Abstract
Research in Chlamydia trachomatis and Chlamydia pneumoniae has gained new traction due to recent advances in molecular biology, namely the widespread use of the metagenomic analysis and the development of a stable genomic transformation system, resulting in a better understanding of Chlamydia pathogenesis. C. trachomatis, the leading cause of bacterial sexually transmitted diseases, is responsible of cervicitis and urethritis, and C. pneumoniae, a widespread respiratory pathogen, has long been associated with several chronic inflammatory diseases with great impact on public health. The present review summarizes the current evidence regarding the complex interplay between C. trachomatis and host defense factors in the genital micro-environment as well as the key findings in chronic inflammatory diseases associated to C. pneumoniae.
1. Introduction
Currently, there is a renewed research interest in Chlamydiae that cause a broad spectrum of pathologies of varying severity in human, mainly Chlamydia trachomatis and Chlamydia pneumoniae [1,2]. Advances in molecular biology and, in particular, the recent advent of metagenomic analysis as well as the development of a stable genomic transformation system in Chlamydiae have significantly contributed to expanding our understanding of Chlamydia pathogenesis [3,4,5].
C. trachomatis is the leading cause of bacterial sexually transmitted diseases with 127 million new cases per year, according to the most recent World Health Organization estimates [6]. In fact, C. trachomatis is well known as common cause of cervicitis and urethritis; however, most genital infections in women are asymptomatic and if untreated can lead to severe reproductive sequelae including pelvic inflammatory disease, ectopic pregnancy, obstructive infertility as well as miscarriages and preterm birth [7,8]. Furthermore, C. trachomatis infection can also be transmitted to infants following the direct contact with infective cervical secretions during delivery, resulting in neonatal conjunctivitis and pneumonitis [1,7,8]. Lastly, there is evidence that C. trachomatis infection increases the risk of acquiring and transmitting human immunodeficiency virus by 3 to 4 times and, more recently, it has been associated with Human Papillomavirus related-cervical cancer [9,10].
C. pneumoniae is a widespread respiratory pathogen responsible for sinusitis, pharyngitis, and pneumonia and its transmission occurs via the aerial route [11]. A peculiar feature of C. pneumoniae is its ability to systematically disseminate from the lungs through peripheral blood mononuclear cells and to localize in several extra-pulmonary tissues including arteries, joints, bone and the central nervous system [12,13,14,15,16,17]. Indeed, C. pneumoniae has long been associated with several chronic inflammatory diseases with great impact on public health, mainly atherosclerosis, Alzheimer’s Disease, and inflammatory arthritis [17,18,19,20,21,22,23,24]. This is unsurprising since C. pneumoniae has been shown to multiply in all cell types involved in the pathogenesis of these conditions, including monocytes/macrophages, synovial cells, vascular endothelial and smooth muscle cells (VSMCs), microglial cells, astrocytes and neurons [17,22,23,24].
The present review summarizes the current evidence regarding the complex interplay between C. trachomatis and host defense factors in the genital micro-environment as well as the key findings in chronic inflammatory diseases associated to C. pneumoniae.
2. Chlamydiae Developmental Cycle
C. trachomatis and C. pneumoniae are Gram-negative obligate intracellular bacteria with a peculiar developmental cycle alternating between two morphologically and functionally distinct forms: the elementary body (EB) and the reticulate body (RB) [25]. The EB is the small (200 nm), extracellular infectious form, classically considered as metabolically inactive, although recent studies have shown that EBs maintain protein translation capabilities, whereas the RB is the large (800 nm), intracellular, metabolically active replicative form [25,26,27].
The developmental cycle begins when EBs attach and enter the host cell by endocytosis (Figure 1). It is thought that the interaction of EBs with the host cell occurs in a two-step process involving a reversible interaction mediated by heparin-sulphate proteoglycans followed by irreversible binding to a wide range of host receptors: mannose receptor, epidermal growth factor receptor, ephrin receptor A2, and β1 integrin [28,29]. Soon after the attachment to host cell, EBs are internalized and confined to a vacuole termed the inclusion, through a process requiring the secretion of Type III secretion system (T3SS) effector proteins (e.g., Incs), as well as other chlamydial proteins, like the chlamydia protease-like activity factor (CPAF) and the high temperature requirement A protein (HtrA) [28,29]. Chlamydial Incs, inserted into the inclusion membrane, allow the escape of EB endosome from the endocytic-lysosomal pathway [30,31]. CPAF, a serine protease, plays a role in maintaining the integrity of the inclusion and promotes virulence by interfering with several host antimicrobial pathways such as apoptosis and complement system [32,33]. Lastly, HtrA, a serine protease as well, has been recognized as a critical factor for intracellular survival of Chlamydiae [34].
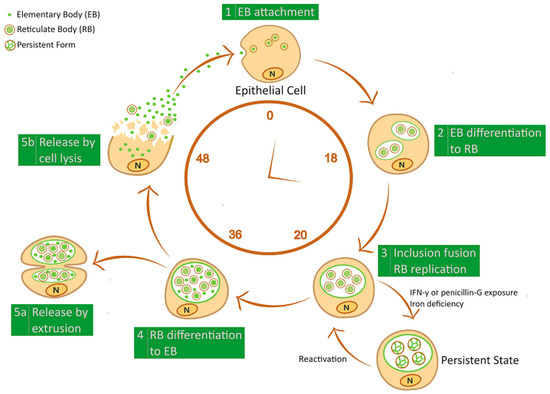
Figure 1.
Schematic representation of Chlamydiae developmental cycle. Infectious elementary body (EB) enters into the host-cell and transforms in the replicative reticulate body (RB); RB re-differentiates into EB, which is released from the host-cell by inclusion extrusion or cell lysis. Exposure to IFN-γ and penicillin G or iron depletion induce Chlamydiae to generate a non-infectious persistent form.
Within the inclusion, EBs then differentiate to RBs, which replicate by binary fission within 24 h post-infection and, as the inclusion expands, RBs begin to transition back to EBs in an asynchronous process. At the end of the developmental cycle, the inclusion occupies most of the host cell’s cytoplasm and, after approximately 48–72 h, the EBs are finally released from the host cell by inclusion extrusion or cell lysis. Thereafter, a multitude of infectious EBs spreads and infects neighboring epithelial cells, perpetuating the infectious process [25].
However, under stressful conditions, Chlamydiae halted the production of infectious EBs leading to viable but non-infectious forms characterized by continued synthesis of unprocessed 16S rRNA and genomic replication. These persistent forms are able to remain for a long-time in the host cell and are frequently associated with the presence of enlarged and morphologically aberrant RBs that retain their ability to resume the normal developmental cycle when the inducer is removed [35,36].
Several factors have been demonstrated to induce persistent forms via in vitro models including the exposure to interferon gamma (IFN)-γ or antibiotics (e.g., penicillin and amoxicillin), and nutrient deprivation (e.g., essential amino-acids or iron) [35,36,37,38,39,40]. Furthermore, it has been evidenced that coinfection with Herpes Simplex Virus type 2 or Toxoplasma gondii induces C. trachomatis persistent forms [36,41,42].
Importantly, these stress conditions may also occur in vivo [43,44] and, most notably, chlamydial persistence is supported by numerous observations of chlamydial aberrant forms in several tissues [45,46].
A relevant feature of chlamydial persistent form is its resistance to first line antibiotics towards Chlamydiae including and azithromycin [44,47]. This aspect alongside their ability to evade the host immune response may favor the long-term survival of Chlamydiae within tissues, resulting in a chronic inflammatory state and the subsequent tissue damage [48].
3. Genomic Modification Approaches in Chlamydiae
In the field of Chlamydia research, the insertion of exogenous DNA has always represented a big challenge [49,50] and, only recently, a reliable and robust transformation system has been developed for C. trachomatis [5], becoming the preferred technique for its recombination. This genomic transformation system has been utilized for the ectopic expression of reporter proteins conferring fluorescence to C. trachomatis, to either visualize live bacteria or investigate the localization of tagged-proteins during the chlamydial developmental cycle, like Incs [51,52]. At first, only promoters for the constitutive expression of target genes were used, then inducible promoter systems for conditional gene expression were developed, like the Tet System [53].
However, methods for the deletion or repression of a target gene, highly needed for investigating the molecular function of gene products, are still in development. Different approaches based on chemical mutagenesis or the TargeTron System were attempted [54,55,56,57], but both tools had important limitations. These were recently overcome, for the most part, by the development of a fluorescence-reported allelic exchange mutagenesis (FRAEM) system, through the engineering of a suicide vector by Mueller et al., 2016 [58], although, further studies will be necessary to optimize and validate these innovative techniques.
To be noted, these molecular tools were developed and applied to different strains of C. trachomatis, and, until recently, none of them were suitable for the genetic manipulation of C. pneumoniae. However, the C. pneumoniae plasmid shuttle vector, engineered by Shima et al. [59], enabled the generation of stable transformants in isolates of C. pneumoniae, providing the first tool for the transformation of this pathogen.
4. C. trachomatis Interaction with Host Defense Factors
The female genital tract is an ecological niche where several aerobe and anaerobe microorganisms coexist in a dynamic balance [4,5,60]. The homeostasis of the genital ecosystem results from complex interactions and synergies among the host and the resident microorganisms [3,4]. Changes in the structure and composition of this microbial ecosystem are influenced by several factors like age, menarche, pregnancy, infections, hormonal contraception, and sexual activity [61].
It is generally accepted that a healthy genital microbiota is typically dominated by Lactobacillus species, but other microorganisms, such as Staphylococcus, Ureaplasma, Corynebacterium, Streptococcus, Peptostreptococcus, Gardnerella, Bacteroides, Mycoplasma, Enterococcus, Escherichia, Veillonella, Bifidobacterium and Candida can be present in much lower amounts [4,5].
Eventually, the depletion of lactobacilli and the overgrowth of Gardnerella vaginalis or Candida spp. is known to lead to numerous clinical conditions, like bacterial vaginosis and candidiasis potentially associated to biofilm formation [62,63,64]. In our recent study, C. trachomatis was demonstrated to survive within the biofilm produced by Candida albicans or G. vaginalis, retaining its infectious properties [65]. This evidence is of clinical relevance since the biofilm, known as a protective niche, might favor C. trachomatis evasion of the host immune system and reduce its antibiotic susceptibility.
Lactobacillus spp. are the main host defense factor against pathogens, like C. trachomatis, within the cervico-vaginal ecosystem; in fact, they are able to limit the growth of genital pathogens through different mechanisms, such as competitive exclusion, anti-microbial compound production (lactic acid, hydrogen peroxide, defensins, etc.), the immune system activation as well as the maintenance of a low vaginal pH [66,67,68].
According to Gong et al. [69], lactic acid and, hence, a low pH, were demonstrated as essential for the anti-chlamydial activity of predominant Lactobacillus species in the cervico-vaginal microbiota. Since then, several studies reported the ability of different vaginal Lactobacillus strains such as Lactobacillus brevis or Lactobacillus crispatus to strongly inhibit early phases of C. trachomatis infection as well as its intracellular replication. In particular, several potential mechanisms interfering with C. trachomatis adhesion to host cell have been described, including the increased production of lactate and consumption of glucose, the co-aggregation with EBs, the changes in lipid composition of the cell membrane as well as the modulation of the α5 integrin subunit [70,71,72]. As a further defense mechanism, L. brevis has been demonstrated to inhibit the development of C. trachomatis persistent forms induced by HSV-2 coinfection [70]. Finally, Lactobacillus species may also protect the genital tract via immunomodulatory mechanisms. Specifically, in C. trachomatis-infected cervical epithelial cells and macrophages, L. crispatus has been shown to down-regulate the production of the cytokines frequently associated to tissue damage, like interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α, and, at the same time, to up-regulate IL-10 expression, an anti-inflammatory cytokine [73].
Alongside the resident lactobacilli, the female genital tract possesses other defense systems known to protect against C. trachomatis. Amongst them, lactoferrin, an 80-kDa multifunctional cationic glycoprotein belonging to the transferrin family, has acquired increasing interest for its marked anti-inflammatory and anti-chlamydial activities [74,75,76,77,78]. In fact, lactoferrin is released in the cervico-vaginal fluid by mucosal epithelial cells and neutrophils following C. trachomatis infection, as evidenced by higher levels of lactoferrin in infected rather than in healthy women [79,80,81].
Particularly interesting, in a clinical scenario, is the observation that the combination of lactoferrin and L. brevis is the most effective in inhibiting the early phases of C. trachomatis infection of cervical epithelial cells and in decreasing inflammatory cytokine synthesis, suggesting an additive effect of both host defense factors [77].
In addition to lactoferrin, other host defense peptides including defensins and cathelicidins, released in the cervico-vaginal fluid from genital epithelial cell and/or recruited neutrophils, have been demonstrated to inhibit C. trachomatis infection by inactivating EBs or by preventing their entry into the host cell as well as their intracellular growth [82,83,84,85]. However, it was recently demonstrated that cathelicidin LL-37 was degraded by the CPAF secreted by C. trachomatis [86]. Such an observation is of pathological significance since it describes one of the potential mechanisms by which C. trachomatis infection can spread into the upper genital tract and, hence, result in severe reproductive sequelae.
Genital Microbiota Characterization by Metagenomic Analysis
Over last few years, culture-independent high-resolution techniques based on the analysis of 16s ribosomal RNA gene sequences have contributed to expanding our knowledge on the composition of the genital microbiota, leading to its classification into five community state types (CSTs I-V) [4,87,88,89,90].
In healthy reproductive women, the cervico-vaginal mucosa is mostly populated by L. crispatus (CST I) and L. gasseri (CST II) dominated microbiota [4,5,68,89]. In fact, L. crispatus, as well as L. gasseri, are known to produce D-lactic acid, bacteriocins and other anti-microbial compounds that provide protection against genital pathogens [68,89]. By contrast, women with C. trachomatis or C. trachomatis/HPV coinfection possess a genital microbiota dominated by L. iners (CSTs III) or by different anaerobic bacterial species (CST-IV) [81,91,92,93,94,95,96,97,98,99]. On this regard, it has been demonstrated that some anaerobes, like Prevotella ssp., frequently observed in dysbiosis conditions, are able to produce indole allowing C. trachomatis to elude the IFN-γ-mediated host immune response [98,100,101]. At the same time, it is very likely that the latter generates chlamydial persistent forms which in presence of indole producing bacteria may revert to active developmental cycle resulting in recurrent infection.
More recently, for the first time, a specific cervical bacterial network including G. vaginalis, Prevotella amnii, Prevotella buccalis, Prevotella timonensis, Aerococcus christensenii and Variovorax guangxiensis has been proposed as a potential biomarker for C. trachomatis infection. This interesting data may add up valuable information to the ongoing research on the cervical microbiota associated to C. trachomatis infection and may help to identify women at risk of infection [81]. In the future, it will be important to perform longitudinal studies in order to monitor the temporal dynamics of the cervical microbiota during C. trachomatis infection.
5. C. pneumoniae and Chronic Inflammatory Diseases
Over the past decades, a growing number of studies have focused on the involvement of C. pneumoniae in chronic inflammatory diseases, mainly atherosclerotic cardiovascular diseases, Alzheimer’s Disease and reactive arthritis (ReA) [17,18,19,20,21,22,23,24]. Recently, the role of the infectious burden, including more infectious agents alongside C. pneumoniae, acquired importance as a novel view for the etiopathogenesis of these diseases [102,103,104].
5.1. Atherosclerotic Cardiovascular Diseases
Atherosclerotic cardiovascular disease (CVD) is the leading cause of death worldwide with over 17 million deaths per year [105] and the main pathological process underlying this disease is the atherosclerosis.
The relationship between C. pneumoniae and CVDs was been first suggested in 1988, by Saikku et al. [106]. Since then, accumulating evidence has supported the involvement of C. pneumoniae in the pathogenesis of CVDs, including seroepidemiological studies, the detection of C. pneumoniae DNA in the atherosclerotic plaque and the isolation of viable bacteria from the atheroma [17,107,108,109,110,111,112,113]. Stronger evidence came from in vivo studies demonstrating a causative role of C. pneumoniae in the pathogenesis of the atherosclerosis. Specifically, C. pneumoniae has been shown to promote endothelial dysfunction in normolipidemic and hyperlipidemic animal models as well as to accelerate the progression of atherosclerotic lesion in hyperlipidemic animals [17,114,115].
Particularly important are the experimental studies that highlighted the molecular mechanisms linking oxidative stress and inflammation to C. pneumoniae-mediated atherosclerosis. Indeed, numerous are the evidence demonstrating the ability of C. pneumoniae to induce oxidative stress and, hence, contribute to the early as well as the late stages of the atherosclerotic process by promoting endothelial dysfunction, foam cell formation and platelet activation [116,117]. Specifically, C. pneumoniae infection of vascular cells has been shown to upregulate multiple enzymatic systems capable of producing reactive oxygen species, including NADPH oxidase, lipoxygenase and cyclooxygenase as previously described [117]. Recently, C. pneumoniae has also been found to interfere with endothelial nitric oxide (NO) synthase impairing NO production and, hence, leading to vascular dysfunction [118].
Concerning inflammatory pathways, several studies have demonstrated the ability of C. pneumoniae to induce in macrophages, platelets, endothelial cells and VSMCs an increased production of pro-inflammatory cytokines and adhesion molecules, such as IL-6, IFN-γ, TNF-α, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, all responsible for the initiation, progression and destabilization of the atherosclerotic plaque [119,120].
More recently, further studies have highlighted that inflammatory and immune mechanisms activated by C. pneumoniae alongside dyslipidemia may play a role in the development and progression of the atherosclerotic plaque. For example, Turmurkhuu et al. [121] found that C. pneumoniae was able to activate Nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome with subsequent increase of IL-1β in macrophages resulting in accumulation of intracellular cholesterol and foam cell formation. Again, Chen et al. [122] observed that C. pneumoniae and lipids engaged the same innate immune signaling pathways (Toll Like receptor-4/myeloid differentiation primary response 88), accelerating the atherosclerotic process.
In addition to the vascular inflammation, there is also the evidence that C. pneumoniae may contribute to CVDs via the systemic inflammation [123]. However, despite the numerous evidence supporting the C. pneumoniae involvement in the pathogenesis of atherosclerosis, its causative role still needs to be assessed due to the failure of clinical antibiotic trials [124].
Of particular relevance is the recent observation that the infectious burden, including C. pneumoniae, may be involved in the development of atherosclerosis and the subsequent cardiovascular events. Potentially, an individual may be exposed to more microbial agents during his lifetime rather than to a single pathogen, since more than half of the world population is seropositive to, for example, C. pneumoniae, Helicobacter pylori or human cytomegalovirus [17,125,126]. Zhu et al. [127] were the first to show the association between a high risk of coronary artery disease and C. pneumoniae alongside other bacterial and/or viral pathogens, and, thereupon, further studies have contributed to strengthen this interesting hypothesis [102,128].
More importantly, the novel idea of a vascular microbiome involved in the development of atherosclerosis has been recently suggested by the detection of a microbiota in the atherosclerotic plaque, hypothesizing the oral cavity and/or the gut as the bacterial source [129,130,131,132].
5.2. Alzheimer’s Disease
Alzheimer’s Disease (AD) is an inflammatory brain disease that affects more 45 million people worldwide and is associated with a combination of genetic and environmental factors, leading to inflammation of the brain, neuronal cell death and progressive dementia [133].
The first evidence that C. pneumoniae may be involved in AD was reported in 1998 by Balin et al. [134] through the detection of C. pneumoniae in brain tissue from patients with late-onset dementia and, later on, by Dreses-Werringloer et al. [135] through the isolation of viable microorganism from post-mortem brain tissue samples of AD patients; since then, a growing number of studies have been published, although with controversial results. However, a recent meta-analysis study confirmed a positive association between C. pneumoniae and AD [20,24,136,137,138].
Further evidence on the involvement of C. pneumoniae in the pathogenesis of AD came from studies showing the ability of this pathogen to disseminate to the brain via the olfactory system or following a vascular infection and, hence, to induce or accelerate the formation of amyloid deposits, a pathological feature typically observed in AD patients [24,139,140,141].
Similar to C. pneumoniae-associated atherosclerosis, inflammation seems to play a role in AD pathogenesis as well. Indeed, it has been demonstrated the ability of C. pneumoniae to elicit the production of IL-6 and TNF-α, responsible for neuronal death, in microglial cells and astrocytes, and the synthesis of IL-1β and IL-8, known to elicit neurodegeneration in AD via the activation of nitric oxide synthase, in persistently infected monocytes [24].
Several other mechanisms by which C. pneumoniae may contribute to the development and progression of AD have also been documented, including the potential interaction with host genetic factors, namely the ApoEɛ4 isoform, a known risk factor for the development of late–onset dementia [24], and the inhibition of apoptosis in neuroblastoma cells, leading to a long-term infection [142].
More recently, the involvement of the infectious burden has also been proposed in the pathogenesis of AD by Bu et al. [104], through the detection of more viral and/or bacterial pathogens, including C. pneumoniae, in AD patients, evidencing a marked inflammatory state. Interestingly, it has been demonstrated that C. pneumoniae and other pathogens expressed proteins with marked homology to amyloid-β (Aβ) and amyloid precursor protein (APP), suggesting that infections may trigger autoantibodies that cross-reacted with membrane bound APP and caused synaptic and neuronal dysfunction and subsequent cognitive decline [104,143].
Despite all the evidence, in the only clinical trial, the antibiotic treatment had some beneficial effects on the cognitive symptoms in AD patients, but it was ineffective against C. pneumoniae [144].
5.3. Reactive Arthritis
Reactive arthritis (ReA), an inflammatory syndrome that arises during or soon after bacterial infections occurring elsewhere in the body and classically related to C. trachomatis, has also been associated to C. pneumoniae in the last two decades [21,22].
To date, evidence for C. pneumoniae involvement in the development of ReA is exclusively based on the detection of chlamydial nucleic acid from synovial fluid or tissue in patients with ReA [145,146,147,148,149,150,151,152,153,154]. Surprisingly, prospective epidemiological studies estimated a much lower occurrence of ReA following C. pneumoniae (2.2%) as compared to its seroprevalence in the general population (80–90%) [148,149,155]. Several factors may explain this apparent disconnection, including the spontaneous resolution of most ReA, the subtle presentation in women and the lack of standardized diagnostic criteria, leading to an underestimation of the impact of ReA following C. pneumoniae infection [22].
In the last decade, the coinfection of C. pneumoniae with C. trachomatis in the synovial fluid of ReA patients has also been evidenced, suggesting the possibility that C. pneumoniae might act synergistically with C. trachomatis in the etiopathogenesis of this disease via the development of chronic inflammation in the joint [156,157]. Thereupon, the hypothesis that the infectious burden, including C. pneumoniae, might be involved in the etiopathogenesis of ReA has acquired importance, suggesting the possibility that multiple infections acted synergistically, increasing the risk of developing ReA [152,154,157].
6. Conclusions and Future Prospective
Important advances in the molecular mechanisms of pathogenicity as well as in the interaction with the host have been achieved in the field of C. trachomatis and C. pneumoniae research, although much more remains to be done.
Concerning C. trachomatis, an interesting finding is the survival of this pathogen within biofilm generated by resident microorganisms of the genital ecosystem [65]; this novel evidence is worthy of further investigation since the biofilm is frequently found on intrauterine devices and may contribute to C. trachomatis transmission as well as dissemination to the upper genital tract [158,159].
Interestingly, the recent characterization of the cervico-vaginal microbiota associated to C. trachomatis infection as well as the anti-chlamydial activity of host defense peptides [77,84,99] will be helpful to develop novel prevention or treatment strategies. In this regard, of pathophysiological and clinical relevance will be the discovery of novel mechanisms underlying the anti-C. trachomatis activity of the host defense factors, like Lactobacillus spp. or lactoferrin. For example, it will be important to clarify how Lactobacillus spp. limited C. trachomatis intracellular replication or how lactoferrin impaired chlamydial invasion.
Differently from C. trachomatis, research on C. pneumoniae in the field of chronic inflammatory diseases did not undergo significant development, due to difficulties in isolating and culturing C. pneumoniae as well as to the multifactorial etiology of these pathological conditions. However, in recent times, the involvement of the infectious burden [102,103,104], including C. pneumoniae, in the etiopathogenesis of chronic inflammatory diseases opened the way to further approaches.
An important issue that remains to be solved for both C. pneumoniae and C. trachomatis is the persistence state into host cell. In fact, Chlamydiae persistence might be one explanation for the failure of clinical trials in C. pneumoniae-associated chronic inflammatory diseases as well as in C. trachomatis recurrent infections [132,160,161].
For years, research on Chlamydiae persistent forms has focused on identifying a distinct transcriptional and protein profile, such as the up-regulation or the down-regulation of the genes, involved in RB division and/or differentiation into EBs [35,162], as well as on the potential survival strategies, such as the production of membrane vesicles [163], an alternative protein delivery system in host cell. However, despite all the efforts, the identification of a common persistence marker during chlamydial infection is still missing.
In the future, the application of the recently engineered transformation system for the insertion of foreign DNA sequences in C. trachomatis and C. pneumoniae will contribute to expanding our knowledge on Chlamydiae pathogenesis. In particular, it will allow us to precisely characterize the temporal dynamics, role and functions of the genes expressed during the different phases of chlamydial developmental cycle, uncovering, for example, the elusive mechanisms underlying the generation of persistent forms. Furthermore, these molecular tools will be needed to decipher the function of essential genes involved in the host cell interaction, providing, for example, novel targets for the development of an effective Chlamydia vaccine.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mylonas, I. Female genital Chlamydia trachomatis infection: Where are we heading? Arch. Ginecol. Obstet. 2012, 285, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Choroszy-Król, I.; Frej-Mądrzak, M.; Hober, M.; Sarowska, J.; Jama-Kmiecik, A. Infections caused by Chlamydophila pneumoniae. Adv. Clin. Exp. Med. 2014, 23, 123–126. [Google Scholar] [CrossRef]
- Wang, Y.; Kahane, S.; Cutcliffe, L.T.; Skilton, R.J.; Lambden, P.R.; Clarke, I.N. Development of a transformation system for Chlamydia trachomatis: Restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011, 7, e1002258. [Google Scholar] [CrossRef] [PubMed]
- Van de Wijgert, J.H.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.M.; Bernstein, K.T.; Aral, S.O. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Shaw, K.; Coleman, D.; O’Sullivan, M.; Stephens, N. Public health policies and management strategies for genital Chlamydia trachomatis infection. Risk Manag. Healthc. Policy 2011, 4, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lanjouw, E.; Ouburg, S.; de Vries, H.J.; Stary, A.; Radcliffe, K.; Unemo, M. 2015 European guideline on the management of Chlamydia trachomatis infections. Int. J. STD AIDS 2016, 27, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Buckner, L.R.; Amedee, A.M.; Albritton, H.L.; Kozlowski, P.A.; Lacour, N.; McGowin, C.L.; Schust, D.J.; Quayle, A.J. Chlamydia trachomatis infection of endocervical epithelial cells enhances early HIV transmission events. PLoS ONE 2016, 11, e0146663. [Google Scholar] [CrossRef]
- Silva, J.; Cerqueira, F.; Medeiros, R. Chlamydia trachomatis infection: Implications for HPV status and cervical cancer. Arch. Gynecol. Obstet. 2014, 289, 715–723. [Google Scholar] [CrossRef]
- Grayston, J.T.; Aldous, M.B.; Easton, A.; Wang, S.P.; Kuo, C.C.; Campbell, L.A.; Altman, J. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J. Infect. Dis. 1993, 168, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Moazed, T.C.; Kuo, C.C.; Grayston, J.T.; Campbell, L.A. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J. Infect. Dis. 1998, 177, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, A.; Abramov, R.; Hammond, C.J.; Hudson, A.P.; Arking, E.J.; Little, C.S.; Appelt, D.M.; Balin, B.J. Chlamydia pneumoniae infection promotes the transmigration of monocytes through human brain endothelial cells. J. Neurosci. Res. 2003, 71, 740–750. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; Schiavoni, G.; Santino, I.; Benedetti-Valentini, F.; Perna, R.; Romano, S.; del Piano, M. Chlamydia pneumoniae DNA in patients with symptomatic carotid atherosclerotic disease. J. Vasc. Surg. 2003, 37, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; di Pietro, M.; Schiavoni, G.; Petrucca, A.; Cipriani, P.; Zagaglia, C.; Nicoletti, M.; Santino, I.; del Piano, M. Measurement of Chlamydia pneumoniae bacterial load in peripheral blood mononuclear cells may be helpful to assess the state of Chlamydial infection in patients with carotid atherosclerotic disease. Atherosclerosis 2007, 195, e224–e230. [Google Scholar] [CrossRef]
- Di Pietro, M.; Schiavoni, G.; Sessa, V.; Pallotta, F.; Costanzo, G.; Sessa, R. Chlamydia pneumoniae and osteoporosis-associated bone loss: A new risk factor? Osteoporos. Int. 2013, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Nicoletti, M.; Di Pietro, M.; Schiavoni, G.; Santino, I.; Zagaglia, C.; Del Piano, M.; Cipriani, P. Chlamydia pneumoniae and atherosclerosis: Current state and future prospectives. Int. J. Immunopathol. Pharmacol. 2009, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, G.; Di Pietro, M.; Ronco, C.; De Cal, M.; Cazzavillan, S.; Rassu, M.; Nicoletti, M.; Del Piano, M.; Sessa, R. Chlamydia pneumoniae infection as a risk factor for accelerated atherosclerosis in hemodialysis patients. J. Biol. Regul. Homeost. Agents. 2010, 24, 367–375. [Google Scholar]
- Campbell, L.A.; Rosenfeld, M.E. Infection and atherosclerosis development. Arch. Med. Res. 2015, 46, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Porritt, R.A.; Crother, T.R. Chlamydia pneumoniae infection and inflammatory diseases. Forum Immunopathol. Dis. Ther. 2016, 7, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, H.; Hudson, A.P. Causality of Chlamydiae in Arthritis and Spondyloarthritis: A Plea for Increased Translational Research. Curr. Rheumatol. Rep. 2016, 18, 9. [Google Scholar] [CrossRef]
- Carter, J.D.; Hudson, A.P. Recent advances and future directions in understanding and treating Chlamydia-induced reactive arthritis. Expert Rev. Clin. Immunol. 2017, 13, 197–206. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Falasca, F.; Turriziani, O.; Sessa, R. Infectious Agents in Atherosclerotic Cardiovascular Diseases through Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 2459. [Google Scholar] [CrossRef]
- Balin, B.J.; Hammond, C.J.; Little, C.S.; Hingley, S.T.; Al-Atrache, Z.; Appelt, D.M.; Whittum-Hudson, J.A.; Hudson, A.P. Chlamydia pneumoniae: An Etiologic Agent for Late-Onset Dementia. Front. Aging. Neurosci. 2018, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.M.; Belland, R.J. The Chlamydial developmental cycle. FEMS Microbiol. Rev. 2005, 29, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Omsland, A.; Sager, J.; Nair, V.; Sturdevant, D.E.; Hackstadt, T. Developmental stage-specific metabolic and transcriptional activity of Chlamydia trachomatis in an axenic medium. Proc. Natl. Acad. Sci. USA 2012, 109, 19781–19785. [Google Scholar] [CrossRef] [PubMed]
- Skipp, P.J.; Hughes, C.; McKenna, T.; Edwards, R.; Langridge, J.; Thomson, N.R.; Clarke, I.N. Quantitative Proteomics of the Infectious and Replicative Forms of Chlamydia trachomatis. PLoS ONE 2016, 11, e0149011. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial intracellular survival strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Dai, W.; Li, Z. Conserved type III secretion system exerts important roles in Chlamydia trachomatis. Int. J. Clin. Exp. Pathol. 2014, 7, 5404–5414. [Google Scholar]
- Nans, A.; Ford, C.; Hayward, R.D. Host-pathogen reorganisation during host cell entry by Chlamydia trachomatis. Microbes Infect. 2015, 17, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Bednar, M.M.; Amin, V.; Davis, B.K.; Ting, J.P.; McCafferty, D.G.; Valdivia, R.H. The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence. Cell Host Microbe 2011, 10, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, L.; Zhou, Z.; Zhong, G. Neutralizing anti-chlamydial activity of complement by Chlamydia-secreted protease CPAF. Microbes Infect. 2016, 18, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lei, L.; Gong, S.; Chen, D.; Flores, R.; Zhong, G. The chlamydial periplasmic stress response serine protease cHtrA is secreted into host cell cytosol. BMC Microbiol. 2011, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.J.; Mathews, S.A.; Mukhopadhyay, S.; Summersgill, J.T.; Timms, P. Chlamydial persistence: Beyond the biphasic paradigm. Infect. Immun. 2004, 72, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Wyrick, P.B. Chlamydia trachomatis persistence in vitro: An overview. J. Infect. Dis. 2010, 201, S88–S95. [Google Scholar] [CrossRef] [PubMed]
- Raulston, J.E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect. Immun. 1997, 65, 4539–4547. [Google Scholar] [PubMed]
- Di Pietro, M.; Tramonti, A.; de Santis, F.; de Biase, D.; Schiavoni, G.; Filardo, S.; Zagaglia, C.; Sessa, R. Analysis of gene expression in penicillin G induced persistence of Chlamydia pneumoniae. J. Biol. Regul. Homeost. Agents. 2012, 26, 277–284. [Google Scholar] [PubMed]
- Kintner, J.; Lajoie, D.; Hall, J.; Whittimore, J.; Schoborg, R.V. Commonly prescribed β-lactam antibiotics induce C. trachomatis persistence/stress in culture at physiologically relevant concentrations. Front. Cell. Infect. Microbiol. 2014, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef]
- Vanover, J.; Kintner, J.; Whittimore, J.; Schoborg, R.V. Interaction of herpes simplex virus type 2 (HSV-2) glycoprotein D with the host cell surface is sufficient to induce Chlamydia trachomatis persistence. Microbiology 2010, 156, 1294–1302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romano, J.D.; de Beaumont, C.; Carrasco, J.A.; Ehrenman, K.; Bavoil, P.M.; Coppens, I. A novel co-infection model with Toxoplasma and Chlamydia trachomatis highlights the importance of host cell manipulation for nutrient scavenging. Cell. Microbiol. 2013, 15, 619–646. [Google Scholar] [CrossRef] [PubMed]
- Phillips Campbell, R.; Kintner, J.; Whittimore, J.; Schoborg, R.V. Chlamydia muridarum enters a viable but non-infectious state in amoxicillin-treated BALB/c mice. Microbes Infect. 2012, 14, 1177–1185. [Google Scholar] [CrossRef][Green Version]
- Phillips-Campbell, R.; Kintner, J.; Schoborg, R.V. Induction of the Chlamydia muridarum stress/persistence response increases azithromycin treatment failure in a murine model of infection. Antimicrob. Agents. Chemother. 2014, 58, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Borel, N.; Summersgill, J.T.; Mukhopadhyay, S.; Miller, R.D.; Ramirez, J.A.; Pospischil, A. Evidence for persistent Chlamydia pneumoniae infection of human coronary atheromas. Atherosclerosis 2008, 199, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.E.; Belland, R.J.; AbdelRahman, Y.M.; Beatty, W.L.; Aiyar, A.A.; Zea, A.H.; Greene, S.J.; Marrero, L.; Buckner, L.R.; Tate, D.J.; et al. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front. Cell. Infect. Microbiol. 2014, 4, 71. [Google Scholar] [CrossRef]
- Wyrick, P.B.; Knight, S.T. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 2004, 54, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Choroszy-Król, I.C.; Frej-Mądrzak, M.; Jama-Kmiecik, A.; Bober, T.; Jolanta Sarowska, J.; Frej-Mądrzak, M.; Jama-Kmiecik, A.; Bober, T.; Jolanta Sarowska, J. Characteristics of the Chlamydia trachomatis species - immunopathology and infections. Adv. Clin. Exp. Med. 2012, 21, 799–808. [Google Scholar]
- Tam, J.E.; Davis, C.H.; Wyrick, P.B. Expression of recombinant DNA introduced into Chlamydia trachomatis by electroporation. Can. J. Microbiol. 1994, 40, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Binet, R.; Maurelli, A.T. The Chlamydial functional homolog of KsgA confers kasugamycin sensitivity to Chlamydia trachomatis and impacts bacterial fitness. BMC Microbiol. 2009, 9, 279. [Google Scholar] [CrossRef]
- Agaisse, H.; Derré, I. A C. trachomatis cloning vector and the generation of C. trachomatis strains expressing fluorescent proteins under the control of a C. trachomatis promoter. PLoS ONE 2013, 8, e57090. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Derré, I. A Co-infection Model System and the Use of Chimeric Proteins to Study Chlamydia Inclusion Proteins Interaction. Front. Cell Infect Microbiol. 2017, 7, 79. [Google Scholar] [CrossRef]
- Wickstrum, J.; Sammons, L.R.; Restivo, K.N.; Hefty, P.S. Conditional gene expression in Chlamydia trachomatis using the tet system. PLoS ONE 2013, 8, e76743. [Google Scholar] [CrossRef]
- Kari, L.; Goheen, M.M.; Randall, L.B.; Taylor, L.D.; Carlson, J.H.; Whitmire, W.M.; Virok, D.; Rajaram, K.; Endresz, V.; McClarty, G.; et al. Generation of targeted Chlamydia trachomatis null mutants. Proc. Natl. Acad. Sci. USA 2011, 108, 7189–7193. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Valdivia, R.H. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc. Natl. Acad. Sci. USA 2012, 109, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Fisher, D.J. Site-specific, insertional inactivation of incA in Chlamydia trachomatis using a group II intron. PLoS ONE 2013, 8, e83989. [Google Scholar] [CrossRef] [PubMed]
- Kokes, M.; Dunn, J.D.; Granek, J.A.; Nguyen, B.D.; Barker, J.R.; Valdivia, R.H.; Bastidas, R.J. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of Chlamydia. Cell Host Microbe 2015, 17, 716–725. [Google Scholar] [CrossRef]
- Mueller, K.E.; Wolf, K.; Fields, K.A. Gene Deletion by Fluorescence-Reported Allelic Exchange Mutagenesis in Chlamydia trachomatis. mBio 2016, 7, e01817-15. [Google Scholar] [CrossRef] [PubMed]
- Shima, K.; Wanker, M.; Skilton, R.J.; Cutcliffe, L.T.; Schnee, C.; Kohl, T.A.; Niemann, S.; Geijo, J.; Klinger, M.; Timms, P.; et al. The Genetic Transformation of Chlamydia pneumoniae. mSphere 2018, 3, e00412-18. [Google Scholar] [CrossRef] [PubMed]
- Mendling, W. Vaginal Microbiota. Adv. Exp. Med. Biol. 2016, 902, 83–93. [Google Scholar]
- Srinivasan, S.; Fredricks, D.N. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 750479. [Google Scholar] [CrossRef] [PubMed]
- Eckert, L.O. Clinical practice. Acute vulvovaginitis. N. Engl. J. Med. 2006, 355, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Cerca, N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Cauchie, M.; Desmet, S.; Lagrou, K. Candida and its dual lifestyle as a commensal and a pathogen. Res. Microbiol. 2017, 168, 802–810. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Tranquilli, G.; Sessa, R. Biofilm in Genital Ecosystem: A Potential Risk Factor for Chlamydia trachomatis Infection. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 1672109. [Google Scholar] [CrossRef] [PubMed]
- Mijac, V.D.; Dukić, S.V.; Opavski, N.Z.; Dukić, M.K.; Ranin, L.T.; Dukić, S.V.; Opavski, N.Z.; Dukić, M.K.; Ranin, L.T. Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 129, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Vielfort, K.; Sjölinder, H.; Roos, S.; Jonsson, H.; Aro, H. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect. 2008, 10, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef]
- Mastromarino, P.; Di Pietro, M.; Schiavoni, G.; Nardis, C.; Gentile, M.; Sessa, R. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int. J. Med. Microbiol. 2014, 304, 654–661. [Google Scholar] [CrossRef]
- Nardini, P.; Ñahui Palomino, R.A.; Parolin, C.; Laghi, L.; Foschi, C.; Cevenini, R.; Vitali, B.; Marangoni, A. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci. Rep. 2016, 6, 29024. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Frisco, G.; Foschi, C.; Giordani, B.; Salvo, M.; Vitali, B.; Marangoni, A.; Calonghi, N. Lactobacillus crispatus BC5 Interferes with Chlamydia trachomatis Infectivity Through Integrin Modulation in Cervical Cells. Front Microbiol. 2018, 9, 2630. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Fiorentino, M.; Buommino, E.; Donnarumma, G.; Losacco, A.; Bevilacqua, N. Lactobacillus crispatus mediates anti-inflammatory cytokine interleukin-10 induction in response to Chlamydia trachomatis infection in vitro. Int. J. Med. Microbiol. 2015, 305, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antiviral Res. 2003, 58, 209–215. [Google Scholar] [CrossRef]
- Naidu, A.S.; Chen, J.; Martinez, C.; Tulpinski, J.; Pal, B.K.; Fowler, R.S. Activated lactoferrin’s ability to inhibit Candida growth and block yeast adhesion to the vaginal epithelial monolayer. J. Reprod. Med. 2004, 49, 859–866. [Google Scholar] [PubMed]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. C. R. Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et., al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Sessa, R.; di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell. Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Otsuki, K.; Mitsukawa, K.; Yakuwa, K.; Nagatsuka, M.; Okai, T. Cervical inflammatory cytokines and other markers inthe cervical mucus of pregnant womenwith lower genital tract infection. Int. J. Gynaecol. Obstet. 2006, 92, 117–121. [Google Scholar] [CrossRef]
- Spear, G.T.; Kendrick, S.R.; Chen, H.Y.; Thomas, T.T.; Bahk, M.; Balderas, R.; Ghosh, S.; Aaron Weinberg, A.; Landay, A.L. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ß and lactoferrin. PLoS ONE 2011, 6, e19560. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Tranquilli, G.; Latino, M.A.; Recine, N.; Porpora, M.; Sessa, R. Selected immunological mediators and cervical microbial signatures in women with Chlamydia trachomatis infection. mSystems 2019. In press. [Google Scholar]
- Yasin, B.; Pang, M.; Lehrer, R.I.; Wagar, E.A. Activity of Novispirin G-10, a novel antimicrobial peptide against Chlamydia trachomatis and vaginosis-associated bacteria. Exp. Mol. Pathol. 2003, 74, 190–195. [Google Scholar] [CrossRef]
- Donati, M.; di Leo, K.; Benincasa, M.; Cavrini, F.; Accardo, S.; Moroni, A.; Gennaro, R.; Cevenini, R. Activity of cathelicidin peptides against Chlamydia spp. Antimicrob. Agents Chemother. 2005, 49, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Favaroni, A.; Donati, M. Host defense peptides: General overview and an update on their activity against Chlamydia spp. Expert Rev. Anti. Infect. Ther. 2013, 11, 1215–1224. [Google Scholar] [CrossRef]
- Noda-Nicolau, N.M.; Bastos, L.B.; Bolpetti, A.N.; Pinto, G.V.S.; Marcolino, L.D.; Marconi, C.; Ferreira, C.S.T.; Polettini, J.; Vieira, E.P.; da Silva, M.G. Cervicovaginal Levels of Human β-Defensin 1, 2, 3, and 4 of Reproductive-Aged Women With Chlamydia trachomatis Infection. J. Low Genit. Tract. Dis. 2017, 21, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, J.; Zhou, Z.; Yu, P.; Yang, Z.; Zhong, G. Chlamydia-secreted protease CPAF degrades host antimicrobial peptides. Microbes Infect. 2015, 17, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Ma, B.; Brotman, R.M.; Gajer, P.; Fadrosh, D.; Mahurkar, A.; White, O.; Terplan, M.; Bavoil, P.; Forney, L.J. Association between Chlamydia trachomatis genital infection and the vaginal microbiome. Sex. Transm. Infect. 2013, 89, A1–A428. [Google Scholar] [CrossRef][Green Version]
- Filardo, S.; di Pietro, M.; Porpora, M.G.; Recine, N.; Farcomeni, A.; Latino, M.A.; Sessa, R. Diversity of Cervical Microbiota in Asymptomatic Chlamydia trachomatis Genital Infection: A Pilot Study. Front. Cell. Infect. Microbiol. 2017, 7, 321. [Google Scholar] [CrossRef]
- van der Veer, C.; Bruisten, S.M.; van der Helm, J.J.; de Vries, H.J.; van Houdt, R. The cervicovaginal microbiota in women notified for Chlamydia trachomatis infection: A case-control study at the sexually transmitted infection outpatient clinic in Amsterdam, The Netherlands. Clin. Infect. Dis. 2017, 64, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Balle, C.; Lennard, K.; Dabee, S.; Barnabas, S.L.; Jaumdally, S.Z.; Gasper, M.A.; Maseko, V.; Mbulawa, Z.Z.A.; Williamson, A.L.; Bekker, L.G.; et al. Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection. Sci. Rep. 2018, 8, 11109. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Porpora, M.G.; Recine, N.; Latino, M.A.; Sessa, R. HPV/Chlamydia trachomatis co-infection: Metagenomic analysis of cervical microbiota in asymptomatic women. New Microbiol. 2018, 41, 34–41. [Google Scholar] [PubMed]
- Parolin, C.; Foschi, C.; Laghi, L.; Zhu, C.; Banzola, N.; Gaspari, V.; D’Antuono, A.; Giordani, B.; Severgnini, M.; Consolandi, C.; et al. Insights Into Vaginal Bacterial Communities and Metabolic Profiles of Chlamydia trachomatis Infection: Positioning Between Eubiosis and Dysbiosis. Front. Microbiol. 2018, 9, 600. [Google Scholar] [CrossRef]
- van Houdt, R.; Ma, B.; Bruisten, S.M.; Speksnijder, A.G.C.L.; Ravel, J.; de Vries, H.J.C. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: A case-control study. Sex. Transm. Infect. 2018, 94, 117–123. [Google Scholar] [CrossRef]
- Ziklo, N.; Vidgen, M.E.; Taing, K.; Huston, W.M.; Timms, P. Dysbiosis of the Vaginal Microbiota and Higher Vaginal Kynurenine/Tryptophan Ratio Reveals an Association with Chlamydia trachomatis Genital Infections. Front. Cell. Infect. Microbiol. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Tamarelle, J.; Thiébaut, A.C.M.; de Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 35–47. [Google Scholar] [CrossRef]
- Aiyar, A.; Quayle, A.J.; Buckner, L.R.; Sherchand, S.P.; Chang, T.L.; Zea, A.H.; Martin, D.H.; Belland, R.J. Influence of the tryptophan-indole-IFNγ axis on human genital Chlamydia trachomatis infection: Role of vaginal co-infections. Front. Cell. Infect. Microbiol. 2014, 4, 72. [Google Scholar] [CrossRef]
- Ziklo, N.; Huston, W.M.; Hocking, J.S.; Timms, P. Chlamydia trachomatis genital tract infections: When host immune response and the microbiome collide. Trends Microbiol. 2016, 24, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; di Pietro, M.; Filardo, S.; Turriziani, O. Infectious burden and atherosclerosis: A clinical issue. World J. Clin. Cases. 2014, 2, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Strelić, N.; Bojović, J.; Pavlica, L.; Cikota-Aleksić, B.; Miličić, B.; Magić, Z. Detection of bacteria and analyses of Chlamydia trachomatis viability in patients with postvenereal reactive arthritis. Intern. Med. J. 2014, 44, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Yao, X.Q.; Jiao, S.S.; Zeng, F.; Liu, Y.H.; Xiang, Y.; Liang, C.R.; Wang, Q.H.; Wang, X.; Cao, H.Y.; et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol. 2015, 22, 1519–1525. [Google Scholar] [CrossRef]
- Mendis, S.; Puska, P.; Norrving, B.; World Health Organization, World Heart Federation. Global Atlas on Cardiovascular Disease Prevention and Control. Available online: http://whqlibdoc.who.int/publications/2011/9789241564373_eng.pdf (accessed on 15 May 2019).
- Saikku, P.; Leinonen, M.; Mattila, K.; Ekman, M.R.; Nieminen, M.S.; Mäkelä, P.H.; Huttunen, J.K.; Valtonen, V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 1988, 2, 983–986. [Google Scholar] [CrossRef]
- Ramirez, J.A. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. The Chlamydia pneumoniae/Atherosclerosis Study Group. Ann. Intern. Med. 1996, 125, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Kuo, C.C.; Rodriguez, D.I.; Lee, A.; Grayston, J.T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J. Infect. Dis. 1997, 176, 292–295. [Google Scholar]
- Apfalter, P.; Loidl, M.; Nadrchal, R.; Makristathis, A.; Rotter, M.; Bergmann, M.; Polterauer, P.; Hirschl, A.M. Isolation and continuous growth of Chlamydia pneumoniae from arterectomy specimens. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 305–308. [Google Scholar] [CrossRef]
- Romano, S.; Penco, M.; Fratini, S.; Di Pietro, M.; Sessa, R.; del Piano, M.; Fedele, F.; Dagianti, A. Chlamydia pneumoniae infection is associated with coronary artery disease but not implicated in inducing plaque instability. Int. J. Cardiol. 2004, 95, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Schiavoni, G.; Galdiero, M.; Cipriani, P.; Romano, S.; Zagaglia, C.; Santino, I.; Faccilongo, S.; Del Piano, M. Chlamydia pneumoniae in asymptomatic carotid atherosclerosis. Int. J. Immunopathol. Pharmacol. 2006, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Alp, N.J. Role of Chlamydia pneumoniae in atherosclerosis. Clin. Sci. 2008, 114, 509–531. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Khandelwal, B.; Joshi, D.; Gupta, O.P. Chlamydophila pneumoniae infection and cardiovascular disease. N. Am. J. Med. Sci. 2013, 5, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Liuba, P.; Pesonen, E.; Paakkari, I.; Batra, S.; Forslid, A.; Kovanen, P.; Pentikäinen, M.; Persson, K.; Sandström, S. Acute Chlamydia pneumoniae infection causes coronary endothelial dysfunction in pigs. Atherosclerosis 2000, 167, 215–222. [Google Scholar] [CrossRef]
- De Kruif, M.D.; van Gorp, E.C.; Keller, T.T.; Ossewaarde, J.M.; ten Cate, H. Chlamydia pneumoniae infections in mouse models: Relevance for atherosclerosis research. Cardiovasc. Res. 2005, 65, 317–327. [Google Scholar] [CrossRef]
- Di Pietro, M.; de Santis, F.; Schiavoni, G.; Filardo, S.; Sessa, R. Resveratrol in Chlamydia pneumoniae-induced foam cell formation and interleukin-17A synthesis. J. Biol. Regul. Homeost. Agents. 2013, 27, 509–518. [Google Scholar] [PubMed]
- Di Pietro, M.; Filardo, S.; de Santis, F.; Mastromarino, P.; Sessa, R. Chlamydia pneumoniae and oxidative stress in cardiovascular disease: State of the art and prevention strategies. Int. J. Mol. Sci. 2014, 16, 724–735. [Google Scholar] [CrossRef]
- Mueller, K.E.; Wolf, K.C. pneumoniae disrupts eNOS trafficking and impairs NO production in human aortic endothelial cells. Cell. Microbiol. 2015, 17, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.A. Growth in vascular cells and cytokine production by Chlamydia pneumoniae. J. Infect. Dis. 2000, 181, S473–S478. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; de Santis, F.; Sessa, R. Chlamydia pneumoniae infection in atherosclerotic lesion development through oxidative stress: A brief overview. Int. J. Mol. Sci. 2013, 14, 15105–15120. [Google Scholar] [CrossRef]
- Tumurkhuu, G.; Dagvadorj, J.; Porritt, R.A.; Crother, T.R.; Shimada, K.; Tarling, E.J.; Erbay, E.; Arditi, M.; Chen, S. Chlamydia pneumoniae hijacks a host autoregulatory IL-1β loop to drive foam cell formation and accelerate atherosclerosis. Cell Metab. 2018, 28, 432–448.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shimada, K.; Crother, T.R.; Erbay, E.; Shah, P.K.; Arditi, M. Chlamydia and lipids engage a common sigOfnaling pathway that promotes atherogenesis. J. Am. Coll. Cardiol. 2018, 71, 1553–1570. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Farcomeni, A.; Schiavoni, G.; Sessa, R. Chlamydia pneumoniae-mediated inflammation in atherosclerosis: A metaanalysis. Mediators Inflamm. 2015, 2015, 378658. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Rosenfeld, M.E. Persistent C. pneumoniae infection in atherosclerotic lesions: Rethinking the clinical trials. Front. Cell. Infect. Microbiol. 2014, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Sotuneh, N.; Hosseini, S.R.; Shokri-Shirvani, J.; Bijani, A.; Ghadimi, R. Helicobacter pylori infection and metabolic parameters: Is there an association in elderly population? Int. J. Prev. Med. 2014, 5, 1537–1542. [Google Scholar] [PubMed]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 31, e2034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Quyyumi, A.A.; Norman, J.E.; Csako, G.; Waclawiw, M.A.; Shearer, G.M.; Epstein, S.E. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am. J. Cardiol. 2000, 85, 140–146. [Google Scholar] [CrossRef]
- Budzyński, J.; Wiśniewska, J.; Marek Ciecierski, M.; Anna Kędzia, A. Association between bacterial infection and peripheral vascular disease: A review. Int. J. Angiol. 2016, 25, 3–13. [Google Scholar]
- Filardo, S.; di Pietro, M.; Schiavoni, G.; Minniti, G.; Ortolani, E.; Romano, S.; Sessa, R. Chlamydia pneumoniae Clinical Isolate from Gingival Crevicular Fluid: A Potential Atherogenic Strain. Front. Cell. Infect. Microbiol. 2015, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Drautz-Moses, D.I.; Alhede, M.; Maw, M.T.; Liu, Y.; Purbojati, R.W.; Yap, Z.H.; Kushwaha, K.K.; Gheorghe, A.G.; Bjarnsholt, T.; et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015, 3, 38. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Hållenius, F.F.; Akrami, R.; Johansson, E.; Wester, P.; Arnerlöv, C.; Bäckhed, F.; Bergström, G. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 2017, 263, 177–183. [Google Scholar] [CrossRef]
- Ascher, S.; Reinhardt, C. The gut microbiota: An emerging risk factor for cardiovascular and cerebrovascular disease. Eur. J. Immunol. 2018, 48, 564–575. [Google Scholar] [CrossRef]
- Wisniewski, T.; Goñi, F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron 2015, 85, 1162–1176. [Google Scholar] [CrossRef]
- Balin, B.J.; Gérard, H.C.; Arking, E.J.; Appelt, D.M.; Branigan, P.J.; Abrams, J.T.; Whittum-Hudson, J.A.; Hudson, A.P. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med. Microbiol. Immunol. 1998, 187, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Dreses-Werringloer, U.; Gérard, H.C.; Whittum-Hudson, J.; Hudson, A.P. Chlamydophila (Chlamydia) pneumoniae infection of human astrocytes and microglia in culture displays an active, rather than a persistent, phenotype. Am. J. Med. Sci. 2006, 332, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Schiavoni, G.; Borriello, G.; Zagaglia, C.; Marinelli, F.; del Piano, M.; Pozzilli, C. Real time PCR for detection of Chlamydophila pneumoniae in peripheral blood mononuclear cells of patients with multiple sclerosis. J. Neurol. 2007, 254, 1293–1295. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.J.; Hallock, L.R.; Howanski, R.J.; Appelt, D.M.; Little, C.S.; Balin, B.J. Immunohistological detection of Chlamydia pneumoniae in the Alzheimer’s disease brain. BMC Neurosci. 2010, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P.; Eslick, G.D. Bacterial infection and Alzheimer’s disease: A meta-analysis. J. Alzheimers Dis. 2015, 43, 957–966. [Google Scholar] [CrossRef]
- Little, C.S.; Hammond, C.J.; MacIntyre, A.; Balin, B.J.; Appelt, D.M. Chlamydia pneumoniae induces alzheimer-like amyloid plaques in brains of BALB/C mice. Neurobiol. Aging 2004, 25, 419–429. [Google Scholar] [CrossRef]
- Little, C.S.; Joyce, T.A.; Hammond, C.J.; Matta, H.; Cahn, D.; Appelt, D.M.; Balin, B.J. Detection of bacterial antigens and Alzheimer’s disease-like pathology in the central nervous system of BALB/C mice following intranasal infection with a laboratory isolate of Chlamydia pneumoniae. Front. Aging Neurosci. 2014, 6, 304. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Cazzavillan, S.; Segala, C.; Bevilacqua, P.; Bonoldi, E.; D’Amore, E.S.; Rassu, M.; Sessa, R. Could past Chlamydial vascular infection promote the dissemination of Chlamydia pneumoniae to the brain? J. Biol. Regul. Homeost. Agents. 2013, 27, 155–164. [Google Scholar]
- Appelt, D.M.; Roupas, M.; Hammond, C.J.; Little, C.S.; Balin, B.J. Apoptotic effects of Chlamydophila pneumoniae following infection of neuronal cells. BMC Neurosci. 2008, 9, 13–23. [Google Scholar] [CrossRef]
- Carter, C. Alzheimer’s disease: APP, gamma secretase, APOE, CLU, CR1, PICALM, ABCA7, BIN1, CD2AP, CD33, EPHA1, and MS4A2, and their relationships with herpes simplex, C. pneumoniae, other suspect pathogens, and the immune system. Int. J. Alzheimers Dis. 2011, 2011, 501862. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M.B.; Molloy, D.; Smieja, M.; Standish, T.; Goldsmith, C.H.; Mahony, J.; Smith, S.; Borrie, M.; Decoteau, E.; Davidson, W. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J. Am. Geriatr. Soc. 2004, 52, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Beaudreuil, J.; Hayem, G.; Meyer, O.; Kahn, M.F. Reactive arthritis ascribed to Chlamydia pneumoniae. Report of a case. Rev. Rhum. Engl. Ed. 1995, 62, 224. [Google Scholar] [PubMed]
- Moling, O.; Pegoretti, S.; Rielli, M.; Rimenti, G.; Vedovelli, C.; Pristerá, R.; Mian, P. Chlamydia pneumoniae--reactive arthritis and persistent infection. Br. J. Rheumatol. 1996, 35, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Hannu, T.; Puolakkainen, M.; Leirisalo-Repo, M. Chlamydia pneumoniae as a triggering infection in reactive arthritis. Rheumatology 1999, 38, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Melby, K.K.; Kvien, T.K.; Glennås, A.; Anestad, G. Chlamydia pneumoniae as a trigger of reactive arthritis. Scand. J. Infect. Dis. 1999, 31, 327–328. [Google Scholar] [PubMed]
- Schumacher, H.R., Jr.; Gérard, H.C.; Arayssi, T.K.; Pando, J.A.; Branigan, P.J.; Saaibi, D.L.; Hudson, A.P. Lower prevalence of Chlamydia pneumoniae DNA compared with Chlamydia trachomatis DNA in synovial tissue of arthritis patients. Arthritis Rheum. 1999, 42, 1889–1893. [Google Scholar] [CrossRef]
- Gérard, H.C.; Schumacher, H.R.; El-Gabalawy, H.; Goldbach-Mansky, R.; Hudson, A.P. Chlamydia pneumoniae present in the human synovium are viable and metabolically active. Microb. Pathog. 2000, 29, 17–24. [Google Scholar]
- Cascina, A.; Marone Bianco, A.; Mangiarotti, P.; Montecucco, C.M.; Meloni, F. Cutaneous vasculitis and reactive arthritis following respiratory infection due to Chlamydia pneumoniae: Report of a case. Clin. Exp. Rheumatol. 2002, 20, 845–847. [Google Scholar]
- Carter, J.D.; Gérard, H.C.; Espinoza, L.R.; Ricca, L.R.; Valeriano, J.; Snelgrove, J.; Oszust, C.; Vasey, F.B.; Hudson, A.P. Chlamydiae as etiologic agents in chronic undifferentiated spondylarthritis. Arthritis Rheum. 2009, 60, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Grilli, A.; Badia, L.; Guardigni, V.; Govoni, M.; Seraceni, S. Detection of Chlamydophila pneumoniae in patients with arthritis: Significance and diagnostic value. Rheumatol Int. 2011, 31, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Domenico, M.D.; Carratelli, C.R.; Paolillo, R. The role of Chlamydia and Chlamydophila infections in reactive arthritis. Intern Med. 2012, 51, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kvien, T.K.; Glennås, A.; Melby, K.; Granfors, K.; Andrup, O.; Karstensen, B.; Thoen, J.E. Reactive arthritis: Incidence, triggering agents and clinical presentation. J. Rheumatol. 1994, 21, 115–122. [Google Scholar]
- Telyatnikova, N.; Hill Gaston, J.S. Prior exposure to infection with Chlamydia pneumoniae can influence the T-cell-mediated response to Chlamydia trachomatis. FEMS Immunol. Med. Microbiol. 2006, 47, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Bojović, J.; Strelić, N.; Pavlica, L. Reiter’s syndrome—Disease of young men—Analysis of 312 patients. Med. Pregl. 2014, 67, 222–230. [Google Scholar] [CrossRef]
- Pal, Z.; Urbán, E.; Dósa, E.; Pál, A.; Nagy, E. Biofilm formation on intrauterine devices in relation to duration of use. J. Med. Microbiol. 2005, 54, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Auler, M.E.; Morreira, D.; Rodrigues, F.F.; Abr Ao, M.S.; Margarido, P.F.; Matsumoto, F.E.; Silva, E.G.; Silva, B.C.; Schneider, R.P.; Paula, C.R. Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis. Med. Mycol. 2010, 48, 211–216. [Google Scholar] [CrossRef]
- Dean, D.; Suchland, R.J.; Stamm, W.E. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J. Infect. Dis. 2000, 182, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Suchland, R.J.; Dimond, Z.E.; Putman, T.E.; Rockey, D.D. Demonstration of persistent infections and genome stability by whole-genome sequencing of repeat-positive, same-serovar Chlamydia trachomatis collected from the female genital tract. J. Infect. Dis. 2017, 215, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; De Santis, F.; Sessa, R. New insights into Chlamydiae persistence: An energy metabolism strategy? Int. J. Immunopathol. Pharmacol. 2013, 26, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, K.M.; Hua, Z.; Quayle, A.J.; Wang, J.; Lewis, M.E.; Chou, C.W.; Luo, M.; Buckner, L.R.; Shen, L. Membrane vesicle production by Chlamydia trachomatis as an adaptive response. Front. Cell. Infect. Microbiol. 2014, 4, 73. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).