Abstract
Bourbon virus (BRBV, family Orthomyxoviridae) is a tickborne virus recently detected in the United States (US). BRBV was first identified from a fatal human case in 2014 in Bourbon County, Kansas. Enhanced surveillance in Kansas and Missouri implicated Amblyomma americanum as the primary vector for BRBV. Historically, BRBV was only detected in the lower midwestern US, but since 2020 it has been reported in North Carolina, Virginia, New Jersey, and New York State (NYS). This study aimed to elucidate genetic and phenotypic characteristics of BRBV strains from NYS through whole genome sequencing and the assessment of replication kinetics in mammalian cultures and A. americanum nymphs. Sequence analysis revealed the existence of two divergent BRBV clades circulating in NYS. BRBV NY21-2143 is closely related to the midwestern BRBV strains but has unique substitutions in the glycoprotein. Two other NYS BRBV strains, BRBV NY21-1814 and BRBV NY21-2666, form a distinct clade unique from previously sequenced BRBV strains. Phenotypic diversification was also detected in NYS BRBV strains compared to each other and midwestern BRBV strains, with BRBV NY21-2143 displaying attenuation in rodent-derived cell culture and a fitness advantage in experimentally infected A. americanum. These data suggest genetic and phenotypic diversification of emergent BRBV strains circulating in NYS that could contribute to increased spread of BRBV in the northeastern US.
1. Introduction
Bourbon virus (BRBV), a negative-sense, segmented RNA virus, belongs to the Orthomyxoviridae family, which consists of seven genera: four genera containing influenza viruses, one genera of salmon-specific viruses, and two genera of arthropod-borne (arbo) viruses [1]. BRBV is a part of the Thogotovirus genus that includes tick-transmitted arboviruses, Dhori, Oz, Thogoto, and Upolu viruses [1,2,3,4]. These viruses are predominantly found in Asia, Africa, and the Middle East, while BRBV and Aransas Bay virus are the only identified thogotoviruses in North America. BRBV is a human pathogen while Aransas Bay virus primarily circulates in wildlife [5].
All arboviral thogotoviruses are maintained between invertebrate and mammalian hosts with differences in the primary vector matching the known geographic range [1]. Thogotoviruses outside of North America are maintained by Hyalomma and Amblyomma spp. ticks and some mosquito species [1,2,4]. Amblyomma americanum have been incriminated as the primary vector of BRBV after expanded surveillance and testing of multiple tick species (Amblyomma spp., Dermacentor spp., Ixodes spp., and Haemaphysalis spp.) in Kansas and Missouri [6,7]. Additionally, laboratory studies demonstrated the vector competence of A. americanum with vertical and cofeeding transmission observed following experimental infection of naïve ticks [8]. While BRBV has primarily been isolated from A. americanum, detection in H. longicornus in Virginia highlights potential roles for other tick genera in BRBV maintenance in nature [9]. A mammalian reservoir for BRBV has not been identified, but serological testing in Missouri and New York identified white-tailed deer (Odocoileus virginianus) and/or raccoons (Procyon lotor) as potential sentinels for tracking BRBV spread [10,11]. Human sera testing has also determined that ~1% of people living in endemic regions of Missouri have neutralizing antibodies against BRBV [12].
The first human case of BRBV was identified in 2014 from Bourbon County, Kansas [13]. Nonspecific symptoms, including severe febrile illness following a reported tick bite, led to hospitalization of the patient. Eleven days after symptom onset, the patient died. Since 2014, four additional human cases have been identified in the midwestern United States (US) [13,14,15,16]. Symptoms in each case were associated with tick exposure and onset of symptoms including weakness, fatigue, and nausea 2–7 days post reported tick bite. Leukopenia and thrombocytopenia were observed in all three fatal cases of BRBV and now act as potential identifiers of BRBV infection in humans [1,13]. Fatal cases are associated with shock, organ failure, cardiac dysregulation, and acute bone marrow suppression [13,16]. BRBV detection in A. americanum and human cases also overlap with the geographic range of Heartland virus (family Phenuiviridae), which similarly presents as nonspecific flu-like symptoms after tick exposure, making clinical diagnosis and treatment difficult [17,18]. The emergence of a human pathogenic thogotovirus has prompted investigation of antivirals and vaccines against BRBV, but no specific preventative or therapeutic interventions are currently available [15,19].
While human cases have only been identified in the midwestern US, BRBV has been detected in A. americanum ticks across the midwestern, southeastern, and northeastern US. Similar to Ixodes spp., Amblyomma spp.’s geographic range has increased northward as a result of climate change and host expansion [20,21]. Historically, BRBV was only detected in Missouri, Kansas, and Oklahoma, but since 2020, BRBV has been reported in North Carolina, Virginia, New Jersey, and New York State (NYS) [9,11,22,23]. Despite these recent detections, the characterization of BRBV strains has been limited to midwestern isolates [24,25]. Therefore, we aimed to assess the genetic and phenotypic diversity of the first BRBV isolates from NYS through whole genome sequencing and phenotypic characterization in vitro and in vivo. Our results demonstrate important strain and host-specific differences that inform our understanding of BRBV evolution and transmission potential.
2. Materials and Methods
2.1. Viruses
All work was conducted in a biosafety level 3 facility at the New York State Department of Health (NYSDOH). Viral positives were identified through the NYSDOH tick surveillance and testing programs [11]. BRBV-positive tick pools were identified using a quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assay as described previously [11]. All NYS BRBV positive tick pools were A. americanum nymphs collected in Suffolk County, NYS, in 2021. Viral isolates were obtained by amplification of positive tick homogenates on African green monkey kidney cells (Vero, ATCC, CCL-81). Virus positive cultures were confirmed by qRT-PCR, harvested, clarified, and supplemented with heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT, USA).
2.2. Sequencing
RNA was extracted from qRT-PCR positive tick pool homogenates using an automated MagMAX nucleic acid extraction kit and associated instrument (ThermoFisher Scientific, Waltham, MA, USA). Full genome sequences were obtained by amplification of each segment (Table S1) using a Superscript III One-Step RT-PCR kit (ThermoFisher Scientific, Waltham, MA, USA). Segment amplicons were pooled for next generation sequencing (NGS) carried out on an Illumina MiSeq platform (2 × 500 bp PE) (Illumina, San Diego, CA, USA) at the Wadsworth Center Advanced Genomics Technologies Core.
Reads were paired, merged, and mapped to a BRBV reference genome (Bourbon virus strain original: KU708248-55) in Geneious Prime 2021.2 to obtain consensus sequences. Individual amino acid substitutions were identified based on MAFFT alignments of full genome consensus sequences for each segment compared to available sequences from Genbank (Table S2) [26]. Phylogenetic trees were generated based on this MAFFT alignment using the Geneious Tree Builder function for neighbor joining trees, Tamura-Nei distances, and 1000 bootstraps. Trees were visualized in iTOL Interactive Tree of Life v6.
2.3. In Vitro Growth Kinetics
African green monkey kidney cells (Vero, ATCC, CCL-81), baby hamster kidney cells (BHK-21, ATCC, CCL-10), and human hepatocellular carcinoma cells (Huh7, ATCC) were grown in minimal essential media (MEM, Gibco, Invitrogen Corp, Carlsbad, CA, USA) with heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and maintained at 37 °C, 5% CO2. Confluent monolayers were generated by seeding six-well plates with the respective cell type and were maintained for 3–4 days prior to experimental infection to reach confluency (1 × 106 cells per well).
Viral growth kinetics in each cell line were carried out as previously described [27]. Briefly, six-well tissue culture plates of confluent monolayers of each respective cell type were inoculated in triplicate with a multiplicity of infection (MOI) of 0.01 plaque-forming units (PFU) per cell. Inoculum was allowed to adsorb for 1 h at 37 °C, 5% CO2 followed by washing the wells with 2 mL of phosphate-buffered saline (PBS) three times. Maintenance media was added, and 0.1 mL of supernatant was collected at 24-h intervals up to 120 h post-infection (HPI). Samples were stored at −70 °C in a 1:10 dilution of BA-1 media (M199 medium with Hank’s salts, 1% bovine albumin, TRIS base (tris [hydroxymethyl] aminomethane, sodium bicarbonate, 20% FBS, and antibiotics) until processing.
Viral load was determined by plaque assay on Vero cells. Confluent monolayers were inoculated with 0.1 mL of sample in duplicate and were allowed to adsorb for 1 h at 37 °C, 5% CO2. An overlay of MEM, 10% FBS, and 0.6% oxoid agar was added to each well after adsorption and allowed to incubate for 5 days before the addition of a second overlay containing MEM, 2% FBS, 0.6% oxoid agar, and 2% neutral red (Sigma-Aldrich Co. St. Louis, MO, USA). After an overnight incubation at 37 °C, 5% CO2, plaques were counted, and viral titers were determined as PFU/mL. Replication curves were generated based on mean titer and were analyzed using paired t-tests. All analyses and visualizations were carried out in GraphPad Prism version 9.0.1.
2.4. Synchronous Infection of Ticks and Viral Detection
The following reagent was provided by the Centers for Disease Control and Prevention for distribution by Biodefense and Emerging Infections (BEI) Resources, National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): Amblyomma americanum Nymph (Live, NR-44124). Ticks were maintained at 95% relative humidity (RH), 20 °C, and in a 16:8 light:dark cycle. Whole nymphal ticks were infected by immersion as previously described [27,28,29]. Briefly, nymphs were held at reduced RH (40–65%) for 48 h prior to infection, followed by suspension in a 1 × 106 dilution of each respective strain. Ticks were immersed for 1 h at 34 °C, washed with PBS twice, and dried individually before storage in Plaster of Paris jars. At 7-, 14-, 21-, and 28-days post-infection (DPI), whole ticks were moved to individual tubes with 5 mm stainless steel BBs (Daisy Outdoor Products, Roger, AR, USA) and were stored at −70 °C until processing.
For processing, whole nymphs were mechanically homogenized in 0.6 mL of diluent (20% FBS in Dulbecco’s PBS, 50 ug/mL penicillin/streptomycin, 5 ug/mL gentamicin, and 2 ug/mL of fungizone (Sigma-Aldrich, St. Louis, MO, USA)) using a Retsch Mixer Mill, MM 301 (Retsch Inc., Newtown, PA, USA) at 30 cycles/s for 4 min. RNA was then extracted from tick homogenates using the MagMAX nucleic acid extraction kit and was tested for BRBV using the qRT-PCR assay described above [11]. Viral load was determined based on a standard curve of ten-fold dilutions of a known BRBV viral stock and was expressed as a relative PFU/mL. Viral loads were compared by nonparametric one-way ANOVA with Friedman’s multiple comparisons post-hoc tests and infection rates were compared using Chi-squared tests conducted in GraphPad Prism version 9.0.1.
3. Results
3.1. NYS BRBV Strains Are Genetically Distinct from Midwestern US BRBV Strains
To determine the genetic profile of recently isolated NYS BRBV strains—BRBV NY21-1814, BRBV NY21-2143, and BRBV NY21-2666—full genome sequencing was conducted and consensus sequences of each segment were compared to available midwestern BRBV isolates from the US and representative Dhori-like and Thogoto-like strains (Figure 1). Compared to other Dhori and Thogoto viruses, all US BRBV strains cluster together and share 93–99% similarity across all segments at the nucleotide level (Table 1). Despite close genetic similarity, BRBV NY21-1814 and BRBV NY21-2666 cluster separately from the midwestern US BRBV strains and BRBV NY21-2143 in segments 1–4. This separation is a result of a ~6% difference at the nucleotide level, while segment 6 (matrix protein) only differs by ~3%. Synonymous mutations account for the majority of the genetic variability in all segments. Synonymous mutations shared between BRBV NY21-1814 and BRBV NY21-2666 were primarily located in segment 1 (PB2) and segment 3 (PA), while BRBV NY21-2143 displayed the largest number of mutations in segment 4 (GP) relative to midwestern BRBV strains. Shared synonymous mutations between all NYS BRBV strains were found in segment 1 (PB2) and segment 3 (PA). The highest percentage of variable nucleotides (6.9%) was identified in segment 3.
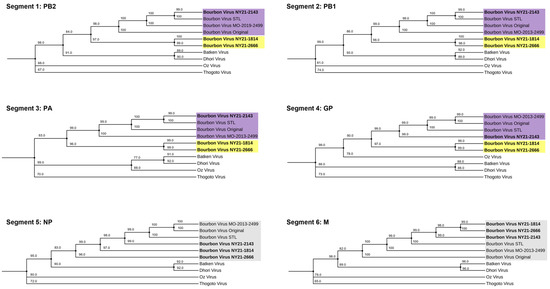
Figure 1.
Maximum likelihood phylogeny of Bourbon virus (BRBV) and related Orthomyxoviruses based on the coding region of segments 1–6. Bootstrap values are displayed at each branchpoint (range: 65–100). Recent BRBV strains from New York State (NYS) cluster separately (yellow) from midwestern BRBV strains (purple) with the exception of BRBV NY21-2143 in segments 1–4. All BRBV strains cluster together in segments 5 and 6 (gray).

Table 1.
Nucleotide identity of Bourbon virus (BRBV) strains isolated in New York State (NYS) compared to midwestern Bourbon virus strains: BRBV Original, BRBV MO2013-2499, and BRBV-STL based on coding sequence. BRBV NY21-2143 shows the highest sequence identity to midwestern BRBV strains.
BRBV NY21-1814 and BRBV NY21-2666 share 33 amino acid substitutions which are distinct from all other US BRBV strains, including BRBV NY21-2143 (Table 2). These substitutions are primarily concentrated in segment 3 (PA), yet some variability exists in all segments. Although BRBV NY21-1814 and 2666 are genetically similar, they contain three and four unique substitutions, respectively. BRBV NY21-2143 has one unique substitution in segment 3 (PA) and two substitutions in segment 4 (GP). The only amino acid substitutions shared between all three NYS BRBV strains are found in segment 3 (PA- I224V and M636I). Notably, segment 5 (NP) is highly conserved across all US BRBV strains except for a single amino acid substitution shared between BRBV NY21-1814 and BRBV NY21-2666 (S300N).

Table 2.
Novel substitutions identified in recent Bourbon virus (BRBV) strains isolated in New York State (NYS) based on coding sequence compared to midwestern BRBV strains BRBV-Original, BRBV-MO2013, and BRBV-STL. Amino acid substitutions present in one strain and shared across multiple strains.BRBV NY21-1814 and BRBV NY21-2666 share many unique substitutions (n = 33) while BRBV NY21-2143 shares only two substitutions with the other NYS strains (bolded). Most substitutions were detected in segment 3 (PA).
3.2. NYS BRBV Strains Display Phenotypic Variability in Mammalian Cells
Viral replication kinetics were determined in distinct mammalian cell lines to better understand phenotypic differences associated with unique genotypes of NYS BRBV strains. BRBV Original was used as a representative strain from the midwestern US BRBV isolates (Table S2). Growth kinetics were assessed in baby hamster kidney (BHK-21), African green monkey kidney (Vero), and human hepatoma (Huh7) cell lines to recapitulate maintenance in small mammalian hosts associated with the tick life cycle (BHK-21 and Vero) and human hosts (Huh7). Kinetics in BHK-21 were significantly different across all BRBV strains assessed (Figure 2A). BRBV Original replicated to the highest levels, followed by BRBV NY21-1814 (Figure 2A, Paired t-test, * p = 0.0314). Of note, BRBV NY21-2143 displayed significant attenuation relative to BRBV Original and BRBV NY21-1814 (Figure 2A, Paired t-test, ** p = 0.0054 and ** p = 0.0072, respectively). However, strain-specific growth kinetics were unique in Vero and Huh7 cultures, as a significant growth deficit was detected for BRBV NY21-1814 relative to BRBV Original and BRBV NY21-2143 (Figure 2B,C, Paired t-test, Vero *** p = 0.0004 and **** p < 0.0001, Huh7 * p = 0.0250 and ** p = 0.0044, respectively).
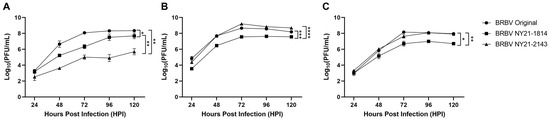
Figure 2.
Growth kinetics of recent Bourbon virus (BRBV) strains from New York State (NYS) compared to the midwestern BRBV Original strain in baby hamster kidney ((A) BHK-21), African green monkey kidney ((B) Vero), and human hepatoma cells ((C) Huh7). Data points represent mean +/− SEM (n = 3 per strain). NYS BRBV strains showed variable kinetics across cell types. BRBV NY21-2143 showed significant attenuation compared to other BRBV strains in BHK-21 ((A) paired t-test, BRBV Original ** p = 0.0054 and BRBV NY21-1814 ** p = 0.0072). There were also significant differences between BRBV Original and BRBV NY21-1814 viral loads in BHK-21 ((A) paired t-test, * p = 0.0314). Replicative fitness displayed similar trends in Vero and Huh7 with BRBV Original and BRBV NY21-2143 exhibiting significantly higher viral loads compared to BRBV NY21-1814 ((B) Vero, paired t-test, *** p = 0.0004 and **** p < 0.0001, (C) Huh7, * p = 0.0250 and ** p =0.0044, respectively).
3.3. Fitness Advantage of NYS BRBV Strain BRBV NY21-2143 in Experimentally Infected Amblyomma americanum
To investigate the influence of BRBV strain on phenotypic variability in invertebrate hosts, nymphal A. americanum were infected by immersion and collected at 7-, 14-, 21-, and 28-days post-infection (DPI). Combined infection rates from all timepoints (~60%) were not significantly different (Chi-squared test, p = 0.09). Despite this, strain had a significant effect on overall viral load (nonparametric one-way ANOVA, * p = 0.0417). BRBV NY21-2143 grew to significantly higher viral loads than BRBV NY21-1814 (Figure 3, nonparametric one-way ANOVA with Friedman’s multiple comparisons post-hoc test, * p = 0.0400). Lower viral loads were also measured for BRBV Original relative to BRBV NY21-2143. While not statistically significant overall (p = 0.23), BRBV NY21-2143 viral load was significantly higher than that of BRBV Original at 28 DPI (t-test, * p = 0.039). These data indicate that BRBV NY21-2143 has an overall fitness advantage in nymphal A. americanum compared to midwestern and NYS BRBV strains.
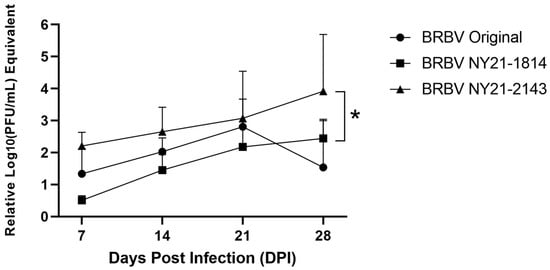
Figure 3.
Growth kinetics of recent Bourbon virus (BRBV) strains from New York State (NYS) relative to the midwestern BRBV Original strain in experimentally infected Amblyomma americanum following immersion. Overall, BRBV NY21-2143 grew to significantly higher viral loads relative to BRBV NY21-1814 (Nonparametric one-way ANOVA with Friedman’s multiple comparisons post-hoc test, * p = 0.0400). BRBV NY21-2143 viral loads were not significantly higher overall relative to BRBV Original but were significantly higher at 28 days post-infection (DPI) (t-test, * p = 0.039).
4. Discussion
This study reported the genotypic and phenotypic characteristics of BRBV strains isolated in the northeastern US. Whole genome sequencing revealed the existence of two distinct clades in NYS, including a novel genotype divergent from previously sequenced midwestern strains. Previous studies indicate that tickborne viruses generally evolve slowly, suggesting this genetic separation is indicative of both distinct historical foci of BRBV and separate introductions into NYS [30]. While BRBV is a recently recognized thogotovirus, it has likely been circulating outside of the midwestern US undetected. However, BRBV may have recently spread to the northeast due to changing environmental conditions that created more suitable habitats for the primary vector A. americanum, sentinel mammals such as white-tailed deer, and anthropogenic land use changes that have altered biodiversity in this region [31,32,33]. A. americanum populations are already established in southern NYS and climate suitability models have predicted a continued expansion northward [21,34,35]. Additionally, white-tailed deer can support all life stages of A. americanum and have seen drastic increases in population density compared to historic counts [33,36,37].
The introduction of BRBV into NYS may have been facilitated by the dispersal of infected immature stages of A. americanum by birds during migration or post-breeding dispersal. Common songbird species including the Northern Cardinal (Cardinalis cardinalis), American Robin (Turdus migratorius), Song Sparrow (Melospiza melodia), Hermit Thrush (Catharus guttatus), and Gray Catbird (Dumetella carolinensis) span the midwestern US and southern NYS and are known to be infested with a diverse range of ectoparasites including A. americanum, I. scapularis, and D. variabilis [38]. This hypothesis is similar to the proposed spread of thogotoviruses from Africa to Europe [39]. The migration and long-distance movement of birds have been implicated in the expansion of other tickborne pathogens, primarily Borrelia burgdorferi, throughout the US and Canada [40,41,42,43,44].
The patterns of genetic variation in NYS BRBV genomes suggest segment-specific evolutionary pressures. Segment 3 (PA) is the most genetically diverse, followed by segments 1 (PB2) and 2 (PB1), two other components of the viral polymerase complex. The diversity of RNA viruses, which can be important for maintenance during host cycling, is largely facilitated by error-prone RNA-dependent RNA polymerases (RdRps), high replication rates, and gene-specific purifying selection [45]. Unlike the variability observed in the polymerase complex, both segments 5 (NP) and 6 (M) are highly conserved across all BRBV strains, despite geographic origin. This is consistent with previous studies that found that both segments act as immune antagonists under strict pressures within mammalian hosts [46,47,48,49,50].
Phenotypic diversity was observed between all NYS BRBV strains as compared to a representative midwestern strain in both in vitro and in vivo systems. Despite all three cell lines being interferon-incompetent (IFN), distinct, strain-specific fitness was observed [51,52,53]. Of note, growth kinetics were similar between the primate-derived cell lines Vero and Huh7, but distinct from the rodent-derived cell line BHK-21. Inherent differences between primates and rodents, including host codon usage, host receptor structures, and gene expression patterns, could contribute to the variability observed [54,55,56]. While BRBV NY21-1814 displayed significant attenuation in Vero and Huh7 compared to BRBV NY21-2143 and BRBV Original, it exhibited an intermediate phenotype in BHK-21. BRBV NY21-2143 grew to similar viral loads as BRBV Original in Vero and Huh7 but showed a significant growth deficit in BHK-21. This difference further supports host-dependent mechanisms leading to differing phenotypes of BRBV NYS strains. Replicative fitness in experimentally-infected A. americanum was also distinct among BRBV strains. BRBV NY21-2143 displayed a fitness advantage compared to BRBV Original and BRBV NY21-1814. Given the attenuation of this strain in BHK-21 cells, these results are consistent with an adaptive trade-off often noted in other arboviral systems [57,58]. To better understand strain-specific fitness, virulence, and the role of distinct substitutions in viral fitness, assessment in an animal model is needed. Animal models could further reveal strain-specific pathogenesis in mammalian hosts and be utilized to assess transmission from tick hosts. This, however, is challenging given that there are currently no established animal models for BRBV [15,24].
Despite BRBV NY21-1814 and BRBV NY21-2666 displaying the most genetic divergence, BRBV NY21-2143 was the most phenotypically distinct compared to other NYS and midwestern BRBV strains, with a majority of substitutions identified in segment 4 (GP). BRBV GP is unique in that thogoto- (THOV) and dhori-like virus (DHOV) GPs are highly homologous to the insect-specific baculovirus glycoprotein 64 (gp64) as compared to the envelope GPs of influenza viruses [59,60]. Baculovirus gp64 plays important roles in insect host cell entry and host range determination [61,62,63]. The unique GP substitutions identified in BRBV NY21-2143 could be responsible for the attenuation exhibited in rodent cell lines and fitness advantage in A. americanum. The GP is important for early viral processes such as host cell attachment, entry, and release into the host cell, which could contribute to this observed phenotypic variation. To further elucidate the role of these GP substitutions in NYS BRBV strain- and host-specific fitness, the development of novel genetic tools is needed. Currently, these tools are limited to reporter systems, and previous work is confined to THOV GPs and structural comparisons of orthomyxovirus GPs [19,59,64]. These studies, however, provide important insights into the potential consequences of these substitutions as THOV GPs have been shown to be physiologically active at tick pH (6–6.5) compared to mammalian pH (human-7.3) [65]. Additionally, when compared to known GP structures of THOV, DHOV, midwestern BRBV, and baculoviruses, the unique BRBV NY21-2143 GP substitutions reside in a domain that is highly sensitive to pH-dependent conformational changes [60]. While the BRBV NY21-2143 substitutions do not align with important cysteine or glycosylation sites identified in these other orthomyxoviruses, there is potential for these substitutions to contribute to the fitness tradeoff observed in experimental mammalian and invertebrate systems [59,60,64,65].
Overall, these studies highlight the need to maintain robust tickborne disease surveillance programs across states in this region. NYS has a widespread collection and testing program that facilitated the detection of BRBV; however, increased surveillance in other states with established A. americanum populations, in concert with genetic and phenotypic characterization, is vital for tracking viral spread and diversity. Additionally, the vector competence of other tick species present in the northeast for BRBV is unknown and spillover events into new transmission cycles could further expand BRBV establishment. These studies emphasize the importance of educational outreach to practitioners and the public to spread awareness of human disease symptoms and individual preventative measures when spending time outdoors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11061590/s1. Table S1: Primer sets used for whole genome sequencing of recent Bourbon virus (BRBV) strains from New York State. Table S2: Bourbon virus (BRBV) strains used in mutational analysis and phylogenetic trees including New York State BRBV strains, midwestern BRBV strains, and distantly related orthomyxoviruses Batken, Dhori, Oz, and Thogoto viruses.
Author Contributions
R.E.L., A.P.D.II and A.T.C. conceptualized the study. R.E.L. conducted all experiments, analysis, and wrote the original draft. R.E.L., A.P.D.II and A.T.C. reviewed and edited the manuscript. Funding for the project was acquired by A.T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Cooperative Agreement Number U01CK000509 funded by the Northeast Center of Excellence. This work was also supported by the National Institutes of Health RNA Science and Technology in Health and Disease Training Grant provided by the Office of Graduate Studies and the RNA Institute of the University of Albany. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Data Availability Statement
Data is contained within the article and Supplementary Material. Sequence data is publically available in NCBI GenBank [accession numbers can be found in Table S2].
Acknowledgments
The authors would like to thank the Wadsworth Center Media and Tissue Culture Core for providing media and cells and the Wadsworth Center Advanced Genomic Technologies Core for sequencing. We also thank the Center for Disease Control and Prevention BEI Resources for providing live ticks.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hao, S.; Ning, K.; Kuz, C.A.; McFarlin, S.; Cheng, F.; Qiu, J. Eight Years of Research Advances in Bourbon Virus, a Tick-borne Thogotovirus of the Orthomyxovirus Family. Zoonoses 2022, 2. [Google Scholar] [CrossRef]
- Anderson, C.R.; Casals, J. Dhori virus, a new agent isolated from Hyalomma dromedarii in India. Indian J. Med. Res. 1973, 61, 1416–1420. [Google Scholar]
- Briese, T.; Chowdhary, R.; da Rosa, A.T.; Hutchison, S.K.; Popov, V.; Street, C.; Tesh, R.B.; Lipkin, W.I. Upolu virus and Aransas Bay virus, two presumptive bunyaviruses, are novel members of the family Orthomyxoviridae. Virol. J. 2014, 88, 5298–5309. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, H.; Lim, C.K.; Isawa, H.; Fujita, R.; Murota, K.; Sato, T.; Kobayashi, D.; Kan, M.; Hattori, M.; Kimura, T.; et al. Characterization of a novel thogotovirus isolated from Amblyomma testudinarium ticks in Ehime, Japan: A significant phylogenetic relationship to Bourbon virus. Virus Res. 2018, 249, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yunker, C.E.; Clifford, C.M.; Keirans, J.E.; Thomas, L.A.; Rice, R.C.A. Aransas Bay virus, a new arbovirus of the Upolu serogroup from Ornithodoros capensis (Acari: Argasidae) in coastal Texas. J. Med. Entomol. 1979, 16, 453–460. [Google Scholar] [CrossRef]
- Savage, H.M.; Burkhalter, K.L.; Godsey, M.S., Jr.; Panella, N.A.; Ashley, D.C.; Nicholson, W.L.; Lambert, A.J. Bourbon virus in field-collected ticks, Missouri, USA. Emerg. Infect. Dis. 2017, 23, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Godsey, M.S., Jr.; Panella, N.A.; Burkhalter, K.L.; Manford, J.; Trevino-Garrison, I.C.; Straily, A.; Wilson, S.; Bowen, J.; Raghavan, R.K. Surveillance for tick-borne viruses near the location of a fatal human case of bourbon virus (Family Orthomyxoviridae: Genus Thogotovirus) in Eastern Kansas, 2015. J. Med. Entomol. 2018, 55, 701–705. [Google Scholar] [CrossRef]
- Godsey, M.S., Jr.; Rose, D.; Burkhalter, K.L.; Breuner, N.; Bosco-Lauth, A.M.; Kosoy, O.I.; Savage, H.M. Experimental infection of Amblyomma americanum (Acari: Ixodidae) with bourbon virus (Orthomyxoviridae: Thogotovirus). J. Med. Entomol. 2021, 58, 873–879. [Google Scholar] [CrossRef]
- Cumbie, A.N.; Trimble, R.N.; Eastwood, G. Pathogen spillover to an invasive tick species: First detection of Bourbon virus in Haemaphysalis longicornis in the United States. Pathogens 2022, 11, 454. [Google Scholar] [CrossRef]
- Jackson, K.C.; Gidlewski, T.; Root, J.J.; Bosco-Lauth, A.M.; Lash, R.R.; Harmon, J.R.; Brault, A.C.; Panella, N.A.; Nicholson, W.L.; Komar, N. Bourbon virus in wild and domestic animals, Missouri, USA, 2012–2013. Emerg. Infect. Dis. 2019, 25, 1752. [Google Scholar] [CrossRef]
- Dupuis, I.I.; Alan, P.; Prusinski, M.A.; O’Connor, C.; Maffei, J.G.; Koetzner, C.A.; Zembsch, T.E.; Zink, S.D.; White, A.L.; Santoriello, M.P.; et al. Bourbon Virus Transmission, New York, USA. Emerg. Infect. Dis. 2023, 29, 145–148. [Google Scholar] [CrossRef]
- Bamunuarachchi, G.; Harastani, H.; Rothlauf, P.W.; Dai, Y.N.; Ellebedy, A.; Fremont, D.; Whelan, S.P.J.; Wang, D.; Boon, A.C.M. Detection of Bourbon virus-specific serum neutralizing antibodies in human serum in Missouri, USA. Msphere 2022, 7, e00164-22. [Google Scholar] [CrossRef]
- Kosoy, O.I.; Lambert, A.J.; Hawkinson, D.J.; Pastula, D.M.; Goldsmith, C.S.; Hunt, D.C.; Staples, J.E. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg. Infect. Dis. 2015, 21, 760. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. Bourbon Virus Linked to Death of Park Official—CDC Testing Tick Samples. The Missourian. 8 March 2018. Available online: https://www.emissourian.com/local_news/county/bourbon-virus-linked-to-deathof-park-official—Cdc-testing-tick-samples/article_057f0f0a-bddd-5f7a-aa0d-735187e4c379.html (accessed on 31 October 2021).
- Bricker, T.L.; Shafiuddin, M.; Gounder, A.P.; Janowski, A.B.; Zhao, G.; Williams, G.D.; Boon, A.C. Therapeutic efficacy of favipiravir against Bourbon virus in mice. PLoS Pathog. 2019, 15, e1007790. [Google Scholar] [CrossRef] [PubMed]
- Roe, M.K.; Huffman, E.R.; Batista, Y.S.; Papadeas, G.G.; Kastelitz, S.R.; Restivo, A.M.; Stobart, C.C. Comprehensive Review of Emergence and Virology of Tickborne Bourbon Virus in the United States. Emerg. Infect. Dis. 2023, 29, 1–7. [Google Scholar] [CrossRef]
- Aziati, I.D.; McFarland, D., Jr.; Antia, A.; Joshi, A.; Aviles-Gamboa, A.; Lee, P.; Harastani, H.; Wang, D.; Adalsteinsson, S.A.; Boon, A.C. Prevalence of Bourbon and Heartland viruses in field collected ticks at an environmental field station in St. Louis County, Missouri, USA. Ticks Tick Borne Dis. 2023, 14, 102080. [Google Scholar] [CrossRef] [PubMed]
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland virus epidemiology, vector association, and disease potential. Viruses 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Ning, K.; Wang, X.; Wang, J.; Cheng, F.; Ganaie, S.S.; Tavis, J.E.; Qiu, J. Establishment of a Replicon Reporter of the Emerging Tick-Borne Bourbon Virus and Use It for Evaluation of Antivirals. Front. Microbiol. 2020, 11, 572631. [Google Scholar] [CrossRef]
- Tokarz, R.; Williams, S.H.; Sameroff, S.; Leon, M.S.; Jain, K.; Lipkin, W.I. Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. Virol. J. 2014, 88, 11480–11492. [Google Scholar] [CrossRef]
- Sagurova, I.; Ludwig, A.; Ogden, N.H.; Pelcat, Y.; Dueymes, G.; Gachon, P. Predicted Northward Expansion of the Geographic Range of the Tick Vector Amblyomma americanum in North America under Future Climate Conditions. Environ. Health Perspect. 2019, 127, 107014. [Google Scholar] [CrossRef]
- Komar, N.; Hamby, N.; Palamar, M.B.; Staples, J.E.; Williams, C. Indirect evidence of Bourbon virus (Thogotovirus, Orthomyxoviridae) infection in North Carolina. N. C. Med. J. 2020, 81, 214–215. [Google Scholar] [CrossRef]
- Egizi, A.; Wagner, N.E.; Jordan, R.A.; Price, D.C. Lone star ticks (Acari: Ixodidae) infected with Bourbon virus in New Jersey, USA. J. Med. Entomol. 2023, tjad052. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Lamkiewicz, K.; Kolesnikova, L.; Hölzer, M.; Marz, M.; Kochs, G. Comparative study of ten thogotovirus isolates and their distinct in vivo characteristics. Virol. J. 2022, 96, e01556-21. [Google Scholar] [CrossRef]
- Lambert, A.J.; Velez, J.O.; Brault, A.C.; Calvert, A.E.; Bell-Sakyi, L.; Bosco-Lauth, A.M.; Staples, J.E.; Kosoy, O.I. Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus Thogotovirus. J. Clin. Virol. 2015, 73, 127–132. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.E.; Dupuis, A.P., II.; Prusinski, M.A.; Maffei, J.G.; Koetzner, C.A.; Ngo, K.; Backenson, B.; Oliver, J.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Identification and characterization of novel lineage 1 Powassan virus strains in New York State. Emerg. Microbes Infect. 2023, 12, 2155585. [Google Scholar] [CrossRef]
- Policastro, P.F.; Schwan, T.G. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J. Med. Entomol. 2003, 40, 364–370. [Google Scholar] [CrossRef]
- Mitzel, D.N.; Wolfinbarger, J.B.; Long, R.D.; Masnick, M.; Best, S.M.; Bloom, M.E. Tick-borne flavivirus infection in Ixodes scapularis larvae: Development of a novel method for synchronous viral infection of ticks. Virology 2007, 365, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Bondaryuk, A.N.; Kulakova, N.V.; Belykh, O.I.; Bukin, Y.S. Dates and Rates of Tick-Borne Encephalitis Virus-The Slowest Changing Tick-Borne Flavivirus. Int. J. Mol. Sci. 2023, 24, 2921. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of Land Use Changes and Habitat Fragmentation on the Eco-epidemiology of Tick-Borne Diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef]
- Rochlin, I.; Egizi, A.; Ginsberg, H.S. Modeling of historical and current distributions of lone star tick, Amblyomma americanum (Acari: Ixodidae), is consistent with ancestral range recovery. Exp. Appl. Acarol. 2023, 89, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Yabsley, M.J. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum—Associated zoonoses in the United States. Curr. Top. Microbiol. 2007, 315, 289–324. [Google Scholar]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.I.; Hamer, S.A.; Han, S.; Sidge, J.L.; Hickling, G.J. The Contribution of Wildlife Hosts to the Rise of Ticks and Tick-Borne Diseases in North America. J. Med. Entomol. 2021, 58, 1565–1587. [Google Scholar] [CrossRef]
- Warren, R.J. Deer overabundance in the USA: Recent advances in population control. Anim. Prod. Sci. 2011, 51, 259–266. [Google Scholar] [CrossRef]
- Loss, S.R.; Noden, B.H.; Hamer, G.L.; Hamer, S.A. A quantitative synthesis of the role of birds in carrying ticks and tick-borne pathogens in North America. Oecologia 2016, 182, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int. J. Environ. 2020, 17, 2117. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef]
- Mukherjee, N.; Beati, L.; Sellers, M.; Burton, L.; Adamson, S.; Robbins, R.G.; Moore, F.; Karim, S. Importation of exotic ticks and tick-borne spotted fever group rickettsiae into the United States by migrating songbirds. Ticks Tick Borne Dis. 2014, 5, 127–134. [Google Scholar] [CrossRef]
- Schneider, S.C.; Parker, C.M.; Miller, J.R.; Fredericks, L.P.; Allan, B.F. Assessing the contribution of songbirds to the movement of ticks and Borrelia burgdorferi in the Midwestern United States during fall migration. Ecohealth 2015, 12, 164–173. [Google Scholar] [CrossRef]
- Brinkerhoff, R.J.; Folsom-O’Keefe, C.M.; Streby, H.M.; Bent, S.J.; Tsao, K.; Diuk-Wasser, M.A. Regional variation in immature Ixodes scapularis parasitism on North American songbirds: Implications for transmission of the Lyme pathogen, Borrelia burgdorferi. J. Med. Entomol. 2011, 48, 422–428. [Google Scholar] [CrossRef]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- Lauring, A.S.; Andino, R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010, 6, e1001005. [Google Scholar] [CrossRef]
- Wagner, V.; Sabachvili, M.; Bendl, E.; Fuchs, J.; Kochs, G. The Antiviral Activity of Equine Mx1 against Thogoto Virus Is Determined by the Molecular Structure of Its Viral Specificity Region. Virol. J. 2023, 97, e01938-22. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.; Preuss, E.; Mayer, D.; Weber, F.; Schwemmle, M.; Kochs, G. The interferon antagonist ML protein of thogoto virus targets general transcription factor IIB. Virol. J. 2008, 82, 11446–11453. [Google Scholar] [CrossRef] [PubMed]
- Jennings, S.; Martínez-Sobrido, L.; García-Sastre, A.; Weber, F.; Kochs, G. Thogoto virus ML protein suppresses IRF3 function. Virology 2005, 331, 63–72. [Google Scholar] [CrossRef]
- Hagmaier, K.; Jennings, S.; Buse, J.; Weber, F.; Kochs, G. Novel gene product of Thogoto virus segment 6 codes for an interferon antagonist. Virol. J. 2003, 77, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Buettner, N.; Vogt, C.; Martínez-Sobrido, L.; Weber, F.; Waibler, Z.; Kochs, G. Thogoto virus ML protein is a potent inhibitor of the interferon regulatory factor-7 transcription factor. J. Gen. Virol. 2010, 91, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Stanwick, T.L.; Hallum, J.V. Role of interferon in six cell lines persistently infected with rubella virus. Infect. Immun. 1974, 10, 810–815. [Google Scholar] [CrossRef]
- Desmyter, J.A.N.; Melnick, J.L.; Rawls, W.E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). Virol. J. 1968, 2, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Kato, N.; Gale, M.; Lemon, S.M. Distinct poly (IC) and virus-activated signaling pathways leading to interferon-β production in hepatocytes. J. Biol. Chem. 2005, 280, 16739–16747. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.; Smith, D.K.; Rabadan, R.; Peiris, M.; Poon, L.L. Codon usage bias and the evolution of influenza A viruses. Codon Usage Biases of Influenza Virus. BMC Evol. Biol. 2010, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Yoon, H.; Lee, H.J.; Lee, J.H.; Chang, B.J.; Song, C.S.; Nahm, S.S. Expression patterns of influenza virus receptors in the respiratory tracts of four species of poultry. J. Vet. Sci. 2011, 12, 7–13. [Google Scholar] [CrossRef]
- Kim, A.S.; Zimmerman, O.; Fox, J.M.; Nelson, C.A.; Basore, K.; Zhang, R.; Durnell, L.; Desai, C.; Bullock, C.; Deem, S.L.; et al. An evolutionary insertion in the Mxra8 receptor-binding site confers resistance to alphavirus infection and pathogenesis. Cell Host Microbe 2020, 27, 428–440. [Google Scholar] [CrossRef]
- Deardorff, E.R.; Fitzpatrick, K.A.; Jerzak, G.V.; Shi, P.Y.; Kramer, L.D.; Ebel, G.D. West Nile virus experimental evolution in vivo and the trade-off hypothesis. PLoS Pathog. 2011, 7, e1002335. [Google Scholar] [CrossRef]
- Ciota, A.T.; Payne, A.F.; Kramer, L.D. West Nile virus adaptation to ixodid tick cells is associated with phenotypic trade-offs in primary hosts. Virology 2015, 482, 128–132. [Google Scholar] [CrossRef]
- Morse, M.A.; Marriott, A.C.; Nuttall, P.A. The glycoprotein of Thogoto virus (a tick-borne orthomyxo-like virus) is related to the baculovirus glycoprotein GP64. Virology 1992, 186, 640–646. [Google Scholar] [CrossRef]
- Bai, C.; Qi, J.; Wu, Y.; Wang, X.; Gao, G.F.; Peng, R.; Gao, F. Postfusion structure of human-infecting Bourbon virus envelope glycoprotein. J. Struct. Biol. 2019, 208, 99–106. [Google Scholar] [CrossRef]
- Kataoka, C.; Kaname, Y.; Taguwa, S.; Abe, T.; Fukuhara, T.; Tani, H.; Moriishi, K.; Matsuura, Y. Baculovirus GP64-mediated entry into mammalian cells. Virol. J. 2012, 86, 2610–2620. [Google Scholar] [CrossRef]
- Katou, Y.; Yamada, H.; Ikeda, M.; Kobayashi, M. A single amino acid substitution modulates low-pH-triggered membrane fusion of GP64 protein in Autographa californica and Bombyx mori nucleopolyhedroviruses. Virology 2010, 404, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.P.; Gu, L.Z.; Lou, Y.H.; Cheng, R.L.; Xu, H.J.; Wang, W.B.; Zhang, C.X. A baculovirus isolated from wild silkworm encompasses the host ranges of Bombyx mori nucleopolyhedrosis virus and Autographa californica multiple nucleopolyhedrovirus in cultured cells. J. Gen. Virol. 2012, 93, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zhang, S.; Cui, Y.; Shi, Y.; Gao, G.F.; Qi, J. Structures of human-infecting Thogotovirus fusogens support a common ancestor with insect baculovirus. Proc. Natl. Acad. Sci. USA 2017, 114, E8905–E8912. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Jones, L.D.; Nuttall, P. Identification of viral structural polypeptides of Thogoto virus (a tick-borne orthomyxo-like virus) and functions associated with the glycoprotein. J. Gen. Virol. 1992, 73, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).