Abstract
This study examined the seropositivity of T. gondii and coinfections with other TORCH pathogens among pregnant women attending 17 Basic Health Units (UBS) in Araçatuba, SP, Brazil. Of the 711 pregnant women seen at these UBS, only 297 were tested for T. gondii. Of the women tested for T. gondii (n = 297), 26.9% had IgG antibodies, 6.7% had IgM, and 32.0% tested positive for either or both. Only 1.4% showed both IgG and IgM antibodies, while 67.7% were non-reactive. The seropositivity was 17.1% for syphilis, 63.2% for rubella, 0.9% for hepatitis C, 0.9% for dengue, 17.9% for COVID-19, and 0.9% for herpes simplex (types 1/2). Coinfections with syphilis, rubella, and herpes simplex were also noted. Higher education levels appeared to protect against T. gondii seropositivity. The findings highlight a significant prevalence of T. gondii among pregnant women, with variation across UBSs, pointing to socioeconomic, behavioral, and environmental factors as influential. We also observed co-occurrence with other infections, such as syphilis, rubella, and herpes simplex. The study underscores the need for targeted public health interventions to reduce the risks of congenital infections.
1. Introduction
Toxoplasmosis is caused by the protozoan Toxoplasma gondii and is part of the TORCH complex, a group of infections that can lead to serious congenital problems when acquired during pregnancy [1,2]. TORCH complex infections include toxoplasmosis (TOX), others (syphilis, hepatitis, varicella-zoster, HIV), rubella (RV), cytomegalovirus (CMV), and herpes simplex (HSV-1/HSV-2) [3].
Infection by T. gondii generally occurs through the ingestion of sporulated oocysts, eliminated by cats, which are its definitive hosts, contaminating the environment, food, or water. Consumption of raw or undercooked meat containing parasite cysts is another common route of infection. Typically, the infection is asymptomatic and self-limiting [4]. This protozoan can infect virtually all warm-blooded animals, including humans [5].
During pregnancy, the infection is particularly serious, as it can be transmitted vertically to the fetus, resulting in congenital toxoplasmosis [6]. The initial infection in humans is often subclinical, but the parasite can persist in the body in a latent form, reactivating in situations of immunosuppression [7].
Congenital toxoplasmosis occurs when the infection is transmitted from the mother to the fetus through the placenta. The consequences of this infection can vary depending on the timing of transmission during pregnancy [8,9]. Infections early in pregnancy tend to result in more severe consequences, such as spontaneous abortion, fetal death, or serious neurological damage to the fetus, including hydrocephalus, intracranial calcifications, and chorioretinitis, which can lead to blindness. Infections acquired later in pregnancy may result in milder or even asymptomatic conditions at birth, with clinical manifestations appearing months or years later, such as hearing problems and delays in neuropsychomotor development [10,11,12,13,14,15].
In developed countries like the U.S.A., about 10% of pregnant women who are infected exhibit symptoms, which are often nonspecific [7]. In contrast, the prevalence of toxoplasmosis among pregnant women in Brazil ranges from 50% to 80% [16]. A meta-analysis estimated a pooled seroprevalence of T. gondii infection among pregnant women at 40.0% (95% CI, 37.0–44.0%), highlighting a significant level of exposure in this demographic group [17]. According to the meta-analysis by Bigna et al. [18], the global IgM seroprevalence of T. gondii infection was 1.9% (95% CI: 1.7–2.3), and the global IgG seroprevalence was 32.9% (95% CI: 29.4–36.4). Among the WHO regions, the Americas had the highest IgG seroprevalence at 45.2% (95% CI: 33.4–53.4), indicating a significant exposure level in this region.
Coinfection with other TORCH complex pathogens, such as syphilis, hepatitis C, dengue virus, COVID-19, rubella, CMV, and herpes simplex, poses additional risks for pregnant women and their fetuses [19,20]. These infections can lead to a range of congenital anomalies and complications, making it imperative to understand their combined impact. Comprehensive epidemiological analyses are necessary to inform public health interventions and develop specific strategies to reduce the incidence and impact of these congenital infections [2].
This study aimed to investigate the seropositivity of T. gondii and the rates of coinfection with other TORCH complex pathogens among pregnant women in Araçatuba, SP, Brazil. By analyzing serological data and examining associated risk factors, it is expected to provide relevant information about these infections and contribute to the development of effective public health strategies to reduce the morbidity and mortality associated with congenital infections.
2. Materials and Methods
2.1. Design and Study Area
This cross-sectional, descriptive, and quantitative study was conducted after approval by the Human Research Ethics Committee of the São Paulo State University (UNESP), under Opinion N° 2.625.160. The research was carried out in the municipality of Araçatuba, located in the northwest of the State of São Paulo (21°12′32′′ S, 50°25′58′′ W), at an altitude of 380 m above sea level, supplied by the Tietê and Ribeirão Baguaçu rivers, and characterized by a semi-humid tropical climate. In 2022, the municipality’s population was estimated at 200,124 inhabitants, with an infant mortality rate of 13.91 deaths per thousand live births.
2.2. Context of Primary Health Care
The municipality has a basic health care network consisting of 20 service units, with 17 located in urban areas and 3 in rural areas. Three of these units do not provide prenatal care and instead refer pregnant women to the nearest facilities. For this study, 17 units were selected, including 16 in urban areas and 1 in a rural area.
2.3. Data Collection
The analysis was carried out in partnership with the Municipal Health Department (SMS) to obtain data on pregnant women treated at Basic Health Units (BHU). In total, 711 pregnant women were treated at the 17 selected BHUs from February 2022 to March 2023. The distribution of pregnant women per unit was as follows: BHU 0 (43), BHU 1 (35), BHU 2 (21), BHU 3 (76), BHU 4 (35), BHU 5 (35), BHU 6 (27), BHU 7 (26), BHU 8 (70), BHU 9 (30), BHU 10 (20), BHU 11 (51), BHU 12 (122), BHU 13 (34), BHU 14 (40), BHU 15 (51), BHU 16 (26), and BHU 17 (4).
Weekly visits were made to the BHU, determined by neighborhoods, for in-person data collection. In this way, the collection was comprehensive and thorough, which was essential for detailed analysis and for preparing the study.
2.4. Data Collection Instrument
Epidemiological data were collected through a transcription of medical records used by health units, using all the information necessary for analysis. The information collected included race, education, basic sanitation conditions, marital status, pregnancy planning and acceptance, high risk and reason for high risk, and IgG and IgM results for T. gondii, syphilis, rubella, COVID-19, and any other TORCH complex disease. To safeguard participant identities, we implemented stringent anonymization procedures. Each participant was assigned a unique code, and all personally identifiable information was either removed or encrypted in the datasets. Additionally, access to the data was strictly limited to authorized personnel, ensuring confidentiality and adherence to ethical standards.
2.5. Laboratory Analyses
The laboratory tests were carried out at the Mahatma Gandhi Clinical Analysis Laboratory, located in Araçatuba, which maintains a partnership with the Municipal Government of Araçatuba. All examinations followed the protocols established by the Ministry of Health [21].
The Microparticle Chemiluminescent Immunoassay (CMIA) system was used to detect antibodies of T. gondii (IgM/IgG) and syphilis (Abbott Ireland, Diagnostics Division, Sligo, Ireland), following the manufacturer’s instructions. Confirmation of syphilis in non-treponemal reactive results was confirmed with a treponemal test, the fluorescent treponemal antibody absorption assay (FTA-ABS), which detects serum antibodies specific for Treponema pallidum (WAMA Diagnostica, São Paulo, Brazil).
Electrochemiluminescence (ECLIA) was used for the diagnosis of rubella (IgM/IgG) and hepatitis C (IgG/IgM) (Roche Diagnostics, Switzerland), while chemiluminescence (CLIA) was used for the detection of herpes simplex type 1 and 2 (IgM/IgG) using kits from Euroimmun (Lübeck, Germany). The diagnostic methods used for dengue and COVID-19 were, respectively, NS1 antigen test and rapid antigen test (RT-Ag) (WAMA Diagnostica, São Paulo, Brazil). All analyses were performed strictly in accordance with the manufacturers’ instructions.
2.6. Data Analysis
Statistical tests were performed using STATA/SE software (version 16.1; Stata Corp LLC, College Station, TX, USA). We used descriptive statistics to characterize the demographic variables that were described in terms of percentages, standard errors, and 95% confidence intervals. Data collected from 711 pregnant women in 17 BHUs were analyzed to determine seropositivity of T. gondii and rates of co-infection with TORCH pathogens in pregnant women.
For inferential statistics, the dependent variable was seropositivity for IgG and/or IgM against T. gondii, while the independent variables included education, race, basic sanitation, pregnancy planning, pregnancy acceptance, and high-risk pregnancy [22]. To explore possible interactions between independent variables and assess whether the effect of one variable on T. gondii seropositivity is modified by another variable, we conducted binary logistic regression analyses with interaction terms. Due to the overrepresentation of urban health units in our study, with only one unit located in a rural area, a meaningful comparison of T. gondii seropositivity between urban and rural areas was not feasible. A p-value less than 0.05 was considered statistically significant.
The georeferencing (Figure 1) of the visited BHUs was performed using a GPS device (GPS Garmin eTrex 30). The data were then transported to the QGIS desktop program (3.26.3) [23], where they were placed on the map of Araçatuba/SP. The database modeling and graph plotting steps were performed at the Animal Parasitology and Zoonoses Laboratory of the Institute of Agricultural Sciences, Federal University of the Jequitinhonha and Mucuri Valleys.
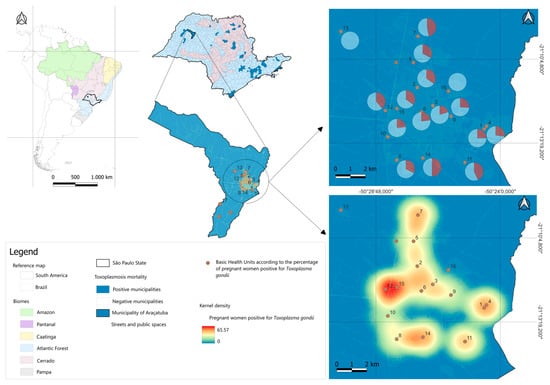
Figure 1.
Distribution of toxoplasmosis seropositivity in pregnant women in Basic Health Units in Araçatuba, SP.
3. Results
Table 1 shows the distribution of pregnant women attended prenatally and the seropositivity of T. gondii in 17 Basic Health Units (BHU) in Araçatuba, SP, Brazil.

Table 1.
Number of pregnant women attended prenatally and tested positive for T. gondii in 17 Basic Health Units (BHU) monitored in Araçatuba/SP, Brazil.
The data presented in this table indicate that, among the 711 pregnant women attended to in 17 BHUs in Araçatuba/SP, only 297 showed results for T. gondii.
Among the 297 pregnant women evaluated, 80 (26.9%) had IgG antibodies to T. gondii, indicating previous exposure to the parasite. Additionally, 20 pregnant women (6.7%) were identified with IgM antibodies to T. gondii. In the combined analysis of IgG or IgM, 95 of the pregnant women (32.0%) were positive for one or both types of antibodies. Simultaneous detection of IgG and IgM occurred in only 4 (1.4%) pregnant women, and 201 (67.7%) pregnant women presented non-reactive IgG and IgM serology, revealing themselves to be susceptible to acquiring the infection.
Data on T. gondii seropositivity among pregnant women in the BHUs of Araçatuba, SP, show variability, which can be attributed to socioeconomic, behavioral and environmental factors. While BUH 13, together with BHU 16 and BHU 17, did not register positive cases among the pregnant women treated, other units presented high infection rates. BHUs 14 and 7 stood out with the highest rates, reaching 50% and 46.7%, respectively, followed by BHU 11 with 42.9% and BHU 12 with 39.4%.
This data can be viewed in detail in Figure 1. The map geographically represents the seropositivity of toxoplasmosis among pregnant women treated at 17 Basic Health Units (BHU) in Araçatuba, São Paulo.
Table 2 shows the demographic characteristics of pregnant women seropositive for T. gondii who attended prenatal care in 17 BHUs in Araçatuba, SP, Brazil.

Table 2.
Demographic characteristics of pregnant women seropositive for T. gondii receiving prenatal care at 17 Basic Health Units (BHU) in Araçatuba/SP, Brazil.
The average age of the participants was 26.2 years. Regarding the level of education, the largest percentage of women (39.4%) completed high school, while 27.6% of women did not respond about their education. Regarding ethnicity, most women did not respond (58.3%), but among those who responded, 23.6% were white and 14.5% were brown.
In terms of basic sanitation, 38.4% considered themselves to have access to adequate sanitation, but 61.3% did not answer this question. Regarding pregnancy planning, 40.7% of women said they did not plan the pregnancy, 26.3% planned it, and 33.0% did not respond. Acceptance of pregnancy shows that 61.6% of women accepted the pregnancy, while 3.0% did not, and 35.4% did not respond. Finally, 25.9% of women were in a high-risk pregnancy, while the majority (74.0%) were not high-risk.
Table 3 shows the univariate logistic regression of T. gondii seropositivity according to the predictors evaluated in pregnant women in Araçatuba/SP.

Table 3.
Univariate logistic regression of T. gondii seropositivity according to risk factors assessed in pregnant women in Araçatuba/SP.
The education variable has an odds ratio of 0.78, which suggests that increases in the level of education are associated with a decrease in the probability of being seropositive for T. gondii. The 95% confidence interval for this odds ratio ranges from 0.62 to 0.98, and the p-value of 0.033 indicates that this effect is statistically significant at the 5% level.
This model suggests that there is no significant difference in seropositivity for T. gondii (IgG/IgM) among the other variables analyzed, such as race, basic sanitation, women with planned pregnancies, acceptance of pregnancy, and presence of high risk.
Table 4 presents the seropositivity of the diseases analyzed and correlations between the presence of antibodies for toxoplasmosis (IgG/IgM) and other infectious diseases in pregnant women, using Spearman’s correlation coefficient (rho) and the respective p-values to assess the statistical significance of these correlations among pregnant women who received prenatal care in 17 Basic Health Units (BHU) in Araçatuba/SP, Brazil.

Table 4.
Seropositivity of the diseases analyzed and Spearman’s correlation coefficient (rho) among pregnant women from 17 Basic Health Units (BHU) in Araçatuba/SP, Brazil.
The data reveal that, of the 711 women monitored, 297 underwent tests to detect T. gondii antibodies (IgG/IgM), while only 117 were evaluated for other diseases such as syphilis, rubella, hepatitis C, dengue virus, COVID-19, and herpes simplex.
Of these, rubella showed the highest seroprevalence, with 63.2% (74/117), followed by COVID-19 with 17.9% (21 of 117). Seropositivity for T. gondii shows that 32.0% of the pregnant women evaluated were seropositive for IgG/IgM. Notably, some diseases show co-occurrence with toxoplasmosis. The non-significant correlations with toxoplasmosis indicate that, despite some weak associations, there is no strong evidence that the presence of antibodies to toxoplasmosis is directly related to other infectious diseases in the sample studied.
Data analysis revealed a significant negative correlation between rubella and syphilis seropositivity in pregnant women, with a Spearman coefficient (rho) of −0.5387 and a significantly low p value (p < 0.001). This result suggests that pregnant women who are seropositive for rubella tend to be less likely to be seropositive for syphilis.
4. Discussion
The prevalence of toxoplasmosis in pregnant women varies considerably between different regions of Brazil, as indicated by several studies. This study identified a prevalence of anti-T. gondii antibodies (IgG or IgM) of around 32.0%, which is lower than the seropositivity reported in the North region, city of Gurupi, TO, with a high prevalence (IgG or IgM) of 68.4% (333/487) among pregnant women [16].
The prevalence of maternal chronic infection (IgG) was 26.9% (80/297); similar results were described by Pereira et al. [24] in Presidente Prudente/SP, with 24.6% (69/280), and by Ferreira et al. [25] with 20.9% (29/139), in Campina Grande/PB. Higher prevalence rates were reported by Figueiró–Filho et al. [26], with 91.6% (29,781/32,512) in the state of Mato Grosso do Sul; by Areal and Miranda [27], with 73.5% (847/1,153) in Vitória/ES; with Gontijo da Silva, Clare Vinaud, and de Castro [16], who reported a prevalence of 63.0% (307/487) in Gurupi/TO; by Castilho–Pelloso et al. [28], with 65.2% (10,882/16,686) in the northwest of the state of Paraná; by Santos et al. [29], with 62.5% (125/280) in Rio Grande/RS; and by Freitas et al. [30], with 59.0% (210/356) in Jaçanã/RN.
Although such variation may be related to the technique used for IgG detection, dietary habits and water sources may play a role in transmission, as previously reported [31].
Infection of pregnant women was diagnosed in 20 (6.7%) 265 cases during the first trimester of gestational age by detecting IgM antibodies, only. Indeed we did not determine IgA levels against T. gondii or assess IgG avidity in IgM-positive females. Similar results were presented by Gontijo da Silva, Clare Vinaud, and de Castro [16], who reported a prevalence of 4.9 (24/487) in Gurupi/TO. Lower results were reported by Castilho–Pelloso, Falavigna, and Falavigna–Guilherme [28], with 0.2% (26/16.686) in the northwest of the state of Paraná; by Figueiró-Filho, Lopes, Senefonte, Souza Júnior, Botelho, Figueiredo, and Duarte [26], with 0.4% (137/32,512) in the state of Mato Grosso do Sul; and by Areal and Miranda [27], with 1.3% (15/1153) in Vitória/ES. In the context of study limitations, it is important to note that we did not determine IgA levels against T. gondii or assess IgG avidity in IgM-positive females.
Recent worldwide systematic review studies have reported global seropositivity of anti-T. gondii antibodies in pregnant women; with an IgG of 32.9% and an IgM of 1.9% [32], Rostami, et al. [32] found a 33.8% global prevalence of latent toxoplasmosis in pregnant women.
Ordinal logistic regression revealed that having a higher educational level was a protective factor against seropositivity for toxoplasmosis, corroborating other studies on T. gondii in pregnant women in Brazil [16,33,34] and other countries [35,36,37]. Pregnant women with a higher level of education were associated with having knowledge related to toxoplasmosis, which may increase their awareness and understanding of the importance of hygiene habits to prevent diseases.
The analysis of the distribution of pregnant women attended prenatally and the seropositivity of T. gondii in 17 Basic Health Units (BHU) in Araçatuba, SP, Brazil, reveals fundamental data for understanding the prevalence of toxoplasmosis and identifying areas that require specific interventions. The variability in the distribution of pregnant women between the BHUs, ranging from 2.8% (BHU 14 and BHU 3) to 17.2% (BHU 12), reflects the disparity in demand and service capacity of the health units. This discrepancy may indicate an unequal distribution of resources and accessibility to prenatal health services, suggesting the need for more equitable allocation and improvement in health infrastructure [38]. Identifying these critical areas allows for more effective allocation of health resources.
TORCH infections are prevalent globally and are a major cause of increased prenatal, perinatal, and postnatal infant morbidity and mortality [39,40,41].
The syphilis positivity rate in this study was 17.1%, within the values found worldwide by Newman et al. [42], in which the estimated number of infected pregnant women by region was 535,203 in Africa (39.3%), 106,500 in the Americas (7.8%), 603,293 in Asia (44.3%), 21,602 in Europe (1.6%), 40,062 in the Mediterranean (3.0%), and 53,825 (4.0%) in the Pacific. There was also no statistically significant association observed between the presence of syphilis and T. gondii in the pregnant women evaluated.
The incidence of congenital rubella syndrome has been decreasing worldwide due to increased rubella vaccination coverage, but it remains a threatening and costly disease in regions where pregnant women are not immunized and do not have protective levels of IgG against rubella virus. In this survey, the overall rate of immunity against rubella among pregnant women was 63.2% (74/117), and similar results were reported by de Melo Inagaki et al. [43], who found 64.3% in Sergipe. Higher seropositivity among women was described at 98.0% in Iowa (USA) [44], 93.0% in Cartagena (Colombia) [45], 97.0% in Brazil [46], 99.5% in Türkiye [47], and 85.2% in India [48].
No pregnant women were detected with acute rubella infection, but 36.8% of women were susceptible to the virus, which reinforces the need for rubella vaccination campaigns to achieve greater coverage and prevent the emergence of congenital rubella syndrome.
The absence of universal prenatal screening for the diagnosis of hepatitis C, with recommendations restricted only to women with risk factors, makes it difficult to accurately estimate its prevalence in the global population of pregnant women. However, in this survey, the overall prevalence of hepatitis C was 0.9%, in line with the results of other studies conducted in Serbia (0.9%) [49], Poland (0.9%) [50], and Canada (0.8%) [51]. In countries like Türkiye (0.07%) [52], Slovenia (0.09%) [53], and Saudi Arabia (0.05%) [54], positivity rates were lower. In contrast, significantly higher prevalences were reported in Yemen (6.0%) [55], India (2.3%) [56], and Ghana (3.4%) [57]. No co-infection of hepatitis C and T. gondii was observed in the pregnant women evaluated.
Infection with herpes simplex types 1 and 2 during pregnancy can result in transmission of the virus to the newborn during delivery. This study revealed a positivity rate of 0.9% (1/117), with a case of coinfection by T. gondii. Rates of herpes in pregnant women vary around the world. In Belgium, an incidence of 0.1% of cases was found [58]. In Norway, Eskild et al. [59] identified a frequency of 4% for acute cases of herpes infection.
The co-occurrence of infections with T. gondii in cases of rubella (20 cases), syphilis (6 cases), and COVID-19 (4 cases) highlights the complexity of the infectious landscape in pregnant women and the need for integrated strategies to monitor and treat multiple infections simultaneously. The concomitant presence of T. gondii with other TORCH complex infections may increase the risks to maternal and fetal health, requiring strict monitoring and immediate therapeutic interventions. While the absence of co-occurrence with hepatitis C, dengue, and herpes simplex is positive, surveillance must continue to ensure that these infections remain under control [3,20].
Complementing seropositivity data with stratified estimates across relevant demographic groups, such as age cohorts and geographic areas, is essential for targeting interventions and assessing program impacts. The limitations of these estimates highlight the urgent need for improved data through stronger national surveillance and monitoring systems. We also understand that resources used in testing for rubella should be allocated to vaccinating susceptible women.
Additional studies on the incidence and prevalence of vertically transmitted infectious diseases, as well as cost–benefit analyses of laboratory tests, are needed to develop effective protocols in Brazil. The lack of standardization in filling out medical records compromises the quality of the data collected, highlighting the need for constant training for health professionals. Improving data collection and record keeping by implementing standardized and consistent systems is crucial for the accurate monitoring of maternal and child health, contributing to reducing morbidity and mortality associated with congenital infections.
5. Conclusions
The results of this study demonstrate significant seropositivity of T. gondii among pregnant women treated at Basic Health Units (BHU) in Araçatuba, SP. This prevalence varies considerably between different BHUs, indicating that socioeconomic, behavioral, and environmental factors may influence exposure to the parasite. Co-occurrence of other infectious diseases, such as syphilis, rubella, and herpes simplex (1 and 2), was observed. These results are essential to guide local public health policies, focusing on educational and preventive strategies that consider the particularities of each BHU. The high prevalence of diseases belonging to the TORCH complex, especially congenital toxoplasmosis, points to the need for more targeted actions, such as awareness campaigns on infection prevention and continuous monitoring of pregnant women.
Author Contributions
Conceptualization, K.D.S.B. and S.S.F.; methodology, I.T.N. and K.D.S.B.; validation, K.D.S.B., S.S.F., V.M.F.S., A.d.N.B., Y.M.B. and T.R.S.-D.; formal analysis, S.S.F. and T.R.S.-D.; investigation, S.S.F., V.M.F.S., A.C.M., M.S.d.S., B.L.H., Y.M.B., J.F.G. and G.Z.T.D.; resources, S.S.F., A.C.M., M.S.d.S., B.L.H. and A.d.N.B.; data curation, S.S.F., A.C.M., A.d.N.B. and T.R.S.-D.; writing—original draft preparation, S.S.F., J.F.G. and G.Z.T.D.; writing—review and editing, J.F.G., I.T.N. and T.R.S.-D.; visualization, T.R.S.-D.; supervision, I.T.N. and K.D.S.B.; project administration, K.D.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Devaraju, M.; Li, A.; Ha, S.; Li, M.; Shivakumar, M.; Li, H.; Nishiguchi, E.P.; Gerardin, P.; Waldorf, K.A.; Al-Haddad, B.J.S. Beyond TORCH: A narrative review of the impact of antenatal and perinatal infections on the risk of disability. Neurosci. Biobehav. Rev. 2023, 153, 105390. [Google Scholar] [CrossRef]
- Jaan, A.; Rajnik, M. TORCH Complex; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Abu Shqara, R.; Or, S.; Abu Zraki, A.; Rizik, J.; Glikman, D.; Rechnitzer, H.; Lowenstein, L.; Frank Wolf, M. The Utility of Maternal TORCH Screening Due to Obstetrical Indications in Detecting Congenital Infections: A Retrospective Observational Study. Pediatr. Infect. Dis. J. 2024, 43, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, S.; Basevi, V.; Gagliotti, C.; Spettoli, D.; Gori, G.; D’Amico, R.; Magrini, N. Prenatal education for congenital toxoplasmosis. Cochrane Database Syst. Rev. 2015, 2015, CD006171. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.; Caceres-Alan, A.; Caceres-Alan, T. Toxoplasma gondii infections in pediatric neurosurgery. Childs Nerv. Syst. 2024, 40, 295–301. [Google Scholar] [CrossRef]
- Melo, M.S.; Cabrera, L.A.A.; Lima, S.; Dos Santos, A.D.; Oliveira, L.; de Oliveira, R.C.; de Sousa Menezes, J.; de Figueiredo, J.A.; de Moura Lane, V.F.; de Lima Junior, F.E.F.; et al. Temporal trend, spatial analysis and spatiotemporal clusters of infant mortality associated with congenital toxoplasmosis in Brazil: Time series from 2000 to 2020. Trop. Med. Int. Health 2023, 28, 476–485. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Hampton, M.M. Congenital Toxoplasmosis: A Review. Neonatal. Netw. 2015, 34, 274–278. [Google Scholar] [CrossRef]
- Strang, A.G.G.F.; Ferrari, R.G.; do Rosário, D.K.; Nishi, L.; Evangelista, F.F.; Santana, P.L.; de Souza, A.H.; Mantelo, F.M.; Guilherme, A.L.F. The congenital toxoplasmosis burden in Brazil: Systematic review and meta-analysis. Acta Trop. 2020, 211, 105608. [Google Scholar] [CrossRef] [PubMed]
- Hotop, A.; Hlobil, H.; Gross, U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2012, 54, 1545–1552. [Google Scholar] [CrossRef]
- Villard, O.; Cimon, B.; L’Ollivier, C.; Fricker-Hidalgo, H.; Godineau, N.; Houze, S.; Paris, L.; Pelloux, H.; Villena, I.; Candolfi, E. Serological diagnosis of Toxoplasma gondii infection: Recommendations from the French National Reference Center for Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2016, 84, 22–33. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F. Congenital Toxoplasmosis: A Plea for a Neglected Disease. Pathogens 2018, 7, 25. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F.; Cornu, C.; Vinault, S.; Abrahamowicz, M.; Kopp, C.B.; Binquet, C. Congenital toxoplasma infection: Monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin. Infect. Dis. 2013, 56, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Bichara, C.N.C.; Canto, G.A.d.C.; Tostes, C.d.L.; Freitas, J.J.d.S.; Carmo, E.L.d.; Póvoa, M.M.; Silveira, E.d.C. Incidence of congenital toxoplasmosis in the city of Belém, state of Pará, northern Brazil, determined by a neonatal screening program: Preliminary results. Rev. Soc. Bras. Med. Trop. 2012, 45, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Gundeslioglu, O.O.; Haytoglu, Z.; Esen, E.; Alabaz, D.; Cay, U.; Ozlu, F.; Kibar, F.; Cetiner, S. Congenital Toxoplasmosis and Long-term Outcomes. Turk. Parazitol Derg. 2024, 48, 8–14. [Google Scholar] [CrossRef]
- Gontijo da Silva, M.; Clare Vinaud, M.; de Castro, A.M. Prevalence of toxoplasmosis in pregnant women and vertical transmission of Toxoplasma gondii in patients from basic units of health from Gurupi, Tocantins, Brazil, from 2012 to 2014. PLoS ONE 2015, 10, e0141700. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, V.; Rahmanian, K.; Jahromi, A.S.; Bokaie, S. Seroprevalence of toxoplasma gondii infection: An umbrella review of updated systematic reviews and meta-analyses. J. Family Med. Prim. Care 2020, 9, 3848–3855. [Google Scholar] [CrossRef]
- Bigna, J.J.; Tochie, J.N.; Tounouga, D.N.; Bekolo, A.O.; Ymele, N.S.; Youda, E.L.; Sime, P.S.; Nansseu, J.R. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: A systematic review, modelling and meta-analysis. Sci. Rep. 2020, 10, 12102. [Google Scholar] [CrossRef]
- Chew, L.C.; Verma, R.P. Fetal Growth Restriction; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ren, X.; Wang, K.; Chang, Z.; Liu, M.; Cheng, F.; Min, B.; Wei, S. Serological Screening of TORCH Pathogen Infections in Infertile Women of Childbearing Age in Northwest China. Reprod. Sci. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Brasil. Guide for Laboratory Diagnosis in Public Health: Guidelines for the National Public Health Laboratory System; Ministério da Saúde: Brasília, Brazil, 2021; p. 363.
- Thrusfield, M.; Christley, R. Veterinary Epidemiology, 4th ed.; Blackwell Science: Cambridge, MA, USA, 2018. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation: Beaverton, OR, USA, 2023. [Google Scholar]
- Pereira, E.; Ferreira, I.B.; Victorino, R.B.; Lescano, S.A.Z.; Giuffrida, R.; Kmetiuk, L.B.; Biondo, A.W.; Santarem, V.A. Serosurvey of Toxoplasma gondii and Toxocara spp. co-infection in pregnant women in low-income areas of Brazil. Front. Public Health 2024, 12, 1340434. [Google Scholar] [CrossRef]
- Ferreira, J.V.; Leite, R.; Holanda, C.; Barbosa, V. Soroprevalência para toxoplasmose em gestantes. Educ. Ciênc. Saúde 2020, 7, 101–116. [Google Scholar]
- Figueiró-Filho, E.A.; Lopes, A.H.A.; Senefonte, F.R.d.A.; Souza Júnior, V.G.d.; Botelho, C.A.; Figueiredo, M.S.; Duarte, G. Toxoplasmose aguda: Estudo da freqüência, taxa de transmissão vertical e relação entre os testes diagnósticos materno-fetais em gestantes em estado da Região Centro-Oeste do Brasil. Rev. Bras. Ginecol. Obs. 2005, 27, 442–449. [Google Scholar] [CrossRef]
- Areal, K.R.; Miranda, A.E. Soroprevalência de toxoplasmose em gestantes atendidas na rede básica de saúde de Vitória, ES. ES NewsLab 2008, 87, 122–129. [Google Scholar]
- Castilho-Pelloso, M.P.; Falavigna, D.L.M.; Falavigna-Guilherme, A.L. Suspected acute toxoplasmosis in pregnant women. Rev. Saúde Pública 2007, 41, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, P.C.; Telmo, P.d.L.; Lehmann, L.M.; Mattos, G.T.; Klafke, G.B.; Lorenzi, C.; Hirsch, C.; Lemos, L.; Berne, M.E.A.; Gonçalves, C.V. Risk and other factors associated with toxoplasmosis and toxocariasis in pregnant women from southern Brazil. J. Helminthol. 2017, 91, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.C.; de Vasconcelos Marques, M.R.; Leite, R.B.d.C.H.; Holanda, C.M.d.C.X.; de Arruda Barbosa, V.S. Seroprevalence of toxoplasmosis in pregnant women in a city in Rio Grande do Norte state, Brazil. J. Trop. Pathol. 2017, 46, 147–158. [Google Scholar]
- Ahmed, M.; Sood, A.; Gupta, J. Toxoplasmosis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 44–50. [Google Scholar] [CrossRef]
- Rostami, A.; Riahi, S.M.; Gamble, H.R.; Fakhri, Y.; Nourollahpour Shiadeh, M.; Danesh, M.; Behniafar, H.; Paktinat, S.; Foroutan, M.; Mokdad, A.H.; et al. Global prevalence of latent toxoplasmosis in pregnant women: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 673–683. [Google Scholar] [CrossRef]
- Barbosa, I.R.; de Carvalho Xavier Holanda, C.M.; de Andrade-Neto, V.F. Toxoplasmosis screening and risk factors amongst pregnant females in Natal, northeastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 377–382. [Google Scholar] [CrossRef]
- Moura, F.L.d.; Goulart, P.R.M.; Moura, A.P.P.d.; Souza, T.S.d.; Fonseca, A.B.M.; Amendoeira, M.R.R. Fatores associados ao conhecimento sobre a toxoplasmose entre gestantes atendidas na rede pública de saúde do município de Niterói, Rio de Janeiro, 2013–2015. Epidemiol. Serv. Saúde 2016, 25, 655–661. [Google Scholar] [CrossRef]
- Mizani, A.; Alipour, A.; Sharif, M.; Sarvi, S.; Amouei, A.; Shokri, A.; Rahimi, M.-T.; Hosseini, S.A.; Daryani, A. Toxoplasmosis seroprevalence in Iranian women and risk factors of the disease: A systematic review and meta-analysis. Trop. Med. Health 2017, 45, 1–13. [Google Scholar] [CrossRef]
- NA, H.H.; Shaapan, R.M.; Hassanain, H. Associated Antenatal Health Risk Factors with Incidence of Toxoplasmosis in Egyptian Pregnant Women. Pak. J. Biol. Sci. 2018, 21, 463–468. [Google Scholar]
- Dambrun, M.; Dechavanne, C.; Guigue, N.; Briand, V.; Candau, T.; Fievet, N.; Lohezic, M.; Manoharan, S.; Sare, N.; Viwami, F. Retrospective study of toxoplasmosis prevalence in pregnant women in Benin and its relation with malaria. PLoS ONE 2022, 17, e0262018. [Google Scholar] [CrossRef] [PubMed]
- Esposti, C.D.D.; Santos-Neto, E.T.d.; Oliveira, A.E.; Travassos, C.; Pinheiro, R.S. Desigualdades sociais e geográficas no desempenho da assistência pré-natal de uma Região Metropolitana do Brasil. Ciênc. Saúde Coletiva 2020, 25, 1735–1750. [Google Scholar] [CrossRef]
- Lynn, M.K.; Aquino, M.S.R.; Self, S.C.W.; Kanyangarara, M.; Campbell, B.A.; Nolan, M.S. TORCH Congenital Syndrome Infections in Central America’s Northern Triangle. Microorganisms 2023, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Ma, N.; Zhang, Q.; Wang, H.; Cui, J.; Wang, S. The association of ToRCH infection and congenital malformations: A prospective study in China. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Neu, N.; Duchon, J.; Zachariah, P. TORCH infections. Clin. Perinatol. 2015, 42, 77–103. [Google Scholar] [CrossRef]
- Newman, L.; Kamb, M.; Hawkes, S.; Gomez, G.; Say, L.; Seuc, A.; Broutet, N. Global Estimates of Syphilis in Pregnancy and Associated Adverse Outcomes: Analysis of Multinational Antenatal Surveillance Data. PLoS Med. 2013, 10, e1001396. [Google Scholar] [CrossRef]
- Inagaki, A.D.d.M.; de Oliveira, L.A.R.; de Oliveira, M.F.B.; Santos, R.C.S.; Araújo, R.M.; Alves, J.A.B.; Pinheiro, K.S.; Gurgel, R.Q.; Mussi-Pinhata, M.M. Seroprevalence of antibodies for toxoplasmosis, rubella, cytomegalovirus, syphilis and HIV among pregnant women in Sergipe. Rev. Soc. Bras. Med. Trop. 2009, 42, 532–536. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Burns, B.A.; Ault, K.A. Does rubella immunity predict measles immunity? A serosurvey of pregnant women. Infect. Dis. Obstet. Gynecol. 2006, 2006, 013890. [Google Scholar] [CrossRef]
- Mora-García, G.J.; Ramos-Clason, E.; Mazenett, E.; Gómez-Camargo, D. The seroprevalence IgG antibodies against rubella (German measles) in 10–49 year-old women from Cartagena, Colombia. Rev. Salud Pública 2011, 13, 288–297. [Google Scholar]
- Costa, G.B.; De Oliveira, M.C.; Gadelha, S.R.; Albuquerque, G.R.; Teixeira, M.; da Silva Raiol, M.R.; Sousa, S.M.B.; Marin, L.J. Infectious diseases during pregnancy in Brazil: Seroprevalence and risk factors. J. Infect. Dev. Ctries. 2018, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Gürses, G.; Yentür Doni, N.; Şimşek, Z.; Aksoy, M.; Hilali, N.G.; Özek, B. Evaluation of T. gondii, rubella, and cytomegalovirus seroprevalences among female Syrian refugees in Sanliurfa, Turkiye. J. Infect. Dev. Ctries. 2024, 18, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, D.; Viswanathan, R.; Winter, A.K.; Agarwal, A.; Roychowdhury, B.; Muliyil, D.; Prasad, G.R.V.; Pushpalatha, K.; Gowda, M.; Singh, P.; et al. Rubella immunity among pregnant women and the burden of congenital rubella syndrome (CRS) in India, 2022. Vaccine 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, B.; Lazarevic, A.; Bokun, R.; Vasiljevic, N. The prevalence of hepatitis B, hepatitis C virus, and human immunodeficiency virus at pregnant women and at person with risky behaviour. Vox Sang. 2013, 105, 65–299. [Google Scholar] [CrossRef]
- Walewska-Zielecka, B.; Religioni, U.; Juszczyk, G.; Czerw, A.; Wawrzyniak, Z.; Soszynski, P. Diagnosis of hepatitis C virus infection in pregnant women in the healthcare system in Poland: Is it worth the effort? Medicine 2016, 95, e4331. [Google Scholar] [CrossRef]
- Biondi, M.J.; Marchand-Austin, A.; Cronin, K.; Nanwa, N.; Ravirajan, V.; Mandel, E.; Goneau, L.W.; Mazzulli, T.; Shah, H.; Capraru, C. Prenatal hepatitis B screening, and hepatitis B burden among children, in Ontario: A descriptive study. Can. Med. Assoc. J. 2020, 192, E1299–E1305. [Google Scholar] [CrossRef]
- Simavli, S.; Ozlu, T.; Kucukbayrak, B. Age specific prevalence of hepatitis B and hepatitis C among pregnant women in the northwestern region of Turkey. Indian J. Gastroenterol. 2014, 33, 293–294. [Google Scholar] [CrossRef]
- Kopilović, B.; Poljak, M.; Seme, K.; Klavs, I. Hepatitis C virus infection among pregnant women in Slovenia: Study on 31,849 samples obtained in four screening rounds during 1999, 2003, 2009 and 2013. Eurosurveillance 2015, 20, 21144. [Google Scholar] [CrossRef][Green Version]
- Al-Mandeel, H.M.; Manahel Alansary, M.A.; Fatimah Algawahmed, F.A.; Hind Al-Mojally, H.A.-M.; Alfaleh, K.M.; Aldakhil, L.O. Seroprevalence of hepatitis B and C, and human immunodeficiency viruses in Saudi pregnant women and rates of vertical transmission. Kuwait Med. J. 2015, 47, 221–224. [Google Scholar]
- Murad, E.A.; Babiker, S.M.; Gasim, G.I.; Rayis, D.A.; Adam, I. Epidemiology of hepatitis B and hepatitis C virus infections in pregnant women in Sana’a, Yemen. BMC Pregnancy Childbirth 2013, 13, 127. [Google Scholar] [CrossRef]
- Goyal, L.D.; Kaur, S.; Jindal, N.; Kaur, H. HCV and pregnancy: Prevalence, risk factors, and pregnancy outcome in north Indian population: A case–control study. J. Obstet. Gynecol. India 2014, 64, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, A.A.; Ofori-Asenso, R.; Mprah, A.; Ashiagbor, G. Epidemiology of hepatitis C virus in Ghana: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- Bodéus, M.; Laffineur, K.; Kabamba-Mukadi, B.; Hubinont, C.; Bernard, P.; Goubau, P. Seroepidemiology of herpes simplex type 2 in pregnant women in Belgium. Sex. Transm. Dis. 2004, 31, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Eskild, A.; Jeansson, S.; Stray-Pedersen, B.; Jenum, P.A. Herpes simplex virus type-2 infection in pregnancy: No risk of fetal death: Results from a nested case-control study within 35,940 women. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 1030–1035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).