Simple Summary
Heat stress is a significant threat to the pigs’ production performance as it greatly affects various body systems, particularly those that are responsible for nutrient digestion and absorption. Heat-stress-induced stressors such as oxidative stress threaten the integrity and functionality of the intestine by negatively affecting its morphology and histology through reduction of villus height, crypt depth, villus height to crypt depth ratio, mucosal surface and villi sloughing. Its protective function is also compromised as heat stress negatively influences the expression of tight junction proteins and disrupts the tight junction barrier function, leading to endotoxemia. These adverse effects of heat stress can be highly mitigated by supplementing dietary antioxidants, as these substances positively influence the intestinal integrity and function of pigs through the improvement of intestinal morphology and histology. Reduction of blood endotoxin through improved tight junction barrier function and depletion of oxidative stress with enhanced mucosal antioxidant capacity is also evident upon such supplementation.
Abstract
Heat stress (HS) significantly affects the performance of pigs by its induced stressors such as inflammation, hypoxia and oxidative stress (OS), which mightily strain the intestinal integrity and function of pigs. As heat stress progresses, several mechanisms in the intestinal epithelium involved in the absorption of nutrients and its protective functions are altered. Changes in these mechanisms are mainly driven by cellular oxidative stress, which promotes disruption of intestinal homeostasis, leading to intestinal permeability, emphasizing intestinal histology and morphology with little possibility of recovering even after exposure to HS. Identification and understanding of these altered mechanisms are crucial for providing appropriate intervention strategies. Therefore, it is this papers’ objective to review the important components for intestinal integrity that are negatively affected by HS and its induced stressors. With due consideration to the amelioration of such effects through nutritional intervention, this work will also look into the capability of dietary antioxidants in mitigating such adverse effects and maintaining the intestine’s integrity and function upon the pigs’ exposure to high environmental temperature.
1. Introduction
Animal welfare and productivity are compromised when livestock animals are exposed to an ambient temperature above their thermoneutral zone [1]. Among the various livestock species, pigs are more sensitive to ambient temperature changes and are susceptible to heat stress (HS). Several types of stressors including inflammation, hypoxia and oxidative stress (OS) are induced by HS in the skeletal muscles and intestine, which adversely affects the pigs’ performance [2,3,4,5]. OS occurs due to an imbalance between the production of reactive oxygen species (ROS) and the available antioxidant defense against them. Various stressors can cause ROS overproduction, and one of them is HS [6,7]. This can then lead to damages to cellular constituents [8]. Physiologically, the organ that is highly sensitive to HS and its induced stressors is the gastrointestinal tract (GIT) [9], as it is the first significant organ being affected [10]. Changes in the pigs’ physiology, metabolism and behavior are evident upon exposure to HS. As an immediate physiological response, pigs tend to increase their peripheral blood flow to promote heat loss; consequently, blood flow to the internal organs, notably the GIT, is reduced [11]. This reduction of blood flow leads to the decrease in the supply of oxygen (hypoxia) and nutrients in the GIT [12], compromising intestinal barrier integrity and function [13]. The intestinal barrier is composed of a single layer of enterocytes and intercellular tight junctions (TJs) and serves as a selective permeable membrane that is responsible for digestion and absorption of nutrients and constitutes the first line of defense against various harmful substances that can enter the intestinal mucosa and systemic circulations [14,15]. HS disrupts TJs in the pigs’ intestine, increasing the circulation of endotoxin [16]. As a result, increased intestinal permeability can occur, leading to a high risk of endotoxemia [17]. Mitigation strategies that pinpoint the root cause of HS-induced intestinal permeability are vital as they can provide an efficient solution to the said matter. Nutritional interventions are proven effective in alleviating HS’s effects on the intestinal integrity of pigs [18]. As HS induces cellular OS in the intestine [5], this review aims to look into the details on how HS and its stressors promote intestinal permeability and the ability of antioxidants to mitigate such adverse effects.
2. Intestinal Epithelium
A healthy gut is important in aiding efficient digestion and absorption of the dietary nutrients ingested by an animal [19]. The intestine’s digestive, absorptive and protective functions are dependent on an intact and functional intestinal epithelium (IE) [20]. As an active barrier, it serves both the uptake of nutrients and prevents harmful substances and potential pathogens from entering into the bloodstream [21,22]. The intestine is inhabited by many microorganisms, which provides an avenue for nutrition, metabolism and immunity [23]. A single layer of intestinal epithelial cells (IECs) bound together by TJs (composed of transmembrane proteins: occludin, claudins and junctional adhesion molecules) makes up the IE [24,25]. This unique composition of the IE is to prevent harmful microorganisms, antigens and toxins from the gut lumen from entering into the circulation [26]. This is achieved through the IEC’s function in creating mucosal barrier (physical and chemical barrier) which maintains symbiosis between the gut microbiota and the host. The barriers maintain homeostasis by segregating gut microbiota and host immune cells, which prevents inflammation due to excessive immune response [27]. IECs also influence the recruitment and activation of immune cells through production of cytokines and chemokines [22,28], thus being appreciated as a central component of innate immunity [29]. As a single cell layer, IE is selectively permeable through transcellular (nutrients passing through the cell) and paracellular (via TJ) pathways [30]. The IEC maintains barrier integrity through weak protein–protein bonding of junctional complexes such as TJ, adherens junction (AJ) and desmosomes, all of which have occlusive properties [31]. Proteins that link adjacent epithelial cells to the actin cytoskeleton are present in the AJ and desmosomes which are essential for mechanical linking of cells [9,32]. The TJ regulates the formation of intestinal barriers by modulating cell proliferation, differentiation and polarization [33]. It is also responsible for the regulation of ions, solutes and water across the intestinal epithelium through paracellular movement [34]. The pigs’ IE renews every two to three days, compelled by the intestinal stem cells (ISCs) [35]. This ensures that only the fittest and metabolically able cells comprise the IE and maintain an impermeable barrier to gut microbiota and luminal contents as well as for nutrient digestion, absorption and secretion of antimicrobial peptides [36]. Another positive influence of ISCs is the generation of highly proliferative transit-amplifying cells. These cells then differentiate into enterocytes and secretory cells upon migration to the villi and into Paneth cells (secrete antimicrobial peptides) upon migration towards the crypt. Although Paneth cells’ existence in the pig’s intestine is debatable [37], several researches have confirmed that it exists [38,39]. Stability of ISC’s self-renewal and differentiation controls the intestinal epithelial homeostasis and is essential for ensuring intestinal epithelial integrity [35].
3. Heat Stress and Its Induced Stressors
HS is experienced by pigs when they can no longer release excess heat as influenced by increase in environmental temperature. The sensitivity of pigs to HS is due to their thick layer of subcutaneous adipose tissue and lack of functional sweat glands, making them less efficient in losing heat when exposed to high environmental temperature [40]. However, ambient temperature (AT) is not the sole culprit in inducing HS to pigs, as studies show that it is also influenced by relative humidity (RH) and the index that combines these factors is called heat index (HI). Knowledge about HI is important as it can alert farmers to provide appropriate response to either prevent or alleviate the consequences of HS on the performance of pigs. HS index charts released by Iowa State University and Ontario Ministry of Agriculture show that pigs are in danger of experiencing HS at AT of 26 °C with an RH of 75 to 90% [41,42]. This indicates that even at temperatures within the desirable limits for pigs, it still makes them susceptible to HS when the RH is high. This can be supported by the fact that, under such temperatures, pigs tend to loss more heat through evaporation (panting); however, it is insufficient as higher RH makes it less effective due to the fact that less moisture can be evaporated. However, when the AT is at 30 °C and above, RH under 50% is enough to cause HS to pigs [42,43]. Several research pieces suggest that HS induces OS in various species of food animals such as poultry [44] and pigs [5,45]. HS is considered cytotoxic and has been proven to induce oxidative damage by disturbing mitochondrial homeostasis and cellular function [4]. Intestinal homeostasis is lost due to OS and associated tissue redox imbalance, which negatively affects the intestine’s digestive and absorptive function and its purpose for stem cell proliferation and immune response [46]. The latter is confirmed by cellular apoptosis inhibition caused by HS [3]. HS induces hypoxia in the intestine, resulting in the imbalance between the production of ROS and the antioxidant defense system, which jeopardizes the intestinal epithelium and enhances inflammatory response which can exacerbate intestinal permeability [10,47]. Such response is mediated by various immune cells (macrophages, dendritic cells, mast cells and lymphocytes) and their mechanisms in antigen processing and presentation and production of regulatory and effector molecules (cytokines, chemokines and immunoglobulins). T lymphocytes identified by a surface cluster of differentiation (CD) molecule named CD3 has two major groups, CD4 (helper T cells) and CD8 (cytotoxic T cells), which are found in the intestinal epithelium and can contribute to the barrier integrity but occasionally become proinflammatory when the balance between the groups is disturbed [48,49]. In the study of Huo et al. [3], pigs subjected to chronic HS expressed significant increase of CD3, but were associated with an imbalance between CD4 and CD8 signifying immune dysfunction. Nevertheless, in other studies inflammatory response to HS involved upregulation of proinflammatory cytokines (tumor necrosis factor alpha (TNFα), interleukin (IL-4 and IL-6) in the skeletal muscle of pigs and in the intestine of cattle and chicken [4,50,51]. Hypoxia-inducible factor (HIF)-1 triggers the cellular response to hypoxia. HIF-1 consists of two subunits: the oxygen-regulated alpha (α) and a constitutively expressed beta (β). The former is responsible for the regulation of energy homeostasis and cellular adaptation to hypoxia. Under normal conditions, HIF-1α is rapidly degraded in the proteasome through hydroxylation by oxygen-sensitive prolyl hydroxylases (PHD). However, hypoxic conditions can lead to HIF-1α’s rapid accumulation, stabilization and transcriptional activity due to the inhibition of PHD activity, which enables the cells to adapt to hypoxic stress. Nevertheless, the consequence of hypoxic signaling is the abnormal accumulation of ROS by cytochrome reductase of the mitochondrial electron transport chain [47,52]. ROS are unstable and oxygen-centered molecules containing unpaired-valence electrons which are highly reactive with proteins, lipids, carbohydrates and nucleic acids in the cell. It includes radical compounds (superoxide (O2−), hydroxyl radicals (HO), lipid hydroperoxides) and reactive non-radical compounds or oxidants (oxygen (1O2), ozone (O3), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), hypobromous acid (HOBr), chloramines (RNHCl) and organic hydroperoxides (ROOH)), which can impair intestinal cells, leading to OS [53,54,55]. The animal body is equipped with antioxidant defense system which is responsible against the deleterious effect of ROS. This system involves endogenous enzymatic antioxidants (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR)) and non-enzymatic antioxidants (glutathione (GSH), thioredoxin (Trx) and melatonin (Mel)) with ability to remove free radicals from the system and inhibit oxidation. Among the various endogenous antioxidant enzymes, SOD and CAT impart substantial antioxidant defenses against ROS [55]. SOD catalyzes dismutation of O2− into O2 and H2O2, it scavenges O2− from mitochondrial intermembranous space and mitochondrial matrix, while CAT dismutates H2O2 to H2O and O2 [56]. Other antioxidant enzymes also have a significant role; GPx is responsible for the conversion of GSH into glutathione disulfide (GSSG), which reduces H2O2 to H2O and lipid hydroperoxides to stable alcohols. Conversely, GR can reduce GSSG to GSH which maintains the balance of cell differentiation, growth and apoptosis [53,57,58]. Non-enzymatic antioxidants such as GSH, Trx and Mel interrupt free radical chain reaction. GSH is ubiquitously expressed together with GSH-Px, GR and glutathione S-transferases (GST) forming the glutathione system which serves as an antioxidant barrier in the gut mucosa. Trx serves as donors of electron to peroxidase to efficiently scavenge ROS, while Mel can efficiently protect the mitochondria from oxidative damage through its direct free radical scavenging capability; whilst other antioxidants can be converted into free radicals, the said conversion does not apply to Mel as its oxidative role involves donation of two electrons. These mechanisms can then result in reduced lipid peroxidation, DNA damage, protein activation and restoration of intestinal injury induced by OS [53,55,59]. Antioxidant to pro-oxidant balance in the intestine is an essential determinant of human [60] and pig gut health [61]. However, this balance is disturbed by HS, as it increases mitochondrial free radicals such as O2− and oxidants (H2O2), and decreases the activities of endogenous antioxidants which are associated with the excessive generation of ROS [7,62]. Normal cellular metabolism produces low to moderate amounts of ROS as its byproducts, which is useful in several physiological processes such as wound healing, tissue repairs as well as killing invading pathogens [55]. However, its overproduction due to HS can be detrimental as heat-induced ROS targets proteins; lipids, polysaccharide and DNA [7]. ROS directly reacts with DNA components which can cause DNA lesions, particularly the mitochondrial DNA (MtDNA) as its location is close to the ROS-generated place. This then leads to mutations that can cause the electron transport chain (ETC) complex dysfunction and lead to more production of ROS and oxidative damage [7,63,64]. Oxidation of proteins can be induced by ROS, as it oxidizes various amino acids causing formation of protein-to-protein cross linkages that can result in protein denaturation and loss of functioning as well as losses in enzyme activity, function receptors and transport proteins [63,65]. Lipids are highly susceptible to oxidation by free radicals such as HO; in excess it can damage cell membrane and lipoproteins through a process called lipid peroxidation and leads to the formation of malondialdehyde (MDA) and production of 4-hydroxenonenal (4-HNE) which are cytotoxic and mutagenic. As this process occurs through radical chain reaction, it spreads rapidly and affects numerous lipid molecules [66]. Thus, excessive ROS can lead to cellular dysfunction, disruption of vital cellular processes and apoptosis through modification of the structure of cellular proteins and the alteration of their function [7,67,68,69].
4. Intestinal Integrity and Function of Pigs under Heat Stress
Harmonized regulation of the mucus layer, TJs, IECs and the enteric immune system influences the intestine’s integrity and function [9,32]. The first line of defense against intestinal injury is provided by the intestinal mucus layer [70], as it serves as a physical barrier against bacteria and antigenic substances in the lumen by coating the interior surface of the intestine and lubricating its luminal contents [71]. It is composed of mucin glycoprotein (MUC2) and bioactive molecules such as epithelial-bound mucins (MUC1, MUC3 and MUC17), which are synthesized by the intestinal goblet cells [72] that are confined to the crypts of Lieberkühn and on the small intestinal villi. In the colon, goblet cells amass at the opening of the colonic crypts and are also found deep within the crypts and on the surface of the colon [73,74]. The high polymeric protein backbone structure of these mucins that are linked to numerous hygroscopic and hydrophilic oligosaccharide side-chains contributes to the mucus layers’ gel-like structure [72,75]. The intestinal mucus layer establishes an active semi-permeable barrier which allows passage of nutrients from the gut lumen towards the epithelium [76] while promoting clearance that separates bacteria from the epithelial cells that inhibit inflammation and infection [77]. HS can directly and indirectly (via reduced feed intake) compromise intestinal integrity of pigs. The former takes effect as HS induces hypoxia and OS in the intestine, as assessed by acute increase of HIF-1α mRNA abundance and increase in lipid oxidation (4-HNE abundance). Moreover, intestinal structures of proteins responsible for cell structure and motility (such as alpha-actinin-1 and myosin regulatory light chain) as well as for cellular proliferation and apoptosis (TNF receptor-associated protein 1 and Erlin-2) are altered by HS along with reduction of endogenous antioxidants (GPx and GSH). This leads to deprivation of oxygen and nutrients to the enterocytes and loosening of TJs. Nutrient restriction due to reduced feed intake also causes alterations in intestinal function and morphology of pigs, thus accompanied by HS’s direct effects exacerbating intestinal permeability [5,17,78,79] with an emphasis on intestinal morphology parameters (Table 1), such as reduced villus height and crypt depth, villus to crypt ratio and decrease in the mucosal surface, sloughing of the intestinal villi as well as undergoing autolysis, regardless of the duration of exposure [17,80,81,82]. This then influences changes in the cellular proliferation and membrane function [83]. Intact morphological structure of the intestine is important for nutrient utilization and absorption and it is associated with longer villi which is a good indicator of a healthy gut; thus, its reduction and damage can be cautiously used to represent increasing intestinal permeability and infiltration of endotoxins [81,84].

Table 1.
Intestinal morphology of pigs under thermal comfort (TC) and heat stress (HS).
Nevertheless, these consequences on intestinal integrity can be influenced by the duration and intensity of the pigs’ exposure to HS. During the first 2 to 4 h of HS (37 °C and 40% RH), the pigs’ intestinal integrity in the ileum declined, associated with decrease in transepithelial resistance (TER), while the colon remained unaffected at this period. Between 6 and 12 h TER rebounds as it increases, however it ultimately declined after 24 h of exposure signifying intestinal permeability [80,86,87]. Under such conditions, Pearce et al. [80] observed increase in ileum MUC2 after 6 h of exposure to HS which can act as a protective barrier for the intestine and may combat the decrease in intestinal integrity. However, in another study, pigs exposed to HS for 3 h showed a reduction of goblet cells in the jejunum and ileum [85], and this was also observed even during the recovery period of 7 days after constant exposure to HS for 3 days [81]. This suggests that mucin glycoprotein (MUC2) production and activity can be reduced as this is produced by the goblet cells which can then potentially compromise intestinal function; that could lead to higher risk of infection caused by increased bacterial adhesion to the epithelium [85,88]. Pearce et al. [16] observed decreased resistance across the intestinal epithelium of pigs under HS, as assessed by reduced TER, which favors increasing intestinal permeability and allows the translocation of lipopolysaccharides (LPS) into the systemic circulation; that leads to endotoxemia and increases inflammation [89,90].
TJ, which is responsible for the intestine’s physical barrier integrity, can be compromised under HS with evidence of its altered expression and localization. Pigs under 24 h of exposure to HS showed increased claudin 3 and occludin in the ileum [16]. Claudin 3, acts as a sealing component of TJ and occludin is essential for TJ integrity [91] Increase in the TJ proteins may indicate improvement of the intestinal barrier during HS as it strives to relieve the stress-induced permeability; this is often associated with heat-induced expression of heat shock proteins (HSPs) [89]. Recovery of the intestine from HS is influenced by HSPs (HSP27, 70 and 90). These molecular chaperones aid in protein folding and cell survival under stress conditions. Expression of these proteins was upregulated in the ileum and colon of pigs within 2 to 4 h of exposure to HS; such upregulation might influence the partial recovery from HS. Liu et al. [92] observed severe damage in the small intestine (epithelium shedding at the tips of the intestinal villi, and shorter villus height and shallower crypt depth for the duodenum and jejunum) of Chinese mini pigs exposed to HS (40 °C) 5 h a day for 3 days. However, they also observed gradual recovery from the pigs exposed to the same condition on the 6th day of exposure until day 10; still, it is incomparable to those pigs in the thermoneutral group. This gradual recovery is possibly influenced by HSPs ability to prevent the activation of conventional protein kinase C (cPKC), resulting in reduced myosin light chain kinase (MLCK) protein phosphorylation of the actin cytoskeleton [80,92]. Noticeably, TJ proteins distribution was altered; as Pearce et al. [16] observed, upregulation of claudin protein was more in the membrane fraction, while occludin was more in the cytosolic fraction. It can be said that the regulation of TJ complexes is disturbed with emphasis on key kinases, particularly in a c-Srcfamily kinases (SFKs) manner [16,93]. Multiple kinases influence on occludin phosphorylation is believed to contribute to the regulation and modification of TJ; involvement of SFKs in the assembly and integrity of TJ are evident by regulating cell proliferation, migration, differentiation and adhesion [94,95]. Disassociation of TJ protein complexes was observed upon activation and upregulation of Casein kinase II-α (CKII-α) in the ileum of pigs under HS which impaired its barrier function [16]. Indeed, as observed in mice, CKII-α plays significant roles: in occludin phosphorylation and as regulator of zonula occluden-1 (ZO-1), claudin-1 and claudin-2 proteins [96]. Moreover, increased expression and activation of MLCK were observed and associated with reduced intestinal integrity [16]. MLCK largely mediates the actin cytoskeleton regulation in the epithelial cells, which affects its role in TJ physiology and intestinal integrity [97]. In a different study, where finishing pigs were subjected to cyclical HS (35 °C for 12 h and 22 °C for 12 h) for 30 days, reduced expression TJ proteins ZO-1 and occludin were observed compromising their epithelial barrier function [98]. Despite the varied intensity of HS, intestinal integrity and function of pigs is compromised as assessed by HS’s effects on the intestinal mucus layer, TJs, enteric immune and antioxidant system which are condensed in Table 2. The condition of the gut under thermoneutral zone (TNZ) and HS is illustrated in Figure 1 along with the role of endogenous antioxidants in suppressing ROS.

Table 2.
Heat stress (HS) effects on the intestinal integrity and function of pigs.
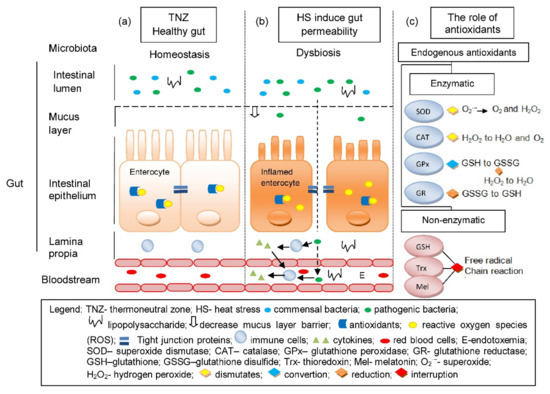
Figure 1.
The condition of the gut under thermoneutral zone (a) is normal and is considered a healthy gut, as there is a homeostasis in microbiota, antioxidant to ROS balance and a fully functional gut epithelium that prevents the entry of bacteria and toxins. (b) HS induces gut permeability, which causes OS due to the imbalance between the endogenous antioxidants and ROS. It also allows translocation of pathogenic bacteria and toxins into the blood circulation via paracellular transport due to the disruptions of TJs and can lead to endotoxemia. (c) The role of endogenous antioxidants in cellular redox balance through elimination of ROS.
5. Mitigation of Heat-Stress-Induced Intestinal Permeability by Antioxidants
Nutrition is one of the promising avenues to aid HS and its induced stressors, particularly to the intestinal integrity and function of pigs [99]. Antioxidants such as vitamins and minerals are a potential nutritional tool to compensate for HS’s harmful effects [100]. These substances can reduce cell damage by neutralizing free radicals [101] during HS-induced oxidation and oxidative stress [7]. Nevertheless, the body is capable of synthesizing antioxidant enzymes, including GPx, SOD and CAT, and non-enzymatic antioxidants (GSH, Trx, melatonin and coenzyme Q); exogenous antioxidants (vitamins A, C, D, E and minerals selenium, iron, copper, zinc and manganese) which are obtained through the diet are still essential as they serve as co-factors of the said antioxidant enzymes for optimum catalytic activity [102]. In vitro studies proved that gastrointestinal cell homeostasis is influenced by vitamins A and D as these substances can modify the expression of TJ molecules and upregulate ZO-1, occludin and other claudins that lead to increased transepithelial resistance (TER) of the intestinal epithelial cells in mice and regulate its barrier function [48,103]. He et al. [104] reported that vitamin A alone can enhance the TER by alleviating LPS-induced intestinal permeability. Vitamin D with known biological functions such as vitamin D receptor (VDR) can maintain the mucosal barrier integrity by reducing MLCK [105]. Vitamin C’s antioxidant function is to protect cellular structures from the harmful effect of free radicals. In contrast, as a lipid-soluble antioxidant, vitamin E can neutralize free radicals induced by HS [106]. Besides its antioxidant function, vitamin C is also responsible for the synthesis of serotonin, which is involved in the proper functioning of the endocrine, nervous, digestive and immune systems [107,108]. Minerals such as Selenium (Se) and Zinc (Zn) have their diverse antioxidant function. Se incorporates into proteins in the form of selenoproteins (antioxidant enzymes) to avert cell damage and OS [109]. Zn delays the oxidative process by inducing metallothionein’s expression (responsible for zinc-related cell homeostasis) and behaves as potent electrophilic scavengers and cytoprotective agents [110]. Furthermore, the antibacterial effects of Zn have beneficial effects on the gut health of pigs [111]. Several research pieces also proved that independent and combined use of these minerals as supplements improved the condition and performance of rabbits, lambs and broilers exposed to high environmental temperature [112,113,114].
With these notable facts about antioxidants, its potential has been explored as a sole supplement and in combination as a means to alleviate HS’s ill effects on the intestinal integrity and function of pigs (Table 3).

Table 3.
Effects of antioxidants on the intestinal integrity and function of pigs under heat stress.
Sanz Fernandez et al. [115] reported that supplementation of 200 mg organic Zn (zinc amino acid complex, ZnAA) could improve the intestinal integrity of pigs under severe HS. Regulated dietary organic zinc (ZnAA 60 mg/kg) with the addition of inorganic Zn (zinc sulfate 60 mg/kg) supplementation improved the intestinal barrier integrity as well as reduced blood endotoxin of pigs under acute heat stress (12 h) by exhibiting higher ileum TER as compared to pigs in the HS condition without ZnAA supplementation. Furthermore, villi height to crypt depth ratio of HS pigs supplemented with ZnAA is comparable to pigs under thermal comfort and with no manifestation of ileal autolysis, unlike the pigs in the HS group without ZnAA supplementation [87]. Liu et al. [116] reported that combinations of Zn oxide and tannins (ZnO + hydrolysable tannins) improved the antioxidant capacity and digestive enzymes of pigs. Organic zinc (Zn glycinate and Zn methionine) also influences the pigs’ antioxidant status and helps maintain a healthy immune system [117,118]. In another study, supplementation of Se and vitamin E at high levels attenuated HS’s impact on the intestinal barrier integrity of pigs, associated with a depletion of OS, as assessed by decrease in antioxidant enzymes [119]. This was in agreement with the previous report of Lv et al. [120], which shows the capability of Se to enhance antioxidant capacity as evidence of increased blood GPx activity and a higher concentration of thyroid hormones that promotes a stable and healthy gastrointestinal ecosystem of pigs under HS.
Recently, a study by Liu et al. [15] revealed that supplementing pigs with dietary selenium-enriched yeast attenuated OS-induced disruption of intestinal mucosa, with elevated mucosal CAT, GPx and total antioxidant capacity (T-AOC) activities and improved intestinal barrier functions with enhanced TJ protein (ZO-1) distribution and abundance as well as enhanced intestinal morphology. Summary of antioxidants’ mitigation on HS’s adverse effects on intestinal integrity and morphology of pigs is shown in Table 4.

Table 4.
Mitigation of heat stress effect on intestinal morphology and TER by antioxidant supplementation.
The supplementation of antioxidants influence on the intestinal integrity and function of pigs under HS positively impacts their performance. The capability of antioxidants to protect the intestinal barrier integrity and increase the levels of antioxidant enzymes can lead to better gut health, intestinal function, alleviation of endotoxemia and OS. Such abatement on the intestinal integrity components signifies better nutrient digestion and absorption which can be summed up as a better production performance of pigs under HS, as supported by the pigs’ manifestation of better growth, intestinal barrier function, enhanced immune function and improved antioxidant system [115,118,119].
6. Conclusions
HS induces intestinal OS in pigs, compromising intestinal homeostasis and jeopardizing the intestinal epithelium. Based on the information from various researches, as HS progresses, duodenal, jejunal and ileal villus height, crypt depth and villus height to crypt depth ratio are reduced by about 11%, 3% and 9%, respectively. This indicates that HS causes intestinal damage mainly in the duodenum and ileum. HS also threatens the intestine’s protective function against harmful substances as it reduces the expression of TJ proteins and the intestine’s TER. This leads to the disruption of the TJ barrier function, allowing translocation of endotoxins into the systemic circulation and leading to endotoxemia. Dietary antioxidants (zinc, vitamin E and selenium) can combat HS-induced OS by neutralizing free radicals and serving as co-factors for antioxidant enzymes. In conclusion, the antioxidant supplementation can partially mitigate the effect of heat stress on the villus height and TER by about 70–85%. Interestingly, the average mitigation of crypt depth shows negative result, but the individual results are variable. We have to point out that only a few studies applied the necessary treatments to allow the calculation of % mitigation, which indicates the necessity of further research. We also have to admit that the dietary interventions in the reviewed manuscripts mainly lasted only for the length of the study. Therefore, it would be worth studying whether the full intestinal recovery can be achieved after HS with longer specific supplementation. Due to the complex nature of the problem, combined approaches may have higher mitigation capacity.
Author Contributions
Conceptualization, C.S.; research, A.D.S.V.O. and C.S.; writing—original draft preparation, A.D.S.V.O.; writing—review and editing, A.D.S.V.O. and C.S.; visualization, A.D.S.V.O. and C.S.; supervision, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
A.D.S.V.O. received funding from the Tempus Public Foundation, Stipendium Hungaricum Scholarship Programme.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The publication is supported by the EFOP-3.6.3-VEKOP-16-2017-00008 project. The project is co-financed by the European Social Fund. The authors are grateful to Malam Abulbashar Mujitabas’ help in proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baumgard, L.H.; Rhoads, R. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Renaudeau, D.; Gourdine, J.L.; St-Pierre, N.R. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 2011, 89, 2220–2230. [Google Scholar] [CrossRef]
- Huo, C.; Xiao, C.; She, R.; Liu, T.; Tian, J.; Dong, H.; Tian, H.; Hu, Y. Chronic heat stress negatively affects the immune functions of both spleens and intestinal mucosal system in pigs through the inhibition of apoptosis. Microb. Pathog. 2019, 136, 103672. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Reynolds, C.; Hollinger, K.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, 288–296. [Google Scholar] [CrossRef]
- Cui, Y.; Gu, X. Proteomic changes of the porcine small intestine in response to chronic heat stress. J. Mol. Endocrinol. 2015, 55, 277–293. [Google Scholar] [CrossRef]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, E.-H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.K.; Pearce, S.C. The impact of heat stress on intestinal function and productivity in grow-finish pigs. Anim. Prod. Sci. 2015, 55, 1403–1410. [Google Scholar] [CrossRef]
- Lambert, G.P.; Gisolfi, C.V.; Berg, D.J.; Moseley, P.L.; Oberley, L.W.; Kregel, K.C. Selected Contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. 2002, 92, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Collin, A.; Lebreton, Y.; Fillaut, M.; Vincent, A.; Thomas, F.; Herpin, P. Effects of exposure to high temperature and feeding level on regional blood flow and oxidative capacity of tissues in piglets. Exp. Physiol. 2001, 86, 83–91. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Ma, Q.; Henderson, J.W.; Siddique, A.; Archer, S.Y.; Hodin, R.A. Enterocyte Response to Ischemia Is Dependent on Differentiation State. J. Gastrointest. Surg. 2002, 6, 403–409. [Google Scholar] [CrossRef]
- Yan, Y.-E.; Zhao, Y.-Q.; Wang, H.; Fan, M. Pathophysiological factors underlying heatstroke. Med. Hypotheses 2006, 67, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, C.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Selenium-Enriched Yeast Alleviates Oxidative Stress-Induced Intestinal Mucosa Disruption in Weaned Pigs. Oxidative Med. Cell. Longev. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal Barrier Integrity and Favors Intestinal Glucose Transport in Growing Pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef]
- Cottrell, J.J.; Liu, F.; Hung, A.T.; Digiacomo, K.; Chauhan, S.S.; Leury, B.J.; Furness, J.B.; Celi, P.; Dunshea, F.R. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1391–1402. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Umar, S. Intestinal Stem Cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef]
- Stromberg, P.E.; Coopersmith, C.M. Epithelium, Proliferation of. In Encyclopedia of Gastroenterology; Johnson, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 725–730. [Google Scholar] [CrossRef]
- Oswald, I.P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Veter. Res. 2006, 37, 359–368. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Williams, J.M.; Duckworth, C.A.; Burkitt, M.D.; Watson, A.J.M.; Campbell, B.J.; Pritchard, D.M. Epithelial Cell Shedding and Barrier Function: A Matter of Life and Death at the Small Intestinal Villus Tip. Veter. Pathol. 2014, 52, 445–455. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stadnyk, A.W. Intestinal Epithelial Cells as a Source of Inflammatory Cytokines and Chemokines. Can. J. Gastroenterol. 2002, 16, 241–246. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Colgan, S.P. Cytokine responses and epithelial function in the intestinal mucosa. Cell. Mol. Life Sci. 2016, 73, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, R.A.; Douglas-Escobar, M.; Li, N.; Neu, J. Intestinal Barrier Function: Implications for the Neonate and Beyond. In Gastroenterology and Nutrition: Neonatology Questions and Controversies; Polin, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 93–110. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Aijaz, S.; Tsapara, A.; Balda, M.S. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 2005, 17, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Verdile, N.; Mirmahmoudi, R.; Brevini, T.; Gandolfi, F. Evolution of pig intestinal stem cells from birth to weaning. Animals 2019, 13, 2830–2839. [Google Scholar] [CrossRef]
- Blander, J.M. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016, 283, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Myer, M.S. The presence of Paneth cells confirmed in the pig. Onderstepoort J. Veter. Res. 1982, 49, 131–132. [Google Scholar]
- Gonzalez, L.M.; Williamson, I.; Piedrahita, J.A.; Blikslager, A.T.; Magness, S.T. Cell Lineage Identification and Stem Cell Culture in a Porcine Model for the Study of Intestinal Epithelial Regeneration. PLoS ONE 2013, 8, e66465. [Google Scholar] [CrossRef] [PubMed]
- Van der Hee, B.; Loonen, L.; Taverne, N.; Taverne-Thiele, J.; Smidt, H.; Wells, J. Optimized procedures for generating an enhanced, near physiological 2D culture system from porcine intestinal organoids. Stem Cell Res. 2018, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.W.; Hale, B.J.; Gabler, N.K.; Rhoads, R.P.; Keating, A.F.; Baumgard, L.H. Physiological consequences of heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1381–1390. [Google Scholar] [CrossRef]
- Xin, H.; Harmon, J. Heat Stress Indices for Livestock. 1998. Available online: https://www.ipic.iastate.edu/info/HeatStressIndicesLivestock.pdf (accessed on 3 April 2021).
- Eastwood, L. Avoiding Production Losses in Swine Due to Heat Stress. 2020. Available online: https://onswine.wordpress.com/2020/07/09/avoiding-production-losses-in-swine-due-to-heat-stress/. (accessed on 3 April 2021).
- Myer, R.; Bucklin, R. Influence of Hot-Humid Environment on Growth Performance and Reproduction of Swine 1. 2001. Available online: https://edis.ifas.ufl.edu/pdffiles/AN/AN10700.pdf (accessed on 3 April 2021).
- Akbarian, A.; Michiels, J.; DeGroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hao, Y.; Li, J.; Bao, W.; Li, G.; Gao, Y.; Gu, X. Chronic Heat Stress Induces Immune Response, Oxidative Stress Response, and Apoptosis of Finishing Pig Liver: A Proteomic Approach. Int. J. Mol. Sci. 2016, 17, 393. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Braber, S.; Garssen, J.; Wichers, H.J.; Folkerts, G.; Fink-Gremmels, J.; Varasteh, S. Beyond Heat Stress: Intestinal Integrity Disruption and Mechanism-Based Intervention Strategies. Nutrients 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Rahiman, S.A.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Van Kaer, L.; Olivares-Villagómez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef]
- Tang, L.-P.; Li, W.-H.; Liu, Y.-L.; Lun, J.-C.; He, Y.-M. Heat stress aggravates intestinal inflammation through TLR4-NF-κB signaling pathway in Ma chickens infected with Escherichia coli O157:H7. Poult. Sci. 2021, 100, 101030. [Google Scholar] [CrossRef] [PubMed]
- Zeitouni, N.E.; Chotikatum, S.; Von Köckritz-Blickwede, M.; Naim, H.Y. The impact of hypoxia on intestinal epithelial cell functions: Consequences for invasion by bacterial pathogens. Mol. Cell. Pediatr. 2016, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The Anti-inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef]
- Moine, L.; Rivoira, M.; De Barboza, G.D.; Pérez, A.; De Talamoni, N.T. Glutathione depleting drugs, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2018, 24, 4979–4988. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Q.; Chen, S.-P.; Sun, J.; Wang, X.-M.; Chen, N.; Zhou, Y.-Q.; Tian, Y.-K.; Ye, D.-W. Berberine protects against ischemia-reperfusion injury: A review of evidence from animal models and clinical studies. Pharmacol. Res. 2019, 148, 104385. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Surai, K.P.; Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Antioxidant-Prooxidant balance in the intestine: Food for thought 2. Antioxidants. Curr. Top. Nutraceutical Res. 2004, 2, 27–46. Available online: https://feedfood.co.uk/download/2_Nutraceuticals_my_main.pdf (accessed on 10 October 2020).
- Liu, P.; Kerr, B.J.; Weber, T.E.; Chen, C.; Johnston, L.J.; Shurson, G.C. Influence of thermally oxidized vegetable oils and animal fats on intestinal barrier function and immune variables in young pigs. J. Anim. Sci. 2014, 92, 2971–2979. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, C.; Hao, Y.; Gu, X.; Wang, H. Chronic Heat Stress Induces Acute Phase Responses and Serum Metabolome Changes in Finishing Pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Cline, S.D. Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim. Biophys. Acta Bioenerg. 2012, 1819, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Koppal, T.; Howard, B.; Subramaniam, R.; Hall, N.; Hensley, K.; Yatin, S.; Allen, K.; Aksenov, M.; Aksenova, M.; et al. Structural and Functional Changes in Proteins Induced by Free Radical-mediated Oxidative Stress and Protective Action of the Antioxidants N-tert-Butyl-alpha-phenylnitrone and Vitamin Ea. Ann. N. Y. Acad. Sci. 1998, 854, 448–462. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3614697/ (accessed on 1 April 2021). [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, K.A.; Bonda, T.A.; Korecki, J.; Musial, W.J. Oxidative stress and neutrophil activation—The two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002, 86, 41–59. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Nyström, E.E.L.; Johansson, M.E.V.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Andrianifahanana, M.; Moniaux, N.; Batra, S.K. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta Bioenerg. 2006, 1765, 189–222. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Mackie, A.R.; Krupa, L. Permeability of the small intestinal mucus for physiologically relevant studies: Impact of mucus location and ex vivo treatment. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009, 87, E101–E108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chauhan, N.R.; Chowdhury, D.; Singh, A.; Meena, R.C.; Chakrabarti, A.; Singh, S.B. Heat stress modulated gastrointestinal barrier dysfunction: Role of tight junctions and heat shock proteins. Scand. J. Gastroenterol. 2017, 52, 1315–1319. [Google Scholar] [CrossRef]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef]
- Abuajamieh, M.; Kvidera, S.K.; Mayorga, E.J.; Kaiser, A.; Lei, S.M.; Seibert, J.T.; Horst, E.A.; Fernandez, M.V.S.; Ross, J.W.; Selsby, J.T.; et al. The effect of recovery from heat stress on circulating bioenergetics and inflammatory biomarkers. J. Anim. Sci. 2018, 96, 4599–4610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bass, B.E.; Bandrick, M.; Loving, C.L.; Brockmeier, S.L.; Looft, T.; Trachsel, J.; Madson, D.M.; Thomas, M.; Casey, T.A.; et al. Fermentation products as feed additives mitigate some ill-effects of heat stress in pigs. J. Anim. Sci. 2017, 95, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited Review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kpodo, K.R.; Duttlinger, A.W.; Maskal, J.M.; Johnson, J.S. Effects of feed removal on thermoregulation and intestinal morphology in pigs recovering from acute hyperthermia. J. Anim. Sci. 2020, 98, 041. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 2012, 90, 257–259. [Google Scholar] [CrossRef]
- Pearce, S.C.; Fernandez, M.-V.S.; Torrison, J.L.; Wilson, M.E.; Baumgard, L.H.; Gabler, N.K. Dietary organic zinc attenuates heat stress–induced changes in pig intestinal integrity and metabolism. J. Anim. Sci. 2015, 93, 4702–4713. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J. Gut barrier function: Effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018, 97, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.K.; Koltes, D.; Schaumberger, S.; Murugesan, G.R.; Reisinger, N. Diurnal heat stress reduces pig intestinal integrity and increases endotoxin translocation. Transl. Anim. Sci. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Günzel, R.; Fromm, M. Claudins and Other Tight Junction Proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar] [CrossRef]
- Liu, F.; Yin, J.; Du, M.; Yan, P.; Xu, J.; Zhu, X.; Yu, J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling1. J. Anim. Sci. 2009, 87, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, S.; Sheth, P.; Kuppuswamy, D.; Balasubramanian, S.; Ray, R.M.; Rao, R.K. Expression of Kinase-inactive c-Src Delays Oxidative Stress-induced Disassembly and Accelerates Calcium-mediated Reassembly of Tight Junctions in the Caco-2 Cell Monolayer. J. Biol. Chem. 2003, 278, 11916–11924. [Google Scholar] [CrossRef] [PubMed]
- Dörfel, M.J.; Huber, O. Modulation of Tight Junction Structure and Function by Kinases and Phosphatases Targeting Occludin. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Okada, M. Regulation of the Src Family Kinases by Csk. Int. J. Biol. Sci. 2012, 8, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, D.R.; Boe, D.M.; Yu, D.; Weber, C.R.; Marchiando, A.M.; Bradford, E.M.; Wang, Y.; Wu, L.; Schneeberger, E.E.; Shen, L.; et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 2011, 193, 565–582. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular Basis of Epithelial Barrier Regulation: From Basic Mechanisms to Clinical Application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Xiong, Y.; Wu, Q.; Wang, M.; Liu, S.; Jiang, Z.; Wang, L. Effects of dietary supplementation with l-arginine on the intestinal barrier function in finishing pigs with heat stress. J. Anim. Physiol. Anim. Nutr. 2019, 104, 1134–1143. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Baumgard, L.H.; Suagee, J.K.; Sanders, S.R. Nutritional Interventions to Alleviate the Negative Consequences of Heat Stress. Adv. Nutr. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Babinszky, L.; Horváth, M.; Remenyik, J.; Verstegen, M. 8: The Adverse Effects of Heat Stress on the Antioxidant Status and Performance of Pigs and Poultry and Reducing These Effects with Nutritional Tools. In Poultry and Pig Nutrition; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 187–208. [Google Scholar] [CrossRef]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I. Antioxidant-Prooxidant Balance in the Intestine: Applications in Chick Placement and Pig Weaning. J. Veter. Sci. Med. 2015, 2, 66. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef]
- Du, J.; Chen, Y.; Shi, Y.; Liu, T.; Cao, Y.; Tang, Y.; Ge, X.; Nie, H.; Zheng, C.; Li, Y.C. 1,25-Dihydroxyvitamin D Protects Intestinal Epithelial Barrier by Regulating the Myosin Light Chain Kinase Signaling Pathway. Inflamm. Bowel Dis. 2015, 21, 2495–2506. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Sinbad, O.O.; Folorunsho, A.A.; Olabisi, O.L.; Ayoola, O.A.; Temitope, E.J. Vitamins as Antioxidants. J. Food Sci. Nutr. Res. 2019, 2, 214–235. Available online: https://www.researchgate.net/publication/335857838_Vitamins_as_Antioxidants (accessed on 5 March 2021).
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Hassan, F.; Mobarez, S.; Mohamed, M.; Attia, Y.; Mekawy, A.; Mahrose, K. Zinc and/or Selenium Enriched Spirulina as Antioxidants in Growing Rabbit Diets to Alleviate the Deleterious Impacts of Heat Stress during Summer Season. Animals 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Dunshea, F.R.; Plozza, T.E.; Hopkins, D.L.; Ponnampalam, E.N. The Impact of Antioxidant Supplementation and Heat Stress on Carcass Characteristics, Muscle Nutritional Profile and Functionality of Lamb Meat. Animals 2020, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Kishawy, A.T.; Khater, S.I.; Arisha, A.H.; Mohammed, H.A.; Abdelaziz, A.S.; El-Rahman, G.I.A.; Elabbasy, M.T. Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross Broiler Chickens. Animals 2019, 9, 342. [Google Scholar] [CrossRef]
- Fernandez, M.V.S.; Pearce, S.C.; Gabler, N.K.; Patience, J.F.; Wilson, M.E.; Socha, M.T.; Torrison, J.L.; Rhoads, R.; Baumgard, L.H. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animals 2014, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, J.; Mahfuz, S.; Piao, X. Effects of Hydrolysable Tannins as Zinc Oxide Substitutes on Antioxidant Status, Immune Function, Intestinal Morphology, and Digestive Enzyme Activities in Weaned Piglets. Animals 2020, 10, 757. [Google Scholar] [CrossRef]
- Holodova, M.; Cobanova, K.; Sefcikova, Z.; Barszcz, M.; Tuśnio, A.; Taciak, M.; Gresakova, L. Dietary Zinc and Fibre Source can Influence the Mineral and Antioxidant Status of Piglets. Animals 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, Q.; Wang, L.; Wang, Y.; Cheng, Z.; Yang, Z.; Yang, W. The Effects of Partially or Completely Substituted Dietary Zinc Sulfate by Lower Levels of Zinc Methionine on Growth Performance, Apparent Total Tract Digestibility, Immune Function, and Visceral Indices in Weaned Piglets. Animals 2019, 9, 236. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.H.; Wang, T.; Regmi, N.; Chen, X.; Huang, K.; Liao, S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1161–1171. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Sa, S.J.; Cho, E.S.; Ko, H.S.; Choi, J.W.; Kim, J.S. Effects of Zinc Oxide and Arginine on the Intestinal Microbiota and Immune Status of Weaned Pigs Subjected to High Ambient Temperature. Animals 2020, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Silva Guillen, Y.V. Antioxidant Supplementation to Alleviate the Negative Effects of Heat and Oxidative Stress on Performance and Health of Nursery and Growing Pigs. Ph.D. Thesis, Graduate Faculty of North Carolina State University, Raleigh, NC, USA, 2019. Available online: https://repository.lib.ncsu.edu/bitstream/handle/1840.20/36925/etd.pdf?sequence=1 (accessed on 15 October 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).