Optimization of Growth Conditions of Desulfovibrio desulfuricans Strain REO-01 and Evaluation of Its Cd(II) Bioremediation Potential for Detoxification of Rare Earth Tailings
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Source and Cultivation
2.2. Biochemical Characterization of Strain REO-01
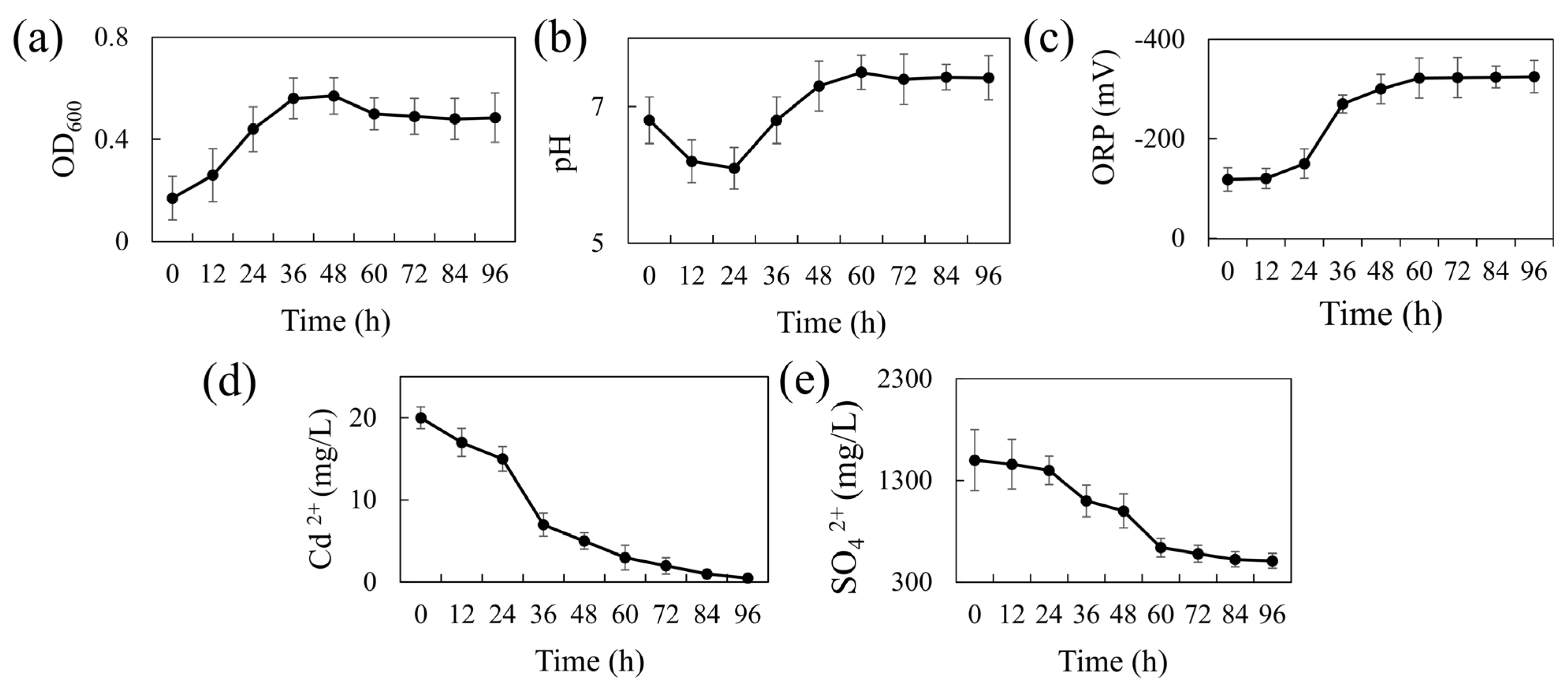
2.3. Growth and Metabolic Dynamics of Strain REO-01
2.4. Optimizing Environmental Conditions for Enhanced Growth and Remediation Efficiency of Strain REO-01
2.5. Comparative Effects of Sodium Lactate, Glycerol, and Ethanol on the Sulfate Reduction Performance of Strain REO-01
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biochemical Characteristics and Bioremediation Capacity of REO-01
3.2. Growth Response of Strain REO-01 to Environmental Factors
3.3. Performance Evaluation of Lactate, Glycerol, and Ethanol in Sulfate-Reducing Bioremediation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, C.; Li, L.; Yang, Y. Readsorption of rare earth elements during leaching process of ion-adsorption-type rare earth ore. Rare Met. 2023, 42, 2113–2120. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Yi, Y. Effect of sulfate erosion on strength and leaching characteristic of stabilized heavy metal contaminated red clay. Trans. Nonferrous Met. Soc. China 2017, 27, 666–675. [Google Scholar] [CrossRef]
- Vizcaíno, R.L.; Yustres, A.; Asensio, L.; Saez, C.; Cañizares, P.; Rodrigo, M.; Navarro, V. Enhanced electrokinetic remediation of polluted soils by anolyte pH conditioning. Chemosphere 2018, 199, 477–485. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Dong, B.; Xu, Z. Use of sludge stabilization products for remediation of heavy metal (loid)s-contaminated mine tailings: Physicochemical, biochemical and microbial mechanisms. Chem. Eng. J. 2024, 488, 150640. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- Wang, F.; Peng, S.; Fan, L.; Li, Y. Improved sulfate reduction efficiency of sulfate-reducing bacteria in sulfate-rich systems by acclimatization and multiple-grouting. Alex. Eng. J. 2022, 61, 9993–10005. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Y.; Zhang, Z.; Tang, Y.; Su, P.; Lin, Z. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. J. Clean. Prod. 2022, 376, 134109. [Google Scholar] [CrossRef]
- Marietou, A.; Røy, H.; Jørgensen, B.B.; Kjeldsen, K.U. Sulfate Transporters in Dissimilatory Sulfate Reducing Microorganisms: A Comparative Genomics Analysis. Front. Microbiol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Rodrigues, C.; Núñez-Gómez, D.; Silveira, D.D.; Lapolli, F.R.; Lobo-Recio, M.A. Chitin as a substrate for the biostimulation of sulfate-reducing bacteria in the treatment of mine-impacted water (MIW). J. Hazard. Mater. 2019, 375, 330–338. [Google Scholar] [CrossRef]
- Salgar-Chaparro, S.J.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Microbiologically influenced corrosion as a function of environmental conditions: A laboratory study using oilfield multispecies biofilms. Corros. Sci. 2020, 169, 108595. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, W.; Wang, J. Simultaneous occurrence of sulfate reduction and nitrate reduction in solid-phase denitrification system. Chem. Eng. J. 2025, 507, 160570. [Google Scholar] [CrossRef]
- Barton, L.L.; Fauque, G.D. Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv. Appl. Microbiol. 2009, 68, 41–98. [Google Scholar] [PubMed]
- Liu, X.Z.; Zhang, L.M.; Prosser, J.I.; He, J.Z. Abundance and community structure of sulfate reducing prokaryotes in a paddy soil of southern China under different fertilization regimes. Soil Biol. Biochem 2009, 41, 687–694. [Google Scholar] [CrossRef]
- Ollivier, B.; Cayol, J.L.; Fauque, G. Sulphate-reducing bacteria from oil fields environments and deep-sea hydrothermal vents. In Sulpate-Reducing Bacteria: Environmental and Engineered Systems; Cambridge University Press: Cambridge, UK, 2007; pp. 305–328. [Google Scholar]
- Lazar, C.S.; Dinasquet, J.; L’Haridon, S.; Pignet, P.; Toffin, L. Distribution of anaerobic methane-oxidizing and sulfate-reducing communities in the G11 Nyegga pockmark, Norwegian Sea. Antonie Van Leeuwenhoek 2011, 100, 639–653. [Google Scholar] [CrossRef]
- Bao, P.; Hu, Z.; Wang, X.; Chen, J.; Ba, Y.; Hua, J.; Zhu, C.; Zhong, M.; Wu, C. Dechlorination of p,p′-DDTs coupled with sulfate reduction by novel sulfate-reducing bacterium Clostridium sp. BXM. Environ. Pollut. 2011, 162, 303–310. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.; Han, F.X. Microbially Induced Carbonate Precipitation Techniques for the Remediation of Heavy Metal and Trace Element–Polluted Soils and Water. Water Air Soil. Pollut. 2021, 232, 268. [Google Scholar] [CrossRef]
- Jiang, Y.; Di, J. Study on the joint treatment of acidic mine wastewater by sulfate reducing bacteria and sulfide oxidizing bacteria under different systems. Process Saf. Environ. Prot. 2025, 198, 107078. [Google Scholar] [CrossRef]
- Liu, H.; Yao, J.; Shi, C.; Liu, D.R.J.; Jiang, S.; Li, M.; Pang, W.; Ma, B.; Cao, Y.; Geoffrey, S. Sulfate-reducing consortium HQ23 stabilizes metal(loid)s and activates biological N-fixation in mixed heavy metal-contaminated soil. Sci. Total Environ. 2024, 946, 174402. [Google Scholar] [CrossRef]
- Tuppurainen, K.; Väisänen, A.; Rintala, J. Zinc removal in anaerobic sulphate-reducing liquid substrate process. Miner. Eng. 2002, 15, 847–852. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, F.; Dai, W.; Wei, C. Variation of sulfate reducing bacteria communities in ionic rare earth tailings and the potential of a single cadmium resistant strain in bioremediation. Chemosphere 2023, 328, 138615. [Google Scholar] [CrossRef]
- Sophia, L.; Jiang, A.K.; Grant, M.R.; Gabriela, A.; Glory, M.N.; Jiang, X.; Brantley, H. Convergent evolution of oxidized sugar metabolism in commensal and pathogenic microbes in the inflamed gut. Nat. Commun. 2025, 16, 1121. [Google Scholar]
- Lucelia, C.; Persinoti, G.F.; Paixão, D.A.A.; Martins, M.P.; Morais, M.A.B.; Mariana, C.; Domingues, M.N.; Sforca, M.L.; Pirolla, R.A.S.; Generoso, W.C.; et al. Gut microbiome of the largest living rodent harbors unprecedented enzymatic systems to degrade plant polysaccharides. Nat. Commun. 2022, 13, 629. [Google Scholar]
- Emma, W.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Use of untargeted metabolomics for assessing soil quality and microbial function. Soil Biol. Biochem. 2020, 143, 107758. [Google Scholar]
- Benavides, M.; Salm, S.; Thompson, C.; Zaitsev, I. Microbiology: A Laboratory Manual; Morton Publishing Company: Englewood, CO, USA, 2016. [Google Scholar]
- Huang, L. Simulation and evaluation of different statistical functions for describing lag time distributions of a bacterial growth curve. Microb. Risk Anal. 2016, 1, 47–55. [Google Scholar] [CrossRef]
- Bölscher, T.; Wadsö, L.; Börjesson, G.; Herrmann, A.M. Differences in substrate use efficiency: Impacts of microbial community composition, land use management, and substrate complexity. Biol. Fertil. Soils 2016, 52, 547–559. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2019, 18, 35–46. [Google Scholar] [CrossRef]
- Fishman, K.S.; Akimov, V.N.; Suzina, N.E.; Vainshtein, M.B.; Liang, X. Sulfate-reducing bacteria Desulfobulbus sp. strain BH from a freshwater lake in Guizhou Province, China. Inland Water Biol. 2013, 6, 13–17. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Puhakka, J.A. Sulfate Reduction Based Bioprocesses for the Treatment of Acid Mine Drainage and the Recovery of Metals. Eng. Life Sci. 2007, 6, 541–564. [Google Scholar] [CrossRef]
- Lin, H.; Shi, J.; Chen, X.; Yang, J.; Chen, Y.; Zhao, Y.; Hu, T. Effects of lead upon the actions of sulfate-reducing bacteria in the rice rhizosphere. Soil Biol. Biochem. 2010, 42, 1038–1044. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Yoo, C.G.; Pu, Y.; Meng, X.; Muchero, W.; Tuskan, G.A.; Tschaplinski, T.; Ragauskas, A.J. Understanding the influences of different pretreatments on recalcitrance of Populus natural variants. Bioresour. Technol. 2018, 265, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kumari, D.; Huang, M.; Achal, V. Biosynthesis of CdS nanoparticles through microbial induced calcite precipitation. Mater. Des. 2016, 98, 209–214. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.; Mu, Z.; Wang, H.; Yu, H.; Li, Y.; Harada, H. Selection of effective methods for extracting extracellular polymeric substances (EPSs) from Bacillus megaterium TF10. Sep. Purif. Technol. 2012, 95, 216–221. [Google Scholar] [CrossRef]
- Cheng, G.; Ding, H.; Chen, G.; Shi, H.; Zhang, X.; Zhu, M.; Tan, W. Effects of cadmium sulfide nanoparticles on sulfate bioreduction and oxidative stress in Desulfovibrio desulfuricans. Bioresour. Bioprocess. 2022, 9, 35. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, F.; Gao, Y.; Mao, X.; Hu, X. Sequestration of nanoparticles by an EPS matrix reduces the particle-specific bactericidal activity. Sci. Rep. 2016, 6, 21379. [Google Scholar] [CrossRef]
- Liang, J.; He, Q.; Zhao, Y.; Yuan, Y.; Wang, Z.; Gao, Z.; Hu, Z.; Zhao, X.; Yue, T. Synthesis of sulfhydryl modified bacterial cellulose gel membrane and its application in adsorption of patulin from apple juice. LWT-Food Sci. Technol. 2022, 158, 113159. [Google Scholar] [CrossRef]
- Boyanov, M.; Kelly, S.; Kemner, K.; Bunker, B.; Fein, J.; Fowle, D. Adsorption of cadmium to Bacillus subtilis bacterial cell walls: A pH-dependent X-ray absorption fine structure spectroscopy study. Geochim. Cosmochim. Acta 2003, 67, 3299–3311. [Google Scholar] [CrossRef]
- Parmar, P.; Shukla, A.; Goswami, D.; Gaur, S.; Patel, B.; Saraf, M. Comprehensive depiction of novel heavy metal tolerant and EPS producing bioluminescent Vibrio alginolyticus PBR1 and V. rotiferianus PBL1 confined from marine organisms. Microbiol. Res. 2020, 238, 126526. [Google Scholar] [CrossRef]
- McMahon, S.; Anderson, R.; Saupe, E.; Briggs, D. Experimental evidence that clay inhibits bacterial decomposers: Implications for preservation of organic fossils. Geology 2016, 44, 867–870. [Google Scholar] [CrossRef]
- Luo, H.; Fu, S.; Liu, G.; Zhang, R.; Bai, Y.; Luo, X. Autotrophic biocathode for high efficient sulfate reduction in microbial electrolysis cells. Bioresour. Technol. 2014, 167, 462–468. [Google Scholar] [CrossRef]
- Teng, W.; Liu, G.; Luo, H.; Zhang, R.; Xiang, Y. Simultaneous sulfate and zinc removal from acid wastewater using an acidophilic and autotrophic biocathode. J. Hazard. Mater. 2015, 304, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Girkin, N.T.; Lopes dos Santos, R.A.; Vane, C.H.; Ostle, N.; Turner, B.L.; Sjögersten, S. Peat Properties, Dominant Vegetation Type and Microbial Community Structure in a Tropical Peatland. Wetlands 2020, 40, 1367–1377. [Google Scholar] [CrossRef]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef]
- Sharma, K.; Derlon, N.; Hu, S.; Yuan, Z. Modeling the pH effect on sulfidogenesis in anaerobic sewer biofilm. Water Res. 2013, 49, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kikot, P.; Viera, M.; Mignone, C.; Donati, E. Study of the effect of pH and dissolved heavy metals on the growth of sulfate-reducing bacteria by a fractional factorial design. Hydrometallurgy 2010, 104, 494–500. [Google Scholar] [CrossRef]
- Koschorreck, M. Microbial sulphate reduction at a low pH. FEMS Microbiol. Ecol. 2008, 64, 329–342. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Meier, J.; Piva, A.; Fortin, D. Enrichment of sulfate-reducing bacteria and resulting mineral formation in media mimicking pore water metal ion concentrations and pH conditions of acidic pit lakes. FEMS Microbiol. Ecol. 2011, 79, 69–84. [Google Scholar] [CrossRef]
- Zhu, N.; Tang, J.; Tang, C.; Duan, P.; Yao, L.; Wu, Y.; Dionysiou, D.D. Combined CdS nanoparticles-assisted photocatalysis and periphytic biological processes for nitrate removal. Chem. Eng. J. 2018, 353, 237–245. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, K.; Jiang, Z.; Wang, J.; Yu, J.C.; Wong, P.K. Biohybrid photoheterotrophic metabolism for significant enhancement of biological nitrogen fixation in pure microbial cultures. Energy Environ. Sci. 2019, 12, 2185–2191. [Google Scholar] [CrossRef]
- Dutta, R.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Villa-Gomez, D.K.; Cassidy, J.; Keesman, K.J.; Sampaio, R.; Lens, P.N.L. Sulfide response analysis for sulfide control using a pS electrode in sulfate reducing bioreactors. Water Res. 2013, 50, 48–58. [Google Scholar] [CrossRef]
- Fernández-Palacios, E.; Lafuente, J.; Mora, M.; Gabriel, D. Exploring the performance limits of a sulfidogenic UASB during the long-term use of crude glycerol as electron donor. Sci. Total Environ. 2019, 688, 184–1192. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, G.; Mao, Z.S.; Li, Y.; Fang, Z.; Yang, C. Influence of electron donors on the growth and activity of sulfate-reducing bacteria. Int. J. Miner. Process. 2012, 106–109, 58–64. [Google Scholar] [CrossRef]
- Guo, W.; Wen, Y.; Chen, Y.; Zhou, Q. Sulfur cycle as an electron mediator between carbon and nitrate in a constructed wetland microcosm. Front. Environ. Sci. Eng. 2020, 14, 57. [Google Scholar] [CrossRef]
- Zhou, J.; Liang, X.; Luo, J.; Chen, W.; Liu, J. Effects of sulfate on activated sludge characteristics and membrane fouling in membrane bioreactor treating penicillin wastewater. J. Water Process. Eng. 2020, 38, 101594. [Google Scholar] [CrossRef]
- Kousi, P.; Remoundaki, E.; Hatzikioseyian, A.; Battaglia-Brunet, F.; Joulian, C.; Kousteni, V.; Tsezos, M. Metal precipitation in an ethanol-fed, fixed-bed sulphate-reducing bioreactor. J. Hazard. Mater. 2011, 189, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Nevatalo, L.M.; Mäkinen, A.E.; Kaksonen, A.H.; Puhakka, J.A. Biological hydrogen sulfide production in an ethanol-lactate fed fluidized-bed bioreactor. Bioresour. Technol. 2009, 101, 276–284. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Viggi, C.C.; Cibati, A.; Uccelletti, D.; Toro, L.; Palleschi, C. Biotreatment of Cr(VI) contaminated waters by sulphate reducing bacteria fed with ethanol. J. Hazard. Mater. 2011, 199–200, 186–192. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Plumb, J.J.; Franzmann, P.D.; Puhakka, J.A. Simple organic electron donors support diverse sulfate-reducing communities in fluidized-bed reactors treating acidic metal- and sulfate-containing wastewater. FEMS Microbiol. Ecol. 2004, 47, 279–289. [Google Scholar] [CrossRef]
- Sahinkaya, E. Biotreatment of zinc-containing wastewater in a sulfidogenic CSTR: Performance and artificial neural network (ANN) modelling studies. J. Hazard. Mater. 2008, 164, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.K.; Jho, E.H. Heavy metal and sulfate removal from sulfate-rich synthetic mine drainages using sulfate reducing bacteria. Sci. Total Environ. 2018, 635, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K.; Huang, J. ORP-based oxygenation for sulfide control in anaerobic treatment of high-sulfate wastewater. Water Res. 2003, 37, 2053–2062. [Google Scholar] [CrossRef]
- van den Brand, T.P.H.; Roest, K.; Chen, G.H.; Brdjanovic, D.; van Loosdrecht, M.C.M. Occurrence and activity of sulphate reducing bacteria in aerobic activated sludge systems. World J. Microbiol. Biotechnol. 2015, 31, 507–516. [Google Scholar] [CrossRef]
- Liamleam, W.; Annachhatre, A.P. Electron donors for biological sulfate reduction. Biotechnol. Adv. 2007, 25, 452–463. [Google Scholar] [CrossRef]
- Nauhaus, K.; Albrecht, M.; Elvert, M.; Boetius, A.; Widdel, F. In vitro cell growth of marine archaeal-bacterial consortia during anaerobic oxidation of methane with sulfate. Environ. Microbiol. 2007, 9, 187–196. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Franzmann, P.D.; Puhakka, J.A. Performance and ethanol oxidation kinetics of a sulfate-reducing fluidized-bed reactor treating acidic metal-containing wastewater. Biodegradation 2003, 14, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wiessner, A.; Rahman, K.Z.; Kuschk, P.; Kästner, M.; Jechorek, M. Dynamics of sulphur compounds in horizontal sub-surface flow laboratory-scale constructed wetlands treating artificial sewage. Water Res. 2010, 44, 6175–6185. [Google Scholar] [CrossRef]
- Hoehler, T.M.; Jørgensen, B.B. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 2013, 11, 83–94. [Google Scholar] [CrossRef]





| Component | Concentration (g/L) | Component | Concentration (g/L) |
|---|---|---|---|
| KH2PO4 | 0.5 | Sodium lactate (60%) | 4 |
| NH4Cl | 1.0 | Yeast extract | 1.0 |
| CaCl2·4H2O | 0.1 | FeSO4·7H2O | 0.5 |
| Na2SO4 | 0.5 | Ascorbic acid | 0.1 |
| MgSO4·7H2O | 2.0 | — | — |
| CSR | Carbon Source Addition (g/L) | ||
|---|---|---|---|
| Sodium Lactate | Glycerin | Ethanol | |
| 0.67 | 2.34 (L1) | 0.49 (G1) | 0.82 (E1) |
| 1.32 | 4.67 (L2) | 0.98 (G2) | 1.64 (E2) |
| 2.01 | 7.01 (L3) | 1.46 (G3) | 2.47 (E3) |
| 2.68 | 9.35 (L4) | 1.95 (G4) | 3.29 (E4) |
| Test | REO-01 Result |
|---|---|
| Hydrogen sulfide | + |
| Indole | + |
| Catalase | + |
| N-acetylglucosamine | + |
| Oxidase | − |
| Gram stain | − |
| Urease | − |
| Methyl red | − |
| Sodium pyruvate | + |
| Glycerol | + |
| Tartarate | + |
| Acetate | + |
| Glucose | − |
| Mannitol | − |
| Arabinose | − |
| Sucrose | − |
| Malonate | − |
| Fructose | − |
| Nitrate reduction | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wei, C.; Yang, F. Optimization of Growth Conditions of Desulfovibrio desulfuricans Strain REO-01 and Evaluation of Its Cd(II) Bioremediation Potential for Detoxification of Rare Earth Tailings. Microorganisms 2025, 13, 1511. https://doi.org/10.3390/microorganisms13071511
Zhang P, Wei C, Yang F. Optimization of Growth Conditions of Desulfovibrio desulfuricans Strain REO-01 and Evaluation of Its Cd(II) Bioremediation Potential for Detoxification of Rare Earth Tailings. Microorganisms. 2025; 13(7):1511. https://doi.org/10.3390/microorganisms13071511
Chicago/Turabian StyleZhang, Ping, Chaoyang Wei, and Fen Yang. 2025. "Optimization of Growth Conditions of Desulfovibrio desulfuricans Strain REO-01 and Evaluation of Its Cd(II) Bioremediation Potential for Detoxification of Rare Earth Tailings" Microorganisms 13, no. 7: 1511. https://doi.org/10.3390/microorganisms13071511
APA StyleZhang, P., Wei, C., & Yang, F. (2025). Optimization of Growth Conditions of Desulfovibrio desulfuricans Strain REO-01 and Evaluation of Its Cd(II) Bioremediation Potential for Detoxification of Rare Earth Tailings. Microorganisms, 13(7), 1511. https://doi.org/10.3390/microorganisms13071511







