superficially dividing the shield into three smaller plate-like regions; a small plate is indicated between setae c3–d3 on dorsal opisthosomal margin; posteroventral body margin often with band of pustulate cuticle. Dorsal opisthosoma with setae c1, c3, d1, d3, e1, e3, f3, h1, h2 present (except setae f3 absent in U. aberrans); setae f2 present or absent, when present then inserted on lateral margin aligned with lateral setae e3, f3, h1, h2; setae c2, d2, e2 absent. Setae h2 not flagellate, similar in form to h1; setae sc2, e3, f2, f3, h1, h2 flattened, lanceolate, oblanceolate, obovate to ovate, with sc2 often falcate; form of other dorsal setae variable (e.g., sc2 and f3 flagellate in U. bunyai). Three pairs of ps (pseudanal) setae present; female with ps3 positioned anteriorly on anal valves and much shorter than ps1–2, which are closely associated with each other and positioned posterolaterad anal valves; setae ps2 usually much longer than ps1; male with ps3 modified into accessory genital stylets and inserted on elongate genitoanal valves, with ps1–2 positioned as in female. Ventral, genital and anal regions membranous, without defined sclerotized plates; flap of ovipore and anus surrounded by strongly plicate and wrinkled membranous cuticle. Genital setae g2 inserted slightly anterior to g1 on reduced genital flap; g1–2 often aligned longitudinally with setae ag. Intercoxal setae (3a, 4a) not multiplied. Palps four segmented; palp tibiae with 1–2 setae; palp tarsi with 1–3 phaneres, with solenidion always present, sometimes curved, often inserted basally on palp tarsus segment at junction with palp tibia. Dorsal setae on legs inserted in lateral position. Femora of legs I–II with four setae (d, l’, v’, bv″); genua I–II with three setae (d, l’, l″) (except some species variously described with two setae—U. acharis (genua I–II with 3–2 setae; possibly d absent), U. pterophilus (genua I–II with 2–2)); tibiae I–II usually with five setae (except two species described with four setae, U. avarua (v″ absent) and U. lacorpuzrarosae (possibly d absent)). Tarsal claws pad-like. Immature stages with setae c1 inserted distinctly anterior to level of setae c3. See also diagnosis of [1].”
Description
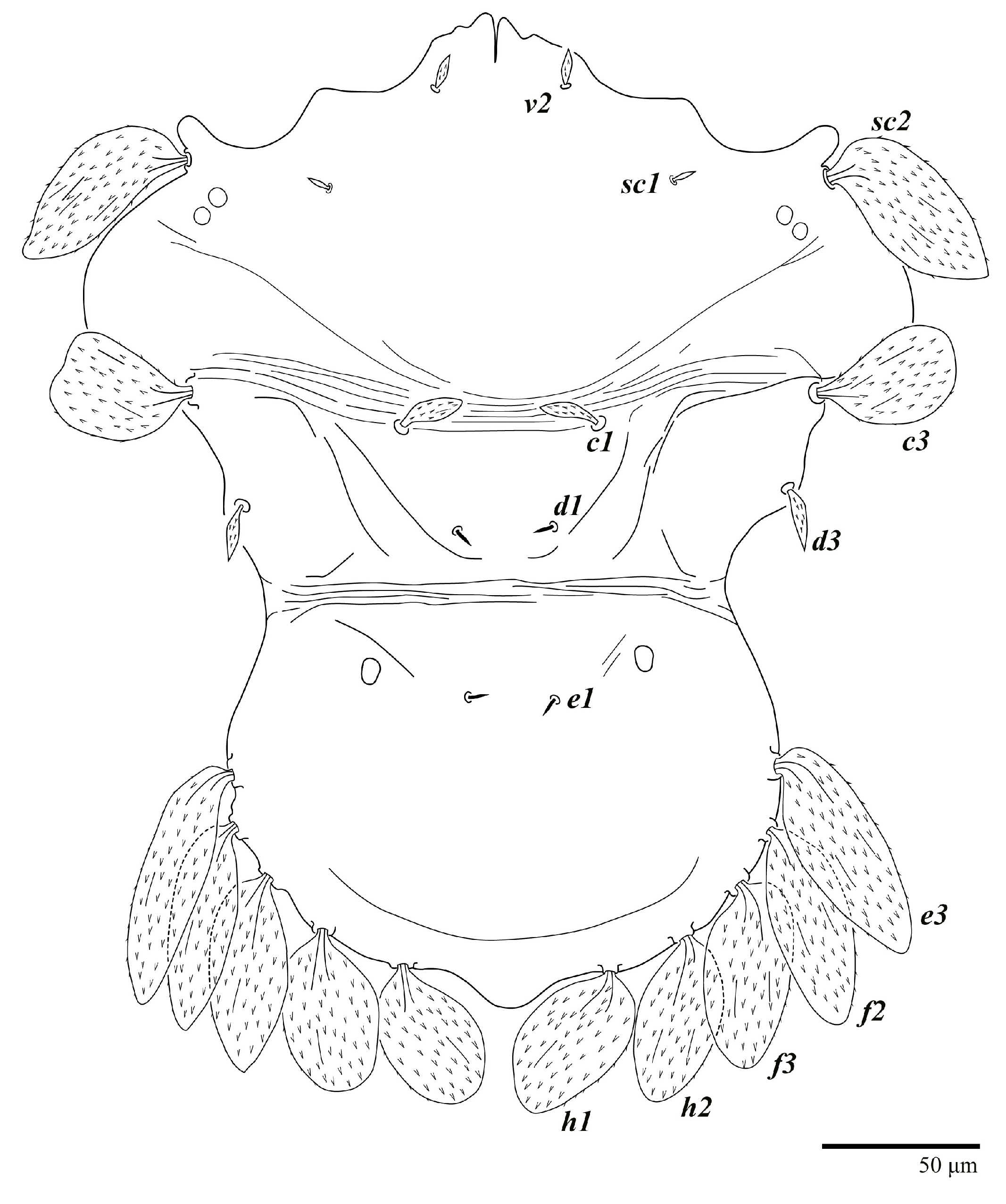
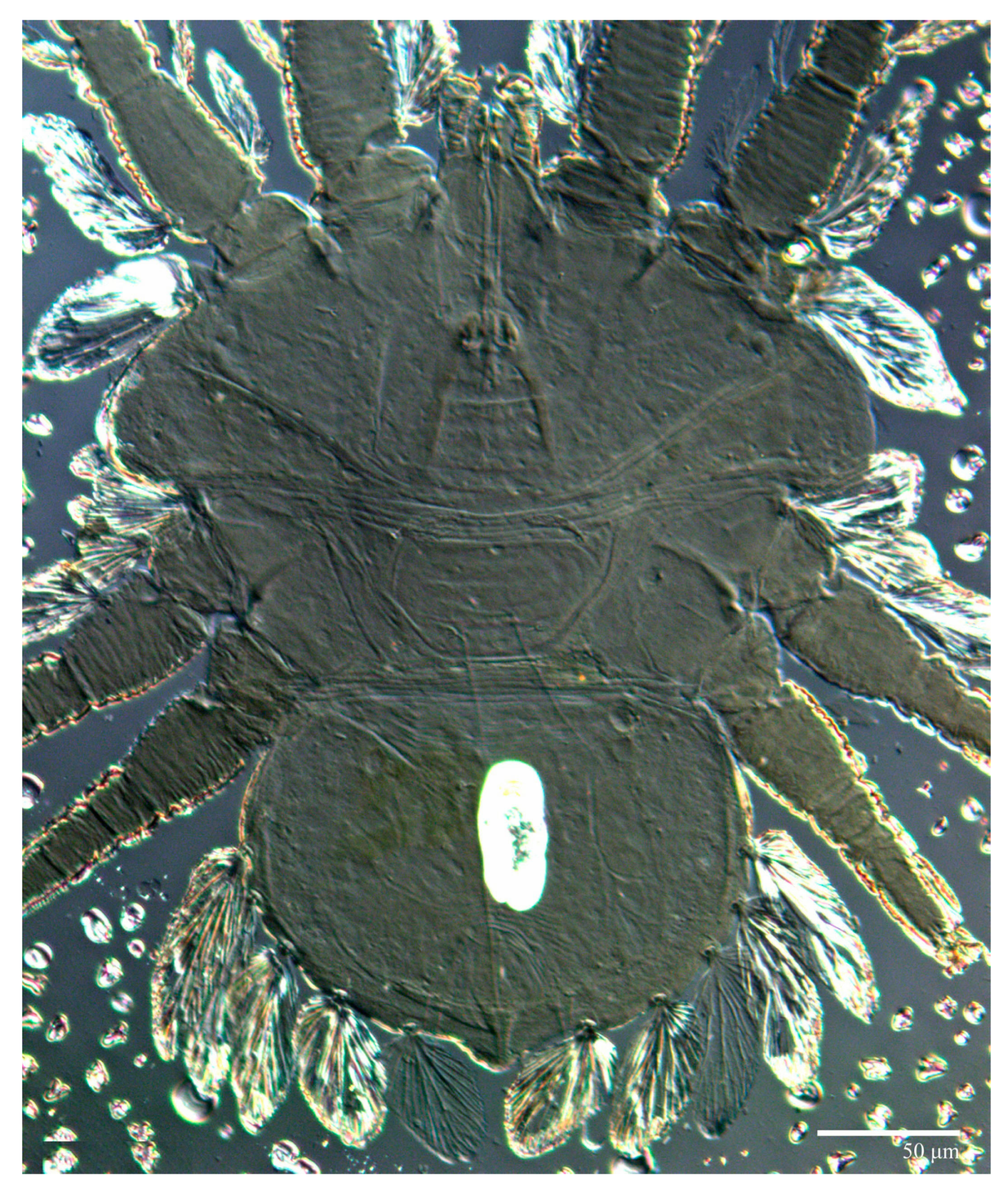
Diagnosis. Female: As per genus, in addition to: prodorsal setae v2, sc1 minute to short, and sc2 large, flattened obovate to ovate; dorsal opisthosoma with 10 pairs of setae (f2 present); most of the dorsal opisthosomal setae large, flattened, obovate to ovate, except setae d3 is distinctly short and c3 almost orbicular; pair lateral body projections anterior to setae sc2 and projection between opisthosomal setae h1 both present; palp four segmented, setal formula 0, 0, 2, 2. Male: Opisthosoma narrower than that of the female, with distinct transverse constriction (waist) between setae d1 and e1; many dorsal setae similar in form to those of females, except c1, d1, and e1 short to minute, d3 longer, and setae along posterior margin of opisthosoma (especially e3) narrower and more elongated than those of the female. Tarsi I–II each with two solenidia (ω′ paraxial and ventrolateral; ω” antiaxial); tarsus III with one solenidion ω′ paraxial and ventrolateral. Immatures: with lateral body projection anterior to setae sc2 present (except absent in larvae); posterior projection between setae h1 absent; dorsal setae similar in general form to those of the female, except setae c1, d1, and e1 short to minute. Larvae with central prodorsum and pygidial region of posterior opisthosoma with finely colliculated integument.
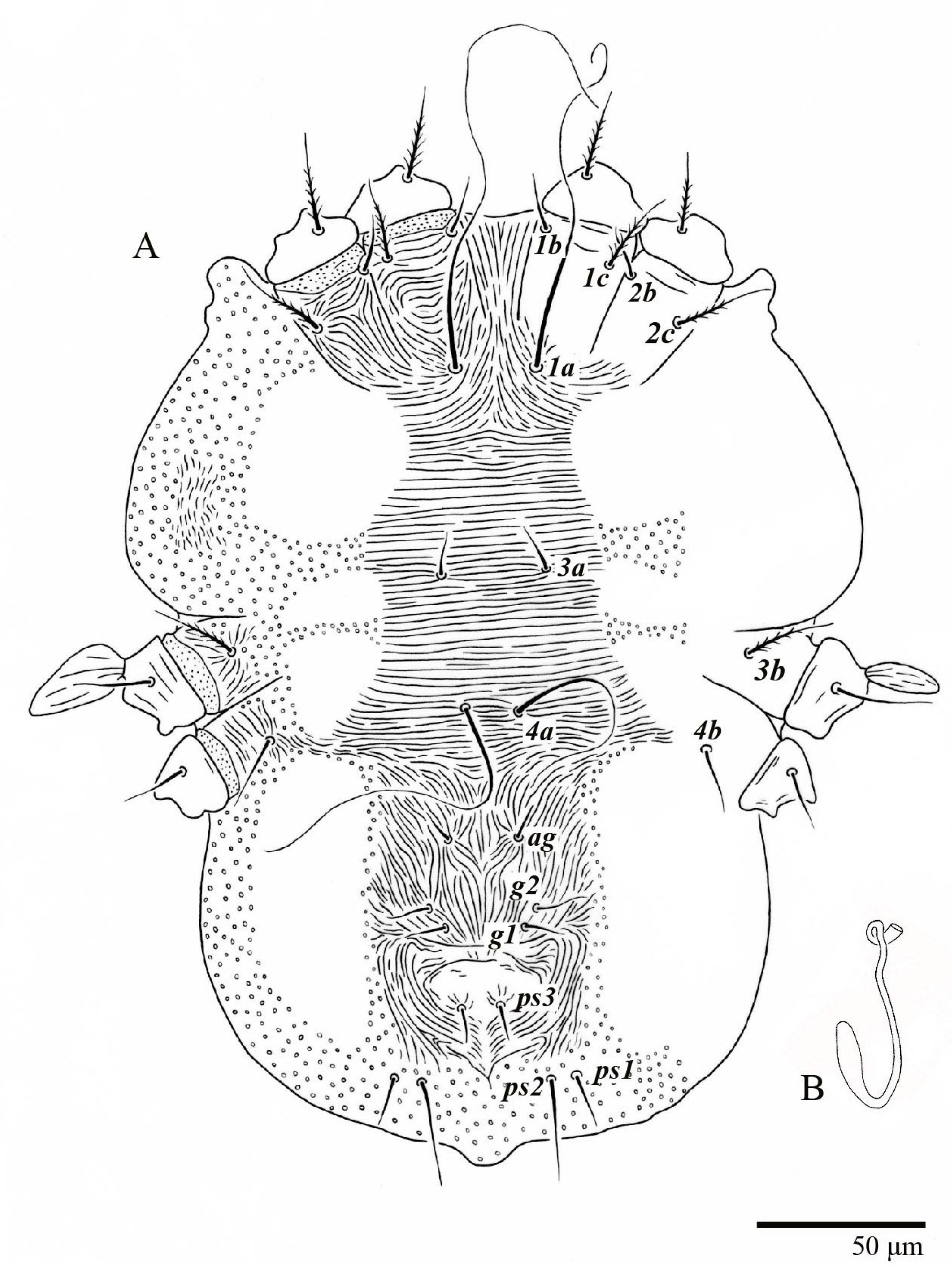
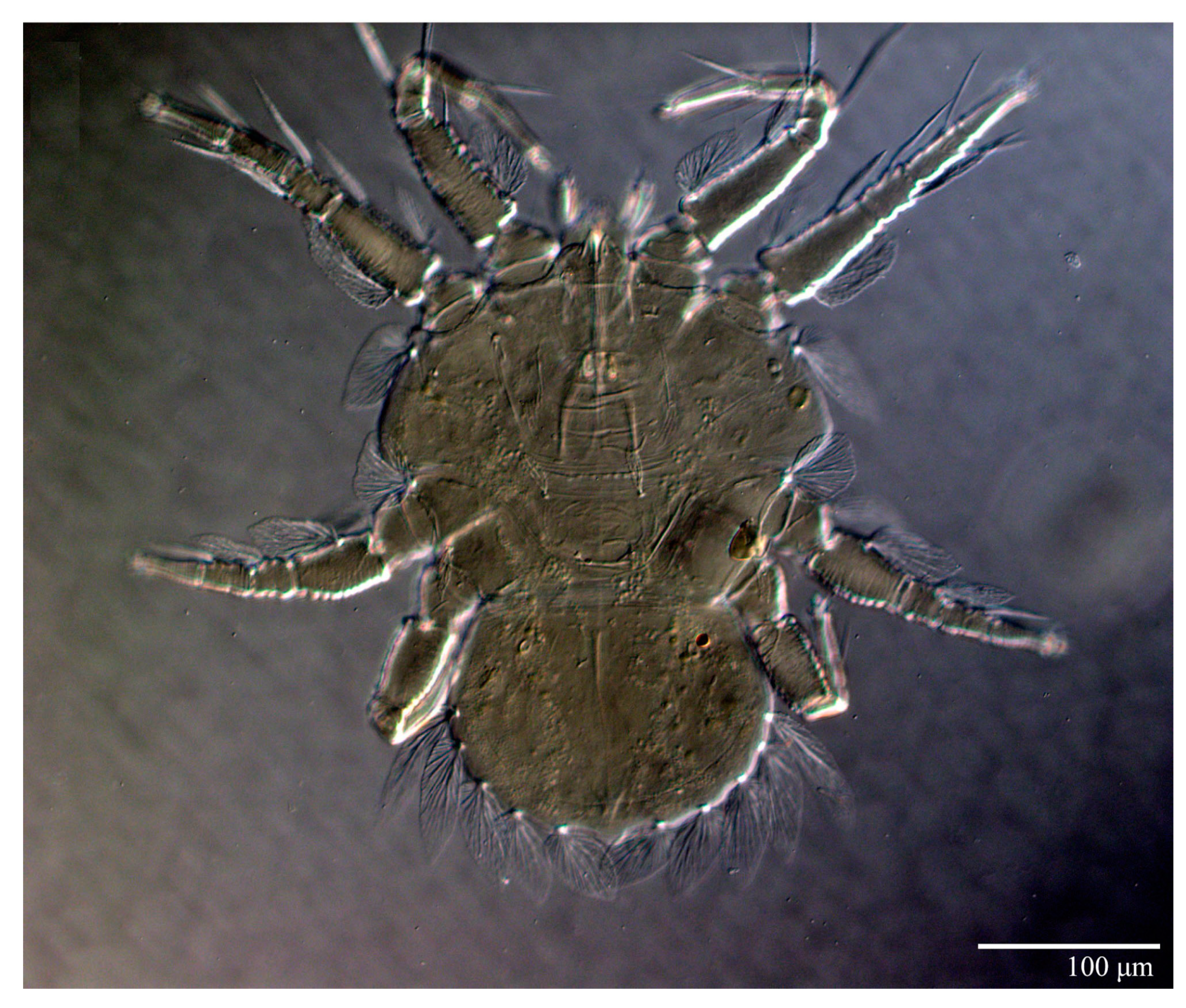
Female (n = 10) (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10)
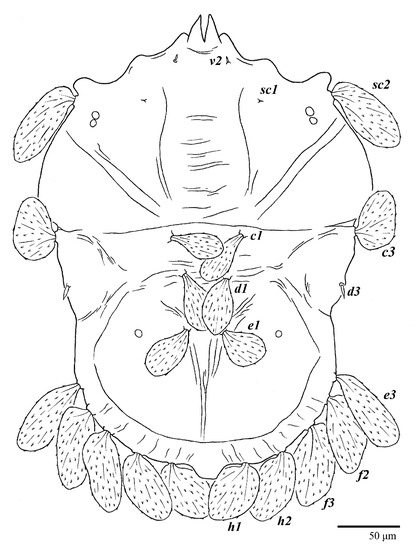
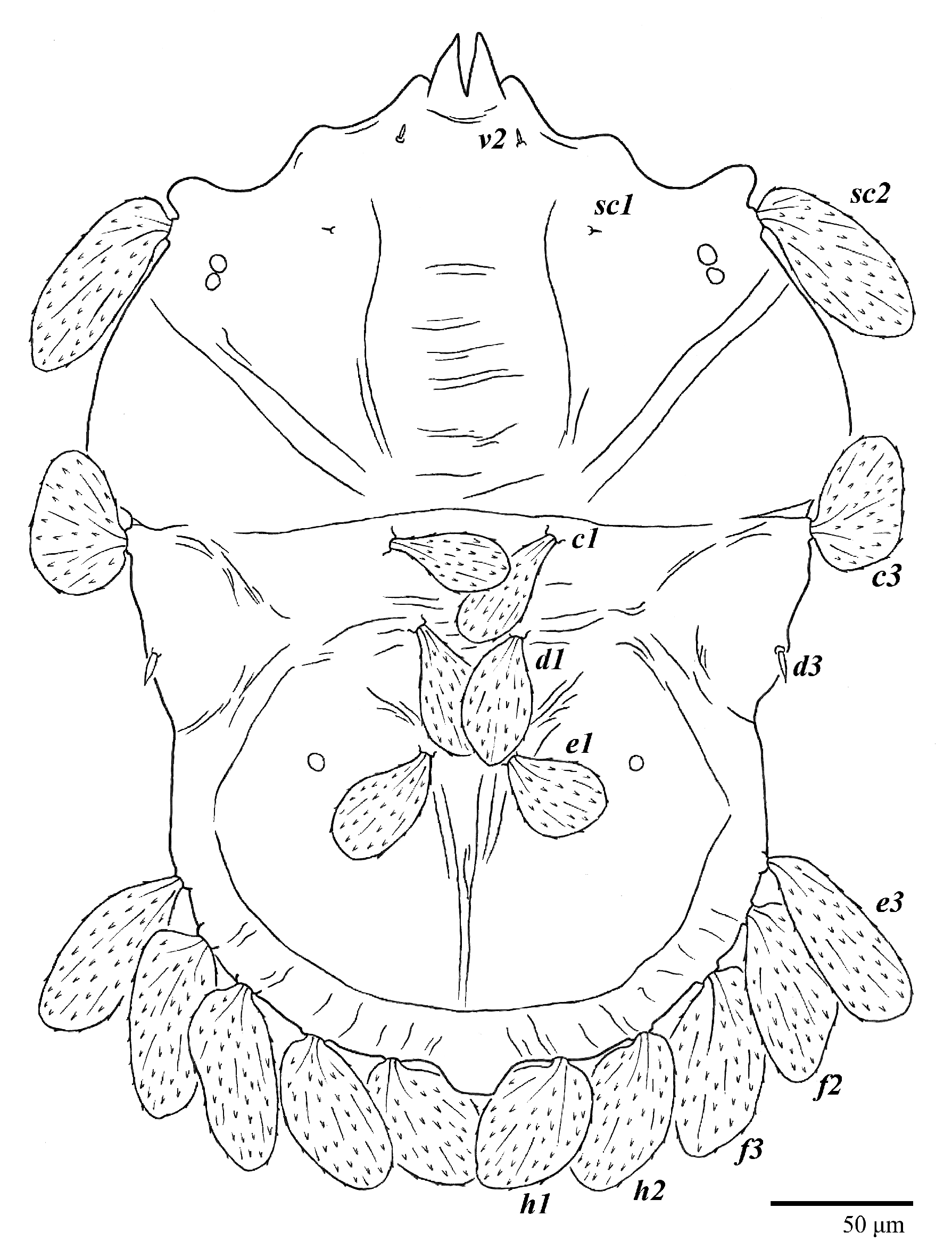
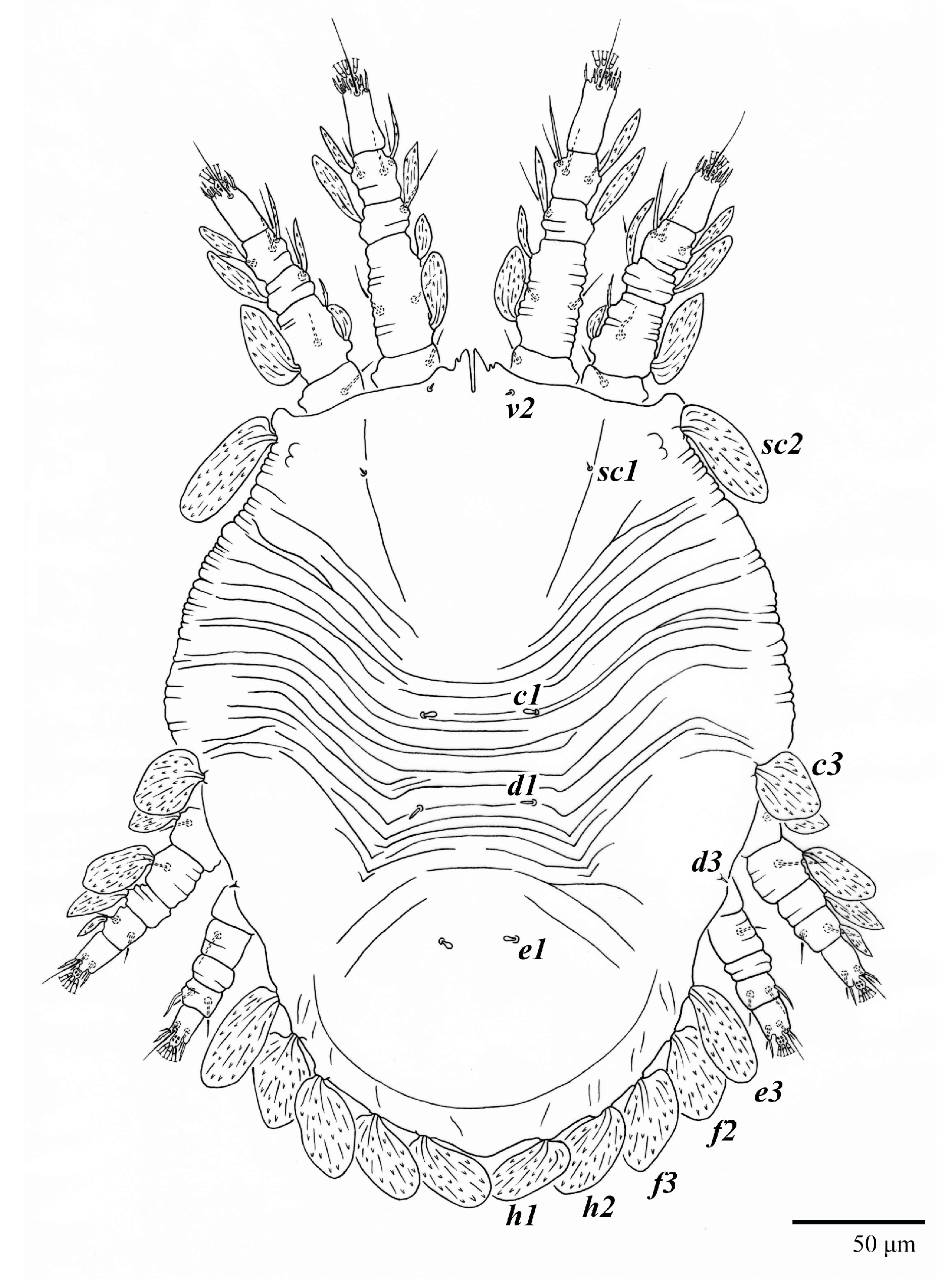
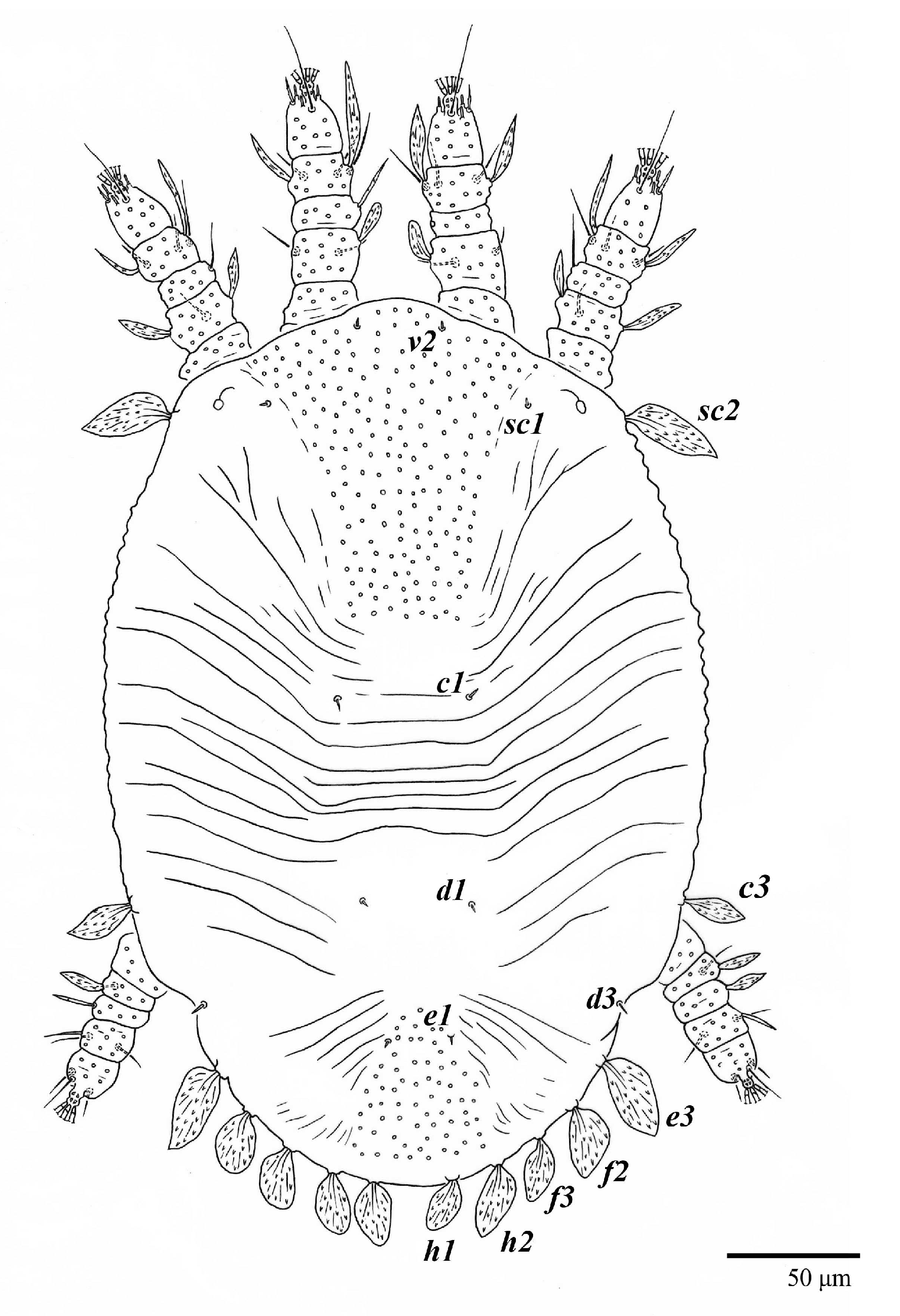
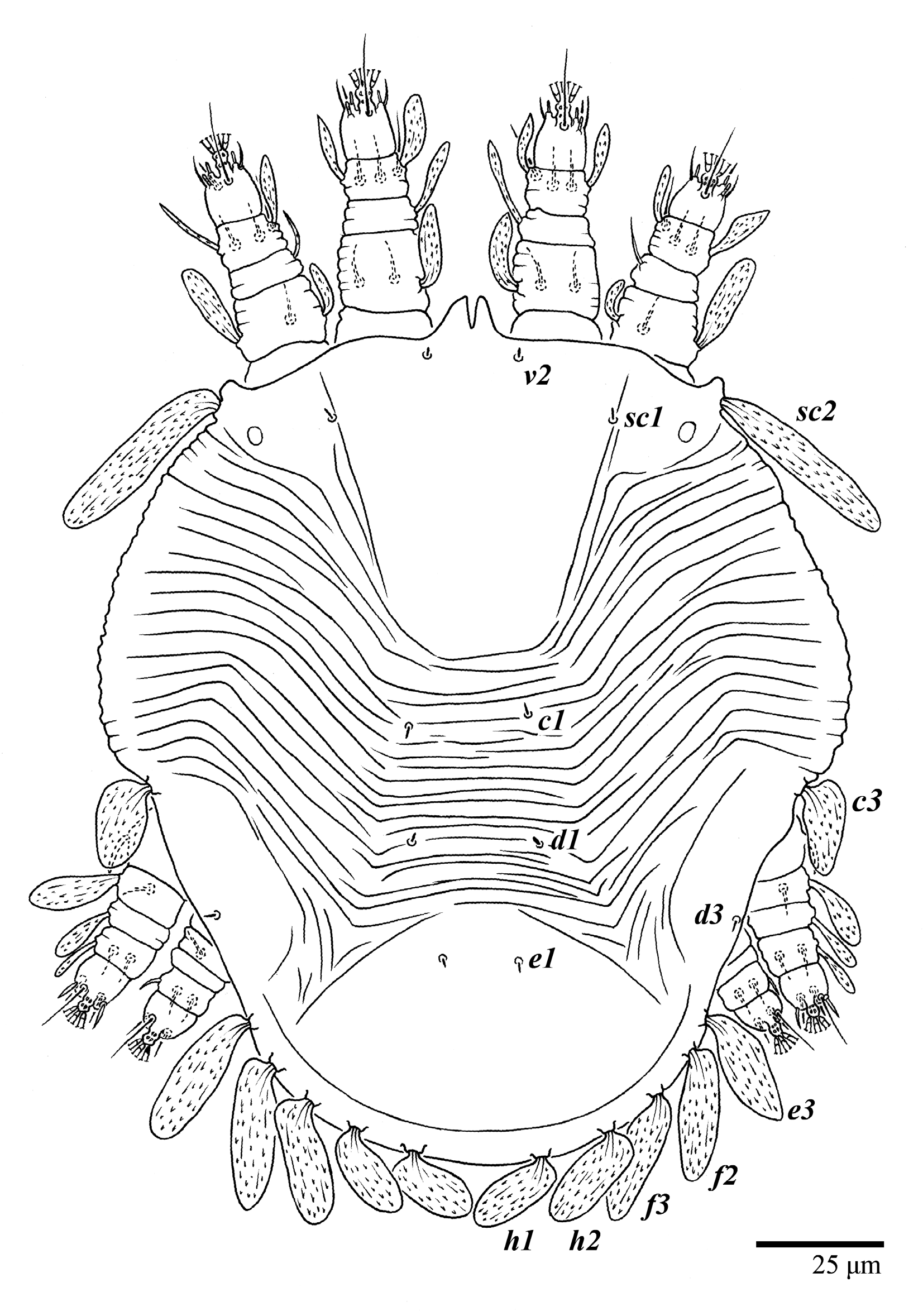
Figure 1.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): view of dorsum.
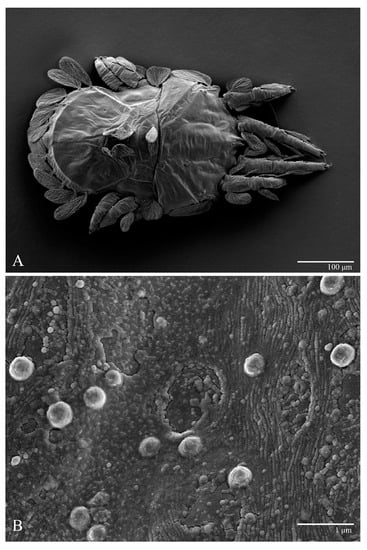
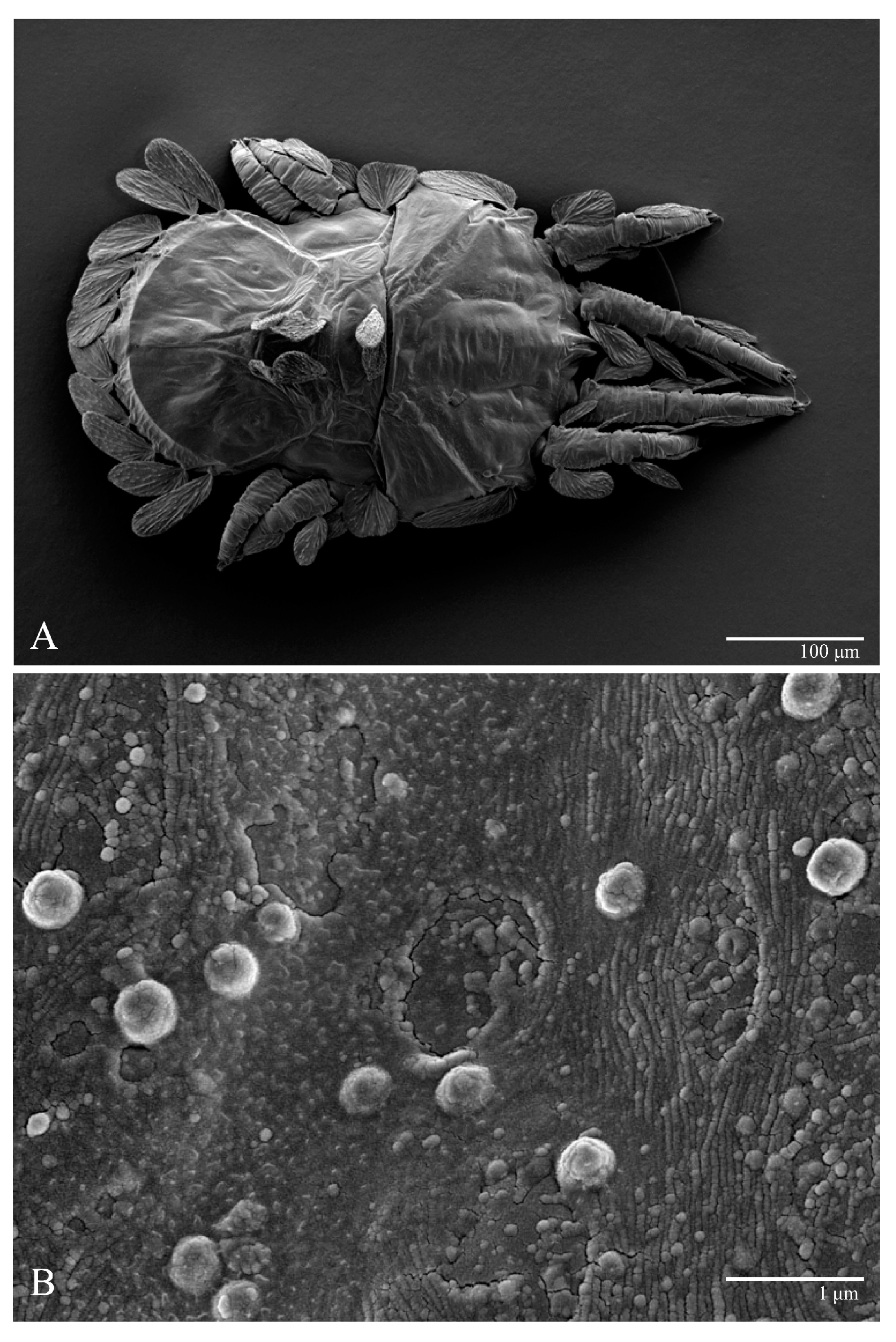
Figure 2.
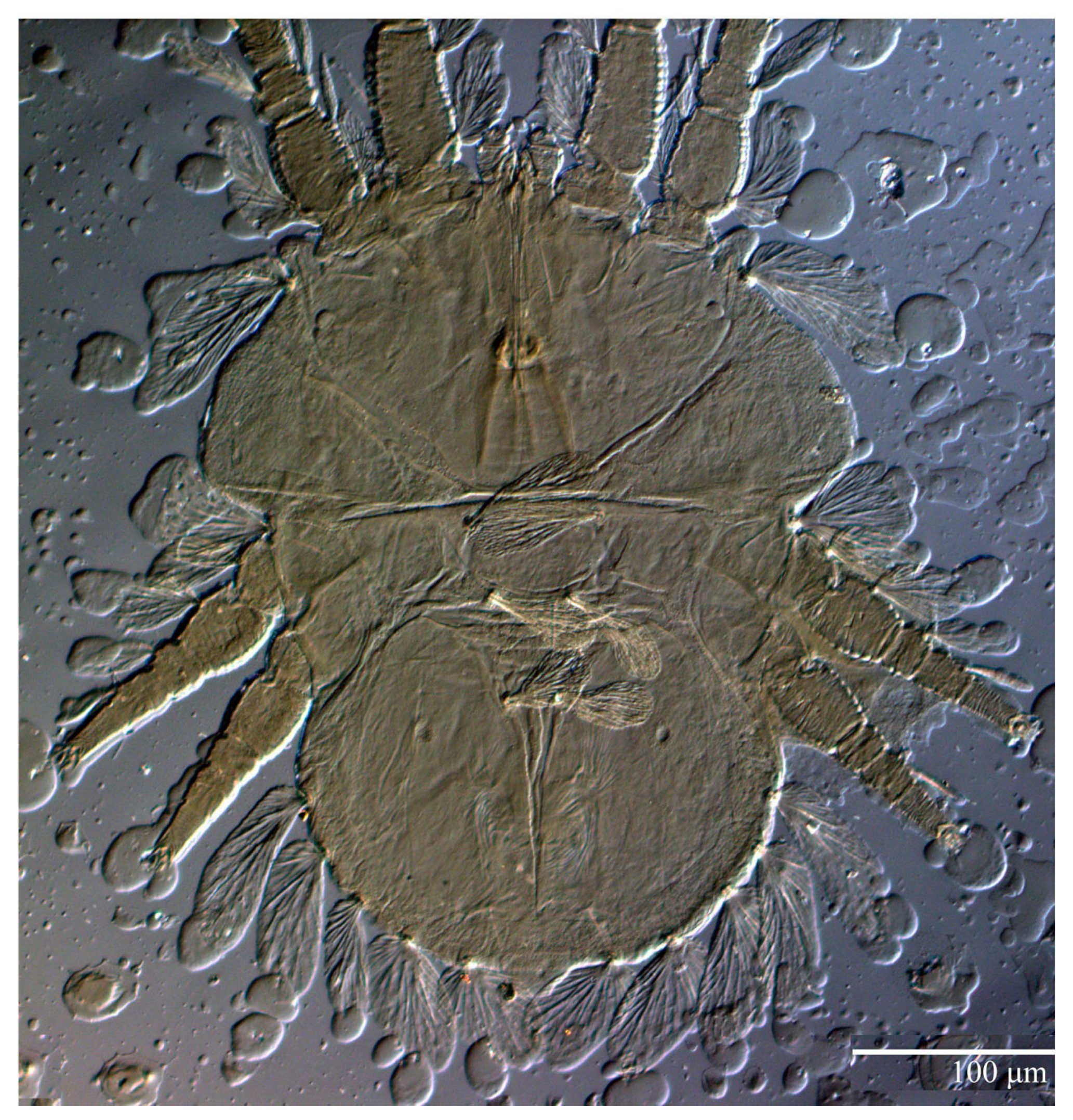
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): (A) dorsal view; (B) view of cuticular microplates on the dorsum.
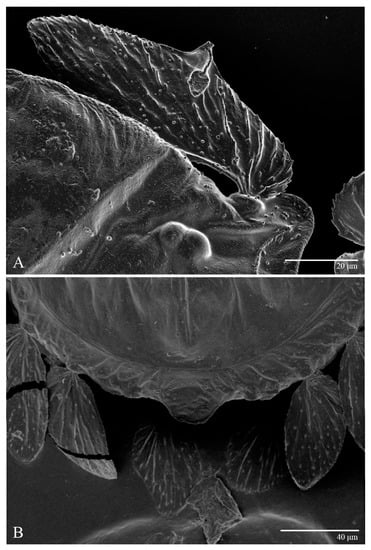
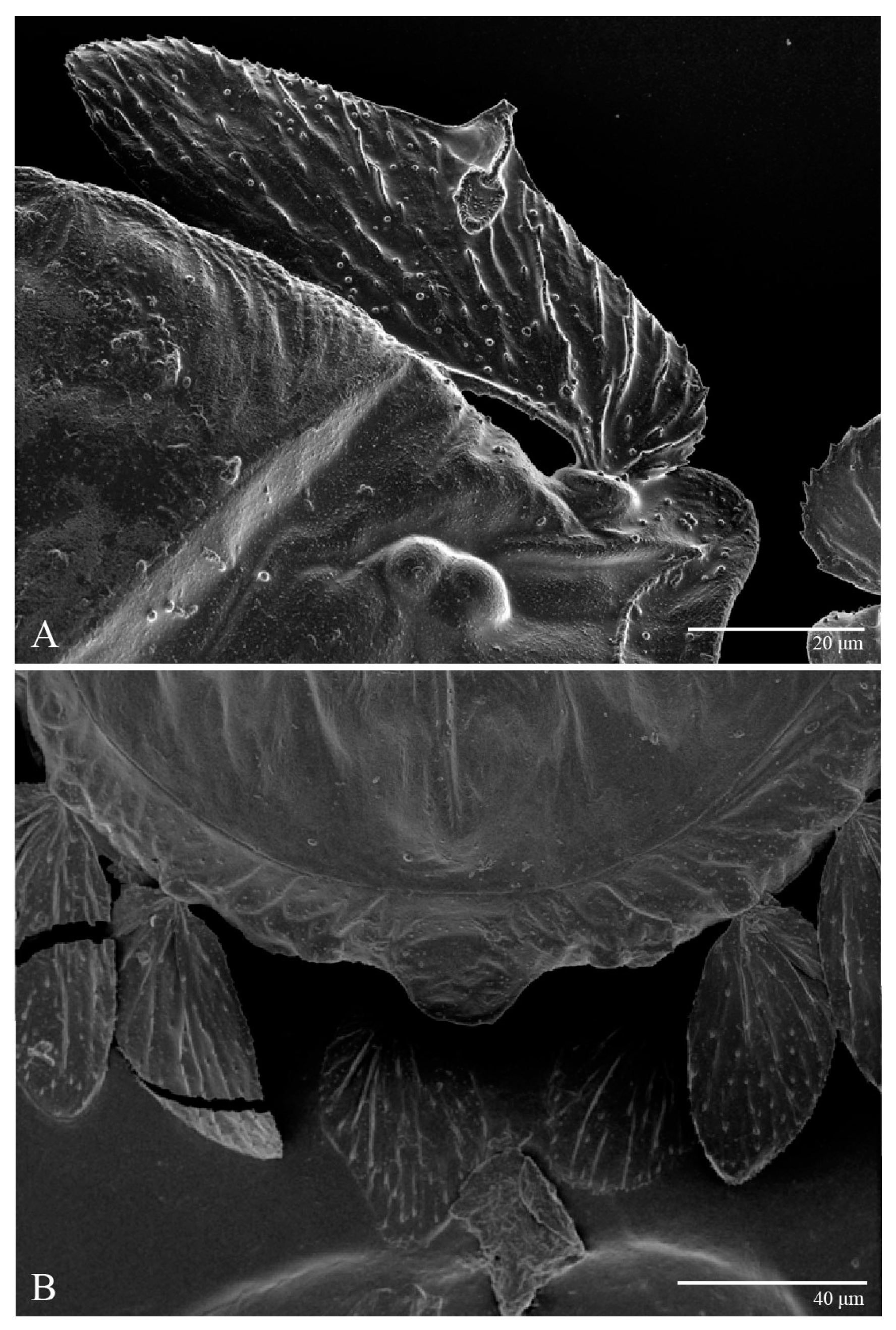
Figure 3.
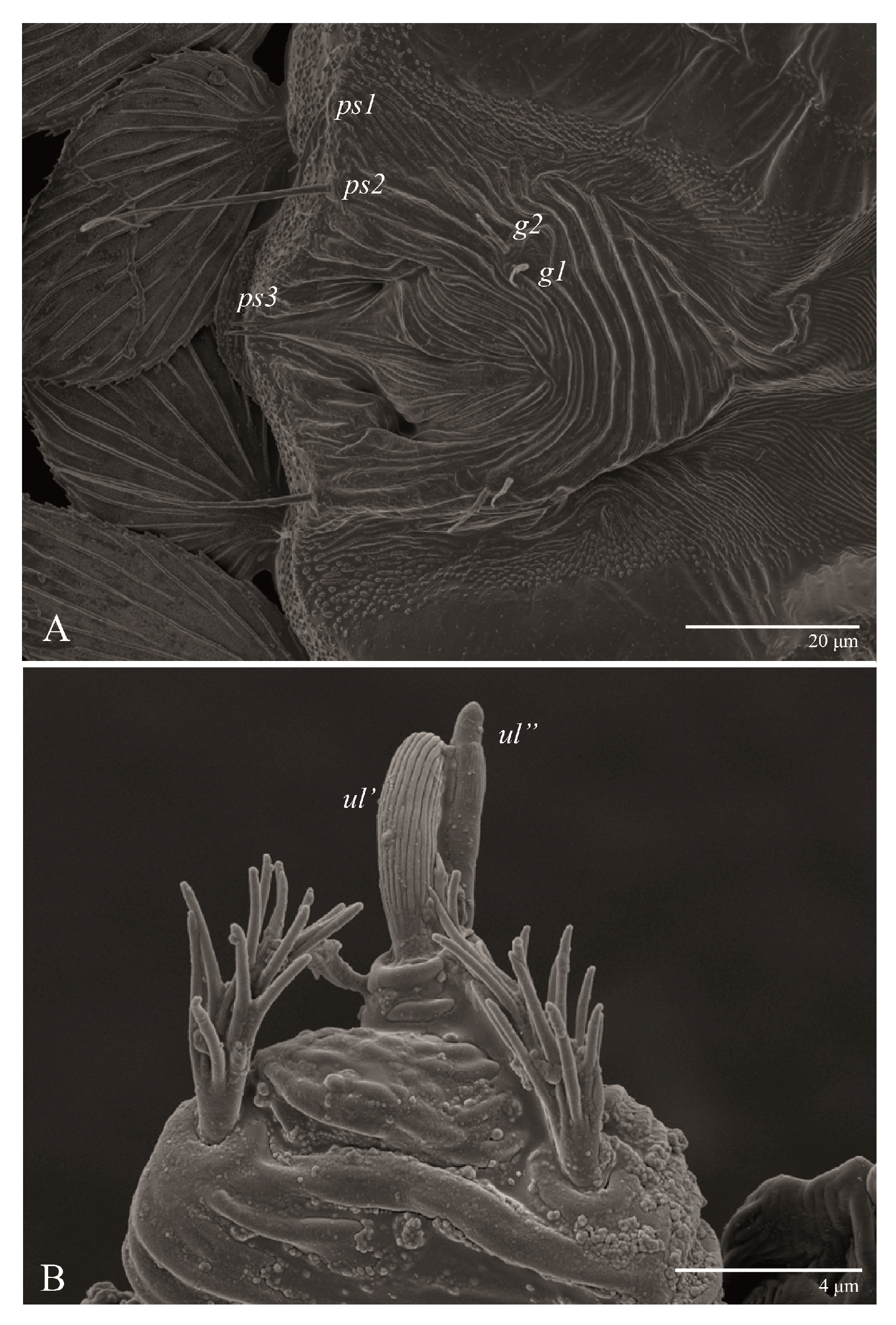
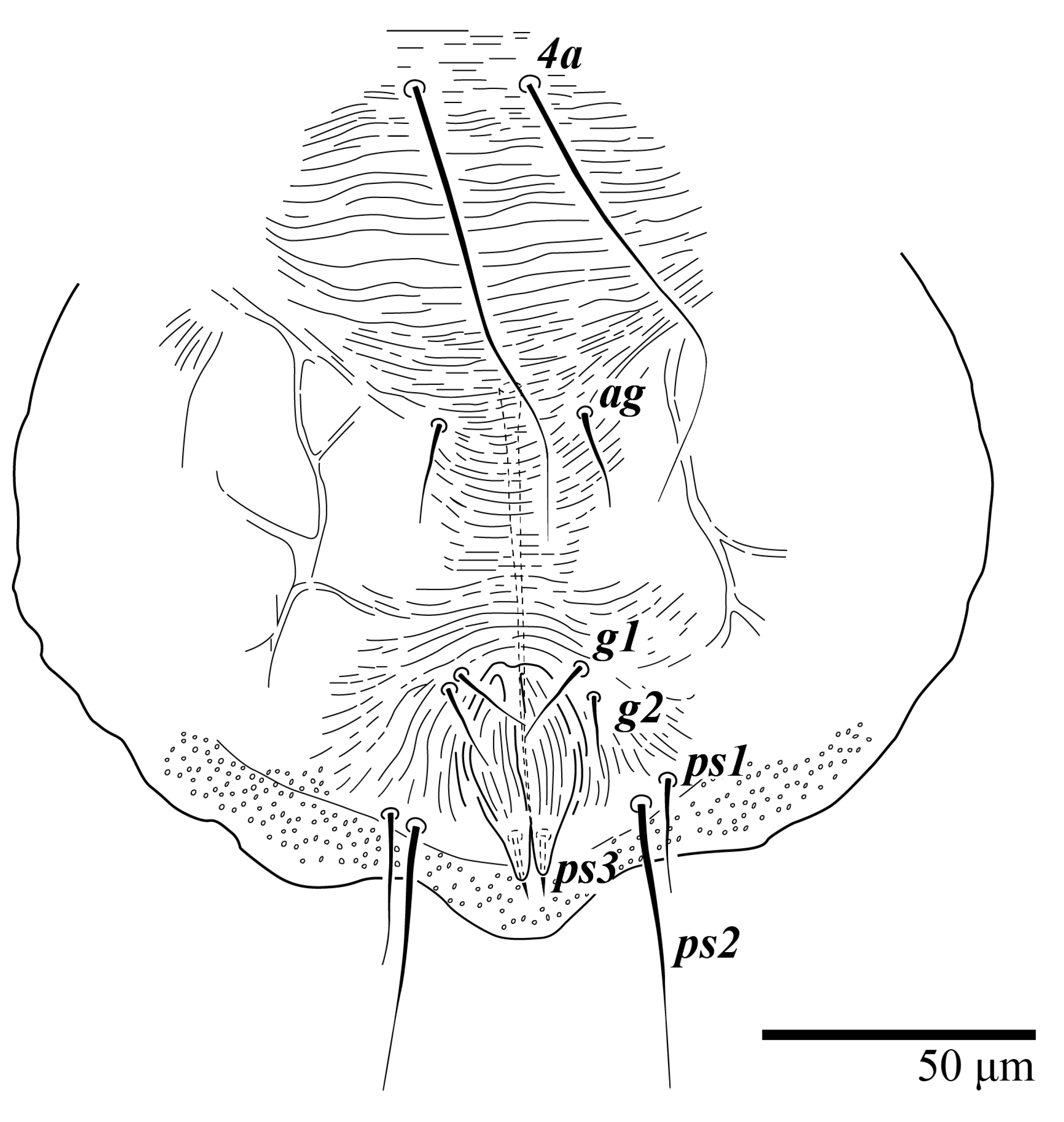
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): (A) detail of the lateral region of prodorsum, with setae sc2. Note the presence of body projection anterior to sc2; (B) posterior region of opisthosoma, indicating the body projection on posterior margin.
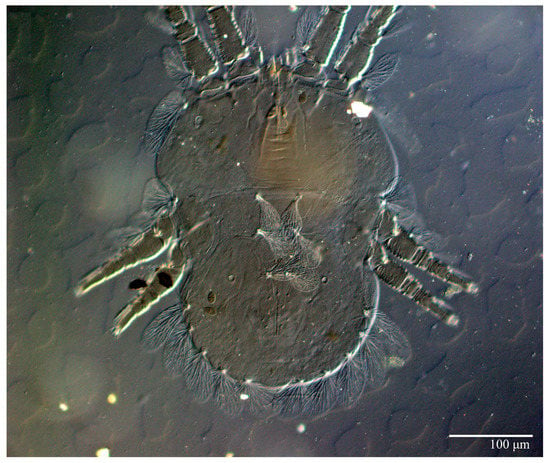
Figure 4.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): view of dorsum.
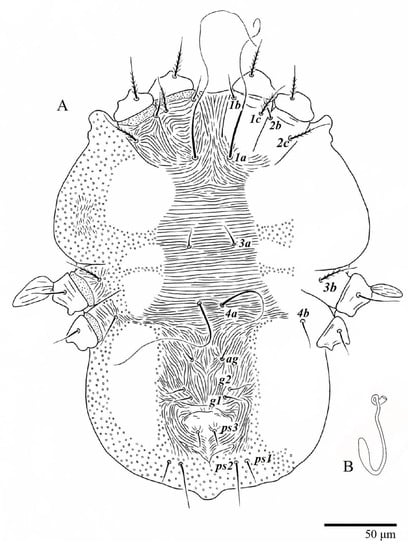
Figure 5.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): (A) view of venter; (B) spermatheca.
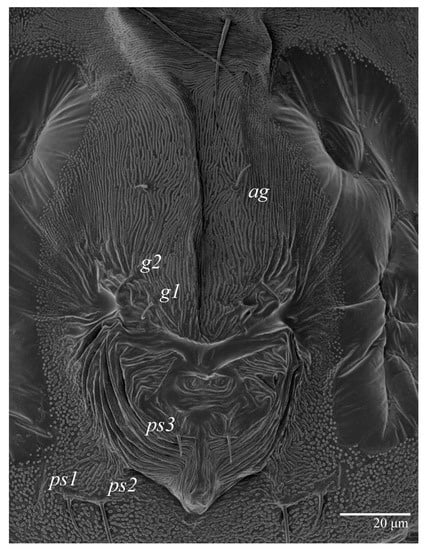
Figure 6.
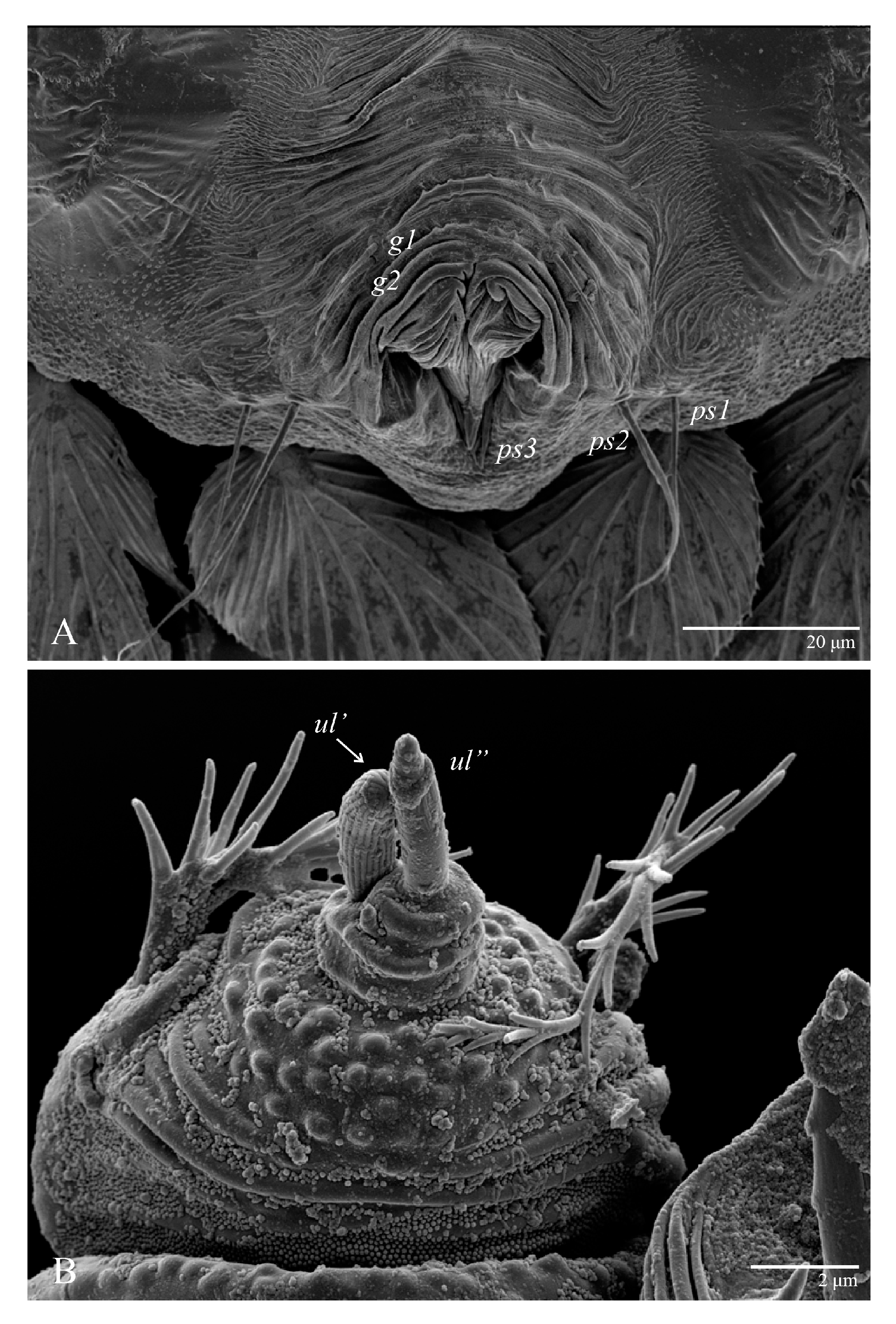
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): posterior ventral opisthosoma.
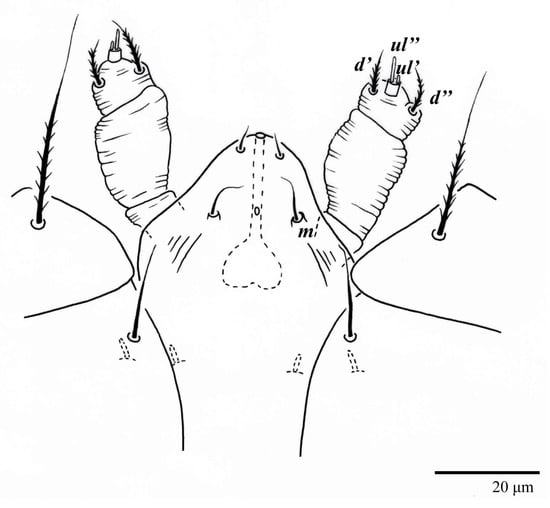
Figure 7.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): ventral infracapitulum.

Figure 8.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): (A) view of ventral infracapitulum; (B) detail of palp.
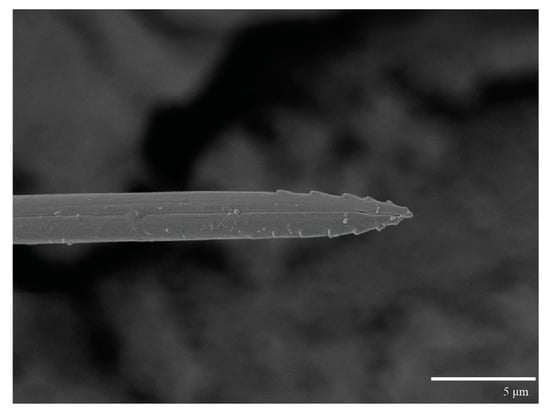
Figure 9.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): detail of stylet tip with lateral serrations.
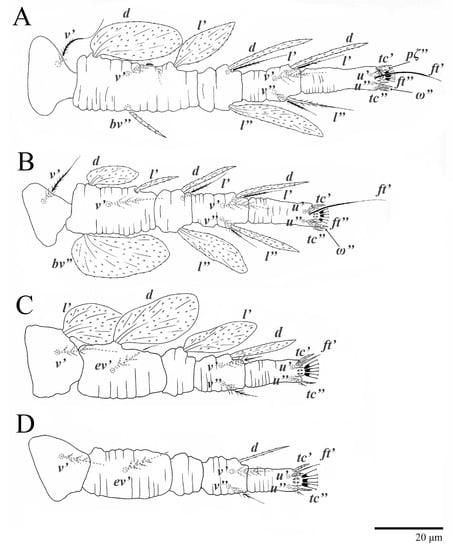
Figure 10.
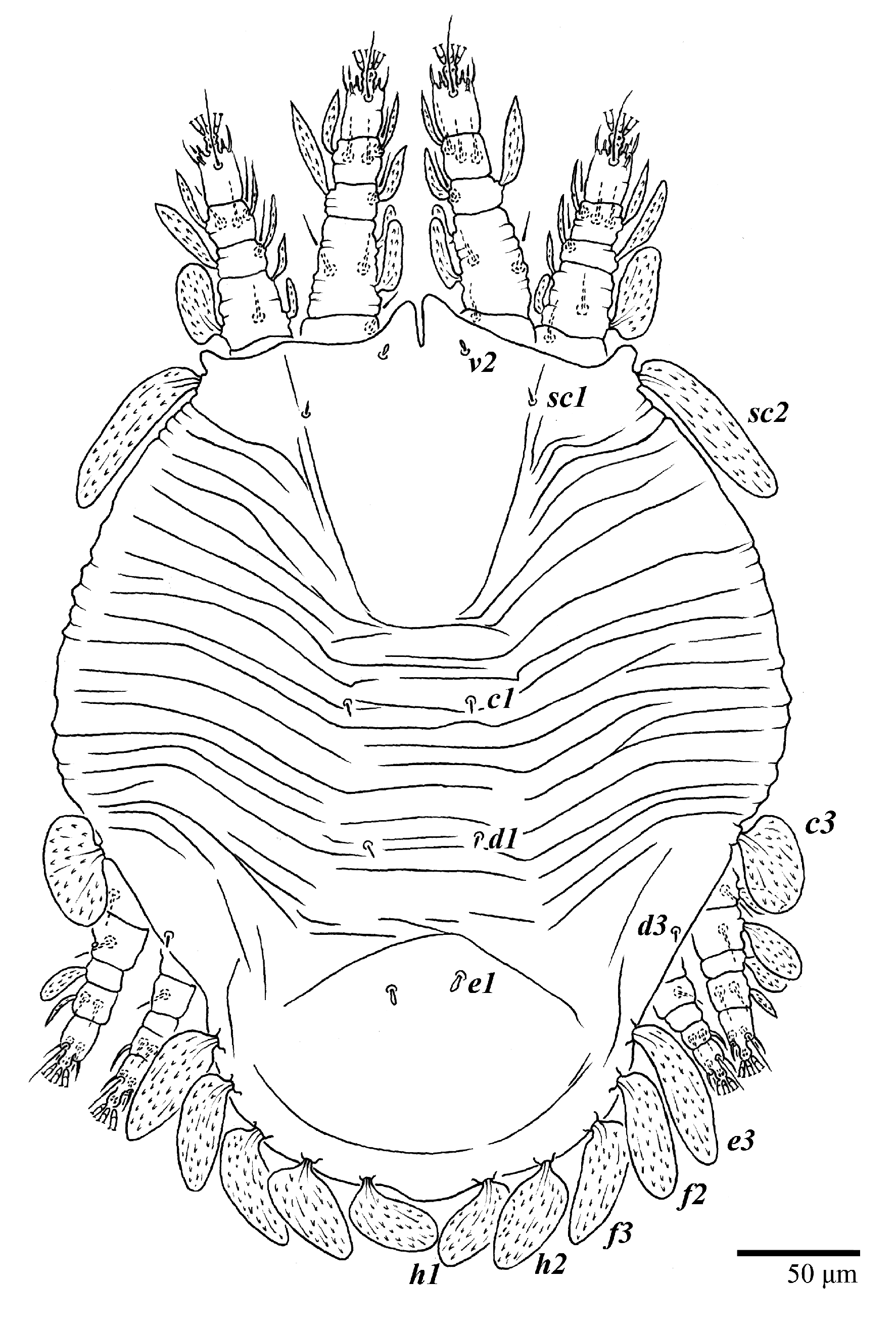
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Female): (A) leg I; (B) leg II; (C) leg III; (D) leg IV. (Right legs).
Body measurements: distance between setae v2–h1 350 (340–375), sc2–sc2 220 (215–230); other measurements: v2–v2 45 (37–45), sc1–sc1 93 (90–110), c1–c1 60 (55–63), c3–c3 260 (245–265), d1–d1 40 (37–45), d3–d3 240 (230–240), e1–e1 32 (27–33), e3–e3 220 (215–225), f2–f2 205 (205–215), f3–f3 170 (170–185), h1–h1 58 (58–68), h2–h2 120 (115–135).
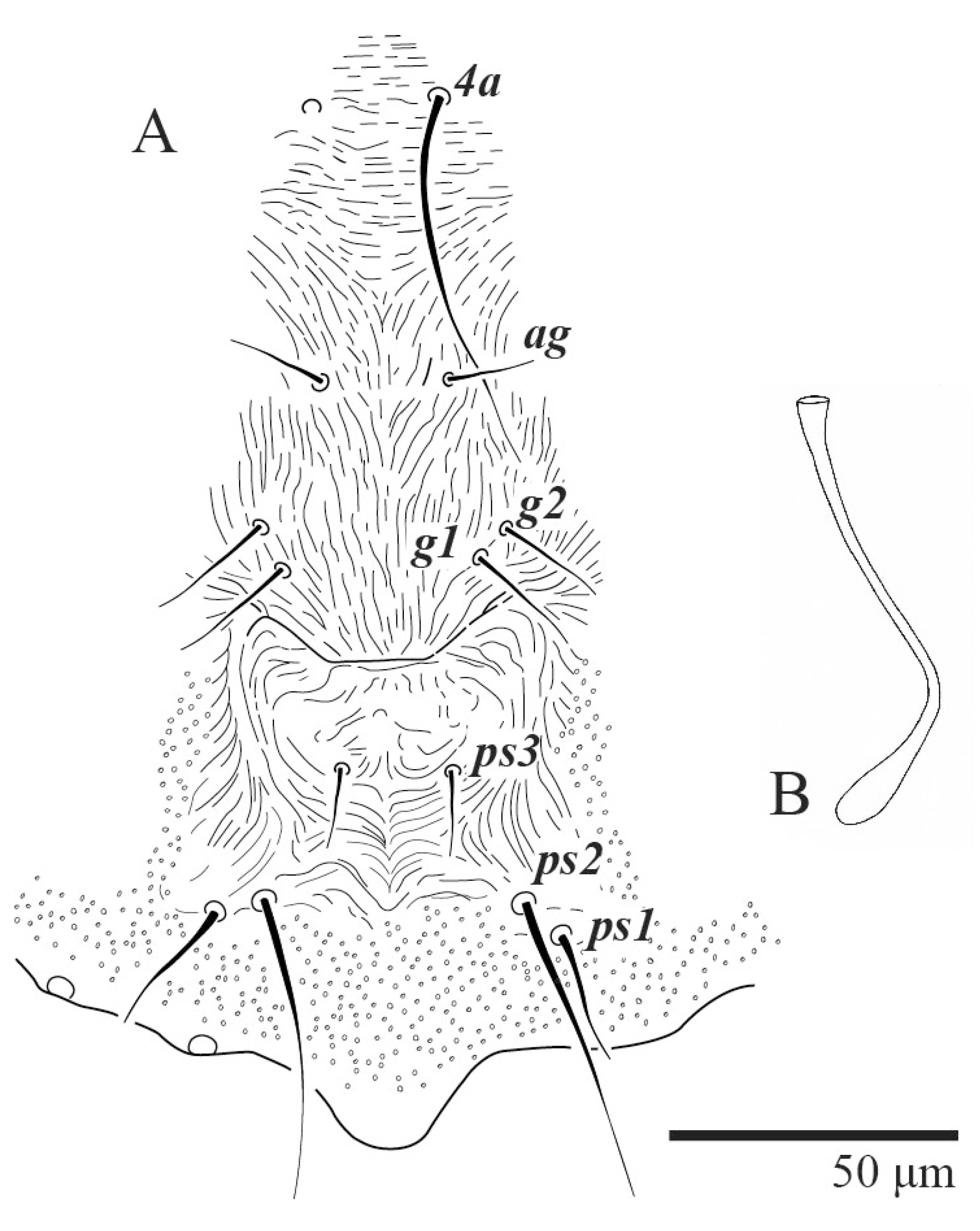
Dorsum (Figure 1, Figure 2, Figure 3 and Figure 4). Anterior margin of prodorsum with a short median forked projection forming a short notch 27 (20–27). Dorsum smooth, with pair lateral projections anterior to setae sc2 and single projection between opisthosomal setae h1 (Figure 3B). A pair of converging folds from the eyes to near the sejugal furrow on the prodorsum posterior margin. Prodorsal setae v2 and sc1 short to minute; sc2 large, flattened elongate obovate (Figure 1 and Figure 3A); most opisthosomal setae similar to prodorsal setae sc2, except d3 is short. Setal measurements: v2 5 (4–7), sc1 3 (3–5), sc2 74 (74–82), c1 52 (52–58), c3 36 (36–45), d1 55 (54–55), d3 10 (8–10), e1 50 (48–55), e3 70 (70–81), f2 65 (65–70), f3 61 (60–68), h1 52 (52–60), h2 58 (58–65).
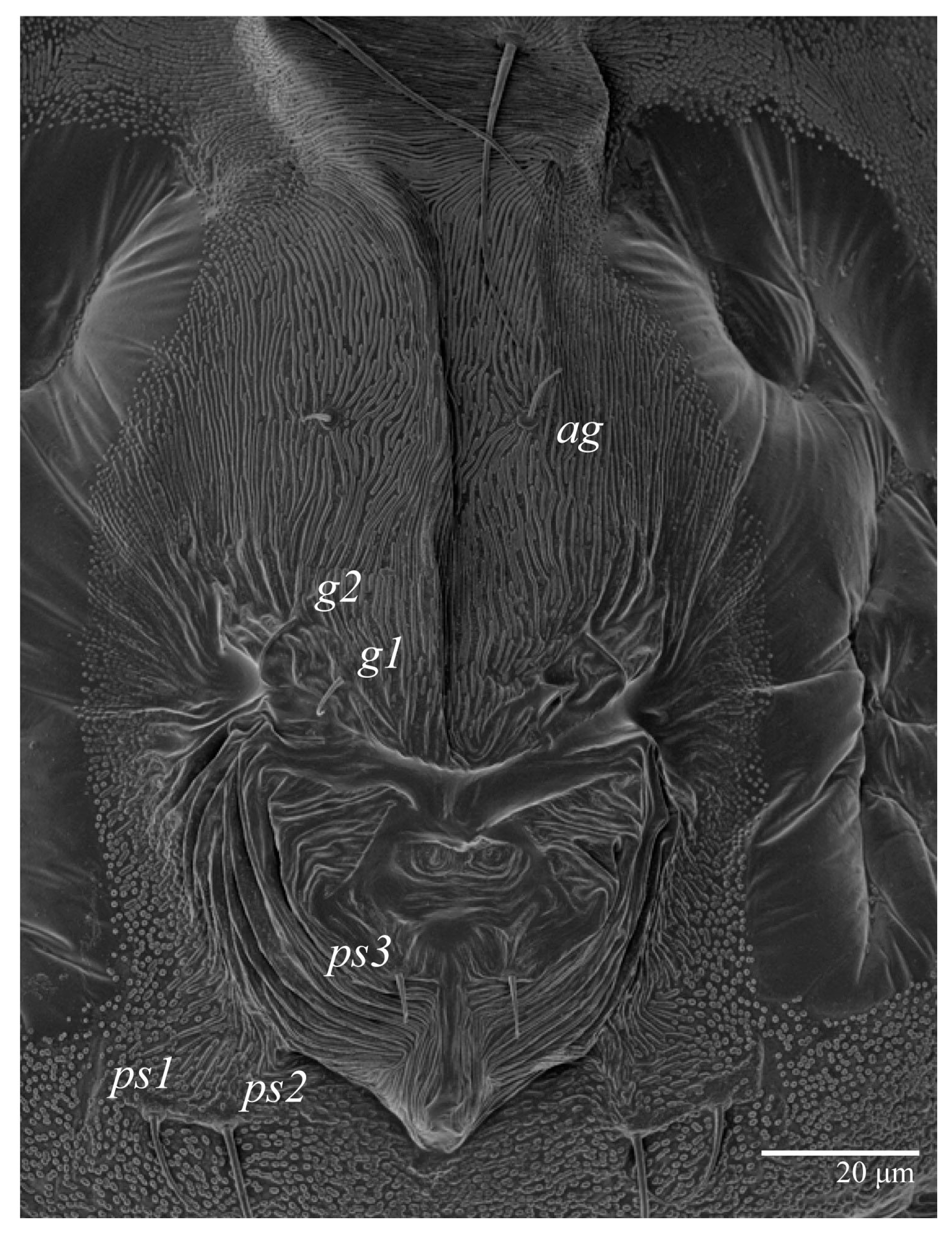
Venter (Figure 5A and Figure 6). Ventral integument weakly striate along central region and densely colliculated around lateral body margin; ventral, genital, and anal plates not developed, and entire region membranous and distinctly plicate; ventral setae filiform, with coxal setae 1c, 2c, and 3b barbed; setae ps2 distinctly longer than ps1. Setal measurements: 1a 105 (100–135), 1b 13 (13–16), 1c 30 (25–30), 2b 22 (22–26), 2c 38 (30–39), 3a 18 (15–18), 3b 32 (31–36), 4a 145 (115–145), 4b 22 (18–22), ag 10 (10–11), g1 16 (14–16), g2 16 (15–18), ps1 28 (22–28), ps2 53 (50–55), ps3 12 (10–13).
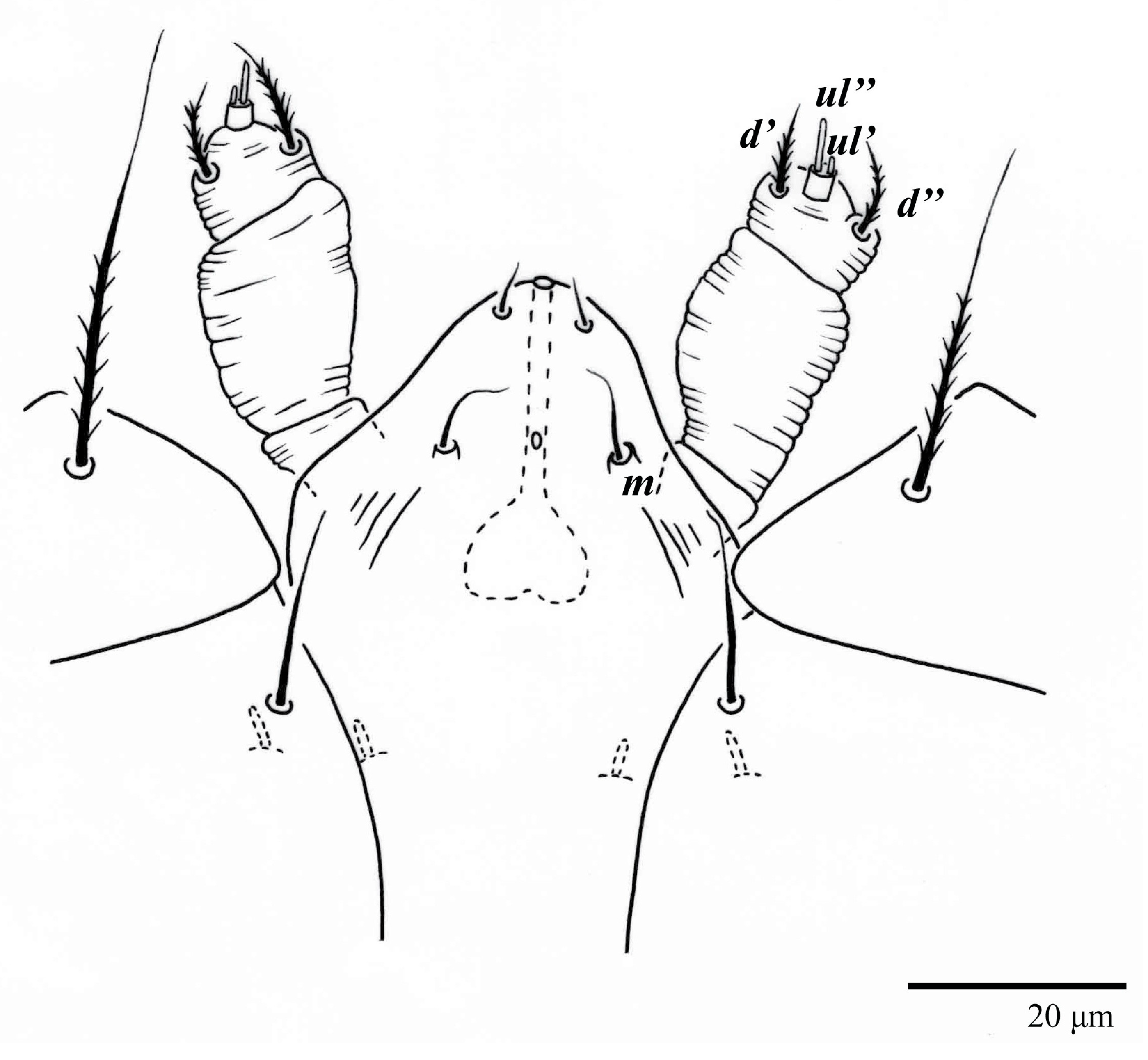
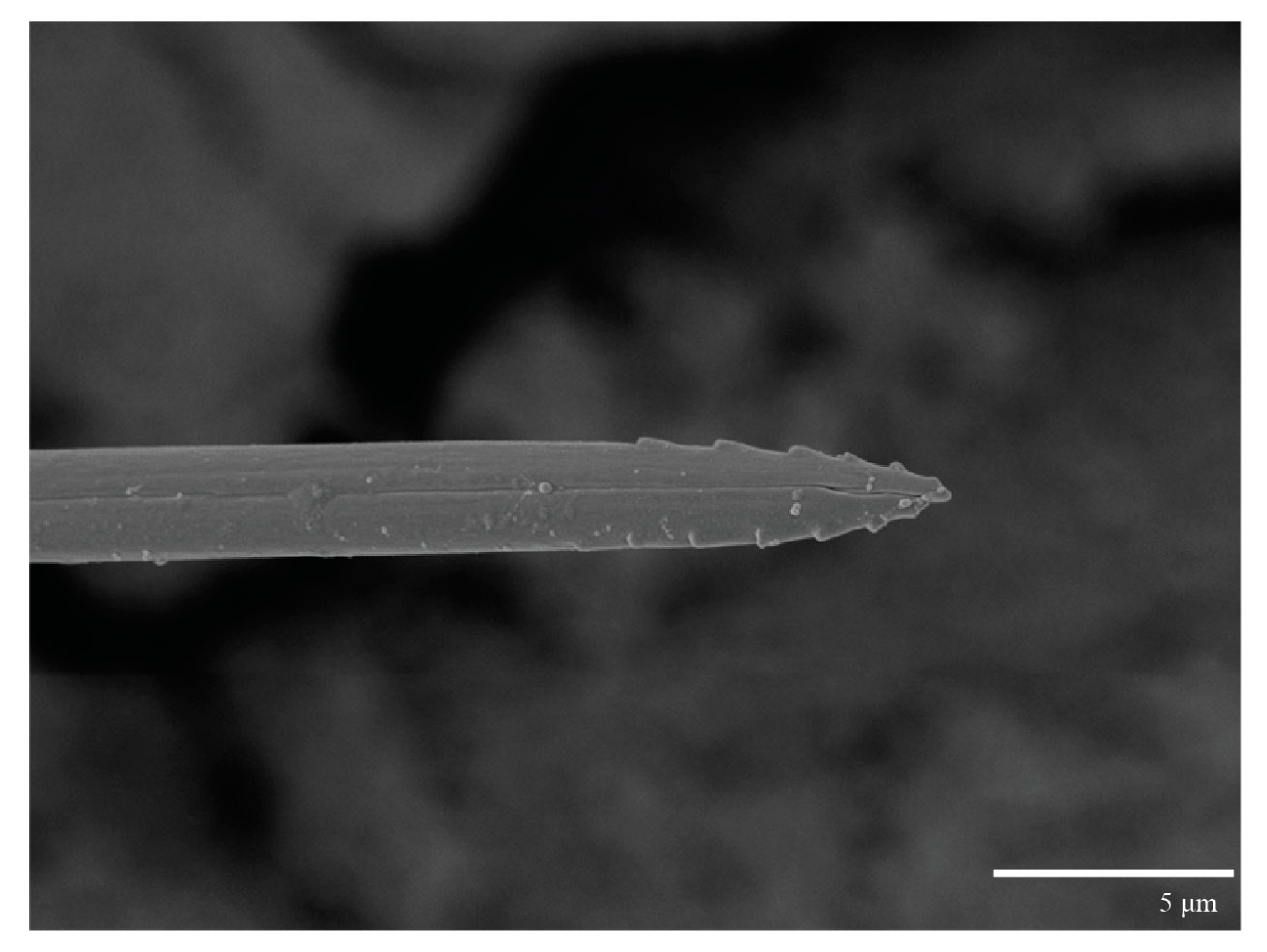
Gnathosoma (Figure 7, Figure 8 and Figure 9). Palps four segmented, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 7 (6–8), d″ 6 (5–6), tarsus with one eupathidium 5 (3–5) and one solenidion 1 (1–2). Ventral setae m 8 (6–8); distance between setae m–m 14 (13–16). Tips of cheliceral stylets with a few bluntly rounded lateral projections (Figure 9).
Spermatheca (Figure 5B). Duct length ca. 75–85, terminating in smooth rounded bulb.
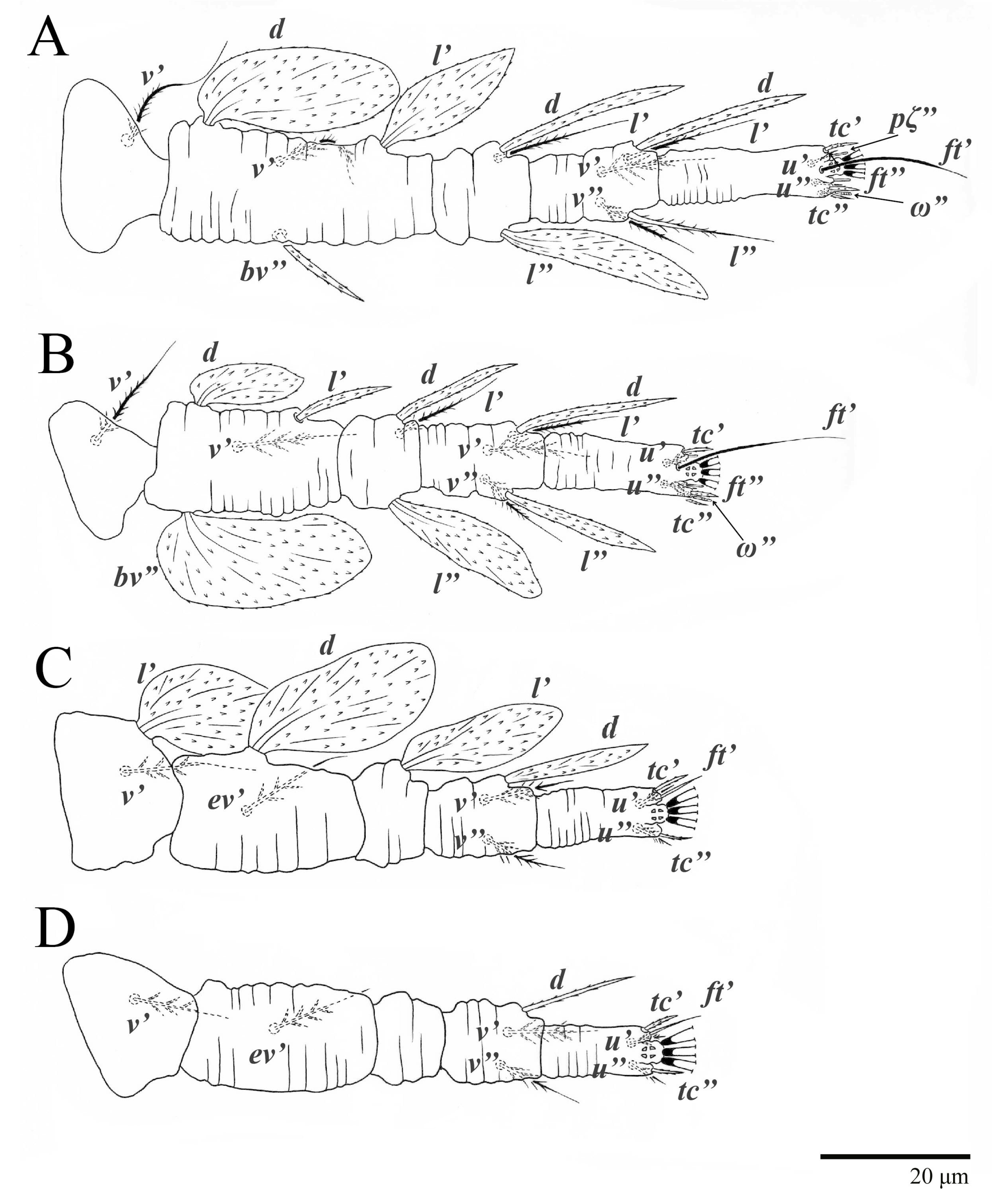
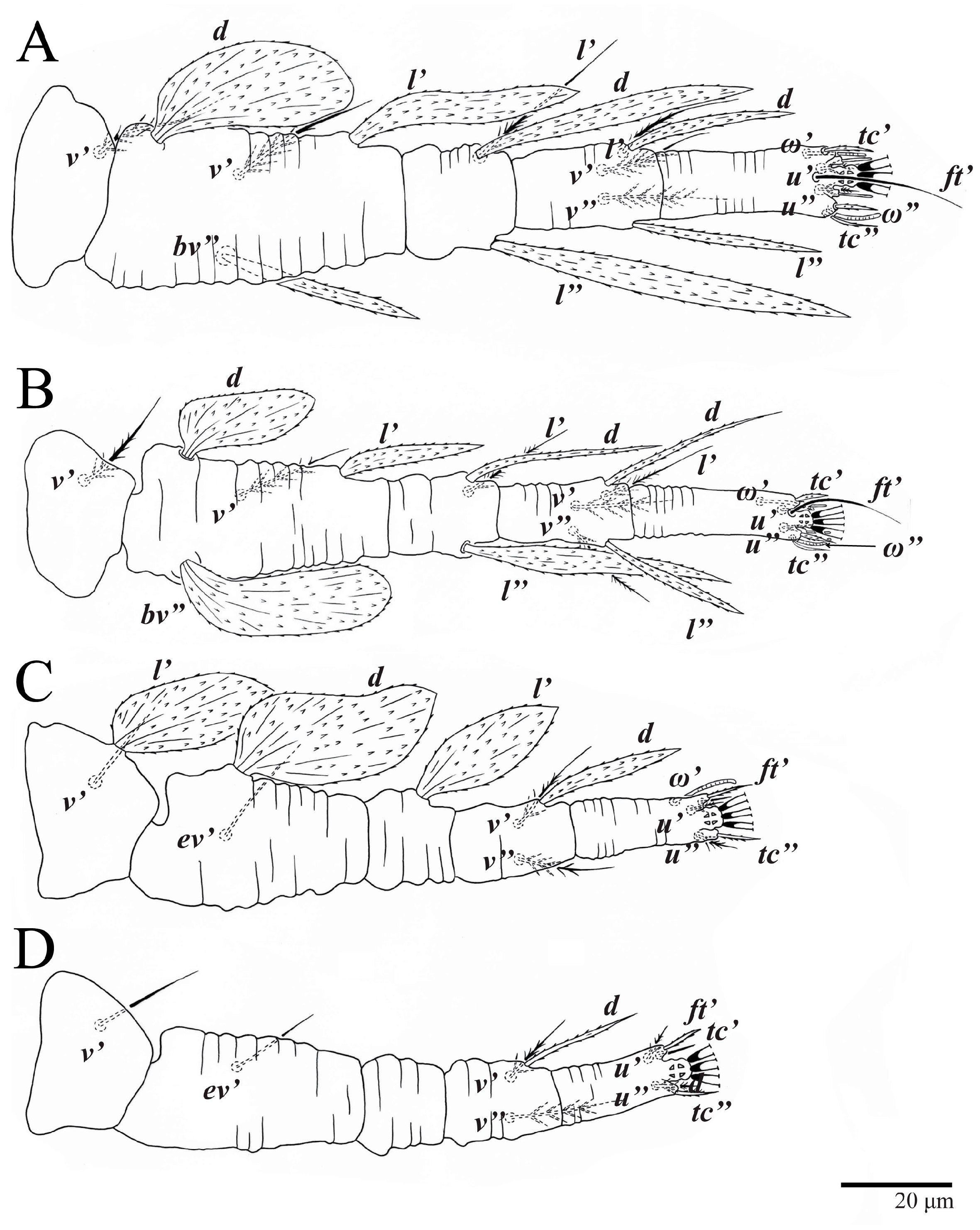
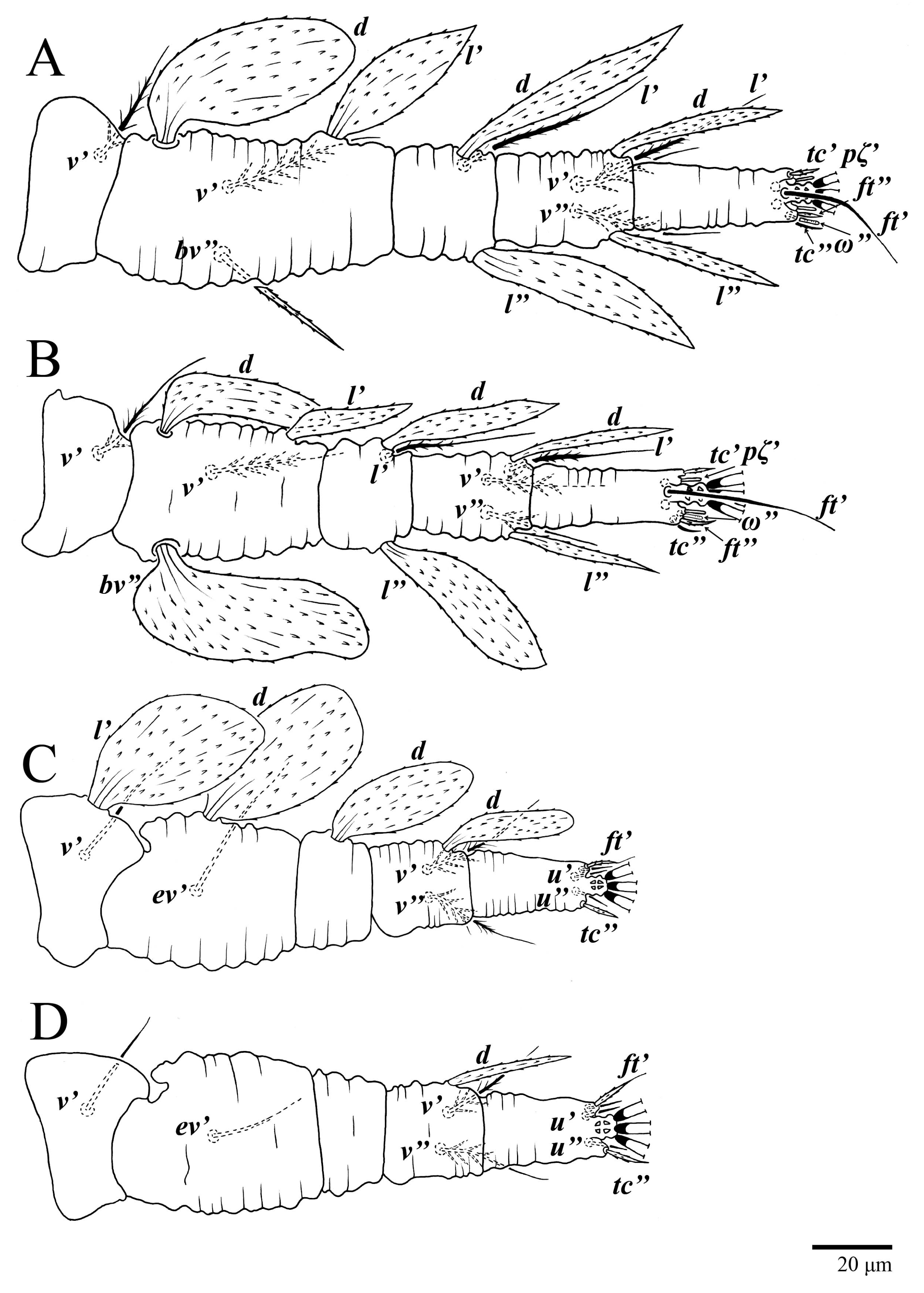
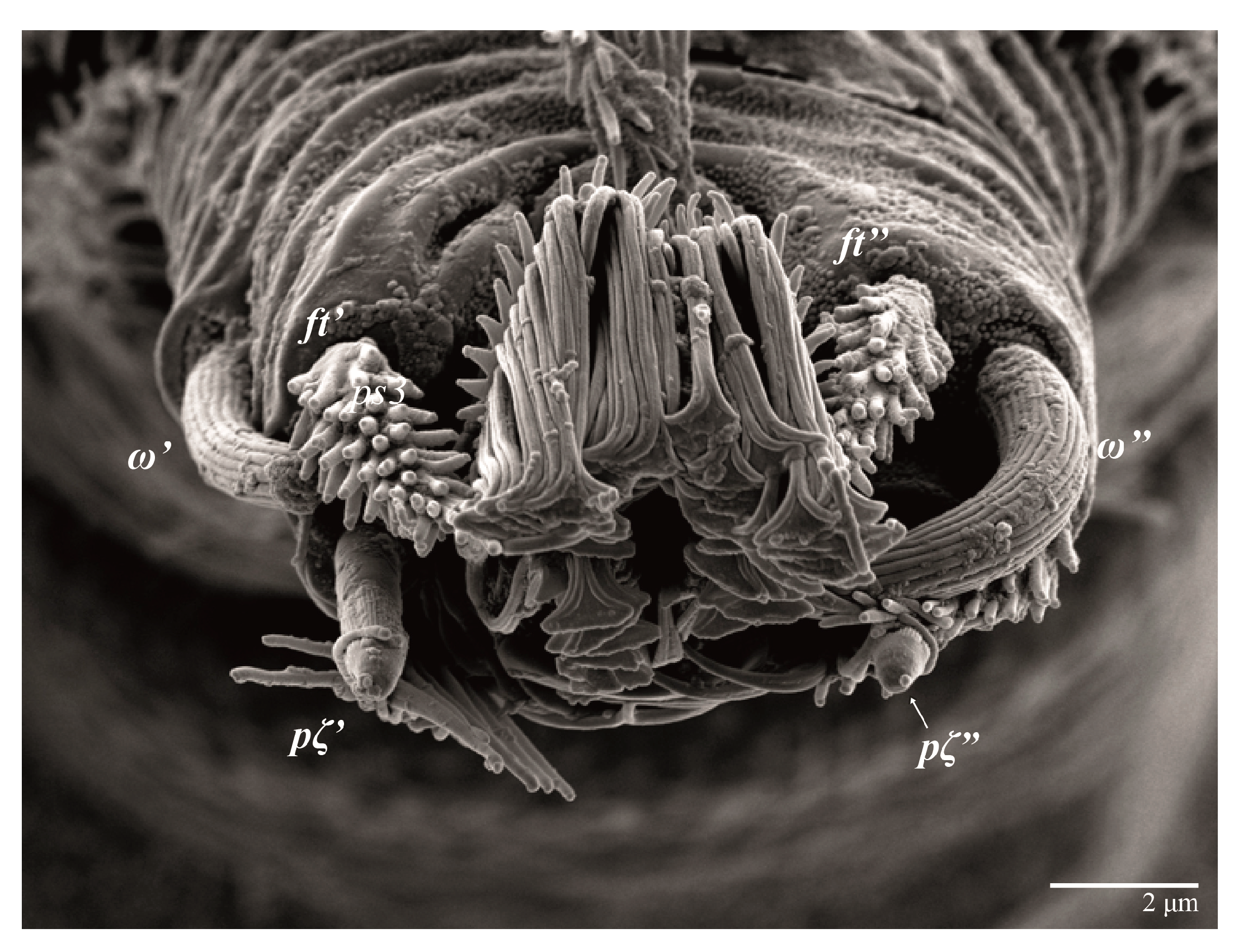
Legs (Figure 10). Setation (from coxae to tarsi): I 3–1–4–3–5–8(1), II 2–1–4–3–5–8(1), III 1–2–2–1–3–5, IV 1–1–1–0–3–5. Tarsi I–II each with one solenidion ω” 7 (6–8) (for both tarsi I and tarsi II) and two eupathidia pζ′–pζ” (5–6, 5–6; 5, 5–6, respectively); femur I with setae d obovate and l′ broadly lanceolate; femur II with setae d narrowly obovate, l′ lanceolate, and bv” obovate to broadly falcate. Femora, genua, and tibiae with setae d inserted in lateral position. Detail of the development of leg chaetotaxy in Table 1.

Table 1.
Additions of leg setae during ontogeny in both Ultratenuipalpus parameekeri Castro, Ochoa & Feres and Ultratenuipalpus meekeri (De Leon). The stage in which each seta first appears is indicated. Setae in parentheses represent pairs.
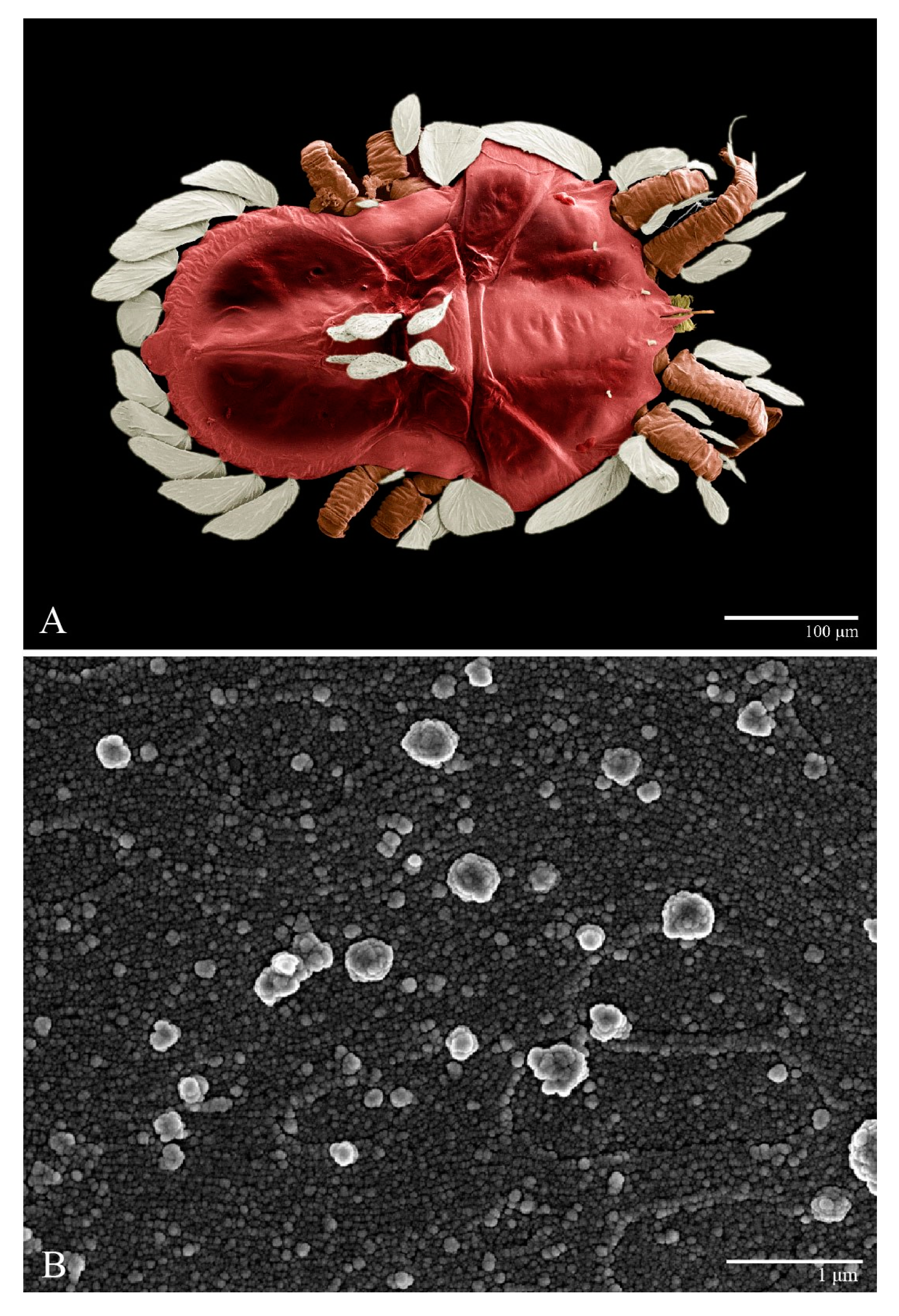
Microplates (Figure 2B). The microplate layer forms a reticulate network of thick ridges covered in small, single, irregularly-shaped wax-like crystals or masses.
Color. The body is mostly orange with the margin of prodorsum and opisthosoma with dark spots, eyes red, and legs orange. The dorsal body setae and leg setae white to translucent.
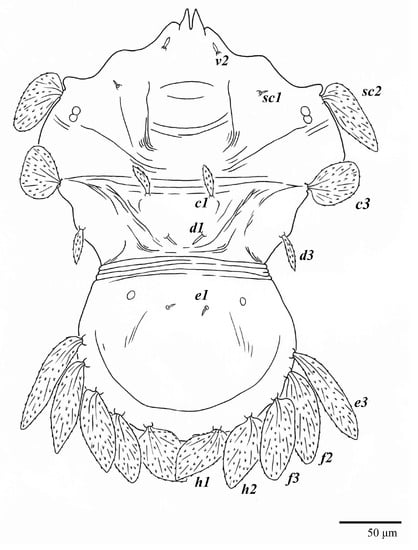
Figure 11.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Male): view of dorsum.

Figure 12.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Male): view of dorsum.
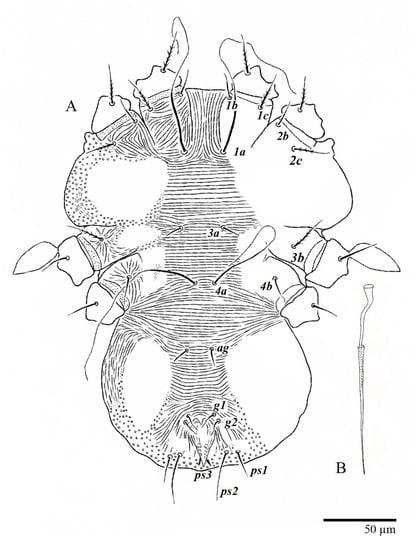
Figure 13.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Male): (A) view of venter; (B) aedeagus.
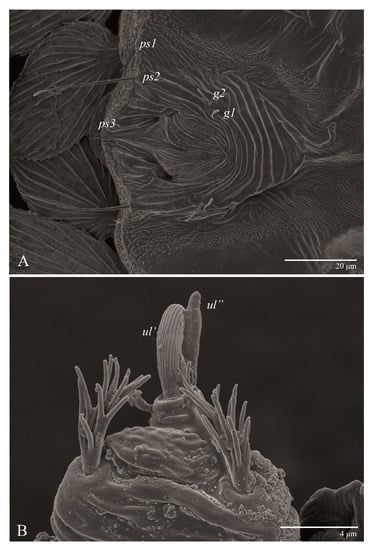
Figure 14.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Male): (A) posterior ventral opisthosoma; (B) detail of palp. Note the well-developed solenidion.
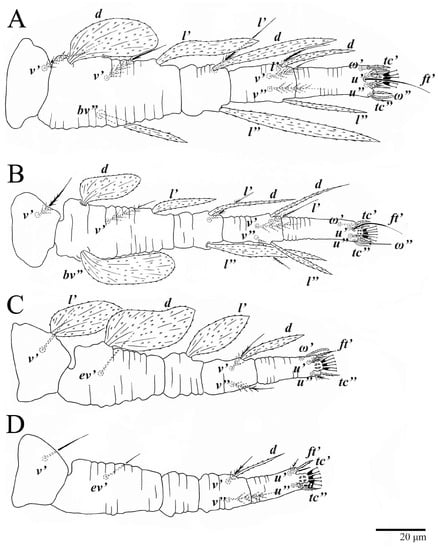
Figure 15.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Male): (A) leg I; (B) leg II; (C) leg III; (D) leg IV. (Right legs).
Body measurements: distance between setae, v2–h1 285–310, sc2–sc2 200–220; other measurements: v2–v2 45–50, sc1–sc1 105–120, c1–c1 50–58, c3–c3 190–215, d1–d1 27–30, d3–d3 155–175, e1–e1 27–33, e3–e3 165–175, f2–f2 160–170, f3–f3 140–145, h1–h1 55–58, h2–h2 105–115.
Dorsum (Figure 11 and Figure 12). Anterior margin of prodorsum with a short median forked projection forming a short notch. The dorsum is smooth, with a pair of lateral projections anterior to setae sc2 and a single projection between opisthosomal setae h1. Many dorsal setae similar in general form to those of female, except c1, d1, and e1 short to minute, d3 longer, and setae along posterior margin of opisthosoma (especially e3) narrower and more elongated than those of the female. Setal measurements: v2 5–7, sc1 4–6, sc2 60–67, c1 29–30, c3 40–45, d1 8–10, d3 19–27, e1 5–7, e3 77–80, f2 60–72, f3 60–63, h1 49–50, h2 53–55.
Venter (Figure 13 and Figure 14A). Ventral integument weakly striate along central region and densely colliculated along lateral body margin; ventral setae filiform, with coxal setae 1c, 2c, and 3b barbed; setae ps2 distinctly longer than ps1; setae ps3 thickened and inserted ventrally on the elongate tapered anal valves. Setal measurements: 1a 100–105, 1b 18–21, 1c 27–30, 2b 23–29, 2c 34–35, 3a 17–23, 3b 40–42, 4a 120–130, 4b 23–30, ag 14–15, g1 12–13, g2 14–17, ps1 21–23, ps2 42–55, ps3 13–14.
Gnathosoma (Figure 14B). Palps four segmented, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 7–8, d″ 7–8, tarsus with one eupathidium 5–6 and one solenidion 6. Ventral setae m 6–7; distance between setae m–m 13–14.
Legs (Figure 15). Setation (from coxae to tarsi): I 3–1–4–3–5–8(2), II 2–1–4–3–5–8(2), III 1–2–2–1–3–5(1), IV 1–1–1–0–3–5. Tarsi I–II each with two solenidia (one abaxial, one adaxial), tarsi I ω″ 10–11, ω′ 16–17, tarsi II ω″ 11–12, ω′ 14–15 and two eupathidia pζ′–pζ” (6–7, 7; 5–6, 5–6), and tarsus III with one solenidion (paraxial and ventrolateral) ω′ 13–15. Leg setae similar to that of the female; seta l” on genu I distinctly elongated. Detail of the development of leg chaetotaxy in Table 1.
Aedeagus (Figure 13B). As figured; ca. 130 long.
Deutonymph (n = 3) (Figure 16)
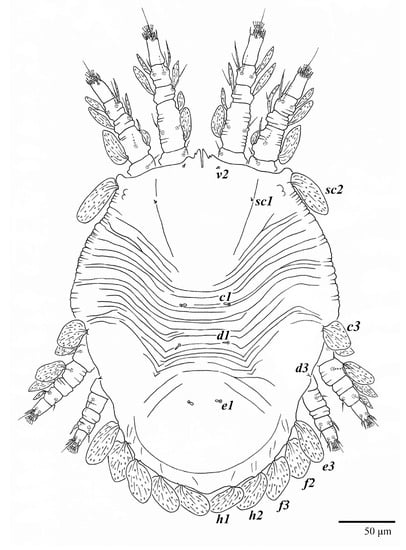
Figure 16.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Deutonymph): dorsum, with detail of legs (unguinal setae u′–u” on tarsus I and II are not included in the drawing).
Body measurements: distance between setae v2–h1 335–365, sc2–sc2 165–180; other measurements: v2–v2 37–40, sc1–sc1 93–105, c1–c1 42–55, c3–c3 235–265, d1–d1 40–50, d3–d3 200–215, e1–e1 18–28, e3–e3 155–175, f2–f2 145–160, f3–f3 120–135, h1–h1 45–50, h2–h2 87–95.
Dorsum (Figure 16). Anterior margin of prodorsum with a short median forked projection forming a short notch; pair of lateral projections anterior and adjacent to setae sc2 present; projection not formed (or rudimentary) between setae h1. Prodorsal region smooth; region between setae sc2–c3 with transverse plicae and folds; region posterior to setae d1–d3 smooth. Dorsal setae similar in general form to that of females, except setae c1, d1 and e1 short to minute. Setal measurements: v2 3–4, sc1 2–3, sc2 60–64, c1 3–5, c3 32–35, d1 2–3, d3 3–4, e1 3–4, e3 45–54, f2 40–42, f3 41–42, h1 35–36, h2 38–45.
Gnathosoma. Palps similar to those of female, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 4–5, d″ 4–5, tarsus with one eupathidium 3–4 and one minute solenidion, 1 long. Ventral setae m 4–5; distance between setae m–m 10–11.
Venter. Cuticle covered with fine and mostly transverse striae. Coxal, genital, and anal setae fine. Setal lengths: 1a 80–100, 1b 10–15, 1c 10–12, 2b 10–14, 2c 18–20, 3a 12–13, 3b 12–17, 4a 50–80, 4b 12–21, ag 7–8, g1 8–9, ps1 12–14, ps2 25–27, ps3 9–10. Setae g2 absent.
Legs (Figure 16). Setation (from coxae to tarsi): I 3–1–4–3–5–8(1), II 2–1–4–3–5–8(1), III 1–2–2–1–3–5, IV 1–0–1–0–3–5. Leg chaetotaxy similar to that of the female, except by trochanter IV nude; tarsi I–II each with one solenidion ω” (tarsi I 4–5 and tarsi II 5), and two eupathidia pζ′–pζ” (4–5, 5; 4–5, 4–5, respectively). Detail of the development of leg chaetotaxy in Table 1.
Protonymph (n = 3) (Figure 17)
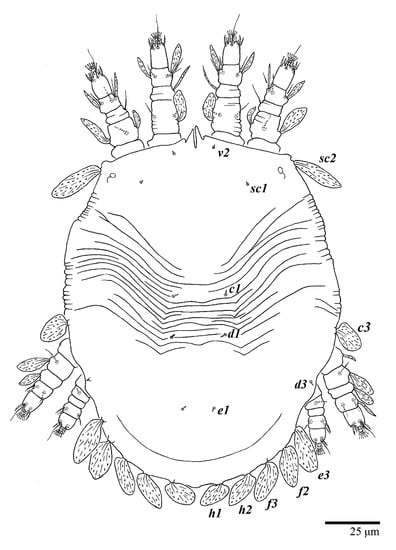
Figure 17.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Protonymph): dorsum, with detail of legs (unguinal setae u′–u” on tarsus I and II are not included in the drawing).
Body measurements: distance between setae v2–h1 275–290, sc2–sc2 135–145; other measurements: v2–v2 32–35, sc1–sc1 80–83, c1–c1 40–43, c3–c3 185–195, d1–d1 35–38, d3–d3 150–155, e1–e1 22–25, e3–e3 120–130, f2–f2 110–115, f3–f3 90–95, h1–h1 30–33, h2–h2 62–65.
Dorsum (Figure 17). Anterior margin of prodorsum with a short median forked projection forming a short notch; pair of lateral body projections anterior and adjacent to setae sc2 present. Prodorsal region smooth; region between setae sc2–c3 with transverse striations and region posterior to setae c3 smooth; dorsal setae similar to that of the female, except setae c1, d1, and e1 short. Setal measurements: v2 2–3, sc1 2–3, sc2 40–44, c1 3–4, c3 24–25, d1 3–4, d3 3–4, e1 2–3, e3 30–32, f2 27–28, f3 24–26, h1 22–25, h2 24–25.
Gnathosoma. Palps similar to those of the female, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 4–5, d″ 3–4, tarsus with one eupathidium 3–4 and one solenidion, 1 long. Ventral setae m 4–5; distance between setae m–m 10–12.
Venter. Cuticle covered with fine and mostly transverse striae. Coxal, genital and anal setae fine. Setal measurements: 1a 65–67, 1b 10–11, 1c 9–12, 2c 12–13, 3a 10–13, 3b 14–17, ag 6–7, ps1 7–9, ps2 13–15, ps3 7–8. Setae 2b, 4a, 4b, g1 and g2 absent.
Legs (Figure 17). Setation (from coxae to tarsi): I 3–0–3–1–5–6(1), II 1–0–3–1–5–6(1), III 1–0–2–0–3–5, IV 0–0–1–0–3–3. Tarsi I–II each with one solenidion ω” 4–5 (for both tarsi I and tarsi II) and two eupathidia pζ′–pζ” (all 3–4). Detail of the development of leg chaetotaxy in Table 1.
Larva (n = 3) (Figure 18)
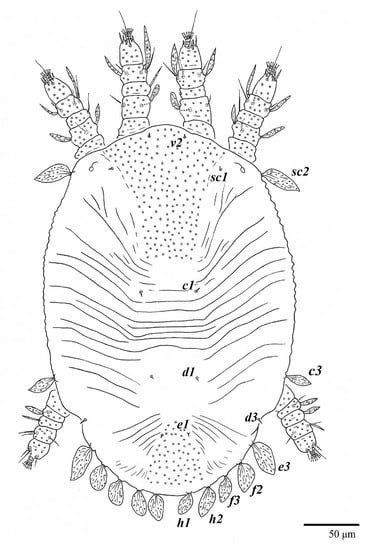
Figure 18.
Ultratenuipalpus parameekeri Castro, Ochoa & Feres sp. nov. (Larva): dorsum, with detail of legs (unguinal setae u′–u” on tarsus I and II are not included in the drawing).
Body measurements: distance between setae v2–h1 220–230, sc2–sc2 115–125; other measurements: v2–v2 22–25, sc1–sc1 70–73, c1–c1 32–38, c3–c3 140–150, d1–d1 30–38, d3–d3 110–115, e1–e1 16–18, e3–e3 100–105, f2–f2 86–88, f3–f3 67–70, h1–h1 20–23, h2–h2 40–45.
Dorsum (Figure 18). Prodorsal region with colliculated integument anteromedially; region between setae sc2–c3 with oblique and transverse folds; pygidial region posterior to setae e1 with colliculated integument; dorsal setae similar in general form to those of females except much smaller and setae c1, d1, and e1 minute. Setal measurements: v2 2–3, sc1 1–2, sc2 25–26, c1 2–4, c3 16–18, d1 2–3, d3 2–3, e1 2–3, e3 22–23, f2 16–20, f3 15–18, h1 15–17, h2 16–17.
Gnathosoma. Palps similar to those of female, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 3–4, d″ 5, tarsus with one eupathidium 3–4 and one minute solenidion, 1 long. Setae m absent.
Venter. Cuticle covered with fine and mostly transverse striae. Coxal, genital, and anal setae fine. Setal measurements: 1a 55–65, 1b 7–8, 3a 10–11, ps1 5–7, ps2 10–11, ps3 5–6. Setae 1c, 2b, 2c, 3b, 4a, 4b, ag, g1, and g2 absent.
Legs (Figure 18). Setation (from coxae to tarsi): I 2–0–3–1–5–6(1), II 0–0–3–1–5–6(1), III 0–0–2–1–3–3. Tarsi I–II each with one solenidion ω” 3–4 (for both tarsi I and II) and two eupathidia pζ′–pζ” (3–4, 3–4; 3–4, 3–4, respectively). Cuticle of all legs covered with colliculated sculpturing. Detail of the development of leg chaetotaxy in Table 1.
Etymology. The specific name parameekeri refers to the morphological similarity of this species and U. meekeri (De Leon), the type species of the genus.
Differential diagnosis. This new species resembles Ultratenuipalpus meekeri (De Leon) (herein redescribed) as they both have dorsal setae of a similar shape and length and the same leg and palp chaetotaxy in all developmental stages. These two species also share several other characteristics, such as the pair of lateral projections anterior to setae sc2 and a single posterior projection between opisthosomal setae h1. However, the two species can be separated: the prodorsum is distinctly broader in adult females and males (measured at the widest point between setae sc1 and c1) in U. meekeri (325–345) than U. parameekeri (290–315) (in females); notch in anterior forked projection is shorter in U. meekeri (8–13) than in U. parameekeri (20–27) (in females); e3 is narrower and more lanceolate on male (and to a lesser extent on females) U. meekeri than in U. parameekeri; l” on ti I on U. meekeri is thicker than on U. parameekeri; d on fe II is longer and more falcate on female U. meekeri than on U. parameekeri; c3 in larvae is narrower and more lanceolate in U. meekeri than U. parameekeri. In addition to the morphological differences, the molecular analyses confirmed that U. parameekeri and U. meekeri represent distinct species, with a 15.7% difference between their COI sequences.
DNA Barcoding. DNA was successfully amplified and the mitochondrial cytochrome C oxidase subunit I gene (COI) sequenced from one specimen of U. parameekeri collected on Cyclosorus interruptus (Thelypteridaceae) from Pindorama, São Paulo, Brazil; sequence data have been deposited in GenBank (https://www.ncbi.nlm.nih.gov/, accessed on 15 January 2023), with the following accession code: female, 398 base pairs (GenBank: OQ533138).
Type material examined. Holotype: female collected on ferns Rumohra adiantiforme (Dryopteridaceae) from Ilha do Cardoso, São Paulo, Brazil, 22 March 2017, coll. G.C.O. Piccoli (DZSJRP). Paratypes: 3 females, 1 protonymph, and 2 larvae, with the same data as the holotype (DZSJRP); 4 females, 3 males, 4 deutonymphs, 5 protonymphs, and 2 larvae collected on Psychotria nuda (Rubiaceae) from Ilha do Cardoso, São Paulo, Brazil, 22 March 2017, coll. G.C.O. Piccoli (DZSJRP); 2 females and 2 males collected on P. nuda from Ilha do Cardoso, São Paulo, Brazil, 22 March 2017, coll. G.C.O. Piccoli (NMNH); 4 females and 1 deutonymph collected on ferns C. interruptus from Pindorama, São Paulo, Brazil, 15 December 2002, coll. R. Kishimoto (DZSJRP).
Other material examined. 1 female and 1 larva collected on ferns C. interruptus from Pindorama, São Paulo, Brazil, 15 March 2003, coll. P. Demite (DZSJRP); 2 females, 2 deutonymphs, 3 protonymphs, and 1 larva collected on ferns C. interruptus from Pindorama, São Paulo, Brazil, 15 December 2002, coll. R. Kishimoto (DZSJRP); 2 females, 2 deutonymphs, 3 protonymphs, and 1 larva collected on ferns C. interruptus from Pindorama, São Paulo, Brazil, 15 March 2005, coll. P. Demite (DZSJRP, USNM).
3.2. Redescription of Ultratenuipalpus meekeri (De Leon, 1957)
- Tenuipalpus meekeri De Leon: De Leon [13]—original designation
- Ultratenuipalpus meekeri (De Leon): Mitrofanov [14]
Redescriptions [4,15,16,17,18,19].
Diagnosis. Female: As per genus, in addition to: prodorsal setae v2, sc1 minute to short, and sc2 large, flattened, obovate to ovate; dorsal opisthosoma with 10 pairs of setae (f2 present); most of the dorsal opisthosomal setae large, flattened, obovate to ovate, except setae d3 is distinctly short and c3 is almost orbicular; pair lateral projections anterior to setae sc2 and single posterior projection between opisthosomal setae h1 present; palp four segmented, setal formula 0, 0, 2, 2. Male: Opisthosoma narrower than that of females, with a distinct transverse constriction (waist) between setae d1 and e1; many dorsal setae similar to those of the female, except c1 much smaller, d1 and e1 short to minute, and v2 and d3 longer. Tarsi I–II each with two solenidia (ω′ paraxial and ventrolateral; ω′ antiaxial); tarsus III with one solenidion ω′ paraxial and ventrolateral. Immatures: with lateral body projections anterior to setae sc2 present (except absent in larvae); single posterior projection between setae h1 absent; dorsal setae similar in general form to those of the female, except c1, d1, and e1 minute. Larvae with anterior margin colliculated and central prodorsum smooth; pygidial region of posterior opisthosoma with colliculated integument.
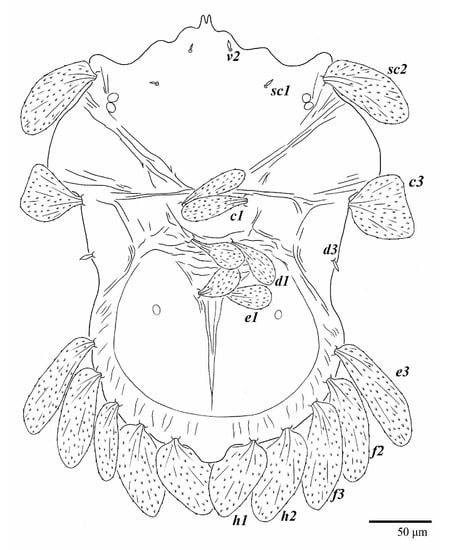
Figure 19.
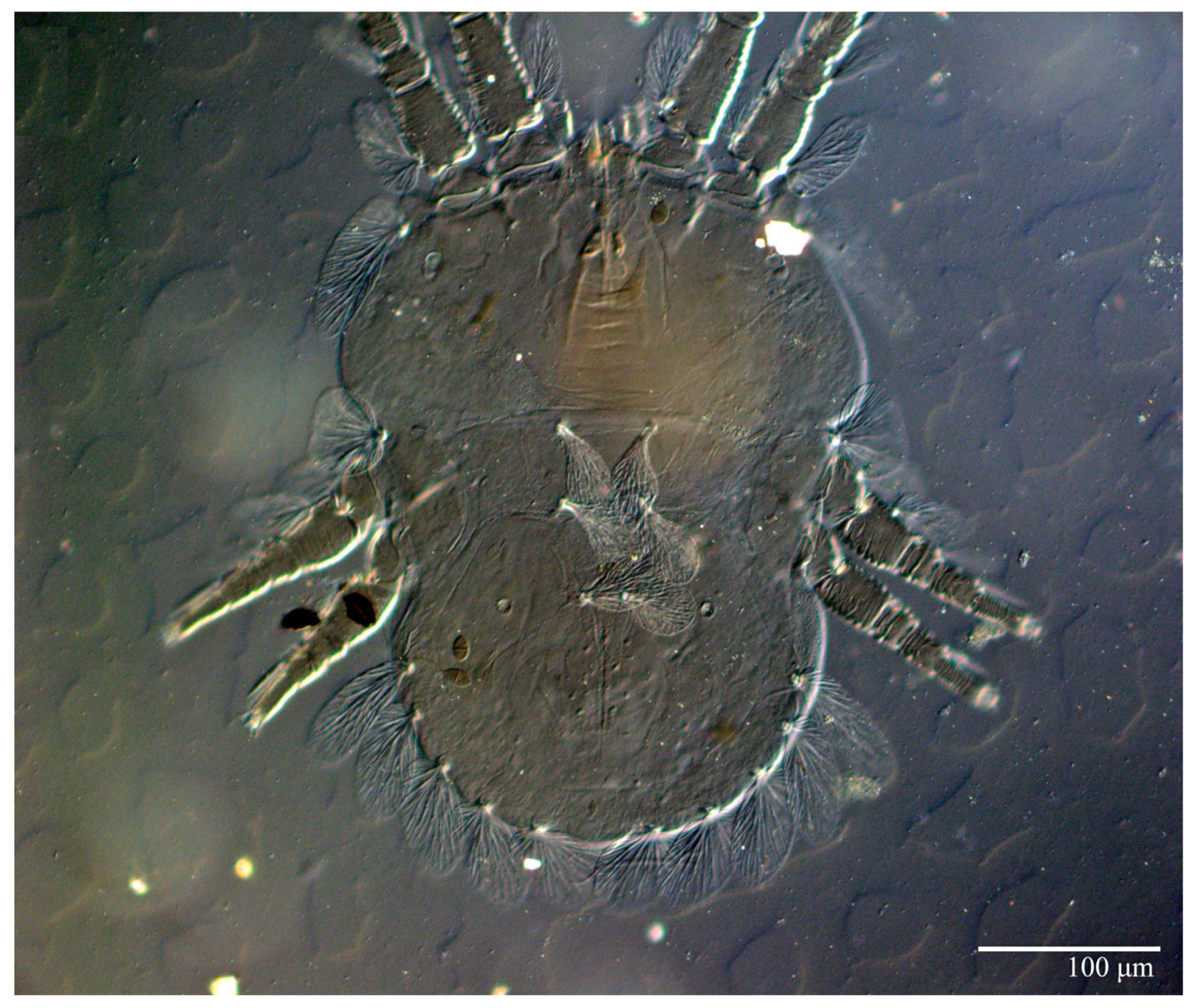
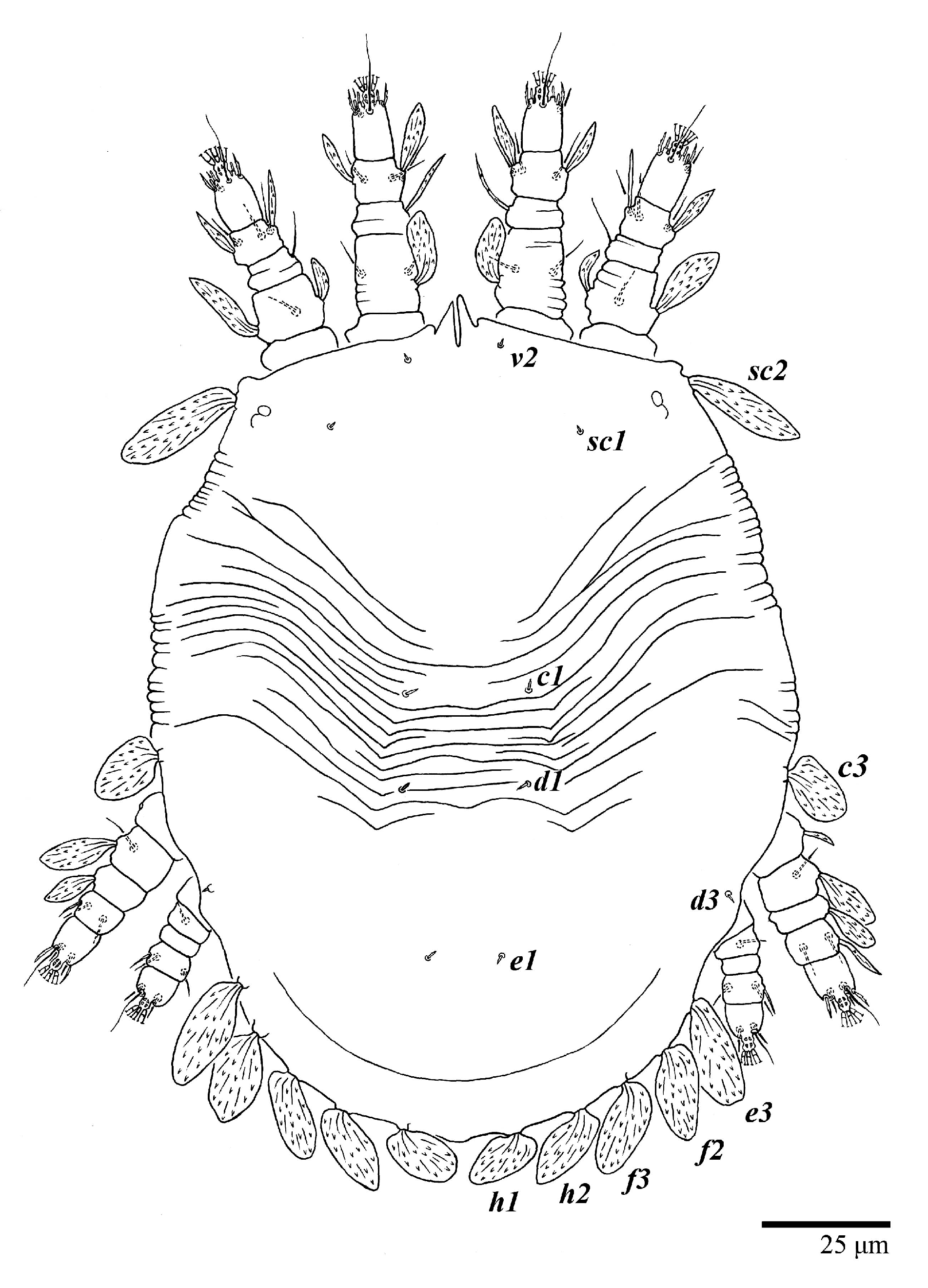
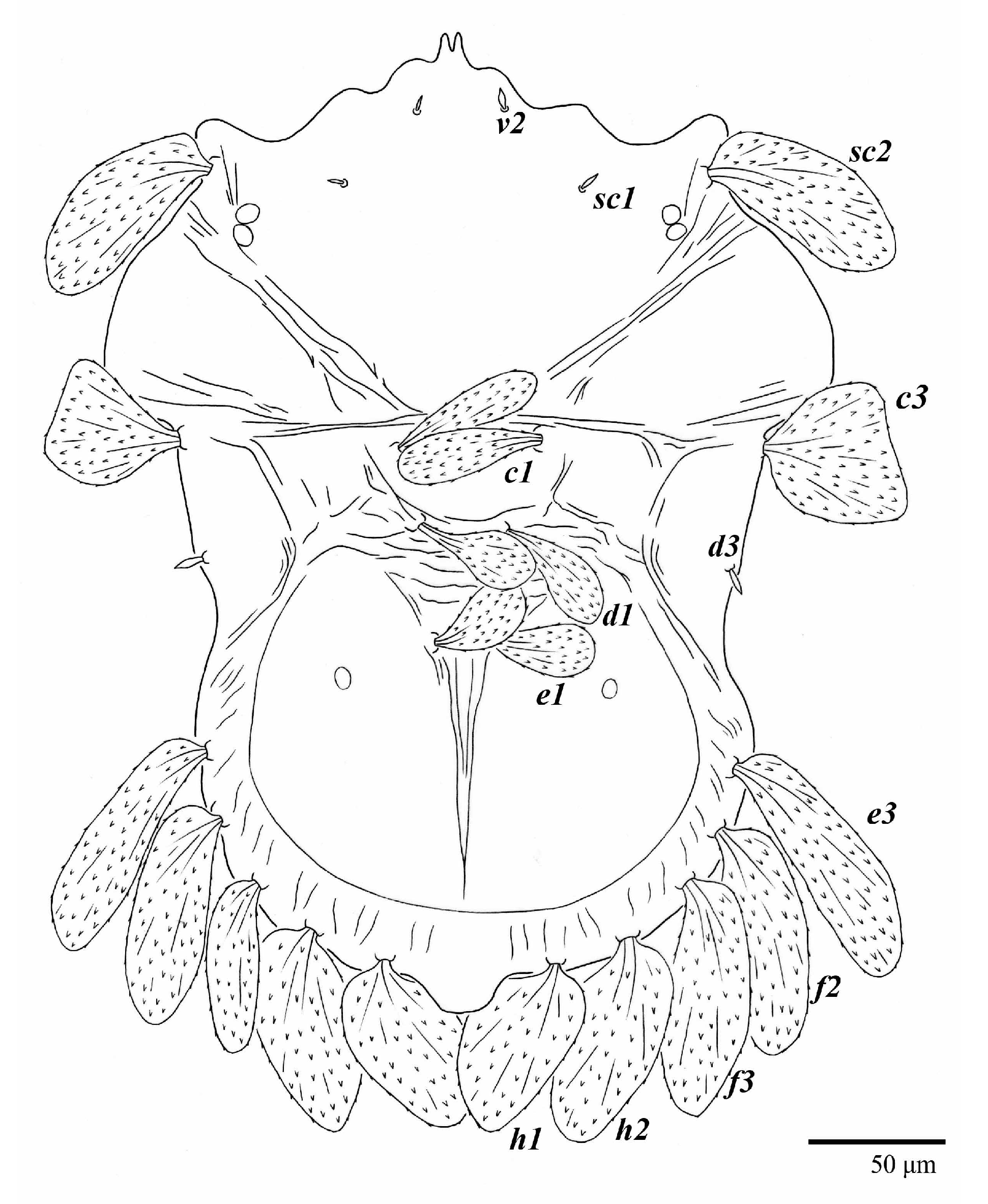
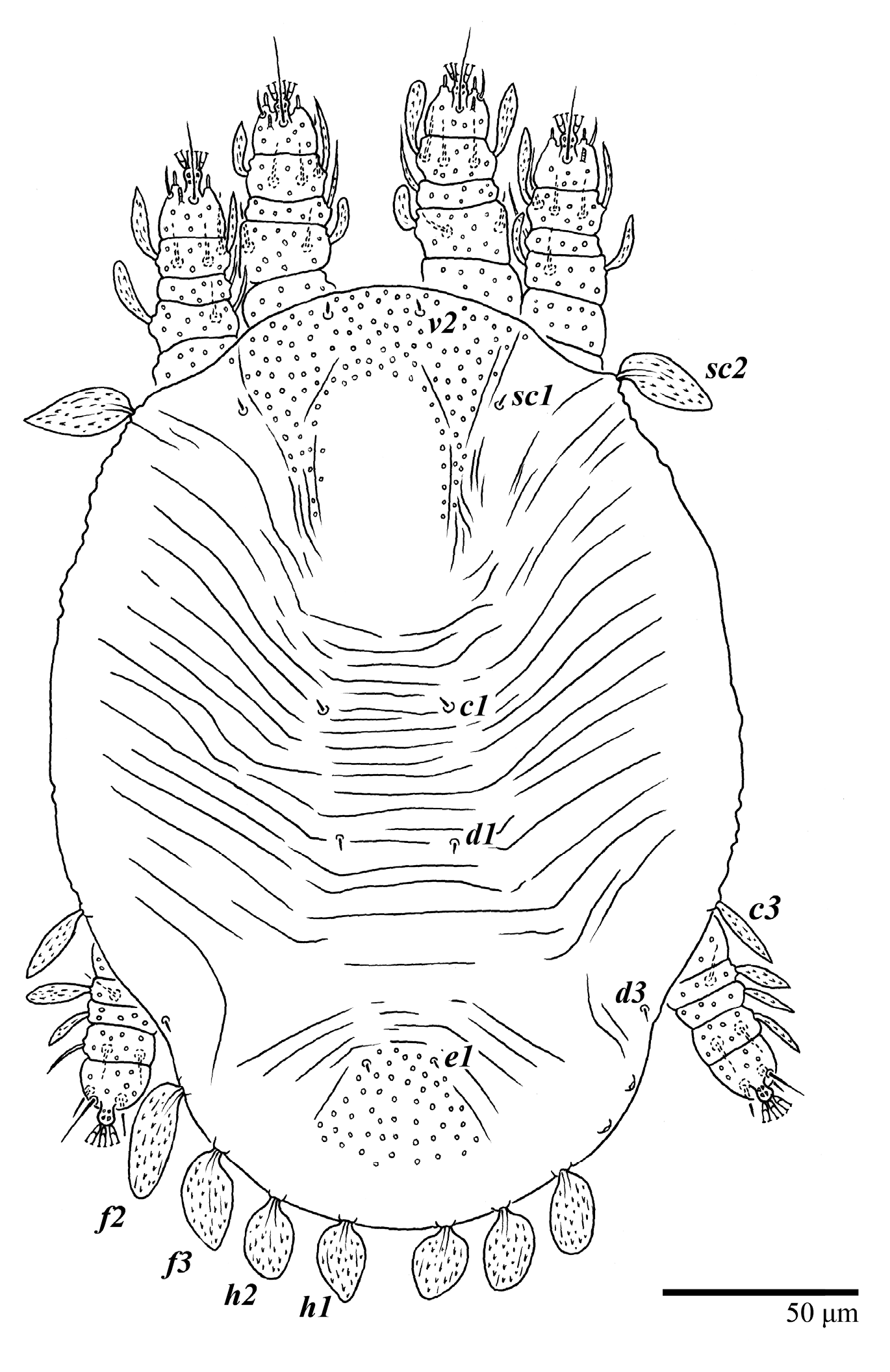
Ultratenuipalpus meekeri (De Leon). (Female, paratype): view of dorsum.
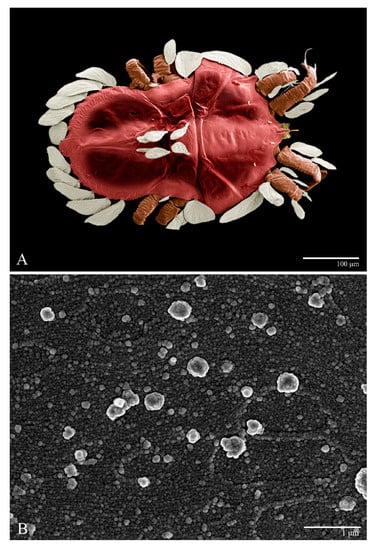
Figure 20.
Ultratenuipalpus meekeri (De Leon). (Female): (A) dorsal view; (B) view of cuticular microplates on the dorsum.
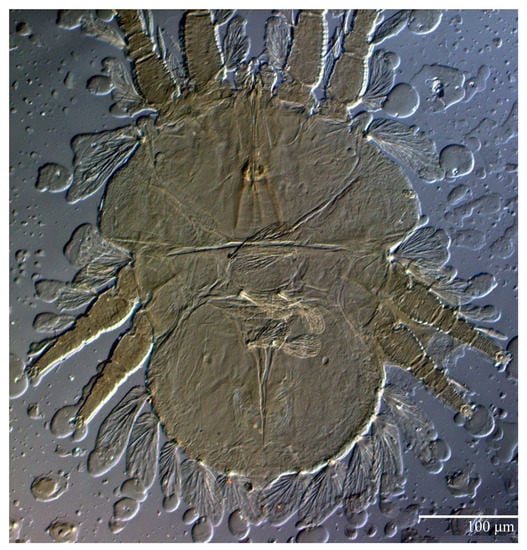
Figure 21.
Ultratenuipalpus meekeri (De Leon). (Female, paratype): view of dorsum.
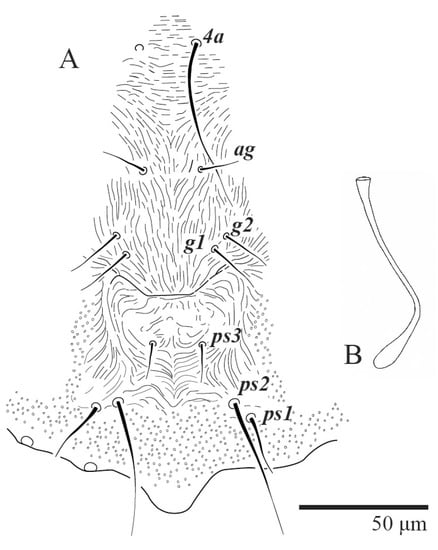
Figure 22.
Ultratenuipalpus meekeri (De Leon). (Female, paratype): (A) posterior ventral opisthosoma; (B) spermatheca.

Figure 23.
Ultratenuipalpus meekeri (De Leon). (Female): (A) view of ventral infracapitulum; (B) detail of palp; note the basal insertion of solenidion.
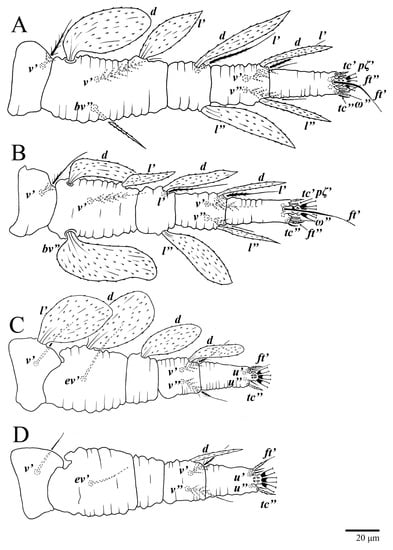
Figure 24.
Ultratenuipalpus meekeri (De Leon). (Female, paratype): (A) leg I; (B) leg II; (C) leg III; (D) leg IV. (Right legs).
Body measurements: distance between setae v2–h1 390 (375–390), sc2–sc2 230 (230–235); other measurements: v2–v2 42 (42–48), sc1–sc1 115 (115), c1–c1 65 (65–68), c3–c3 290 (260–290), d1–d1 43 (37–43), d3–d3 250 (235–250), e1–e1 28 (25–30), e3–e3 235 (230–240), f2–f2 225 (215–225), f3–f3 190 (185–195), h1–h1 72 (72–78), h2–h2 135 (130–140).
Dorsum (Figure 19, Figure 20 and Figure 21). Anterior margin of prodorsum with a short median forked projection forming a short notch 8 (8–13). Dorsum smooth, with pair of lateral projections anterior to setae sc2 and a single projection between opisthosomal setae h1 present. A pair of converging folds from the eyes to near the sejugal furrow on the prodorsum posterior margin. Prodorsal setae v2 and sc1 short to minute; sc2 large, flattened elongated obovate (Figure 19 and Figure 20A); most opisthosomal setae similar to prodorsal setae sc2, except d3 short. Setal measurements: v2 8 (4–8), sc1 4 (4–10), sc2 83 (83–94), c1 65 (65–69), c3 57 (54–57), d1 55 (55–58), d3 14 (14–15), e1 48 (39–48), e3 95 (92–95), f2 80 (80–84), f3 77 (77–83), h1 63 (62–66), h2 70 (70–73).
Venter (Figure 22A). Ventral integument weakly striate along central region and densely colliculated around lateral body margin; ventral, genital, and anal plates not developed, entire region membranous and distinctly plicate; ventral setae filiform, with coxal setae 1c, 2c, and 3b barbed; setae ps2 distinctly longer than ps1. Setal measurements: 1a 105 (105–115), 1b 19 (12–19), 1c 29 (26–29), 2b 27 (27–28), 2c 47 (41–47), 3a 20 (20–21), 3b 43 (37–43), 4a 105 (95–115), 4b 26 (23–26), ag 15 (15–17), g1 19 (15–19), g2 17 (17–20), ps1 15 (12–15), ps2 48 (48–60), ps3 31 (23–31).
Gnathosoma (Figure 23). Palps four segmented, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 7 (7–11), d″ 8 (7–8), tarsus with one eupathidium 5 (5) and one solenidion, 1 (1) long. Ventral setae m 7 (7–8); distance between setae m–m 15 (13–15).
Spermatheca (Figure 22B). Duct length ca. 70–85, terminating in smooth rounded bulb.
Legs (Figure 24). Setation (from coxae to tarsi): I 3–1–4–3–5–8(1), II 2–1–4–3–5–8(1), III 1–2–2–1–3–5, IV 1–1–1–0–3–5. Tarsi I–II each with one solenidion ω” 9 (8–9) (for both tarsi I and tarsi II) and two eupathidia pζ′–pζ” (7, 7; 7, 6–7, respectively); femur I with setae d obovate and l′ broadly lanceolate; femur II with setae d elongate obovate to weakly falcate, l′ lanceolate and bv” obovate to broadly falcate. Femora, genua, and tibiae with setae d inserted in lateral position. Detail of the development of leg chaetotaxy in Table 1.
Color (Figure 20A). The body is reddish with the central region becoming darker, eyes red, and legs orange. Dorsal body setae and legs setae are white.
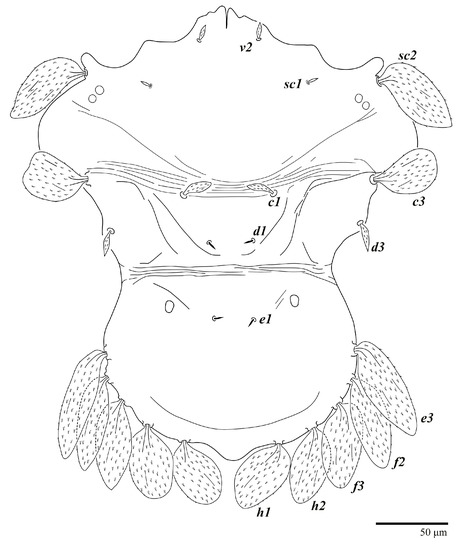
Figure 25.
Ultratenuipalpus meekeri (De Leon). (Male, paratype): view of dorsum.
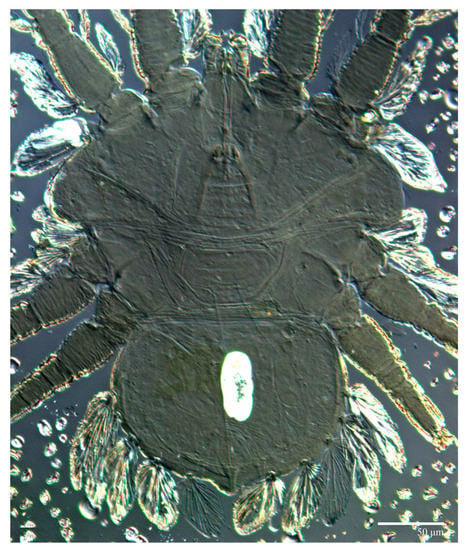
Figure 26.
Ultratenuipalpus meekeri (De Leon). (Male, paratype): view of dorsum.
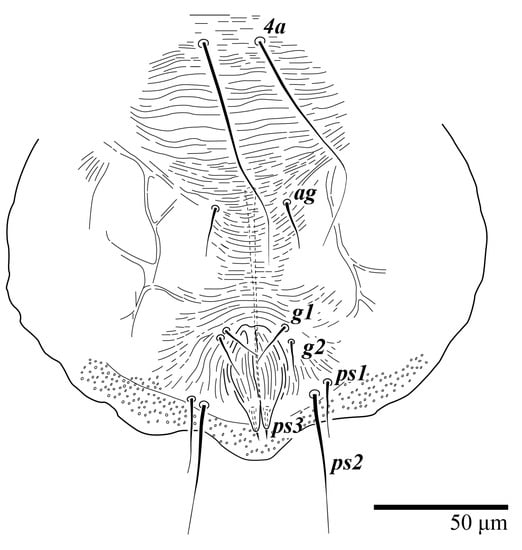
Figure 27.
Ultratenuipalpus meekeri (De Leon). (Male, paratype): posterior ventral opisthosoma.
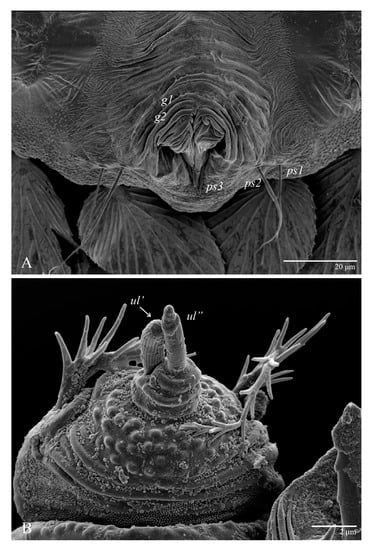
Figure 28.
Ultratenuipalpus meekeri (De Leon). (Male): (A) posterior ventral opisthosoma; (B) detail of palp; note the basal insertion of solenidion.
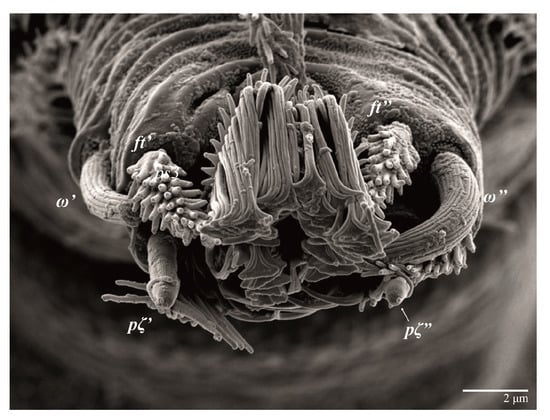
Figure 29.
Ultratenuipalpus meekeri (De Leon). (Male): detail of tarsus II. Note the presence of solenidion ω’ paraxial and ventrolateral.
Body measurements: distance between setae v2–h1 280, sc2–sc2 210; other measurements: v2–v2 43, sc1–sc1 110, c1–c1 65, c3–c3 205, d1–d1 30, d3–d3 165, e1–e1 28, e3–e3 175, f2–f2 170, f3–f3 150, h1–h1 63, h2–h2 110.
Dorsum (Figure 25 and Figure 26). Anterior margin of prodorsum with a short median forked projection forming a short notch. Dorsum smooth, with pair lateral projections anterior to setae sc2 and a single projection between opisthosomal setae h1 present. Prodorsum with a pair of converging folds from the eyes to near the sejugal furrow on the posterior margin. Dorsal setae similar in general form to those of the female, except c1, d1, and e1 small to minute, and d3 longer. Setal measurements: v2 10, sc1 8, sc2 65, c1 23, c3 49, d1 6, d3 18, e1 5, e3 74, f2 70, f3 66, h1 53, h2 57.
Venter (Figure 27 and Figure 28A). Ventral integument weakly striated along central region and densely colliculated around lateral margin of body; ventral setae filiform; coxal setae 1c, 2c, and 3b barbed; setae ps2 distinctly longer than ps1; setae ps3 thickened and inserted ventrodistally on elongated, tapered anal valves. Setal measurements: 1a 85, 1b 22, 1c 28, 2b 26, 2c 35, 3a 21, 3b 35, 4a 90, 4b 23, ag 20, g1 19, g2 16, ps1 24, ps2 60, ps3 16.
Gnathosoma (Figure 28B). Palps four segmented, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 8, d″ 7, tarsus with one eupathidium 5 and one solenidion 6. Ventral setae m 8; distance between setae m–m 14.
Legs. Setation (from coxae to tarsi): I 3–1–4–3–5–8(2), II 2–1–4–3–5–8(2), III 1–2–2–1–3–5(1), IV 1–1–1–0–3–5. Tarsi I–II (Figure 29) each with two solenidia (one abaxial, one adaxial), tarsi I ω′ 12, ω″ 9, tarsi II ω′ 13, ω″ 9, and two eupathidia pζ′–pζ” (all 6–7), and tarsus III with one solenidion (paraxial and ventrolateral) ω′ 12. Leg setae similar to that of the female. Detail of the development of leg chaetotaxy in Table 1.
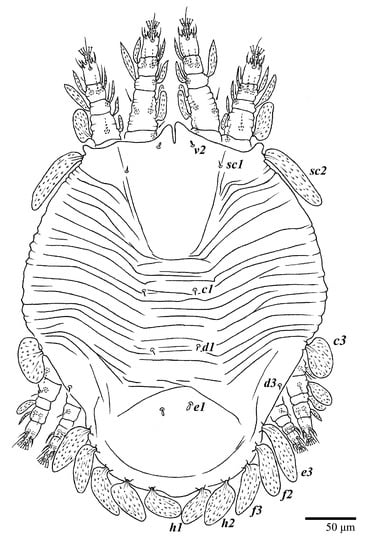
Figure 30.
Ultratenuipalpus meekeri (De Leon). (Deutonymph, paratype): dorsum, with detail of legs (unguinal setae u′–u” on tarsus I and II are not included in the drawing).
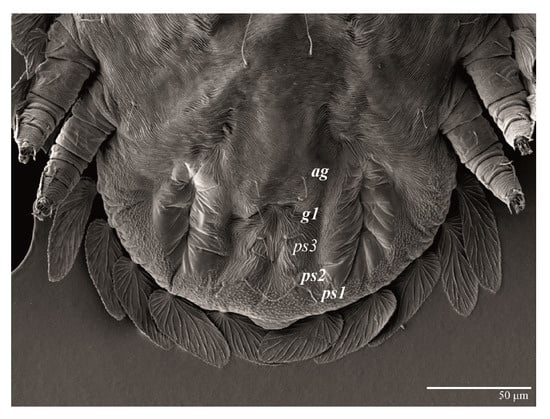
Figure 31.
Ultratenuipalpus meekeri (De Leon). (Deutonymph): posterior ventral opisthosoma.
Body size measurements: distance between setae v2–h1 310–350, sc2–sc2 170–190; other measurements: v2–v2 37–40, sc1–sc1 95–105, c1–c1 43–53, c3–c3 220–270, d1–d1 45–55, d3–d3 200–225, e1–e1 25–30, e3–e3 160–180, f2–f2 145–170, f3–f3 120–145, h1–h1 40–55, h2–h2 83–105.
Dorsum (Figure 30). Anterior margin of prodorsum with a short median forked projection forming a short notch; a pair of body projections anterior and adjacent to setae sc2 present; posterior projection between setae h1 absent. Prodorsum with central region smooth; region between setae sc2–c3 with transverse folds and plicae; region posterior to setae e1 smooth. Dorsal setae similar in general form to those of the female, except setae c1, d1, and e1 are short to minute. Setal measurements: v2 3–5, sc1 4–5, sc2 64–78, c1 6–12, c3 36–41, d1 5–7, d3 5–7, e1 4–8, e3 52–64, f2 51–55, f3 47–55, h1 36–42, h2 43–51.
Gnathosoma. Palps similar to those of female, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 6–7, d″ 5–6, tarsus with one eupathidium 4–5 and one minute solenidion 1. Ventral setae m 5–7; distance between setae m–m 12–13.
Venter (Figure 31). Cuticle covered with fine and mostly transverse striae; with band of a colliculated cuticle around posterior body margin. Coxal, genital, and anal setae fine. Setal lengths: 1a 75–90, 1b 9–12, 1c 11–15, 2b 10–18, 2c 13–16, 3a 10–15, 3b 16–17, 4a 60–80, 4b 11–12, ag 11–15, g1 8–11, ps1 9–10, ps2 27–33, ps3 15–17. Setae g2 absent.
Legs (Figure 30). Setation (from coxae to tarsi): I 3–1–4–3–5–8(1), II 2–1–4–3–5–8(1), III 1–2–2–1–3–5, IV 1–0–1–0–3–5. Leg chaetotaxy similar to that of the female, except by trochanter IV nude; tarsi I–II each with one solenidion ω” (tarsi I 5–6 and tarsi II 5, 6) and two eupathidia pζ′–pζ” (5–6, 5–6; 5, 5 respectively). Detail of the development of leg chaetotaxy in Table 1.
Protonymph (n = 1) (Figure 32)
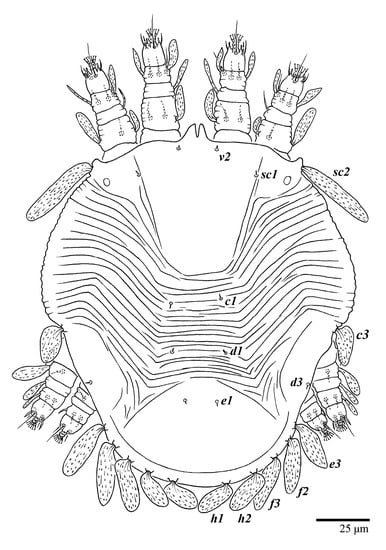
Figure 32.
Ultratenuipalpus meekeri (De Leon). (Protonymph, paratype): dorsum, with detail of legs (unguinal setae u′–u” on tarsus I and II are not included in the drawing).
Body size measurements: distance between setae v2–h1 230, sc2–sc2 150; other measurements: v2–v2 28, sc1–sc1 85, c1–c1 35, c3–c3 190, d1–d1 25, d3–d3 155, e1–e1 23, e3–e3 130, f2–f2 120, f3–f3 100, h1–h1 38, h2–h2 73.
Dorsum (Figure 32). Anterior margin of prodorsum with a short median forked projection forming a short notch; a pair of lateral body projections anterior and adjacent to setae sc2 present; posterior projection between setae h1 absent. Prodorsum with central region smooth; region between setae sc2–c3 with transverse folds and plicae; region posterior to setae e1 smooth; dorsal setae similar in general form to those of the female, except setae c1, d1, and e1 short to minute. Setal measurements: v2 3, sc1 2, sc2 54, c1 4, c3 27, d1 4, d3 4, e1 4, e3 45, f2 38, f3 35, h1 30, h2 35.
Gnathosoma. Palps similar to those of the female, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 4, d″ 4, tarsus with one eupathidium 3 and one minute solenidion, 1 long. Ventral setae m 5; distance between setae m–m 12.
Venter. Cuticle covered with fine and mostly transverse striae. Coxal, genital, and anal setae fine. Setal measurements: 1a 70, 1b 9, 1c 8, 2c 13, 3a 14, 3b 11, ag 10, ps1 8, ps2 16, ps3 10. Setae 2b, 4a, 4b, g1, and g2 absent.
Legs (Figure 32). Setation (from coxae to tarsi): I 3–0–3–1–5–6(1), II 1–0–3–1–5–6(1), III 1–0–2–1–3–5, IV 0–0–1–0–3–3. Tarsi I–II each with one solenidion ω” 4 (for both tarsi I and tarsi II) and two eupathidia pζ′–pζ” (all 5). Detail of the development of leg chaetotaxy in Table 1.
Larva (n = 1) (Figure 33)
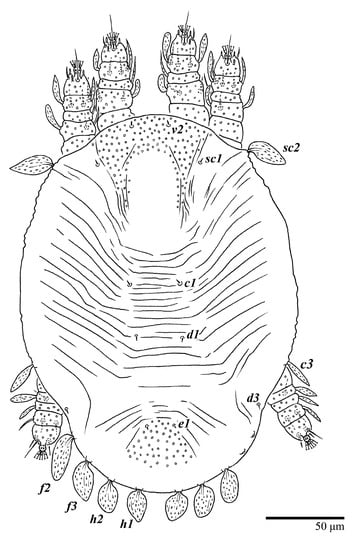
Figure 33.
Ultratenuipalpus meekeri (De Leon). (Larva, paratype): dorsum, with detail of legs (unguinal setae u′–u” on tarsus I and II are not included in the drawing).
Body size measurements: distance between setae v2–h1 225, sc2–sc2 120; other measurements: v2–v2 33, sc1–sc1 68, c1–c1 33, c3–c3 160, d1–d1 30, d3–d3 120, e1–e1 18, e3–e3 115, f2–f2 105, f3–f3 88, h1–h1 23, h2–h2 58.
Dorsum (Figure 33). Prodorsal region with broad band of a colliculated integument anteromedially between setae sc1; region between setae sc2–c3 with oblique and transverse folds and plicae; pygidial region posterior to setae e1 with a small region of colliculated integuments; dorsal setae similar in general form to those of the female, except setae c1, d1, and e1 are short to minute. Setal measurements: v2 3, sc1 3, sc2 28, c1 4, c3 23, d1 2, d3 3, e1 3, e3 missing, f2 30, f3 25, h1 21, h2 21.
Gnathosoma. Palps similar to those of female, setal formula: 0, 0, 2, 2; tibia with two setae, d′ 3, d″ 5, tarsus with one eupathidium 3 and one minute solenidion, 1 long. Setae m absent.
Venter. Cuticle covered with fine and mostly transverse striae. Coxal, genital, and anal setae fine. Setal measurements: 1a 50, 1b 7, 3a 10, ps1 7, ps2 11, ps3 6. Setae 1c, 2b, 2c, 3b, 4a, 4b, ag, g1, and g2 absent.
Legs (Figure 33). Setation (from coxae to tarsi): I 2–0–3–1–5–6(1), II 0–0–3–1–5–6(1), III 0–0–2–1–3–3. Tarsi I–II each with one solenidion ω” 3 (for both tarsi I and II) and two eupathidia pζ′–pζ” (5, 5; 4, 4, respectively). Cuticles of all legs covered with colliculated cuticles. Detail of the development of leg chaetotaxy in Table 1.
Remarks. The new specimens examined in this study have similar body and setal measurements to those of the type specimens. In addition, the palp and leg chaetotaxy of those specimens match those of the type specimens.
DNA Barcoding. DNA was successfully amplified and the mitochondrial cytochrome C oxidase subunit I gene (COI) sequenced from one specimen of U. meekeri collected on Acrostichum danaeifolium (Pteridaceae) from Tecpan de Galeana, Guerrero State, Mexico; sequence data have been deposited in GenBank (https://www.ncbi.nlm.nih.gov/, accessed on 15 January 2023), with the following accession code: female, 446 base pairs (GenBank: OQ533137).
Type material examined: Holotype: the female collected on a fern in a mangrove swamp, from San Blas, Nayarit State, Mexico, 21 March 1957, coll. D. De Leon, was deposited in the Museum of Comparative Zoology (MCZ), Harvard University. Paratypes: 2 females, 1 male, 3 deutonymphs, 1 protonymph, and 1 larva, with the same data as the holotype, were deposited in the National Insect and Mite Collection, National Museum of Natural History (NMNH), Smithsonian Institution.
Other material examined: Non-type material: 5 females collected on ferns A. danaeifolium in a mangrove swamp, from Tecpan de Galeana, Guerrero State, Mexico, 5 September 2017, coll. G. Otero-Colina (USNM, DZJSRP).
4. Discussion
4.1. Ontogeny
Studies on possible patterns of ontogenetic development of chaetotaxy provide information potentially useful for understanding mite taxonomy, phylogeny, and biology [20]. The family Tenuipalpidae has the highest number of ontogenetic studies among all Trombidiformes mites [21], with ontogenetic data available for 60 species in 20 genera [22]. However, ontogenetic development is known only for one species of Ultratenuipalpus, U. jubatus Otley, Beard & Seeman [2,22]. Here, we discuss the ontogeny of the two species, U. parameekeri and U. meekeri, which share the same pattern of additions of leg setae (Table 1).
Trochanters. Setae v′ are added to trochanters I, II, and III in the deutonymph and on trochanter IV in the adults. This is the standard pattern for other flat mites [22,23,24], and also for Tetranychidae [10]. Setae l′ are added to trochanters III in the protonymph, and this addition also occurs in U. jubatus; although the expression of setae l′ and v′ varies within the family, this pattern has been commonly reported [2,22,23,25,26].
Femora. Setae l′ on femora I and II are added in the deutonymph. The expression of setae l′ on the deutonymph also occurs in U. jubatus and in many species of Tenuipalpus [2,22,23]. Setae d and ev′ are present on femora III in the larva. This pattern is common in the Tenuipalpidae [22], but in U. jubatus, the addition of setae ev′ is delayed until the protonymph. Setae ev′ are added on the femora IV in the protonymph of U. parameekeri and U. meekeri (n.b., femora IV are not nude as described for U. meekeri in [17] and in the keys of [1,2]).
Genua. There is great variation in the chaetotaxy of genua I and II in the Tenuipalpidae [22,23]. Here, setae l′ is present on genua I and II in the larva, and setae d and l” are added on genua I and II in the deutonymph. This pattern also occurs in U. jubatus and is common in the Tenuipalpus [22,23]. Setae l′ is present on the genu III in the larva of the new species, and the same pattern occurs in U. jubatus; while many species of Tenuipalpus add setae l′ or d on genu II in the deutonymph [22,23].
Tibiae. Although the number of tibial setae varies across the family, there are no post-larval additions made to the tibiae in the Tenuipalpidae [10]. Here, the number of tibial setae for both species is 5–5–3–3, as is also seen on U. jubatus, whereas setae l′ are suppressed on tibiae III and IV on U. avarua Xu, Fan & Zhang [1,2,22].
Tarsi. Ultratenuipalpus parameekeri and U. meekeri have a pair of tectal setae added to tarsi I–III in the protonymph, as occurs in U. jubatus. However, many species of the Tenuipalpus added these setae in the deutonymph. As is the case for many additions to leg IV, the addition of the tectal setae on tarsi IV is delayed to the deutonymph. This same pattern also occurs in U. jubatus, while many Tenuipalpus add tectal setae to tarsi IV in the adults [22,23].
The solenidion ω′ is added on each tarsi I–III in males of U. parameekeri and U. meekeri. Similarly, the male of U. jubatus has this solenidion added to tarsus I and II, but not to tarsus III [2]. This characteristic also occurs in other tenuipalpid genera, such as Tenuipalpus, Prolixus, and Acaricis [26,27,28], with some species of Acaricis also bearing one solenidion ω’ on tarsus IV.
In response to the detailed work of Lindquist [10] on the patterns of setal additions to the legs in the family Tetranychidae, ontogenetic studies regarding the family Tenuipalpidae have received increasing attention in recent years. For example, the genus Raoiella has ontogenetic data available for 13 of the 22 known species [22]. However, despite this increase in attention, there are still many tenuipalpid genera that have received little or no such focused research [22], such as the Ultratenuipalpus. Only three of the 26 known species of the Ultratenuipalpus have so far been studied ontogenetically, and it is one of the genera that should receive priority in future studies. Filling these gaps may allow an adequate comparison of ontogenetic data between species and genera of the flat mite family, and as Lindquist [10] suggests, may contribute to our further understanding of the superfamily as a whole.
4.2. Distribution, Taxonomy and Systematic
The genus Ultratenuipalpus is known from all zoogeographic regions of the world with the exception of the Nearctic and Western Palearctic regions [2,3,4]. To date, six species of the Ultratenuipalpus have been described from the Neotropical region: the new species herein described from Brazil (which represents the first record of the genus for the country), in addition to two species from Mexico (U. meekeri and U. younguisti Baker & Tuttle) and three species from Chile (U. acharis (Gonzalez), U. canelae (Gonzalez), and U. charlini (Gonzalez)) [3,29].
According to Beard et al. [2], the presence or absence of opisthosomal setae f2 may indicate a biogeographic pattern within the genus. Those species that lack f2 show a putative Gondwanan distribution, being found in Chile, Australia, New Zealand, and the Cook Islands. Those species with setae f2 present are found in China, the Philippines, Mexico, and now with the new species herein described, in Brazil. The unique exception for this pattern is U. younguisti, which lacks the setae f2 and was described from Mexico (based on specimens intercepted in the USA).
The presence of a pair of lateral body projections anterior to setae sc2 in some species of the Ultratenuipalpus (e.g., U. meekeri, U. parameekeri, and U. avarua) and the Tenuipalpus sensu stricto group could indicate a strong relationship between these two genera. Within the Ultratenuipalpus, the presence of a single posterior body projection between the setae h1 may be an important character for separating a subgrouping, since it is shared by at least six species of the genus: U. avarua, U. hainanensis (Wang), U. lacorpuzrarosae Rimando, U. meekeri, U. parameekeri, and U. umtataensis Meyer.
5. Conclusions
The study of body morphology, spermathecae, geographic distribution, and plant associations will allow a broader and deeper understanding of the internal relationships within the genus Ultratenuipalpus, as well its relationships with other related genera (e.g., Tenuipalpus, Extenuipalpus, Acaricis, and Prolixus). In addition, we believe that the study of possible patterns of ontogenetic additions of leg setae can provide important insights into the systematics and origin of these taxa.
Author Contributions
Conceptualization, E.B.C., J.J.B., R.O., G.R.B., G.O.-C., R.J.F.F. and A.C.L.; methodology, E.B.C., J.J.B., R.O., G.R.B., G.O.-C., R.J.F.F. and A.C.L.; molecular analysis, A.P.G.D.; data curation, R.J.F.F., A.C.L. and R.O.; writing—original draft preparation, E.B.C.; writing—review and editing, E.B.C., J.J.B., R.O., G.R.B., G.O.-C., R.J.F.F. and A.C.L.; supervision, R.J.F.F., A.C.L. and R.O.; project administration, R.J.F.F. and A.C.L.; funding acquisition, R.J.F.F., A.C.L. and E.B.C. All authors have read and agreed to the published version of the manuscript.
Funding
E.B.C. was supported by a FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) Post-doctoral Scholarship, grant numbers 2016/01193-5 and 2017/00458-8; and by a UNESP Post-doctoral Scholarship, with project number 4324 (Edital PROPe 13/2022). Part of the studied specimens of the new species was collected with financial support of the FAPESP, grant numbers 2004/04820-3, 2006/55725-6 and 2006/57868-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in this paper.
Acknowledgments
The authors would like to thank the late Debra Creel and Andrew Ulsamer (SEL-USDA) for their help with references and technical support. We also thank Laura Leibensperger, Department of Invertebrate Zoology, Museum of Comparative Zoology (MCZ), Harvard University, for lending specimens for study; and the Smithsonian Natural History Museum (NMNH) and National Agricultural Library (NAL-USDA), SEL-USDA for support and assistance with specimens and references. The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Y.; Fan, Q.-H.; Zhang, Z.-Q. A new species of Ultratenuipalpus (Acari: Tenuipalpidae) from Cook Islands, with a key to the known species. Zootaxa 2013, 3731, 223–233. [Google Scholar] [CrossRef]
- Beard, J.J.; Otley, J.; Seeman, O.D. A review of Ultratenuipalpus (Trombidiformes: Tenuipalpidae) and related genera, with a new species from forest oak Allocasuarina torulosa (Aiton) (Casuarinaceae). Int. J. Acarol. 2016, 42, 285–302. [Google Scholar] [CrossRef]
- Tenuipalpidae Database. Available online: http://www.tenuipalpidae.ibilce.unesp.br (accessed on 12 February 2023).
- Mesa, N.C.; Ochoa, R.; Welbourn, W.C.; Evans, G.A.; de Moraes, G.J. A catalog of the Tenuipalpidae (Acari) of the World with a key to genera. Zootaxa 2009, 2098, 1–185. [Google Scholar] [CrossRef]
- Lindquist, E.E. Anatomy, phylogeny and systematics, Chapter 1.1.3. Diagnosis and phylogenetic relationships. In Spider Mites: Their Biology, Natural Enemies and Control; Helle, W., Sabelis, M.W., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1985; Volume 1A, pp. 63–74. [Google Scholar]
- Castro, E.B.; Kane, E.C.; Feres, R.J.F.; Ochoa, R.; Bauchan, G.R. Definition of Tenuipalpus sensu stricto (Acari, Tenuipalpidae), with redescription of Tenuipalpus caudatus (Dugès) and description of a new species from Costa Rica. Int. J. Acarol. 2016, 42, 106–126. [Google Scholar] [CrossRef]
- Castro, E.B.; Ochoa, R.; Feres, R.J.F.; Beard, J.J.; Bauchan, G.R. Reinstatement of the genus Colopalpus Pritchard and Baker (1958) and re-description of Colopalpus matthyssei Pritchard and Baker (1958), the type species of the genus (Acari, Tenuipalpidae). Int. J. Acarol. 2015, 41, 310–328. [Google Scholar] [CrossRef]
- Navajas, M.; Lagnel, J.; Gutierrez, J.; Boursot, P. Species-wide homogeneity of nuclear ribosomal ITS2 sequences in the spider mite Tetranychus urticae contrasts with extensive mitochondrial COI polymorphism. Heredity 1998, 80, 742–752. [Google Scholar] [CrossRef]
- Rodrigues, J.C.V.; Childers, C.C.; Gallo-Meagher, M.; Ochoa, R.; Adams, B.J. Mitochondrial DNA and RAPD polymorphisms in the haploid mite Brevipalpus phoenicis (Acari: Tenuipalpidae). Exp. Appl. Acarol. 2004, 34, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, E.E. Anatomy, phylogeny and systematics, Chapter 1.1.1. External anatomy. In Spider Mites: Their Biology, Natural Enemies and Control; Helle, W., Sabelis, M.W., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1985; Volume 1A, pp. 3–28. [Google Scholar]
- Xu, Y.; Fan, Q.-H. Tenuipalpus orilloi Rimando, a new record to the Chinese fauna (Acari: Tenuipalpidae). Syst. Appl. Acarol. 2010, 15, 135–138. [Google Scholar] [CrossRef]
- Seeman, O.D.; Beard, J.J. A new species of Aegyptobia (Acari: Tenuipalpidae) from Myrtaceae in Australia. Syst. Appl. Acarol. 2011, 16, 73–89. [Google Scholar] [CrossRef]
- De Leon, D. The genus Tenuipalpus in Mexico (Acarina: Tenuipalpidae). Fla. Entomol. 1957, 40, 81–93. [Google Scholar] [CrossRef]
- Mitrofanov, V.I. Revision of the system of phytophagous mites of the subfamily Tenuipalpinae s. str. (Trombidiformes, Tenuipalpidae). Zool. Zhurnal 1973, 52, 1315–1320. [Google Scholar]
- Corpuz-Raros, L.A. New Philippine Tetranychoidea (Acarina). Kalikasan Philipp. J. Biol. 1978, 7, 211–230. [Google Scholar]
- Meyer, M.K.P. The Tenuipalpidae (Acari) of Africa with Keys to the World Fauna; Entomology Memoir; Department of Agricultural Technical Services: Pretoria, South Africa, 1979; Volume 50, pp. 1–135. [Google Scholar]
- Baker, E.W.; Tuttle, D.M. The False Spider Mites of Mexico (Tenuipalpidae: Acari); Technical Bulletin; United States Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1987; Volume 1706, pp. 1–236. [Google Scholar]
- Meyer, M.K.P. The South African species of Ultratenuipalpus Mitrofanov (Acari: Tenuipalpidae), with a key to the species of the genus. Int. J. Acarol. 1993, 19, 39–43. [Google Scholar] [CrossRef]
- Rimando, L.C. A review of the genus Ultratenuipalpus Mitrofanov (Acari: Tenuipalpidae) with descriptions of two new species from the Philippines. Philipp. Entomol. 2001, 15, 101–113. [Google Scholar]
- Zhang, Z.-Q. Accelerating studies on the ontogeny and morphological diversity in immature mites. Zootaxa 2018, 4540, 5–6. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhang, Z.-Q. A survey of descriptions of immature instars of mites during the last three years. Zootaxa 2018, 4540, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-Y.; Zhang, Z.-Q. Ontogenetic development of chaetotaxy in Tenuipalpidae: A survey with special reference to sexual dimorphism. Zootaxa 2020, 4900, 154–200. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, W.C.; Beard, J.J.; Bauchan, G.R.; Ochoa, R. Description of a new species of Tenuipalpus (Acari: Trombidiformes) from succulent plants in Florida, USA, and a redescription of T. crassulus Baker and Tuttle. Int. J. Acarol. 2017, 43, 112–136. [Google Scholar] [CrossRef]
- Castro, E.B.; Beard, J.J.; Ochoa, R.; Bauchan, G.R.; Feres, R.J.F. Two new species of Tenuipalpus sensu stricto (Acari: Tenuipalpidae) from Brazil, with a discussion on the ontogeny of leg setae. Zootaxa 2018, 4540, 178–210. [Google Scholar] [CrossRef]
- Khanjani, M.; Khanjani, M.; Seeman, O.D. The flat mites of the genus Tenuipalpus Donnadieu (Acari: Tenuipalpidae) from Iran. Int. J. Acarol. 2013, 39, 97–129. [Google Scholar] [CrossRef]
- Castro, E.B.; Ramos, F.A.M.; Feres, R.J.F.; Ochoa, R. A new species of Tenuipalpus Donnadieu (Acari: Tenuipalpidae) from Brazil, with ontogeny of chaetotaxy. Syst. Appl. Acarol. 2015, 20, 339–356. [Google Scholar]
- Beard, J.J.; Fan, Q.H.; Walter, D.E. A new genus and two new species of Tenuipalpidae (Prostigmata: Tetranychoidea) from an Australian sedge. Acarologia 2005, 45, 161–181. [Google Scholar]
- Castro, E.B.; Beard, J.J.; Ochoa, R.; Feres, R.J.F. Two species of Acaricis (Acari: Tenuipalpidae) from New Zealand, moved from the genus Tenuipalpus, with a key to the known species. Acarologia 2018, 58, 855–867. [Google Scholar] [CrossRef]
- Flat Mites of the World. Available online: https://idtools.org/tools/1074/index.cfm (accessed on 12 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).