Abstract
Campylobacter jejuni is one of the most common causes of foodborne human gastroenteritis in the developed world. This bacterium colonizes in the ceca of chickens, spreads throughout the poultry production chain, and contaminates poultry products. Despite numerous on farm intervention strategies and developments in post-harvest antimicrobial treatments, C. jejuni is frequently detected on broiler meat products. This indicates that C. jejuni is evolving over time to overcome the stresses/interventions that are present throughout poultry production and processing. The development of aerotolerance has been reported to be a major survival strategy used by C. jejuni in high oxygen environments. Recent studies have indicated that C. jejuni can enter a viable but non-culturable (VBNC) state or develop biofilm in response to environmental stressors such as refrigeration and freezing stress and aerobic stress. This review provides an overview of different stressors that C. jejuni are exposed to throughout the poultry production chain and the genotypic and phenotypic survival mechanisms, with special attention to aerotolerance, biofilm formation, and development of the VBNC state.
1. Introduction
In the United States, it is estimated that Campylobacter is responsible for 1.5 million illnesses each year [1] and is one of the most common causes of foodborne gastroenteritis in humans [2]. Consumption of contaminated poultry meat is the primary mode of transmission for Campylobacter and is estimated that poultry is responsible for up to 30% of human Campylobacter infections [3,4]. Although there are 16 species of Campylobacter, C. jejuni is responsible for the majority of human infections [3]. Campylobacteriosis is characterized by symptoms such as diarrhea, cramps, fever, and vomiting and has been associated as a major predisposing causes of Guillain-Barre syndrome, a nervous system disorder affecting peripheral nerves [5]. Campylobacter is a microaerophilic pathogen (requires 5–10% oxygen) with optimum growth at 42 °C [6,7]. Historically, it has been found that Campylobacter lacks the mechanisms present in other bacteria to remove reactive oxygen species (ROS) to defend against oxidative stress. Therefore, they are highly sensitive to environmental oxygen [8]. Despite these physiological restrictions and numerous pre-and post-harvest intervention strategies during poultry processing, C. jejuni is frequently detected in retail broiler products [9]. C. jejuni tend to survive harsh environmental conditions in poultry processing facilities, which include an aerobic environment, hot water treatments (scalding), chilling and freezing, and usage of antimicrobials such as chlorine [10]. The evolution of survival strategies against these stressors makes it difficult to eliminate C. jejuni from food processing surfaces.
One of the major reported mechanisms that enable Campylobacter to survive in poultry production and processing environments is the development of aerotolerance. Despite requiring microaerophilic conditions for survival, Campylobacter has been reported to exhibit tolerance to oxidative stress [11]. Oxidative stress arises when ROS levels in bacteria surpass the cell’s ability to protect itself. Given that the number of human intestinal campylobacteriosis cases is increasing, it is reasonable to assume that Campylobacter has adapted to survive oxidative stress across the food chain. In fact, a high aerotolerance has been reported in Campylobacter strains involved in human infections [12,13]. Out of 121 C. jejuni strains studied, it was reported that 65 were hyper-aerotolerant (HAT) and 46 were moderately aerotolerant (IAT) [13]. Similarly, High occurrence (63%) of HAT (able to survive more than 24 h of aerobic exposure) isolates in broiler carcass, dairy products, and clinical samples have also been observed, rather than aero-sensitive strains (AS) [14]. Historically, the environmental survivability of C. jejuni has been underestimated because of its specific growth requirements and environmental sensitivity. However, the recent reports of aerotolerant strains suggests that increased monitoring of this pathogen is essential in poultry production and processing.
One of the key survival mechanisms of Campylobacter may be biofilms. Biofilm formation is the mechanism that various bacteria use to survive environmental stress. Biofilms are defined as ‘multicellular communities of bacteria embedded within a matrix of extracellular polymeric substances that consist of various proteins, polysaccharides, and water’ [15]. Bacteria in biofilms exhibit greater resistance to external stressors than their planktonic counterparts [16]. Recent studies have shown that C. jejuni can form biofilm on various abiotic surfaces like acrylonitrile butadiene styrene and polyvinyl chloride plastics commonly used in watering systems and stainless steel commonly used in processing facility [17,18,19]. In food processing facilities, the formation of biofilms may protect Campylobacter from cleaning and disinfection protocols and facilitates continued dissemination. This leads to prolonged cross-contamination of food products, thus increasing its potential to cause disease [20]. A particularly concerning aspect of the biofilm formation of Campylobacter is its increased survivability at low temperatures [21]. Prolonged survival of C. jejuni biofilm at refrigeration temperatures (4 °C and 10 °C) was observed by Dykes et al. [22]. These results suggest that temperature is an important factor that impacts the ability of Campylobacter to form biofilms. Similarly, biofilm formation is also accelerated when food surfaces or organic substances are involved. C. jejuni biofilm formation was accelerated in brucella broth with chicken meat exudate on glass and polystyrene surfaces [19]. However, compared to other foodborne pathogens such as Salmonella, biofilm forming attributes of Campylobacter are still unclear in terms of genetic determinants.
Viable but non culturable state (VBNC) is another mechanisms used by many bacteria in response to environmental stressors such as nutrient starvation, osmotic shock, temperature, and pH fluctuations [23,24,25]. In the VBNC state, the ability to culture bacteria is lost even though the bacteria is alive and metabolically active [26]. This state allows bacteria to survive harsh conditions until the environment becomes favorable for growth and cell division [27]. In poultry processing facilities, the bacteria face post-harvest stress and may enter a VBNC state, thereby making their detection difficult during quality control inspection. The ability of C. jejuni to enter a VBNC state was first described in 1986 by Rollins and Colwell [28]. Studies have been reported that C. jejuni can enter the VBNC state in the presence of acid or cold stress [23,29]. However, the VBNC forming ability of C. jejuni and its ability to cause infection in humans is still not clear. C. jeuni ability to become non-culturable when exposed to stresses such as heat stress, osmotic stress, acid stress, and refrigeration in the processing facility, as well as its potential to revive and cause illness when consumed by people, needs to be investigated.
The purpose of this review is to summarize the stressors that C. jejuni is exposed to in poultry processing environment and to critically analyze the role of various survival mechanisms that are used by this pathogen to overcome the stressors. Brief descriptions of the specific stressors encountered, the potential regions of exposure in the processing plant, and the survival mechanisms used under the given circumstances, have been provided. Potential genes implicated in survival under various stress scenarios are also listed. Understanding the effect of each stressor and the survival processes adopted by this pathogen in overcoming stressors may aid in identifying future avenues for building effective C. jejuni control measures in the food chain.
2. Physiology of Campylobacter
Campylobacter requires microaerobic conditions and an optimum temperature of 37–42 °C to grow [30]. The term “microaerobic” refers to oxygen (O2) concentrations ranging from 3 to 15%. Although most Campylobacter strains are cultured with about 10% oxygen, the usual environment for C. jejuni/C. coli isolation comprises of approximately 5% oxygen, 10% carbon dioxide (CO2), and 85% nitrogen (N2) [31].
Campylobacter spp. are helical bacteria that may change shape to become rod or coccoid when exposed to specific environmental factors [32]. Spiral shapes are more common in young cultures, but coccoid patterns are more common in older ones [33,34]. The older cells of C. jejuni, according to some researchers, are degenerated to the coccoid form and resemble similar coccoid forms that have been observed in several chemoheterotrophic spirilla species [35]. Growing C. jejuni in liquid culture resulted in gross morphological changes over time, and that long culture incubation (late decline phase of growth curve) resulted in the formation of a coccal population [36]. In fact, C. jejuni becomes coccoid due to an increase in peptidoglycan dipeptides and a reduction in tri- and tetrapeptides. The hydrolases DL-carboxypeptidase Pgp1 and LD-carboxypeptidase Pgp2 separate tri and tetrapeptides and remodel the peptide glycan (PG) structure, and the loss of any of these hydrolases affects the structure and form of the PG, thus compromising the stress tolerance of C. jejuni [37].
Further, Campylobacter species are unique from other foodborne pathogens in that they have a particular appearance, physiological needs, and a small genome. The C. jejuni (NCTC11168 strain) genome is 1.6 Mbp in size, with a low number of repetitive sequences and no insertion or phage-related regions. Interestingly, it also it lacks the antioxidant stress defense regulators SoxRS and OxyR, which are found in Salmonella and E. coli [38]. In a similar manner, C. jejuni possesses only one superoxide dismutase gene (sodB), unlike E. coli, which possesses three of these genes (sodA, sodB and sodC), respectively [39]. C. jejuni has just one subunit of alkyl hydroperoxide reductase (AhpC), in contrast to Salmonella Enterica, which has two subunits of alkyl hydroperoxide reductase [40]. The function of these genes and their role in overcoming stress has been discussed below.
3. Incidence of Campylobacter in Poultry Processing Facilities
Campylobacter is typically introduced into a manufacturing facility via birds (up to 109 cells/g cecal material) [41]. Campylobacter is a commensal organism of many avian species and may be traced back to the farm despite numerous on-farm treatments. It can spread to poultry meat after it reaches the slaughter line, especially during defeathering and evisceration [42]. Poultry meat becomes contaminated with Campylobacter due to intestinal breakage, loss of feces from the cloaca, and contact with contaminated equipment, water, or other carcasses. The birds are unloaded, shackled, slaughtered, scalded, defeathered, eviscerated, washed, cooled, and packaged inside a processing facility. The cross contact between the birds, employees, and equipment during these processing steps could contribute to Campylobacter contamination. A number of reports disclose the incidence of C. jejuni at various stages of processing which can be seen in Table 1.
The persistence of Campylobacter in the plant environment and its significance as a source of contamination are unclear. A Campylobacter positive flock that arrives at a processing plant can contaminate successive batches of birds that arrive at the same processing plant and are slaughtered prior to the cleaning and sanitation shift at the plant [43,44]. In addition, despite thorough cleaning and disinfection, C. jejuni can survive overnight on food processing equipment surfaces [45]. These findings indicate that some C. jejuni isolates may be able to survive longer in the processing plant, which could infect successive batches of carcasses from flocks that are not positive for Campylobacter.
4. Stress Encountered by Campylobacter inside Poultry Processing Facilities
Immersion of broiler carcasses in hot water (scalding), rapid cooling (chilling), use of anti-microbials (spraying/dipping), and active packaging (modified atmospheric condition) are some of the hurdles (Figure 1) that Campylobacter is subjected to inside the processing facility. Detailed explanations of C. jejuni response to each of these stress condition has been discussed below. Involvement of genes for Campylobacter to overcome these stressors has been highlighted in Table 2.
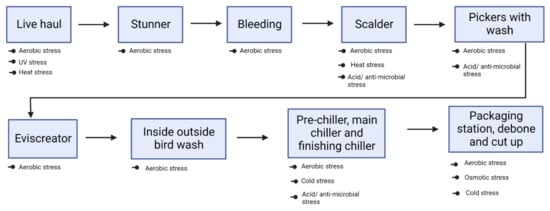
Figure 1.
Potential stressors encountered by C. jejuni at various stages of broiler processing plant.
4.1. Aerobic Stress
The primary stress that C. jejuni faces as a microaerophilic bacterium is the oxygen level in the surrounding environment. Presence of oxygen can lead to the formation and accumulation of a wide range of hazardous intermediate products, which are known as ROS including the superoxide anion radical (O2), hydrogen peroxide (H2O2), and the hydroxyl radical (OH) [46]. When ROS levels in bacteria reach a level that exceeds the cell’s ability to defend itself, oxidative stress occurs. Despite its susceptibility to oxidative stress, the rising prevalence of human intestinal campylobacteriosis suggests that Campylobacter has adapted to withstand oxidative stress across the entire food chain.
Recent studies iterate that HAT strains of C. jejuni are common in human infections that have been linked to poultry meat [12,13,14] C. jejuni strains from both retail chicken and duck meat were found tolerant to aerobic stress. [47]. High occurrence (63%) of HAT C. jejuni isolates in broiler carcass, dairy products, and clinical samples have been observed rather than aero-sensitive strains (AS) [14]. In fact, several parameters have been linked to C. jejuni survival in oxygen-rich environments. For example, pyruvate has been shown to protect C. jejuni from severe oxidative stress in aerobic circumstances and a combination of ferrous sulfate, sodium metabisulfite, and sodium pyruvate promotes C. jejuni viability in culture media [11]. Furthermore, metabolic commensalism with Pseudomonas spp. in the rotting microbiota of chicken flesh has been shown to support C. jejuni survival under aerobic conditions [48].
4.2. Heat Stress
The ideal temperature for growth of Campylobacter spp is between 42 °C and 45 °C. It has been observed that below the minimal growth temperature (31 °C), there is a sudden decrease in the growth rate of C. jejuni [49]. In poultry processing plants, scalding occurs at approximately 53 °C to 62 °C in order to loosen feather and aid in their removal. While these temperatures would normally hinder Campylobacter growth, some clinical strains, which are tolerant to high temperature pasteurization for both short time (72 °C for 15 S) and long time (72 °C for 30 S) exposure, would not be affected by these scalding temperatures [13]. In addition, the ability to survive heat stress varies with genotypic diversity. For example, C. jejuni assigned to clonal complex CC-21 indicated better survivability after exposure to heat stress when compared to CC-45 when the heat stress inside a broiler processing facility was mimicked in the study [50].
Despite having an optimum temperature requirement for growth, Campylobacter appears to be resistant to high temperatures, and several studies have been conducted to understand the mechanism of heat stress survival in Campylobacter [49,50,51]. In one study, twenty four proteins were more abundant in Campylobacter when cells were heat-shocked at temperatures ranging from 43 °C to 48 °C, including a chaperone protein named DnaJ [51]. DnaJ is a heat shock protein also known as Hsp40 which coordinates with other heat shock proteins such as DnaK and GrpE in the folding of polypeptides and several other cellular functions [52]. A temperature-responsive signal transduction system called RacR-RacS was also identified in response to temperature changes with C. jejuni [53]. The two component regulators RacR-RacS consists of cytoplasmic sensory histidine kinase and a cytoplasmic membrane response regulator [26]. In most bacteria, this two component regulator system acts as a survival mechanism to protect against environmental changes by affecting the expression of stress response genes [54].
4.3. Refrigeration and Freezing Stress
Historically, C. jejuni is not able to survive in cold environments and this inability may be explained by the lack of cold shock proteins [38]. However, the ability of Campylobacter to survive at 4 °C or colder has been reported by various researchers. Survival of C. jejuni on raw chicken skin fragments at freezing temperatures (−20 °C) for 14 days and −70 °C for 56 days was reported by Lee et al. [55]. The ability to survive in cold temperature varies due to strain. Chan and others (2001) reported that strains that were isolated from humans can better survive at 4 °C compared to poultry isolates [56]. Similarly, some strains survive better at 4 °C than at 30 °C at different pHs and NaCl concentrations [57].
C. jejuni when incubated at 5 °C, genes involved in energy metabolism, particularly tricarboxylic acid cycle, oxidative phosphorylation, glycolysis and gluconeogenesis were found upregulated [58]. This indicates that C. jejuni utilizes energy for metabolism and survival in refrigeration temperature. Similarly, the superoxide dismutase gene (SOD) provides resistance to freeze–thaw that is induced by oxidative damage in C. coli [59]. In the same study, it was found that the SOD mutant was more sensitive to freezing and thawing compared to wild type.
The ability of C. jejuni to tolerate refrigeration and freezing temperatures is a food safety and public health challenge. However, C. jejuni’s mechanism for survival when exposed to cold temperatures has not been elucidated. Although this bacterium lacks cold shock proteins to tolerate and adapt to cold environments, it has still been isolated from chicken meat that was stored at 4 °C. Since most of the research has used single strains to understand the mechanism of C. jejuni survival, more comprehensive studies involving different strains are needed to fully understand the pattern of cold resistance, and and further genetic approaches should be explored to understand the mechanism of survival in cold environments.
4.4. Osmotic Stress
Osmotic homoeostasis is important for proper cell growth and maintenance. Slight imbalances in osmotic pressure or turgor pressure can disrupt the physiological balance of a cell and induce a VBNC state [26]. For Gram-negative bacteria, the minimum solute concentration in the cytoplasm that is necessary for growth is around 300 milliosmole [60]. Sodium chloride (NaCl) is the most common food preservative used and C. jejuni may encounter changes in osmolarity during food processing. While the role of osmotic stress is not fully understood, it has been reported that C. jejuni is sensitive to NaCl. C. jejuni was incapable of surviving in Mueller- Hinton (MH) medium that was supplemented with 2% NaCl but was able to survive in the presence of 0.5% to 1.5% NaCl [61]. In contrast, 44.6% (54/121) of C. jejuni (clinical strains) survived 2% NaCl, and 35.5% (43/121) of the strains were tolerant, even in 4% NaCl [13]. This indicates that osmotic tolerance might be strain specific. C. jejuni survive longer in the presence of 4.5% NaCl (w/v) in 4 °C environment when compared to 42 °C [61]. Similarly, no substantial decrease in C.jejuni was seen in 1.5% NaCl-treated minced poultry meat stored at 4 °C [62]. Likewise, C. jejuni was found to be able to survive for up to 96 h in non-growth temperatures (25 °C & 4 °C) in high osmolality (>175 mosmol) media [63]. This shows that C. jejuni ability to tolerate and grow in the presence of salt is mostly determined by temperature.
Several Gram-negative bacteria, including E. coli contain a high functioning potassium transporter system that responds to osmotic stress. However, C. jejuni lacks this osmotic change adaptation mechanism. It lacks the osmoregulatory betaine and trehalose biosynthetic pathways [64] observed in other bacteria, hence they cannot synthesize suitable solutes. Therefore, they must rely on other mechanisms to survive during osmotic changes. Filamentation has occurred in C. jejuni in response to hyper-osmotic stress [65]. Filamentous bacteria have historically been thought of as abnormal, but evidence suggests that they also play an important role in bacterial survival and pathogenicity [66].
4.5. UV Stress
Enteric pathogen survival can be affected by light. Ultraviolet radiation (UVR) is emitted from wavelengths of 10–400 nm and is subdivided into UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm) [67]. The FDA has approved UV-C (with 90% emission at 253.7 nm) as a method of controlling surface bacteria on food products [68]. Research had been carried out to study the effect of natural sunlight on C. jejuni survival in sea and river water. It was found that C. jejuni was sensitive to sunlight with a high inactivation rate [69]. The effect of UV-C irradiation was studied in E. coli, Yersinia and Campylobacter (human clinical isolates), and the application of UV-C resulted in a 3 log CFU/mL reduction in all bacteria with a dosage of at least 1.8 mWs/cm2 required for Campylobacter reduction In vitro [70]. C. jejuni was inactivated by high intensity blue light (405 nm) on micro-well plate, which was more effective towards C. jejuni than Salmonella Enteritidis and E. coli O157: H7 [71]. While doing predictive modeling study, combination of different wavelengths of UV light particularly 280 and 300 nm was found to be effective in inactivating C. jejuni in vitro [72], whereas when using chicken meat (breast fillet) inoculated with C. jejuni instead of artificial growth media and exposing to UV treatment (0.192 J/cm2) resulted in 0.76 log CFU/g reduction [73]. In addition, UV-C reduced C. jejuni initially inoculated between 6.4–7.5 CFU/mL on broiler meat and skin by 0.7 log and 0.8 log, respectively, at 32.9 mWs/cm2 [74]. This demonstrates that UV is efficient against Campylobacter in vitro; however, when it comes to chicken meat, the opacity of the meat could prevent the penetration of UV light, hence reducing the effectiveness of the treatment. Additionally, the natural populations of C. jejuni were found to be more UV-resistant than culture collection strains, indicating that natural populations could be better equipped for stress survival [75].
UV disinfection has shown to induce VBNC in E. coli, P. aeruginosa and S. aureus [76,77]. Unfortunately, no study has analyzed if the UV light can induce VBNC in C. jejuni. Likewise, very limited study has analyzed the effect of UV light against C. jejuni in genetic level. RecA, a protein that helps DNA repair, was found to be expressed higher in C. jejuni after it was exposed to UV light, which suggests C. jejuni could be resistant to UV light [78].
4.6. Acid Stress
The ideal pH range for C. jejuni growth is 6.5 to 7.5 [23]. However, it has been found that C. jejuni (strain NCTC 11168) remained viable after 20 min exposure to pH 4.5 [79]. Campylobacter is sensitive to several organic acids. Exposure to 1% lactic acid for 5 min in broth at low temperature (5 °C) reduced C. jejuni by 2.1 log CFU/mL [80]. Organic acids such as acetic acid, citric acid, lactic acid, tartaric acid and malic acid of 0.5% final concentration decreased C. jejuni populations in broth (chicken juice and brain heart infusion broth) by 4 to 6 log CFU/mL (after 24 h); tartaric acid was the most effective [81]. In contrast to studies with broth, enteric pathogens like Salmonella and Campylobacter are protected from inactivation in extreme acid conditions (pH 2.5) when they are in food [82]. Foodborne pathogens may be more tolerable of acid stress in foods due to the widespread use of low pH settings for food preservation. On-farm therapies such as acidification of drinking water with organic acid, acidification of litter, and inclusion of feed additives (2 percent formic acid) are routinely used to reduce campylobacter colonization in poultry. Similarly, antimicrobials such as peracetic acid (PAA) and cetylpyridinium chloride (CPCL) are applied in chiller tanks and post-chill rinses to minimize the amount of Campylobacter and other food-borne pathogens in the processing plant. Continuous exposure of Campylobacter to low doses of acids may induce an adaptive tolerance response in Campylobacter. For example, a sublethal dose of stress provides protection towards subsequent lethal dose. This is shown in a study in which C. jejuni was exposed to pH 5.5 prior to challenge with a lethal dose of 4.5, it enabled 100-fold greater survival when compared to uninduced culture [83,84].
The ability of C. jejuni to overcome acid stress still needs to be explored. A new epidemiological pathway has been proposed for C. jejuni to survive a harsh environment. Protozoa have served as a natural vehicle to disseminate pathogens, such as Helicobacter pylori [85]. Protozoa are abundant in water systems. When co-cultured with Acanthamoeba polyphaga, C. jejuni were able to survive pHs well below their typical range, surviving for 20 h at pH 4 and 5 h at pH 2 [86]. This shows that a host can protect in severe environments and be a contributing factor to C. jejuni infections.
Certain genes, such as the clpB gene contribute to acid resistance [79]. Similarly, thioredoxin-disulfide (TrxB) and 19 KDa periplasmic protein (p9) genes were upregulated when C. jejuni was exposed to hydrochloric acid (HCl) and acetic acid [87]. Exposure to HCl or acetic acid also increased the expression of oxidative stress defense genes, such as dps, sodB, trxB, and ahpC in C. jejuni [87]. The Ferric uptake regulator gene (Fur) is important to the survival of C. jejuni when exposed to acid. C. jejuni fur mutant was more sensitive to acid than wild type, which shows that Fur is an important regulator during acid response [88].
5. Potential Survival Mechanisms
As previously mentioned, C. jejuni has confirmed to thrive several extreme environmental stressors like heat stress, acid stress and aerobic stress. However, how it manages to overcome stressors is still a question. Some of the potential survival meachnisms C. jejuni has been adopting to counteract environmental stressors has been discussed below.
5.1. Aerotolerance Development
The development of aerotolerance is one of the key documented mechanisms that allows Campylobacter to survive in harsh environments. Campylobacter strains linked to human illnesses have been found to exhibit significantly greater aerotolerance [13]. Different levels of aerotolerance have been used to describe the survivability of Campylobacter in oxygen rich environments [13,14,89]. Strains that survive for more than 24 h of oxygen exposure are referred to as Hyper aerotolerant strains and it’s prevalence is higher in both retail chicken and duck meat [47] as well as in humans [13]. Bacterial growth in the presence of oxygen produces ROS that are detoxified by the oxidative stress defense system. Unlike aerobes, microaerophiles and anaerobes exist in low-oxygen environments, yet have conserved oxidative stress resistance systems. The most common ROS-detoxification enzymes are alkyl hydroperoxide reductase, catalase, and superoxide dismutase. C. jejuni has single gene copies of alkyl hydroperoxide reductase, catalase, and superoxide dismutase (ahpC, katA and sodB) that play important roles in survival and morphological changes of C. jejuni under aerobic conditions [90]. Research shows that C. jejuni with mutation in key antioxidant genes, including ahpC, katA, and sodB exhibited growth reduction under aerobic conditions [11]. Proteins including alkyl hydroperoxide reductase AhpC [11], ferredoxin FdxA, protease HtrA, and a shortened hemoglobin Ctb expressed are also involved in oxygen tolerance in C. jejuni [91,92,93,94]. Previously annotated as a hypothetical protein, Cj1556 was discovered as a MarR family transcriptional regulator after reannotation of the C. jejuni NCTC11168 genome sequence, and additional investigation revealed a potential involvement in regulating the oxidative stress response [95] Increased sensitivity to oxidative and aerobic stress was observed in a C. jejuni 11168H transcriptional regulator Cj1556 mutant which demonstrated that transcriptional regulator Cj1556 has role in the regulation of aerobic stress [95]. Thiol peroxidase genes, tpx and bcpb encode for enzymes Tpx and Bcp. These enzymes demonstrated a role in regulating oxidative stress responses in C. jejuni, most specially those related to molecular oxygen [96]. Aerotolerance and oxidative stress response may be closely related given that there are common genes expressed in both of these survival mechanisms (Table 3).
5.2. Biofilm Formation
Biofilms are bacterial communities, which are encased in a matrix that are irreversibly adhered to one another and/or to surfaces or interfaces [97]. Biofilms help bacteria survive by protecting them from environmental stresses such as dehydration and antibacterial and sanitizing agents, which makes their elimination difficult. Biofilms created on food processing surfaces shield bacteria from washing and sanitation procedures, which can lead to food contamination and food borne illnesses. It is believed that biofilm formation is one of the mechanisms contributing towards C. jejuni persistence in the environment [98]. While most investigations of C. jejuni biofilm development have been conducted under microaerobic circumstances, limited research have been conducted to investigate C. jejuni biofilm production under aerobic conditions. C. jejuni form a stronger biofilm under lower oxygen conditions [17]. Likewise, C. jejuni showed enhanced biofilm formation by use of Fe2+ or Fe3+ or both [99]. Iron has shown to enhances the biofilm formation of C. jejuni through increasing oxidative stress. The iron molecules may create a microaerobic environment that is conducive to their life and development. More research on biofilm development by C. jejuni is needed since many aspects remain unclear, especially C. jejuni’s capacity to build biofilms under environmental oxidative stress.
C. jejuni forms biofilms on stainless steel, nitrocellulose, and glass fiber filters [100]. The ability to form biofilm is enhanced when it is linked with food surfaces or organic components in food. Chicken meat exudate increases C. jejuni biofilm formation on glass, polystyrene, and stainless steel when supplemented with brucella broth [19]. Interestingly, Campylobacter isolated from retail food samples was able to form more biofilm in co-culture with E. coli or Pseudomonas aeruginosa than in pure culture [101]. Similar results occurred when C. jejuni multilocus sequence type ST-47, a dominant poultry and human-associated type in New Zealand, exhibited greater biofilm formation when grown in mixed microbial population with Enterococcus faecalis and Staphylococcus simulans. It could be because these microorganisms derived from chicken may create a favorable habitat for C. jejuni survival and proliferation in poultry processing plant settings.
C. jejuni has a minimum growth temperature requirement of 37 °C to 42 °C [102,103]. The failure to replicate and develop below 30 °C may be explained in part by a lack of stress adaptive response components, such as cold stress proteins. Nonetheless, C. jejuni is metabolically active at temperatures lower than its lowest growth temperature. It has been shown that survival of planktonic C. jejuni is improved at low temperature (4 °C) [56]. Moreover, C. jejuni cultured as planktonic cells and biofilm cells lived longer at lower temperatures (4 °C and 10 °C) than at higher temperatures (25 °C and 37 °C) [22]. C. jejuni’s ability to build biofilms may alter depending on temperature. However, research on the influence of temperature on C. jejuni biofilm production is sparse. A greater knowledge of the processes involved in biofilm development is critical in establishing cleaning procedures, which will reduce Campylobacter persistence and transmission. Strategies to eradicate Campylobacter reservoirs during food manufacturing would benefit our understanding of how to limit the incidence and transmission of Campylobacteriosis and other food-borne diseases throughout the food chain.
Genes Involved in Biofilm Formation by Campylobacter
Campylobacter strains differ in their ability to form biofilms [104], which impact their ability to survive outside of the host, as well as transmission and colonization of various host species [105]. Genetic determinants of biofilm formation vary between species. However, strains of the same species with variation in homologous regions differ in biofilm phenotype [18]. The ability of 102 C. jejuni isolates to form biofilms and the associated genetic factors were investigated by Pasoce et al. [18]. They reported a total of 46 biofilm-related genes, including those involved in adhesion, motility, glycosylation, capsule formation, and oxidative stress. They found four genes, trxA, trxB, ilvE, and nuoC, associated with oxidative stress sensing, which is strongly linked to biofilm formation. Studies show that genes involved in motility (flgA) [106], quorum sensing (luxS) [17] and adhesion (cadF) and stress response genes such as dnaJ, cbrA, htrA, and sodB [107] are involved in biofilm formation. Similar to this, C. jejuni mutant of AhpC gene, which is involved in oxidative stress, had greater biofilm formation [107]. Cell binding proteins, Peb1A and Peb4 (also known as CBF1 and CBF2), play important roles in adhesion and in vivo colonization of Campylobacter [108]. Deletion of the peb4 gene impaired cell adhesion and biofilm formation [109]. Briefly, the list of potential genes involved in C. jejuni biofilm formation along with their functions are in Table 4. C. jejuni induce biofilm formation by activation of various stress regulators [109]. Campylobacter’s ability to form biofilms is dependent upon strain, and Campylobacter forms multispecies biofilm with other bacteria to thrive in harsh environments.
Several strategies have been evaluated to target Campylobacter biofilms. Extra cellular polymeric substance produced by the bacteria is an essential component of biofilm and it is composed of extracellular DNA (eDNA) [110]. Use of DNase can degrade the eDNA and provide a promising measure to inhibit biofilm [111]. Zinc nano particles (0.5 mg/mL) reduces C. jejuni biofilm formation by both in mono and multispecies culture under aerobic condition [101]. Natural compounds, such as carvacrol (66.56 mM), attenuate C. jejuni colonization and biofilm mass by greater than 7 log CFU/mL reduction [112]. Similarly, plant derived compounds like citrus extract at the concentration of 75% of minimum bactericidal concentration of 130–250 µg/mL [113], cinnamaldehyde at concentration greater than 15.63 µg/mL [114], eugenol at concentration of 60.9 mM [115] has shown promising effect in decreasing Campylobacter biofilms. In summary, several strategies to control Campylobacter biofilms have been studied but the practical aspect of using them in food industry needs to be further investigated. Some treatments are successful in the lab when bacteria are cultivated in culture plates, and most treatments are focused on a single strain of Campylobacter spp. However, the substantial genetic diversity among Campylobacter strains complicates treatment effectiveness on food contact surfaces in food plants.
5.3. Viable but non Culturable (VBNC) State Formation
Rollins and Colwell (1986) first described VBNC state in C. jejuni when they found that increasing cultivation temperature resulted in decreased spread plate culture counts when compared to direct viable counts with epifluorescence microscopy and assaying protein synthesis [28]. Prolonged incubation of C. jejuni at 4 °C leads to the formation of VBNC state which retain the ability to invade CaCo-2 human intestinal epithelial cells in vitro as reported by Chaisowwong et al. [29]. This might explain why, despite better sanitation and food preparation standards, campylobacteriosis infections and outbreaks are still common in developed nations.
Several genes are involved in VBNC state formation in C. jejuni (Table 5). Transcription of virulence genes, including cadF, ciaB, flaA, flaB, cdtA, cdtB, and cdtC, were down regulated in C. jejuni during the VBNC state [29]. An outer-membrane protein (CadF) expression was found to retain the ability of VBNC C. jejuni to adhere to CaCo-2 cells [116]. During the VBNC state (when incubated at 5 °C), gene expression involved in energy metabolism increased [58], which indicates that they conserve energy by decreasing their virulence activity and utilize energy for metabolism and survival.
The VBNC form of C. jejuni can resuscitate [24,117,118,119]. Non culturable Campylobacter in water sources were able to colonize in hens following water intake [120]. There are several other studies that shows the ability of C. jejuni to resuscitate in laboratory animals [24,117,118,119]. In contrast, oral inoculation of laboratory animals and volunteers with non-culturable C. jejuni did not show any symptoms and no campylobacter was detected in the stool [121]. Similarly, C. jejuni was not isolated from the caeca of chicks 14d after oral inoculation of non-culturable C. jejuni [102]. This suggests that the resuscitation mechanism might differ between strains, hosts, and/or transmission vector. Further investigation is needed to understand the possible mechanisms fully for C. jejuni to shift from the non-culturable state to the culturable state.
C. jejuni passes through different levels of environmental stressors when a bird is processed. Since shifting to the VBNC state is a response to an unfavorable environment, C. jeuni could shift to the non-culturable state after passing through all stressors, but remain in the chicken. Viable cells were detected up to 7 months later when incubated at 4 °C [122]. Studies have been reported that C. jejuni can form VBNC due to acid stress [23] and cold stress [29]. However, the VBNC forming ability of Campylobacter after passing through stressors, and its ability to cause infection in humans is still not clear. Therefore, there is a need to understand the mechanism underlying environmental stress resistance in Campylobacter that leads to the transition to the VBNC state.
6. Future Research to Fill Current Knowledge Gaps
Researchers have predominantly addressed the effect of various stressors such as aerobic stress, acidity, osmotic imbalance, freeze–thaw, high temperatures, and UV stress at the phenotypic level [11,94] and identified genes that are associated with different phenotypes. However, limited research has been conducted to determine the expression level of those genes when exposed to stress. The focus of future study should be on understanding the underlying adaptive responses, which may be accomplished by gene expression profiling.
Furthermore, understanding the ability to shift from a non-culturable to a culturable state, as well as the substantial genetic diversity among Campylobacter strains (evolutionary adaptation), may aid in the discovery of additional pathways involved in stress resistance, which may ultimately lead to the development of effective measures to control the risk of Campylobacter in the food supply chain. The biofilm-forming characteristics of Campylobacter are still unknown compared to other foodborne pathogens such as Salmonella. Thus, knowing genetic diversity and its involvement in biofilm development is critical to the removal of Campylobacter from food processing and manufacturing plants.
7. Conclusions
While there have been a number of technological food safety advances in chicken meat processing, Campylobacter remains the most common pathogen found in poultry meat, which results in foodborne illnesses and outbreaks in the United States. There are a variety of interventions that commercial processing facilities employ to decrease the incidence of Campylobacter in retail food products. Use of hot water treatments (scalding), chilling and freezing, as well as use of antimicrobials are the most utilized treatments in the processing plant. However, Campylobacter still poses a threat to food safety (Table 1) and public health due to its ability to adapt to stress, form biofilms, and shift to the VNBC state.

Table 1.
Incidence of Campylobacter jejuni at various stages inside processing plants.
Table 1.
Incidence of Campylobacter jejuni at various stages inside processing plants.
| Source of Contamination | Country of Occurrence | Incidence of C. jejuni | References |
|---|---|---|---|
| Birds with feathers, birds at rehang, birds after evisceration, immediately after entering chiller, after exiting chlorinated chill tank | USA—Georgia | 87.27%, (n = 55) | [123] |
| Rehang and post chill whole carcass rinse | USA—Alabama, Arkansas, California, Delaware, Georgia, Indiana, Missouri, North Carolina, South Carolina, Tennessee, Texas, Virginia, and West Virginia | Rehang—74.5%, (n = 800) Post-chill—34.90%, (n = 800) | [124] |
| Feces, pasture soil, whole carcass rinse directly after processing (WCR-P), final product whole carcass rinse after chilling and storage time (WCR-F), and ceca samples collected during processing from each farm | Southeastern USA from March 2014 to November 2017 | 39.08%, (n = 2305) | [125] |
| Air, feces-litter, feed pans and water lines | USA—Virginia | 26.66%, (n = 120) | [126] |
| Fecal and environmental samples | USA—North Carolina | Fecal: 29.50%; (n = 400) Environmental sample: 1%, (n = 500) | [127] |
| Pre-scald (feather and skin) | USA—Delmarva Peninsula | 77%, (n = 48) | [128] |
| Evisceration | USA | 96–100%, (n = 48) | [129] |
| Slaughterhouse | Brazil- Parana, santa Catarina and Rio Grande do Sul | Campylobacter spp.—35.84% (n = 816) C. jejuni—78.47% (n = 144) | [130] |
| Defeathering, Evisceration, Shackles, Converyor belt | North of Spain | Defeathering—80% (n = 30) Eviseration—100% (n = 39) Shackles—100% (n = 23) Converyor belt—96.6% (n = 29) | [131] |
| Defeathering machine, evisceration machine, conveyor belts, scald tank, water | France | 87% (34/39) | [45] |
C. jejuni utilizes numerous mechanisms to adapt to environmental stressors (Table 2 and Table 3). C. jejuni induces biofilm formation (Table 4) in some strains by activation of various stress regulators. There have been several control strategies to remove biofilms from processing facilities. However, there is substantial genetic diversity among campylobacter strains, which complicates treatment effectiveness.

Table 2.
Cluster of genes and their potential functions in overcoming multiple stress that C. jejuni encounters in processing facility.
Table 2.
Cluster of genes and their potential functions in overcoming multiple stress that C. jejuni encounters in processing facility.
| Stressors | Gene Involved | Gene Function | References |
|---|---|---|---|
| Heat stress |
| Express DnaJ chaperone protein-protein folding and heat shock response | [51] |
| Component of RacR-RacS temperature-responsive signal transduction system | [53] | |
| High temperature requirement A (HtrA)-like protease and chaperones | [91] | |
| Express iron sequestration ferritin protein (Dps) | [132] | |
| Express DnaK chaperonin- protein folding | ||
| Refrigeration and freezing stress |
| Superoxide dismutase | [59] |
| S-ribosylhomocysteinase-catalyzes the formation of autoinducer-2 (AI-2) molecules and homocysteine | [133] | |
| Osmotic stress |
| Capsule export gene-encodes capsule export apparatus | [65] |
| Acid stress |
| ATP-dependent Protease-heat shock gene | [79] |
| Thioredoxin-disulfide reductase-protein folding | [87] | |
| Ferric uptake regulator gene | [88] | |
| UV stress | recA | Express recA protein involved in DNA repair | [78] |

Table 3.
Cluster of genes involved, and its potential function in aerotolerance mechanism in Campylobacter jejuni.
Table 3.
Cluster of genes involved, and its potential function in aerotolerance mechanism in Campylobacter jejuni.
| Gene | Gene Product or Function | Involved Function in Aerotolerance | References |
|---|---|---|---|
| ahpC | Alkyl hydroperoxide reductase-Antioxidant | Antioxidant | [40,90] |
| katA | Catalase | Peroxide detoxification | [90] |
| sodB | Iron co-factored superoxide dismutase- | Antioxidant | [90] |
| fdxA | Ferredoxin A-cyclophilin gene | Antioxidant | [93] |
| htrA | High temperature requirement-A protease | Removal of misfolded proteins as a result of oxygen stress | [92] |
| tpx | Thiol peroxidase- | Scavenges molecular oxygen | [96] |
| bcpb | Express Bacterioferritin comigratory protein | Regulate oxidative stress by attacking molecular oxygen | [96] |
| ctb | Truncated hemoglobin | Oxygen-protective physiological role by increasing oxygen uptake rates | [94] |
| Cj1556 | MarR family transcriptional regulator | Oxidative stress response | [95] |

Table 4.
Cluster of genes involved and its function in biofilm formation in Campylobacter jejuni.
Table 4.
Cluster of genes involved and its function in biofilm formation in Campylobacter jejuni.
| Gene | Gene Product or Function | Involved Function in Biofilm | References |
|---|---|---|---|
| flaA | Glycosylated structural flagellins-A | Involved in cell associated with motility | [106] |
| luxS | S-ribosylhomocysteianse | Quorum sensing | [17] |
| cadF | Campylobacter adhesion to fibronectin | Adhesion | [17] |
| dnaJ | Chaperone DnaJ | Stress response | [107] |
| cbrA | Campylobacter bile resistance regulator | Stress response | [107] |
| htrA | High temperature requirement A | Stress response | [107] |
| sodB | Superoxide dismutase | Stress response | [107] |
| ahpC | Alkyl hydroperoxide reductase | Involved in oxidative stress response | [107] |
| peb4 | encode homolog of cluster 3 binding protein | adhesion | [109] |
| trxA | Thioredoxin A | Involved in oxidative stress response | [18] |
| trxB | Thioredoxin B | Involved in oxidative stress response | [18] |
| ilvE | Branched chain amino transferases for leucine, isoleucine, and valine | Involved in oxidative stress response | [18] |
| nuoC | NuoC- subunit of complex I (ubiquinone oxidoreductase) | Assembly or the stability of ubiquinone oxidoreductase | [18] |
VBNC state is another response mechanism to unfavorable environment condition (Table 5). C. jejuni cand shift to the non-culturable state after passing through stressors and remain in food, with a potential to cause human infection. However, the mechanism underlying this adaptive response is still not clear. Further research needs to be conducted to understand the genetic aspect of the survival mechanism so that effective control measures can be developed.

Table 5.
Cluster of genes involved and its function in VBNC formation in Campylobacter jejuni.
Table 5.
Cluster of genes involved and its function in VBNC formation in Campylobacter jejuni.
| Gene | Gene Product or Function | Involved Function in VBNC | References |
|---|---|---|---|
| ppk1 | Poly phosphate kinase 1-codes for PPK1 enzyme mediates the synthesis of poly P | Mutant of ppk1 decreased the accumulation of poly P-decreased stress response | [134] |
| cadF | Code 37 kDa adhesin-bind to fibronectin and mediates bacteria-host interaction | Retain ability to adhere to host cells | [116] |
| flaA, flab | Flagellin-Involved bacteria internalization | Decreased expression- conserve energy for metabolism | [29] |
| cdtA, cdtB and cdtC | Cytolethal distending toxins-arrest G2/M phase of cell cycle causing cell death | Decreased expression-conserve energy for metabolism | [29] |
| ciaB | Campylobacter invasion antigen B | Decreased expression-conserve energy for metabolism | [29] |
Author Contributions
A.T.S. and D.P. contributed towards the conceptualization. A.T.S. developed the idea and supervised the project. D.P. participated in the outline, design, drafted the review, and made modifications together with A.T.S. to form the final manuscript. Critical reviews were conducted by A.T.S., D.P., H.T.T., R.R., S.B.W., W.S., T.T.N.D. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The funding for this study was provided by the Mississippi Agriculture and Forestry Experiment Station through USDA-NIFA Hatch Project (MIS-322380).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no conflict of interest.
References
- Centers for Disease Control and Prevention (CDC). Campylobacter (Campylobacteriosis). (Final Update). Available online: https://www.cdc.gov/campylobacter/index.html (accessed on 10 September 2022).
- Tam, C.C.; Rodrigues, L.C.; Viviani, L.; Dodds, J.P.; Evans, M.R.; Hunter, P.R.; Gray, J.J.; Letley, L.H.; Rait, G.; Tompkins, D.S.; et al. Longitudinal Study of Infectious Intestinal Disease in the UK (IID2 Study): Incidence in the Community and Presenting to General Practice. Gut 2012, 61, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Mongodin, E.F.; Mandrell, R.E.; Miller, W.G.; Rasko, D.A.; Ravel, J.; Brinkac, L.M.; Deboy, R.T.; Parker, C.T.; Daugherty, S.C.; et al. Major Structural Differences and Novel Potential Virulence Mechanisms from the Genomes of Multiple Campylobacter Species. PLoS Biol. 2005, 3, e15. [Google Scholar] [CrossRef] [PubMed]
- Dogan, O.B.; Clarke, J.; Mattos, F.; Wang, B. A Quantitative Microbial Risk Assessment Model of Campylobacter in Broiler Chickens: Evaluating Processing Interventions. Food Control 2019, 100, 97–110. [Google Scholar] [CrossRef]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter Species and Guillain-Barre Syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef]
- Shane, S.M. The Significance of Campylobacter Jejuni Infection in Poultry: A Review. Avian Pathol. 1992, 21, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Stanley, K.; Cunningham, R.; Jones, K. Isolation of Campylobacter Jejuni from Groundwater. J. Appl. Microbiol. 1998, 85, 187–191. [Google Scholar] [CrossRef]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of Environmental Survival in Transmission of Campylobacter Jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Mangen, M.J.J.; De Koeijer, A.A.; Bogaardt, M.J.; Evers, E.G.; Jacobs-Reitsma, W.F.; Van Pelt, W.; Wagenaar, J.A.; De Wit, G.A.; Van Der Zee, H.; et al. Effectiveness and Efficiency of Controlling Campylobacter on Broiler Chicken Meat. Risk Anal. 2007, 27, 831–844. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.; Jeon, B. Impact of Oxidative Stress Defense on Bacterial Survival and Morphological Change in Campylobacter Jejuni under Aerobic Conditions. Front. Microbiol. 2015, 6, 295. [Google Scholar] [CrossRef]
- Uzunović-Kamberović, S.; Zorman, T.; Heyndrickx, M.; Možina, S.S. Role of Poultry Meat in Sporadic Campylobacter Infections in Bosnia and Herzegovina: Laboratory-Based Study. Croat. Med. J. 2007, 48, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Chui, L.; Bae, J.; Li, V.; Ma, A.; Mutschall, S.K.; Taboada, E.N.; McMullen, L.M.; Jeon, B. Frequent Implication of Multistress-Tolerant Campylobacter Jejuni in Human Infections. Emerg. Infect. Dis. 2018, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Mouftah, S.F.; Cobo-Díaz, J.F.; Álvarez-Ordóñez, A.; Mousa, A.; Calland, J.K.; Pascoe, B.; Sheppard, S.K.; Elhadidy, M. Stress Resistance Associated with Multi-Host Transmission and Enhanced Biofilm Formation at 42 °C among Hyper-Aerotolerant Generalist Campylobacter Jejuni. Food Microbiol. 2021, 95, 103706. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, H.P.; Parijs, I.; Foster, K.R.; Vanderleyden, J. Experimental Evolution in Biofilm Populations. FEMS Microbiol. Rev. 2016, 40, 373–397. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Reeser, R.J.; Medler, R.T.; Billington, S.J.; Jost, B.H.; Joens, L.A. Characterization of Campylobacter Jejuni Biofilms under Defined Growth Conditions. Appl. Environ. Microbiol. 2007, 73, 1908–1913. [Google Scholar] [CrossRef]
- Pascoe, B.; Méric, G.; Murray, S.; Yahara, K.; Mageiros, L.; Bowen, R.; Jones, N.H.; Jeeves, R.E.; Lappin-Scott, H.M.; Asakura, H.; et al. Enhanced Biofilm Formation and Multi-Host Transmission Evolve from Divergent Genetic Backgrounds in Campylobacter Jejuni. Environ. Microbiol. 2015, 17, 4779–4789. [Google Scholar] [CrossRef]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H.M. Chicken Juice Enhances Surface Attachment and Biofilm Formation of Campylobacter Jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef]
- Thames, H.T.; Sukumaran, A.T. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- Teh, A.H.T.; Lee, S.M.; Dykes, G.A. Does Campylobacter Jejuni Form Biofilms in Food-Related Environments? Appl. Environ. Microbiol. 2014, 80, 5154–5160. [Google Scholar] [CrossRef]
- Dykes, G.A.; Sampathkumar, B.; Korber, D.R. Planktonic or Biofilm Growth Affects Survival, Hydrophobicity and Protein Expression Patterns of a Pathogenic Campylobacter Jejuni Strain. Int. J. Food Microbiol. 2003, 89, 1–10. [Google Scholar] [CrossRef]
- Chaveerach, P.; Huurne, A.A.H.M.T.; Lipman, L.J.A.; van Knapen, F. Survival and Resuscitation of Ten Strains Of Campylobacter Jejuni and Campylobacter coli under Acid Conditions. Society 2003, 69, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Baffone, W.; Casaroli, A.; Citterio, B.; Pierfelici, L.; Campana, R.; Vittoria, E.; Guaglianone, E.; Donelli, G. Campylobacter Jejuni Loss of Culturability in Aqueous Microcosms and Ability to Resuscitate in a Mouse Model. Int. J. Food Microbiol. 2006, 107, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Guzej, B.; Jamnik, P.; Vučković, D.; Abram, M.; Možina, S.S. Stress Response and Pathogenic Potential of Campylobacter Jejuni Cells Exposed to Starvation. Res. Microbiol. 2009, 160, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.N.; Davis, B.; Tirado, S.M.; Duggal, M.; Van Frankenhuyzen, J.K.; Deaville, D.; Wijesinghe, M.A.K.; Tessaro, M.; Trevors, J.T. Survival Mechanisms and Culturability of Campylobacter Jejuni under Stress Conditions. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2009, 96, 377–394. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The Importance of the Viable but Non-Culturable State in Human Bacterial Pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Rollins, D.M.; Colwell, R.R. Viable but Nonculturable Stage of Campylobacter Jejuni and Its Role in Survival in the Natural Aquatic Environment. Appl. Environ. Microbiol. 1986, 52, 531–538. [Google Scholar] [CrossRef]
- Chaisowwong, W.; Kusumoto, A.; Hashimoto, M.; Harada, T.; Maklon, K.; Kawamoto, K. Physiological Characterization of Campylobacter Jejuni under Cold Stresses Conditions: Its Potential for Public Threat. J. Vet. Med. Sci. 2012, 74, 43–50. [Google Scholar] [CrossRef]
- Khan, I.U.H.; Hill, S.; Nowak, E.; Edge, T.A. Effect of Incubation Temperature on the Detection of Thermophilic Campylobacter Species from Freshwater Beaches, Nearby Wastewater Effluents, and Bird Fecal Droppings. Appl. Environ. Microbiol. 2013, 79, 7639–7645. [Google Scholar] [CrossRef]
- Oyarzabal, O.A.; Carrillo, C.D. Isolation, Identification, and Typing of Campylobacter Strains from Food Samples; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Esson, D.; Mather, A.E.; Scanlan, E.; Gupta, S.; De Vries, S.P.W.; Bailey, D.; Harris, S.R.; McKinley, T.J.; Méric, G.; Berry, S.K.; et al. Genomic Variations Leading to Alterations in Cell Morphology of Campylobacter spp. Sci. Rep. 2016, 6, 38303. [Google Scholar] [CrossRef]
- Ng, L.K.; Sherburne, R.; Taylor, D.E.; Stiles, M.E. Morphological Forms and Viability of Campylobacter Species Studied by Electron Microscopy. J. Bacteriol. 1985, 164, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Tangwatcharin, P.; Chanthachum, S.; Khopaibool, P.; Griffiths, M.W. Morphological and Physiological Responses of Campylobacter Jejuni to Stress. J. Food Prot. 2006, 69, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Buck, G.E.; Parshall, K.A.; Davis, C.P. Electron Microscopy of the Coccoid Form of Campylobacter Jejuni. J. Clin. Microbiol. 1983, 18, 420–421. [Google Scholar] [CrossRef]
- Griffiths, P.L. Morphological Changes of Campylobacter Jejuni Growing in Liquid Culture. Lett. Appl. Microbiol. 1993, 17, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Frirdich, E.; Biboy, J.; Adams, C.; Lee, J.; Ellermeier, J.; Gileda, L.D.; DiRita, V.J.; Girardin, S.E.; Vollmer, W.; Gaynor, E.C. Peptidoglycan-Modifying Enzyme Pgp1 Is Required ForHelical Cell Shape and Pathogenicity Traits In Campylobacter Jejuni. PLoS Pathog. 2012, 8, e1002602. [Google Scholar] [CrossRef]
- Parkhill, J.; Wren, B.W.; Mungall, K.; Ketley, J.M.; Churcher, C.; Basham, D.; Chillingworth, T.; Davies, R.M.; Feltwell, T.; Holroyd, S.; et al. The Genome Sequence of C. Jejuni. Nature 2000, 403, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Pesci, E.C.; Cottle, D.L.; Pickett, C.L. Genetic, Enzymatic, and Pathogenic Studies of the Iron Superoxide Dismutase of Campylobacter Jejuni. Infect. Immun. 1994, 62, 2687–2694. [Google Scholar] [CrossRef]
- Baillon, M.L.A.; Van Vliet, A.H.M.; Ketley, J.M.; Constantinidou, C.; Penn, C.W. An Iron-Regulated Alkyl Hydroperoxide Reductase (AhpC) Confers Aerotolerance and Oxidative Stress Resistance to the Microaerophilic Pathogen Campylobacter Jejuni. J. Bacteriol. 1999, 181, 4798–4804. [Google Scholar] [CrossRef]
- Hue, O.; Allain, V.; Laisney, M.J.; Le Bouquin, S.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.Y.; Picherot, M.; et al. Campylobacter Contamination of Broiler Caeca and Carcasses at the Slaughterhouse and Correlation with Salmonella Contamination. Food Microbiol. 2011, 28, 862–868. [Google Scholar] [CrossRef]
- Berrang, M.E.; Buhr, R.J.; Cason, J.A.; Dickens, J.A. Broiler Carcass Contamination with Campylobacter from Feces during Defeathering. J. Food Prot. 2001, 64, 2063–2066. [Google Scholar] [CrossRef]
- Borges, K.A.; Cisco, I.C.; Furian, T.Q.; Tedesco, D.C.; Rodrigues, L.B.; do Nascimento, V.P.; dos Santos, L.R. Detection and Quantification of Campylobacter spp. In Brazilian Poultry Processing Plants. J. Infect. Dev. Ctries. 2020, 14, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Perez-Arnedo, I.; Gonzalez-Fandos, E. Prevalence of Campylobacter spp. In Poultry in Three Spanish Farms, a Slaughterhouse and a Further Processing Plant. Foods 2020, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Peyrat, M.B.; Soumet, C.; Maris, P.; Sanders, P. Recovery of Campylobacter Jejuni from Surfaces of Poultry Slaughterhouses after Cleaning and Disinfection Procedures: Analysis of a Potential Source of Carcass Contamination. Int. J. Food Microbiol. 2008, 124, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Pocheron, A.L.; Hernould, M.; Haddad, N.; Tresse, O.; Cappelier, J.M. Description of Campylobacter Jejuni Bf, an Atypical Aero-Tolerant Strain. Gut Pathog. 2015, 7, 30. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Kim, J.; Kim, J.H.; Jung, J.I.; Cho, S.; Ryu, S.; Jeon, B. Comparative Analysis of Aerotolerance, Antibiotic Resistance, and Virulence Gene Prevalence in Campylobacter Jejuni Isolates from Retail Raw Chicken and Duck Meat in South Korea. Microorganisms 2019, 7, 433. [Google Scholar] [CrossRef]
- Hilbert, F.; Scherwitzel, M.; Paulsen, P.; Szostak, M.P. Survival of Campylobacter Jejuni under Conditions of Atmospheric Oxygen Tension with the Support of Pseudomonas spp. Appl. Environ. Microbiol. 2010, 76, 5911–5917. [Google Scholar] [CrossRef]
- Habib, I.; Uyttendaele, M.; De Zutter, L. Survival of Poultry-Derived Campylobacter Jejuni of Multilocus Sequence Type Clonal Complexes 21 and 45 under Freeze, Chill, Oxidative, Acid and Heat Stresses. Food Microbiol. 2010, 27, 829–834. [Google Scholar] [CrossRef]
- Lindqvist, R.; Upadhyay, A.; Överby, A.K. Tick-Borne Flaviviruses and the Type I Interferon Response. Viruses 2018, 10, 340. [Google Scholar] [CrossRef]
- Konkel, M.E.; Kim, B.J.; Klena, J.D.; Young, C.R.; Ziprin, R. Characterization of the Thermal Stress Response of Campylobacter Jejuni. Infect. Immun. 1998, 66, 3666–3672. [Google Scholar] [CrossRef]
- Stewart, G.R.; Robertson, B.D.; Young, D.B. Analysis of the Function of Mycobacterial DnaJ Proteins by Overexpression and Microarray Profiling. Tuberculosis 2004, 84, 180–187. [Google Scholar] [CrossRef]
- Brás, A.M.; Chatterjee, S.; Wren, B.W.; Newell, D.G.; Ketley, J.M. A Novel Campylobacter Jejuni Two-Component Regulatory System Important for Temperature-Dependent Growth and Colonization. J. Bacteriol. 1999, 181, 3298–3302. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Carroll, C.; Jordan, K.N. Environmental Survival Mechanisms of the Foodborne Pathogen Campylobacter Jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Snith, S.C.; Coloe, P.J. Survival and Growth of Campylobacter Jejuni after Artificial Inoculation onto Chicken Skin as a Function of Temperature and Packaging Conditions. J. Food Prot. 1998, 61, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Tran, H.L.; Kanenaka, R.Y.; Kathariou, S. Survival of Clinical and Poultry-Derived Isolates of Campylobacter Jejuni at a Low Temperature (4 °C). Appl. Environ. Microbiol. 2001, 67, 4186–4191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelana, L.C.; Griffiths, M.W. Use of an Autobioluminescent Campylobacter Jejuni to Monitor Cell Survival as a Function of Temperature, PH, and Sodium Chloride. J. Food Prot. 2003, 66, 2032–2037. [Google Scholar] [CrossRef]
- Moen, B.; Oust, A.; Langsrud, Ø.; Dorrell, N.; Marsden, G.L.; Hinds, J.; Kohler, A.; Wren, B.W.; Rudi, K. Explorative Multifactor Approach for Investigating Global Survival Mechanisms of Campylobacter Jejuni under Environmental Conditions. Appl. Environ. Microbiol. 2005, 71, 2086–2094. [Google Scholar] [CrossRef]
- Stead, D.; Park, S.F. Roles of Fe Superoxide Dismutase and Catalase in Resistance of Campylobacter Coli to Freeze-Thaw Stress. Appl. Environ. Microbiol. 2000, 66, 3110–3112. [Google Scholar] [CrossRef][Green Version]
- White, D.; Drummond, J.; Fuqua, C. The Physiology and Biochemistry of the Prokaryotes; Oxford University Press: New York, NY, USA, 2011; p. 656. [Google Scholar]
- Doyle, M.P.; Roman, D.J. Response of Campylobacter Jejuni to Sodium Chloride. Appl. Environ. Microbiol. 1982, 43, 561–565. [Google Scholar] [CrossRef]
- Sampers, I.; Habib, I.; De Zutter, L.; Dumoulin, A.; Uyttendaele, M. Survival of Campylobacter spp. in Poultry Meat Preparations Subjected to Freezing, Refrigeration, Minor Salt Concentration, and Heat Treatment. Int. J. Food Microbiol. 2010, 137, 147–153. [Google Scholar] [CrossRef]
- Reezal, A.; Mcneil, B.; Anderson, J.G. Effect of Low-Osmolality Nutrient Media on Growth and Culturability of Campylobacter Species. Appl. Environ. Microbiol. 1998, 64, 4643–4649. [Google Scholar] [CrossRef]
- Strom, A.R.; Kaasen, I. Trehalose Metabolism in Escherichia Coli: Stress Protection and Stress Regulation of Gene Expression. Mol. Microbiol. 1993, 8, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Frirdich, E.; Huynh, S.; Parker, C.T.; Gaynor, E.C. Hyperosmotic Stress Response of Campylobacter Jejuni. J. Bacteriol. 2012, 194, 6116–6130. [Google Scholar] [CrossRef] [PubMed]
- Kempf, B.; Bremer, E. Uptake and Synthesis of Compatible Solutes as Microbial Stress Responses to High-Osmolality Environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 21348 Definitions of Solar Irradiance Spectral Categories. Environment 2007, 5, 6–7. [Google Scholar]
- Zajac, A. Irradiation in The Pro-Duction, Processing and Handling of Food; Food and Drug Administration: Silver Spring, MD, USA, 2007; Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? (accessed on 10 September 2022).
- Sinton, L.; Hall, C.; Braithwaite, R. Sunlight Inactivation of Campylobacter Jejuni and Salmonella Enterica, Compared with Escherichia Coli, in Seawater and River Water. J. Water Health 2007, 5, 357–365. [Google Scholar] [CrossRef]
- Butler, R.C.; Lund, V.; Carlson, D.A. Susceptibility of Campylobacter Jejuni and Yersinia Enterocolitica to UV Radiation. Appl. Environ. Microbiol. 1987, 53, 375–378. [Google Scholar] [CrossRef]
- Murdoch, L.E.; Maclean, M.; MacGregor, S.J.; Anderson, J.G. Inactivation of Campylobacter Jejuni by Exposure to High-Intensity 405-Nm Visible Light. Foodborne Pathog. Dis. 2010, 7, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Modelling the Effect of UV Light at Different Wavelengths and Treatment Combinations on the Inactivation of Campylobacter Jejuni. Innov. Food Sci. Emerg. Technol. 2021, 69, 102626. [Google Scholar] [CrossRef]
- Haughton, P.N.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J.; Fanning, S.; Whyte, P. Efficacy of UV Light Treatment for the Microbiological Decontamination of Chicken, Associated Packaging, and Contact Surfaces. J. Food Prot. 2011, 74, 565–572. [Google Scholar] [CrossRef]
- Isohanni, P.M.I.; Lyhs, U. Use of Ultraviolet Irradiation to Reduce Campylobacter Jejuni on Broiler Meat. Poult. Sci. 2009, 88, 661–668. [Google Scholar] [CrossRef]
- Obiri-Danso, K.; Paul, N.; Jones, K. The Effects of UVB and Temperature on the Survival of Natural Populations and Pure Cultures of Campylobacter Jejuni, Camp. Coli, Camp. Lari and Urease-Positive Thermophilic Campylobacters (UPTC) in Surface Waters. J. Appl. Microbiol. 2001, 90, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ye, C.; Cui, L.; Wan, K.; Chen, S.; Zhang, S.; Yu, X. Population and Single Cell Metabolic Activity of UV-Induced VBNC Bacteria Determined by CTC-FCM and D2O-Labeled Raman Spectroscopy. Environ. Int. 2019, 130, 104883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, C.; Lin, H.; Lv, L.; Yu, X. UV Disinfection Induces a Vbnc State in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 2015, 49, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Guerry, P.; Pope, P.M.; Burr, D.H.; Leifer, J.; Joseph, S.W.; Bourgeois, A.L. Development and Characterization of RecA Mutants of Campylobacter Jejuni for Inclusion in Attenuated Vaccines. Infect. Immun. 1994, 62, 426–432. [Google Scholar] [CrossRef]
- Reid, A.N.; Pandey, R.; Palyada, K.; Naikare, H.; Stintzi, A. Identification of Campylobacter Jejuni Genes Involved in the Response to Acidic PH and Stomach Transit. Appl. Environ. Microbiol. 2008, 74, 1583–1597. [Google Scholar] [CrossRef]
- Stern, N.J.; Rothenberg, P.J.; Stone, J.M. Enumeration and Reduction of Campylobacter Jejuni in Poultry and Red Meats. J. Food Prot. 1985, 48, 606–610. [Google Scholar] [CrossRef]
- Birk, T.; Grønlund, A.C.; Christensen, B.B.; Knøchel, S.; Lohse, K.; Rosenquist, H. Effect of Organic Acids and Marination Ingredients on the Survival of Campylobacter Jejuni on Meat. J. Food Prot. 2010, 73, 258–265. [Google Scholar] [CrossRef]
- Waterman, S.R.; Small, P.L.C. Acid-Sensitive Enteric Pathogens Are Protected from Killing under Extremely Acidic Conditions of PH 2.5 When They Are Inoculated onto Certain Solid Food Sources. Appl. Environ. Microbiol. 1998, 64, 3882–3886. [Google Scholar] [CrossRef]
- Goodson, M.; Rowbury, R.J. Habituation to Normally Lethal Acidity by Prior Growth of Escherichia Coli at a Sub-Lethal Acid PH Value. Lett. Appl. Microbiol. 1989, 8, 77–79. [Google Scholar] [CrossRef]
- Murphy, C.; Carroll, C.; Jordan, K.N. Induction of an Adaptive Tolerance Response in the Foodborne Pathogen, Campylobacter Jejuni. FEMS Microbiol. Lett. 2003, 223, 89–93. [Google Scholar] [CrossRef][Green Version]
- winiecka-krusnell, J.; Wreiber, K.; von Euler, A.; Engstrand, L.; Linder, E. Free-Living Amoebae Promote Growth and Survival of Helicobacter Pylori. Scand. J. Infect. Dis. 2002, 34, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Axelsson-Olsson, D.; Svensson, L.; Olofsson, J.; Salomon, P.; Waldenström, J.; Ellström, P.; Olsen, B. Increase in Acid Tolerance of Campylobacter Jejuni through Coincubation with Amoebae. Appl. Environ. Microbiol. 2010, 76, 4194–4200. [Google Scholar] [CrossRef] [PubMed]
- Birk, T.; Wik, M.T.; Lametsch, R.; Knøchel, S. Acid Stress Response and Protein Induction in Campylobacter Jejuni Isolates with Different Acid Tolerance. BMC Microbiol. 2012, 12, 174. [Google Scholar] [CrossRef]
- Askoura, M.; Sarvan, S.; Couture, J.F.; Stintzi, A. The Campylobacter Jejuni Ferric Uptake Regulator Promotes Acid Survival and Cross-Protection against Oxidative Stress. Infect. Immun. 2016, 84, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Andrews, K.J.; McMullen, L.M.; Jeon, B. Tolerance to Stress Conditions Associated with Food Safety in Campylobacter Jejuni Strains Isolated from Retail Raw Chicken. Sci. Rep. 2019, 9, 11915. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Oh, E.; Hwang, S.; Ryu, S.; Jeon, B. Non-Selective Regulation of Peroxide and Superoxide Resistance Genes by PerR in Campylobacter Jejuni. Front. Microbiol. 2015, 6, 126. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Miller, W.G.; De Reuse, H.; Mendz, G.L. Oxygen Requirement and Tolerance of Campylobacter Jejuni. Res. Microbiol. 2007, 158, 644–650. [Google Scholar] [CrossRef]
- Brøndsted, L.; Andersen, M.T.; Parker, M.; Jørgensen, K.; Ingmer, H. The HtrA Protease of Campylobacter Jejuni Is Required for Heat and Oxygen Tolerance and for Optimal Interaction with Human Epithelial Cells. Appl. Environ. Microbiol. 2005, 71, 3205–3212. [Google Scholar] [CrossRef]
- Van Vliet, A.H.M.; Baillon, M.L.A.; Penn, C.W.; Ketley, J.M. The Iron-Induced Ferredoxin FdxA of Campylobacter Jejuni Is Involved in Aerotolerance. FEMS Microbiol. Lett. 2001, 196, 189–193. [Google Scholar] [CrossRef]
- Wainwright, L.M.; Elvers, K.T.; Park, S.F.; Poole, R.K. A Truncated Haemoglobin Implicated in Oxygen Metabolism by the Microaerophilic Food-Borne Pathogen Campylobacter Jejuni. Microbiology 2005, 151, 4079–4091. [Google Scholar] [CrossRef]
- Gundogdu, O.; Mills, D.C.; Elmi, A.; Martin, M.J.; Wren, B.W.; Dorrell, N. The Campylobacter Jejuni Transcriptional Regulator Cj1556 Plays a Role in the Oxidative and Aerobic Stress Response and Is Important for Bacterial Survival In Vivo. J. Bacteriol. 2011, 193, 4238–4249. [Google Scholar] [CrossRef] [PubMed]
- Atack, J.M.; Harvey, P.; Jones, M.A.; Kelly, D.J. The Campylobacter Jejuni Thiol Peroxidases Tpx and Bcp Both Contribute to Aerotolerance and Peroxide-Mediated Stress Resistance but Have Distinct Substrate Specificities. J. Bacteriol. 2008, 190, 5279–5290. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Buswell, C.M.; Herlihy, Y.M.; Lawrence, L.M.; McGuiggan, J.T.M.; Marsh, P.D.; Keevil, C.W.; Leach, S.A. Extended Survival and Persistence of Campylobacter spp. Water and Aquatic Biofilms and Their Detection by Immunofluorescent-Antibody and -RRNA Staining. Appl. Environ. Microbiol. 1998, 64, 733–741. [Google Scholar] [CrossRef]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced Biofilm Formation by Ferrous and Ferric Iron through Oxidative Stress in Campylobacter Jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.-L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic Analysis of Campylobacter Jejuni 11168 Biofilms Reveals a Role for the Motility Complex in Biofilm Formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wu, Q.; Zhang, J.; Ma, Z.; Wang, J.; Nie, X.; Ding, Y.; Xue, L.; Chen, M.; Wu, S.; et al. Campylobacter Jejuni Biofilm Formation Under Aerobic Conditions and Inhibition by ZnO Nanoparticles. Front. Microbiol. 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Van de Giessen, A.W.; Heuvelman, C.J.; Abee, T.; Hazeleger, W.C. Experimental Studies on the Infectivity of Non-Culturable Forms of Campylobacter spp. in Chicks and Mice. Epidemiol. Infect. 1996, 117, 463–470. [Google Scholar] [CrossRef]
- Hazeleger, W.C.; Janse, J.D.; Koenraad, P.M.F.J.; Beumer, R.R.; Rombouts, F.M.; Abee, T. Temperature-Dependent Membrane Fatty Acid and Cell Physiology Changes in Coccoid Forms of Campylobacter Jejuni. Appl. Environ. Microbiol. 1995, 61, 2713–2719. [Google Scholar] [CrossRef]
- Asakura, H.; Brüggemann, H.; Sheppard, S.K.; Ekawa, T.; Meyer, T.F.; Yamamoto, S.; Igimi, S. Molecular Evidence for the Thriving of Campylobacter Jejuni ST-4526 in Japan. PLoS ONE 2012, 7, e48394. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; Strachan, N.J.C.; MacRae, M.; McCarthy, N.D.; Wilson, D.J.; Gormley, F.J.; Falush, D.; Ogden, L.D.; Maiden, M.C.J.; et al. Campylobacter Genotyping to Determine the Source of Human Infection. Clin. Infect. Dis. 2009, 48, 1072–1078. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, C.; Kim, Y.J. Role of FlgA for Flagellar Biosynthesis and Biofilm Formation of Campylobacter Jejuni NCTC11168. J. Microbiol. Biotechnol. 2015, 25, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Jeon, B. Role of Alkyl Hydroperoxide Reductase (AhpC) in the Biofilm Formation of Campylobacter Jejuni. PLoS ONE 2014, 9, e87312. [Google Scholar] [CrossRef]
- Pei, Z.; Burucoa, C.; Grignon, B.; Baqar, S.; Huang, X.-Z.; Kopecko, D.J.; Bourgeois, A.; Fauchere, J.-L.; Blaser, M.J. Mutation in the Peb1A Locus Of Campylobacter Jejuni Reduces Interactions with Epithelial Cells and Intestinal Colonization of Mice. Infect. Immun. 1998, 66, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Yamasaki, M.; Yamamoto, S.; Igimi, S. Deletion of Peb4 Gene Impairs Cell Adhesion and Biofilm Formation in Campylobacter Jejuni. FEMS Microbiol. Lett. 2007, 275, 278–285. [Google Scholar] [CrossRef]
- Brown, H.L.; Hanman, K.; Reuter, M.; Betts, R.P.; van Vliet, A.H.M. Campylobacter Jejuni Biofilms Contain Extracellular DNA and Are Sensitive to DNase I Treatment. Front. Microbiol. 2015, 6, 699. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, C.; Lee, E.J.; Bang, W.S.; Kim, Y.J.; Kim, J.S. Biofilm Formation of Campylobacter Strains Isolated from Raw Chickens and Its Reduction with DNase I Treatment. Food Control 2017, 71, 94–100. [Google Scholar] [CrossRef]
- Wagle, B.R.; Donoghue, A.M.; Shrestha, S.; Upadhyaya, I.; Arsi, K.; Gupta, A.; Liyanage, R.; Rath, N.C.; Donoghue, D.J.; Upadhyay, A. Carvacrol Attenuates Campylobacter Jejuni Colonization Factors and Proteome Critical for Persistence in the Chicken Gut. Poult. Sci. 2020, 99, 4566–4577. [Google Scholar] [CrossRef]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus Extracts as Inhibitors of Quorum Sensing, Biofilm Formation and Motility of Campylobacter Jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Yu, H.H.; Song, Y.J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Investigating the Antimicrobial and Antibiofilm Effects of Cinnamaldehyde against Campylobacter spp. Using Cell Surface Characteristics. J. Food Sci. 2020, 85, 157–164. [Google Scholar] [CrossRef]
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S. Trans-Cinnamaldehyde, Eugenol and Carvacrol Reduce Campylobacter Jejuni Biofilms and Modulate Expression of Select Genes and Proteins. Front. Microbiol. 2019, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Campana, R.; Vallorani, L.; Dominici, S.; Federici, S.; Casadei, L.; Gioacchini, A.M.; Stocchi, V.; Baffone, W. CadF Expression in Campylobacter Jejuni Strains Incubated under Low-Temperature Water Microcosm Conditions Which Induce the Viable but Non-Culturable (VBNC) State. Antonie Leeuwenhoek 2013, 103, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M.; Sutcliffe, E.M.; Curry, A. Recovery of Viable but Non-Culturable Campylobacter Jejuni. J. Gen. Microbiol. 1991, 137, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Saha, S.; Sanyal, S.C. Recovery of Injured Campylobacter Jejuni Cells after Animal Passage. Appl. Environ. Microbiol. 1991, 57, 3388–3389. [Google Scholar] [CrossRef]
- Cappelier, J.M.; Magras, C.; Jouve, J.L.; Federighi, M. Recovery of Viable but Non-Culturable Campylobacter Jejuni Cells in Two Animal Models. Food Microbiol. 1999, 16, 375–383. [Google Scholar] [CrossRef]
- Pearson, A.D.; Greenwood, M.; Healing, T.D.; Rollins, D.; Shahamat, M.; Donaldson, J.; Colwell, R.R. Colonization of Broiler Chickens by Waterborne Campylobacter Jejuni. Appl. Environ. Microbiol. 1993, 59, 987–996. [Google Scholar] [CrossRef]
- Beumer, R.R.; de Vries, J.; Rombouts, F.M. Campylobacter Jejuni Non-Culturable Coccoid Cells. Int. J. Food Microbiol. 1991, 15, 153–163. [Google Scholar] [CrossRef]
- Lázaro, B.; Cárcamo, J.; Audícana, A.; Perales, I.; Fernández-Astorga, A. Viability and DNA Maintenance in Nonculturable Spiral Campylobacter Jejuni Cells after Long-Term Exposure to Low Temperatures. Appl. Environ. Microbiol. 1999, 65, 4677–4681. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Thayer, S.G.; Law, B.F.; Mild, R.M.; Hofacre, C.L.; Singer, R.S. Enumeration of Salmonella and Campylobacter spp. in Environmental Farm Samples and Processing Plant Carcass Rinses from Commercial Broiler Chicken Flocks. Appl. Environ. Microbiol. 2013, 79, 4106–4114. [Google Scholar] [CrossRef]
- Berrang, M.E.; Bailey, J.S.; Altekruse, S.F.; Patel, B.; Shaw, W.K.; Meinersmann, R.J.; Fedorka-Cray, P.J. Prevalence and Numbers of Campylobacter on Broiler Carcasses Collected at Rehang and Postchill in 20 U.S. Processing Plants. J. Food Prot. 2007, 70, 1556–1560. [Google Scholar] [CrossRef]
- Xu, X.; Rothrock, M.J.; Mohan, A.; Kumar, G.D.; Mishra, A. Using Farm Management Practices to Predict Campylobacter Prevalence in Pastured Poultry Farms. Poult. Sci. 2021, 100, 101122. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.W.; Eifert, J.D.; Ponder, M.A.; Schmale, D.G. Association of Campylobacter spp. Levels between Chicken Grow-out Environmental Samples and Processed Carcasses. Poult. Sci. 2014, 93, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Brake, J.; Keelara, S.; Zou, M.; Susick, E. Farm and Environmental Distribution of Campylobacter and Salmonella in Broiler Flocks. Res. Vet. Sci. 2013, 94, 33–42. [Google Scholar] [CrossRef]
- Kotula, K.L.; Pandya, Y. Bacterial Contamination of Broiler Chickens before Scalding. J. Food Prot. 1995, 58, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, J.K.; Berrang, M.E.; Dickens, J.A.; Fletcher, D.L.; Cox, N.A. Effect of Broiler Age, Feed Withdrawal, and Transportation on Levels of Coliforms, Campylobacter, Escherichia Coli and Salmonella on Carcasses before and after Immersion Chilling. Poult. Sci. 2003, 82, 169–173. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Armendaris, P.M.; de Sá, C.V.G.C.; Haddad, J.P.A.; de Melo, C.B. Prevalence of Campylobacter spp. in Chicken Carcasses in Slaughterhouses from South of Brazil. Curr. Microbiol. 2021, 78, 2242–2250. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Jaime, I.; Hänninen, M.L.; Rossi, M.; Rovira, J. Campylobacter Jejuni Survival in a Poultry Processing Plant Environment. Food Microbiol. 2017, 65, 185–192. [Google Scholar] [CrossRef]
- Kim, J.C.; Oh, E.; Kim, J.; Jeon, B. Regulation of Oxidative Stress Resistance in Campylobacter Jejuni, a Microaerophilic Foodborne Pathogen. Front. Microbiol. 2015, 6, 751. [Google Scholar] [CrossRef]
- Ligowska, M.; Cohn, M.T.; Stabler, R.A.; Wren, B.W.; Brøndsted, L. Effect of Chicken Meat Environment on Gene Expression of Campylobacter Jejuni and Its Relevance to Survival in Food. Int. J. Food Microbiol. 2011, 145 (Suppl. 1), S111–S115. [Google Scholar] [CrossRef]
- Gangaiah, D.; Kassem, I.I.; Liu, Z.; Rajashekara, G. Importance of Polyphosphate Kinase 1 for Campylobacter Jejuni Viable-but-Nonculturable Cell Formation, Natural Transformation, and Antimicrobial Resistance. Appl. Environ. Microbiol. 2009, 75, 7838–7849. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).