Simple Summary
Lower survival rates have been reported in foals than adults with severe colic lesions obstructing blood flow to the small intestine, but this has not been compared directly. These survival rates are important to horse owners making medical decisions surrounding colic, for both the foal’s wellbeing and the owner’s finances. In this retrospective case-control study, hospital records of surgical colic cases were collected from five US academic referral hospitals to directly compare foal and adult survival following surgery for specific colic lesions. It was hypothesized that foals would exhibit lower survival than case-matched adults. This study was limited by incomplete medical and surgical records, relatively small sample size, and lack of long-term follow-up. Short-term survival in foals was not significantly different than in adults with comparable colic lesions and may have been partly driven by decision-making on the farm prior to referral. More optimism toward surgical treatment of foals with suspected SISO may be warranted.
Abstract
Lower survival has been reported in foals than adults with small intestinal strangulating obstruction (SISO), but age-dependent outcomes have not been examined directly. Hospital records were collected from five US academic referral hospitals. It was hypothesized that foals would exhibit lower survival than case-matched adults. Foal cases 6-months-of-age or younger, and adult cases between 2- and 20-years-of-age were collected. Data revealed 24 of 25 (96.0%) foals and 66 of 75 (88.0%) adults that were recovered from surgery for SISO survived to hospital discharge. Sixteen of the total 41 (39.0%) foals studied were euthanized intraoperatively, whereas 30 of 105 (28.6%) adults were euthanized intraoperatively. Common lesions in foals that were recovered from surgery were volvulus (n = 13) and intussusception (n = 5), whereas common lesions in adults were volvulus (n = 25) and strangulating lipoma (n = 23). This study was limited by incomplete medical records, relatively small sample size, and lack of long-term follow-up. Unexpectedly, short-term survival tended to be higher in foals than adults and may have been partly driven by case selection prior to referral or surgery or decision-making intraoperatively. More optimism toward surgical treatment of foals with SISO may be warranted.
1. Introduction
Colic is the leading cause of death in adult horses, accounting for over 30% of deaths in horses from 1- to 20-years-of-age [1,2,3]. The most fatal form of colic in adult horses is strangulating obstruction [4], including strangulating lipomas of the small intestine where the reported mean age of affected horses is 19.2 years, and epiploic foramen entrapment of the small intestine where the reported mean age of affected horses is 9.6 years of age [5]. Reported rates of short-term survival from surgery to hospital discharge for these diseases range from 50–80% [5]. Alternatively, short-term survival rates for foals experiencing strangulating lesions of the small intestine, most commonly small intestinal volvulus, range from 27–50% [6]. While age-dependent outcomes have been examined in adult horses [7], survival rates have not been directly compared between foals and adults [6,8]. Reported long-term survival rates of foals that survived to hospital discharge following exploratory celiotomy ranges from 33–75%, depending on the lesion and the foal’s age at surgery [9]. Recently, reported survival rates for surgical correction of colic lesions in adult horses have risen to between 80–95% depending on the lesion, and horses have been shown to return to use more frequently than previously reported [10,11].
The decision to recommend referral for surgery is based on the horse’s apparent level of pain on presentation, vital signs (particularly the heart rate) [12,13], peritoneal fluid and lactate [14,15], the duration of colic symptoms, response to analgesia [12], evidence of reflux with nasogastric intubation, and findings on palpation per rectum [16]. The speed of referral is pivotal in optimizing survival rates of equids with small intestinal obstructions [17], with a 6-h duration of strangulating obstruction having a favorable prognosis, but a marked reduction in survival rates after a 12 h duration of strangulating obstruction [6,10]. The referral process is complicated in the foal due to varying degrees of visible or recognizable pain in young horses, and the inability to perform rectal palpation in the foal [8]. Abdominal radiographs and ultrasound are useful for detecting possible lesions in foals, but these modalities may not be commonly used in equine primary care practice, especially in the foal [6,8,18]. The communication process between the referring veterinarian, surgeon, and foal owner is further complicated by previous reports of lower survival rates in foals undergoing colic surgery compounded with overestimation of the cost of colic surgery, and whether or not the foal is insured [19]. Misconceptions and outdated literature may bias the way in which veterinarians present referral options to foal owners. Furthermore, an owner’s decision-making may be affected by their previous experiences with colic, or by shared stories from other horse owners [19].
Recent studies have reported increased survival rates for horses of all ages following colic surgery, and higher than expected return to use has been reported as well [10,11]. However, age-dependent outcomes following surgical management of colic have not been compared directly. This retrospective case-control study aimed to examine differences in clinical outcomes between foal and case-matched adult SISO patients, with the objective of generating knowledge pertinent to both clinicians and horse owners, to facilitate a dialogue regarding more accurate expectations prior to referral.
2. Materials and Methods
2.1. Inclusion Criteria
Hospital records from five institutions were reviewed to find surgical SISO cases. These records were obtained from North Carolina State University, University of California, Davis, Colorado State University, University of Pennsylvania and The Ohio State University. Horses were included if SISO was confirmed during laparotomy, with adults defined as horses between 2- and 20-years-of-age, and foals defined as 6-months-of-age or younger. Records were collected for both horses that were recovered from surgery and those that were euthanized intraoperatively, though the cases euthanized intraoperatively were analyzed separately. Three adult cases recovered from surgery were matched to each foal case that was recovered from surgery. Attempts were made to match adult cases to foals by institution, year of surgery, type and location of lesion, and whether a resection was performed, based on the medical records available at each referral hospital (Figure 1). Epiploic foramen entrapments were excluded from analysis due to previous research indicating a much poorer prognosis compared to other SISO lesions [20]. Types of lesions matched were approximate, as some cases present with lesions not seen in the other age group. For example, a foal was included with SI strangulation secondary to an ascarid impaction (an uncommon impaction etiology in adult horses) and was matched to adults that also had strangulated SI secondary to impactions of feed material. Foals and adults with SISO euthanized intraoperatively were excluded from survival analyses and therefore not case-matched, but were used to examine possible differences in intraoperative decision-making between foals and adults with SISO.
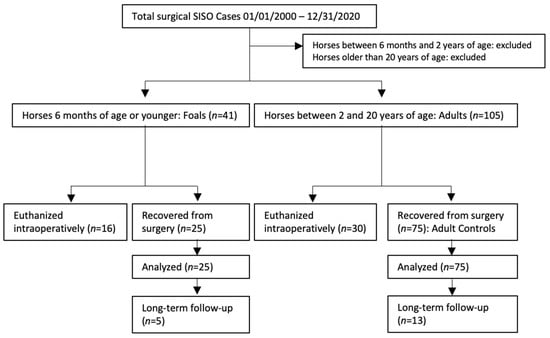
Figure 1.
Case selection and inclusion flow chart.
2.2. Statistical Analyses
GraphPad Prism software was utilized for all statistical analyses. Age distributions were evaluated for normality using Shapiro–Wilk normality tests. Univariate analyses were performed to assess selected factors with survival. Factors assessed included age (foal or adult), sex, breed, lesion, and whether or not a resection was performed. In addition, the distribution of breed and of intraoperative euthanasia decisions was assessed in foals as compared to adults using simple logistic regression or Fisher’s exact tests when appropriate. p < 0.05 was considered significant.
3. Results
3.1. Age Distribution
The population of foal cases collected was not normally distributed by age (p = 0.0002) (Figure 2a). The foal population appeared to have a right skew (mean = 67.34 days-of-age, median = 42 days-of-age), with younger foals presenting more frequently than older foals, particularly foals under 60-days-of-age (Figure 2a). When broken down by intraoperative decision, the population of foals euthanized during surgery was normally distributed (p = 0.07) (mean = 65.34 days of age, median = 40 days of age), with a trend toward intraoperative euthanasia of younger foals to achieve a more normal distribution from an originally right-skewed distribution (Figure 2b). Similar to the foal population, the adult population was not normally distributed (p < 0.0001) but is bimodal in appearance, with older and younger adult horses presenting more frequently than middle-aged horses (n = 20 horses between 17 and 18 years of age, n = 22 horses between 6 and 8 years of age) (Figure 2c). The distribution of adults euthanized during surgery was not normally distributed and followed the same bimodal appearance of the full population (p = 0.02) (Figure 2d).
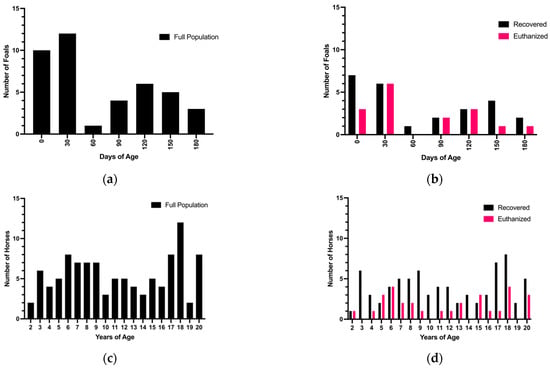
Figure 2.
Age affects intraoperative decision in foals. (a) shows the frequency distribution of foals by days of age, binned by months. The full population of all foal cases collected was not normally distributed, and appears to be skewed right, with more younger foals presenting than older foals (mean = 67.34 days-of-age, median = 42 days-of-age) (Shapiro–Wilk normality test p = 0.0002, W = 0.8655). (b) When separated based on intraoperative decision, the population of foals euthanized during surgery was normally distributed, while the population of foals recovered from surgery was not (mean = 65.34 days of age, median = 40 days of age) (Shapiro–Wilk normality test p= 0.071, W = 0.8966 and p = 0.0021, W = 0.8490, respectively). (c) The full population of adult cases collected was not normally distributed, and appears to have a bimodal shape, with younger and older adults presenting more frequently than adult horses between 10 and 16 years of age (n = 20 horses between 17 and 18 years of age, n = 22 horses between 6 and 8 years of age) (Shapiro–Wilk normality test p < 0.0001, W = 0.9326). (d) When separated by intraoperative decision, both the population of adults that were euthanized in surgery and recovered from surgery were not normally distributed, (Shapiro–Wilk normality test p = 0.0175, W = 0.9127 and p = 0.0009, W = 0.9358, respectively).
3.2. Breed Distribution
Some breeds presented more frequently with SISO, including Thoroughbreds, the warmblood breed group, Arabians, and Standardbreds. Of note, Standardbred foals were overrepresented in the total population when compared to the adult full population (p = 0.02), but were no longer overrepresented when comparing foals and adults that were recovered from surgery (p = 0.08). In total, 25 of 41 foals were recovered from surgery, including 9 Thoroughbreds, 6 Standardbreds, 4 warmbloods, 2 Arabians, 2 Andalusians, 1 Quarter Horse, and 1 Percheron. A total of 75 of the 105 adults were recovered from surgery, including 22 Thoroughbreds, 12 Quarter Horses, 10 warmbloods, 7 Arabians, 7 Standardbreds, 2 ponies of unspecified breed, 2 Morgans, 2 American Saddlebreds, 2 Tennessee Walking Horses, 1 Shire, 1 Percheron, 1 Quarab (Arabian-Quarter Horse cross), 1 Gypsy Cob, and 5 others of unspecified or unknown breed. Further information on breed distributions can be found in the Supplementary Materials (Table S1).
3.3. Lesion Distribution
Lesions that presented in foals that were recovered from surgery included small intestinal volvulus, mesenteric rents, inguinal hernias, intussusceptions, umbilical Richter’s hernias, ischemia secondary to an impaction, and strangulation by a mesodiverticular band.
Lesions that presented in adults recovered from surgery included small intestinal volvulus, mesenteric rents, inguinal hernias, omental rents, strangulating lipomas, gastrosplenic ligament entrapments, nephrosplenic ligament rents, and SI entrapment in the abdominal wall. Detailed case-matching by age and lesion is available in the Supplementary Materials (Table S2).
3.4. Survival Analysis
Sixteen of 41 (39.0%) foals and 30 of 105 (28.6%) adults were euthanized during surgery (p = 0.2; 95% CI = 0.3 to 1.4) (Figure 3a). Assessment of survival revealed that 24 of 25 (96.0%) foals and 66 of 75 (88.0%) adults that were recovered from surgery for SISO survived to hospital discharge (Figure 3b). Of the foals that survived to hospital discharge, 9 (37.5%) had resections, and 27 (40.9%) of the surviving adults had resections (p = 0.5; 95% CI 0.8 to 2.7). Of the 9 adults euthanized postoperatively, 5 (55.6%) had resections. The 1 foal that was euthanized postoperatively did not have a resection. When analyzing cases recovered from surgery, there was a trend toward higher survival in foals as compared to adults (p = 0.4; 95% CI 0.4 to 37.3) (Figure 3b).
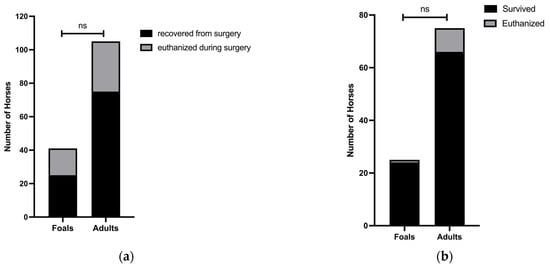
Figure 3.
Similar proportions of foals and adults were euthanized intraoperatively, and short-term survival was higher in foals than adult case-controls. (a) Of 41 foals, 16 (39.0%) were euthanized intraoperatively, while 30 of 105 (28.6%) adults were euthanized intraoperatively. (b) Of 25 foals, 24 (96.0%) survived to hospital discharge, while only 66 of 75 (88.0%) adult case-controls survived to hospital discharge.
Long-term follow-up was available for 13 adults and 5 foals, showing that there was a 60% long-term survival in foals and 84.62% long-term survival in adults, though the cases available were too few for statistical analysis (Figure 4). Of those available for long term follow up, 2 foals and 2 adults were euthanized 1 month post-operatively due to the formation of adhesions.
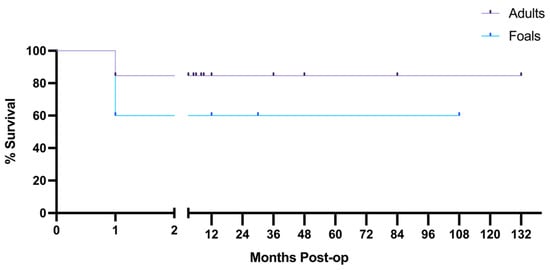
Figure 4.
Long-term follow-up shows higher survival rates in adult case-controls. Of 13 adults and 5 foals, 2 adults and 2 foals were euthanized 1-month post-operatively for adhesions. Follow-up shows 84.62% survival in available adults, and 60% survival in available foals. The break in the x-axis spans time in which no horses died or were lost to follow-up. Tick marks on the survival curve indicate a horse that was lost to follow-up.
3.5. Assessment of Factors Associated with Survival
Factors evaluated for their association with survival were age, breed, and lesion (Table 1). No significant risk factors were identified in the univariate analyses.

Table 1.
Univariate analyses of risk factors affecting survival.
4. Discussion
There were no significant differences in the number of foals and adults euthanized at surgery, and a similar number of foals and adults underwent intestinal resection. The short-term survival of foals with SISO was higher but not significantly different than case-matched adult horses (96% vs. 88%, p = 0.4) despite a similar proportion of intra-operative euthanasia decisions and resections between the two age groups. This contrasts with previous studies examining foal survival rates [6,8,9], and is suggestive of a more favorable short-term prognosis for foals with surgical colic lesions as compared to prior reports.
Long-term follow-up was incomplete in this study, so no conclusions can be drawn regarding foal or adult prognoses past discharge from the hospital. This may be a target for future research, as updated long-term survival statistics would be of similar interest to horse owners and veterinarians.
This retrospective study examines data regarding survival of foals and adult horses with surgical colic lesions. Regardless of the pathophysiology involved in the lesion and the prognosis provided by the referral veterinarian or surgeon, horse owners must make their own decisions to either continue pursuit of treatment or elect for humane euthanasia. Human decision-making undoubtedly biases the survival outcomes, which would likely be different if there was no human intervention involved, and the sociological factors in the decision-making process of horse owners and veterinarians may not be ignored.
The relatively higher rate of survival in foals may have been partly driven by case-selection prior to referral, or following initial workup in the referral hospital, but these data were not available as the clinical records evaluated were of horses that had owners that elected referral for further colic work-up. Many of the decisions to pursue referral and surgery are financially driven, regardless of the animals’ age or prognoses provided [19]. While it is known that surgeons expect foals to form intrabdominal adhesions more readily than adult horses [21,22,23,24,25], recent studies are showing this may not be the case [25,26]. Regardless, this knowledge is commonly held and may further bias the way surgeons view foal cases intraoperatively. It is also possible that fewer foals are referred because they are not yet proven as, for example, adult show horses in their intended discipline. There is literature focusing on primary care veterinarians’ decision-making around recommending referral [19,27] and on surgeons’ intraoperative decision-making [10], although this has not focused on decision-making in foal cases. Sociological research conducted in the UK and Europe focused on horse owners’ abilities to recognize serious colic symptoms and their views on referring a horse for further workup and possible surgery. Researchers found that most owners can recognize signs of colic in their horse, but the immediate actions taken were dependent on the extent of their experience with colic, and on their ability to identify symptoms of more severe cases [28]. The decisions to refer for further workup and possible surgery can also be impacted by other factors, including geographic region relative to a referral hospital, and whether horse owners view their horses more as companion animals (pets) or as working animals and a source of income [28]. Similar sociological research is lacking in the US and identifies an important gap in the knowledge that undoubtedly impacts colic survival rates [28,29]. Without adequate qualitative data from horse owners and primary care veterinarians in the US, this study cannot draw conclusions about the proportion of foals or horses that may have been euthanized after initial workup on the farm or at the hospital, nor what the leading reason was for decisions on euthanasia.
Furthermore, the inherent differences in geographical location likely had some impact on the demographics seen in this study. For example, Standardbred foals were overrepresented in one population, but it is worth noting that many of the Standardbreds included in the study were admitted to the University of Pennsylvania (UPenn), where this distribution is an adequate representation of the surrounding population, as there are many Standardbred racehorse breeding farms in the Pennsylvania area. UPenn tends to see fewer Standardbred adults compared to Thoroughbred adults. Anecdotally, this is because Standardbred horses are more likely to be sold to Amish families following their racing career, so they are less likely to have a show career post-racing than a Thoroughbred and fewer referral options as adults than other breeds, which leads to the abundance of Standardbred foals referred relative to a lack of Standardbred adults.
In addition, differences in lesion types likely had some impact on the survival rates seen in this study. Foals and adult horses often experience lesions that are specific to certain age groups. For example, aged geldings are predisposed to developing pedunculated lipomas which encircle and strangulate sections of intestine, while foals do not develop these lipomas [30]. In contrast, foals more commonly experience ascarid impactions, an uncommon lesion in adult horses [31]. With inclusion of these age-specific lesions, efforts were made to case-match lesions that were comparable in pathophysiology. While these efforts did not produce perfectly matched control cases, it was possible to identify control lesions similar enough in severity and pathophysiology to be compared. For example, an adult with ischemic injury secondary to an impaction of feed material was compared to a foal with ischemic injury secondary to the ascarid impaction. While it has previously been noted that ascarid impactions have poorer outcomes than other types of impactions [31], surgical notes were referenced to validate similarity of lesion severity, though this is ultimately difficult to guarantee due to frequently incomplete surgical records.
Finally, the age range for enrolled adult colic cases was capped at 20-years-of-age to limit impact of age-related comorbidities on adult survival. Widening the age range for adult controls might have increased the number of adults that could have served as better controls in some instances.
Overall, limitations of this study include a relatively small sample size and lack of follow up on the majority of cases, rendering long-term survival analyses and conclusions regarding long-term foal survival impossible.
5. Conclusions
The results of this study demonstrate no significant difference in short-term outcome of surgically treated SISO foals compared with adults. The clinical application of such findings supports more optimism toward surgical treatment of foals with SISO. First opinion veterinarians should be encouraged to discuss a surgical referral option for owners of foals with suspected SISO more favorably given these updated short-term survival rates. More research is required in this area, as more data and updated representative statistics regarding foal survival for referring veterinarians will allow foal owners to make the most appropriate decision for the foal’s wellbeing and allow for judicious use of the owner’s finances. Future work might focus on elucidating the factors that influence the decision-making of surgeons and foal owners.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12111374/s1, Table S1: Breeds of foals and adults recovered from surgery; Table S2: Case-matched Lesions.
Author Contributions
Conceptualization, S.J.E., A.L.Z., and A.T.B.; methodology, S.J.E., A.L.Z., and A.T.B.; validation, A.T.B.; visualization, S.J.E.; formal analysis, S.J.E. and M.E.C.; investigation, S.J.E., M.E.C., J.E.D., M.R.A., and D.M.H.; data curation, M.E.C.; writing—original draft preparation, S.J.E.; writing—review and editing, A.L.Z., A.T.B., J.E.D., M.R.A., and D.M.H.; project administration, S.J.E., A.L.Z., and A.T.B.; funding acquisition, A.L.Z. and A.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health K01 OD 028207, National Institutes of Health P30 DK 034987, National Institutes of Health R01 HD095876, United States Department of Agriculture- AFRI-NIFA 2019-67017-29372, NCSU CVM Veterinary Faculty Practice Plan.
Institutional Review Board Statement
Ethical review and approval were waived due to the retrospective nature of this case-control study.
Informed Consent Statement
Not applicable.
Data Availability Statement
De-identified case data may be obtained from the corresponding author by reasonable request.
Acknowledgments
The authors thank Emily Hellstrom for her assistance collecting long-term follow up data. The authors also thank Megan Burke for coordinating case collection with the Ohio State University, and Alison Gardner at the Ohio State University for providing relevant cases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United States Department of Agriculture (USDA). Equine 2015, “Baseline Reference of Equine Health and Management in the United States, 2015”; USDA–APHIS–VS–CEAH–NAHMS: Fort Collins, CO, USA, 2016.
- Wormstrand, B.H.; Ihler, C.F.; Diesen, R.; Krontveit, R.I. Surgical treatment of equine colic—A retrospective study of 297 surgeries in Norway 2005–2011. Acta Vet. Scand. 2014, 56, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzerro, D.M.; Southwood, L.L.; Lindborg, S. Short-term complications after colic surgery in geriatric versus mature non-geriatric horses. Vet. Surg. 2015, 44, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, M.J.; Denagamage, T.N.; Freeman, D.E. Effects of age, disease and anastomosis on short- and long-term survival after surgical correction of small intestinal strangulating diseases in 89 horses. Equine Vet. J. 2022, 1–8, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.E.; Schaeffer, D.J. Age distributions of horses with strangulation of the small intestine by a lipoma or in the epiploic foramen: 46 cases (1994–2000). J. Am. Vet. Med. Assoc. 2001, 219, 87–89. [Google Scholar] [CrossRef]
- Vatistas, N.J.; Snyder, J.R.; Wilson, W.D.; Drake, C.; Hildebrand, S. Surgical treatment for colic in the foal (67 cases): 1980–1992. Equine Vet. J. 1996, 28, 139–145. [Google Scholar] [CrossRef]
- Krista, K.M.; Kuebelbeck, K.L. Comparison of survival rates for geriatric horses versus nongeriatric horses following exploratory celiotomy for colic. J. Am. Vet. Med. Assoc. 2009, 235, 1069–1072. [Google Scholar] [CrossRef]
- Bryant, J.E.; Gaughan, E.M. Abdominal surgery in neonatal foals. Vet. Clin. N. Am. Equine Pract. 2005, 21, 511–535. [Google Scholar] [CrossRef]
- Mackinnon, M.C.; Southwood, L.L.; Burke, M.J.; Palmer, J.E. Colic in equine neonates: 137 cases (2000–2010). J. Am. Vet. Med. Assoc. 2013, 243, 1586–1595. [Google Scholar] [CrossRef]
- Gardner, A.; Dockery, A.; Quam, V. Exploratory Celiotomy in the Horse Secondary to Acute Colic: A Review of Indications and Success Rates. Top. Companion Anim. Med. 2019, 34, 1–9. [Google Scholar] [CrossRef]
- Davis, W.; Fogle, C.A.; Gerard, M.P.; Levine, J.F.; Blikslager, A.T. Return to use and performance following exploratory celiotomy for colic in horses: 195 cases (2003–2010). Equine Vet. J. 2013, 45, 224–228. [Google Scholar] [CrossRef]
- White, N.A.; Elward, A.; Moga, K.S.; Ward, D.L.; Sampson, D.M. Use of web-based data collection to evaluate analgesic administration and the decision for surgery in horses with colic. Equine Vet. J. 2005, 37, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Parry, B.W.; Gay, C.C.; Anderson, G.A. Assessment of the necessity for surgical intervention in cases of equine colic: A retrospective study. Equine Vet. J. 1983, 15, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Yamout, S.Z.; Nieto, J.E.; Beldomenico, P.M.; Dechant, J.E.; leJeune, S.; Snyder, J.R. Peritoneal and plasma D-lactate concentrations in horses with colic. Vet. Surg. 2011, 40, 817–824. [Google Scholar] [CrossRef]
- Peloso, J.G.; Cohen, N.D. Use of serial measurements of peritoneal fluid lactate concentration to identify strangulating intestinal lesions in referred horses with signs of colic. J. Am. Vet. Med. Assoc. 2012, 240, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.J.; Curtis, C.R.; Salman, M.D.; Stashak, T.S.; Reif, J.S. Development and validation of multivariable models to predict the need for surgery and prognosis in equine colic patients. Acta Vet. Scand. Suppl. 1988, 84, 329–332. [Google Scholar]
- Southwood, L.L. Practical Guide to Equine Colic; Wiley-Blackwell: Ames, IA, USA, 2012. [Google Scholar]
- Curtis, L.; Trewin, I.; England, G.C.; Burford, J.H.; Freeman, S.L. Veterinary practitioners’ selection of diagnostic tests for the primary evaluation of colic in the horse. Vet. Rec. Open 2015, 2, e000145. [Google Scholar] [CrossRef] [Green Version]
- Archer, D.C. Colic surgery: Keeping it affordable for horse owners. Vet. Rec. 2019, 185, 505–507. [Google Scholar] [CrossRef]
- van Bergen, T.; Haspeslagh, M.; Wiemer, P.; Swagemakers, M.; van Loon, G.; Martens, A. Surgical treatment of epiploic foramen entrapment in 142 horses (2008–2016). Vet. Surg. 2019, 48, 291–298. [Google Scholar] [CrossRef]
- Lundin, C.; Sullins, K.E.; White, N.A.; Clem, M.F.; Debowes, R.M.; Pfeiffer, C.A. Induction of peritoneal adhesions with small intestinal ischaemia and distention in the foal. Equine Vet. J. 1989, 21, 451–458. [Google Scholar] [CrossRef]
- Baxter, G.M.; Broome, T.E.; Moore, J.N. Abdominal adhesions after small intestinal surgery in the horse. Vet. Surg. 1989, 18, 409–414. [Google Scholar] [CrossRef]
- Ellis, H.; Harrison, W.; Hugh, T.B. The Healing of Peritneum under Normal and Pathological Conditions. Br. J. Surg. 1965, 52, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Sullins, K.E.; White, N.A.; Lundin, C.S.; Dabareiner, R.; Gaulin, G. Prevention of ischaemia-induced small intestinal adhesions in foals. Equine Vet. J. 2004, 36, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Santschi, E.; Slone, D.; Embertson, R. Colic surgery in 206 juvenile Thoroughbreds: Survival and racing results. Equine Vet. J. 2000, 32, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.E.; Fubini, S.L.; Todhunter, R.J.; Brooks, M.B. Comparison of plasma and peritoneal indices of fibrinolysis between foals and adult horses with and without colic. Am. J. Vet. Res. 2011, 72, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Bowden, A.; England, G.C.W.; Brennan, M.L.; Mair, T.S.; Furness, W.A.; Freeman, S.L.; Burford, J.H. Indicators of ‘critical’ outcomes in 941 horses seen ‘out-of-hours’ for colic. Vet. Rec. 2020, 187, 492. [Google Scholar] [CrossRef]
- Scantlebury, C.E.; Perkins, E.; Pinchbeck, G.L.; Archer, D.C.; Christley, R.M. Could it be colic? Horse-owner decision making and practices in response to equine colic. BMC Vet. Res. 2014, 10, S1. [Google Scholar] [CrossRef] [Green Version]
- Bowden, A.; Burford, J.H.; Brennan, M.L.; England, G.C.W.; Freeman, S.L. Horse owners’ knowledge, and opinions on recognising colic in the horse. Equine Vet. J. 2020, 52, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Seco, E.; Wilson, D.A.; Kramer, J.; Keegan, K.G.; Branson, K.R.; Johnson, P.J.; Tyler, J.W. Prevalence and risk factors associated with outcome of surgical removal of pedunculated lipomas in horses: 102 cases (1987–2002). J. Am. Vet. Med. Assoc. 2005, 226, 1529–1537. [Google Scholar] [CrossRef]
- Tatz, A.J.; Segev, G.; Steinman, A.; Berlin, D.; Milgram, J.; Kelmer, G. Surgical treatment for acute small intestinal obstruction caused by Parascaris equorum infection in 15 horses (2002–2011). Equine Vet. J. Suppl. 2012, 44, 111–114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).