Abstract
The spread of antibiotic resistance genes (ARGs) that are present in livestock manures, which are discharged into the environment, is a severe threat to human and animal health. Here, we used 16S rRNA gene profiling and metagenomic analysis to characterize microbial community composition and antibiotic resistance in a manure storage lagoon from a large-scale swine finishing facility. Manure samples were collected at intervals of two years. Both the prokaryotic community and the resistome were dominated by the Firmicutes, Proteobacteria and Bacteroidota. Metagenomic analysis of two samples revealed 726 and 641 ARGs classified into 59 and 46 AMR gene families. Besides multidrug efflux pumps, the predominating ARGs potentially encoded resistance to tetracyclines, macrolide–lincosamide–streptogramin, aminoglycosides, peptide antibiotics, rifamycin, chloramphenicol, and beta-lactams. Genes from all predominant AMR gene families were found in both samples indicating overall long-term stability of the resistome. Antibiotic efflux pumps were the primary type of ARGs in the Proteobacteria, while antibiotic target alteration or protection was the main mechanism of resistance in the Firmicutes, Actinobacteriota and Bacteroidota. Metagenome-assembled genomes (MAG) of four multidrug-resistant strains were assembled. The first MAG, assigned to Escherichia flexneri, contained 46 ARGs, including multidrug efflux pumps, modified porins, beta-lactamases, and genes conferring resistance to peptide antibiotics. The second MAG, assigned to the family Alcaligenaceae, contained 18 ARGs encoding resistance to macrolide–lincosamide–streptogramin, tetracyclines, aminoglycosides and diaminopyrimidins. Two other MAGs representing the genera Atopostipes and Prevotella, contained four and seven ARGs, respectively. All these MAGs represented minor community members and accounted for less than 0.3% of the whole metagenome. Overall, a few lineages originated from the gut but relatively rare in the manure storage lagoon, are the main source of ARGs and some of them carry multiple resistance determinants.
1. Introduction
Antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARG) are a global threat to both humans and animals. Antimicrobials are traditionally used to prevent and treat bacterial infections in livestock. Antibiotics are not fully degraded by the animals, but rather excreted with urine and feces, which are often used as organic fertilizers on arable land. This agricultural practice causes the dissemination of ARB and ARG in the environment [1,2]. Swine manure is considered to be a “hotspot” of ARB [3,4], and its discharge has been referred to as the ARG pollution of soil [5]. Swine farms have been indicated as important sources of tetracycline and sulfonamide resistance reservoirs in the environment [6,7].
The best-studied bacteria in the resistome are enteric, which are associated with food- and water-borne diseases [8,9]. It is broadly agreed that multidrug-resistant Enterobacteriaceae can spread or disseminate ARGs from animal farms or clinics/hospitals and can adversely affect agriculture and public health [10]. While numerous studies have explored the ARG distribution in the model enteric bacterial pathogens [6,11], less attention has been paid to the resistome of free-living environmentally relevant bacteria receiving ARG from gut ARB. Manure storage lagoons used for the collection and primary treatment of swine manure is a system in which gut microbes may transmit ARG to environmentally relevant bacteria. Non-pathogenic environmental microorganisms have been suggested as a massive reservoir of antibiotic resistance genes [12]. Previously, we demonstrated the occurrence of ARG in Desulfovibrio desulfuricans L4, a sulfate-reducing bacterium, isolated from waste of a large-scale swine finishing facility [13]. Strain L4 contained a plasmid with a multidrug-resistance gene cassette, containing tetracycline-, streptomycin-, and sulfonamide-resistance genes. The horizontal transfer of this plasmid was suggested from Enterobacteriaceae, namely Shigella flexneri, which harbored a nearly identical plasmid.
The aim of the present study was to perform a metagenomics analysis of manure slurry from the same large-scale finishing facility to profile antibiotic resistome and predict ARG dissemination in the environmentally relevant prokaryotes. Here, we report microbial community composition and resistomes of manure samples collected in different years.
2. Materials and Methods
2.1. Study Site, Sampling, and Physicochemical Parameters Measurements
Swine manure was collected from a large-scale swine finishing facility, ‘Tomskii’, with a capacity of 176,000 hogs a year and located in the close vicinity of Tomsk, the capital city of the Tomsk region in Western Siberia, Russia. Manure was sampled on 2 November 2021 from the manure storage lagoon receiving cumulative slurry from the facility (the Tom2 sample). The second sample, the near-bottom manure sediment slurry, was collected from the same storage lagoon (the Tom3 sample) on 18 October 2019 and used for Desulfovibrio isolation [13]. Total genomic DNA from manure slurry samples was extracted using a Power Soil DNA isolation kit (Qiagen, Hilden, Germany) and stored at −20 °C.
2.2. Sequencing of the 16S rRNA Gene Fragments and Bioinformatics Analysis of Microbial Community Composition
PCR amplification of 16S rRNA gene fragments comprising the V3–V4 variable regions was carried out using the universal prokaryotic primers PRK 341F (5′-CCTAYG GGDBGCWSCAG) and PRK 806R (5′-GGA CTA CNVGGG THTCTAAT) [14]. The PCR fragments were sequenced on Illumina MiSeq in a paired reads format (2 × 300 nt). Pairwise overlapping reads were merged using FLASH v.1.2.11 [15]. The 16S rRNA gene sequences were clustered into operational taxonomic units (OTUs) at 97% identity using the USEARCH v. 11 program [16]. Low-quality reads, chimeric sequences, and singletons were removed by the USEARCH algorithm. A total of 24,237 (Tom2) and 26,062 (Tom3) sequences passed all filters. To calculate OTU abundances, all obtained reads were mapped to OTU sequences at a 97% global identity threshold by USEARCH. The taxonomic assignment of OTUs was performed by searching against the SILVA v.138 rRNA sequence database using the VSEARCH v. 2.14.1 algorithm [17].
2.3. Metagenomic Sequencing and Identification of ARG
Total DNA isolated form manure slurry sample was sequenced using the Illumina HiSeq2500 platform according to the manufacturer’s instructions (Illumina Inc., San Diego, CA, USA). The sequencing of a paired-end (2 × 150 bp) TruSeq DNA library generated 192,103,014 read pairs (~57.6 Gb) for the Tom2 sample and 108,273,070 read pairs (~32.5 Gb) for the Tom3 sample. Adapter removal and trimming of low-quality sequences (Q < 30) were performed using Cutadapt v.3.4 [18] and Sickle v.1.33 (https://github.com/najoshi/sickle, accessed on 14 October 2022), respectively. The reads were de novo assembled into contigs using the MEGAHIT v.1.2.9 program [19].
The obtained contigs were binned into metagenome-assembled genomes (MAGs) using MetaBAT v. 2.15 (Kang et al., 2019). The assembled MAGs were taxonomically classified using the Genome Taxonomy Database Toolkit (GTDB-Tk) v.1.5.0 [20] and Genome Taxonomy database (GTDB) [21]. CheckM 1.1.3 [22] was used to check completeness and contamination values of obtained MAGs. The open reading frames (ORFs) in contigs were predicted using Prodigal v2.6.3 (Hyatt et al., 2010). To identify putative ARGs, we aligned the predicted protein sequences of ORFs against the Comprehensive Antibiotic Resistance Database (CARD, https://card.mcmaster.ca/, accessed on 14 October 2022) [23,24,25] using the BLASTP tool with e-value < 1 × 10−5 [23]. An ORF was annotated as an ARG if the best BLASTP hit showed at least 80% identity over a query coverage of 85% [23]. The identified ARG-like ORFs were then assigned to various AMR gene families, drug classes and resistance mechanisms categories according to the CARD. The taxonomy of the ARG-carrying contigs was assigned using the Kaiju webserver with the NCBI NR protein database as a reference [26].
3. Results
3.1. Composition of Tom2 Manure Slurry Microbiome Revealed by 16S rRNA Gene Profiling
To characterize the taxonomic compositions of microbial communities a total of 37,335 sequences of 16S rRNA gene fragments were determined and clustered into 593 OTUs at the level of 97% sequence identity. Taxonomic assignment of OTUs revealed the presence of 17 phylum-level lineages of Bacteria and Archaea, recognized in the Genome Taxonomy Database (GTDB). Archaea were represented by a single OTU assigned to Candidatus Methanoplasma, which accounted for only 0.01% of all 16S rRNA gene sequences. Bacterial community was dominated by the Firmicutes (41.4% of 16S rRNA gene reads), other major groups were the Proteobacteria (27.6%), Bacteroidota (16.9%), Actinobacteriota (8.2%), Desulfobacterota (2.5%), Deinococcota (1.6%) and Campylobacterota (0.8%) (Figure 1). Other phyla altogether accounted for only 1% of the community.
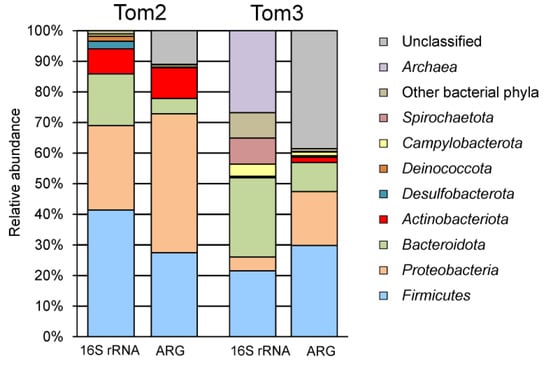
Figure 1.
Compositions of microbial communities (16S rRNA) and resistomes (ARG) in the manure storage lagoon at the domain/phylum level. Taxonomic composition of the resistome is shown according to predicted taxonomy of the ARG-carrying contigs.
Most of Firmicutes were assigned to the class Bacilli (37.0%), which was dominated by unclassified lineages of the family Bacillaceae (about 28%) phylogenetically distant from cultured species, and the genus Atopostipes (Carnobacteriaceae, Lactobacillales) accounting for about 7.5%. Atopostipes sp. are typical inhabitants of various composts [27,28]. Classes Clostridia and Limnochordia were less numerous (2.2% and 2.1%, respectively) and most of the corresponding OTUs were not classified even at the family level representing phylogenetically distant uncultured groups.
Proteobacteria mostly belonged to the class gamma (27.3%) and comprised 157 OTUs. However, the majority of proteobacterial 16S rRNA gene sequences belonged to only two OTUs. One of them, comprising 16.1% of all 16S rRNA gene reads, was assigned to the family Alcanivoracaceae (Pseudomonadales), while the second one (5.1%) belonged to the Alcaligenaceae (Burkholderiales); both OTUs were not classified at the genus level.
The phylum Bacteriodetes was mostly represented by the families Flavobacteriaceae (6.6%), Sphingobacteriaceae (5.5%) and Marinilabiliaceae (3.4%). Most abundant OTUs belonged to uncultured candidate genera. The phylum Actinobacteriota mostly comprised the members of Corynebacteriales and Micrococcales, and nearly a half of Actinobacteriota belonged to the genus Corynebacterium (4.6%), which was reported in swine manure compost [29]. Desulfobacterota was represented by four OTUs phylogenetically distant from cultured lineages of this phylum. As revealed by GenBank searches, the most abundant OTU (2.3%) showed only 82.5% sequence identity to the closest cultured relative, Natronanaerobius thermophilum, and 96.6% identity to environmental clone OTU_112 (GenBank MH312167) found in river sediments. Conversely, all Deinococcota OTUs belonged to the known genus Truepera, frequently found in the bacterial communities during swine manure composting [30]. Finally, the phylum Campylobacterota mostly comprised known genera of sulfur-oxidizing bacteria, Sulfurimonas and Sulfurospirillum. Their growth was probably supported by hydrogen sulfide generated by sulfate reducing bacteria [13].
3.2. Composition of Tom3 Manure Slurry Microbiome
The structure of the microbial community of the near-bottom sediment slurry, the Tom3 sample, was briefly reported in [13] and will be presented in more detail below. Unlike Tom2, in the Tom3 sample, Archaea accounted for about 27% of all 16S rRNA gene sequences and were represented mostly by methanogens of the orders Methanobacteriales, Methanomicrobiales and Methanomassiliicoccales. The high relative abundance of methanogens is probably related to anaerobic conditions in the sediments. Bacterial community was dominated by the Bacteroidota (25.9%) and the Firmicutes (21.5%), and other major groups were the Spirochaetota (8.6%), Proteobacteria (4.5%) and Campylobacterota (3.9%), while Actinobacteriota and Desulfobacterota were detected in minor amounts (Figure 1).
The phylum Bacteriodetes was mostly represented by taxa typical of animal digestive tracts, assigned to the families Bacteroidaceae, Rikenellaceae, Prevotellaceae and Dysgonomonadaceae. Most of Firmicutes were assigned to the class Clostridia (13.5%) which was dominated by the families Ruminococcaceae and Lachnospiraceae, and various uncultured lineages. Classes Bacilli and Negativicutes were less numerous (7.5% and 0.5%, respectively). Proteobacteria mostly belonged to the class gamma (4.3%) of the orders Enterobacterales (2.4%) and Pseudomonadales (1.4%). Interestingly, two proteobacterial OTUs highly abundant in the Tom2 sample were not found in Tom3. Spirochaetota were mostly represented by the genus Sphaerochaeta (5.4%) and Treponema (3.0%), typically found in pig gut microbiomes [31]. Finally, the phylum Campylobacterota mostly comprised the family Arcobacteraceae (3.5%), while sulfur-oxidizing bacteria of the genera Sulfurimonas and Sulfurospirillum were less numerous.
3.3. Characterization of the Tom2 Manure Resistome
Metagenome sequencing of the Tom2 manure sample resulted in assembly of 1,424,703 contigs with N50 size of 1452 bp and a total length of 1359 Mb, the maximal contig length was 749,306 bp. In total, 726 ARGs classified into 59 AMR gene families recognized in CARD were identified in 673 contigs (Supplementary Table S1). The most numerous AMR gene families were resistance-nodulation-division (RND) antibiotic efflux pumps (141 genes), major facilitator superfamily (MFS) antibiotic efflux pumps (96 genes), Erm 23S ribosomal RNA methyltransferase (70 genes), tetracycline-resistant ribosomal protection protein (54 genes), Pmr phosphoethanolamine transferase (42 genes), vga-type ABC-F protein (41 genes) and rifamycin-resistant beta-subunit of RNA polymerase (33 genes). The detected resistance genes represented all major resistance mechanisms: antibiotic inactivation (147 genes), antibiotic efflux or reduced permeability to antibiotic (267 genes), and antibiotic target alteration, replacement or protection (312 genes).
Tetracyclines are the most commonly used antibiotics for animal growth promotion and disease control [7,32,33,34] and ARG profiles obtained from our sample indicated that resistance to this antibiotic was most frequent among the detected ARGs (109 genes) (Figure 2). Other frequently found ARG drug classes included MLS antibiotics (macrolide–lincosamide–streptogramin, 82 genes), aminoglycosides (73 genes), peptide antibiotic (54 genes), rifamycin (36 genes), phenicol antibiotics (e.g., chloramphenicol, 23 genes), and beta-lactams (21 genes). Nevertheless, many ARGs encoded multidrug efflux pumps and were predicted to confer resistance to several classes of antibiotics.
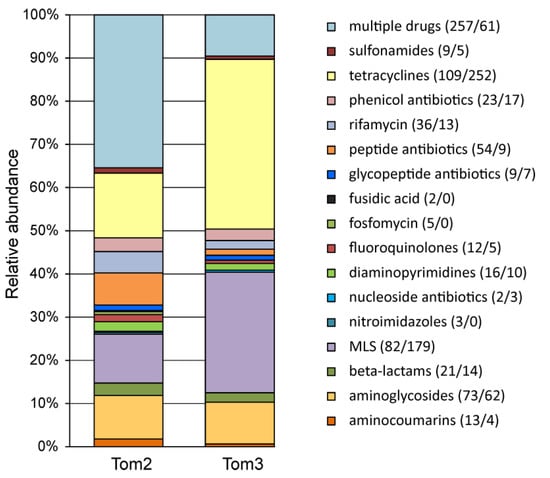
Figure 2.
Relative abundances of ARG identified in manure samples Tom2 and Tom3 categorized by class of resistance. Numbers of genes are shown in parentheses (Tom2/Tom3).
Taxonomic assignment of contigs carrying ARGs was estimated by Kaiju. Most ARG were assigned to the Proteobacteria (330 genes, 45% of the total) followed by Firmicutes (199, 27%), Actinobacteriota (73, 10%), Bacteroidota (36, 5%), Campylobacterota (5, 0.7%), Desulfobacterota (2, 0.3%) and Acidobacteriota (1, 0.1%); taxonomic affiliation of other 80 ARG remained uncertain (Figure 1).
The majority of ARG assigned to the Proteobacteria represented antibiotic efflux pumps of the RND (129 genes), MFS (66 genes) and ATP-binding cassette (ABC) (10 genes) families (Figure 3, full data are shown in Supplementary Table S1).
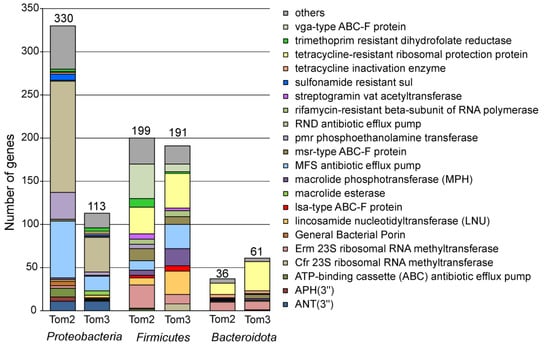
Figure 3.
Abundances of ARG identified in the Tom2 and Tom3 manure samples categorized by AMR gene family. Numbers of ARG assigned to particular family are shown for the Proteobacteria, Firmicutes and Bacteroidota. Others, ARG families comprising less than 5 ARG in these phyla.
They were predicted to confer resistance to various compounds, including fluoroquinolones, tetracyclines, macrolides, diaminopyrimidine antibiotics, phenicol antibiotics, beta-lactams, aminoglycosides, aminocoumarin antibiotics, disinfecting agents and antiseptics. Resistance to aminoglycosides based on antibiotic inactivation could be conferred by ARGs of AAC(3), AAC(6′), APH(3′), ANT(3″), ANT(4′), APH(4) and APH(6) families (30 genes in total). A total of 31 ARG of the Pmr phosphoethanolamine transferase family (mostly ugd genes) were predicted to determine resistance to peptide antibiotics such as polymyxin. Resistance to beta lactams such as carbapenem, cephalosporin and penam is probably determined by various beta-lactamases (15 genes of ampC, CARB, CMY, CTX-M, EC, OXA and SRT types), while seven sul genes (sul1, sul2, and sul3) could enable resistance to sulfonamides. Although MFS family efflux pump were most numerous among tetracycline resistance determinants, two tet(X6) genes determined antibiotic inactivation and tet(57) gene coding for tetracycline-resistant ribosomal protection protein were found as well. Considering other drugs, specific resistance genes were found for lincosamides (lincosamide nucleotidyltransferase, two genes), macrolides (macrolide phosphotransferase, 2 genes), chloramphenicol (chloramphenicol acetyltransferase, four genes), fluoroquinolones (quinolone resistance protein Qnr, two genes), diaminopyrimidine antibiotics (trimethoprim resistant dihydrofolate reductase Dfr, three genes), and streptothricin (streptothricin acetyltransferase).
More than a quarter of detected ARGs were assigned to Firmicutes (Figure 3). Contrary to the Proteobacteria, antibiotic target alteration/protection was the most frequent resistance mechanism (146 genes), while antibiotic inactivation and efflux were less common (39 and 14 genes, respectively). The most numerous were genes of the ABC-F protein families, conferring resistance through ribosomal protection in Gram-positive bacteria [35]. A total of 40 genes of the vga-type ABC-F proteins and three genes encoding the lsa-type ABC-F proteins are known to provide resistance to lincosamides and streptogramin A antibiotics. Fourteen genes of the msr-type ABC-F protein family are responsible for resistance to macrolides and group B streptogramins, and one optrA gene confers resistance to oxazolidinones and phenicols [35]. The lsa-type and vga-type ABC-F proteins were found only in the Firmicutes. Resistance to tetracyclines could be enabled by 31 genes of tetracycline-resistant ribosomal protection protein family (tetT, tetS, tetO, tetM and tet32) and three genes encoding MFS family efflux pumps (tet(L) and tet(40)). The presence of 27 genes encoding Erm 23S ribosomal RNA methyltransferase, three genes for non-erm 23S ribosomal RNA methyltransferase (G748) and one gene for Cfr 23S ribosomal RNA methyltransferase indicates resistance to several antibiotics, such as lincosamides, macrolides, and streptogramins through target alteration. Resistance to aminoglycosides based on antibiotic inactivation could be conferred by ARGs of AAC(6′), APH(2″), ANT(4′), ANT(6), ANT(9), APH(2″), APH(3′) families (11 genes in total). There are 10 genes for trimethoprim resistant dihydrofolate reductase Dfr, eight genes for lincosamide nucleotidyl transferase (LNU), five ugd genes for phosphoethanolamine transferase (Pmr), four genes for chloramphenicol acetyltransferase, six genes for macrolide phosphotransferase, six genes for streptogramin Vat acetyltransferase, and two genes of the glycopeptide resistance gene cluster (vanR). Resistance to rifamycin could be conferred by rifamycin-resistant beta-subunit of RNA polymerase (6 rpoB genes) and rifampin phosphotransferase (two rphB genes). The presence of two genes for FusB-type target protecting proteins, fosfomycin thiol transferase gene fosD, and blaZ beta-lactamase gene indicates resistance to fusidic acid, fosfomycin and penam. Genes encoding antibiotic efflux pumps were less numerous among ARGs of the Firmicutes. Addition to aforementioned three tetracycline efflux pumps, there are 10 other genes encoding the MFS superfamily transporters predicted to confer resistance to macrolides, fosfomycin, chloramphenicol, and various disinfecting agents and antiseptics. A single bcrA gene encoding an ABC transporter probably confers bacitracin resistance.
Among 73 actinobacterial ARGs, the most numerous was rifamycin-resistant beta-subunit of RNA polymerase rpoB (24 genes), followed by MFS tetracycline efflux pumps (tet(33), tet(Z) and tetB(48), a total of 14 genes), and Erm 23S ribosomal RNA methyltransferase conferring resistance to lincosamides and macrolides (11 genes) (Figure 3). RbpA gene encoding RNA polymerase-binding protein also provides resistance to rifamycin. Resistance to macrolides could be determined by MtrA efflux pump of the RND family (10 genes) and macrolide phosphotransferase MphO (three genes). Six vanR glycopeptide resistance gene of the Van cluster probably confer resistance to drugs of this class. The presence of two cmx genes for MFS superfamily exporter indicates resistance to chloramphenicol.
Interestingly, all 36 ARGs assigned to the members of the Bacteroidota confer resistance via antibiotic inactivation or target alteration/protection, while drug efflux pumps were not found (Figure 3). Resistance to tetracyclines was the most common, it was determined by tetracycline-resistant ribosomal protection proteins (two tetQ and 11 tet36 genes) or through tetracycline inactivation (tet(X4), tet(X5), tet(X6), and tetX). Ten erm(35) genes encoding the Erm 23S ribosomal RNA methyltransferase likely determine resistance to lincosamides and macrolides. Four aadS genes (ANT(6) family) could be responsible for resistance to aminoglycoside antibiotics. Other resistance determinants were MphG macrolide phosphotransferase, EreD macrolide esterase, msr-type ABC-F protein (confers resistance to macrolides and streptogramin), streptogramin Vat acetyltransferase and sulfonamide resistance protein Sul2.
3.4. Diversity of ARG from the Tom3 Manure Sample
Metagenomic analysis of the Tom3 manure sample revealed 641 ARG classified into 46 AMR gene families (Supplementary Table S2). Resistance to tetracyclines was most frequent in the Tom3 resistome (252 genes) followed by MLS antibiotics (179 genes) and aminoglycosides (62 genes) (Figure 2). Interestingly, ARGs encoding multidrug efflux pumps were less frequent than in the Tom2 sample (61 genes), as well as ARGs conferring resistance to aminocoumarins, fluoroquinolones, peptide antibiotics and rifamycin (Figure 2).
Taxonomic assignment of contigs carrying ARGs by Kaiju enabled to classify 394 of 641 detected ARG (Figure 1). Most of them were assigned to the Firmicutes (191, 30% of the total), Proteobacteria (113 genes, 18%) and Bacteroidota (61 genes, 10%), while none represented archaea. For the Firmicutes, the most frequent resistance mechanism was antibiotic target alteration/protection (98 genes), followed by antibiotic inactivation (63 genes) and efflux (30 genes). ARGs enabling resistance to MLS antibiotics were most numerous and included lincosamide nucleotidyltransferase LNU (27 genes), Erm and Cfr 23S ribosomal RNA methyltransferases (11 and 8 genes, respectively), vga-type (nine genes), lsa-type (six genes) and msr-type (nine genes) ABC-F proteins, macrolide phosphotransferase MPH (20 genes) and streptogramin Vat acetyltransferase. Resistance to tetracyclines could be conferred by tetracycline-resistant ribosomal protection proteins (40 genes, mostly tet(T)) and MFS superfamily efflux pumps (24 genes). ARGs of AAC(6′), APH(2″), ANT(4′), ANT(6), and ANT(9) families (10 in total) could be responsible for inactivation of aminoglycoside antibiotics. The presence of seven rpoB genes for the rifamycin-resistant beta-subunit of RNA polymerase indicates resistance to this drug. In addition, there are three genes for chloramphenicol acetyltransferase (CAT), four genes of glycopeptide resistance van cluster, two genes for trimethoprim resistant dihydrofolate reductase Dfr, two genes for streptothricin acetyltransferase (SAT), two optrA genes encoding ABC-F subfamily ATP-binding cassette ribosomal protection proteins, and six efflux pumps.
Like in the metagenome of Tom2 sample, the majority of ARG assigned to the Proteobacteria represented antibiotic efflux pumps (61 genes) predicted to confer resistance to various drugs. Seventeen genes of the AAC(3), AAC(6′), ANT(3″), APH(3′) and APH(4) families could enable inactivation of aminoglycosides. Resistance to MLS antibiotics could be determined by lincosamide nucleotidyltransferase (three genes), Erm and Cfr 23S ribosomal RNA methyltransferases (two genes), macrolide esterase (five genes) and phosphotransferase (one gene). Specific resistance genes were also found for diaminopyrimidines, fluoroquinolones, beta lactams, peptide antibiotics, chloramphenicol, sulfonamide, rifamycin and tetracycline, like in the Tom2 manure sample.
The inventory of ARGs among the Bacteroidetes was similar to that observed in the Tom2 sample. The most common was resistance to tetracyclines determined by tetracycline-resistant ribosomal protection proteins (34 genes, mostly tet(Q) and tet(36)) or tetracycline inactivation enzyme (three genes). Genes encoding the Erm 23S ribosomal RNA methyltransferase (10 genes), msr-type ABC-F protein (five genes), macrolide phosphotransferase (two genes) and macrolide esterase (one gene) likely determine resistance to MLS antibiotics. Other resistance determinants were aminoglycoside phosphotransferases (APH(6) and APH(3″)), CfxA beta-lactamase (three genes) and sulfonamide resistance protein Sul2.
3.5. Multidrug Resistant Strains
To obtain MAGs of microbial community members, we sequenced the metagenomes of manure samples using Illumina technique and binned obtained contigs into MAGs. Since we were interested in genomes containing genetic determinants of multiple drug resistance, only MAGs with more than 50% completeness and less than 25% contamination were selected for further analysis. Of the 140 such MAGs obtained for the Tom2 sample, 24 contained at least one ARG which can serve as a rough estimate of the proportion of resistant strains in the community. Twenty MAGs contained one gene each, two MAGs contained two ARGs and two genomes contained 18 and 46 resistance genes, apparently representing multidrug-resistant strains (Table 1).

Table 1.
ARGs in multidrug-resistant strains in the Tom2 sample.
The first genome, MAG-8, with an estimated 63% completeness and 2% possible contamination harbored 46 ARGs. This MAG accounted for only 0.03% of the whole metagenome. Analysis of the taxonomic affiliation of this MAG showed that it belongs to the genus Escherichia and showed 98.50% ANI with Escherichia (Shigella) flexneri strain 2a (GCA_002950215.1) therefore representing this species. Most of resistance genes encode antibiotic efflux pumps of RND (22 genes), MFS (12 genes) and ABC (three genes) families, conferring resistance to various drugs including aminocoumarins, aminoglycosides, fluoroquinolones, and macrolides. Three genes encode porins with reduced permeability to beta-lactams. In addition, there are pmrF and eptA genes (phosphoethanolamine transferase), and bacA gene (undecaprenyl pyrophosphate related protein) conferring resistance to peptide antibiotics, and three beta-lactamase genes (EC-14, EC-5 and ampC) predicted to confer resistance to cephalosporins.
MAG-42, carrying 18 ARGs, was estimated to be 81% complete, with 25% possible contamination. The share of this MAG in the whole metagenome was estimated in 0.3%. Although such a high contamination did not allow its taxonomic identification using GTDB tools, 123 of 133 conservative marker genes showed best BLASTP hits with proteins form the members of the family Alcaligenaceae (Proteobacteria, Burkholderiales). Although Alcaligenaceae were among the dominant groups according to 16S rRNA gene profiling data (see above), this MAG represented a low abundance phylotype of this family. Eight ARGs were predicted to determine resistance to macrolide–lincosamide–streptogramin antibiotics, namely lincosamide nucleotidyltransferase (lnuA) enabling inactivation of lincosamide, Erm 23S (five genes) and non-erm (two genes) ribosomal RNA methyltransferases protecting their target. Resistance to tetracyclines could be provided by tetracycline-resistant ribosomal protection proteins (three genes) and an MFS efflux pump (tet(L)). Two aminoglycoside nucleotidyltransferases (ANT(6) and ANT(9)) and two aminoglycoside phosphotransferases (APH(2″) and APH(3′)) probably determine resistance to aminoglycoside antibiotics. In addition, this MAG contains two trimethoprim resistant dihydrofolate reductase genes (dfrG) responsible for resistance to diaminopyrimidins.
Similarly, in the case of the Tom3 sample, of the 172 MAGs with more than 50% completeness and less than 25% of contamination, 22 contained at least one ARG: 18 MAGs contained single ARG, two MAGs contained two ARGs, and two MAGs representing multidrug-resistant strains harbored four and seven ARGs. MAG Tom3-16, carrying four ARGs and obtained with 88% completeness and 5% contamination, was assigned to the genus Atopostipes (Firmicutes, Bacilli). This genome contains genes for tetracycline-resistant ribosomal protection protein (tet(M)), lincosamide nucleotidyltransferase LNU, chloramphenicol acetyltransferase CAT and kanamycin nucleotidyltransferase (ANT(4′)-Ib). The second MAG Tom3-259, assigned to the genus Prevotella (Bacteroidota), was estimated to be 90% complete, with 12% possible contamination. This MAG contained two genes for lincosamide nucleotidyltransferase LNU, two genes for tetracycline-resistant ribosomal protection protein, macrolide phosphotransferase gene (mphB), an aminoglycoside nucleotidyltransferase gene (ANT(6)-Ia) and tetA(P) gene of tetracycline efflux pump. Each of these MAGs accounted for less than 0.2% of the whole metagenome and therefore represented a low abundance phylotypes.
4. Discussion
In this work, we characterized the composition of the microbial community and the resistome of two swine manure samples collected from a storage lagoon. In the fecal swine matter Firmicutes is known to be the dominating phylum followed by the Bacteroidota and Actinobacteriota [35,36,37]. Likewise, Firmicutes and Bacteroidota predominated in the analyzed storage lagoon, but the second most numerous bacterial phylum in the Tom2 sample was the Proteobacteria (27.6%). Since the storage lagoon contains manure at different stages of degradation, occurring both under aerobic and anaerobic conditions, it can be assumed that particular lineages of Proteobacteria predominantly multiply in the lagoon, and only a minor part of Proteobacteria represented the microbiome of the swine gut. An increase in the relative abundance of the Proteobacteria during composting of swine manure has been reported in other studies [33,37,38]. Interestingly, in the Tom2 sample, most of the proteobacterial 16S rRNA gene sequences (21.2%) belonged to only two OTUs. The most numerous OTU was only distantly related to both cultured and uncultured sequences (the best GenBank hit was from Alcanivorax limicola JB21 with 94.2% identity). The corresponding MAG was assigned to the family Alcanivoracaceae, but was not classified at the genus level. Members of this family are known to be involved in degradation of alkanes (e.g., [39]), and are not typical members of gut microbiomes. This phylotype probably originated from the environment and proliferated already in the storage lagoon. These two phylotypes were absent in the Tom3 sample and, accordingly, the share of Proteobacteria in this sample was significantly lower (4.5% vs. 27.6%, Figure 1). Nevertheless, most other proteobacterial lineages identified in the Tom2 and Tom3 samples (e.g., Burkholderiales, Pseudomonadales, Enterobacterales, etc.) are typical gut bacteria.
A distinctive feature of the microbiome of the Tom3 sample was a significant proportion of archaea, mainly methanogens. Probably, the high relative abundance of methanogens reflects local anaerobic conditions in the near-bottom sediment. This probably also explains the relatively high abundance of anaerobes of the phylum Spirochaetota, which was almost absent in the Tom2 sample.
Compared to the composition of the microbiomes revealed by 16S rRNA gene profiling, the resistomes represented the same main bacterial lineages, but the proportion of Proteobacteria was higher in the resistomes (Figure 1). Of the 726 resistance genes identified in the Tom2 sample, 121 genes were assigned to the family Enterobacteriaceae (mostly Escherichia and Klebsiella), whose share in the community, according to 16S RNA gene profiling data, was only 0.06%. Likewise, Enterobacteriaceae accounted for 26 ARG in the Tom3 sample (4% of the total), although this family accounted for only 0.03% of 16S rRNA gene sequences. Enterobacteriaceae are typical intestinal inhabitants and are considered as markers of fecal contamination [40]. Most of these genes code for an efflux pumps, but genes of enzymes that ensure the inactivation of aminoglycosides and beta-lactam antibiotics were also found.
One of detected strains carrying multiple resistance determinants (MAG-8), Escherichia (Shigella) flexneri, also represented Enterobacteriaceae. Shigella can cause moderate to acute gastrointestinal tract infection, known as shigellosis [41]. Shigella isolates could be resistant to various antibiotics, including sulfonamides, tetracycline, chloramphenicol, ampicillin, quinolones, and the third-generation cephalosporins [42,43,44,45], and several multidrug-resistant isolates have been characterized [41]. In particular, β-lactamases could provide high level resistance to penicillin and first-generation, second-generation and third-generation cephalosporins and monoamide antibiotics [46]. The presence of three class C beta-lactamases in MAG-8 along with numerous efflux pumps indicates that this bacterium is probably a multidrug-resistant strain that could disseminate ARGs in the manure storage lagoon.
The same is probably true for the second potential multidrug-resistant strain, although its taxonomic affiliation and origin is less clear. This strain, represented by MAG-42, was affiliated with the Proteobacteria, the family Alcaligenaceae. Although this family accounted for about 6.4% of the whole microbiome, the share of this strain was much lower (~0.3%), while MAGs representing dominant Alcaligenaceae phylotypes lacked recognizable ARGs. Multi-drug resistant pathogens from this family, e.g., Alcaligenes faecalis, have been repeatedly described [47,48,49,50]. The most common was resistance to beta-lactams, aminoglycosides, fluoroquinolone and tetracyclines. Particularly, ARGs were identified in Alcaligenes strains isolated form livestock manure [51], pigsties and manured soil [52]. Likewise, ARGs predicted to confer resistance to aminoglycosides, diaminopyrimidines, macrolide–lincosamide–streptogramin and tetracyclines were identified in MAG-42.
To assess the stability of the composition of microbial communities and their resistomes, we conducted a comparative analysis of manure samples taken from the same storage lagoon with an interval of two years. In both samples, three phyla, Firmicutes, Proteobacteria and Bacteroidota, accounted for more than half of the communities. A specific feature of the Tom2 sample is the presence of a significant fraction of methanogenic archaea, which is probably associated with the presence of anaerobic sediments in this sample. The composition of the resistome turned out to be more stable. Thus, genes from all 21 dominant AMR gene families (Figure 3) were found in both samples. The same can be said about the drug classes for which ARGs were found (Figure 2). The observed differences in the representation of different ARGs mainly reflected differences in the content of individual taxonomic groups. For example, the share of the RND antibiotic efflux pumps characteristic of Proteobacteria was higher in the resistome of the Tom2 sample, in which the relative abundance of Poteobacteria themselves was also higher.
5. Conclusions
16S rRNA gene profiling and metagenomic analysis revealed that both the prokaryotic community and the resistome were dominated by the Firmicutes, Proteobacteria, and Bacteroidota. However, the ARG relative abundance was higher in the Proteobacteria than in the Firmicutes and especially the Bacteroidota. Besides multidrug efflux pumps, the predominating ARGs potentially encoded resistance to tetracyclines, macrolide–lincosamide–streptogramin, aminoglycosides, peptide antibiotics, rifamycin, chloramphenicol, and beta-lactams. Genes from all predominant AMR gene families were found in samples collected with 2 years interval indicating overall stability of the resistome. Antibiotic efflux was the dominant mechanism of resistance in the Proteobacteria, while the Firmicutes, Actinobacteriota and Bacteroidota mostly rely on antibiotics inactivation, target alteration or protection. Metagenomics analysis indicated that the most numerous phylotypes lacked ARGs, while a limited number of lineages that presumably originated from the gut, but were relatively rare in the manure storage lagoon, were the main source of ARGs. Some of these strains are potential pathogens carrying multiple resistance determinants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10112301/s1, Table S1: ARGs identified in the metagenome of manure the Tom2 sample; Table S2: ARGs identified in the metagenome of manure the Tom3 sample.
Author Contributions
Conceptualization, N.V.R. and O.V.K.; investigation, S.B., E.V.G., L.B.G. and A.V.M.; resources, O.V.K.; data curation, S.B., A.V.B. and N.V.R.; writing—original draft preparation, S.B., O.V.K. and N.V.R.; writing—review and editing, S.B., O.V.K. and N.V.R.; supervision, N.V.R. and O.V.K.; funding acquisition, O.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation in the framework of the Federal scientific-technical program of the genetic technologies development for 2019–2027 (Agreement № 075-15-2021-1401, 3 November 2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) under the accession numbers SRR21185896 (16S rRNA gene fragments, Tom2 sample), SRR21203432 (metagenome, Tom2 sample), SRR13442339 (16S rRNA gene fragments, Tom3 sample), and SRR13442943 (metagenome, Tom3 sample) within the BioProject PRJNA785864.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Hamscher, G.; Pawelzick, H.T.; Höper, H.; Nau, H. Different behavior of tetracyclines and sulfonamides in sandy soils after repeated fertilization with liquid manure. Environ. Toxicol. Chem. 2005, 24, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Innes, G.K.; Randad, P.R.; Korinek, A.; Davis, M.F.; Price, L.B.; So, A.D.; Heaney, C.D. External societal costs of antimicrobial resistance in humans attributable to antimicrobial use in livestock. Annu. Rev. Public Health 2020, 41, 141–157. [Google Scholar] [CrossRef]
- Bassitta, R.; Nottensteiner, A.; Bauer, J.; Straubinger, R.K.; Hölzel, C.S. Spread of antimicrobial resistance genes via pig manure from organic and conventional farms in the presence or absence of antibiotic use. J. Appl. Microbiol. 2022, 133, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Xiao, D.; Xie, L.; Yang, J.; Zhao, R.; Hao, J.; Huo, Z.; Zeng, Z.; Xiong, W. Swine manure facilitates the spread of antibiotic resistome including tigecycline-resistant tet(X) variants to farm workers and receiving environment. Sci. Total Environ. 2022, 808, 152157. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231 Pt 2, 1621–1632. [Google Scholar] [CrossRef]
- Hölzel, C.; Harms, K.; Küchenhoff, H.; Kunz, A.; Müller, C.; Meyer, K.; Schwaiger, K.; Bauer, J. Phenotypic and genotypic bacterial antimicrobial resistance in liquid pig manure is variously associated with contents of tetracyclines and sulfonamides. J. Appl. Microbiol. 2010, 108, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, H.; Li, S.; Li, C.; Li, J.; Ma, Y. The presence of tetracyclines and sulfonamides in swine feeds and feces: Dependence on the antibiotic type and swine growth stages. Environ. Sci. Pollut. Res. Int. 2020, 27, 43093–43102. [Google Scholar] [CrossRef] [PubMed]
- Stange, C.; Sidhu, J.P.S.; Tiehm, A.; Toze, S. Antibiotic resistance and virulence genes in coliform water isolates. Int. J. Hyg. Environ. Health 2016, 219, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; Török, M.E. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb. Genom. 2018, 4, e000197. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of antibiotic resistance genes in the environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Wallace, M.J.; Fishbein, S.R.S.; Dantas, G. Antimicrobial resistance in enteric bacteria: Current state and next-generation solutions. Gut. Microbes 2020, 12, 1799654. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Karnachuk, O.V.; Rusanov, I.I.; Panova, I.A.; Grigoriev, M.A.; Zyusman, V.S.; Latygolets, E.A.; Kadyrbaev, M.K.; Gruzdev, E.V.; Beletsky, A.V.; Mardanov, A.V.; et al. Microbial sulfate reduction by Desulfovibrio is an important source of hydrogen sulfide from a large swine finishing facility. Sci. Rep. 2021, 11, 10720. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022, 50, D785–D794. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Pehrsson, E.C.; Tsukayama, P.; Patel, S.; Mejía-Bautista, M.; Sosa-Soto, G.; Navarrete, K.M.; Calderon, M.; Cabrera, L.; Hoyos-Arango, W.; Bertoli, M.T. Interconnected microbiomes and resistomes in low-income human habitats. Nature 2016, 533, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016, 7, 11257. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Li, J.; Xin, Y.; Hao, Z.; Chen, C.; Li, H.; Wang, B.; Ding, M.; Li, W.; et al. Insights into bacterial diversity in compost: Core microbiome and prevalence of potential pathogenic bacteria. Sci. Total Environ. 2020, 718, 137304. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cui, X.; Stinner, W.; Zhang, L.; Ju, X.; Guo, J.; Dong, R. Ensiling excessively wilted maize stover with biogas slurry: Effects on storage performance and subsequent biogas potential. Bioresour. Technol. 2020, 305, 123042. [Google Scholar] [CrossRef]
- Wu, N.; Xie, S.; Zeng, M.; Xu, X.; Li, Y.; Liu, X.; Wang, X. Impacts of pile temperature on antibiotic resistance, metal resistance and microbial community during swine manure composting. Sci. Total Environ. 2020, 744, 140920. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, J.; Wang, X.; Tuo, X.; Zhang, K.; Zhang, L.; Guo, A.; Zhang, X. Effects of copper on the composition and diversity of microbial communities in laboratory-scale swine manure composting. Can. J. Microbiol. 2018, 64, 409–419. [Google Scholar] [CrossRef]
- Xie, C.; Teng, J.; Wang, X.; Xu, B.; Niu, Y.; Ma, L.; Yan, X. Multi-omics analysis reveals gut microbiota-induced intramuscular fat deposition via regulating expression of lipogenesis-associated genes. Anim. Nutr. 2021, 9, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.T.; Chen, C.Y.; Young, C.W.; Chao, W.L.; Li, M.H.; Liu, Y.H.; Lin, C.M.; Ying, C. Prevalence of sulfonamide-resistant bacteria, resistance genes and integron-associated horizontal gene transfer in natural water bodies and soils adjacent to a swine feedlot in northern Taiwan. J. Hazard. Mater. 2014, 277, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Jin, H.; Ye, X.; Liu, W.; Li, D.; Shah, G.G.M.; Zhu, Y. Fate and driving factors of antibiotic resistance genes in an integrated swine wastewater treatment system: From wastewater to soil. Sci. Total Environ. 2020, 721, 137654. [Google Scholar] [CrossRef]
- Græsbøll, K.; Damborg, P.; Mellerup, A.; Herrero-Fresno, A.; Larsen, I.; Holm, A.; Nielsen, J.P.; Christiansen, L.E.; Angen, Ø.; Ahmed, S.; et al. Effect of tetracycline dose and treatment mode on selection of resistant coliform bacteria in nursery pigs. Appl. Environ. Microbiol. 2017, 83, e00538-17. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.K.; Edwards, T.A.; O’Neill, A.J. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio 2016, 7, e01975. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Yang, S.H.; Kim, B.S.; Lee, E.Y. Comparison of microbial communities in swine manure at various temperatures and storage times. Asian-Australas J. Anim. Sci. 2018, 31, 1373–1380. [Google Scholar] [CrossRef]
- Gao, Q.; Dong, Q.; Wu, L.; Yang, Y.; Hale, L.; Qin, Z.; Xie, C.; Zhang, Q.; Van Nostrand, J.D.; Zhou, J. Environmental antibiotics drives the genetic functions of resistome dynamics. Environ. Int. 2020, 135, 105398. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, Z.; Zhang, Y. Evolution of physicochemical properties and bacterial community in aerobic composting of swine manure based on a patent compost tray. Bioresour. Technol. 2022, 343, 126136. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, C.B.; Ram, R.M.; Tiwari, O.K.; Titus, S.; Lodha, T. Genome sequence of an obligate hydrocarbonoclastic bacterium Alcanivorax marinus NMRL4 isolated from oil polluted seawater of the Arabian Sea. Mar. Genom. 2021, 60, 100875. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; Wanjugi, P.; Brooks, L.; Cao, Y.; Harwood, V.J. Persistence and decay of fecal microbiota in aquatic habitats. Microbiol. Mol. Biol. Rev. 2019, 83, e00005-19. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Molecular characterization of multidrug-resistant Shigella spp. of food origin. Int. J. Food Microbiol. 2015, 194, 78–82. [Google Scholar] [CrossRef]
- DeLappe, N.; O’Halloran, F.; Fanning, S.; Corbett-Feeney, G.; Cheasty, T.; Cormican, M. Antimicrobial resistance and genetic diversity of Shigella sonnei isolates from western Ireland, an area of low incidence of infection. J. Clin. Microbiol. 2003, 41, 1919–1924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, J.C.; Ye, R.; Meng, D.M.; Zhang, W.; Wang, H.Q.; Liu, K.Z. Molecular characteristics of class 1 and class 2 integrons and their relationships to antibiotic resistance in clinical isolates of Shigella sonnei and Shigella flexneri. J. Antimicrob. Chemother. 2006, 58, 288–296. [Google Scholar] [CrossRef][Green Version]
- Farrar, W.E., Jr.; Eidson, M. Antibiotic resistance in Shigella mediated by R factors. J. Infect. Dis. 1971, 123, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Kundu, M. Molecular characterization of the SHV-11 beta-lactamase of Shigella dysenteriae. Antimicrob. Agents Chemother. 1999, 43, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Yao, M.; Fu, H.; Yuan, G.; Wu, S.; Sun, Y. Resistance characteristics of CTX-M type Shigella flexneri in China. Biosci. Rep. 2019, 39, BSR20191741. [Google Scholar] [CrossRef]
- Huang, C. Extensively drug-resistant Alcaligenes faecalis infection. BMC Infect. Dis. 2020, 20, 833. [Google Scholar] [CrossRef]
- Adesoji, A.T.; Ogunjobi, A.A.; Olatoye, I.O.; Call, D.R. Prevalence of tetracycline resistance genes among multi-drug resistant bacteria from selected water distribution systems in southwestern Nigeria. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 35. [Google Scholar] [CrossRef]
- Majewski, P.; Majewska, P.; Gutowska, A.; Piszcz, J.; Sacha, P.; Wieczorek, P.; Żebrowska, A.; Radziwon, P.; Tryniszewska, E. Molecular characterisation of clinical pandrug-resistant Alcaligenes faecalis strain MUB14. Int. J. Antimicrob. Agents 2020, 55, 105939. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Li, Y.; Yang, W.; Dong, R.; Liang, Y.; Liu, J.; Chen, L.; Wang, W.; Ji, B.; Tian, G.; et al. Genomic and resistome analysis of Alcaligenes faecalis strain PGB1 by Nanopore MinION and Illumina Technologies. BMC Genom. 2022, 23, 316. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, T.; Niu, T.; Wang, P. Molecular characterization of antibiotic resistance in cultivable multidrug-resistant bacteria from livestock manure. Environ. Pollut. 2017, 229, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Agersø, Y.; Sandvang, D. Class 1 integrons and tetracycline resistance genes in Alcaligenes, Arthrobacter, and Pseudomonas spp. isolated from pigsties and manured soil. Appl. Environ. Microbiol. 2005, 71, 7941–7947. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).