Abstract
Salmonella Enteritidis (SE) can spread from the intestines to cause systemic infection, mainly involving macrophages. Intramacrophage Salmonella exits and reinfects neighboring cells, leading to severe disease. Salmonella genes involved in exiting from macrophages are not well understood or fully identified. A focA::Tn5 mutant was identified by an in vitro assay, with increased ability to exit from macrophages. A defined SEΔfocA mutant and its complemented derivative strain, SEΔfocA::focA, were constructed to confirm this phenotype. Although the lethal ability of focA mutants was similar to that of the parental SE in mice, it was isolated earlier from the liver and spleen than the parental SE. focA mutants induced higher levels of proinflammatory IL-12 and TNF-α compared with the parental SE and SEΔfocA::focA. focA mutants showed higher cytotoxicity and lower formate concentrations than SE and SEΔfocA::focA, whereas there was no change in pyroptosis, apoptosis and flagella formation ability. These current data suggest that the focA gene plays an important role in regulating intramacrophage Salmonella exiting and extraintestinal spread in mice, although the specific mechanism requires further in-depth studies.
1. Introduction
Salmonellaenterica spp. remain a major foodborne zoonotic pathogen causing serious public health problems and economic losses [1,2]. During systemic infection in the host, Salmonella infects intestinal epithelial cells after entering the gut, where it proliferates and induces inflammation. This is followed by extracellular [3] and intracellular dissemination [4,5,6,7], the latter generally occurring in macrophages, where multiplication takes place. Transmission between macrophages is clearly important to spread infection within organs, such as the lymph nodes, spleen and liver, where further multiplication appears to take place. Multiplication within macrophages involves accumulation of relatively small numbers of bacteria before dissemination to adjacent susceptible macrophages occurs [8].
Exiting macrophages is as important as invasion for pathogens, which cycle between intra- and extracellular stages [9]. The exit phase is well understood for pathogens such as Shigella and Listeria, which exist predominantly within the cell cytoplasm, and intercellular transfer occurs via actin-mediated protrusions projecting into neighboring cells [10,11]. Other mechanisms expressed by pathogens, such as Chlamydia [12] and Legionella [13], have also been described.
Although the abilities of invasion and replication within cells have been the major focus of research on Salmonella pathogenicity, the ability of intramacrophage Salmonella exiting has received little attention. The means by which bacteria exit the cell and whether this involves some form of cell death [14] remains unclear. It is well known that Salmonella has the capacity to induce cell death at different times after infection [15,16]. The SipB protein induces rapid cell death through activation of caspase-1, with fragmentation of chromatin and cytoplasmic membrane blebbing [17,18,19]. Pyroptosis is also caspase-1-dependent. In contrast, apoptosis is known to be caspase-3-dependent [20]. The cell swelling that precedes necrosis and bacterial release induced by motile Salmonella is thought to be flagella-related [21].
Whether genes such as sifA are involved is unclear, as sifA is involved information of the Salmonella-containing vacuole (SCV), preventing Salmonella from entering the cytoplasm; sifA mutants diminish the integrity of the SCV [22]. The prgJ gene may also be involved in the exit process [23], possibly due to its involvement in pyroptosis caused by Salmonella also involving flagella, although in this case, it is thought to be a defense mechanism in pig lymph nodes [24].
Because very few bacterial genes have been identified in relation to Salmonella exiting from macrophages, we decided to screen a mini-Tn5 transposon mutant library of Salmonella Enteritidis C50041 to identify genes required for Salmonella to exit from macrophages. Derivatives of the mini-Tn5 transposon-carrying selectable antibiotic resistance markers are powerful tools for mining bacterial genes related to phenotypes and functions [25]. We found that the focA gene [26], expressed as the formate transporter FocA, is involved in regulating the ability of Salmonella to exit from macrophages, as its loss quantitatively increased the exiting ability, which boosted early extraintestinal spread for systemic infection in mice.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Cells
The bacterial strains, plasmids and cells used in this study are listed in Table A1. The defined bacterial deletion mutant was produced according the method of scarless–markerless genome genetic modification [27].
2.2. Mice and Animal Ethics
Specific pathogen-free (SPF) female BALB/c mice (8 week; 20 ± 2 g) were obtained from the Comparative Medical Center of Yangzhou University (Yangzhou, China). All animal experiments were approved by the Animal Welfare and Ethics Committees of Yangzhou University and complied with the guidelines of the Institutional Administrative Committee and Ethics Committee of Laboratory Animals.
2.3. Construction of Tn5 Mutant Library and Mutant Screen
SE C50041 was used for random transposon mutagenesis by a mini-Tn5 transposon delivered on suicide vector pUT with a kanamycin-resistant gene, as described in [28]. A Tn5 mutant library was constructed by conjugating E. coli χ7213 (mini-Tn5) as donor strain with C50041, which is sensitive to kanamycin, as recipient. The transconjugants were isolated on LB agar containing 50 mg/mL chloramphenicol and 100 μg/mL kanamycin.
The mutant screen was performed as described previously. Briefly, RAW264.7 cells (5.0 × 105 cells/well) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (GenDEPOT Inc, Barker, TX, USA) for 12 h at 37 °C in 24-well plates. Mutants (MOI = 100:1) were added to the culture medium, and the plates were centrifuged at 1000 rpm for 10 min for Salmonella to be deposited onto the surface of RAW264.7 cell s. The cells were then incubated at 37 °C for 1 h, washed twice with sterile phosphate-buffered saline (PBS) and incubated in DMEM plus 100 mg/mL gentamicin (GM, Sigma Aldrich, St. Louis, MO, USA) for 1 h. The culture medium was changed to DMEM with 10 mg/mL gentamicin. After 8 h, the medium with gentamicin was removed, and new DMEM without antibiotics was added for another 1 h. The cells were lysed with 0.2% Triton X-100 for 10 min at 37 °C. The loads of Salmonella in the culture medium and inside cells were counted, and their ratios were calculated and compared.
2.4. Identification of Sequence Flanking Tn5 Inserted in Bacterial Genome
The sequence-flanking Tn5 inserted in the bacterial genomes was amplified by PCR [27]. The primers are listed in Table A2. Briefly, bacterial genomic DNA was isolated from mutants and digested with Nla III (New England Biolabs, Hitchin, Herts, UK). An adaptor of double-stranded DNA was ligated to the genomic DNA, a special PCR was performed once with primer set Y linker/P6U and twice with Y linker/Tn5-p, and the PCR product was sequenced by a Tn5-p primer. Homology searches were performed using the public databases BLASTn and BLASTx at http://www.ncbi.nlm.nih.gov, accessed on 6 July 2020.
2.5. Cosntruction of SEΔfocA and SEΔfocA::focA
According the protocol based on pGMB152 suicide plasmid [28], SEΔfocA was constructed by the chloramphenicol resistance gene replacing the focA gene, and the complemented strain SEΔfocA::focA was generated with pBR322-focA using a method described in [29].
2.6. In Vitro Exiting Ability of Intramacrophage Salmonella focA Mutants
The assay was performed as the Salmonella mutant screen described in Section 2.3.
2.7. Virulence Analysis of Salmonella focA Mutant in Mice
2.7.1. Extraintestinal Spread
The capacity for the extraintestinal spread of Salmonella mutants was analyzed by monitoring Salmonella loads in the murine liver and spleen. Mice were infected orally with 1.0 × 107 CFU in 100 μL of each SE strain/mutant. Three mice in each group were euthanized at 1, 4 and 7 dpi, and a section of the liver and spleen were removed aseptically, weighed and homogenized individually in 1 mL PBS. Dilutions of the homogenates (100 μL each) were plated on XLT4 agar and incubated at 37 °C overnight. Bacterial colonies were counted and expressed as Log10 CFU/g, with negative samples reported as 0 CFU/g.
2.7.2. Lethal Ability
The lethal ability of the focA mutant for mice was performed as described in [30]. Mice were inoculated orally with 0.2 mL of Salmonella mutant (approximately 2.0 × 106 CFU). The mouse survival rate of each group (n = 10, 5 group) was calculated after two weeks.
2.7.3. mRNA Level of Cytokines in Murine Spleen
The spleens of mice infected with Salmonella were collected on post-infection days 1, 4 and 7, and total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA, USA). cDNA synthesis was carried out using reverse transcriptase PCR (Master cycler, Eppendorf, Hamburg, Germany). cDNA was diluted 5 times as a template for qRT-PCR, and the SYBR method was used to detect the mRNA level of cytokines with the primers listed in Table A3.
2.8. Biological Feature Analysis for Possible Mechanisms
2.8.1. Formate Level in the Bacteria
Formate concentrations were measured by a formate assay kit (Abcam, Cambridge, MA, USA). The formate level was detected in the bacteria at 3, 5 and 9 h.
With the Abcam formate assay kit, formate is oxidized to generate a product resulting in color formation (λ = 450 nm) proportional to the formate concentration. In brief, formate assay buffer, enzyme mix and substrate mix were added to each standard and test sample in 96-well plates and incubated for 60 min at 37 °C.
The OD450nm was measured in a microplate reader proportional to the formate concentration [31].
2.8.2. Formate Level in Salmonella-Infected Macrophages
The steps for Salmonella infection to macrophage were the same as those described Section 2.3. After 9 h, the culture medium with 10 mg/mL gentamicin was removed, washed twice with sterile PBS and lysed in formate assay buffer at a ratio of 1.0 × 106 cells per 100 μL buffer. Lysates were transferred into Eppendorf tubes and centrifuged for 10 min at 14,000 rpm. The supernatant was used to detect the formate level with a formate assay kit (Abcam, USA) [31].
2.8.3. LDH Assay for Cytotoxicity
The cytotoxicity of the focA mutants was evaluated by LDH release from Salmonella-infected cells. Cell culture and bacterial infection were performed as in the mutant screen described above. DMEM with 10 μg/mL gentamicin was added for 3 h, and the LDH level released in the cell medium was detected by an LDH cytotoxicity assay detection kit (Beyotime, Nantong, China).
2.8.4. Pyroptosis and Apoptosis Assessment for Cell Death
Analysis of pyroptosis: J774A.1 cells were seeded into 12-well plates at a density of 5.0 × 105 cells per well and infected with Salmonella as described above. After harvesting the supernatants, the remaining cells were lysed directly with 300 μL cell lysis buffer per well, and the supernatant and lysate from each well were mixed. The mixtures were centrifuged at 2000 rpm for 5 min to remove cell debris. An equal volume of methanol and a 0.25 volume of chloroform were added, vortexed vigorously and centrifuged at 12,000 rpm for 5 min. The supernatant was aspirated completely. An equal volume of methanol was added to each sample, vortexed vigorously and centrifuged at 12,000 rpm for 5 min. The protein pellets were dried at 55 °C for 10 min, resuspended with 40 μL of 1 × SDS-PAGE sample-loading buffer (Beyotime, China) and boiled for 10 min at 95 °C. The samples were loaded onto 15% Tris-glycine gels and analyzed by Western blot [32]. The primary antibody used in this study was anti-caspase-1 p10 antibody (AG-20B-0042-C100, AdipoGen, San Diego, CA, USA). The secondary antibodies were goat anti-mouse IgG-HRP.
Analysis of apoptosis: An annexin V-FITC/PI double staining method was used to detect apoptosis. After RAW264.7 cells were infected with SE and incubated in DMEM plus 10 mg/mL gentamicin for 3 h, the cells were stained with an annexin V-FITC kit (Miltenyi) and analyzed by flow cytometry. The specific operation was as follows: 1.0 × 106 cells were collected and washed in 1 mL of 1 × binding buffer and centrifuged at 12,000 rpm for 10 min. After the supernatant was removed completely, cells were resuspended in 100 µL of 1× binding buffer and 10 µL of annexin V-FITC and incubated for 15 min in the dark at room temperature. Cells were washed again by adding 1 mL of 1× binding buffer and centrifuged at 12,000 rpm for 10 min. Supernatant was removed completely, and cells were resuspended in 500 µL of 1× binding buffer. After 5 µL of PI solution was added, cells were immediately analyzed by flow cytometry [33].
2.8.5. Motility Analysis for Flagella
Bacterial motility was analyzed by U tube and semisolid agar plate.
U tube: Fresh cultured single colonies of the SE strains were selected from a solid LB plate and used to inoculate one side of a U tube containing 6 mL semisolid LB medium. The U tube was incubated at 37 °C for 8 h, and the growth of the bacteria was observed from the other side of the U tube.
Semisolid agar plate: SE strains were cultured in LB liquid broth to their logarithmic phase. After washing the bacterial cells twice with PBS, the bacterial density was adjusted to OD600 nm = 1.0. A freshly prepared semisolid LB plate containing 0.5% agar was used to detect motility. A volume of 10 μL of the bacterial suspension was pipetted to the center of the semisolid plate. The plate was allowed to dry for 20 min and incubated at 37 °C for 20 h. Motility was evaluated by the diameter of the visible bacterial growth.
Electron microscope: Salmonella focA mutant cultures were negatively stained with 0.1% phosphotungstic acid solution for 1 min. The flagella were observed under an electron microscope.
2.9. Statistical Analysis
The bacterial CFUs, mouse survival and morphometric analysis data were analyzed using GraphPad Prism 7 (GraphPad Software, LaJolla, CA, USA). Analysis of variance (ANOVA) was performed to compare the mutant groups with the C50041 control, as well as to compare the mutant groups with the PBS control. All results are expressed as the mean ± SEM. Statistical significance was assigned at p values <0.05 (*), <0.01 (**) or <0.001 (***) based on a Student’s t-test.
3. Results
3.1. focA::Tn5 Mutant with Imrpoved Exiting Ability from Macrophages
A total of 887 conjugants were screened from the Tn5 mutant library of C50041, and one mutant showed improved exiting ability from the RAW264.7 macrophages. Following amplification of the Tn5-flanking sequence by PCR and BLAST analysis, the Tn5-inserted gene was identified as focA.
3.2. SEΔfocA Mutant Reconfirmed
To confirm that the exiting ability of the SE focA mutant improved, the SEΔfocA-deletant and SEΔfocA::focA-complemented strain were constructed. RAW264.7 cells were infected with focA::Tn5, SEΔfocA, SEΔfocA::focA and C50041, and their exiting abilities from RAW264.7 cells were analyzed. As shown in Figure 1, 1 h after removal of antibiotics, the numbers of SEΔfocA in the culture medium increased significantly compared to C50041 (p < 0.05), whereas the intracellular numbers were very similar. The ratio of extracellular/intracellular bacterial count of SEΔfocA was also increased significantly (p < 0.05) compared to that of C50041. The exiting ability of SEΔfocA was similar that of focA::Tn5 (p < 0.01), and the exiting ability of SEΔfocA::focA was similar to that of C50041 (p > 0.05).
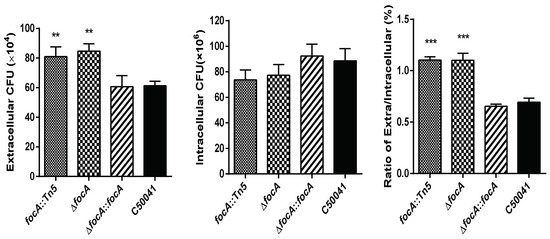
Figure 1.
Exiting ability of intracellular SE focA mutants from RAW264.7. Data are presented as mean ± SEM of three independent experiments; ** p < 0.01, *** p < 0.001.
3.3. Virulence Analysis of Salmonella focA Mutants in Mice
3.3.1. Improved Early Extraintestinal Spreading Ability
After 1.0 × 107 CFU in 100 μL focA::Tn5, SEΔfocA, SEΔfocA::focA and C50041 were administered orally to mice. The in vivo dynamics of the four SE strains showed that focA mutants could be isolated from the livers and spleens one day after inoculation, whereas C50041 was not isolated at this time, and higher loads of focA mutants could be isolated from the liver and spleen compared to C50041 (Figure 2).
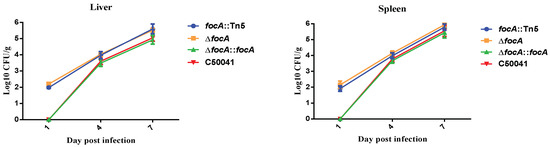
Figure 2.
Extraintestinal spread of SE focA mutants by bacterial loads in murine liver and spleen. Data are presented as mean ± SEM of three independent experiments.
3.3.2. No Obvious Change in Lethal Ability in Mice
The survival rates of the four SE strains were compared after oral inoculation of mice with 2.0 × 106 CFU. The survival of mice infected with the focA mutants did not differ significantly from that of the mice infected with C50041 (Figure 3).
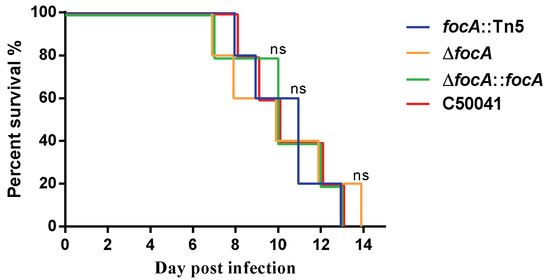
Figure 3.
The survival of mice infected by SE focA mutants. Data are presented as mean ± SEM of three independent experiments.
3.3.3. Increased Ability to Promote Murine Proinflammatory Cytokines
The mRNA levels of the proinflammatory cytokines IL-12 (focA::Tn5: p < 0.05 and ΔfocA: p < 0.05) and TNF-a (focA::Tn5, p < 0.05 and ΔfocA, p < 0.05) exceeded those induced by C50041 at 1 dpi (Figure 4).
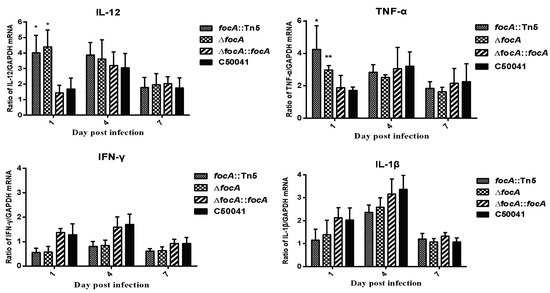
Figure 4.
mRNA levels of cytokines in murine spleen caused by SE focA mutants. Data are presented as mean ± SEM of three independent experiments; * p < 0.05, ** p < 0.01.
3.4. Biological Phenotype Analysis of focA Mutant for Possible Mechanisms
3.4.1. Less Formate Produced by focA Mutant
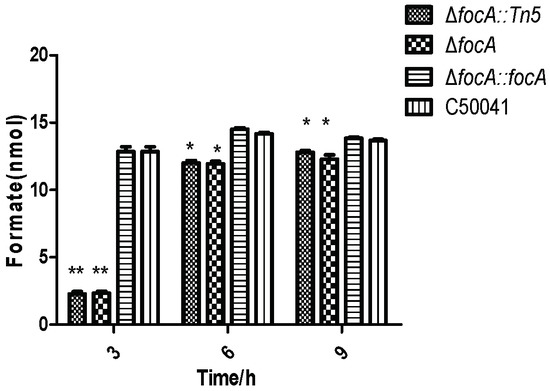
Figure 5.
Formate concentration in SE focA mutant at different culture times. Data are presented as mean ± SEM of three independent experiments; * p < 0.05, ** p < 0.01.
3.4.2. Less Formate in focA Mutant-Infected RAW264.7
According to analysis by formate assay kit, the formate concentration in focA mutant-infected RAW264.7 cells was (p < 0.05) significantly lower than that in C50041-infected RAW264.7 cells, as shown in Figure 6.
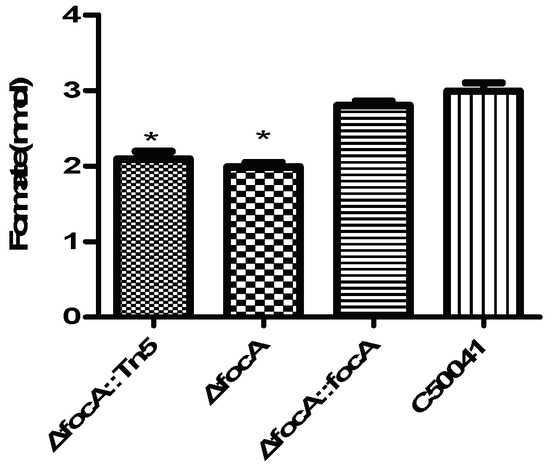
Figure 6.
Formate concentration in Salmonella-infected RAW264.7 (MOI = 100, T = 9). Data are presented as mean ± SEM of three independent experiments; * p < 0.05.
3.4.3. Increased Cytotoxicity by focA Mutants
The cytotoxicity of Salmonella was analyzed by LDH level in the culture medium of infected cells. As shown in Figure 7, LDH levels induced by focA::Tn5 (p < 0.05) and ΔfocA (p < 0.05) were significantly higher than those induced by C50041. LDH levels induced by SEΔfocA::focA and C50041 were similar.
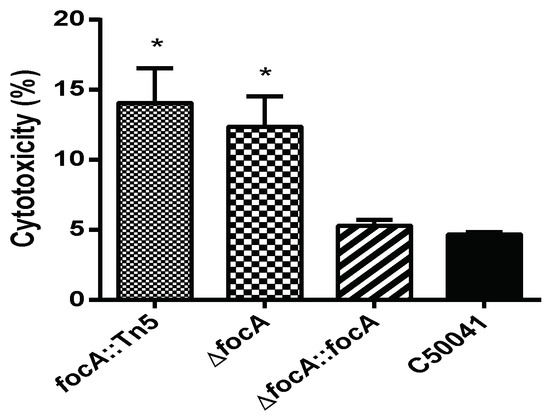
Figure 7.
The cytotoxicity of SE focA mutants to RAW264.7. Data are presented as mean ± SEM of three independent experiments; * p < 0.05.
3.4.4. No Obvious Change in Cellular Pyroptosis Based on Caspase-1 Protein Measurement by focA Mutants
Western blotting was used to detect caspase-1 expression in J774A.1 macrophage-like cells after infection with the SE strains. The results are shown in Figure 8. Compared with the C50041 group, expression of P45 (Procaspase-1), caspase-1 and GAPDH in the SEΔfocA group was not altered significantly, suggesting that the mutation of the focA gene had no obvious effect on the ability of SE to cause pyroptosis.
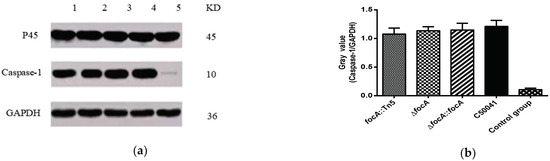
Figure 8.
The ability of SE focA mutants to induce expression of caspase-1 protein: (a) Western blot analysis of caspase-1 protein: 1: focA::Tn5, 2: ΔfocA, 3: ΔfocA::focA, 4: C50041, 5: control group; (b) gray analysis of caspase-1 protein. Data are presented as mean ± SEM of three independent experiments.
3.4.5. No Obvious Change in Cellular Apoptosis Based on Annexin V-FITC/PI Staining by focA Mutants
Apoptosis of infected RAW264.7 cells was detected by flow cytometry. The results are shown in Figure 9. Compared to the C50041 group (4.5% (early), 51.9% (late)), the apoptosis rate induced by focA::Tn5, ΔfocA and ΔfocA::focA did not change significantly (2.3% (early), 46.9% (late) for focA::Tn5; 3.1% (early), 46.1% (late) for ΔfocA; and 3.6% (early), 45.3% (late) for ΔfocA::focA). The results indicate that the focA gene is not involved in apoptosis induced by SE.
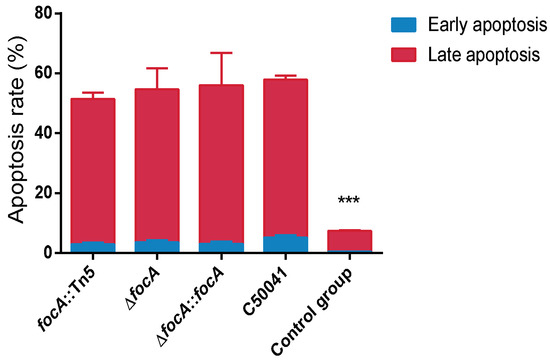
Figure 9.
The apoptosis of the Salmonella-infected RAW264.7 was detected by flow cytometry. Data are presented as mean ± SEM of three independent experiments. *** p < 0.001.
3.4.6. No Obvious Change of Flagella Based on Bacterial Motility by focA Mutants
U tubes and semisolid agar plates were used to detect the motility of the SE focA mutants (Figure 10). U tubes showed no alterations in motility. In the semisolid agar plate, the diameter of the bacterial colony of SEΔfocA was 54 ± 2 mm, and that of C50041 was 61 ± 2 mm. Statistical analysis (Figure 10) showed that the size of the bacterial colony did not differ significant (p > 0.05), proving that the focA gene has no significant effect on SE motility. Normal flagella around the focA mutants were observed by electron microscopy.
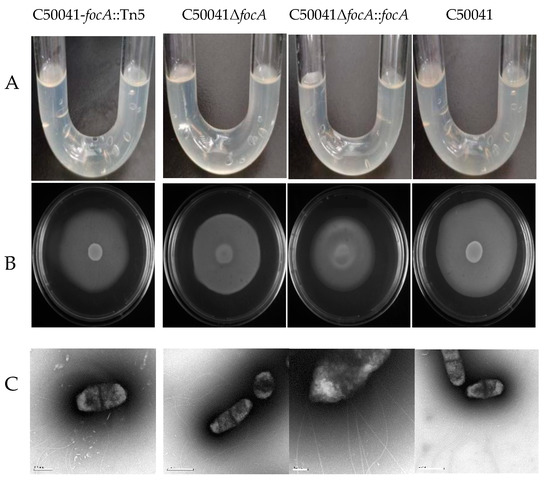
Figure 10.
Motility analysis and flagellar observation of Salmonella focA mutants: (A) by U tube, (B) by semisolid agar plate, (C) by electron microscopy. The experiment was repeated in triplicate, and the results were consistent.
4. Discussion
The ability of virulent Salmonella organisms to exit from infected macrophages, by whatever means, is clearly a key step in the process of bacterial spread from one susceptible cell to another, as occurs with some other intracellular pathogens [9,10,11,12]. This is also indicated by the fact that the number of bacteria within infected macrophages remains low and infection spreads to more susceptible macrophages [4]. In healthy individuals, the host can recognize and eliminate pathogens through innate and acquired immunity. Salmonella clearly face the challenge of immune mediators during their escape from the intracellular environment. Once in macrophages, invasive Salmonella are able to evade immune surveillance by manipulation of the intracellular environment, including the SCV, using sophisticated strategies [5,6,7,8].
Several S. Typhimurium genes have been identified, the mutations of which have been shown to reduce the ability to exit from macrophages and which also attenuate virulence. These genes include SipB, which induces rapid cell death through activation of caspase-1 [20], flagella [22] and possibly also SifA and PrgJ, although the case for these is less clear [14,23]
Extracellular escape is affected by many factors and, if it involves cell death, may take many forms [15,16,17].
We identified a mutation of focA in S. enteritidis, which increased the ability to exit from macrophages, suggesting that the FocA protein plays a key role in suppressing premature release of Salmonella bacteria. Given that the number of bacterial cells that accumulate in macrophages before release is relatively low, the dynamics of multiplication, together with release and exiting, could form an important study that could shed light on its role in this stage of the infection process. The means by which this protein is involved in exiting remain unclear. It is an important component in mixed acid fermentation and in metabolic switching, depending on carbon source and redox level [26,31].
In addition to the phenotype of the initial transposon mutant, we demonstrated that the focA mutant colonized the liver and spleen more rapidly following oral inoculation of mice in the early stages of infection, although it did not affect the bacterial load in these organs at 4 and 7 days post infection. As a result of this latter observation, the lethality of the focA mutant for mice was also not changed from that of the current parent strain. The results were obtained initially with a Tn5 insertional mutation and confirmed by producing a defined mutant. In addition, a focA-complemented strain was constructed using the pBR322-focA construct. The phenotype of the complemented strain was very similar to that of the parent strain. A role of the focA gene in negative regulation of the escaping ability of Salmonella Enteritidis in macrophages must be considered highly likely.
Attempts were made to ascertain the basic mechanism behind this phenotype. Key proinflammatory cytokines IL-12 and TNF-α were measured in vitro. The focA mutation led to increases in IL-12 and TNF-α, indicating that the FocA protein plays a role during infection in suppressing these early indicators of the immune response. Small changes were observed in the levels of IFNγ and IL-1β, but the differences were not statistically significant. Early indicators of innate immunity, including suppression of proinflammatory chemokines, are a key characteristic of typhoid (acute systemic disease)-producing Salmonella serovars invading from the intestine [34]. This is facilitated by the absence of flagella in serovars such as Salmonella Gallinarum and Salmonella Pullorum and suppression of flagellation and other virulence genes in Salmonella Typhi [35]. Thus, suppression of components of the early innate response may facilitate bacterial survival during transfer between susceptible macrophages.
The non-functional formate transport activity of the focA mutant [36] coupled with more rapid exiting capacity and cytotoxicity suggests that FocA is actively involved in regulating bacterial virulence in the early stages of systemic infection, possibly by or including suppression of inflammatory host signals. It has been reported that quantities of formate are secreted by E. coli and Salmonella during stationary-phase growth and that this contributes to increased resistance to antimicrobial peptides by virtue of their oxidation via the respiratory chain bypassing the site of inhibition between NADH dehydrogenase and quinone [37]. There is also an interesting relationship between formate accumulation in epithelial cells and Shigella virulence, with a mutation in pyruvate-format lyase reducing plaque production and virulence, which is restored by the addition of formate [38]. Koestler et al. also believe that intracellular formate is a signal for modulation of bacterial virulence factors.
Although the mechanism of Salmonella exiting from the intramacrophage environment currently remains unclear, programmed cell death, including pyroptosis, does not seem to be involved. Intracellular Salmonella activates the NLRC4 inflammasome, mainly through the Salmonella pathogenicity island-1 type III secretion system (T3SS) and flagella, which further activates caspase-1 and causes pyroptosis [39,40]. Apoptosis could be activated by Salmonella pathogenicity island-1 effectors through activated caspase-3-induced pathways, including both intrinsic and extrinsic pathways in Salmonella-infected macrophages [41], and it could be a possible strategy for induced intracellular Salmonella bacteria to exit from macrophages. Although we found that focA mutants induced increased cytotoxicity, further analysis by Western blot and flow cytometry showed that there was no change in induction of pyroptosis and apoptosis. Although flagella may facilitate bacterial escape from macrophages [22], in this study, we observed no change in motility or physical appearance by electron microscopy.
Our preliminary results show that the focA gene plays a significant role in the ability of intracellular Salmonella to exit from macrophages in vitro and increases early-stage extraintestinal spread in systemic infection without affecting lethal ability. The nature of the interaction between the metabolic function of FocA and its contribution to the early stages of systemic infection clearly require further investigation.
5. Conclusions
In this study, a focA mutation was identified from a mini-Tn5 transposon mutant library of S. enteritidis C50041, which displayed stronger exiting ability from macrophages and boosted early extraintestinal spread in mice. This result indicates that the focA gene negatively regulated the S. enteritidis exiting ability from macrophages, although this mechanism requires further in-depth studies.
Author Contributions
Conceptualization, R.G., J.Z. and S.G.; methodology, R.G., H.G. and Y.W.; software, R.G. and J.Z.; formal analysis, R.G., J.Z. and Y.W.; data curation, X.K., R.G. and J.Z.; supervision, X.J., P.B. and S.G.; project administration, X.J. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program Special Project (2016YFD0501607), the Natural Science Foundation of Jiangsu Province of China (BK20151306) and the Special Project on Science and Technology in North Jiangsu (SZ-SQ2021046).
Institutional Review Board Statement
Animal experiments were approved by the Animal Welfare and Ethics Committees of Yangzhou University (SYXK[Su] 2017-0044) and were maintained in accordance with the guidelines of the Yangzhou University Institutional Animal Care and Use Committee (IACUC).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank all colleagues who made suggestion and revisions to this paper.
Conflicts of Interest
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A

Table A1.
Bacterial strains, plasmids and cells used in this study.
Table A1.
Bacterial strains, plasmids and cells used in this study.
| Strains or Plasmids | Characteristic | Reference |
|---|---|---|
| Strains | ||
| SE C50041 | Wild-type Salmonella Enteritidis C50041 | Lab collection |
| SE C50041focA::Tn5 | C50041 with Tn5 inserted in focA gene | This study |
| SE C50041ΔfocA | C50041 with a defined deletion of the focA gene | This study |
| SE C50041ΔfocA::focA | C50041∆focA with pBR322 expressing the focA gene | This study |
| E.coli χ7213 | Its growth for pGMB152 with DAP, as conjugal donor | [27] |
| Plasmids | ||
| pUT mini-Tn5Km2(Cm) | Transposon delivery vector, Cmr, Kmr | [28] |
| pGMB152 | pGMB151 derivative, suicide vector, Ampr, Smr, LacZYA | [27] |
| pBR322 | For construction of C50041ΔfocA::focA, Cmr | [29] |
| pBR322-focA | pPR322 derivative containing focA, Ampr and Tetr | This study |
| Cell | ||
| RAW264.7 J774A.1 | Murine macrophages Murine macrophages | This study This study |
Appendix B

Table A2.
Primers used in this study.
Table A2.
Primers used in this study.
| Primer Name | Primer Sequences (5′-3′) | Target |
|---|---|---|
| Y-linker | CTGCTCGAATTCAAGCTTCT | PCR of sequence-flanking Tn5 in bacterial genome |
| P6U | CGAGCTCGAATTCGGCCTAG | |
| Tn5-P | GGCCAGATCTGACAAGAGA | |
| Adapter | TTTCTGCTCGAATTCAAGCTTC TAACGATGTACGGGGACACATG | |
| TGTCCCCGTACATCGTTAGAACTACTCGTACCATCCACAT | ||
| focA-up-F | CCCCCCCTGCAGGTCGACGTGCGTCTTGCTCGGTGAT | Construction of SEΔfocA |
| focA-up-R | CAGCCTACACAATCGCTCAA GATGCCCATTACACGCAGTAA | |
| focA-Cm-F | TTACTGCGTGTAATGGGCATC TTGAGCGATTGTGTAGGCTG | |
| focA-Cm-R | CTTTGTTAGTATCTCGTCGCCG ATGGGAATTAGCCATGGTCC | |
| focA-down-F | GGACCATGGCTAATTCCCAT CGGCGACGAGATACTAACAAAG | |
| focA-down-R | CTTATCGATACCGTCGACTGCGTGAACTGTTGGGTCTG | |
| focA-in-F | ACGCAGGTAAATGACCCAGT | |
| focA-in-R | TTTTCGTGTTACTGATGTGGC | |
| pGMB152-F | CGTGGAGGCCATCAAACCAC | |
| pGMB152-R | CGCGAAATAAACGACCGGGA | |
| R-focA-F | TTATCATCGATAAGCTTTTGTTAGTATCTCGTCGCCGACT | Construction of SEΔfocA::focA |
| R-focA-R | TCCGGCGTAGAGGATCCTGCGTGTAATGGGCATCAAC |
“Underline” indicates the homologous sequence for one-step ligation of recombinant plasmid.
Appendix C

Table A3.
Primers used in this study.
Table A3.
Primers used in this study.
| Primer Name | Primer Sequence (5′-3′) | Size (bp) |
|---|---|---|
| IL-1β-F | TGGCCTTCAAAGGAAAGAATCTATACCTGTCC | 167 |
| IL-1β-R | GTTGGGGAACTCTGCAGACTCAAACTCCAC | |
| IL-12-F | TGCCCCCACAGAAGACGTCTTTGATGAT | 138 |
| IL-12-R | GATGGCCACCAGCATGCCCTTGTC | |
| TNF-α-F | CAGGCCTTCCTACCTTCAGACCTTTCCAGAT | 122 |
| TNF-α-R | ACACCCCGCCCTTCCAAATAAATACATTCAT | |
| IFN-γ-F | GCCAAGACTGTGATTGCGGGGTTGTATCT | 198 |
| IFN-γ-R | TAAAGCGCTGGCCCGGAGTGTAGACA | |
| GAPDH-F | CAGCCTCGTCCCGTAGACAA | 156 |
| GAPDH-R | ACCCCGTCTCCGGAGTCCATCACAAT |
References
- WHO. Critically Important Antimicrobials for Human Medicine; World Health Organization: Geneva, Switzerland, 2017.
- Ao, T.T.; Feasey, N.A.; Gordon, M.A.; Keddy, K.H.; Angulo, F.; Crump, J.A. Global burden of invasive nontyphoidal Salmonella disease. Emerg. Infect. Dis. 2010, 21, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, G.D.; Paulin, S.M.; Charleston, B.; Watson, P.R.; Bowen, A.J.; Dziva, F.; Morgan, E.; Villarreal-Ramos, B.; Wallis, T.S.; Stevens, M.P. Systemic translocation of Salmonella enterica serovar Dublin in cattle occurs predominantly via efferent lymphatics in a cell-free niche and requires type III secretion system 1 (T3SS-1) but not T3SS-2. Infect. Immun. 2007, 75, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, P.; Grant, A.J. Spread of Salmonella enterica in the body during systemic infection: Unravelling host and pathogen determinants. Expert. Rev. Mol. Med. 2011, 13, e12. [Google Scholar] [CrossRef]
- García-del Portillo, F. Salmonella intracellular proliferation: Where, when and how? Microbes. Infect. 2001, 3, 1305–1311. [Google Scholar] [CrossRef]
- Lathrop, S.K.; Binder, K.A.; Starr, T.; Cooper, K.G.; Chong, A.; Carmody, A.B.; Steele-Mortimer, O. Replication of Salmonella enterica Serovar Typhimurium in Human Monocyte-Derived Macrophages. Infect. Immun. 2015, 83, 2661–2671. [Google Scholar] [CrossRef]
- Pucciarelli, M.G.; García-Del Portillo, F. Salmonella intracellular lifestyles and their impact on host-to-host transmission. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Gog, J.R.; Murcia, A.; Osterman, N.; Restif, O.; McKinley, T.J.; Sheppard, M.; Achouri, S.; Wei, B.; Mastroeni, P.; Wood, J.L. Dynamics of Salmonella infection of macrophages at the single cell level. J. R. Soc. Interface 2012, 9, 2696–2707. [Google Scholar] [CrossRef]
- Hybiske, K.; Stephens, R.S. Exit strategies of intracellular pathogens. Nat. Rev. Microbiol. 2008, 6, 99–110. [Google Scholar] [CrossRef]
- Jennison, A.V.; Verma, N.K. Shigella flexneri infection: Pathogenesis and vaccine development. FEMS Microbiol. Rev. 2003, 28, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Mounier, J.; Ryter, A.; Coquis-Rondon, M.; Sansonetti, P.J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect. Immun. 1990, 58, 1048–1058. [Google Scholar] [CrossRef]
- Hybiske, K.; Stephens, R.S. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. USA 2007, 104, 11430–11435. [Google Scholar] [CrossRef]
- Alli, O.A.; Gao, L.Y.; Pedersen, L.L.; Zink, S.; Radulic, M.; Doric, M.; Abu Kwaik, Y. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 2000, 68, 6431–6440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Guiney, D.G. The role of host cell death in Salmonella infections. Curr. Top. Microbiol. Immunol. 2005, 289, 131–150. [Google Scholar]
- Hueffer, K.; Galan, J.E. Salmonella-induced macrophage death: Multiple mechanisms, different outcomes. Cell. Microbiol. 2004, 6, 1019–1025. [Google Scholar] [CrossRef]
- Chen, L.M.; Kaniga, K.; Galan, J.E. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 1996, 21, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Monack, D.M.; Raupach, B.; Hromockyj, A.E.; Falkow, S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 9833–9838. [Google Scholar] [CrossRef]
- Hersh, D.; Monack, D.M.; Smith, M.R.; Ghori, N.; Falkow, S.; Zychlinsky, A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 1999, 96, 2396–2401. [Google Scholar] [CrossRef]
- Brennan, M.A.; Cookson, B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000, 38, 31–40. [Google Scholar] [CrossRef]
- Sano, G.-I.; Yasunari, T.; Sinichi, G.; Matuyama, K.; Shindo, Y.; Oka, K.; Matsui, H.; Matsuo, K. Flagella facilitate escape of Salmonella from oncotic macrophages. J. Bacteriol. 2007, 189, 8224–8232. [Google Scholar] [CrossRef]
- Beuzón, C.R.; Méresse, S.; Unsworth, K.E.; Ruíz-Albert, J.; Garvis, S.; Waterman, S.R.; Ryder, T.A.; Boucrot, E.; Holden, D.W. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000, 19, 3235–3249. [Google Scholar] [CrossRef] [PubMed]
- Sukhan, A.; Kubori, K.; Galan, J.E. Synthesis and Localization of the Salmonella SPI-1 Type III Secretion Needle Complex Proteins PrgI and PrgJ. J. Bacteriol. 2003, 185, 3480–3483. [Google Scholar] [CrossRef]
- Martins, R.P.; Aguilar, C.; Graham, J.E.; Carvajal, A.; Bautista, R.; Claros, M.G.; Garrido, J.J. Pyroptosis and adaptive immunity mechanisms are promptly engendered in mesenteric lymph-nodes during pig infections with Salmonella enterica serovar Typhimurium. Vet. Res. 2013, 44, 120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Watarai, M.; Kondo, Y.; Erdenebaatar, J.; Makino, S.; Shirahata, T. Isolation and characterization of mini-Tn5Km2 insertion mutants of Brucella abortus deficient in internalization and intracellular growth in HeLa cells. Infect. Immun. 2003, 71, 3020–3027. [Google Scholar] [CrossRef][Green Version]
- Lü, W.; Du, J.; Schwarzer, N.J.; Gerbig-Smentek, E.; Einsle, O.; Andrade, S.L. The formate channel FocA exports the products of mixed-acid fermentation. Proc. Natl. Acad. Sci. USA 2012, 109, 13254–13259. [Google Scholar] [CrossRef]
- Geng, S.Z.; Tian, Q.; An, S.M.; Pan, Z.M.; Chen, X.; Jiao, X.A. High-efficiency, two-step scarless-markerless genome genetic modification in Salmonella enterica. Curr. Microbiol. 2016, 72, 700–706. [Google Scholar] [CrossRef]
- Geng, S.Z.; Jiao, X.A.; Barrow, P.; Pan, Z.M.; Chen, X. Virulence determinants of Salmonella Gallinarum biovar Pullorum identified by PCR signature-tagged mutagenesis and the spiC mutant as a candidate live attenuated vaccine. Vet. Microbiol. 2014, 168, 388–394. [Google Scholar] [CrossRef]
- Geng, S.Z.; Wang, Y.N.; Xue, Y.; Wang, H.Q.; Cai, Y.; Zhang, J.; Barrow, P.; Pan, Z.M.; Jiao, X.A. The SseL protein inhibits the intracellular NF-κB pathway to enhance the virulence of Salmonella Pullorum in a chicken model. Microb. Pathog. 2019, 129, 1–6. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, Z.Y.; Wu, H.; Mei, C.Y.; Shen, P.C.; Pan, Z.M.; Jiao, X.A. Chromosomally located fosA7 in Salmonella isolates from China. Front. Microbiol. 2021, 12, 781306. [Google Scholar] [CrossRef]
- Oizel, K.; Tait-Mulder, J.; Fernandez-de-Cossio-Diaz, J.; Pietzke, M.; Brunton, H.; Lilla, S.; Dhayade, S.; Athineos, D.; Blanco, G.R.; Sumpton, D. Formate induces a metabolic switch in nucleotide and energy metabolism. Cell. Death. Dis. 2020, 11, 310. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, H.L.; Weng, C.C.; Janapatla, R.P.; Chen, C.L.; Chiu, C.H. Activation of apoptosis by Salmonella pathogenicity island-1 effectors through both intrinsic and extrinsic pathways in Salmonella-infected macrophages. J. Microbiol. Immunol. Infect. 2021, 54, 616–626. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell. Res. 2015, 12, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Rothwell, L.; Galyov, E.E.; Barrow, P.A.; Burnside, J.; Wigley, P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 2000, 146, 3217–3226. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Rafatellu, M.; Wilson, R.P.; Russmann, H.; Baumler, A.J. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 2008, 10, 247–261. [Google Scholar] [CrossRef]
- Doberenz, C.; Zorn, M.; Falke, D.; Nannemann, D.; Hunger, D.; Beyer, L.; Ihling, C.H.; Meiler, J.; Sinz, A.; Sawers, R.G. Pyruvate formate-lyase interacts directly with the formate channel FocA to regulate formate translocation. J. Mol. Biol. 2014, 426, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.C.; Kinsella, N.; Jaspe, N.A.; Friedrich, A.T.; O’Connor, C.D. Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Mol. Microbiol. 2000, 35, 1518–1529. [Google Scholar] [CrossRef]
- Koestler, B.; Fisher, C.R.; Payne, S.M. Formate promotes Shigella intercellular spread and virulence gene expression. mBio 2018, 9, e01777-18. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 2007, 9, 2562–2570. [Google Scholar] [CrossRef]
- Brokatzky, D.; Mostowy, S. Pyroptosis in host defence against bacterial infection. Dis. Model. Mech. 2022, 7, dmm049414. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Abu Kwaik, Y. Hijacking of apoptotic pathways by bacterial pathogens. Microbes. Infect. 2000, 14, 1705–1719. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).