Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase
Abstract
1. Introduction
2. Leptospira—A Particular Type of Bacteria
3. Outer Envelope Toxins
3.1. Lipopolysaccharides (LPS)
3.2. The Lipid A
3.3. Proteins
3.4. LipL32 and HlyX
3.5. Glycolipoprotein (GLP)
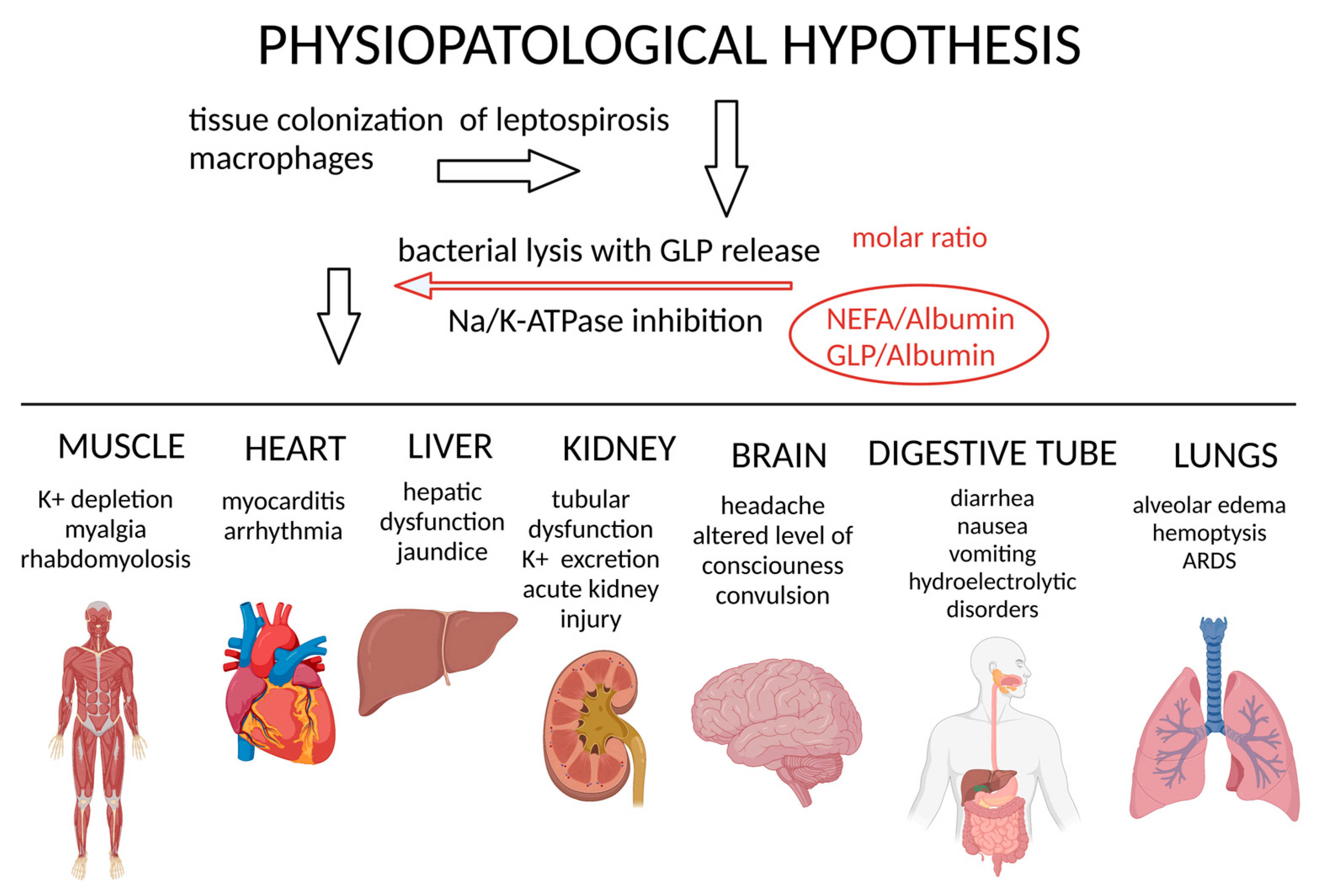
4. Pathogenesis
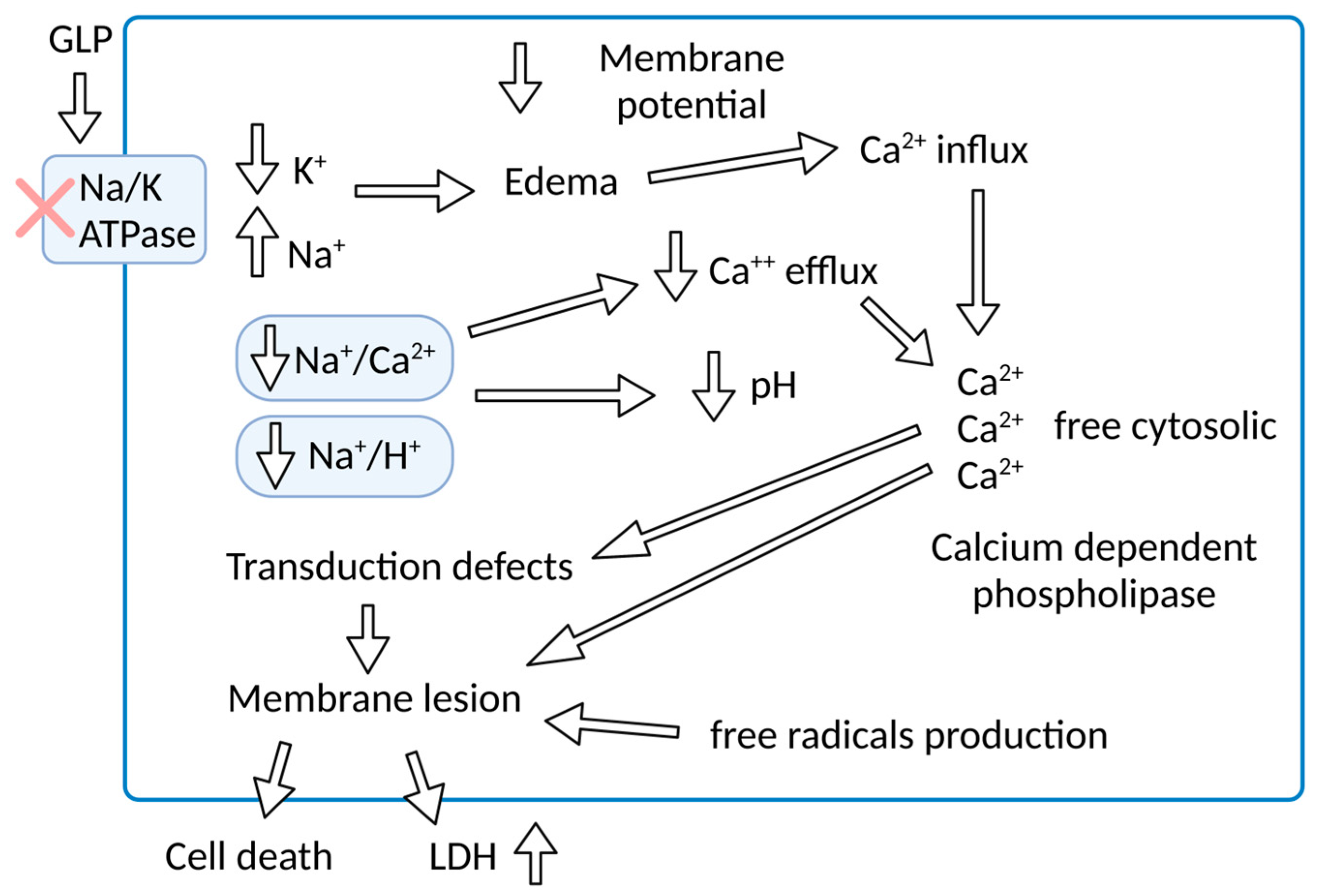
5. Pathophysiology—Na/K-ATPase and GLP—A Molecular Target
6. Organ Impact of Molecular Mechanisms
7. Na/K-ATPase Signalosome
Signalosome in Leptospirosis
8. Final Remarks
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Zanzi, C.; Groene, E.; Morawski, B.M.; Bonner, K.; Costa, F.; Bertherat, E.; Schneider, M.C. A systematic literature review of leptospirosis outbreaks worldwide, 1970–2012. Rev. Panam Salud. Publica 2020, 44, e78. [Google Scholar] [CrossRef] [PubMed]
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.; Zinsstag, J.; Hattendorf, J. Environmental and behavioural determinants of leptospirosis transmission: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003843. [Google Scholar] [CrossRef] [PubMed]
- Thibeaux, R.; Geroult, S.; Benezech, C.; Chabaud, S.; Soupe-Gilbert, M.E.; Girault, D.; Bierque, E.; Goarant, C. Seeking the environmental source of Leptospirosis reveals durable bacterial viability in river soils. PLoS Negl. Trop. Dis. 2017, 11, e0005414. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Santana, R.; de Oliveira, D.; Palma, F.; Lustosa, R.; Eyre, M.T.; Carvalho-Pereira, T.; Reis, M.G.; Ko, A.I.; Diggle, P.J.; et al. Poverty, sanitation, and Leptospira transmission pathways in residents from four Brazilian slums. PLoS Negl. Trop. Dis. 2021, 15, e0009256. [Google Scholar] [CrossRef]
- Casanovas-Massana, A.; Costa, F.; Riediger, I.N.; Cunha, M.; de Oliveira, D.; Mota, D.C.; Sousa, E.; Querino, V.A.; Nery, N., Jr.; Reis, M.G.; et al. Spatial and temporal dynamics of pathogenic Leptospira in surface waters from the urban slum environment. Water Res. 2018, 130, 176–184. [Google Scholar] [CrossRef]
- Chadsuthi, S.; Chalvet-Monfray, K.; Wiratsudakul, A.; Modchang, C. The effects of flooding and weather conditions on leptospirosis transmission in Thailand. Sci. Rep. 2021, 11, 1486. [Google Scholar] [CrossRef]
- Taylor, A.J.; Paris, D.H.; Newton, P.N. A systematic review of the mortality from untreated Leptospirosis. PLoS Negl. Trop. Dis. 2015, 9, e0003866. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar]
- Naing, C.; Reid, S.A.; Aung, K. Comparing antibiotic treatment for leptospirosis using network meta-analysis: A tutorial. BMC Infect. Dis. 2017, 17, 29. [Google Scholar] [CrossRef]
- CDC. Leptospirosis. Available online: https://www.cdc.gov/leptospirosis/treatment/index.html (accessed on 5 March 2023).
- Sznajder, J.I.; Ridge, K.M.; Harris, Z.L.; Olivera, W.; Curiel, C.; Rutschman, D.H. Alveolar type II cell Na,K-ATPase is upregulated during mechanical ventilation-induced pulmonary edema. Chest 1994, 105, 116S–117S. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H. Morphological characteristics and nomenclature of leptospira (spirochaeta) icterohaemorrhagiae (inada and ido). J. Exp. Med. 1918, 27, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Haake, D.A. Spirochaetal lipoproteins and pathogenesis. Microbiology 2000, 146 Pt 7, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.W.; Broom, J.C. The genus leptospira noguchi, 1917; problems of classification and a suggested system based on antigenic analysis. Doc. Med. Geogr. Trop. 1954, 6, 78–95. [Google Scholar] [PubMed]
- Nauman, R.K.; Holt, S.C.; Cox, C.D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J. Bacteriol. 1969, 98, 264–280. [Google Scholar] [CrossRef]
- Younes-Ibrahim, M.; Barlet-Bas, C.; Buffin-Meyer, B.; Cheval, L.; Rajerison, R.; Doucet, A. Ouabain-sensitive and -insensitive K-ATPases in rat nephron: Effect of K depletion. Am. J. Physiol. 1995, 268, F1141–F1147. [Google Scholar] [CrossRef]
- Ren, S.X.; Fu, G.; Jiang, X.G.; Zeng, R.; Miao, Y.G.; Xu, H.; Zhang, Y.X.; Xiong, H.; Lu, G.; Lu, L.F.; et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 2003, 422, 888–893. [Google Scholar] [CrossRef]
- Picardeau, M.; Bulach, D.M.; Bouchier, C.; Zuerner, R.L.; Zidane, N.; Wilson, P.J.; Creno, S.; Kuczek, E.S.; Bommezzadri, S.; Davis, J.C.; et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE 2008, 3, e1607. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef]
- Mejia, L.; Prado, B.; Cardenas, P.; Trueba, G.; Gonzalez-Candelas, F. The impact of genetic recombination on pathogenic Leptospira. Infect. Genet. Evol. 2022, 102, 105313. [Google Scholar] [CrossRef]
- Guglielmini, J.; Bourhy, P.; Schiettekatte, O.; Zinini, F.; Brisse, S.; Picardeau, M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007374. [Google Scholar] [CrossRef]
- Thibeaux, R.; Girault, D.; Bierque, E.; Soupe-Gilbert, M.E.; Rettinger, A.; Douyere, A.; Meyer, M.; Iraola, G.; Picardeau, M.; Goarant, C. Biodiversity of environmental leptospira: Improving identification and revisiting the diagnosis. Front Microbiol. 2018, 9, 816. [Google Scholar] [CrossRef]
- Ellinghausen, H.C., Jr. Growth, cultural characteristics, and antibacterial sensitivity of Leptospira interrogans serovar hardjo. Cornell Vet. 1983, 73, 225–239. [Google Scholar]
- Guedes, I.B.; Souza, G.O.; Castro, J.F.P.; Cavalini, M.B.; Filho, A.F.S.; Aizawa, J.; Cortez, A.; Heinemann, M.B. Improvement of the enrichment used in the emjh medium (ellinghausen-mccullough-johnson-harris) for the cultivation of Leptospira spp. Rev. Argent Microbiol. 2022, 54, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Vinh, T.; Adler, B.; Faine, S. Glycolipoprotein cytotoxin from Leptospira interrogans serovar copenhageni. J. Gen. Microbiol. 1986, 132, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, J.A.; VanEseltine, W.P. Isolation and chemical characterization of outer envelope of Leptospira pomona. Can. J. Microbiol. 1975, 21, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Yang, C.W. Insight into the structure, functions, and dynamics of the leptospira outer membrane proteins with the pathogenicity. Membranes 2022, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Auran, N.E.; Johnson, R.C.; Ritzi, D.M. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect. Immun. 1972, 5, 968–975. [Google Scholar] [CrossRef]
- Van Why, S.K.; Mann, A.S.; Ardito, T.; Siegel, N.J.; Kashgarian, M. Expression and molecular regulation of Na+-K+-ATPase after renal ischemia. Am. J. Physiol. 1994, 267, F75–F85. [Google Scholar] [CrossRef]
- Vinh, T.; Adler, B.; Faine, S. Ultrastructure and chemical composition of lipopolysaccharide extracted from Leptospira interrogans serovar copenhageni. J. Gen. Microbiol. 1986, 132, 103–109. [Google Scholar] [CrossRef]
- Isogai, E.; Isogai, H.; Fujii, N.; Oguma, K. Macrophage activation by leptospiral lipopolysaccharide. Zentralbl. Bakteriol. 1990, 273, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Kitagawa, H.; Isogai, H.; Matsuzawa, T.; Shimizu, T.; Yanagihara, Y.; Katami, K. Effects of leptospiral lipopolysaccharide on rabbit platelets. Zentralbl. Bakteriol. 1989, 271, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.J.; Adler, B.; Faine, S. Antigens recognised by the human immune response to infection with Leptospira interrogans serovar hardjo. J. Med. Microbiol. 1988, 25, 269–278. [Google Scholar] [CrossRef]
- Adler, B.; Faine, S. Serological and protective-antibody responses of rabbits to leptospiral antigens. J. Med. Microbiol. 1978, 11, 401–409. [Google Scholar] [CrossRef]
- Jost, B.H.; Adler, B.; Vinh, T.; Faine, S. A monoclonal antibody reacting with a determinant on leptospiral lipopolysaccharide protects guinea pigs against leptospirosis. J. Med. Microbiol. 1986, 22, 269–275. [Google Scholar] [CrossRef]
- Segers, R.P.; De Nijs, A.; van Kooten, P.J.; Gaastra, W.; van der Zeijst, B.A. The use of immunodeficient male (CBA/N x BALB/c) F1 mice to produce monoclonal antibodies directed to proteins of Leptospira interrogans rather than to immunodominant lipopolysaccharides. Hybridoma 1990, 9, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.P.; Choudhury, B.; Matthias, M.M.; Baga, S.; Bandyopadhya, K.; Vinetz, J.M. Comparative analysis of lipopolysaccharides of pathogenic and intermediately pathogenic Leptospira species. BMC Microbiol. 2015, 15, 244. [Google Scholar] [CrossRef]
- Werts, C.; Tapping, R.I.; Mathison, J.C.; Chuang, T.H.; Kravchenko, V.; Saint Girons, I.; Haake, D.A.; Godowski, P.J.; Hayashi, F.; Ozinsky, A.; et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2001, 2, 346–352. [Google Scholar] [CrossRef]
- Bonhomme, D.; Santecchia, I.; Vernel-Pauillac, F.; Caroff, M.; Germon, P.; Murray, G.; Adler, B.; Boneca, I.G.; Werts, C. Leptospiral LPS escapes mouse TLR4 internalization and TRIF-associated antimicrobial responses through O antigen and associated lipoproteins. PLoS Pathog. 2020, 16, e1008639. [Google Scholar]
- Cagliero, J.; Vernel-Pauillac, F.; Murray, G.; Adler, B.; Matsui, M.; Werts, C. Pathogenic leptospires limit dendritic cell activation through avoidance of TLR4 and TRIF signaling. Front Immunol. 2022, 13, 911778. [Google Scholar] [CrossRef]
- Shimizu, T.; Matsusaka, E.; Takayanagi, K.; Masuzawa, T.; Iwamoto, Y.; Morita, T.; Mifuchi, I.; Yanagihara, Y. Biological activities of lipopolysaccharide-like substance (LLS) extracted from Leptospira interrogans serovar canicola strain Moulton. Microbiol. Immunol. 1987, 31, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Isogai, H.; Kurebayashi, Y.; Ito, N. Biological activities of leptospiral lipopolysaccharide. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1986, 261, 53–64. [Google Scholar] [CrossRef]
- Nunes-Edwards, P.L.; Thiermann, A.B.; Bassford, P.J., Jr.; Stamm, L.V. Identification and characterization of the protein antigens of Leptospira interrogans serovar hardjo. Infect. Immun. 1985, 48, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Zuerner, R.L.; Knudtson, W.; Bolin, C.A.; Trueba, G. Characterization of outer membrane and secreted proteins of Leptospira interrogans serovar pomona. Microb. Pathog. 1991, 10, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lingrel, J.B.; Orlowski, J.; Shull, M.M.; Price, E.M. Molecular genetics of Na,K-ATPase. Prog. Nucleic. Acid. Res. Mol. Biol. 1990, 38, 37–89. [Google Scholar] [PubMed]
- Ratet, G.; Santecchia, I.; Fanton d’Andon, M.; Vernel-Pauillac, F.; Wheeler, R.; Lenormand, P.; Fischer, F.; Lechat, P.; Haake, D.A.; Picardeau, M.; et al. LipL21 lipoprotein binding to peptidoglycan enables Leptospira interrogans to escape NOD1 and NOD2 recognition. PLoS Pathog. 2017, 13, e1006725. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.L. The lipoprotein LipL32, an enigma of leptospiral biology. Vet. Microbiol. 2013, 162, 305–314. [Google Scholar] [CrossRef]
- Hauk, P.; Macedo, F.; Romero, E.C.; Vasconcellos, S.A.; de Morais, Z.M.; Barbosa, A.S.; Ho, P.L. In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect. Immun. 2008, 76, 2642–2650. [Google Scholar] [CrossRef]
- Choy, H.A.; Kelley, M.M.; Chen, T.L.; Moller, A.K.; Matsunaga, J.; Haake, D.A. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect. Immun. 2007, 75, 2441–2450. [Google Scholar] [CrossRef]
- Robbins, G.T.; Hahn, B.L.; Evangelista, K.V.; Padmore, L.; Aranda, P.S.; Coburn, J. Evaluation of cell binding activities of Leptospira ECM adhesins. PLoS Negl. Trop. Dis. 2015, 9, e0003712. [Google Scholar] [CrossRef]
- Diament, D.; Brunialti, M.K.; Romero, E.C.; Kallas, E.G.; Salomao, R. Peripheral blood mononuclear cell activation induced by Leptospira interrogans glycolipoprotein. Infect. Immun. 2002, 70, 1677–1683. [Google Scholar] [CrossRef]
- De Maria, R.; Cifone, M.G.; Trotta, R.; Rippo, M.R.; Festuccia, C.; Santoni, A.; Testi, R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J. Exp. Med. 1994, 180, 1999–2004. [Google Scholar] [CrossRef]
- Dorigatti, F.; Brunialti, M.K.; Romero, E.C.; Kallas, E.G.; Salomao, R. Leptospira interrogans activation of peripheral blood monocyte glycolipoprotein demonstrated in whole blood by the release of IL-6. Braz. J. Med. Biol. Res. 2005, 38, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Lamande, S.; d’Andon, M.F.; Michel, E.; Ratet, G.; Philpott, D.J.; Girardin, S.E.; Boneca, I.G.; Vandewalle, A.; Werts, C. Downregulation of the Na/K-ATPase pump by leptospiral glycolipoprotein activates the NLRP3 inflammasome. J. Immunol. 2012, 188, 2805–2814. [Google Scholar] [CrossRef] [PubMed]
- Younes-Ibrahim, M.; Buffin-Meyer, B.; Cheval, L.; Burth, P.; Castro-Faria, M.V.; Barlet-Bas, C.; Marsy, S.; Doucet, A. Na,K-ATPase: A molecular target for Leptospira interrogans endotoxin. Braz. J. Med. Biol. Res. 1997, 30, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-de-Albuquerque, C.F.; Burth, P.; Silva, A.R.; de Moraes, I.M.; Oliveira, F.M.; Santelli, R.E.; Freire, A.S.; de Lima, G.S.; da Silva, E.D.; da Silva, C.I.; et al. Murine lung injury caused by Leptospira interrogans glycolipoprotein, a specific Na/K-ATPase inhibitor. Respir. Res. 2014, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Ratet, G.; Veyrier, F.J.; Fanton d’Andon, M.; Kammerscheit, X.; Nicola, M.A.; Picardeau, M.; Boneca, I.G.; Werts, C. Live imaging of bioluminescent Leptospira interrogans in mice reveals renal colonization as a stealth escape from the blood defenses and antibiotics. PLoS Negl. Trop. Dis. 2014, 8, e3359. [Google Scholar] [CrossRef]
- Athanazio, D.A.; Silva, E.F.; Santos, C.S.; Rocha, G.M.; Vannier-Santos, M.A.; McBride, A.J.; Ko, A.I.; Reis, M.G. Rattus norvegicus as a model for persistent renal colonization by pathogenic leptospira interrogans. Acta Trop. 2008, 105, 176–180. [Google Scholar] [CrossRef]
- Lau, C.L.; Townell, N.; Stephenson, E.; van den Berg, D.; Craig, S.B. Leptospirosis: An important zoonosis acquired through work, play and travel. Aust. J. Gen. Pract. 2018, 47, 105–110. [Google Scholar] [CrossRef]
- Morgan, J.; Bornstein, S.L.; Karpati, A.M.; Bruce, M.; Bolin, C.A.; Austin, C.C.; Woods, C.W.; Lingappa, J.; Langkop, C.; Davis, B.; et al. Outbreak of leptospirosis among triathlon participants and community residents in springfield, illinois, 1998. Clin. Infect. Dis. 2002, 34, 1593–1599. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.; Park, S.C.; Kim, M.J. Cytotoxic activities of Leptospira interrogans hemolysin sphh as a pore-forming protein on mammalian cells. Infect. Immun. 2002, 70, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Monahan, A.M.; Callanan, J.J.; Nally, J.E. Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis. Vet. Pathol. 2009, 46, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Cheville, N.F.; Huhn, R.; Cutlip, R.C. Ultrastructure of renal lesions in pigs with acute leptospirosis caused by Leptospira pomona. Vet. Pathol. 1980, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.R.; Seguro, A.C.; Spichler, A.; Magaldi, A.J.; Volpini, R.A.; De Brito, T. Acute kidney injury in human leptospirosis: An immunohistochemical study with pathophysiological correlation. Virchows Arch. 2010, 456, 367–375. [Google Scholar] [CrossRef]
- Andrade, L.; Rodrigues, A.C., Jr.; Sanches, T.R.; Souza, R.B.; Seguro, A.C. Leptospirosis leads to dysregulation of sodium transporters in the kidney and lung. Am. J. Physiol. Renal. Physiol. 2007, 292, F586–F592. [Google Scholar] [CrossRef]
- Cesar, K.R.; Romero, E.C.; de Braganca, A.C.; Blanco, R.M.; Abreu, P.A.; Magaldi, A.J. Renal involvement in leptospirosis: The effect of glycolipoprotein on renal water absorption. PLoS ONE 2012, 7, e37625. [Google Scholar] [CrossRef]
- Skubnik, J.; Pavlickova, V.; Rimpelova, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Burth, P.S.M.; Castro-Faria, M.V.; Younes-Ibrahim, M. Acute renal failure and toxic nephropathies (M133-M156). Nephrol. Dial. Transplant. 2002, 17, 87–93. [Google Scholar]
- Ghasemian, R.; Shokri, M.; Makhlough, A.; Suraki-Azad, M.A. The course and outcome of renal failure due to human leptospirosis referred to a hospital in north of iran; A follow-up study. Casp. J. Intern. Med. 2016, 7, 7–12. [Google Scholar]
- Younes-Ibrahim, M.; Burth, P.; Castro-Faria, M.; Cheval, L.; Buffin-Meyer, B.; Marsy, S.; Doucet, A. Effect of Leptospira interrogans endotoxin on renal tubular Na,K-ATPase and H,K-ATPase activities. Ann. N. Y. Acad. Sci. 1997, 834, 684–686. [Google Scholar] [CrossRef]
- Beguin, P.; Wang, X.; Firsov, D.; Puoti, A.; Claeys, D.; Horisberger, J.D.; Geering, K. The gamma subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J. 1997, 16, 4250–4260. [Google Scholar] [CrossRef] [PubMed]
- Ewart, H.S.; Klip, A. Hormonal regulation of the Na+-K+-ATPase: Mechanisms underlying rapid and sustained changes in pump activity. Am. J. Physiol. 1995, 269, C295–C311. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.L. Purification and characterization of (Na+ plus K+)-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim. Biophys. Acta 1974, 356, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Younes-Ibrahim, M.; Burth, P.; Faria, M.V.; Buffin-Meyer, B.; Marsy, S.; Barlet-Bas, C.; Cheval, L.; Doucet, A. Inhibition of Na,K-ATPase by an endotoxin extracted from Leptospira interrogans: A possible mechanism for the physiopathology of leptospirosis. C. R. Acad. Sci. III 1995, 318, 619–625. [Google Scholar] [PubMed]
- Adler, B.; Faine, S. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans Serovar pomona. Infect. Immun. 1976, 14, 703–708. [Google Scholar] [CrossRef]
- Dos Santos Mda, C.; Burth, P.; Younes-Ibrahim, M.; Goncalves, C.F.; Santelli, R.E.; Oliveira, E.P.; de Castro Faria, M.V. Na/K-ATPase assay in the intact guinea pig liver submitted to in situ perfusion. Anal. Biochem. 2009, 385, 65–68. [Google Scholar] [CrossRef]
- Burth, P.; Younes-Ibrahim, M.; Goncalez, F.H.; Costa, E.R.; Faria, M.V. Purification and characterization of a Na+, K+ ATPase inhibitor found in an endotoxin of Leptospira interrogans. Infect. Immun. 1997, 65, 1557–1560. [Google Scholar] [CrossRef]
- Davis, B.D.; Dubos, R.J. The binding of fatty acids by serum albumin, a protective growth factor in bacteriological media. J. Exp. Med. 1947, 86, 215–228. [Google Scholar] [CrossRef]
- Burth, P.; Younes-Ibrahim, M.; Santos, M.C.; Castro-Faria Neto, H.C.; de Castro Faria, M.V. Role of nonesterified unsaturated fatty acids in the pathophysiological processes of leptospiral infection. J. Infect. Dis. 2005, 191, 51–57. [Google Scholar] [CrossRef]
- Carter, E.P.; Duvick, S.E.; Wendt, C.H.; Dunitz, J.; Nici, L.; Wangensteen, O.D.; Ingbar, D.H. Hyperoxia increases active alveolar Na+ resorption in vivo and type II cell Na,K-ATPase in vitro. Chest 1994, 105, 75S–78S. [Google Scholar] [CrossRef]
- Matalon, S. Mechanisms and regulation of ion transport in adult mammalian alveolar type II pneumocytes. Am. J. Physiol. 1991, 261, C727–C738. [Google Scholar] [CrossRef] [PubMed]
- Nici, L.; Dowin, R.; Gilmore-Hebert, M.; Jamieson, J.D.; Ingbar, D.H. Upregulation of rat lung Na-K-ATPase during hyperoxic injury. Am. J. Physiol. 1991, 261, L307–L314. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-de-Albuquerque, C.F.; Silva, A.R.; Burth, P.; Castro-Faria, M.V.; Castro-Faria-Neto, H.C. Acute respiratory distress syndrome: Role of oleic acid-triggered lung injury and inflammation. Mediators. Inflamm. 2015, 2015, 260465. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xie, Z. Protein interaction and Na/K-ATPase-Mediated signal transduction. Molecules 2017, 22, 990. [Google Scholar] [CrossRef]
- De Brito, T.; Silva, A.; Abreu, P.A.E. Pathology and pathogenesis of human leptospirosis: A commented review. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e23. [Google Scholar] [CrossRef]
- Nicodemo, A.C.; Duarte-Neto, A.N. Pathogenesis of pulmonary hemorrhagic syndrome in human leptospirosis. Am. J. Trop. Med. Hyg. 2021, 104, 1970–1972. [Google Scholar] [CrossRef]
- Goncalves-de-Albuquerque, C.F.; Burth, P.; Silva, A.R.; de Moraes, I.M.; de Jesus Oliveira, F.M.; Santelli, R.E.; Freire, A.S.; Bozza, P.T.; Younes-Ibrahim, M.; de Castro-Faria-Neto, H.C.; et al. Oleic acid inhibits lung Na/K-ATPase in mice and induces injury with lipid body formation in leukocytes and eicosanoid production. J. Inflamm. 2013, 10, 34. [Google Scholar] [CrossRef]
- Goncalves-de-Albuquerque, C.F.; Burth, P.; Silva, A.R.; de Moraes, I.M.; de Oliveira, F.M.; Santelli, R.E.; Freire, A.S.; Younes-Ibrahim, M.; de Castro-Faria-Neto, H.C.; de Castro-Faria, M.V. Na/K-ATPase assay in the intact mice lung subjected to perfusion. BMC Res. Notes 2014, 7, 798. [Google Scholar] [CrossRef]
- Goncalves de Albuquerque, C.F.; Burth, P.; Younes Ibrahim, M.; Garcia, D.G.; Bozza, P.T.; Castro Faria Neto, H.C.; Castro Faria, M.V. Reduced plasma nonesterified fatty acid levels and the advent of an acute lung injury in mice after intravenous or enteral oleic acid administration. Mediators. Inflamm. 2012, 2012, 601032. [Google Scholar] [CrossRef]
- Mayol, V.; Duran, M.J.; Gerbi, A.; Dignat-George, F.; Levy, S.; Sampol, J.; Maixent, J.M. Cholesterol and omega-3 fatty acids inhibit Na, K-ATPase activity in human endothelial cells. Atherosclerosis 1999, 142, 327–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, H.; Zhao, Z.; Peng, Z.; Wang, Z.; Liu, G.; Li, X. Non-esterified fatty acids over-activate the TLR2/4-NF-Kappab signaling pathway to increase inflammatory cytokine synthesis in neutrophils from ketotic cows. Cell Physiol. Biochem. 2018, 48, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-de-Albuquerque, C.F.; Silva, A.R.; Burth, P.; de Moraes, I.M.; Oliveira, F.M.; Younes-Ibrahim, M.; dos Santos Mda, C.; D’Avila, H.; Bozza, P.T.; Faria Neto, H.C.; et al. Oleic acid induces lung injury in mice through activation of the ERK pathway. Mediators. Inflamm. 2012, 2012, 956509. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Santos, M.; Goncalves-de-Albuquerque, C.F.; Castro-Faria-Neto, H.C.; Castro-Faria, M.V.; Burth, P.; Younes-Ibrahim, M. The relationship of oleic acid/albumin molar ratio and clinical outcomes in leptospirosis. Heliyon 2021, 7, e06420. [Google Scholar] [CrossRef] [PubMed]
- Goncalves-de-Albuquerque, C.F.; Barnese, M.R.C.; Soares, M.A.; Castro Faria, M.V.; Silva, A.R.; Castro Faria Neto, H.C.; Burth, P.; Younes-Ibrahim, M. Serum albumin-fatty acid saturation test. MethodsX 2019, 6, 1871–1875. [Google Scholar] [CrossRef]
- Goncalves-de-Albuquerque, C.F.; Barnese, M.R.C.; Soares, M.A.; Castro-Faria, M.V.; Silva, A.R.; de Castro-Faria-Neto, H.C.; Burth, P.; Younes-Ibrahim, M. Serum albumin saturation test based on non-esterified fatty acids imbalance for clinical employment. Clin. Chim. Acta 2019, 495, 422–428. [Google Scholar] [CrossRef]
- Edwards, C.N.; Nicholson, G.D.; Everard, C.O. Thrombocytopenia in leptospirosis. Am. J. Trop. Med. Hyg. 1982, 31, 827–829. [Google Scholar] [CrossRef]
- El-Mallakh, R.S.; Li, R. Is the Na+)-K+-ATPase the link between phosphoinositide metabolism and bipolar disorder? J. Neuropsychiatry Clin. Neurosci. 1993, 5, 361–368. [Google Scholar]
- el Mernissi, G.; Barlet-Bas, C.; Khadouri, C.; Marsy, S.; Cheval, L.; Doucet, A. Characterization and localization of ouabain-insensitive Na-dependent ATPase activities along the rat nephron. Biochim. Biophys. Acta 1991, 1064, 205–211. [Google Scholar] [CrossRef]
- Elger, M.; Bankir, L.; Kriz, W. Morphometric analysis of kidney hypertrophy in rats after chronic potassium depletion. Am. J. Physiol. 1992, 262, F656–F667. [Google Scholar] [CrossRef]
- Ellis, W.A.; O’Brien, J.J.; Bryson, D.G.; Mackie, D.P. Bovine leptospirosis: Some clinical features of serovar hardjo infection. Vet. Rec. 1985, 117, 101–104. [Google Scholar] [CrossRef]
- Emanuel, J.R.; Graw, S.; Housman, D.; Levenson, R. Identification of a region within the Na,K-ATPase alpha subunit that contributes to differential ouabain sensitivity. Mol. Cell. Biol. 1989, 9, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.W. Digitalis. Mechanisms of action and clinical use. N. Engl. J. Med. 1988, 318, 358–365. [Google Scholar] [PubMed]
- Feraille, E.; Barlet-Bas, C.; Cheval, L.; Rousselot, M.; Carranza, M.L.; Dreher, D.; Arystarkhova, E.; Doucet, A.; Favre, H. Presence of two isoforms of Na, K-ATPase with different pharmacological and immunological properties in the rat kidney. Pflugers. Arch. 1995, 430, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers. Arch. 2009, 457, 635–644. [Google Scholar] [CrossRef]
- Rognant, S.; Kravtsova, V.V.; Bouzinova, E.V.; Melnikova, E.V.; Krivoi, I.I.; Pierre, S.V.; Aalkjaer, C.; Jepps, T.A.; Matchkov, V.V. The microtubule network enables Src kinase interaction with the Na,K-ATPase to generate Ca2+ flashes in smooth muscle cells. Front Physiol. 2022, 13, 1007340. [Google Scholar] [CrossRef]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.Y.; Xie, Z.J. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell. 2006, 17, 317–326. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, Z.; Hu, W.; Lou, M.; Zhai, B.; Mei, S.; Hu, Z.; Zhang, L.; Liu, D.; Liu, Z.; et al. Attenuation of the Na/K-ATPase/Src/ROS amplification signaling pathway by astaxanthin ameliorates myocardial cell oxidative stress injury. Mol. Med. Rep. 2020, 22, 5125–5134. [Google Scholar] [CrossRef]
- Madan, N.; Xu, Y.; Duan, Q.; Banerjee, M.; Larre, I.; Pierre, S.V.; Xie, Z. Src-independent ERK signaling through the rat alpha3 isoform of Na/K-ATPase. Am. J. Physiol. Cell Physiol. 2017, 312, C222–C232. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, J. The Na/K-ATPase-mediated signal transduction as a target for new drug development. Front Biosci. 2005, 10, 3100–3109. [Google Scholar] [CrossRef]
- Gable, M.E.; Abdallah, S.L.; Najjar, S.M.; Liu, L.; Askari, A. Digitalis-induced cell signaling by the sodium pump: On the relation of Src to Na(+)/K(+)-ATPase. Biochem. Biophys. Res. Commun. 2014, 446, 1151–1154. [Google Scholar] [CrossRef]
- Silva, C.I.D.; Goncalves-de-Albuquerque, C.F.; Moraes, B.P.T.; Garcia, D.G.; Burth, P. Na/K-ATPase: Their role in cell adhesion and migration in cancer. Biochimie 2021, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Felippe Goncalves-de-Albuquerque, C.; Ribeiro Silva, A.; Ignacio da Silva, C.; Caire Castro-Faria-Neto, H.; Burth, P. Na/K Pump and Beyond: Na/K-ATPase as a modulator of apoptosis and autophagy. Molecules 2017, 22, 578. [Google Scholar] [CrossRef] [PubMed]
- Souza-Souza, K.F.C.; Goncalves-de-Albuquerque, C.F.; Cirne-Santos, C.; Paixao, I.; Burth, P. Alphavirus replication: The role of cardiac glycosides and ion concentration in host cells. Biomed. Res. Int. 2020, 2020, 2813253. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.S.K.F.C.; Moraes, B.P.T.; Paixao, I.; Burth, P.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Na+/K+-ATPase as a target of cardiac glycosides for the treatment of SARS-CoV-2 infection. Front Pharmacol. 2021, 12, 624704. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves-de-Albuquerque, C.F.; Cunha, C.M.C.d.; Castro, L.V.G.d.; Martins, C.d.A.; Barnese, M.R.C.; Burth, P.; Younes-Ibrahim, M. Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase. Microorganisms 2023, 11, 1695. https://doi.org/10.3390/microorganisms11071695
Gonçalves-de-Albuquerque CF, Cunha CMCd, Castro LVGd, Martins CdA, Barnese MRC, Burth P, Younes-Ibrahim M. Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase. Microorganisms. 2023; 11(7):1695. https://doi.org/10.3390/microorganisms11071695
Chicago/Turabian StyleGonçalves-de-Albuquerque, Cassiano Felippe, Carolina Medina Coeli da Cunha, Léo Victor Grimaldi de Castro, Caroline de Azevedo Martins, Marcos Roberto Colombo Barnese, Patrícia Burth, and Mauricio Younes-Ibrahim. 2023. "Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase" Microorganisms 11, no. 7: 1695. https://doi.org/10.3390/microorganisms11071695
APA StyleGonçalves-de-Albuquerque, C. F., Cunha, C. M. C. d., Castro, L. V. G. d., Martins, C. d. A., Barnese, M. R. C., Burth, P., & Younes-Ibrahim, M. (2023). Cellular Pathophysiology of Leptospirosis: Role of Na/K-ATPase. Microorganisms, 11(7), 1695. https://doi.org/10.3390/microorganisms11071695






