Abstract
Introduction. Because patients with a suspicion of Lyme borreliosis (LB) may have experienced difficult care paths, the Tick-Borne Diseases Reference Center (TBD-RC) was started in 2017. The aim of our study was to compare the clinical features of patients according to their final diagnoses, and to determine the factors associated with recovery in the context of multidisciplinary management for suspected LB. Methods. We included all adult patients who were seen at the TBD-RC (2017–2020). Four groups were defined: (i) confirmed LB, (ii) possible LB, (iii) Post-Treatment Lyme Disease Syndrome (PTLDS) or sequelae, and (iv) other diagnoses. Their clinical evolution at 3, 6, and 9–12 months after care was compared. Factors associated with recovery at 3 and at 9–12 months were identified using logistic regression models. Results. Among the 569 patients who consulted, 72 (12.6%) had confirmed LB, 43 (7.6%) possible LB, 58 (10.2%) PTLDS/sequelae, and 396 (69.2%) another diagnosis. A favorable evolution was observed in 389/569 (68.4%) at three months and in 459/569 (80.7%) at 12 months, independent of the final diagnosis. A longer delay between the first symptoms and the first consultation at the TBD-RC (p = 0.001), the multiplicity of the diagnoses (p = 0.004), and the inappropriate prescription of long-term antibiotic therapy (p = 0.023) were negatively associated with recovery, reflecting serial misdiagnoses. Conclusions. A multidisciplinary team dedicated to suspicion of LB may achieve a more precise diagnosis and better patient-centered medical support in the adapted clinical sector with a shorter delay, enabling clinical improvement and avoiding inappropriate antimicrobial prescription.
1. Introduction
Lyme borreliosis (LB) is the most common tick-borne disease in Europe and the USA, caused by spirochetes of the Borrelia burgdorferi sensu lato complex [1,2]. In 2018, the annual incidence in France was 104 cases/100,000 inhabitants (95% Confidence Interval (CI) 91–117), demonstrating a constant increase since 2014 [3,4].
Clinical diagnosis of LB may be difficult because of its wide range of clinical pictures, sometimes resembling other pathologies (rheumatological diseases, auto-immune diseases, neurological disorders etc.) The most frequent clinical manifestations in Europe are erythema migrans (EM) and Lyme neuroborreliosis (LNB) [5]. Some functional symptoms may be present at all stages, which can further complicate the diagnosis [6,7]. Such symptoms may persist after a well-conducted treatment following the guidelines [post-treatment Lyme disease syndrome (PTLDS)] [5,6,8]. Rare sequelae causing definitive impairment may occur [5,6,9]. Rare coinfections with LB, transmitted by a tick-bite, are also described [10,11].
Microbiological diagnosis of LB relies on a two-tier serological test and PCR, for which sensitivities and specificities depend on the disease stage and the anatomical site sampled [12,13,14,15,16]. Current diagnostic tools are performant if their indication and interpretation are well-respected; otherwise, they may lead to an incorrect diagnosis.
Treatment of LB relies on antibiotic therapy for 10–28 days according to its stage and its clinical manifestation [15,17]. No studies have yet proven to be of benefit for longer treatment [18,19,20,21,22]. Nonetheless, there are no clear guidelines for the management of functional, persistent symptoms, which sometimes leaves patients unrelieved.
Therefore, because patients with a suspicion of LB may have experienced diagnostic delay and difficult care paths [23,24,25,26], we started a multidisciplinary LB center at the end of 2017, which is a joint endeavor of the departments of infectious diseases, internal medicine, rheumatology, neurology, algology, dermatology, psychiatry, microbiology, and physical rehabilitation. Our center was named the Tick-borne Diseases Reference Center (TBD-RC) for Paris and Northern Region in July 2019 by the French Ministry of Health. Other teams in other countries have also created such care organizations [27,28,29,30], showing a European awareness for the management of complex LB and its differential diagnoses. Several studies have been published to describe these new care organizations, but none have compared the clinical features of patients according to their diagnosis nor have they described patient care paths and outcomes after multidisciplinary and patient-centered medical support.
The aim of our study was to compare the clinical features of patients attending the TBD-RC according to their diagnosis (LB or not), to describe their care paths and outcomes, and to determine the factors associated with recovery in the context of multidisciplinary management for suspected LB.
2. Materials and Methods
We conducted a prospective descriptive and analytical cohort study, including all adult patients who consulted the TBD-RC for a suspicion of LB, from 1 December 2017 to 1 December 2020.
2.1. Population, Setting, and Intervention
For management at the TBD-RC, a medical file and a letter from a physician who referred the patient was requested prior to consultation, enabling the team to analyze all previous consultations, hospitalizations, and performed tests. Data on previous treatments were collected. Non-recommended treatments were defined as an antibiotic therapy longer than eight weeks and/or associated antimicrobials (≥2 prescribed concomitantly). There were no limitative criteria to receive patients, especially regarding positive or negative Borrelia serology. After a dedicated and multidisciplinary one-hour consultation with a meticulous physical exam, a medical summary was made and a first orientation offered in a one-day hospitalization, a conventional hospitalization, or an outpatient management. If indicated, a serological test for Borrelia was prescribed as well as a cerebrospinal fluid analysis for LNB or articular analysis for Lyme arthritis [16,31]. Other tests (a search for other tick-borne diseases, autoimmune disorders, etc.) were performed if clinically relevant. A complementary expert medical evaluation was requested if needed.
Patients with LB-associated symptoms were classified as follows [5,30,32,33]: (i) confirmed LB (tick-exposure, typical clinical signs, and a positive two-tiered serology), (ii) possible LB (tick exposure and/or prior EM, evocative clinical signs, and marked clinical improvement after 21 days of antibiotics), (iii) PTLDS (asthenia/polyalgia/cognitive complaints) or sequelae (objective and definitive impairment after a LNB, an ACA or a Lyme arthritis) persisting for more than six months after proven LB had been treated as recommended [15,33]. Patients not fulfilling these definitions were considered in the group “other diagnoses”. All diagnoses were made by a physician who specialized in the corresponding field. All the complex cases were discussed during a multidisciplinary consultation meeting to refine the diagnosis.
Finally, patient-centered care in the adapted medical department was offered to all patients to treat any disease/symptoms—even without a definitive diagnosis. Antibiotic therapy was prescribed if the patient presented an untreated confirmed or possible LB according to the guidelines [15,33]. Management was re-evaluated through a medical consultation at 3, 6, and 9–12 months to confirm its accuracy and adapt it if necessary. Patients who were living far away and could have adapted care from their general practitioner (GP) or a specialized physician were only re-evaluated at 9–12 months at the TBD-RC (consultation or teleconsultation). At each evaluation, a clinical statement was made by the doctor according to the patient’s point of view (joint oral conclusion): complete recovery, partial improvement (persistent clinical signs or symptoms allowing resumption of daily and professional activities), stagnation, or deterioration.
2.2. Patient Data
We collected patient data in standardized medical files (tick-exposure, past history of tick-bite, past-history of erythema migrans, delay between the tick-exposure and the symptoms, detailed clinical signs and symptoms, serological results for LB, past history of treatments etc.) at the TBD-RC independently of the study. Anxiety and sadness were measured with the MADRS scale, STAI form, and QIDS-SR16 scale, and asthenia with the FSS-11 score [34,35,36].
2.3. Statistical Analysis
The four groups of patients were compared according to socio-demographic, clinical and microbiological characteristics, and 3- and 9–12-month outcomes after multidisciplinary care.
Categorical variables are reported as proportions and percentages, and continuous variables as median with interquartile range (IQR). Categorical variables were compared by chi-squared or Fischer’s exact test as appropriate. Continuous variables were compared between groups by ANOVA as appropriate.
Factors associated with rapid recovery (evaluated at three months) and with recovery at a later point in time (evaluated at 9–12 months) were identified using logistic regression models. In both analyses, factors associated with the outcome with a p-value < 0.25 in univariate analysis were considered in the multivariate model. A stepwise backward regression was used to identify factors that remained independently associated with the outcome. Gender, age, and “group of patient” were forced in the models.
A p-value < 0.05 was defined for statistical significance. All analyses were performed using Stata version 16 (College Station, TX, USA).
2.4. Approval of the Ethics Committee
The local ethics committee of the University Intercommunal Hospital of Créteil, France, gave its approval for this research. All included patients gave their consent to use their medical data for research purposes prior to their management at the TBD-RC. The research sponsor signed a commitment to comply with the “Reference Methodology MR004” of the French Data Protection Authority, CNIL declaration number 2216096v0 (10 December 2019).
3. Results
3.1. Comparison of the Clinical and Epidemiological Characteristics of the Patients
During the study period, 569 patients consulted the TBD-RC of Paris and Northern region for suspicions of LB. Based on clinical and biological criteria and a multidisciplinary evaluation, 72/569 (12.7%) fulfilled criteria for confirmed LB, 43/569 (7.6%) possible LB, 58/569 (10.2%) PTLDS/sequelae, and 396/569 (69.2%) another diagnosis (Table 1 and Table 2, among whom 51 (9.0%) had no specific diagnosis but confirmed LB, possible LB, and PTLDS/sequelae were ruled out. Moreover, other diagnoses associated with confirmed LB (but unrelated with LB) were found in 36/72 (50%), with possible LB in 30/43 (69.8%), and with PTLDS/sequelae in 34/58 (58.6%). These differential and associated diagnoses are described Table 2. Among the 569 patients, 298 (52.4%) had a single diagnosis, 159 (27.9%) had two diagnoses, and 99 (17.4%) had more than three diagnoses retained. A mean of 1.7 diagnoses/patient was made with a median delay of 15.5 [IQ25,75 = 0;82] days at the TBD-RC.

Table 1.
Description of the final diagnoses made at the TBD-RC of Paris and Northern region.

Table 2.
Detailed description of the differential or associated diagnoses made at the TBD-RC of Paris and Northern region.
The comparative epidemiological characteristics of the patients are presented in Table 3. The median (IQR) delay between onset of symptoms and first consultation at the TBD-RC was 1.4 (0.4–3.8) years and was significantly shorter for confirmed LB (0.3 years or 4 months) (p < 0.001). Only 180/569 (31.6%) patients had a two-tiered positive serological test for Borrelia. Patients with a negative test and confirmed or possible LB had symptoms for less than six weeks. Prior to consulting the TBD-RC, 5/569 (0.9%) had a positive test only in Western-Blot, and all of these had another diagnosis. Prior to care at the TBD-RC, at least one antibiotic therapy had been prescribed in 369/569 (64.9%), and a non-recommended antibiotic therapy in 101/569 (17.8%). The median (IQR) duration of previous antibiotic therapy was three (two to seven) weeks. The proportion of patients who underwent non-recommended antibiotic therapy was significantly larger in the PTLDS/sequelae group (p = 0.001). The main clinical signs are presented in Table 4.

Table 3.
Comparison of the epidemiological characteristics of the patients consulting the TBD-RC of Paris and Northern region.

Table 4.
Comparison of the main clinical signs and symptoms presented by the patients consulting at TBD-RC of Paris and Northern region at baseline in the four different groups.
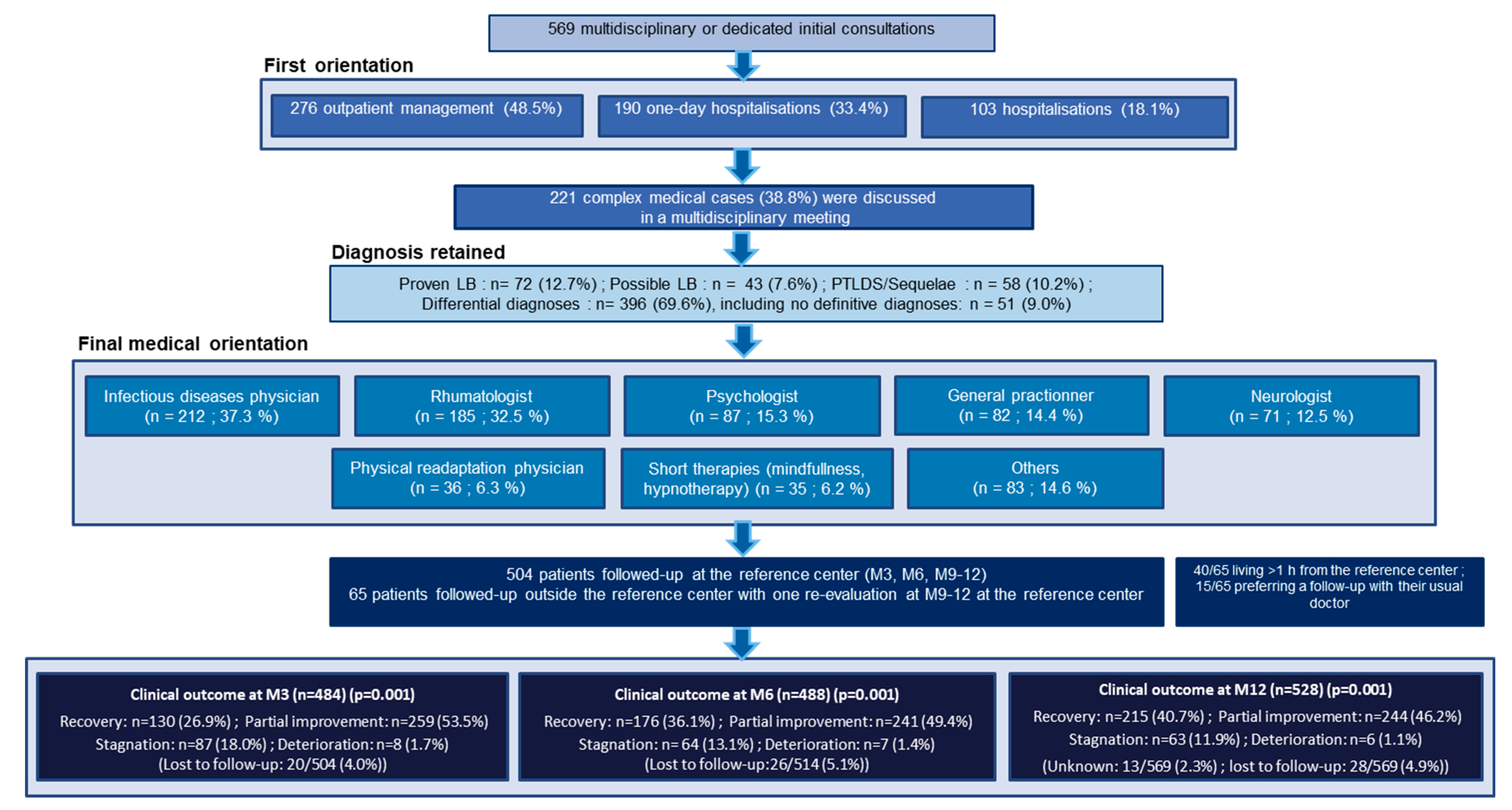
Care paths for the 569 patients are presented Figure 1. All patients were offered patient-centered care in the adapted medical department, mainly in the departments of infectious diseases, rheumatology, and psychology. Antibiotic therapy was prescribed at the TBD-RC for 148/569 (26%) patients for a median (IQR) duration of three (three to four) weeks. A total of 504/569 patients had a planned follow-up at the TBD-RC at three, six, and 9–12 months, and 65/569 had just one reevaluation at 9–12 months (Figure 1 and Figure 2).
Figure 1.
Care paths at the Tick-Borne Diseases Reference Center-Paris and Northern Region.
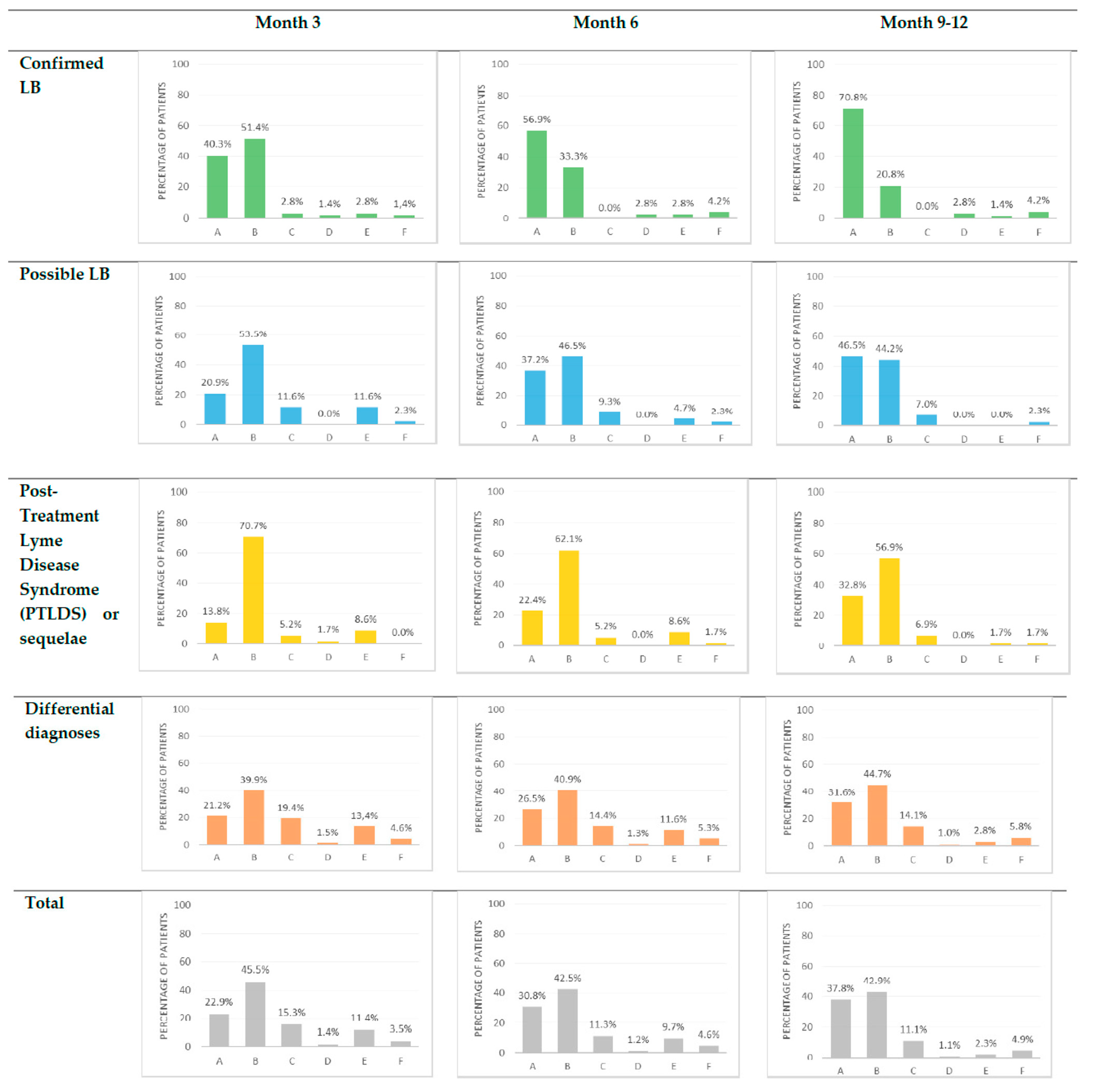
Figure 2.
Clinical outcome of the patients consulting at TBD-RC of Paris and Northern region at three, six and 9–12 months. A = Recovery; B = Partial improvement; C = Stagnation; D = Deterioration; E = Unknown; F = Lost to follow up.
3.2. Factors Associated with Rapid Recovery
At three months, 484/504 (96.0%) patients were evaluated. The proportion of patients with complete recovery, partial improvement, stagnation, or deterioration differed among the four diagnostic groups (p = 0.001). The proportions of patients with rapid recovery were 29/72 (40.3%), 9/43 (20.9%), 8/58 (13.8%), and 84/396 (21.2%) in confirmed LB, possible LB, PTLDS/sequelae, and other diagnoses, respectively (Figure 2).
Factors associated with rapid recovery in univariate and multivariate analysis are presented in Table 5 Factors independently associated with lower odds of rapid recovery were longer delay between onset of symptoms and the first consultation at the TBD-RC (p = 0.001), longer delay to final diagnosis (p < 0.001), multiplicity of diagnoses (p = 0.004), and a history of non-recommended antibiotic therapy (p = 0.023). A history of antibiotics use was not associated with recovery (p = 0.50 in univariate analysis). A first line of antibiotics prescribed at the TBD-RC was associated with recovery (p = 0.036 in univariate analysis). The odds of rapid recovery did not differ among study groups.

Table 5.
Univariate and multivariate analyses of the associated factors with recovery versus partial improvement or stagnation or deterioration at three months after care at the TBD-RC-Paris and Northern region.
In a sensitivity analysis at three months, patients with rapid recovery were compared to those with partial improvement only. Results were similar to the ones described above (Supplementary Table S1).
3.3. Factors Associated with Recovery at a Later Point in Time
At 9–12 months, 528/569 (92.8%) patients were evaluated. Twenty-eight patients were lost to follow-up, and 13 had an unknown status as they had not yet been re-evaluated at 9–12 months. In the absence of clinical evaluation at 9–12 months, patients with a complete recovery at three and/or six months were considered in recovery at 9–12 months. The proportions of patients with complete recovery, partial improvement, stagnation, or deterioration differed among the four groups (p = 0.001). The proportions of patients with recovery at a later point in time were 51/72 (70.8%), 20/43 (46.5%), 19/58 (32.8%), and 125/396 (31.6%) in confirmed LB, possible LB, PTLDS/sequelae, and other diagnoses, respectively (Figure 2).
Factors associated with a recovery at a later point in time in univariate and multivariate analysis are presented in Table 6. Factors independently associated with lower odds of recovery were longer delay between onset of symptoms and the first consultation at the TBD-RC (p < 0.001), and a history of non-recommended antibiotic therapy (p = 0.05). The odds of recovery were significantly higher for those identified as having confirmed LB (p = 0.004). The odds of recovery were significantly higher for those identified as having confirmed LB (p = 0.004) and in those with a history of EM (p = 0.020). After adjusting for other factors, multiplicity of diagnoses was no longer associated with lower odds of recovery, neither was antibiotic treatment provided at the TBD-RC with higher odds of recovery. A history of antibiotics use before consulting the TBD-RC was not associated with recovery (p = 0.52 in univariate analysis) as well as the prescription of a second line of antibiotics at the TBD-RC (p = 0.64 in univariate analysis).

Table 6.
Univariate and multivariate analyses of the associated factors with recovery versus partial improvement or stagnation or deterioration at 12 months after care at the TBD-RC-Paris and Northern region.
3.4. Description of Patients with Stagnation or Deterioration in the Groups with a Primary Diagnosis of LB at 3 Months
In the group “confirmed LB”: two presented stagnation and one deterioration (compressive neurinoma, misuse of doxycycline, and rapid progression of a lymphoma). In the group “possible LB”: five presented stagnation (two previous neurodegenerative diseases that worsened, a rheumatoid arthritis, a Biermer’s disease, and a cirrhosis). In the group “PTLDS/sequelae”: three presented stagnation and one deterioration (three still had persistent neuropathic pains two years after confirmed LNB treated as recommended, with a negative repeated lumbar punction; and an acute arthrosis). These patients could not improve because of another co-existing disease than LB, diagnosed thanks to the initial multidisciplinary management.
4. Discussion
4.1. Summary of the Principal Findings
Among the 569 patients who consulted the TBD-RC for a suspicion of LB, 72 (12.6%) had confirmed LB, 43 (7.6%) possible LB, 58 (10.2%) PTLDS/sequelae, and 396 (69.2%) another diagnosis. Over the entire follow-up, favorable evolution was observed in most of the patients: 389/569 (68.4%) had completely recovered or partially improved allowing resumption of daily and professional activities at three months, and 459/569 (80.7%) at 9–12 months, independent of the diagnosis. Patients with partial improvement, stagnation, or deterioration presented associated diagnoses, explaining the absence of complete recovery. The main factors negatively associated with rapid recovery were longer delay between onset of symptoms and the first consultation at the TBD-RC, multiplicity of diagnoses, and inappropriate prescription of antibiotic therapy, reflecting serial misdiagnosis and the complexity of the cases. The diagnostic delay and the history of non-recommended antibiotic therapy as negative factors for recovery were confirmed at 9–12 months. A confirmed LB was associated with a better recovery at 9–12 months.
4.2. Similar Multidisciplinary Experiences in France and Europe
Patients seeking care at the TBD-RC presented similar epidemiological and clinical characteristics to those in other studies in other settings describing a multidisciplinary care organization for suspected LB [27,28,29,30]. As previously found in these studies, ~10–20% of suspected cases had confirmed LB.
The multiplicity of other diagnoses found in all these studies highlights the complexity of diagnosing LB without disregarding other diagnoses [27,28,29,30]. There is not one disease that presents one picture. This multiplicity was associated with lower odds of recovery at three months. A multidisciplinary care organization may achieve a more precise diagnosis and better patient-centered medical support, with a shorter delay as demonstrated here and in other studies [29], enabling clinical improvement.
Moreover, on one hand, there were not any statistical differences among the recovery of the four groups at three months, which suggests that a multidisciplinary approach is beneficial for all groups and not just one. On the other hand, confirmed LB was associated with better recovery at 9–12 months, suggesting that LB needs a long follow-up for better management of the patients and that patients with confirmed LB have more chances to be cured even if for some presentations it may take more than three months until improvement.
Few patients (9%) had no specific diagnoses compared to other studies varying from 12.1% to 38.5% [27,28,29,30], probably because they had a regular follow-up in the adapted care sector since the beginning of their management, which allowed for clinical re-evaluations and the confirmation of the diagnosis for a longer period. For patients that have experienced diagnostic delay or serial misdiagnosis, a multidisciplinary approach could be a major answer, helping with acceptance of the diagnosis and offered care [23].
4.3. Meaning of the Study and Implication for Practice
Functional symptoms were the most frequent in the four groups of patients (asthenia, polymyalgia, and polyarthralgia) with no significant difference, but objective symptoms significantly differentiated them (Table 4). The predominance of functional symptoms in patients with a suspicion of LB had been demonstrated in previous studies, as well as had facial palsy in confirmed LB [7,27,28,29,30], but no studies had compared the clinical signs of the four groups. Arthritis of small joints in PTLDS suggests an interesting connection between the inflammatory processes linked to previous LB (reversible) or chronic inflammatory rheumatism [37,38].
4.3.1. Serology Does Not Rule the Diagnosis of LB
One third of the patients had a two-tiered positive serology. Among them, 74/180 (41.1%) had another diagnosis, and 34/180 (18.9%) PTLDS. Among patients with negative serology, 7/276 (2.5%) had proven LB, and 15/276 (5.4%) possible LB, and all had presented symptoms for less than six weeks. Our results are similar to the other descriptive cohort studies of multidisciplinary management [29,30,31,32]. Diagnosis of LB also relies on tick exposure and evocative clinical signs that are inseparable within the serology to confirm or refute the diagnosis [5,13,33].
4.3.2. Non-Recommended Antibiotic Therapies Are Associated with a Poorer Clinical Evolution
If past antibiotic use was not associated with recovery, prolonged or associated antibiotic therapies were deleterious. The proportion of patients with non-recommended antibiotic therapy was significantly higher in the group PTLDS/sequelae, reflecting the difficulty of their management and suggesting a causative role of long-term antibiotics on persistent symptoms. Patients clinically improved when antibiotics were stopped and multidisciplinary care started. Five randomized trials have demonstrated the absence of benefit of prolonged antibiotic therapies [18,19,20,21,22].
A favorable evolution for the majority of the patients lets the physicians reassure them despite previous misdiagnosis or diagnostic delay. We specifically focused on complete recovery, which was more common in those with confirmed LB, probably because their disease was well identified and well taken care of. However, the majority of patients in all groups experienced partial recovery after seeking care at the TBD-RC, indicating the benefit of multidisciplinary care (Figure 2).
4.3.3. A Longer Delay between the Onset of Symptoms and the First Consultation at the TBD-RC Is Associated with a Poorer Evolution
Early medical management enables better clinical outcome of the patients. Physicians should address their patients as soon as possible in these multidisciplinary structures, which seem to allow a better management and end diagnostic delay, taking time to listen to the patients, to examine, explore, and consult the expertise of different specialists. This raises the question whether or not these structures should be expanded for other diseases responsible for diagnostic delay, especially when we see here the low number of LB and TBD. General multidisciplinary clinics might also be an answer.
4.4. Strengths and Limitations of the Study
To our knowledge, this is the first study that compares clinical and epidemiological characteristics of patients who presented with suspected LB, based on the final diagnosis, to describe their clinical evolution within a multidisciplinary care center and to determine the factors associated with recovery. Moreover, we have presented one of the largest cohort studies of patients, and few were lost to follow-up, providing higher quality statistical analyses.
The first limitation is the study’s monocentric aspect, but it is qualified, as our results are similar to other multidisciplinary experiences in different European settings [27,29,30]. The second limitation is the inclusion of patients during the COVID-19 pandemic, which interrupted the flux of the patients between 15 March and 30 April 2020. We had no medical demand within this period, which reflected the dramatic decrease in the number of outpatient consultations for all medical fields. Normal activities resumed in May 2020. During the second lockdown (November 2020), 13 new patients with clinical emergencies (e.g., neurological signs) were evaluated, and we continued to follow up with all previous patients.
5. Conclusions
In our study, patients presented similar epidemiological and clinical characteristics to those in other studies in other settings describing a multidisciplinary care organization for suspected LB, but this is the first study which compared the clinical features and evolution of these four groups of patients (confirmed LB, possible LB, PTLDS/sequelae, and other diagnoses). Confirmed LB represented only a small percentage of patients (12.6%) who attended the TBD-RC of Paris and Northern Region. A favorable evolution was observed in the majority of the patients (80.7%) at 12 months, independent of the final diagnosis. A multidisciplinary care organization may achieve a more precise diagnosis and patient-centered medical support in the adapted clinical sector, with a shorter delay and avoiding inappropriate antibiotic therapies. For patients that experience serial misdiagnosis or diagnostic delay, a multidisciplinary approach could be a major answer, which would help with the acceptance of the diagnosis and the offered care.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms10030607/s1, Table S1: Sensibility analysis: Univariate and multivariate analyses of the associated factors of rapid recovery versus partial improvement at 3 months after care at the TBD-RC-Paris and Northern region.
Author Contributions
Conceptualization: A.R., Y.M., N.V.; methodology: A.R., Y.M., N.V.; validation: A.R., Y.M.; formal analysis: A.R., Y.M.; Investigation: A.R., A.C., S.N., J.S., P.C.-P., G.P., A.B., FM, C.F.; writing—original draft preparation: A.R., S.N.; writing—review and editing: A.R., J.S., S.N., P.C.-P., G.P., A.B., F.M., C.F., S.G., N.V., Y.M.; visualization: A.R., J.S., S.N., P.C.-P., G.P., A.B., F.M., C.F., S.G., N.V., Y.M.; supervision: Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of University Intercommunal Hospital of Créteil (protocol code 2021-02-03 and date of approval: 8 February 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available by sending an email at alice.raffetin@chiv.fr.
Acknowledgments
We are grateful to the following persons for their contribution and support to the TBD-RC and this study: The Tick-Borne Diseases Reference Center-Paris and Northern Region Working Group (Agathe Bounhiol, Kevin Diallo, Danielle Jaafar, Claudine Badr, Soline de Monteynard: Department of Infectious Diseases; Romain Jouenne, Emmanuel Dossou: Department of Internal Medicine; Stéphanie Emilie, Christine Shenouda: Department of Rheumatology; Lydie Lim, Navaneethan Nindulan: Department of Neurology; Sylvie Le Berre, Corinne Canu, Vincent Robin: Department of Algology; Sophie Dellion, Floriane Kouby: Department of Dermatology; Jonas Bantsimba: Department of Geriatrics; Jacques Breuil, Eric Hernandez, Camille Corlouer, Laurence Ghisalberti: Department of Microbiology; Eric Meinadier, Arthur Lefort: Department of Physical Rehabilitation; Aurélie Garraffo, Anne Chace, Emilie Georget: Department of Pediatrics; Ilia Pustilnicov: Department of Health Economics). We are grateful to the French Ministry of Health who has provided an annual budget allowance for the development of the TBD-RC since July 2019, and to the Regional Health Agency of Paris region who created a position for an infectious diseases physician to work at the TBD-RC in 2020.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme Disease-United States, 2008–2015. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Santé Publique France: Borréliose de Lyme. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-a-transmission-vectorielle/borreliose-de-lyme/donnees/#tabs (accessed on 11 March 2022).
- Septfons, A.; Goronflot, T.; Jaulhac, B.; Roussel, V.; De Martino, S.; Guerreiro, S.; Launay, T.; Fournier, L.; de Valk, H.; Figoni, J.; et al. Epidemiology of Lyme borreliosis through two surveillance systems: The national Sentinelles GP network and the national hospital discharge database, France 2005 to 2016. Euro Surveill. 2019, 24, 1800134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanek, G.; Fingerle, V.; Hunfeld, K.-P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupprecht, T.A.; Birnbaum, T.; Pfister, H.-W. Pain and neuroborreliosis: Significance, diagnosis and treatment. Schmerz. Berl. Ger. 2008, 22, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Voitey, M.; Bouiller, K.; Chirouze, C.; Fournier, D.; Bozon, F.; Klopfenstein, T. Functional signs in patients consulting for presumed Lyme borreliosis. Med. Mal. Infect. 2019, 50, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.; Bernasconi, E.; Heininger, U.; Abbas, M.; Nadal, D.; Strahm, C.; Erb, S.; Zimmerli, S.; Furrer, H.; Delaloye, J.; et al. Update of the Swiss guidelines on post-treatment Lyme disease syndrome. Swiss Med. Wkly. 2016, 146, w14353. [Google Scholar] [CrossRef] [Green Version]
- Müllegger, R.R.; Glatz, M. Skin manifestations of Lyme borreliosis: Diagnosis and management. Am. J. Clin. Dermatol. 2008, 9, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.; Kieffer, P.; De Martino, S.; Zilliox, L.; Vogel, J.; Jaulhac, B.; Hansmann, Y. Borrelia burgdorferi sl and tick-borne encephalitis virus coinfection in Eastern France. Med. Mal. Infect. 2018, 48, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Figoni, J.; Chirouze, C.; Hansmann, Y.; Lemogne, C.; Hentgen, V.; Saunier, A.; Bouiller, K.; Gehanno, J.; Rabaud, C.; Perrot, S.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): Prevention, epidemiology, diagnosis. Med. Mal. Infect. 2019, 49, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Lohr, B.; Fingerle, V.; Norris, D.; Hunfeld, K.-P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 219–245. [Google Scholar] [CrossRef]
- Leeflang, M.M.G.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Van Bortel, W.; Brandenburg, A.H.; Van Burgel, N.D.; Van Dam, A.P.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talagrand-Reboul, E.; Raffetin, A.; Zachary, P.; Jaulhac, B.; Eldin, C. Immunoserological Diagnosis of Human Borrelioses: Current Knowledge and Perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Jaulhac, B.; Saunier, A.; Caumes, E.; Bouiller, K.; Gehanno, J.; Rabaud, C.; Perrot, S.; Eldin, C.; de Broucker, T.; Roblot, F.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Med. Mal. Infect. 2019, 49, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Raffetin, A.; Bouiller, K.; Hansmann, Y.; Roblot, F.; Raoult, D.; Parola, P. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med. Mal. Infect. 2019, 49, 121–132. [Google Scholar] [CrossRef]
- Nguala, S.; Baux, E.; Patrat-Delon, S.; Saunier, F.; Schemoul, J.; Tattevin, P.; Cazorla, C.; Eldin, C.; Bouiller, K.; Raffetin, A. Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review. Pathogens 2021, 10, 972. [Google Scholar] [CrossRef]
- Berende, A.; Ter Hofstede, H.J.; Vos, F.J.; van Middendorp, H.; Vogelaar, M.L.; Tromp, M.; Hoogen, F.H.V.D.; Donders, A.R.T.; Evers, A.; Kullberg, B.J. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N. Engl. J. Med. 2016, 374, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.; Johnson, G.M.; Trevino, R.P.; Norton, D.; Levy, L.; Wall, D.; McCall, J.; et al. Two Controlled Trials of Antibiotic Treatment in Patients with Persistent Symptoms and a History of Lyme Disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.K.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.F.; Trevino, R.P.; Johnson, G.M.; Levy, L.; Dornbush, R.; Hu, L.T.; Evans, J.; Weinstein, A.; Schmid, C.; Klempner, M.S. Cognitive function in post-treatment Lyme disease Do additional antibiotics help? Neurology 2003, 60, 1916–1922. [Google Scholar] [CrossRef]
- Raffetin, A.; Barquin, A.; Nguala, S.; Paoletti, G.; Rabaud, C.; Chassany, O.; Caraux-Paz, P.; Covasso, S.; Partouche, H. Perceptions, Representations, and Experiences of Patients Presenting Nonspecific Symptoms in the Context of Suspected Lyme Borreliosis. Microorganisms 2021, 9, 1515. [Google Scholar] [CrossRef]
- Drew, D.; Hewitt, H. A Qualitative Approach to Understanding Patients’ Diagnosis of Lyme Disease. Public Health Nurs. 2006, 23, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.R.; Lloyd, V.K.; Gould, O.N. Motivations and Experiences of Canadians Seeking Treatment for Lyme Disease Outside of the Conventional Canadian Health-Care System. J. Patient Exp. 2017, 5, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Vitulano, L.; Lee, R.; Weiss, T.R.; Colson, E.R. Experiences of patients identifying with chronic Lyme disease in the healthcare system: A qualitative study. BMC Fam. Pr. 2014, 15, 79. [Google Scholar] [CrossRef] [Green Version]
- Coumou, J.; Herkes, E.; Brouwer, M.; van de Beek, D.; Tas, S.; Casteelen, G.; van Vugt, M.; Starink, M.; de Vries, H.; de Wever, B.; et al. Ticking the right boxes: Classification of patients suspected of Lyme borreliosis at an academic referral center in the Netherlands. Clin. Microbiol. Infect. 2015, 21, 368.e11–368.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquet, C.; Goehringer, F.; Baux, E.; Conrad, J.; Devonec, M.G.; Schmutz, J.; Mathey, G.; Tronel, H.; Moulinet, T.; Chary-Valckenaere, I.; et al. Multidisciplinary management of patients presenting with Lyme disease suspicion. Med. Mal. Infect. 2019, 49, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Gynthersen, R.M.; Tetens, M.M.; Ørbæk, M.; Haahr, R.; Fana, V.; Hansen, K.; Mens, H.; Andersen, A.B.; Lebech, A.-M. Classification of patients referred under suspicion of tick-borne diseases, Copenhagen, Denmark. Ticks Tick-Borne Dis. 2021, 12, 101591. [Google Scholar] [CrossRef] [PubMed]
- Kortela, E.; Kanerva, M.; Kurkela, S.; Oksi, J.; Järvinen, A. Suspicion of Lyme borreliosis in patients referred to an infectious diseases clinic: What did the patients really have? Clin. Microbiol. Infect. 2020, 27, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Dessau, R.; Van Dam, A.; Fingerle, V.; Gray, J.; Hovius, J.; Hunfeld, K.-P.; Jaulhac, B.; Kahl, O.; Kristoferitsch, W.; Lindgren, P.-E.; et al. To test or not to test? Laboratory support for the diagnosis of Lyme borreliosis—Author’s reply. Clin. Microbiol. Infect. 2018, 24, 211–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primer. 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé (HAS). Borréliose de Lyme et Autres Maladies Vectorielles à Tiques; Haute Autorité de Santé: Saint-Denis, France, 2018; pp. 1–52. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2018-06/reco266_rbp_borreliose_de_lyme_cd_2018_06_13__recommandations.pdf (accessed on 11 March 2022).
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.; Kornstein, S.; Manber, R.; et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Vagg, P.R.; Jacobs, G.A. State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Singh, S.K.; Girschick, H.J. Lyme borreliosis: From infection to autoimmunity. Clin. Microbiol. Infect. 2004, 10, 598–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvikar, S.L.; Steere, A.C. Diagnosis and Treatment of Lyme Arthritis. Infect. Dis. Clin. North Am. 2015, 29, 269–280. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).