1. Introduction
Prion diseases are lethal neurodegenerative disorders that can affect both humans and animals. The pathogenesis of prion diseases requires the conversion of normal cellular prion protein (PrPC) to its disease-associated isoform (PrPSc) and is accompanied by its accumulation in the brains of affected organisms. [
1]. The presence of PrPSc correlates with infectivity [
2], and hence, it is believed to be the main agent of prion diseases. PrPSc exhibits partial proteolytic resistance against proteinase K (PK), and proteinase-resistant PrPSc (PrPres) has been considered a surrogate marker for prion infection.
Several studies have shown that prion infectivity does not always correlate well with PrPres levels [
3]. In previous temporal correlative studies on infectious titers and PrPres levels in prion-infected animals, a continuous increase in PrPres levels was observed even after the infectious titer reached a plateau [
4,
5,
6,
7]. In addition, high levels of infectivity with undetectable or extremely low PrPres were observed in transgenic mice expressing murine PrP P101L (Tg 101LL) with Gerstmann–Sträussler–Scheinker syndrome [
8,
9] or 263K scrapie [
10].
Real-time quaking-induced conversion (RT-QuIC) is one of the novel in vitro amplification assays that was developed based on the prion-seeded conversion of recombinant prion proteins (recPrP) to the amyloid fibrillar form by shaking [
11,
12] rather than the sonication used in its original assay [
13]. There is a strong correlation between RT-QuIC positivity and prion infection. The RT-QuIC assay has been shown to detect prion seeding activity in tissues and body fluids of animals and humans with prion diseases, with sensitivities higher than those of animal bioassays [
11]. Thus, RT-QuIC can act as a possible antemortem test for prion disease [
12], an evaluation tool for decontamination methods [
14], and a risk assessment tools for iatrogenic prion transmission via tissues [
15].
Considering the aforementioned discrepancies between PrPres and prion infectivity, it is important to establish the relationship between prion seeding activity and infectivity. A previous study reported a closer correlation of prion seeding activity with infectivity than with PrPres levels, as judged by the similar seeding activities in the brains of 139A or 263K scrapie-affected Tg 101LL mice containing equivalent infectious titers but discrepant PrPres levels [
16]. On the other hand, it has also been reported that prion infectivity is eliminated by a disinfectant without altering seeding activity [
17]. Hence, the controversial relationship between seeding activity and infectivity remains to be elucidated. Although time-course prion seeding activity in scrapie-infected hamsters has been described [
18], the temporal relationship between prion seeding dose and infectious titer has not yet been determined.
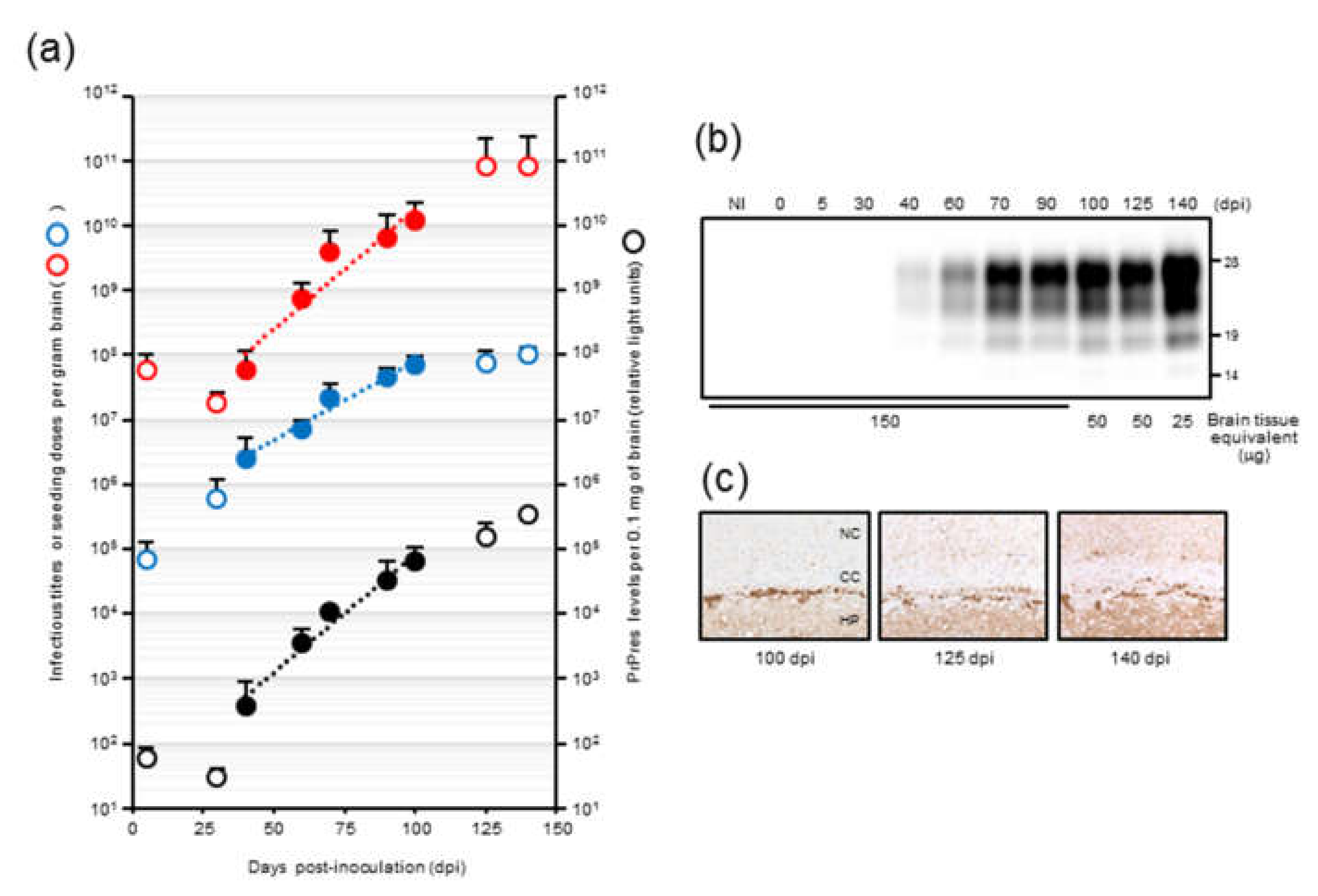
In our previous temporal study on mouse-adopted classical bovine spongiform encephalopathy (mBSE) prion, we reported that the infectious titer reached a plateau at 100 days postinfection (dpi) whereas PrPres levels were continuously elevated towards the end of the incubation period, suggesting the uncoupling of prion titer and PrPres levels [
19]. In this study, we attempted to determine the kinetics of prion seeding activity in the brains of these mice. We applied endpoint dilution RT-QuIC on brain homogenates to obtain a detailed measurement of seeding activities at defined intervals during the incubation period in mice and assessed the temporal relationships among seeding dose, PrPres levels, and prion titer. In addition, we investigated the alterations in seeding doses associated with the reduction of PrPres levels after high-speed centrifugation of brain homogenates.
3. Discussion
In the present study, we used the modified RT-QuIC method to assess prion amyloid formation and established the time course of seeding doses in the brains of mBSE-affected wild-type mice. Although the time course of seeding doses in scrapie-infected hamsters has been described [
18], the temporal relationship between seeding dose and infectious titer has not yet been determined. Our temporal correlative study revealed that seeding activity correlated more tightly with a PrPres subset than prion infectivity in this model.
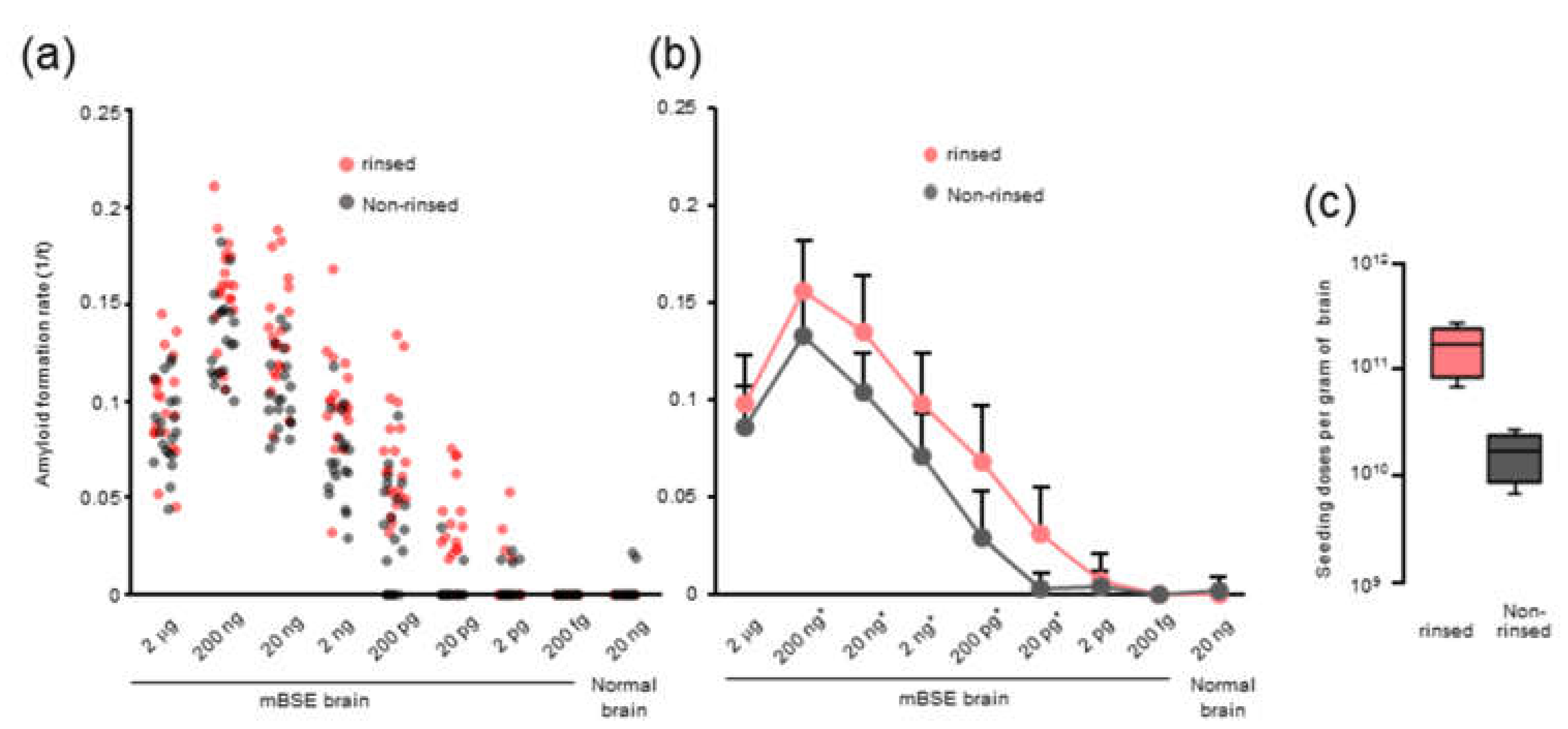
We observed that rinsing the 96-well plates with an acetone-ethanol mixture improved the RT-QuIC detection limit and the amyloid formation of mBSE and CWD prions. However, the precise role of organic solvents remains unclear. Possibly, the acetone-ethanol mixture might remove inhibitory factors from the surface of 96-well plates. Alternatively, a study has shown that the inhibitory effect of brain lipids on prion amyloid formation can be abrogated by alcohol-based extraction [
21]. Thus, it is plausible that rinsing with the acetone-ethanol mixture might change the surface properties of 96-well plates and allows them to absorb more lipids from the samples, which would inhibit prion amyloid formation in vitro.
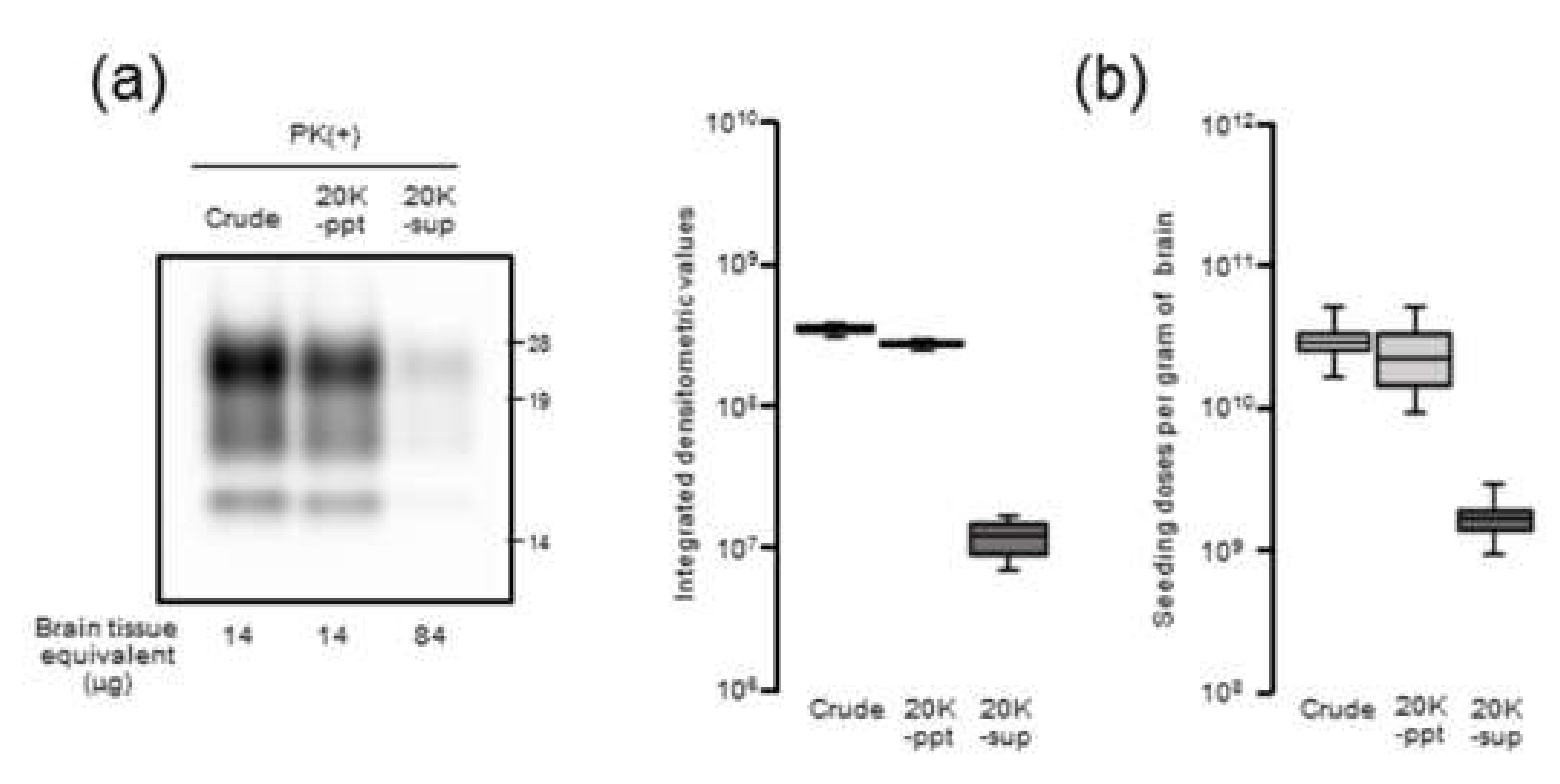
Our results demonstrated that there is a close relationship between prion seeding activity and PrPres levels, except for in late-stage disease, in an mBSE-affected wild-type mouse model. We followed prion seeding activity after mBSE prion infection using end-point dilution RT-QuIC. At the early stage, the lag phases were followed by an exponential phase of seeding dose as well as PrPres amplification whereas no lag phase in prion titer was observed. In the exponential phase (from 40 to 100 days), the calculated doubling time of 8.1 days for seeding dose was closer to that of 8.3 days for PrPres levels than to that of 12.4 days for infectious titer. In addition, by 100 days, the prion titer reached a plateau whereas the seeding dose and PrPres levels remained elevated. Furthermore, our RT-QuIC analysis of supernatants and pellets obtained by high-speed centrifugation of BHs suggested potential discrepancy between seeding activity and prion infectivity. These characteristics of temporal changes in seeding dose, infectious titer, and PrPres levels led us to conclude that RT-QuIC seeding activity correlates more closely with PrPres levels than with prion infectivity.
Nonetheless, at end-stage disease, an uncoupling of seeding doses and PrPres levels was observed. One possible explanation is that PrPSc that accumulated at late-stage disease might contribute little to the increase in seeding activities. Hughson and colleagues reported that a disinfectant neutralizes prion infectivity without eliminating seeding activity, implying that infectious PrPSc comprises only a subset of PrP particles with RT-QuIC activity [
17]. Considering our present data together with this previous finding, it is likely that PrPSc with RT-QuIC seeding activity has a substantial but not complete overlap with proteinase-resistant PrPSc rather than infectious PrPSc.
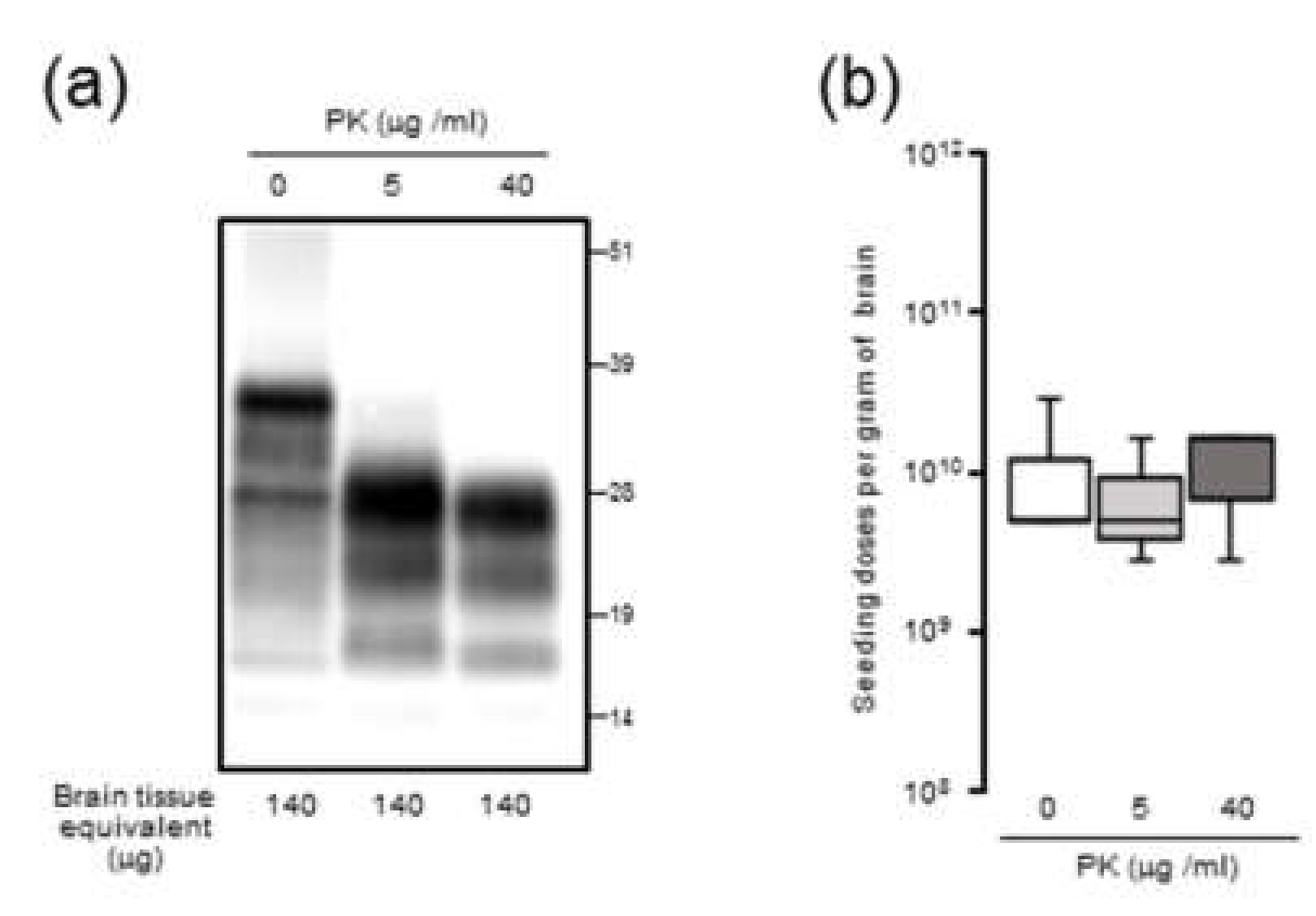
Since it is plausible that the formation of large PrPSc aggregates at late-stage disease might affect seeding activity, BH was processed by RT-QuIC with or without sonication. Contrary to our expectations, the seeding doses of the sonicated BHs were not significantly altered compared to those of the untreated BHs, suggesting that the extent of PrPSc aggregation might exert little influence on RT-QuIC seeding activity. Although the partial fragmentation of PrPSc aggregates after the sonication of BHs was confirmed by sucrose density gradient centrifugation, we cannot exclude the possibility that our sonication treatments were insufficient to collapse the PrPSc aggregate to enhance seeding activities. Alternatively, since it is known that amyloid fibril growth begins with binding of monomers to the growing ends [
23], at late-stage disease, larger aggregates of PrPSc might be mainly formed by amorphous aggregates with PK resistance which have less growing ends rather than amyloid fibrils. Including the involvement of proteinase-sensitive PrPSc and the effect of the hamster recPrP as RT-QuIC substrate, the precise mechanisms underlying an uncoupling of seeding doses and PrPres levels for PrPSc are yet to be determined.
Our present findings appear to be inconsistent with the data reported for 139A or 263K scrapie-affected Tg101LL mice, where an equivalent prion seeding dose and infectious titer but discrepant PrPres levels were observed, indicating a close correlation between prion seeding activity and infectivity [
16]. One possible reason for this discrepancy might be the differences in the mice analyzed in these studies. It is plausible that, unlike PrPres in wild-type mice, only a small population of PrPres in Tg101LL mice might be responsible for seeding activity. In other words, although substantial amounts of PrPres are present in Tg 101LL mice inoculated with 139A scrapie, most of the PrPres might not be relevant for amyloid formation. Alternatively, the PrPres that accumulated in Tg101LL mice affected by 263K possibly possess higher amyloid-formation abilities than in those affected by 139A, resulting in a similar seeding activity, albeit with low amounts of detectable PrPres. To address this discrepancy, it might be necessary to conduct temporal correlative studies on seeding dose, infectious titer, and PrPres levels in Tg101LL mice affected by 139A and 263K scrapie strains.
More detailed temporal correlative studies of various prion strains are required to elucidate the relationship among prion seeding activity, infectivity, and PrPres levels. We performed a temporal correlative study with only one prion strain using wild-type mice, and thus, we cannot exclude the possibility that another prion strain in other host species might show a different correlative relationship between seeding dose and infectious titer. Indeed, in a scrapie-affected hamster model where a continuous increase in both PrPSc levels and infectious titer to the end of disease is observed [
24], the seeding dose also increased toward the end of the incubation period with no plateau phase [
18].
To the best of our knowledge, we believe that this is the first correlative study on the kinetics of prion seeding dose, infectious titer, and PrPres levels in parallel. We demonstrated that amyloidogenic PrPSc substantially but not completely overlaps with PrPres, as compared to infectious PrPSc, in this model. Further studies will be required to clarify the relationship among different types of PrPSc based on various prion strains in another host species.
4. Materials and Methods
4.1. Mouse-Adapted Classical BSE Brain Homogenates
Mouse-adapted classical BSE prions were propagated first in RIII mice and then sequentially in 3-week-old specific pathogen-free female CD-1 mice 14 times. In our previous study [
19], 20 µL of mBSE brain homogenates (BHs), which contained 10
5.9 LD
50 of mBSE prion, was intracerebrally inoculated into CD-1 mice. Three recipient mice were sacrificed at designated time points (5, 30, 40, 60, 70, 90, 100, 125, and 140 days postinfection), and the brains were collected. The brains at each time point were homogenized in phosphate buffered saline (PBS) and stored at −80 °C until further use. In the current study, we used these BHs for the kinetic analysis of seeding dose, infectious titer, and PrPres levels. The data regarding infectious titers which were estimated by incubation-time assay using four to six CD-1 mice were obtained from our previous report [
19] with the permission of its authors including the corresponding author.
4.2. Recombinant Prion Protein
Recombinant Syrian hamster prion protein (recPrP; amino acids 90–231) constructs were expressed in
Escherichia coli strain BL21-CodonPlus (DE3)-RIPL (Stratagene, La Jolla, CA, USA) and purified as previously described with slight modifications [
25]. The solubilized inclusion body containing recPrP was applied to an Ni-NTA Superflow resin (Qiagen, Venlo, Netherland) and loaded onto a XK16-20 column (GE Healthcare, Buckinghamshire, England) connected to an AKTA
FPLC system. The elution peak was pooled and dialyzed in dialysis buffer containing 100 mM NaCl in 10 mM NaPO
4, pH 5.5, overnight at 4 °C. Concentrations of recPrP were estimated by its absorbance at 280 nm. Aliquots of proteins were stored at −80 °C until further use. After thawing, aliquots of proteins were centrifuged at 20,000×
g for 30 min at 4 °C and the soluble recPrP was used.
4.3. Rinsing of 96-Well Plate
Optical-bottom black 96-well plates (#265301, Thermo Fisher Scientific, Waltham, MA, USA) were rinsed with an acetone-ethanol 1/9 (v/v) mixture. Each well was filled with 320 μL of acetone–ethanol mixture and aspirated completely. The rinsed 96-well plate was dried in a safety cabinet for at least 15 min before use.
4.4. Real-Time QUIC
Real-time QuIC (RT-QuIC) assays were performed as reported previously with slight modifications [
25]. Briefly, 98 µL of the RT-QuIC reaction substrate mixture containing 0.1 mg/mL Syrian hamster recPrP (aa 90–231), 320 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), and 10 µM thioflavin T (Th T) in 100 mM phosphate (pH 7.4) was loaded into an optical-bottom black 96-well plate. The reaction substrate mixtures were seeded with 2 μL of BH 10-fold serially diluted with 0.075% sodium dodecyl sulfate (SDS) in PBS. The seeded brain tissue equivalents from an mBSE-inoculated mouse were diluted to 2 µg to 200 fg (10
−2 to 10
−9 dilutions of 10% weight per volume of the BH), and those from a normal mouse were diluted to 20 ng. Each brain homogenate was assayed in quadruplicate wells at least three times using independent reaction substrate mixtures. The plates were sealed and incubated in a BMG FLUOstar Omega plate reader (BMG LABTECH, Ortenberg, Germany) at 37 °C with 1 min of shaking (1100 r.p.m., double-orbital) and 1 min of rest for 62 h. ThT fluorescent readings (450-nm excitation and 480-nm emission, bottom-read, 20 flashes per well, gain of 1500) were recorded every 15 min. The threshold for positivity of each BH was calculated as four-fold greater than the average of the initial fluorescence of all samples. The lag phase (h) was defined as the reaction time required to exceed the threshold. Amyloid formation rate (AFR; 1/h), which is the inverse of lag phase, was used as an indicator of amyloid seeding activity. RT-QuIC conditions such as the concentration of SDS, reaction temperature, and shaking speed were optimized to minimize the false positive rate without a loss of sensitivity.
4.5. Calculation of Seeding Dose
Seeding doses of 50% (SD50) were determined using end-point dilution RT-QuIC. The log-dose of the dilution yielding 50% positive replicates per gram wet weight equivalent (indicated as log SD50 per gram) was calculated using the Spearman–Kärber method, with 10-fold dilutions of each of the positive and negative seeding activity ratios.
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
The BHs were processed for ELISA using the Seprion ligand (Microsens Biotechnologies, London, UK) to semi-quantify the levels of PrPres [
22]. The Seprion ligand is a group of polymeric compounds with specificity for PrPSc and can be immobilized directly on the wells of a microplate as a substitute for capturing antibodies. Briefly, the BHs were lysed in capture buffer (1% N-lauroyl sarcosine, 1% triton X-100, 1% bovine serum albumin, and 0.5% trypsin in 50 mM tris–HCl, pH 8.3) and applied to a 96-well microplate (FluorNunc Black, Thermo Fisher Scientific) coated with the Seprion ligand. After incubation for 2 h, PrPres captured on the well was denatured with 4 M guanidine thiocyanate in 20% polyethylene glycol 8000 (Fisher Scientific, Hampton, NH, USA) and detected using anti-PrP mAb T2 conjugated with horse radish peroxidase (HRP) [
26]. The plates were developed using a chemiluminescence solution (Supersignal West Dura Extended Duration Substrate; Pierce Biotechnology, Rockford, IL, USA), and the signal was determined using an ARVO SX 1420 multilabel counter (Wallac, Turku, Finland). Four-fold serial dilutions of the BH from a terminally ill mouse with mBSE were subjected to ELISA to confirm its linear dynamic range (
Figure S1).
4.7. Western Blot Analysis
The 10% (w/v) BHs of mBSE-affected mice were centrifuged at 20,000× g for 10 min at 4 °C. After collecting the supernatant (20K-sup), the pellet was reconstituted with the same volume of PBS with brief sonication (20K-ppt). Each sample was diluted 20-fold with lysis buffer containing 10 mM tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5% triton X-100, and 0.5% sodium deoxycholate. For PrPres detection, each sample was treated with 40 µg proteinase K (PK) for 30 min at 37 °C. For proteinase-sensitive PrPSc analyses, the 10% (w/v) BHs of mBSE-affected mice were pretreated with 0.5% triton X-100 (final concentration) and digested with 0, 5, and 40 µg/mL of PK for 30 min at 37 °C. PK digestion was terminated by adding Pefabloc (Roche Diagnostics, Mannheim, Germany) at a final concentration of 4 mM. All samples were suspended in three times their volumes of 4 × lithium dodecyl sulfate sample loading buffer (Thermo Fisher Scientific) and boiled. The samples were electrophoresed on 12% NOVEX pre-cast gels (Thermo Fisher Scientific) and electrotransferred onto Durapore (Merck Millipore, Burlington, MA, USA) polyvinylidene fluoride membranes. The blots were probed with HRP-conjugated PrP-specific antibody T2. Chemilumi One Super (Nakarai Chemical, Kyoto, Japan) was used for immunodetection. The blots were visualized with a Fluorchem (Alpha Innotech, San Leandro, CA, USA) and analyzed using image reader software (AlphaEaseFC; Alpha Innotech) according to the manufacturer’s instructions.
4.8. Immunohistochemistry
Formalin-fixed left hemispheres were embedded in paraffin wax following immersion in 98% formic acid to decrease infectivity. After epitope retrieval, PrPSc immunocytochemistry was performed using the anti-PrP monoclonal antibody SAF84. As the secondary antibody, the anti-mouse universal immunoperoxidase polymer (Nichirei Histofine Simple Stain MAX-PO (M); Nichirei, Tokyo, Japan) was used. PrPSc was visualized with 3,3′-diaminobenzedine tetrachloride as the chromogen.
4.9. Statistical Analysis
Values in the text are expressed as means ± SD. To determine statistical significance, a Student’s t test for paired samples was used. Statistical analysis of the data was performed using Excel and R software programs.