Simple Summary
MALDI-ToF MS analysis demonstrates major differences between Mycoplasma agalactiae type strain PG2 and strain GM139. Evaluation of the Vpma (variable proteins of M. agalactiae) phenotypic profiles of these two strains reveals that unlike PG2, GM139 expresses a single Vpma, namely VpmaV, and without any visible phase variation. Although the presence of only one Vpma seems to be sufficient for the pathogenesis of the GM139 strain, the absence of phase variation can compromise immune system evasion and spread to other anatomical sites in the host.
Abstract
Although mycoplasmas have a reduced genome and no cell wall, they have important mechanisms for the antigenic variation in surface lipoproteins that modulate their interactions with the host. Mycoplasma agalactiae, the main etiological agent of contagious agalactia, has a multigene family involved in the high-frequency phase variation in surface lipoproteins called variable proteins of M. agalactiae (Vpmas). The Vpma lipoproteins are involved in the immune evasion, colonization, dissemination, and persistence of M. agalactiae in the host. In this paper, we evaluate the Vpma phenotypic profiles of two different strains of M. agalactiae, namely, GM139 and the type strain PG2, to assess possible correlations between Vpma phase variability and the geographic localization, animal origin, and pathogenicity of these two strains. Using monospecific Vpma antibodies against individual Vpmas in immunoblots, we demonstrate that, unlike PG2, which expresses six Vpma proteins with high-frequency phase variation, colonies of GM139 predominantly express VpmaV and do not exhibit any sectoring phenotype for any Vpma. Since VpmaV is one of the most important Vpmas for cell adhesion and invasion, its predominant sole expression in GM139 without high-frequency variation may be the basis of the differential pathogenicity of GM139 and PG2. Additionally, MALDI-ToF MS analysis also demonstrates significant differences between these two strains and their relatedness with other M. agalactiae strains.
1. Introduction
M. agalactiae is the main etiological agent of the contagious agalactia (CA) syndrome notifiable to the OIE. CA affects small ruminants in several countries of the world, but has a far greater impact in the Mediterranean countries [1] with a prevalence of up to 66.7% among herds in an endemic area of Spain [2]. In the United States, M. agalactiae is associated with mastitis and arthritis in goats [3], and only sporadic cases of its isolation were reported in the recent years [4]. In South America, this pathogen has been identified in goat herds causing mastitis, polyarthritis, conjunctivitis, fever, anorexia, high-morbidity and mortality [5,6,7,8,9,10]. It has been isolated from milk [6,7,8,9,11], semen [11], and nasal swabs [10]. Congenital infections in goats were also described [12]. Asymptomatic forms of the disease in goats also occurred, and it was observed that the etiologic agent is able to be disseminated into the environment for twelve months to eight years in the absence of clinical signs [13,14].
M. agalactiae strain GM139 was isolated from the mastitic milk of a goat in the USA [15], whereas the pathogenic type strain PG2 was isolated from an infected sheep in Spain by Dr. C. Lopez of Madrid [16]. Experimental infections with PG2 caused reduced milk production, excretion of mycoplasmas in milk, as well as pathogen spread and survival in lymph nodes [17,18,19]. On the other hand, infections caused by GM139 seem to be restricted to the udder, although there are no more detailed data concerning the infection caused by this isolate [15].
Although mycoplasmas have reduced genomes (580–1350 Kbp), many of their genes are affected by the diversification of their cell surfaces [20]. The use of variable genes organized in families allows for the generation of an extensive repertoire of antigenic variation, maximizing the potential of a limited genome [21].
Mycoplasma hyorhinis was the first mycoplasma where phenotypic switching of diverse surface lipoproteins was described in the year 1990 [22]. The gene family encoding these phase- and size-variable lipoproteins (Vlps) was also shown to be involved in the adhesion to host cells [23,24]. The lipoprotein VlhA, a hemagglutinin in M. synoviae, is notable as one of the most well-studied phase-variable proteins in mycoplasmas [25]. There are several other membrane-variable lipoproteins in mycoplasmas, such as the pMGA in M. gallisepticum [26], Vmm in M. mycoides subsp. mycoides [27], and Vaa in M. hominis [28]. Similarly, M. bovis, a very close phylogenetic relative of M. agalactiae, exhibits the phase variation in the Vsp family of surface lipoproteins, which is considered to be homologous to the Vpmas of M. agalactiae and provides specific structural and antigenic characteristics to the pathogen [29,30].
So far, only one multigene family in M. agalactiae involved in the high-frequency DNA rearrangements of surface lipoproteins has been described, and it is known as the vpma [31] or the avg locus [32]. This sophisticated system of high-frequency antigenic phase variation affects the expression (phase variation) and/or the structure of genes in the multigene family [17,31,33] mediated by the Xer1 site-specific recombinase [34,35] or by non-Xer1 recombination under the selective pressure induced by the host’s immune response [17]. In the M. agalactiae type strain PG2, six genes are present in the vpma locus (vpmaV, vpmaX, vpmaY, vpmaU, vpmaW, and vpmaZ), together with one xer1 recombinase gene [36]. Although 23 CDS related to the vpma locus were identified in the field strain 5632 at two different genomic loci, the majority of the other analyzed strains (89 out of 92 strains) possess a vpma profile similar to PG2, and the duplication of the vpma locus was considered a rare event [37,38]. Analyses of the vpma genes of strain 5632 showed that the 23 vpma genes are present on two different loci. Locus I contains 16 vpma genes, whereas Locus II contains 7 vpma genes that are duplicated on Locus I. Out of these 23 genes, vpmaW and vpmaX are allelic versions of vpmas in PG2, and 7 others, namely, vpmaA, -B, -C, -D1, -D2, -E, and -F1, are duplicated. Additionally, alleles of vpmaD (vpmaD1 and -D2) and vpmaF (vpmaF1 and -F2) varying in the C-terminal repeats are also present [37]. Therefore, the 5632 strain has 12 different vpma genes compared to PG2 [37]. However, information about the vpma genes, their structure or organization, and their expression in strain GM139 is missing from the literature.
In the M. agalactiae type strain PG2 [16,39], Vpma surface proteins (VpmaV, VpmaU, VpmaX, VpmaW, VpmaY, and VpmaZ) as well as their antigenic phase variations, were shown to be involved in host immune evasion [33], pathogen dissemination [18], and M. agalactiae adhesion/invasion to host cells [40]. Due to the importance of the roles played by these phase-variable lipoproteins, in the current study, we evaluated the Vpma phenotypic profiles of the type strain PG2 [16] and the GM139 strain [15] to assess the correlations of the antigenic variability between these strains from different geographical localizations and animal origins, and any possible implications of the differences in disease characteristics.
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions
Mycoplasma agalactiae strain GM139 [15] isolated from mastitic goat milk (CA, USA) and type strain PG2 [16] isolated from an infected sheep (Madrid, Spain) were grown at 37 °C in SP4 medium for 48 h or an SP4 agar for 4–5 days supplemented with 500 U/mL of penicillin, pyruvate (0.5%, w/v), and phenol red (0.005%, w/v), as described previously [41]. The strains were submitted to DNA extraction [21] and M. agalactiae species-specific PCR [42] before further analysis.
2.2. Western and Colony Immunoblots
Vpma expression on the surface of M. agalactiae colonies was evaluated by the colony immunoblots described previously [34]. Briefly, nitrocellulose membrane discs of 0.22 µm (Amersham™ Protran™, Cytiva, Marlborough, MA, USA) were placed on freshly grown mycoplasma colonies on the surface of agar plates for 5 min before being detached and dried at room temperature (RT). The membranes were rinsed three times in TBS (10 mM Tris and 154 mM NaCl, at pH 7.4) and incubated overnight at 4 °C in the six different Vpma-specific antisera (dilution of each Vpma antisera was performed as described by Chopra-Dewasthaly et al. [34]). After overnight incubation, the nitrocellulose membranes were washed three times for 10 min each with TBS and then incubated with secondary antibody anti-rabbit IgG conjugated to horseradish peroxidase (1:2000, DakoCytomation, Glostrup, Denmark). After three washes (10 min each), the colony blots were developed with 4-chloro-1-naphthol (Bio-Rad Laboratories, Vienna, Austria) and hydrogen peroxide. Colonies of M. gallisepticum and M. ovipneumoniae were used as negative controls. The reaction was stopped by washing the blots in water and counterstained using Ponceau S staining (Carl Roth, Vienna, Austria). The blots were photographed using a Nikon SMZ-U stereomicroscope (Nikon Corp., Tokyo, Japan).
The Vpma phenotypes of the two M. agalactiae strains were also checked by Western blot analysis of their Triton X-114 (Sigma, Vienna, Austria) fractions as described elsewhere [34]. Briefly, samples were treated at 95 °C for 5 min under reducing conditions and subjected to SDS-PAGE. Separated proteins were transferred to nitrocellulose membranes (Amersham™ Protran™, Cytiva, Marlborough, MA, USA) using a blotting buffer (48 mM Tris, 39 mM glycine, 0.037% SDS, and 20% methanol) and incubated separately in the six Vpma-specific antisera. Immunoreactive proteins were stained and visualized as described above for colony immunoblots. M. gallisepticum Triton X-114 extracts were used as negative controls.
2.3. MALDI-ToF MS Analysis
Matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-ToF MS) of the late-exponential-phase broth cultures of M. agalactiae GM139 and of type strain PG2 was performed on a Microflex LT Biotyper (Bruker Daltonics, Bremen, Germany) configured with Bruker flexControl 3.4 software and compared against an in-house mycoplasma reference database containing more than 900 main spectrum profiles (MSPs), as described previously [43].
For protein extraction and the generation of spectra, 1 mL of GM139 and PG2 culture was grown for 3 days and centrifuged at 20,000× g for 7 min. The pellet was washed with 100 µL of HPLC-grade water (Sigma-Aldrich, Vienna, Austria) and centrifuged at 20,000× g for 5 min. The pellet was then subjected to extraction with equal volumes of 70% formic acid and acetonitrile. After centrifugation at 20,000× g for 2 min, the protein extract was spotted onto a 96-target polished steel plate (Bruker Daltonics, Bremen, Germany), air-dried, and overlaid with α-cyano-4-hydroxycinnamic acid matrix solution. MSPs were created and a score-oriented dendrogram was constructed based on the correlation distance measure with the average linkage algorithm (Bruker Daltonics), determined by comparing MSPs to the reference spectra of M. agalactiae strains integrated in the in-house database. Mass spectra were checked visually for characteristic strain-discriminating peaks using FlexAnalysis 3.4 software (Bruker Daltonics, Bremen, Germany).
3. Results and Discussion
Colony immunoblot analyses of M agalactiae type strain PG2 and strain GM139 were performed to evaluate their Vpma expression profiles. Figure 1 demonstrates the differences in the Vpma profiles of strains PG2 and GM139 of M. agalactiae. The PG2 strain shows sectors corresponding to the high-frequency phase variation in the expression of all six Vpmas, amongst which VpmaV is the least expressed (Figure 1A) [34]. On the contrary, GM139 does not exhibit a sectoring phenotype for any of the six tested Vpmas and shows a predominant expression of the VpmaV phenotype. The Western blot results in Figure 1B further demonstrate the expression of only VpmaV in strain GM139 and the absence of all other five Vpma products in PG2 (Original whole Western blot figures see Supplementary Figures S1 and S2). Although the sole presence of VpmaV in the GM139 strain could imply a compromised host immune evasion due to a lack of Vpma phase variation, it highlights the significance of this Vpma protein, which appears to be sufficient for causing infection of the mycoplasma. In a previous study demonstrating Vpmas as major cytadhesins of M. agalactiae, phase-locked mutant V (PLM V) expressing a single stable VpmaV product exhibited the highest adhesion rate to HeLa cells, as well as sheep mammary epithelial (MEC) and stromal (MSC) cells [40]. Interestingly, VpmaV carries many cytoadhesion motifs that are involved in the host adhesion of the homologous Vsp proteins of M. bovis [44]. The 5′ untranslated region and the N-terminal-coding region of vpma genes bear 90% homology to the same regions of the vspA-coding sequence in M. bovis [32], which has been suggested to be involved in host cytoadhesion [36,44]. Although initially regarded as a predominantly extracellular pathogen, in vitro and in vivo infection experiments in recent years clearly demonstrated that M. agalactiae is capable of invading non-phagocytic cells such as the primary sheep mammary and uterine cells [45], HeLa, bovine endometrial cells (BEND), and buffalo lung fibroblast (BLF) cells, thus causing systemic spread inside the host [46]. VpmaU is the protein with the least invasive capacity and VpmaV indicated the highest rate of invasion in HeLa cells [40]. The expression of VpmaV in strain GM139 may indicate a level of invasiveness similar to that of PG2 clonal variants expressing VpmaV.
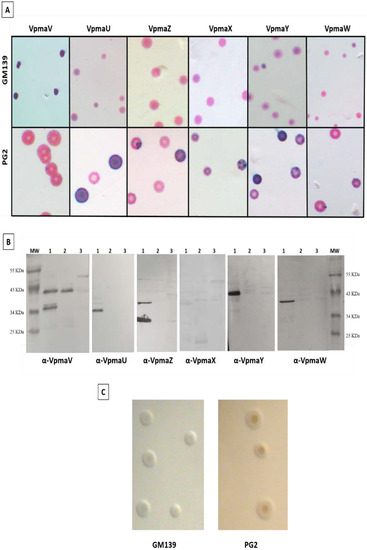
Figure 1.
Vpma expression profiles of Mycoplasma agalactiae strains PG2 and GM139. (A) Colony immunoblot analyses of strain GM139 (upper row) and PG2 (lower row) using the six Vpma-specific antibodies recognizing specific surface-exposed epitopes. The purple color corresponds to specific antibody staining and indicates the expression of Vpmas on the surface of the mycoplasma cells, whereas the pink color corresponds to the non-specific protein counter-staining with Ponceau. (B) Western blot analysis demonstrating recognition of Vpmas by mono-specific Vpma antisera after extraction of lipoprotein fractions by Triton X-114. Lane 1: M. agalactiae strain PG2; Lane 2: M. agalactiae strain GM139; Lane 3: M. gallisepticum (negative control); MW: molecular weight protein marker. (C) Representative colonies of GM139 and PG2 strains after growth on SP4 agar at 37 °C. All micrographs were made using a Nikon SMZ-U stereomicroscope.
The lack of expression of any vpma genes other than vpmaV in the strain GM139, i.e., vpmaX, vpmaY, vpmaW, vpmaU, and vpmaZ, as observed in the PG2 type strain, cannot be explained currently, as nothing is known concerning the genetic organization of the vpma locus in this strain. GM139 genome sequencing and thorough genetic analysis are underway and are anticipated to fill in these gaps in our knowledge. A previous study revealed the enrichment of VpmaX and VpmaW expressors both during experimental and natural M. agalactiae infections [17], thereby indicating the importance of and the selective advantage conferred by these phenotypes inside of the host. The lack of these Vpmas in the GM 139 strain is likely to affect its disease characteristics, especially considering the visible absence of sectoring or high-frequency phase variation among the Vpmas. The latter was shown to be necessary for the expression of Vpma immune evasion proteins in order to escape the immune response and to survive and persist inside the immunocompetent host [17,18,33].
Both the GM139 and the PG2 strains have similar morphological characteristics when grown on SP4 agar (Figure 1C), despite different geographical and host origins [15,16], and different vpma profiles (Figure 1A,B). During experimental subcutaneous infections in the shoulders of lactating ewes, PG2 led to hypogalactia with a reduction in milk production, the excretion of mycoplasmas in milk, and the dissemination and survival of the pathogen in the lymph nodes, even in infections at low concentrations (103 CFU/mL), demonstrating the ability of PG2 to establish a systemic infection [19], as was also witnessed in the experimental conjunctival and intramammary route infections of PG2 [17,18]. On the other hand, infections caused by GM139 seemed to be restricted to the udder, even when large amounts of mycoplasmas were recovered from the mammary secretions, although there are no more detailed data on the infection caused by this isolate [15].
Molecular analysis of GM139 demonstrated a completely different hybridization profile compared to those of PG2 and some other isolates from Israel from both goat and sheep with keratoconjunctivitis, arthritis, or contagiousa agalactia [32]. In another molecular typing study of Spanish M. agalactiae samples (n = 410), genomic homogeneity characterizing the circulation of a single endemic clonal population was revealed using PFGE (pulsed-field gel electrophoresis) analysis. In this study, PG2 shared the same pulsotype with the isolate Teramo (Italy) and the isolates from Valladolid (Spain) in the PFGE analysis. PG2 also clustered with the Teramo isolate, together with the strain 10123 (USA) during multilocus sequence typing (MLST) analysis [47]. However, a previous molecular typing study indicated an unexpected genetic diversity in an endemic area of Spain, whereby PG2 demonstrated the same pulsetype as bulk tank milk isolates with similar Vpma profiles. However, isolates from semen samples did not show hybridization bands for vpmaU, vpmaV, vpmaY, or vpmaZ, demonstrating that they had a different vpma repertoire than isolates from milk samples and from joints (5632 strain) [48]. Furthermore, previous studies evaluating the expression of surface epitopes of M. agalactiae isolates from 10 different countries also demonstrated high phenotypic diversification, which is also related to the geographical origin of the isolates [49].
We used MALDI-ToF MS analysis to compare the MSPs of GM139 and of PG2 strains against the reference spectra of eight selected M. agalactiae strains integrated into the in-house database. In the constructed dendrogram, two clearly separated clusters are evident. M. agalactiae GM139 groups together with M. agalactiae strains from Spain, whereas M. agalactiae PG2 forms a cluster with Mongolian M. agalactiae strains (Figure 2), which further corroborates the subtyping capability of MALDI-ToF MS for M. agalactiae [50]. Spectral concordance between GM139 and PG2 expressed by a log score value of 1.75 demonstrated major differences between these two strains, whereas comparison between GM139 and strain M3 (isolated in Spain) resulted in a high log score of 2.35.
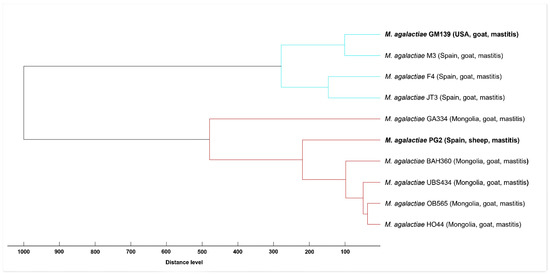
Figure 2.
Score-oriented dendrogram based on distances between MSPs created from M. agalactiae GM139 and PG2 compared to MSPs of M. agalactiae strains from Spain and Mongolia integrated in the in-house mycoplasma library.
In addition, eight strain-specific peaks were found in the peptide mass fingerprints (PMF) that discriminate M. agalactiae GM139 from M. agalactiae PG2, with peaks at m/z 2316, 2410, 4827, 6118, and 12,235 Da present in GM139 and absent in PG2, and 3938, 4037, and 7878 Da present in PG2 and absent in GM139 (Figure 3). PMFs in the mass range of 2–20 kDa are considered to represent mainly ribosomal proteins along with a few housekeeping proteins. However, further in-depth analyses of several clonal variants are needed before correlating any of the respective peaks with specific Vpma phenotypes.
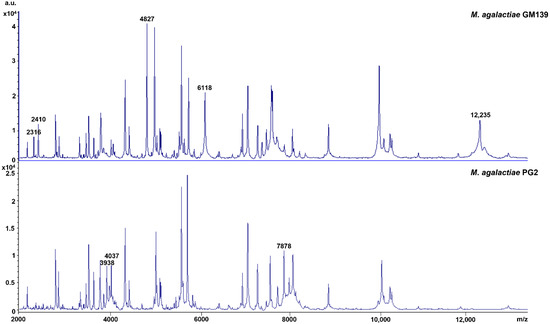
Figure 3.
Peptide mass fingerprints of M. agalactiae GM139 and PG2 highlighting strain-specific peaks.
4. Conclusions
In conclusion, the Vpma expression profile of M. agalactiae strain GM139 is peculiar, as it expresses a single Vpma, namely VpmaV, without any visible phase variation. Although VpmaV has the greatest adhesion capacity and invasiveness compared to all other Vpmas [40], these important pathogenicity characteristics are seemingly sufficient for the pathogenesis of the M. agalactiae strain GM139 strain, as this strain is known to cause mastitis in goats [15]. Yet, the absence of other Vpmas and, especially, the lack of Vpma phase variation might be a factor compromising its immune evasion characteristics and its systemic spread during an infection. Further studies, including molecular analyses, should be carried out on a large number of more recent M. agalactiae field isolates to check for the presence of different vpma genes and their expression. In addition, the genomic sequencing of more isolates of this mycoplasma species is expected to provide further information concerning these observations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12030265/s1, Figure S1: Whole Western blot analysis demonstrating recognition of Vpmas by mono-specific Vpma antisera after extraction of lipoprotein fractions by Triton X-114. Lane 1: M. agalactiae strain PG2; Lane 2: M. agalactiae strain GM139; Lane 3: M. gallisepticum (negative control); MW: molecular weight protein marker. Figure S2: Densitometric analyses using ImageJ software. Western blotting band densities of Vpmas presented in Figure 1B. Band densities were quantified as a ratio to Vpma from PG2 strain.
Author Contributions
Conceptualization, R.C.-D.; experimental work, M.S.B. and R.C.-D.; MALD.-ToF M.S. analysis, J.S.; writing—original draft preparation, M.S.B. and R.C.-D.; writing—review and editing, M.S.B., R.C.-D., L.M.M., J.T., R.R. and J.S.; supervision, R.C.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) grant numbers 2016/23306-6 and 2019/03425-9; the latter covered the research and stay of M.S.B. at the University of Veterinary Medicine Vienna, Austria, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) (Finance Code 001). Open Access Funding by the University of Veterinary Medicine Vienna.
Institutional Review Board Statement
Not applicable. The study did not involve humans or animals.
Data Availability Statement
All relevant data are already present in this manuscript or in the Supplementary Materials.
Acknowledgments
The authors would like to thank Munkhtsetseg Kargl and Ana Ramírez for providing strains for reference spectra generation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, A.; Rahal, A.; Chakraborty, S.; Verma, A.K.; Dhama, K. Mycoplasma agalactiae, an Etiological Agent of Contagious Agalactia in Small Ruminants: A Review. Vet. Med. Int. 2014, 2014, 286752. [Google Scholar] [CrossRef] [PubMed]
- Amores, J.; Sánchez, A.; Gómez-Martín, Á.; Corrales, J.C.; Contreras, A.; de la Fe, C. Surveillance of Mycoplasma agalactiae and Mycoplasma mycoides subsp. capri in dairy goat herds. Small Rumin. Res. 2012, 102, 89–93. [Google Scholar] [CrossRef]
- Kinde, H.; DaMassa, A.J.; Wakenell, P.S.; Petty, R. Mycoplasma Infection in a Commercial Goat Dairy Caused by Mycoplasma Agalactiae and Mycoplasma Mycoides subsp. Mycoides (Caprine Biotype). J. Vet. Diagn. Investig. 1994, 6, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Migliore, S.; Puleio, R.; Nicholas, R.A.J.; Loria, G.R.; Rodriguez, L.F.; Gómez-Martin, Á. Mycoplasma agalactiae: The Sole Cause of Classical Contagious Agalactia. Animals 2021, 11, 1782. [Google Scholar] [CrossRef]
- De Azevedo, E.O.; De Alcântara, M.D.B.; Do Nascimento, E.R.; Tabosa, I.M.; Barreto, M.L.; De Almeida, J.F.; Araújo, M.D.O.; Rodrigues, A.R.O.; Riet-Correa, F.; De Castro, R.S. Contagious agalactia by Mycoplasma agalactiae in small ruminants in Brazil: First report. Braz. J. Microbiol. 2006, 37, 576–581. [Google Scholar] [CrossRef]
- Bandeira, D.A.; Castro, R.S.; Azevedo, E.O.; Nascimento, E.R.; Melo, L.S.S.; Melo, C.B. Infection by Mycoplasma agalactiae in dairy goat herds in the microregions of Cariri in Paraíba State, Brazil. Arq. Bras. Med. Vet. Zootec 2008, 60, 1255–1258. [Google Scholar] [CrossRef]
- Peixoto, R.M.; Andrioli, A.; Pinheiro, R.R.; Alves, F.S.F.; Dos Santos, V.W.S.; De Sousa, M.M.; De Azevedo, D.A.A.; Damasceno, E.M.; Teixeira, M.F.D.S. Mycoplasma agalactiae in dairy goat flocks bred in state of Ceará in association with Caprine Arthritis Encephalitis virus. Acta Sci. Vet. 2018, 46, 1–7. [Google Scholar] [CrossRef]
- Lopes, L.F.V.; Da Silva, E.C.; De Moraes, A.C.A.; Da Silva, E.R.; Santoro, K.R.; Batista, Â.M.V.; Barbosa, S.B.P. Mycoplasma agalactiae and the Mycoplasma mycoides cluster in goat herds in the states of Pernambuco and Paraíba, Brazil. Semin. Agrar. 2019, 40, 2261–2270. [Google Scholar] [CrossRef]
- Matos, R.A.T.; Santos, S.B.; Alves, R.V.; Silva, E.J.; Marinho, M.L.; José Wilton, P.; Mota, R.A.; Júnior, F.G. Ocurrence and risk factors associated with Mycoplasma agalactiae infection in dairy goat herds of Paraíba State, Brazil. Pesqui. Vet. Bras. 2019, 39, 93–98. [Google Scholar] [CrossRef]
- Castilho, R.E., Jr.; de Almeida, C.A.S.; Santos, V.M.; Amorim, A.T.; Gaeta, N.C.; Souza, I.R.; Santos, M.B.; Campos, G.B.; de Souza, L.E.B.; da Cruz, J.F.; et al. Detecting Mollicutes by PCR in goats in southwestern Bahia, Brazil. Braz. J. Microbiol. 2021, 52, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.H.L.S.; Silva, J.G.; Mota, A.R.; Campos, A.C.; Júnior, J.W.P.; Santos, B.; Mota, R.A. Mycoplasma agalactiae in semen and milk of goat from. Pesq. Vet. Bras. 2013, 33, 1309–1312. [Google Scholar] [CrossRef]
- Silva, N.S.; Azevedo, E.O.; Campos, A.C.; Cordeiro, A.A.; Mamede, A.G.; Silva, R.B.S.; Castro, R.S.; Nascimento, E.R.; Marinho, M.L. Infecção congênita em cabritos por Mycoplasma agalactiae. Arq. Bras. Med. Veterinária e Zootec. 2014, 66, 631–634. [Google Scholar] [CrossRef]
- Bergonier, D.; Berthelot, X.; Poumarat, F. Contagious agalactia of small ruminants: Current knowledge concerning epidemiology, diagnosis and control. Rev. Sci. Tech. Off. int. Epiz 1997, 16, 848–873. [Google Scholar] [CrossRef] [PubMed]
- Madanat, A.; Zendulková, D.; Pospíšil, Z. Contagious agalactia of sheep and goats. A review. Acta Vet. Brno 2001, 70, 403–412. [Google Scholar] [CrossRef]
- DaMassa, A.J. Recovery of Mycoplasma agalactiae from mastitic goat milk. J. Am. Vet. Med. Assoc. 1983, 183, 548–549. [Google Scholar]
- Edward, D.G.; Freundt, E.A. Type strains of species of the order Mycoplasmatales, including designation of neotypes for Mycoplasma mycoides subsp. mycoides, Mycoplasma agalactiae subsp. agalactiae, and Mycoplasma arthritidis. Int. J. Syst. Bacteriol. 1973, 23, 55–61. [Google Scholar] [CrossRef][Green Version]
- Chopra-Dewasthaly, R.; Spergser, J.; Zimmermann, M.; Citti, C.; Jechlinger, W.; Rosengarten, R.; Lewinsohn, D.M. Vpma phase variation is important for survival and persistence of Mycoplasma agalactiae in the immunocompetent host. PLoS Pathog. 2017, 13, e1006656. [Google Scholar] [CrossRef]
- Chopra-Dewasthaly, R.; Baumgartner, M.; Gamper, E.; Innerebner, C.; Zimmermann, M.; Schilcher, F.; Tichy, A.; Winter, P.; Jechlinger, W.; Rosengarten, R.; et al. Role of Vpma phase variation in Mycoplasma agalactiae pathogenesis. FEMS Immunol. Med. Microbiol. 2012, 66, 307–322. [Google Scholar] [CrossRef]
- Baranowski, E.; Bergonier, D.; Sagné, E.; Hygonenq, M.; Ronsin, P.; Berthelot, X.; Citti, C. Experimental infections with Mycoplasma agalactiae identify key factors involved in host-colonization. PLoS ONE 2014, 9, e93970. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.; Yogev, D.; Naot, Y. Molecular Biology and Pathogenicity of Mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef] [PubMed]
- Rottem, S. Interaction of mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef]
- Rosengarten, R.; Wise, K.S. Phenotypic switching in mycoplasmas: Phase variation of diverse surface lipoproteins. Science 1990, 247, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Watson-McKown, R.; Droesse, M.; Wise, K.S. Gene families encoding phase- and size-variable surface lipoproteins of Mycoplasma hyorhinis. J. Bacteriol. 2000, 182, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Wang, J.; Ji, Y.; Ni, B.; Zhang, B.; Ma, Q.; Wei, Y.; Xiao, S.; Feng, Z.; Liu, M.; et al. The functions of the variable lipoprotein family of Mycoplasma hyorhinis in adherence to host cells. Vet. Microbiol. 2016, 186, 82–89. [Google Scholar] [CrossRef]
- Khiari, A.B.; Mardassi, B.B.A. Characterization of the antigenic and functional domains of a Mycoplasma synoviae variant vlhA gene. Vet. Microbiol. 2012, 156, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Glew, M.D.; Browning, G.F.; Markham, P.F.; Walker, I.D. pMGA Phenotypic Variation in Mycoplasma gallisepticum Occurs In Vivo and Is Mediated by Trinucleotide Repeat Length Variation. Infect. Immun. 2000, 68, 6027–6033. [Google Scholar] [CrossRef] [PubMed]
- Hamsten, C.; Westberg, J.; Bölske, G.; Ayling, R.; Uhlén, M.; Persson, A. Expression and immunogenicity of six putative variable surface proteins in Mycoplasma mycoides subsp. mycoides SC. Microbiology 2008, 154, 539–549. [Google Scholar] [CrossRef]
- Boesen, T.; Emmersen, J.; Jensen, L.T.; Ladefoged, S.A.; Thorsen, P.; Birkelund, S.; Christiansen, G. The Mycoplasma hominis vaa gene displays a mosaic gene structure. Mol. Microbiol. 1998, 29, 97–110. [Google Scholar] [CrossRef]
- Behrens, A.; Heller, M.; Kirchhoff, H.; Yogev, D.; Rosengarten, R. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect. Immun. 1994, 62, 5075–5084. [Google Scholar] [CrossRef] [PubMed]
- Bürki, S.; Gaschen, V.; Stoffel, M.H.; Stojiljkovic, A.; Frey, J.; Kuehni-Boghenbor, K.; Pilo, P. Invasion and persistence of Mycoplasma bovis in embryonic calf turbinate cells. Vet. Res. 2015, 46, 1–14. [Google Scholar] [CrossRef][Green Version]
- Glew, M.D.; Papazisi, L.; Poumarat, F.; Bergonier, D.; Rosengarten, R.; Citti, C. Characterization of a multigene family undergoing high-frequency DNA rearrangements and coding for abundant variable surface proteins in Mycoplasma agalactiae. Infect. Immun. 2000, 68, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Flitman-Tene, R.; Levisohn, S.; Lysnyansky, I.; Rapoport, E.; Yogev, D. A chromosomal region of Mycoplasma agalactiae containing vsp-related genes undergoes in vivo rearrangement in naturally infected animals. FEMS Microbiol. Lett. 2000, 191, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Czurda, S.; Hegde, S.M.; Rosengarten, R.; Chopra-Dewasthaly, R. Xer1-independent mechanisms of Vpma phase variation in Mycoplasma agalactiae are triggered by Vpma-specific antibodies. Int. J. Med. Microbiol. 2017, 307, 443–451. [Google Scholar] [CrossRef]
- Chopra-Dewasthaly, R.; Citti, C.; Glew, M.D.; Zimmermann, M.; Rosengarten, R.; Jechlinger, W. Phase-locked mutants of Mycoplasma agalactiae: Defining the molecular switch of high-frequency Vpma antigenic variation. Mol. Microbiol. 2008, 67, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Czurda, S.; Jechlinger, W.; Rosengarten, R.; Chopra-Dewasthaly, R. Xer1-mediated site-specific DNA inversions and excisions in Mycoplasma agalactiae. J. Bacteriol. 2010, 192, 4462–4473. [Google Scholar] [CrossRef]
- Glew, M.D.; Marenda, M.; Rosengarten, R.; Citti, C. Surface diversity in Mycoplasma agalactiae is driven by site-specific DNA inversions within the vpma multigene locus. J. Bacteriol. 2002, 184, 5987–5998. [Google Scholar] [CrossRef]
- Nouvel, L.X.; Marenda, M.; Sirand-Pugnet, P.; Sagné, E.; Glew, M.; Mangenot, S.; Barbe, V.; Barré, A.; Claverol, S.; Citti, C. Occurrence, plasticity, and evolution of the vpma gene family, a genetic system devoted to high-frequency surface variation in Mycoplasma agalactiae. J. Bacteriol. 2009, 191, 4111–4121. [Google Scholar] [CrossRef]
- Nouvel, L.X.; Sirand-Pugnet, P.; Marenda, M.S.; Sagné, E.; Barbe, V.; Mangenot, S.; Schenowitz, C.; Jacob, D.; Barré, A.; Claverol, S.; et al. Comparative genomic and proteomic analyses of two Mycoplasma agalactiae strains: Clues to the macro- and micro-events that are shaping mycoplasma diversity. BMC Genom. 2010, 11, 86. [Google Scholar] [CrossRef]
- Solsona, M.; Lambert, M.; Poumarat, F. Genomic, protein homogeneity and antigenic variability of Mycoplasma agalactiae. Vet. Microbiol. 1996, 50, 45–58. [Google Scholar] [CrossRef]
- Hegde, S.; Zimmermann, M.; Rosengarten, R.; Chopra-Dewasthaly, R. Novel role of Vpmas as major adhesins of Mycoplasma agalactiae mediating differential cell adhesion and invasion of Vpma expression variants. Int. J. Med. Microbiol. 2018, 308, 263–270. [Google Scholar] [CrossRef]
- Chopra-Dewasthaly, R.; Zimmermann, M.; Rosengarten, R.; Citti, C. First steps towards the genetic manipulation of Mycoplasma agalactiae and Mycoplasma bovis using the transposon Tn4001mod. Int. J. Med. Microbiol. 2005, 294, 447–453. [Google Scholar] [CrossRef]
- González, Y.R.C.; Bascuñana, C.R.; Bölske, G.; Mattsson, J.G.; Molina, C.F.; Johansson, K.-E. In vitro amplification of the 16S rRNA genes from Mycoplasma bovis and Mycoplasma agalactiae by PCR. Vet. Microbiol. 1995, 47, 183–190. [Google Scholar] [CrossRef]
- Spergser, J.; Hess, C.; Loncaric, I.; Ramírez, A.S. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Is a Superior Diagnostic Tool for the Identification and Differentiation of Mycoplasmas Isolated from Animals. J. Clin. Microbiol. 2019, 57, e00316-19. [Google Scholar] [CrossRef] [PubMed]
- Sachse, K.; Helbig, J.H.; Lysnyansky, I.; Grajetzki, C.; Müller, W.; Jacobs, E.; Yogev, D. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect. Immun. 2000, 68, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Gabriel, C.; Kragl, M.; Chopra-Dewasthaly, R. Sheep primary cells as in vitro models to investigate Mycoplasma agalactiae host cell interactions. Pathog. Dis. 2015, 73, ftv048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hegde, S.; Hegde, S.; Spergser, J.; Brunthaler, R.; Rosengarten, R.; Chopra-Dewasthaly, R. In vitro and in vivo cell invasion and systemic spreading of Mycoplasma agalactiae in the sheep infection model. Int. J. Med. Microbiol. 2014, 304, 1024–1031. [Google Scholar] [CrossRef]
- Ariza-Miguel, J.; Rodríguez-Lázaro, D.; Hernández, M. Molecular characterization of Mycoplasma agalactiae reveals the presence of an endemic clone in Spain. J. Clin. Microbiol. 2013, 51, 656–660. [Google Scholar] [CrossRef]
- De la Fe, C.; Amores, J.; Tardy, F.; Sagne, E.; Nouvel, L.-X.; Citti, C. Unexpected genetic diversity of Mycoplasma agalactiae caprine isolates from an endemic geographically restricted area of Spain. BMC Vet. Res. 2012, 8, 146. [Google Scholar] [CrossRef]
- Bergonier, D.; De Simone, F.; Russo, P.; Solsona, M.; Lambert, M.; Poumarat, F. Variable expression and geographic distribution of Mycoplasma agalactiae surface epitopes demonstrated with monoclonal antibodies. FEMS Microbiol. Lett. 1996, 143, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Pereyre, S.; Tardy, F.; Renaudin, H.; Cauvin, E.; Del Prá Netto Machado, L.; Tricot, A.; Benoit, F.; Treilles, M.; Bébéar, C. 2013. Identification and subtyping of clinically relevant human and ruminant mycoplasmas by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 3314–3323. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).