Abstract
Marine bacterial species contribute to a significant part of the oceanic population, which substantially produces biologically effectual moieties having various medical and industrial applications. The use of marine-derived bacterial pigments displays a snowballing effect in recent times, being natural, environmentally safe, and health beneficial compounds. Although isolating marine bacteria is a strenuous task, these are still a compelling subject for researchers, due to their promising avenues for numerous applications. Marine-derived bacterial pigments serve as valuable products in the food, pharmaceutical, textile, and cosmetic industries due to their beneficial attributes, including anticancer, antimicrobial, antioxidant, and cytotoxic activities. Biodegradability and higher environmental compatibility further strengthen the use of marine bio-pigments over artificially acquired colored molecules. Besides that, hazardous effects associated with the consumption of synthetic colors further substantiated the use of marine dyes as color additives in industries as well. This review sheds light on marine bacterial sources of pigmented compounds along with their industrial applicability and therapeutic insights based on the data available in the literature. It also encompasses the need for introducing bacterial bio-pigments in global pigment industry, highlighting their future potential, aiming to contribute to the worldwide economy.
1. Introduction
1.1. Microbial Pigments
The production of bio-pigments from bacterial species is being conducted globally with soaring interest under the research of microbial autecology. A massive array of these compounds, also referred to as “bioactive pigmented molecules”, can be derived from both Gram-positive and Gram-negative bacterial species. Production of these pigments in the marine environment is mediated through the complex mechanism of “quorum sensing” [1] or also can be induced through exposure to different stress conditions in external environments. Quorum sensing is the mechanism whereby individual bacterial cells can coordinate with others in their colony to carry out constitutive functions especially involving the secretion of numerous specific chemical compounds. These compounds can help them with survival, competence, bioluminescence, biofilm formation, and even sporulation, etc. Bio-pigments can be produced by triggering regulatory quorum sensing mechanisms of these species and can be extensively used in various bio-medical and bio-industrial sectors, including textiles, food, pharmaceutical, and cosmetic industries, owing to their beneficial attributes and biological activities [2,3]. These are moreover convenient to harvest in large volumes through utilizing simple gene manipulating strategies. The rising consumer concerns regarding safety and quality of industrial products holds a significant ground as to why scientists are shifting their focus towards naturally derived, non-toxic, and eco-friendly pigment alternatives [4].
1.2. Bacterial Pigments as Natural Colorants
The use of synthetic pigments goes back to the 1850s when these were put in trend for the first time due to their supercilious coloring properties, lower prices, and easy production strategies [5], the significance of which remains empirically the same to this day. The importance of artificial/synthetic coloring agents is still based on the fact that the appearance of food items influences consumer’s emotions, attitudes, and preferences. Let us say, if a carrot is not red, the consumer is most probably expected to reject it. The same can be applied in regards with the cosmetic industry, where the product apparel decides its fate. Thus, need for “synthetic pigments” cannot be overseen if client orientation is to be fulfilled [6]. The only progress made today is the shift towards naturally derived pigments rather than continuing the use of artificially synthesized ones, which have been denounced for their serious threat to consumer’s well-being [7]. Cancers of skin, liver, and bladder have been found positively related to the use of artificial pigments because of their high azo-dye/heavy metal compositions. Furthermore, the precursors involved and the waste generated through their production process is environmentally hazardous as well [8,9]. The outcry against the use of synthetic colorants in many health-conscious countries has already caused the ban of several artificial colorants, including Blue NO 1, Blue NO 2, Blue FCF, and Yellow NO 6 [10].
Bio-pigments, however, are eco-friendly and proved additionally propitious as antitoxic, antitumor, antioxidant, anticancer, and antimicrobial agents [2]. Other advantages include fast and economic extraction techniques, higher yield, and time- and cost-efficient production. Moreover, the production of microbial pigments can also be made more convenient by the optimization capacity of their growth parameters [11]. Keeping the capacity of bio-pigments into consideration, many biotech industries are now developing protocols for efficient extraction of natural pigments as a replacement to synthetic counterparts. For instance, natural pigments such as zeaxanthin, saproxanthin, myxol and many others which illicit antioxidant activities are being instigated against artificial antioxidants such as butylated hydroxyl toluene and butylated hydroxyl acids [12,13].
1.3. Marine Ecosystem as a Source of Pigment Producing Bacterial Species
The study of a likely natural ecosystem serves as the initial-most important research step needed to find an environment that can entertain the diversity of bio-pigment sources. The marine environment is a habitat for almost 80% of all life forms [14]. It serves as a rich source of aquatic microbial species that exhibit comparatively more augmented diversity than their telluric counterparts [15]. The marine environment is presently being considered as an attractive fount for bio-pigment sources [16]. Numerous bacterial isolates from such biotopes have already been tested for pigment production. At the same time, many of them are also being utilized for various industrial purposes as well [15]. The preference of pigments produced by marine microorganisms is based on their ability to persist in extremities such as highly acidic/alkaline environments (pH < 4 and >9), extreme temperatures (−2–15 °C and 60–110 °C), and under limited substrate availability [17,18]. Apart from bacterial isolates, halophilic archaea are extensively disseminated in the marine ecosystem. Pigmented compounds from marine archaea are also prioritized owing to their ability to tolerate hyper saline and basic pH environments, besides their potential to withstand osmolytes (such as 2-sulfotrehalose) or high ionic strength [19,20].
Concerns regarding environmental conservation and consumers’ preferences have stimulated the interests of researchers and stake holders in exploring nontoxic, eco-friendly, and biodegradable commodities. Bacterially produced bio-pigments (bpBPs) have growing importance not only on account of their dyeing potential, but also due to their medicinal properties. Likewise, awareness regarding the carcinogenic and other pernicious effects of synthetic colorants has kindled a fresh enthusiasm towards the utilization of bacterial pigments in the food industry as safer alternatives to use as antioxidants, color intensifiers, flavor enhancers, and food additives.
Extraction of natural pigments from microorganisms populating environments exclusive of soil is a topic of current interest. Marine environment has become a captivating subject matter for microbiologists, pharmacologists, and biochemists in order to extract water based bacterial pigments. With the recent increase in awareness towards the benefits of natural over synthetic products, the bio-pigment industry is likely to increase its global market. The review aims at discussing the therapeutic and industrial significance of marine derived bacterial pigments helping to delineate the consequence of furthering the scope of these studies. It provides a comprehensive overview of potentiality and competence of marine bacteria as a source of bio-pigments by critically summarizing the scientific researches and accumulated data in the literature and the prominence of these bio-pigments in strengthening the overall pigment market by reviewing latest industry market research, reports, and statistics.
2. Marine Bacterial Species as Sources of Bio-Pigments
The marine environment has been investigated for almost 300,000 known species, which constitutes only a small fraction of the total number of explorable pigment producing bacterial species. Bacterial species isolated from marine sediments or seawater such as Streptomyces sp., Pontibacter korlensis sp., Pseudomonas sp., Bacillus sp., and Vibrio sp. produce an array of pigmented compounds including prodigiosin, astaxanthin, pyocyanin, melanin, and beta carotene, respectively (Table 1). These pigments belong to a range of compound classes, for instance, carotenes are a subclass of carotenoids that have unsaturated polyhydrocarbon structures, prodiginines have a pyrrolyldipyrromethene core structure, tambjamines are alkaloid molecules, while violacein compounds are indole derivatives derived from tryptophan metabolism (Figure 1) [1,2,21]. These and other such pigments, despite their class diversity, share a functional likeness due to the presence of aromatic rings in their structures.

Table 1.
Marine bacterial sources of colored pigmented compounds.
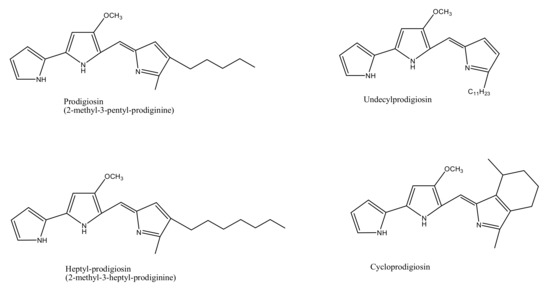
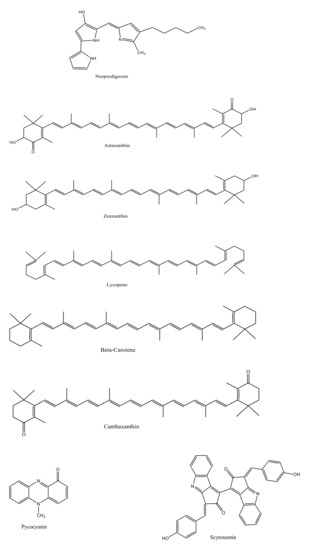
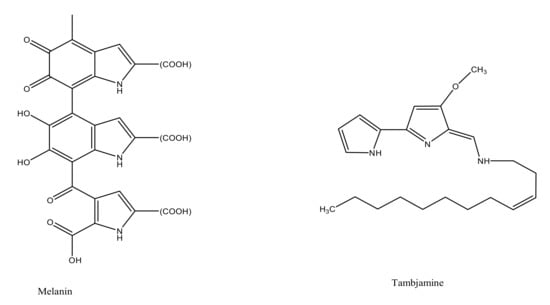
Figure 1.
Chemical structures of various bacterial pigments.
3. Biosynthesis of Bacterial Pigments
The potential of marine bacterial isolates as a leading source of bio-pigments demands an extensive understanding of bio-mechanisms responsible for yielding pigmented molecules. Different studies have reported the proposed biosynthetic pathways of pigment production by marine bacterial isolates along with biochemically characterized enzymatic transformations (Figure 2). However, it is still unclear if the proposed pathways are distinct for marine or terrestrial bacterial species, or may be the same in both cases.
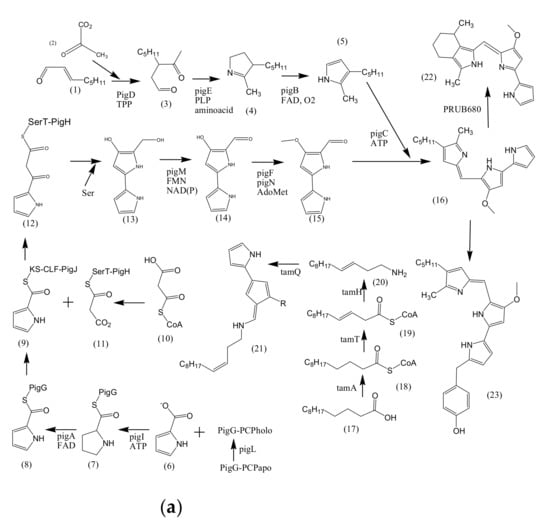
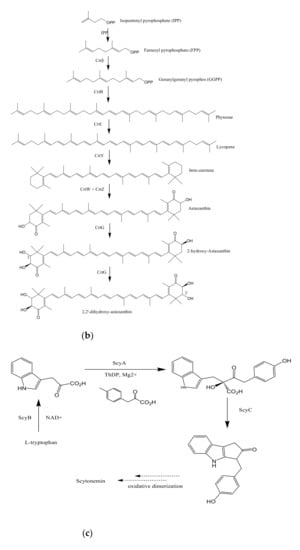
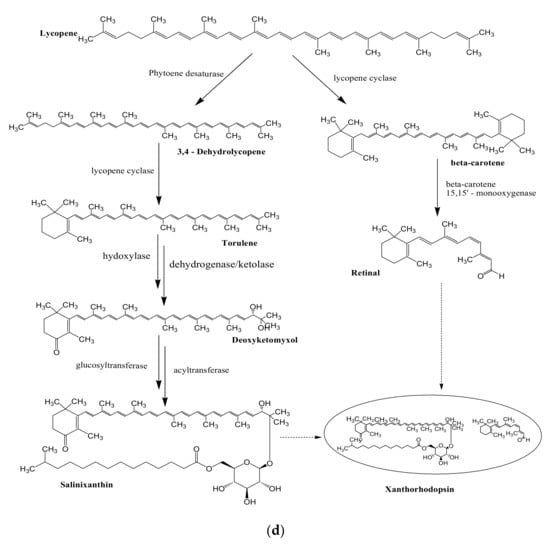
Figure 2.
Proposed biosynthetic pathways of few bacterially produced bio-pigments. (a) Biosynthesis of Prodiginine analogs; MAP biosynthesis; MBC biosynthesis; Tambjamine biosynthesis; Cyloprodigiosin biosynthesis; 2-(p-hydroxybenzyl)prodigiosin (HBPG) biosynthesis. (b) Biosynthesis of carotenoids. (c) Biosynthesis of scytonemin. (d) Biosynthesis of salinixanthin and retinal pigments. (a) Biosynthesis of prodigioinine analogs. MAP Biosythesis (Green): (1) 2octenal, (2) Pyruvate, (3) 3-acetyloctanal, (4) H2MAP, (5) MAP. MBC Biosynthesis (Blue), (6) L-proline, (7) L-prolyl-S-PCP intermediate, (8) Pyrrolyl2-carboxyl-S-PCP, (9) Pyrrole-2-carboxyl thioester, (10) Malonyl-CoA, (11) Bound malonyl, (12) pyrrolyl-β-ketothioester on PigH, (13) 4-hydroxy-2,20-bipyrrole-5methanol (HBM), (14) 4-hydroxy-2,20-bipyrrole-5-carbaldehyde (HBC), (15) MBC, (16) Prodigiosin. Tambjamine Biosynthesis, (17) Dodecenoic acid, (18) Activated fatty acid, (19) CoA-ester, (20) Enamine, (21) Tambjamine, (22) Cycloprodigiosin (cPrG) &, (23) 2-(p-hydroxybenzyl)prodigiosin(HBPG) Biosynthesis. (b). Biosynthesis of carotenoids: CrtE: GGPP synthase, IPP: Isopentenyl pyrophosphate, GGPP: Geranylgeranyl pyrophos, CrtB: Phytoene synthase, CrtI: Phytoene desaturase, CrtY: lycopene β-cyclase, CrtW: β-carotene ketolase, CrtZ: β-carotene hydroxylase, CrtG: Astaxanthin 2,2′-β-ionone ring hydroxylase gene. (c). Biosynthesis of scytonemin: Scytonemin biosynthetic enzymes: ScyA, ScyB, ScyC (ScyA: a thiamin-dependent enzyme, ScyC: enzyme annotated as a hypothetical protein), ThDP: Thiamine diphosphate, NAD: Nicotinamide adenine dinucleotide, Mg2+: Magnesium ion.
3.1. Biosynthesis of Prodiginine Analogs
2-methyl-3-n-amyl-pyrrole (MAP) biosynthesis: This pathway involves three genes; pigB, pigD, and pigE. At first, PigD carries out the addition of pyruvate to 2-octenal in the presence of coenzyme thiamine pyrophosphate (TPP). As a result, 3-acetyloctanal formation occurs along with the release of CO2 molecule. PigE catalyzes the transfer of an amino group to the aldehyde, followed by cyclization, resulting in the formation of H2MAP. PigB carries out further oxidation to form MAP (Figure 2a) [80,81].
4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC) biosynthesis: This pathway involves seven genes: pigA, pigF-J, pigL, and pigM. 4′-phosphopantetheinyl transferase (PigL) carries out the activation of peptidyl carrier protein (PCP) domain of PigG by introducing 4′-phosphopantetheinyl group. Formation of L-prolyl-S-PCP intermediate occurs by the transfer of L-prolyl group of L-proline to the thiol group of phosphopantetheine, carried out by PigI and ATP. PigA further catalyzes the oxidation of the intermediate to pyrrolyl-2-carboxyl-S-PCP. Pyrrole-2-carboxyl thioester is generated by the transfer of pyrrole-2-carboxyl group of PigG to the cysteine active site at PigJ. Phosphopantetheinylated ACP domains of PigH provide binding sites for malonyl group of malonyl-CoA. Decarboxylation of bound malonyl results in condensation with pyrrole-2-carboxyl thioester and leads to the formation of pyrrolyl-β-ketothioester on PigH. Generation of 4-hydroxy-2,2′-bipyrrole-5-methanol (HBM) occurs by decarboxylation between serine and pyrrolyl-β-ketothioester, catalyzed by PigH [80,82]. 4-hydroxy-2,2′-bipyrrole-5-carbaldehyde (HBC) is formed when PigM oxidizes the alcohol group of HBM. Methyltransferase (PigF) and oxidoreductase (PigN) further carries out the methylation of HBC hydroxyl group to form MBC [81]. After the formation of MAP and MBC, PigC utilizes ATP to perform terminal condensation of these pyrroles, synthesizing prodigiosin.
Cycloprodigiosin (cPrG) biosynthesis: The cyclization of undecylprodiginine in order to form metacycloprodigiosin and butyl-meta-cycloheptylprodiginine is carried out by mcpG and redG, respectively [83]. Studies also revealed that a homologus gene (PRUB680) encodes an alkylglycerol monooxygenase-like protein away from pig biosynthetic gene cluster [84]. The respective enzyme demonstrates regiospecificity through C-H activation, resulting in cyclization of prodigiosin to form cPrG [78].
Tambjamine (tam) biosynthesis: Tambjamines have MBC moiety but lack MAP moiety, rather have an enamine group. Enamine biosynthetic pathway involves three genes; tamT, tamH, and afaA [85]. Acyl CoA synthetase (TamA) activates dodecenoic acid [86]. Dehydrogenase (TamT) carries out the oxidation of activated fatty acid, incorporating a π-bond to the fatty acyl side chain at its C-3 carbon. Further, the reduction of CoA-ester, followed by transamination to dodec-3-en-1-amine is facilitated by reductase/aminotransferase (TamH). MBC and enamine then undergoes condensation in order to form tambjamine, catalyzed by TamQ [85].
3.2. Biosynthesis of Carotenoids
Carotenoids are yellow, orange, and red colored pigmented compounds that are further subdivided into carotenes and xanthophylls. So far, 700 carotenoids have been reported, and among them beta-carotene, lutein, canthaxanthin, astaxanthin, lycopene, and zeaxanthin are the highly valued carotenoids [87]. Universal precursors for C40 and C50 carotenoid biosynthesis are two 5 C subunits: isopentenyl diphosphate (IPP) plus its isomeric form dimethylallyl diphosphate (DMAPP). IPP/DMAPP isomerase (IDI) carries out the isomerization of IPP into DMAPP. Geranylgeranyl diphosphate (GGPP) synthase further catalyzes the addition of one DMAPP molecule with three IPP molecules to generate an immediate precursor, i.e., C20 geranylgeranyl diphosphate (GGPP) [88]. Phytoene synthase carries out the first committed step of carotenoid biosynthesis i.e., condensing two GGPP molecules to form phytoene (C40), which is further desaturated by phytoene desaturase by the incorporation of four double bonds in its structure. This desaturated structure is a red-colored compound unlike its colorless parent molecule, and is called lycopene. Lycopene further undergoes several modifications to produce different carotenoids. Beta-carotene is generated by the cyclization of lycopene, carried out by lycopene beta-cyclase. It is then converted into canthaxanthin and zeaxanthin by catalytic activity of two protein classes: beta-carotene ketolase and beta-carotene hydroxylase, respectively, next to the formation of astaxanthin [30]. Beta-carotene ketolase represented by CrtW and CrtO types adds the ketone group to carbon 4/40 of the b-ionone ring. However, beta-carotene hydroxylase, encompassed by CrtR, CrtZ, and P450 types carries out the hydroxylation of carbon 3/30 of the b-ionone ring [89]. 2,2′-β-ionone ring hydroxylase introduces hydroxyl group to the β-ionone ring of astaxanthin and results in the formation of 2,2′-dihydroxy-astaxanthin (Figure 2b) [33].
3.3. Biosynthesis of Scytonemin
Biosynthesis of scytonemin involves three scytonemin biosynthetic enzymes; ScyA, ScyB, and ScyC. ScyB carries out the conversion of L-tryptophan to 3-indole pyruvic acid. ScyA (thiamin-dependent enzyme) performs the coupling of 3-indole pyruvic acid with p-hydroxyphenylpyruvic acid and results in the formation of b-ketoacid, whose cyclization is further carried out by ScyC (enzyme annotated as hypothetical protein) [90]. The resulting tricyclic ketone resembles half of the skeleton of scytonemin (Figure 2c) [91].
3.4. Biosynthesis of Salinixanthin and Retinal
Retinal: Lycopene cyclase converts lycopene into β-carotene. Breakdown of β-carotene into two retinal molecules is further catalyzed by a gene annotated as β-carotene 15,15′-monooxygenase (orf4) (Figure 2d) [92,93].
Salinixanthin: Xanthorhodopsin (orf2) (a light-driven proton pump) has two chromophores; retinal and salinixanthin [94,95]. Phytoene desaturase (CrtI) converts lycopene to 3,4-dehydrolycopene, which is further converted to torulene by lycopene cyclase [92]. Subsequently, conversion of torulene to salinixanthin is catalyzed by hydroxylase, ketolase or dehydrogenase, glucosyltransferase, and acyltransferase, having reactions involved similar to that of biosynthetic reactions of myxol and canthaxanthin [96,97].
4. Industrial and Therapeutic Applications
4.1. Therapeutic Applications
4.1.1. Antibacterial Activity
Antibacterial properties of various bacterially produced bio-pigments of marine origin have been reported against an array of bacterial species, e.g., prodigiosin, cycloprodiogisin (from Z. rubidus sp. S1-1), and the yellow pigment (extracted from Micrococcus sp. strain MP76) have shown antibacterial activity against Staphylococcus aureus sp. and Escherichia coli sp. [98,99]. Other bacterial strains that are reportedly inhibited by prodigiosin and cycloprodigiosin are Bacillus subtilis sp. and Salmonella enterica serovar Typhimurium [98]. Likewise, the yellow pigment has shown activity against P. aeruginosa sp. as well [99]. Norprodigiosin synthesized by marine Serratia sp. has also been reported to exhibit inhibition activity against Vibrio paraheamolyticus sp. and B. subtilis sp. [32]. These studies strengthen the utilization of bpBPs as potential alternatives to synthetic medicinal compounds.
Furthermore, inhibition activities recorded against Citrobacter sp. by pyocyanin and pyorubin [58] and P. aeruginosa sp. by violacein pigment (purified from Antarctic Iodobacter sp.) [100], further stretches the range of marine-derived bpBP’s potential against pathogenic bacterial species to opportunistic bacterial species. There are numerous correspondingly published studies. The pigment “melanin” from marine Streptomyces sp., for instance, demonstrated antibacterial activity against E. coli sp., S. typhi sp., S. paratyphi sp., Proteus mirabilis sp., Vibrio cholera sp., S. aureus sp., and Klebsiella oxytoca sp. [68]. A bright pink-orange colored pigment extracted from Salinicoccus sp. (isolated from Nellore sea coast) also showed antimicrobial potential against several bacterial strains including E.coli sp., Klebsiella pneumoniae sp., B. subtilis sp., Proteus vulgaris sp., P. aeruginosa sp., and S. aureus sp. [101]. Hence, these and similar other studies all indicate the exploration of marine bacterial species as a dynamic approach to derive antibacterial compounds.
A few studies also seemingly suggest that a single pigment from different species may exhibit activities against various target microorganisms. One example is violacein, a violet colored pigment extracted from Antarctic bacterium Janthinobacterium sp. SMN 33.6, which showed antibacterial activity against multi-resistant bacteria: S. aureus sp. ATCC 25923, E. coli sp. ATCC 25922, Kocuria rhyzophila sp. ATCC 9341, and S. typhimurium sp. ATCC 14028 [102], and that extracted from Collimonas sp. showed antibacterial activity against Micrococcus luteus sp. [67].
4.1.2. Antifungal Activity
Studies have also been carried out to determine the antifungal potential of natural pigmented compounds. Several studies have reported the antifungal activity of marine-derived bacterial pigments, among which violacein from Chromobacterium sp. and prodiginine pigments (prodigiosin and cycloprodigiosin) extracted from Indonesian marine bacterium P. rubra sp. reported to exhibit antagonistic activity against Candida albicans sp. [23,103]. Violacein also inhibited several other fungal strains, including Penicillium expansum sp., Fusarium oxysporum sp., Rhizoctonia solani sp., and Aspergillus flavus sp. Studies have also reported that violacein (extracted from a pure Chromobacterium sp.) shows comparable antifungal activity to that of bavistin and amphotericin B, highlighting the potential of marine-derived bpBPs as effective antifungal agents over existing synthetic antifungal compounds [103].
4.1.3. Anticancer Activity
Exploring anticancer compounds from marine microbes has been considered a hot spot in natural product research. Several studies have been carried out in order to examine the antitumor ability of marine bacterial pigments. Anticancer activity of marine-derived bpBPs has been explored against several cancerous cell lines. Astaxanthin and 2-(p-hydroxybenzyl) prodigiosin (HBPG) isolated from P. kolensis sp. and P. rubra sp. displayed significant cytotoxicity against human breast cancer cell line (MCF-7) and human ovarian adenocarcinoma cell line, respectively [38,104]. PCA (Phenazine -1-carboxylic acid) pigment extracted from marine P. aeruginosa sp. GS-33 correspondingly showed inhibition against SK-MEL-2 (human skin melanoma cell line) [105]. Another pigment violacein extracted from Antarctic bacterium isolate, identified as a member of the genus Janthinobacterium (named as Janthinobacterium sp. strain UV13), revealed its antiproliferative activity in HeLa cells. Studies further confirmed the potential of violacein as an anticancer agent to cisplatin drug (anticancer chemotherapy drug) in cervix cell carcinoma [106]. It has also been reported that a single pigment can express anticancer activity against multiple cancerous cell lines. Synthetically derived tambjamines isolated from the marine bacterium P. tunicata sp. have shown significant apoptosis inducing effects against various cancer cell lines including glioblastoma cell line (SF-295), M14 melanoma cell line (MDA-MB-435), ileocecal colorectal adenocarcinoma cell line (HCT-8), and promyelocytic leukemia cells (HL-60) [107]. Carotenoid pigments extracted from marine Arthrobacter sp. G2O (isolated from the Caspian Sea) exhibited antitumor activity on esophageal squamous cancerous cells [108]. Likewise, prodigiosin homolog extracted from marine bacterium Serratia proteamacula sp. was also found to exhibit high antitumor activity [109], indicating the potential of marine-derived bpBPs in antitumor therapy.
4.1.4. Antioxidant Activity
Marine-derived bpBPs are also being explored for their antioxidant activity. 3R saproxanthin and myxol pigments (from marine bacterium belonging to genus Flavobacteriacae) exhibited antioxidant activity against lipid peroxidation and also showed neuroprotective activity against L-glutamate toxicity [110]. The antioxidant activities of zeaxanthin (extracted from marine bacterium of genus Muricauda) [111] and melanin (from marine Pseudomonas stutzeri sp.) [112] have also been identified. Another pigment, phycocyanin extracted from marine bacterium Geitlerinema sp TRV57, demonstrated appreciable antioxidant activity [113]. Crude pigment extracted from the marine bacterium Streptomyces bellus sp. MSA1 also displayed 82% of DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) activity and said to exhibit radical scavenging activity [114]. Likewise, pigment crude extract from Zobellia laminarie sp. 465 (isolated from sea sponge) reported to exhibit high antioxidant values for ABTS-L (capture of the 2,2-azino-bis(3-ethylbenzothiazoline)-6-sulphonic acid (ABTS+) radical of the lipophilic fraction) [115], suggesting the importance of marine derived bacterial pigments in pharmaceutical and medicinal industries.
4.1.5. Antiviral Activity
The advancing viral pandemics have taken a toll over the limited pool of existing antiviral agents, which has led to a rigorous search for newer, natural compounds with better antiviral capacities. Various studies on marine bpBPs suggest them as potential candidates. Prodigiosin extracted from Serratia rubidaea sp. RAM_Alex showed antiviral activity against hepatitis C virus (HCV) upon injecting HepG2 (human liver cancer cell line) cells with 2% of HCV infected serum (Table 2) [116]. Other carotenoid pigments (from Natrialba sp. M6) have also displayed complete elimination of HCV and clearance of 89.42% of hepatitis B virus (HBV) [117], indicating the use of marine pigments as availing antiviral agents.

Table 2.
Therapeutic applications of bio-pigments extracted from marine bacterial isolates.
4.2. Industrial Applications
4.2.1. Bio-Pigments as Food Colorants
Researchers have concluded that marine-derived bpBPs can be utilized to provide full-scale commercial production of food-grade pigments, owing to their little or no threats to consumer health. They also showed pleasant colors at low concentrations. Pyorubrin and pyocyanin, for example, extracted from P. aeruginoasa sp., when assessed for their utilization as food colorings with agar, gave pleasing colors at 25 mg mLG−1 [58]. The utilization of bpBPs was also suggested as a feed additive to promote growth and enhance the coloration of ornamental fishes [119]. Furthermore, prodigiosin (from marine bacterium Zooshikella sp.) has been reported to exhibit good staining properties and a three months shelf life [120], which hints toward a sustainable aspect of marine-derived pigmented molecules as food colorants.
4.2.2. Bio-Pigments as Dyeing Agents
The worldwide demand for clothes is rising exponentially. Newly, there is an increase in the insistence of incorporating antimicrobial properties in fabrics. Lee et al. identified a novel marine bacterium Z. rubidus sp. S1-1 that produced two significant pigments, i.e., prodigiosin and cycloprodigiosin. These were used to dye cotton and silk fabrics. Results revealed that the application of red-pigmented extract solution on fabrics reduced the growth rate of S. aureus sp. KCTC 1916 by 96.62% to 99.98% and E. coli sp. KCTC 1924 by 91.37% to 96.98% [98]. Furthermore, Vibrio sp. isolated from marine sediment produced a bright red colored prodiginine pigment that was used to dye nylon 66, silk, wool, acrylic, and modacrylic fabrics to obtain a pretty deep-colored shade. The dyed silk and wool fabrics also showed antibacterial activity against E. coli sp. and S. aureus sp. [121]. Researchers at Ulsan National Institute have also reported the synthesis of antibacterial fabric by using violacein pigment extracted from C. violacea sp. [122,123]. Prodigiosin pigment extracted from Serratia sp. BTWJ8 effectively dyed paper, PMMA (Polymethyl methacrylate sheets), and rubber latex. Rubber is commonly used in day to day life either in houses or industries. PMMA have been widely utilized for the construction of lenses for exterior lights of automobiles. Different concentrations of prodigiosin produced variable color shades that revealed its affectivity as a coloring agent [124].
4.2.3. Use in Cosmetics
The cosmetic industry is an expeditiously emerging global business market. About 2000 companies in the United States of America are cosmetic manufacturers. It is estimated that American adults use seven different skincare products per day for everyday grooming [125]. The cosmetic industry has a worth of 10.4, 10.6, and 13.01 billion euros in the UK, France, and Germany, respectively [126]. Considering the cosmetic market value worldwide, researchers have also made efforts to explore the use of marine-derived bpBPs in skincare products. The addition of the pigment PCA in a solution of commercial sunscreen enhanced its UV-B (ultraviolet B-rays) protection and increased the SPF (sun protection factor) values up to 10% to 30% [105].
Similarly, melanin incorporated cream (named cream F3) was synthesized by concentrates of seaweed (Gelidium spinosum) and melanin pigment (extracted from marine bacterium Halomonas venusta sp.). Cream F3 showed high SPF values and photoprotective activity and demonstrated great effectivity in wound healing as well. Moreover, the formulated cream also exhibited antibacterial activity against skin pathogens; Streptococcus pyogenes sp. (MTCC 442), and S. aureus sp. (MTCC 96) [127]. Another research reported the effectivity of melanin (extracted from marine bacterium Vibrio natriegens sp.) in protecting mammalian cells from UV irradiation. Results revealed 90% survival rate of HeLa cells in melanized cell culture [128]. In another report, Bio lip balm made from crude pigment (extracted from S. bellus sp. MSA1) in a mixture of coconut oil, lanolin, and shredded bee wax [114] suggested the use of melanin pigment as a significant ingredient in several beauty care products as well.
4.2.4. Antifouling Agent
Billions of dollars have been spent each year to control fouling activities on different objects placed in the marine environment. Biofouling on ships such as dreadnoughts increased the roughness of the hull, which promotes frictional resistance, ultimately leading to an increase in fuel consumption and other corresponding environmental compliances. Heavy metal-based antifoulants cause severe environmental complications, which further mandate the need for “eco-friendly” antifouling agents. Researchers have also revealed the use of marine-derived bpBPs for their role as an antifouling agent, for instance, prodigiosin extracted from Serratia. sp. was reported to exhibit antifouling activity against marine fouling bacterial species such as Gallionella sp. and Alteromonas sp. It also inhibited the adhesion of Cyanobacterium sp. on the glass surface [129]. Likewise, another pigment, polymelanin synthesized by the marine bacterium P. lipolytica sp., prevented metamorphosis and decreased the invertebrate larval settlement [130], hence indicating the role of marine bacterial pigments as potential antifoulants.
4.2.5. Photosensitizers
The use of prodigiosin has also been reported as photosensitizers in solar cells. The high photostability of extracted prodigiosin demonstrated its use as a sensitizer in dye-sensitized solar cells (DSSC) (Table 3) [131]. This study suggests the viability of bpBPs in addition to that of prodigiosin for the construction of cost-effective and low tech industrially produced DSSC.

Table 3.
Industrial applications of bio-pigments extracted from marine bacterial isolates.
5. Industrial Importance and Global Market Trends of Pigmented Compounds
Pigments are already utilized in various nutritional supplements, antibiotics, skin care, and other industrial products (Table 4). The most valuable pigments in the global market are beta-carotene, lutein, and astaxanthin (Figure 3). Astaxanthin has its wide use in nutraceutical industries owing to its antioxidant properties and numerous health benefits. It has also been in wide use in cosmetic industries due to its antiaging activity. Moreover, astaxanthin is being utilized in aquaculture industries to carry out the pigmentation of shrimps, trouts, and salmons. At the industrial scale, astaxanthin production is accomplished using Paracoccus sp. It was predicted that the sales volume of astaxanthin by the year 2020 would be 1.1 billion US dollars [132], and the astaxanthin market is estimated to reach up to 3.4 billion US dollars with CAGR (compound annual growth rate) of 16.2% in 2027 [133].

Table 4.
Different industrial products and nutritional supplements utilizing pigmented compounds along with manufacturers, product brands, suppliers and company coverage.
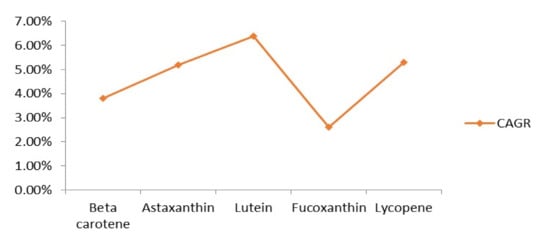
Figure 3.
Prospective compound annual growth rate (CAGR) of several pigments by the year 2026 [161,162,163,164,165].
FDA accepted the use of beta-carotene as a color additive in food products in the year 1964. Additionally, in 1977, the use of beta-carotene got approved in cosmetics also. The E-Number allotted to beta-carotene is E160a. Over and above, canthaxanthin use in food and broiler chicken feed got authorized in 1969, and the E-Number assigned to it is E161g [159]. Lycopene is also being utilized for many industrial purposes, and it is reckoned that the lycopene market will grow at a rate of 5.3% CAGR, by the end of 2026 [160].
The global market potential of carotenoid pigments is estimated to reach up to 5.7% by 2022 [166]. Europe and USA are the key business markets for carotenoid pigments [118]. It is expected that the global carotenoid market will increase from 1397.59 million US$ in 2018 to 2124.68 million dollars by the end of 2025, at the CAGR of 6.16% [167]. For a long time, different classes of pigmented compounds have occupied the entire market due to their wide range of applications in different industries (Figure 4). It is anticipated that by the year 2022, the global market of food colorants will reach up to 3.75 billion US$, along with farming colorant market, to touch 2.03 billion US$ by the year 2022 [166]. Europe holds the forefront for cutting synthetic colorants’ economy by utilizing natural dyes, which make up 85% of total dyes produced. It is evaluated that growing interests towards ready to eat and pre-packaged food items in China, India, and Middle-East countries will also drive the market of food colorants in the Asia Pacific as well [168].
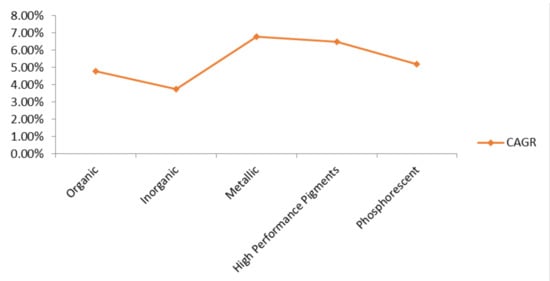
Figure 4.
Expected market worth of different classes of pigments during, the forecasting period: 2019 to 2026 [169,170,171,172,173].
6. Conclusions
Marine bacterial pigments can be a potential substitute for synthetic products to fulfill market demand and to ensure the public well-being. Putting aside the fact that synthetic medicines combat bacterial infections, they also pose adverse effects in terms of health. Likewise, artificial colorants due to the presence of azo dyes and heavy metals can also ignite cancer and other allergies. Microbial pigments derived from marine bacteria can be a promising approach to tackle the detrimental effects of these synthetic compounds.
Furthermore, marine bacteria tolerate a vast range of environmental conditions. Due to their unique biological properties, natural pigments from the marine environment also have wide range of applications in pharmaceutical, food, cosmetics, paper, and textile industries [1]. Marine bacterial species can be cultured in vitro and are genetically modified to get the desired level of pigments. Anyhow, there are certain limitations in implementing these naturally derived marine bacterial pigments on an industrial scale significantly: less percentage annual production, technological imperfections, low stability, high need for cost investments, and health complications [11]. For replacing synthetic products, efforts are required to explore new microbial sources and finding better optimization techniques to enhance the production of pigmented compounds. Genetic engineering and other strain development techniques should also be further studied to harvest bioactive pigments from marine bacterial species.
Author Contributions
Conceptualization: I.u.H.; formal analysis: Z.S. and M.F.; investigation: A.N., R.C., and Z.S.; methodology, A.N., R.C., and M.F.; resources: M.F. and I.u.H.; supervision: L.D. and H.M.; validation: L.D. and H.M.; writing—original draft: A.N., R.C., and Z.S.; writing—review and editing: L.D, H.M., and I.u.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R. Marine Pigmented Bacteria: A Prospective Source of Antibacterial Compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Saviola, B. Pigments and Pathogenesis. J. Mycobact. Dis. 2014, 4, 5. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Azman, A.-S.; Mawang, C.-I.; Abubakar, S. Bacterial Pigments: The Bioactivities and as an Alternative for Therapeutic Applications. Nat. Prod. Commun. 2018, 13, 1747–1754. [Google Scholar] [CrossRef]
- Dufossé, L. Pigments, Microbial. In Reference Module in Life Sciences; Elsevier: London, UK, 2016. [Google Scholar]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Kumar, A.; Vishwakarma, H.S.; Singh, J.; Dwivedi, S.; Kumar, M. Microbial Pigments: Production and Their Applications in Various Industries. Int. J. Pharm. Chem. Biol. Sci. 2015, 5, 203–212. [Google Scholar]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.; Khan, A.L.; Shinwari, Z.K.; Al‑Harrasi, A. Therapeutic Applications of Bacterial Pigments: A Review of Current Status and Future Opportunities. 3Biotech. 2018, 8, 207. [Google Scholar] [CrossRef]
- The Menace of Synthetic Non-Food Colours. Business Recorder. Available online: https://fp.brecorder.com/2008/02/20080216695580/ (accessed on 4 June 2020).
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Petruk, G.; Roxo, M.; Lise, F.D.; Mensitieri, F.; Notomista, F.; Wink, M.; Izzo, V.; Monti, D.M. The Marine Gram-Negative Bacterium Novosphingobium sp. PP1Y as a Potential Source of Novel Metabolites with Antioxidant Activity. Biotechnol. Lett. 2019, 41, 273–281. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing Microalgae for Skin Care. Cosmet. Toilet. 2015, 120, 99–106. [Google Scholar] [CrossRef]
- Bruckner, A.W. Life-Saving Products from Coral Reefs. Issues Sci. Technol. 2002, 18, 35. [Google Scholar]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid. Based Complement. Altern. Med. 2011, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Mohandass, C.; Rajasabapathy, R.; Meena, R.M. Molecular Diversity of Marine Pigmented Bacteria in the Central Arabian Sea with Special Reference to Antioxidant Properties. Cah. Biol. Mar. 2018, 59, 409–420. [Google Scholar]
- Baharum, S.N.; Beng, E.K.M.; Mokhtar, M.A.A. Marine Microorganisms: Potential Application and Challenges. J. Biol. Sci. 2010, 10, 555–564. [Google Scholar] [CrossRef]
- Podar, M.; Reysenbach, A.-L. New Opportunities Revealed by Biotechnological Explorations of Extremophiles. Curr. Opin. Biotechnol. 2006, 17, 250–255. [Google Scholar] [CrossRef]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef]
- Oren, A. Halophilic Microbial Communities and Their Environments. Curr. Opin. Biotechnol. 2015, 33, 119–124. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and Function of Phenazines in Bacteria: Impact on the Behavior of Bacteria in the Environment and Biotechnological Process. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.F.; Yim, J.H.; Kwon, S.-K.; Lee, C.H.; Lee, H.K. Red to Red—The Marine Bacterium Hahella chejuensis and Its Product Prodigiosin for Mitigation of Harmful Algal Blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629. [Google Scholar]
- Setiyono, E.; Adhiwibawa, M.A.S.; Indrawati, R.; Prihastyanti, M.N.U.; Shioi, Y.; Brotosudarmo, T.H.P. An Indonesian Marine Bacterium, Pseudoalteromonas rubra, Produces Antimicrobial Prodiginine Pigments. ACS Omega 2020, 5, 4626–4635. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, G.; Li, J.; Huang, H.; Zhang, X.; Zhang, H.; Ju, J. Cytotoxic and Antibacterial Angucycline- and Prodigiosin-Analogues from the Deep-Sea Derived Streptomyces sp. SCSIO 11594. Mar. Drugs 2015, 13, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Thomas, M.J.; Walstrom, K.M.; Warrick, E.C.; Gasper, B.J. Characterization of Prodiginine Compounds Produced by a Vibrio species Isolated from Salt Flat Sediment along the Florida Gulf Coast. Fine Focus 2016, 3, 33–51. [Google Scholar] [CrossRef]
- Ibrahim, D.; Nazari, T.F.; Kassim, J.; Lim, S.-H. Prodigiosin—An Antibacterial Red Pigment Produced by Serratia marcescens IBRL USM 84 Associated with a Marine Sponge Xestospongia testudinaria. J. Appl. Pharm. Sci. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Yi, H.; Chang, Y.-H.; Oh, H.W.; Bae, K.S.; Chun, J. Zooshikella ganghwensis gen. nov., sp. nov., Isolated from Tidal Flat Sediments. Int. J. Syst. Evol. Microbiol. 2003, 53, 1013–1018. [Google Scholar] [CrossRef]
- Abidin, Z.A.Z.; Ahmad, A.; Latip, J.; Usup, G. Marine Streptomyces sp. UKMCC_PT15 Producing Undecylprodigiosin with Algicidal Activity. J. Teknol. 2016, 78, 11-2. [Google Scholar] [CrossRef][Green Version]
- Bramhachari, P.V.; Mutyala, S.; Bhatnagar, I.; Pallela, R. Novel Insights on the Symbiotic Interactions of Marine Sponge-Associated Microorganisms: Marine Microbial Biotechnology Perspective. In Marine Sponges: Chemicobiological and Biomedical Applications, 1st ed.; Pallela, R., Ehrlich, H., Eds.; Springer: New Delhi, India, 2016; pp. 69–95. [Google Scholar] [CrossRef]
- Huang, Z.; Dong, L.; Lai, Q.; Liu, J. Spartinivicinus ruber gen. nov., sp. nov., a Novel Marine Gamma proteobacterium Producing Heptylprodigiosin and Cycloheptylprodigiosin as Major Red Pigments. Front. Microbiol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Xie, B.-B.; Shu, Y.-L.; Qin, Q.-L.; Rong, J.-C.; Zhang, X.-Y.; Chen, X.-L.; Zhou, B.-C.; Zhang, Y.-Z. Genome Sequence of the Cycloprodigiosin-Producing Bacterial Strain Pseudoalteromonas rubra ATCC 29570 T. J. Bacteriol. 2012, 194, 1637–1638. [Google Scholar] [CrossRef]
- Jafarzade, M.; Yahya, N.A.; Shayesteh, F.; Usup, G.; Ahmad, A. Influence of Culture Conditions and Medium Composition on the Production of Antibacterial Compounds by Marine Serratia sp. WPRA3. J. Microbiol. 2013, 51, 373–379. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Zhang, X.; Tana, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629. [Google Scholar] [CrossRef]
- Henke, N.A.; Heider, S.A.E.; Peters-Wendisch, P.; Wendisch, V.F. Production of the Marine Carotenoid Astaxanthin by Metabolically Engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Asker, D. Isolation and Characterization of a Novel, Highly Selective Astaxanthin-Producing Marine Bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109. [Google Scholar] [CrossRef] [PubMed]
- Shahina, M.; Hameed, A.; Lin, S.-Y.; Hsu, Y.-H.; Liu, Y.-C.; Cheng, I.-C.; Lee, M.-R.; Lai, W.-A.; Lee, R.-J.; Young, C.-C. Sphingomicrobium astaxanthinifaciens sp. nov., an Astaxanthin-Producing Glycolipid-Rich Bacterium Isolated from Surface Seawater and Emended Description of the Genus Sphingomicrobium. Int. J. Syst. Evol. Microbiol. 2013, 63, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Mukoyama, D.; Takeyama, H.; Kondo, Y.; Matsunaga, T. Astaxanthin Formation in the Marine Photosynthetic Bacterium Rhodovulum sulfidophilum Expressing crtI, crtY, crtW and crtZ. FEMS Microbiol. Lett. 2006, 265, 69–75. [Google Scholar] [CrossRef]
- Pachaiyappan, A.; Sadhasivam, G.; Kumar, M.; Muthuvel, A. Biomedical Potential of Astaxanthin from Novel Endophytic Pigment Producing Bacteria Pontibacter korlensis AG6. Waste Biomass Valoriz. 2020, 1–11. [Google Scholar] [CrossRef]
- Balraj, J.; Pannerselvam, K.; Jayaraman, A. Isolation of Pigmented Marine Bacteria Exiguobacterium sp. from Peninsular Region of India and a Study on Biological Activity of Purified Pigment. Int. J. Sci. Techol. Res. 2014, 3, 375–384. [Google Scholar]
- Shi, X.-L.; Wu, Y.-H.; Cheng, H.; Zhang, X.-Q.; Wang, C.-S.; Xu, X.-W. Complete Genome Sequence of Astaxanthin-Producing Bacterium Altererythrobacter ishigakiensis. Mar. Genom. 2016, 30, 77–79. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.T. Cloning and Characterization of the Astaxanthin Biosynthesis gene Cluster from the Marine Bacterium Paracoccus haeundaensis. Gene 2006, 370, 86–95. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, Y.M.; Baik, K.S.; Choi, K.S.; Ka, J.-O.; Seong, C.N. Mesoflavibacter aestuarii sp. nov., a Zeaxanthin Producing Marine Bacterium Isolated from Seawater. Int. J. Syst. Evol. Microbiol. 2014, 64, 1932–1937. [Google Scholar] [CrossRef]
- Hameed, A.; Shahina, M.; Lin, S.-Y.; Lai, W.-A.; Hsu, Y.-H.; Liu, Y.-C.; Young, C.-C. Aquibacter zeaxanthinifaciens gen. nov., sp. nov., a Zeaxanthin-Producing Bacterium of the Family Flavobacteriaceae Isolated from Surface Seawater, and Emended Descriptions of the Genera Aestuariibaculum and Gaetbulibacter. Int. J. Syst. Evol. Microbiol. 2014, 64, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Beppu, T.; Ueda, K. Zeaxanthinibacter enoshimensis gen. nov., sp. nov., a Novel Zeaxanthin-Producing Marine Bacterium of the Family Flavobacteriaceae, Isolated from Seawater Off Enoshima Island, Japan. Int. J. Syst. Evol. Microbiol. 2007, 57, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Shahina, M.; Hameed, A.; Lin, S.-Y.; Lee, R.-J.; Lee, M.-R.; Young, C.-C. Gramella planctonica sp. nov., a Zeaxanthin-Producing Bacterium Isolated from Surface Seawater, and Emended Descriptions of Gramella aestuarii and Gramella echinicola. Antonie van Leeuwenhoek 2014, 105, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Beppu, T.; Ueda, K. Mesoflavibacter zeaxanthinifaciens gen. nov., sp. nov., a Novel Zeaxanthin Producing Marine Bacterium of the Family Flavobacteriaceae. Syst. Appl. Microbiol. 2007, 30, 291–296. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N.M. Carotenoid Production by Formosa sp. KMW, Marine Bacteria of Flavobacteriaceae Family: Influence of Culture Conditions and Nutrient Composition. Biocatal. Agric. Biotechnol. 2015, 4, 559–567. [Google Scholar] [CrossRef]
- Thawornwiriyanun, P.; Tanasupawat, S.; Dechsakulwatana, C.; Techkarnjanaruk, S.; Suntornsuk, W. Identification of Newly Zeaxanthin-Producing Bacteria Isolated from Sponges in the Gulf of Thailand and Their Zeaxanthin Production. Appl. Biochem. Biotechnol. 2012, 167, 2357–2368. [Google Scholar] [CrossRef]
- Hameed, A.; Arun, A.B.; Ho, H.-P.; Chang, C.-M.J.; Rekha, P.D.; Lee, M.-R.; Young, C.-C. Supercritical Carbon Dioxide Micronization of Zeaxanthin from Moderately Thermophilic Bacteria Muricauda lutaonensis CC-HSB-11T. J. Agric. Food Chem. 2011, 59, 4119–4124. [Google Scholar] [CrossRef]
- Seto, R.; Takaichi, S.; Kurihara, T.; Kishi, R.; Honda, M.; Takenaka, S.; Tsukatani, Y.; Madigan, M.T.; Wang-Otomo, Z.Y.; Kimura, Y. Lycopene-Family Carotenoids Confer Thermostability on Photocomplexes from a New Thermophilic Purple Bacterium. Biochemistry 2020, 59, 2351–2358. [Google Scholar] [CrossRef]
- Ramanathan, G.; Ramalakshmi, P. Studies on Efficacy of Marine Bacterium Salinicoccus roseus Pigment for Their Bioactive Potential. Eur. J. Biomed. Pharm. Sci. 2017, 4, 330–334. [Google Scholar]
- Montero, O.; Macías-Sánchez, M.D.; Lama, C.M.; Lubián, L.M.; Mantell, C.; Rodríguez, M.; De la Ossa, E.M. Supercritical CO2 Extraction of â-Carotene from a Marine Strain of the Cyanobacterium Synechococcus Species. J. Agric. Food Chem. 2005, 53, 9701–9707. [Google Scholar] [CrossRef]
- Hamidi, M.; Kozani, P.S.; Kozani, P.S.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria Versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs. 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Sibero, M.T.; Bachtiarini, T.U.; Trianto, A.; Lupita, A.H.; Sari, D.P.; Igarashi, Y.; Harunari, E.; Sharma, A.R.; Radjasa, O.K.; Sabdono, A. Characterization of a Yellow Pigmented Coral-Associated Bacterium Exhibiting Anti-Bacterial Activity against Multidrug Resistant (MDR) Organism. Egypt. J. Aquat. Res. 2018, 45, 81–87. [Google Scholar] [CrossRef]
- Teramoto, M.; Nishijima, M. Flavicella marina gen. nov., sp. nov., a Carotenoid-Producing Bacterium from Surface Seawater. Int. J. Syst. Evol. Microbiol. 2015, 65, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.L.C.; Huang, K.-C.; Ng, H.S.; Lan, J.C.-W. Exploring the Fermentation Characteristics of a Newly Isolated Marine Bacteria Strain, Gordonia terrae TWRH01 for Carotenoids Production. J. Biosci. Bioeng. 2020, 130, 187–194. [Google Scholar] [CrossRef]
- Saha, S.; Thavasi, T.R.; Jayalakshmi, S. Phenazine Pigments from Pseudomonas aeruginosa and Their Application as Antibacterial Agent and Food Colourants. Res. J. Microbiol. 2008, 3, 122–128. [Google Scholar] [CrossRef]
- Fulton, J.M.; Arthur, M.A.; Freeman, K.H. Subboreal Aridity and Scytonemin in the Holocene Black Sea. Org. Geochem. 2012, 49, 47–55. [Google Scholar] [CrossRef]
- Soule, T.; Palmer, K.; Gao, Q.; Potrafka, R.M.; Stout, V.; Garcia-Pichel, F. A Comparative Genomics Approach to Understanding the Biosynthesis of The sunscreen Scytonemin in Cyanobacteria. BMC Genom. 2009, 10, 336. [Google Scholar] [CrossRef]
- Thøgersen, M.S.; Delpin, M.W.; Melchiorsen, J.; Kilstrup, M.; Månsson, M.; Bunk, B.; Spröer, C.; Overmann, J.; Nielsen, K.F.; Gram, L. Production of the Bioactive Compounds Violacein and Indolmycin Is Conditional in a maeA Mutant of Pseudoalteromonas luteoviolacea S4054 Lacking the Malic Enzyme. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Aye, A.M.; Bonnin-Jusserand, M.; Brian-Jaisson, F.; Ortalo-Magne, A.; Culioli, G.; Nevry, R.K.; Rabah, N.; Blache, Y.; Molmeret, M. Modulation of Violacein Production and Phenotypes Associated with Biofilm by Exogenous Quorum Sensing N-acylhomoserine Lactones in the Marine Bacterium Pseudoalteromonas ulvae TC14. Microbiology 2015, 161, 2039–2052. [Google Scholar] [CrossRef]
- Dang, H.T.; Yotsumoto, K.; Enomoto, K. Draft Genome Sequence of Violacein-Producing Marine Bacterium Pseudoalteromonas sp. 520P1. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Ballestriero, F.; Daim, M.; Penesyan, A.; Nappi, J.; Schleheck, D.; Bazzicalupo, P.; Schiavi, E.D.; Egan, S. Antinematode Activity of Violacein and the Role of the Insulin/IGF-1 Pathway in Controlling Violacein Sensitivity in Caenorhabditis elegans. PLoS ONE 2014, 9, e109201. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Cheng, H.; Xu, L.; Jin, X.-B.; Wang, C.-S.; Xu, X.-W. Physiological and Genomic Features of a Novel Violacein-Producing Bacterium Isolated from Surface Seawater. PLoS ONE 2017, 12, e0179997. [Google Scholar] [CrossRef] [PubMed]
- Yada, S.; Wang, Y.; Zou, Y.; Nagasaki, K.; Hosokawa, K.; Osaka, I.; Arakawa, R.; Enomoto, K. Isolation and Characterization of Two Groups of Novel Marine Bacteria Producing Violacein. Mar. Biotechnol. 2008, 10, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hakvåg, S.; Fjærvik, E.; Klinkenberg, G.; Borgos, S.E.F.; Josefsen, K.D.; Ellingsen, T.E.; Zotchev, S.B. Violacein-Producing Collimonas sp. from the Sea Surface Microlayer of Coastal Waters in Trøndelag, Norway. Mar. Drugs 2009, 7, 576–588. [Google Scholar] [CrossRef]
- Vasanthabharathi, V.; Lakshminarayanan, R.; Jayalakshmi, S. Melanin Production from Marine Streptomyces. Afr. J. Biotechnol. 2011, 10, 11224–11234. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production, Characterization and Analysis of Melanin from Isolated Marine Pseudomonas sp. Using Vegetable Waste. Res. J. Eng. Sci. 2013, 2, 40–46. [Google Scholar]
- Lucas-Elío, P.; Goodwin, L.; Woyke, T.; Pitluck, S.; Nolan, M.; Kyrpides, N.C.; Detter, J.C.; Copeland, A.; Teshima, H.; Bruce, D.; et al. The Genomic Standards Consortium Complete Genome Sequence of the Melanogenic Marine Bacterium Marinomonas mediterranea Type Strain (MMB-1T). Stand. Genom. Sci. 2012, 6, 63–73. [Google Scholar] [CrossRef]
- Manirethan, V.; Raval, K.; Balakrishnan, R.M. Adsorptive Removal of Trivalent and Pentavalent Arsenic from Aqueous Solutions Using Iron and Copper Impregnated Melanin Extracted from the Marine Bacterium Pseudomonas stutzeri. Environ. Pollut. 2019, 257, 113576. [Google Scholar] [CrossRef]
- Kurian, N.K.; Nair, H.P.; Bhat, S.G. Evaluation of Anti-Inflammatory Property of Melanin from Marine Bacillus spp. BTCZ31. Asian J. Pharm. Clin. Res. 2015, 8, 251–255. [Google Scholar]
- Sivaperumal, P.; Kamala, K.; Rajaram, R.; Mishra, S.S. Melanin from Marine Streptomyces sp. (MVCS13) with Potential Effect against Ornamental Fish Pathogens of Carassius auratus. Biocatal. Agric. Biotechnol. 2014, 3, 134–141. [Google Scholar] [CrossRef]
- Kurian, N.K.; Bhat, S.G. Photoprotection and Anti-Inflammatory Properties of Non–Cytotoxic Melanin from Marine Isolate Providencia rettgeri Strain BTKKS1. Biosci. Biotechnol. Res. Asia 2017, 14, 1475–1484. [Google Scholar] [CrossRef]
- Kurian, N.K.; Nair, H.P.; Bhat, S.G. Characterization of Melanin Producing Bacteria Isolated from 96m depth Arabian Sea Sediments. Res. J. Biotechnol. 2019, 14, 64–71. [Google Scholar]
- Kamarudheen, N.; Naushad, T.; Rao, K.V.B. Biosynthesis, Characterization and Antagonistic Applications of Extracellular Melanin Pigment from Marine Nocardiopsis Sps. Indian J. Pharm. Educ. Res. 2019, 53, 112–120. [Google Scholar] [CrossRef]
- Kurian, N.K.; Bhat, S.G. Food, Cosmetic and Biological Applications of Characterized DOPA-Melanin from Vibrio alginolyticus strain BTKKS3. Appl. Biol. Chem. 2018, 61, 163–171. [Google Scholar] [CrossRef]
- Sakai-Kawada, F.E.; Ip, C.G.; Hagiwara, K.A.; Awaya, J.D. Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review. Front. Microbiol. 2019, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Picott, K.J.; Deichert, J.A.; De Kemp, E.M.; Schatte, G.; Sauriol, F.; Ross, A.C. Isolation and Characterization of Tambjamine MYP1, a Macrocyclic Tambjamine Analogue from Marine Bacterium Pseudoalteromonas citrea. MedChemComm 2019, 10, 478–483. [Google Scholar] [CrossRef]
- Harris, A.K.P.; Williamson, N.R.; Slater, H.; Cox, A.; Abbasi, S.; Foulds, I.; Simonsen, H.T.; Leeper, F.J.; Salmond, G.P.C. The Serratia Gene Cluster Encoding Biosynthesis of the Red Antibiotic, Prodigiosin, Shows Species- and Strain-Dependent Genome Context Variation. Microbiology 2004, 150, 3547–3560. [Google Scholar] [CrossRef]
- Williamson, N.R.; Simonsen, H.T.; Ahmed, R.A.A.; Goldet, G.; Slater, H.; Woodley, L.; Leeper, F.J.; Salmond, G.P.C. Biosynthesis of the Red Antibiotic, Prodigiosin, in Serratia: Identification of a Novel 2-methyl-3-n-amyl-Pyrroie (MAP) Assembly Pathway, Definition of the Terminal Condensing Enzyme, and Implications for Undecylprodigiosin Biosynthesis in Streptomyces. Mol. Microbiol. 2005, 56, 971–989. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Dorrestein, P.C.; Kelleher, N.L.; Walsh, T.C. Protein Assembly Line Components in Prodigiosin Biosynthesis: Characterization of PigA, G, H, I, J. J. Am. Chem. Soc. 2006, 128, 12600–12601. [Google Scholar] [CrossRef]
- Kimata, S.; Izawa, M.; Kawasaki, T.; Hayakawa, Y. Identification of a Prodigiosin Cyclization Gene in the Roseophilin Producer and Production of a New Cyclized Prodigiosin in a Heterologous Host. J. Antibiot. 2017, 70, 196–199. [Google Scholar] [CrossRef]
- De Rond, T.; Stow, P.; Eigl, I.; Johnson, R.E.; Chan, L.J.G.; Goyal, G.; Baidoo, E.E.K.; Hillson, N.J.; Petzold, C.J.; Sarpong, R.; et al. Oxidative Cyclization of Prodigiosin by an Alkylglycerol Monooxygenase-Like Enzyme. Nat. Chem. Biol. 2017, 13, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.; Thomas, T.; Egan, S.; Kjelleberg, S. The Use of Functional Genomics for the Identification of a Gene Cluster Encoding for the Biosynthesis of an Antifungal Tambjamine in the Marine Bacterium Pseudoalteromonas tunicata: Brief Report. Environ. Microbiol. 2007, 9, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.M.; Kelly, V.; Simpson, J.P.; Ward, M.; Campopiano, D.J. The Carbon chain-Selective Adenylation Enzyme TamA: The Missing Link between Fatty Acid and Pyrrole Natural Product Biosynthesis. Org. Biomol. Chem. 2018, 16, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Rapid and Selective Screening Method for Isolation and Identification of Carotenoid-Producing Bacteria. Methods Mol. Biol. 2018, 1852, 143–170. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017. [Google Scholar] [CrossRef]
- Scaife, M.A.; Burja, A.M.; Wright, P.C. Characterization of Cyanobacterial β-Carotene Ketolase and Hydroxylase Genes in Escherichia coli, and Their Application for Astaxanthin Biosynthesis. Biotechnol. Bioeng. 2009, 103, 944–955. [Google Scholar] [CrossRef]
- Soule, T.; Stout, V.; Swingley, W.D.; Meeks, J.C.; Garcia-Pichel, F. Molecular Genetics and Genomic Analysis of Scytonemin Biosynthesis in Nostoc punctiforme ATCC 29133. J. Bacteriol. 2007, 189, 4465–4472. [Google Scholar] [CrossRef]
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The Biosynthesis of Cyanobacterial Sunscreen Scytonemin in Intertidal Microbial Mat Communities. FEMS Microbiol. Ecol. 2011, 77, 322–332. [Google Scholar] [CrossRef]
- Estrada, A.F.; Maier, D.; Scherzinger, D.; Avalos, J.; Al-Babili, S. Novel Apo Carotenoid Intermediates in Neurospora crassa Mutants Imply a New Biosynthetic Reaction Sequence Leading to Neurosporaxanthin Formation. Fungal Genet. Biol. 2008, 45, 1497–1505. [Google Scholar] [CrossRef]
- Liao, L.; Su, S.; Zhao, B.; Fan, C.; Zhang, J.; Li, H.; Chen, B. Biosynthetic Potential of a Novel Antarctic Actinobacterium Marisediminicola antarctica ZS314T Revealed by Genomic Data Mining and Pigment Characterization. Mar. Drugs. 2019, 17, 388. [Google Scholar] [CrossRef]
- Balashov, S.P.; Imasheva, E.S.; Boichenko, V.A.; Antón, J.; Wang, J.M.; Lanyi, J.K. Xanthorhodopsin: A Proton Pump with a Light-Harvesting Carotenoid Antenna. Science 2005, 309, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Lanyi, J.K.; Balashov, S.P. Xanthorhodopsin: A Bacteriorhodopsin-Like Proton Pump with a Carotenoid Antenna. Biochim. Biophys. Acta 2008, 1777, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Bryant, D.A. The Biosynthetic Pathway for Myxol-2’fucoside (Myxoxanthophyll) in the Cyanobacterium Synechococcus sp. Strain PCC 7002. J. Bacteriol. 2009, 191, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Lorquin, J.; Dortoli, N.A.; Garcia, N.; Chaintreuil, C.; Massonboivin, C.; Dreyfus, B.; Giraud, E. Isolation and Characterization of Canthaxanthin Biosynthesis Genes from the Photosynthetic Bacterium Bradyrhizobium sp. Strain ORS278. J. Bacteriol. 2000, 182, 3850–3853. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, Y.-S.; Park, S.; Kim, J.; Kang, S.-J.; Lee, M.-H.; Ryu, S.; Choi, J.M.; Oh, T.-K.; Yoon, J.-H. Exceptional Production of Both Prodigiosin and Cycloprodigiosin as Major Metabolic Constituents by a Novel Marine Bacterium, Zooshikella rubidus S1-1. Appl. Environ. Microbiol. 2011, 77, 4967–4973. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei-Heidari, H.R.; Partovifar, M.; Memarpoor-Yazdi, M. Evaluation of the Bioactive Potential of Secondary Metabolites Produced by a New Marine Micrococcus Species Isolated from the Persian Gulf. Avicenna J. Med. Biotechnol. 2020, 12, 61–65. [Google Scholar] [PubMed]
- Atalah, J.; Blamey, L.; Muñoz‑Ibacache, S.; Gutierrez, F.; Urzua, M.; Encinas, M.V.; Páez, M.; Sun, J.; Blamey, J.M. Isolation and Characterization of Violacein from an Antarctic Iodobacter: A Non‑Pathogenic Psychrotolerant Microorganism. Extremophiles 2019, 24, 43–52. [Google Scholar] [CrossRef]
- Srilekha, V.; Krishna, G.; Srinivas, V.S.; Charya, M.A.S. Antimicrobial evaluation of bioactive pigment from Salinicoccus sp. isolated from Nellore sea coast. Int. J. Biotechnol. Biochem. 2017, 13, 211–217. [Google Scholar]
- Asencio, G.; Lavin, P.; Alegría, K.; Domínguez, M.; Bello, H.; González-Rocha, G.; González-Aravena, M. Antibacterial Activity of the Antarctic Bacterium Janthinobacterium sp. SMN 33.6 against Multi-Resistant Gram-Negative Bacteria. Electron. J. Biotechnol. 2014, 17, 1–5. [Google Scholar] [CrossRef]
- Sasidharan, A.; Sasidharan, N.K.; Amma, D.B.N.S.; Vasu, R.K.; Nataraja, A.V.; Bhaskaran, K. Antifungal Activity of Violacein Purified from a Novel Strain of Chromobacterium sp. NIIST (MTCC 5522). J. Microbiol. 2015, 53, 694–701. [Google Scholar] [CrossRef]
- Fehér, D.; Barlow, R.S.; Lorenzo, P.S.; Hemscheidt, T.K. A 2-Substituted Prodiginine, 2-(p-Hydroxybenzyl) Prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008, 71, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Paradeshi, J.; Chaudhari, B. Anti-Melanoma and UV-B Protective Effect of Microbial Pigment Produced by Marine Pseudomonas aeruginosa GS-33. Nat. Prod. Res. 2016, 30, 2835–2839. [Google Scholar] [CrossRef] [PubMed]
- Alem, D.; Marizcurrena, J.J.; Saravia, V.; Davyt, D.; Martinez‑Lopez, W.; Castro‑Sowinski, S. Production and Antiproliferative Effect of Violacein, a Purple Pigment Produced by an Antarctic Bacterial Isolate. World J. Microbiol. Biotechnol. 2020, 36, 120. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, D.M.; Banwell, M.G.; Garson, M.J.; Kumar, N.; De Moraes, M.O.; Cavalcanti, B.C.; Pessoa, C. Antimicrobial and Cytotoxic Activities of Synthetically Derived Tambjamines C and E - J, BE-18591, and a Related Alkaloid from the Marine Bacterium Pseudoalteromonas tunicata. Chem. Biodivers. 2010, 7, 1311–1324. [Google Scholar] [CrossRef]
- Afra, S.; Makhdoumi, A.; Matin, M.M.; Feizy, J. A Novel Red Pigment from Marine Arthrobacter sp. G20 with Specific Anticancer Activity. J. Appl. Microbiol. 2017, 123, 1228–1236. [Google Scholar] [CrossRef]
- Miao, L.; Wang, X.; Jiang, W.; Yang, S.; Zhou, H.; Zhai, Y.; Zhou, X.; Dong, K. Optimization of the Culture Condition for an Antitumor Bacterium Serratia proteamacula 657 and Identification of the Active Compounds. World J. Microbiol. Biotechnol. 2012, 29, 855–863. [Google Scholar] [CrossRef]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare Carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, Isolated from Novel Marine Bacteria (Flavobacteriaceae) and Their Antioxidative Activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef]
- Prabhu, S.; Rekha, P.D.; Young, C.C.; Hameed, A.; Lin, S.-Y.; Arun, A.B. Zeaxanthin Production by Novel Marine Isolates from Coastal Sand of India and Its Antioxidant Properties. Appl. Biochem. Biotechnol. 2013, 171, 817–831. [Google Scholar] [CrossRef]
- Kumar, G.; Sahu, N.; Reddy, G.N.; Prasad, R.B.N.; Nagesh, N.; Kamal, A. Production of Melanin Pigment from Pseudomonas stutzeri Isolated from Red Seaweed Hypneamusci formis. Lett. Appl. Microbiol. 2013, 57, 295–302. [Google Scholar] [CrossRef]
- Renugadevi, K.; Nachiyar, C.V.; Sowmiya, P.; Sunkar, S. Antioxidant Activity of Phycocyanin Pigment Extracted from Marine Filamentous Cyanobacteria Geitlerinema sp TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242. [Google Scholar] [CrossRef]
- Srinivasan, M.; Keziah, S.M.; Hemalatha, M.; Devi, C.S. Pigment from Streptomyces bellus MSA1 Isolated from Marine Sediments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263. [Google Scholar] [CrossRef]
- Silva, T.R.; Tavares, R.S.N.; Canela-Garayoa, R.; Eras, J.; Rodrigues, M.V.N.; Neri-Numa, I.A.; Pastore, G.M.; Rosa, L.H.; Schultz, J.A.A.; Debonsi, H.M.; et al. Chemical Characterization and Biotechnological Applicability of Pigments Isolated from Antarctic Bacteria. Mar. Biotechnol. 2019, 21, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Metwally, R.A.; Abeer, A.; El-Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.; Sabry, S. Biological Activity of Prodigiosin from Serratia rubidaea RAM_Alex. Res. J. Biotechnol. 2019, 14, 100. [Google Scholar]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; García-Galbis, M.R.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef]
- Dharmaraj, S.; Ashokkumar, B.; Dhevendaran, K. Food-Grade Pigments from Streptomyces sp. Isolated from the Marine Sponge Callyspongia diffusa. Food Res. Int. 2009, 42, 487–492. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Applications of Prodigiosin Extracted from Marine Red Pigmented Bacteria Zooshikella sp. and Actinomycete Streptomyces sp. Microorganisms 2020, 8, 556. [Google Scholar] [CrossRef]
- Alihosseini, F.; Ju, K.-S.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial Colorants: Characterization of Prodiginines and Their Applications on Textile Materials. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef]
- Anti-Bacterial Fabric Holds Promise for Fighting Superbug. Science Daily. Ulsan National Institute of Science and Technology (UNIST). Available online: https://www.sciencedaily.com/releases/2016/03/160308091646.htm (accessed on 8 March 2020).
- Michael, R. New Antibiotic Dye May Help Prevent Infectious Diseases. Contagion Live. Available online: https://www.contagionlive.com/news/new-antibiotic-dye-may-help-prevent-infectious-diseases (accessed on 4 April 2020).
- Krishna, J.G.; Jacob, A.; Kurian, P.; Elyas, K.K.; Chandrasekaran, M. Marine Bacterial Prodigiosin as Dye for Rubber Latex, Polymethyl Methacrylate Sheets and Paper. Afr. J. Biotechnol. 2013, 12, 2266–2269. [Google Scholar] [CrossRef]
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial Metabolites as a Source of Sunscreens and Moisturizers: A Comparison with Current Synthetic Compounds. Eur. J. Phycol. 2017, 52, 43–56. [Google Scholar] [CrossRef]
- Consumption Value of Cosmetics and Personal Care in Europe in 2018, by Country (in Million Euros). Available online: https://www.statista.com/statistics/382100/european-cosmetics-market-volume-by-country/ (accessed on 24 June 2020).
- Poulose, N.; Sajayan, A.; Ravindran, A.; Sreechitra, T.; Vardhan, V.; Selvin, J.; Kiran, G.S. Photoprotective Effect of Nanomelanin-Seaweed Concentrate in Formulated Cosmetic Cream: With Improved Antioxidant and Wound Healing Properties. J. Photochem. Photobiol. B Biol. 2020, 205. [Google Scholar] [CrossRef]
- Wang, Z.; Tschirhart, T.; Schultzhaus, Z.; Kelly, E.E.; Chen, A.; Oh, E.; Nag, O.; Glaser, E.R.; Kim, E.; Lloyd, P.F.; et al. Characterization and Application of Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens Through Heterologous Biosynthesis. Appl. Environ. Microbiol. 2019, 86. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.A.; Satheesh, S.; Balasubramaniem, A.K.; Varalakshmi, P.; Gopal, S.; Natesan, S. Antifouling Activity of Prodigiosin from Estuarine Isolate of Serratia marcescens CMST 07. In Microbiological Research In Agroecosystem Management, 1st ed.; Velu, R.K., Ed.; Springer: New Delhi, India, 2013; pp. 11–21. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, X.-P.; Cai, X.; Wang, P.; Li, B.; Yang, J.-L.; Wang, X. Pyomelanin from Pseudoalteromonas lipolytica Reduces Biofouling. Microbiol. Biotechnol. 2017, 10, 1718–1731. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Velasco, P.; Morales-Atilano, I.; Rodríguez-Delgado, M.; Rodríguez-Delgado, J.M.; Luna-Moreno, D.; Ávalos-Alanís, F.G.; Villarreal-Chiu, J.F. Photoelectric Evaluation of Dye-Sensitized Solar Cells Based on Prodigiosin Pigment Derived from Serratia marcescens 11E. Dyes Pigment. 2020, 177, 108278. [Google Scholar] [CrossRef]
- Global Astaxanthin Market—Sources, Technologies and Applications. Available online: https://www.marketresearch.com/Industry-Experts-v3766/Global-Astaxanthin-Sources-Technologies-Applications-8827191/ (accessed on 30 March 2020).
- Astaxanthin Market Size, Share & Trends Analysis Report by Source, by Product, by Application and Segment Forecasts, 2020–2027. Available online: https://www.reportlinker.com/p05868776/Astaxanthin-Market-Size-Share-Trends-Analysis-Report-By-Source-By-Product-By-Application-And-Segment-Forecasts.html (accessed on 1 June 2020).
- Prodigiosin Serratia marcescens - CAS 82-89-3 - Calbiochem. Merck: A Leader in Life Sciences. Available online: https://www.merckmillipore.com/INTL/en/product/Prodigiosin-Serratia-marcescens-CAS-82-89-3-Calbiochem,EMD_BIO-529685?ReferrerURL=https%3A%2F%2Fwww.google.com%2F&bd=1 (accessed on 6 June 2020).
- Paracoccus Powder (Astaxanthin Powder) 2OZ. Brine Shrimp Direct. Available online: https://www.brineshrimpdirect.com/paracoccus-powder-astaxanthin-powder-2-ounce (accessed on 6 June 2020).
- Paracoccus Powder, Natural Source of Astaxanthin, 50g. NoCoast Aquatics. Available online: https://www.nocoastaquatics.com/products/paracoccus-powder-astaxanthin (accessed on 7 June 2020).
- Violacein (V9389)—Datasheet—Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/content/dam/sigmaaldrich/docs/Sigma/Datasheet/2/v9389dat.pdf (accessed on 6 August 2020).
- Prodigiosin antibiotic. My Biosource. Available online: https://www.mybiosource.com/antibiotic/prodigiosin/651317 (accessed on 6 August 2020).
- Suppliers for Prodigiosin 25c. BuyersGuideChem. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=121199138105 (accessed on 15 June 2020).
- Suppliers for Prodigiosin. BuyersGuideChem. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=553227439869&modus=einprod (accessed on 21 June 2020).
- Lucantin® Pink (Astaxanthin). Animal Nutrition Products. BASF. Available online: https://nutrition.basf.com/global/en/animal-nutrition/products/lucantin-pink.html (accessed on 15 June 2020).
- AstaSana™ 10% FS. UL Prospector. Available online: https://www.ulprospector.com/pt/eu/Food/Detail/15324/541591/AstaSana-10-FS (accessed on 15 June 2020).
- J-Bio™ Astxanthin. GMP Global Marketing, Inc. Available online: https://www.gmpglobalmarketing.com/portfolio-items/j-bio-astaxanthin/ (accessed on 27 June 2020).
- BioAstin® Hawaiian Astaxanthin. Cyanotech Corporation. Foods of Hawaii. Available online: https://www.foodsofhawaii.com/author/cyanotech-corporation/?user=132 (accessed on 27 June 2020).
- Suppliers for Astaxanthin: BuyersGuideChem. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=244231380730 (accessed on 1 July 2020).
- ZeaGold®, Zeaxanthin. Kalsec. Available online: https://www.kalsec.com/products/zeaxanthin/ (accessed on 1 July 2020).
- MacuShield® softgel capsule. AGP Limited. Available online: https://agp.com.pk/agp_products/macushield/ (accessed on 3 July 2020).
- OPTISHARP® (Zeaxanthin) 20% FS by DSM Nutritional Products, Inc. Prospector. Available online: https://www.ulprospector.com/en/na/Food/Detail/6295/239896/OPTISHARP-Zeaxanthin-20-FS (accessed on 1 July 2020).
- Suppliers for Zeaxanthin. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=750686624198 (accessed on 1 July 2020).
- Redivivo® (Lycopene) 10% FS by DSM Nutritional Products, Inc. UL Prospector. Available online: https://www.ulprospector.com/en/na/Food/Detail/6295/239893/redivivo-Lycopene-10-FS (accessed on 3 September 2020).
- Lyc-O-Mato® -Packed with Powder of Tomato by LycoRed Ltd. NUTRA Ingredients. Available online: https://www.nutraingredients.com/Product-innovations/Lyc-O-Mato-R-Packed-with-the-Power-of-the-Tomato (accessed on 3 September 2020).
- Suppliers for CAS 502-65-8. BuyersGuideChem. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=130817126231 (accessed on 2 June 2020).
- Food, Beverage and Nutrition. Barrington Nutritionals. Available online: https://www.ulprospector.com/en/na/Food/Suppliers/2252/Barrington-Nutritionals?st=1 (accessed on 3 September 2020).
- CaroCare®, Beta-Carotene by DSM Nutritional Products, Inc. New Hope Network. Available online: https://www.newhope.com/ingredients-general/carocare-dsm-natural-choice-carotene (accessed on 7 September 2020).
- Lyc-O-Beta 7.5% VBA by LycoRed Ltd. Available online: https://www.lycored.com/lyc-o-beta-7-5-vbaf/ (accessed on 7 September 2020).
- Suppliers for CAS 7235-40-7. BuyersGuideChem. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=180131918792 (accessed on 7 September 2020).
- FloraGLO® Lutein 20% SAF by DSM Nutritional Products, Inc. UL Prospector. Available online: https://www.ulprospector.com/en/na/Food/Detail/6295/239891/FloraGLO-Lutein-20-SAF (accessed on 10 August 2020).
- Suppliers for Lutein. BuyersGuideChem. Available online: https://www.buyersguidechem.com/AliefAus.php?pnumm=330278064387&modus=einprod&anzahl_produkte_cas=4 (accessed on 1 July 2020).
- Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices, U.S. Food and Drug Administration. Available online: https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices (accessed on 15 November 2017).
- Global Lycopene Market Forecast Report to 2026: Includes Company Profiling with Detailed Strategies, Financials and Recent Developments—Research and Markets.com. Available online: https://www.businesswire.com/news/home/20190430005701/en/Global-Lycopene-Market-Forecast-Report-2026-Includes (accessed on 30 November 2020).
- Beta Carotene Market Size, Share, Trends, & Industry Analysis Report by Source (Algae, Fruits & Vegetables, & Synthetic); by Application (Food & Beverages, Dietary Supplements, Cosmetics, & Animal Feed); by Regions: Segment Forecast, 2018–2026. Available online: https://www.polarismarketresearch.com/industry-analysis/beta-carotene-market (accessed on 1 December 2020).
- Astaxanthin Market 2020: CAGR of 5.2% with top Countries Data, Latest Trends, Market Size, Share, Global Industry Analysis & Forecast to 2026. Press Release. Available online: https://www.wfmj.com/story/42684950/astaxanthin-market-2020-cagr-of-52-with-top-countries-data-latest-trends-market-size-share-global-industry-analysis-amp-forecast-to-2026 (accessed on 27 September 2020).
- Lutein Market to Reach USD 454.8 million by 2026. Reports and Data. Available online: https://www.prnewswire.com/news-releases/lutein-market-to-reach-usd-454-8-million-by-2026--reports-and-data-300941985.html (accessed on 1 December 2020).
- Fucoxanthin (CAS 3351-86-8) Market 2020 Is Expected to See Magnificent Spike in CAGR with Global Industry Brief Analysis by Top Countries Data Which Includes Driving Factors by Manufacturers Growth and Forecast 2026. Press Release. Available online: https://www.marketwatch.com/press-release/fucoxanthin-cas-3351-86-8-market-2020-is-expected-to-see-magnificent-spike-in-cagr-with-global-industry-brief-analysis-by-top-countries-data-which-includes-driving-factors-by-manufacturers-growth-and-forecast-2026-2020-09-17 (accessed on 2 December 2020).
- Lycopene—Global Market Outlook (2017–2026). Research and Markets. Available online: https://www.researchandmarkets.com/reports/4765062/lycopene-global-market-outlook-2017-2026 (accessed on 1 December 2020).
- Saini, R.K.; Keum, Y.-S. Microbial Platforms to Produce Commercially Vital Carotenoids at Industrial Scale: An Updated Review of Critical Issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Global Carotenoids Market—Premium Insight, Competitive News Feed Analysis, Company Usability Profiles, Market Sizing & Forecasts to 2025. Available online: https://www.marketresearch.com/360iResearch-v4164/Global-Carotenoids-Premium-Insight-Competitive-13036149/ (accessed on 4 July 2020).
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Global Organic Pigments Market Is Segmented by Type (Azo Pigments, Phthalocyanine Pigments, High Performance Pigments (HPPs), Others), by Application (Printing Inks, Paints & Coatings, Plastics, Others (Textiles, Cosmetics, Food)), by Source (Natural Organic Pigments, Synthetic Organic Pigments), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa)—Share, Size, Outlook, and Opportunity Analysis, 2019–2026. Available online: https://www.datamintelligence.com/research-report/organic-pigments-market (accessed on 4 December 2020).
- Global Inorganic Pigments Market Is Driven by Rising Demand from End Users and Expanding Paint and Coating Industry. Data Bridge Market Research. Available online: https://www.databridgemarketresearch.com/news/global-inorganic-pigments-market (accessed on 4 December 2020).
- Global Metallic Pigments Market Is Driven by Prevailing Demand of Packaging Applications Mainly in the Food and Beverages, Tobacco Industry, Gifts Wraps among Others. Data Bridge Market Research. Available online: https://www.databridgemarketresearch.com/news/global-metallic-pigments-market (accessed on 5 December 2020).
- Global High-Performance Pigment Market Is Segmented by Type (Organic High-Performance Pigments, In-Organic High-Performance Pigments), by Application (Coatings, Plastics, Cosmetic Products, Others (Includes Inks)), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa)—Share, Size, Outlook, and Opportunity Analysis, 2019–2026. Available online: https://www.datamintelligence.com/research-report/high-performance-pigment-market (accessed on 5 December 2020).
- Global Phosphorescent Pigments Market—Industry Trends and Forecast to 2026. Data Bridge Market Research. Available online: https://www.databridgemarketresearch.com/reports/global-phosphorescent-pigments-market (accessed on 6 December 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
