Abstract
Anthropogenic pressures in the Amazon Basin are reshaping human–animal–environment interactions and increasing zoonotic disease risk. Within this One Health context, domestic dogs and cats are underrecognized contributors to pathogen circulation at the human–wildlife interface. We conducted a PRISMA-compliant systematic review of zoonotic pathogens reported in companion animals across Amazonian territories in nine countries, including literature published between 2000 and 2025 in four languages. Zoonotic pathogens showed a heterogeneous yet widespread distribution, with parasitic infections, particularly Leishmania spp., Toxoplasma gondii, and vector-borne protozoa, being the most frequently reported. A pronounced geographic bias was evident, with studies concentrated in Brazil and selected areas of the western Amazon, while large portions of the Basin remain understudied. Methodological limitations included reliance on cross-sectional designs and heterogeneous diagnostic approaches, often based solely on serology. These findings highlight the need to strengthen One Health-oriented governance frameworks that integrate animal health surveillance into environmental and public health policies. Priority actions include expanding surveillance to underrepresented regions, harmonizing diagnostic protocols, investing in regional laboratory capacity, and promoting community-based monitoring. Strengthened cross-sectoral and transboundary coordination is essential to reduce zoonotic risk and support evidence-based disease prevention in Amazonian ecosystems.
1. Introduction
The Amazon political, biogeographical and hydrographic region covers almost 8.5 million square kilometers [1], encompassing territories of Guyana, French Guiana, Ecuador, Bolivia, Colombia, Peru, Suriname, and Venezuela, with most of its area located within Brazil [2]. This biome harbors unparalleled biodiversity: for vertebrates alone, it holds the greatest species richness on Earth [3]. Preservation of this ecosystem has profound implications for human well-being and economic stability, owing to its ability to meet human needs at both local and global scales [4]. These so-called ecosystem services [5] include evident benefits, such as direct resource extraction, as well as less tangible functions such as climate regulation, carbon and nitrogen cycling (critical for nutrient production), maintenance of an intricate hydrological network, acting as a natural detoxicant essential for planetary homeostasis [6], and trophic balance, which serves as a mediator and barrier against disease outbreaks [5]. The Amazon is among the regions with the greatest potential to harbor zoonotic agents capable of generating outbreaks [7,8]. This circumstance means that, if the ecological balance is disrupted as a result of anthropogenic stressors linked to resource overexploitation—particularly due to habitat modification through deforestation—enzootic agents begin to affect a broader range of species, vectors proliferate, and their susceptibility to carrying pathogens increases [9,10,11,12].
Currently, humans occupy approximately 50% of the planet’s terrestrial surface; of this, an estimated 37% of natural areas remain under limited human influence, largely due to the presence of Indigenous peoples [13]. The Amazon is home to 47.4 million inhabitants, of whom 2.2 million are recognized as Indigenous peoples [1]. Indigenous communities of the Amazon, through their cultural traditions and subsistence practices, maintain close interaction with their environment, drawing directly on natural resources, ranging from basic needs such as food and shelter to specialized resources derived from empirical, inherited knowledge, such as medicinal practices [6,12,13]. Although Indigenous peoples worldwide are increasingly acknowledged in contemporary policy and discourse, persistent inequalities remain. In some regions, these communities face a dual burden of infectious and chronic diseases, exacerbated by structural barriers including poverty, geographic isolation, and systemic discrimination [14].
The impacts of anthropogenic transformations on the natural environment are profound, driving substantial biodiversity loss, particularly in intact territories [13]. Broadly, human activity includes three major negative impacts: (1) loss and alteration of natural habitats and their biodiversity; (2) overexploitation of resources such as through deforestation, mining, land-use change, and territorial occupation [15]; and (3) introduction of invasive species into native ecosystems [5]. Additional effects often cited include the proliferation or imbalance of pathogen populations, accumulation of environmental toxins (pollution), and climate change [5].
Although multiple studies assess the immune status of human populations, there is a widespread lack of information regarding the condition of the pets they live with. These animals can act as sources of direct infection, transmitting or extending the infective phase of the agent, or indirect infection, by serving as multipliers of the pathogen or attractants for vectors, thereby increasing the risk of transmission [16,17]. They also interact with wildlife, generating a flow of pathogen transmission or of strains typical of the wild environment [18].
The introduction of exotic and/or invasive species exerts particularly severe effects on ecosystems. An invasive alien species is one that becomes established in an ecosystem (outside its original range) and subsequently acts as an agent of change and a threat to native biological diversity [19]. In this context, domestic dogs (Canis lupus familiaris) and cats (Felis catus) pose threats in Amazonian ecosystems. Although now common, dogs only arrived in most parts of the Amazon relatively recently [20]. Historical accounts suggest that some Indigenous groups once kept domesticated foxes to assist in hunting large rodents. These animals disappeared following European contact, as larger introduced canids quickly replaced native species, aided by their greater hunting efficiency and the spread of diseases [20].
In the Amazon Basin, close and frequent contact between humans and their domestic dogs and cats constitutes an important factor in zoonotic risk, particularly in communities with limited health education and infrastructure. A recent study conducted in Rondônia reported that more than 74% of household dogs were infected with at least one endoparasite of zoonotic importance, including Ancylostoma spp. and Toxocara canis, while nearly half were infested with ectoparasites such as Rhipicephalus sanguineus and Ctenocephalides felis. These findings highlight both the high exposure of companion animals and the substantial potential for pathogen transmission to humans in rural Amazonian communities [21]. Consistent with these observations, the broader scientific literature recognizes dogs and cats as reservoirs and source of multiple zoonotic agents, including parasites acquired through predation on wildlife, thereby sustaining infection cycles among wildlife, domestic animals, and humans with important ecological and public health implications [22].
Companion animals such as dogs and cats in the Amazon are often not subject to consistent control or owner supervision. Their management is commonly classified into two categories: (1) free-roaming dogs and cats, which are owned and associated with a household but allowed to wander freely; and (2) stray dogs and cats, which lack ownership and live entirely in public spaces. While risk assessments traditionally emphasize pathogen transmission from animals to humans, reverse transmission from humans to animals (anthroponosis) is also possible and may have significant consequences for the health of domestic and wild species [18]. Accordingly, the introduction and maintenance of dogs and cats as companion animals in the Amazonian biome may pose risks not only of zoonotic disease transmission to humans but also to native carnivore species through pathogen spillover and competition [19].
This complex network of transmission routes and enzootic interactions has the potential to drive wildlife population declines and human disease outbreaks, particularly in fragmented landscapes where human activity increases contact between domestic and wild animals [20]. Such interactions can negatively affect native fauna and disrupt ecosystem balance [21,22]. These dynamics are especially critical in the Amazon, where exceptional biodiversity combined with expanding human–wildlife interfaces amplifies the risk of pathogen emergence and spillover. Consequently, integrating One Health approaches that jointly consider human, animal, and environmental health is essential for understanding and mitigating zoonotic risks associated with pet ownership in this region [23]. In this context, the present study compiles and synthesizes current evidence on pet-mediated zoonotic pathogens in the Amazon, emphasizing their potential role as bridges for pathogen transmission to the human populations with which they coexist [21,22,24,25,26,27,28].
2. Materials and Methods
2.1. Literature Search Protocol
In this study, we conducted a systematic search of zoonoses related to dog and cat ownership in the Amazon over the last quarter-century, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol (Figure 1) following PRISMA 2020 guidelines. The literature search was carried out between September and October 2025 through queries in the PubMed, Google Scholar, and Scopus databases.
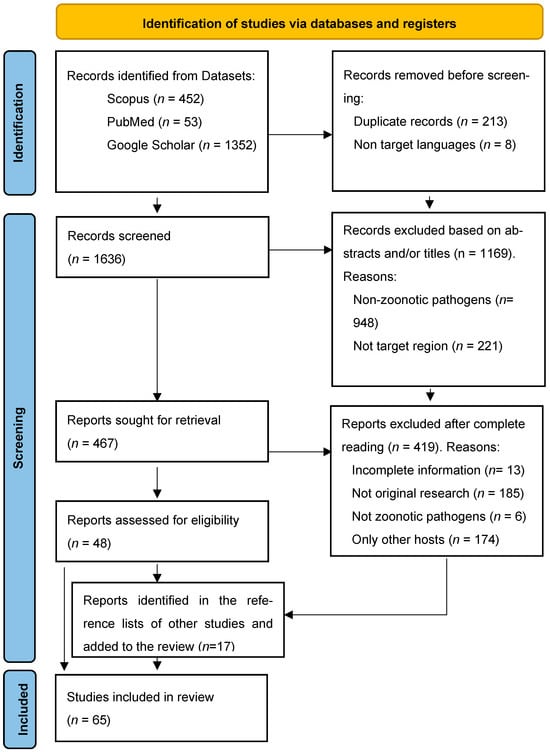
Figure 1.
PRISMA Diagram, applying the inclusion and exclusion criteria to the results found in the selected databases. Notably, 17 results were added manually after reviewing other selected sources.
We systematically searched for relevant literature published in cat and dog zoonoses in this specific geographical framework. Search items were “zoonosis”, “zoonotic”, “emerging infectious disease”, “EID”, “neglected tropical disease”, “NTD”, “Amazon” and derivates (Amazonic, Amazonas, Amazonia, Amazonian), the list of the 9 countries with territories in the Amazon and finally a mention of “dog” or “canine”, “cat” or “feline”. The complete search string, including the corresponding Boolean operators, can be consulted in the Appendix A Section.
2.1.1. Study Eligibility Criteria
Inclusion Criteria
Eligible studies were required to address one or more zoonotic diseases and to fall within the defined geographical scope, which, according to RAISG criteria [1], corresponds to the maximum area of overlap between the Amazon biome, the hydrographic basin, and administrative boundaries (Figure 2A,B). Studies published from 2000 to October 2025, the time of manuscript preparation, and written in English, Spanish, Portuguese, or French were considered eligible. Expanding the search strategy beyond English to include other official languages of the region enabled the identification of additional relevant data and enhanced the comprehensiveness of this review.
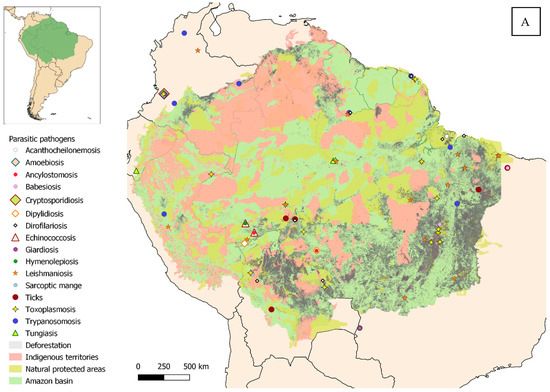
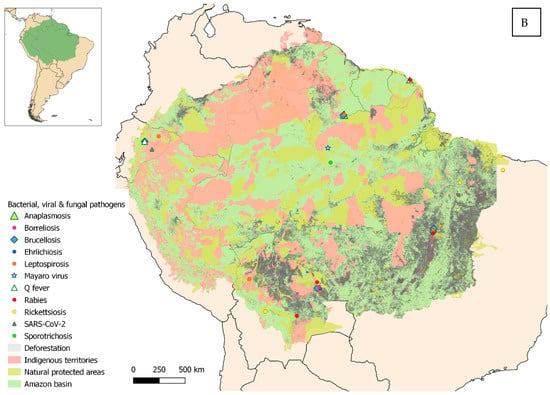
Figure 2.
Geographical distribution of the studies reviewed, within the Amazon and surrounding areas. (A) Parasitic diseases. (B) Bacterial, viral and fungal diseases.
The primary sources consisted of peer-reviewed journal articles; however, additional materials, including doctoral theses, master dissertations, and graduate monographs, were also included when they reported original data. Although such sources are often excluded from systematic reviews, their inclusion enabled the incorporation of relevant data that may not have been fully disseminated through peer-reviewed journals, thereby contributing to a more comprehensive assessment of pathogen prevalence. The methodological quality of non-peer-reviewed studies was evaluated using the same eligibility criteria applied to peer-reviewed publications, and only records reporting original data, clearly defined study populations, and extractable prevalence estimates were included. Studies with insufficient methodological detail were excluded. With exceptions of peer-reviewed case reports, studies were required to provide a clear estimate of prevalence in dogs and/or cats, even when other animal species were also investigated [1].
Exclusion Criteria
Records were excluded if they met any of the following criteria: duplicate entries retrieved from multiple databases (Duplicate records); articles not published in the predefined target languages (Non-target languages); studies conducted outside the predefined geographic scope (Amazon basin and peripheral area) (Non-target region); studies investigating host species other than dogs and cats (Non-target species); articles not addressing pathogens transmissible among animals and humans (Non-zoonotic pathogens); studies lacking sufficient methodological detail or extractable data to allow critical appraisal or to provide a clear estimate of pathogen prevalence (Incomplete information); or publications that did not report original primary research data (Non-original research).
Artificial intelligence-assisted tools were employed to support the identification of some exclusion criteria during screening (language); however, all records were subsequently subjected to manual verification to ensure accuracy and to identify relevant exclusions not detected automatically.
Data Extraction
All authors independently extracted data and discrepancies were resolved by discussion. Quantitative and qualitative data extraction from the included studies was presented as a table in an Excel spreadsheet. The extracted components encompassed the name of the pathogen/disease, country and region, prevalence, detection method, species tested (dog or cat), the primary author along with the year of publication and observations related with the relevance of the disease.
2.2. Review Registration
This review was not registered in any database of systematic review protocols.
2.3. Risk of Bias and Methodological Limitations
No formal risk of bias tool was applied due to heterogeneity of study designs; limitations are acknowledged in the Discussion.
3. Results
The literature search yielded a large volume of results but reading the titles and/or the abstracts allowed us to eliminate many of them in an initial phase, mainly because they were conducted outside our geographical scope, not addressing pathogens transmissible between animals and humans and/or focused on species other than those of interest. A significant number of relevant studies (19) were included manually, often as a result of reading the content of articles that referred to these investigations.
The analysis comprised 65 publications, predominantly peer-reviewed journal articles, including 52 original research studies and 7 case reports, which together accounted for 90.7% (59/65) of the included literature. In addition, six non-peer-reviewed academic works were incorporated, consisting of three doctoral dissertations, one master’s thesis, and two postgraduate monographs (9.3%; 6/65). The majority of the publications were written in English (n = 57), followed by Portuguese (n = 7) and Spanish (n = 1).
Most of the selected resources come from Brazil (41/65; 63.1%), followed by French Guiana (7/65; 10.7%) and Peru (6/65; 9.2%). Notably, two countries in the region (Venezuela and Suriname) are absent, contributing no research to this review. Many of the studies evaluated multiple agents simultaneously, with fungi being the least represented (only one study, 0.86%), followed by mentions of viruses (6/116; 5.1%), bacteria (25/116; 21.5%), and a clear majority of parasites (84/116; 72.4%).
Table 1, Table 2 and Table 3 summarize zoonotic viral, fungal, bacterial, and parasitic pathogens detected in domestic dogs and cats across Amazonian territories, highlighting prevalence, host species, geographic distribution, and diagnostic approaches.

Table 1.
Zoonotic viral and fungal pathogens.

Table 2.
Zoonotic bacterial pathogens.

Table 3.
Zoonotic parasitic pathogens.
Viral and fungal pathogens: Mayaro virus showed high seroprevalence in Brazil, with 60.5% of dogs and 46.1% of cats testing positive north of Manaus (ELISA-ICC). Rabies virus prevalence varied regionally: absent in French Guiana, moderate in Brazil (8–11%), and high in Bolivia (50–56%). SARS-CoV-2 was detected in Ecuadorian Amazon dogs (66.6%, RT-qPCR), indicating active circulation. Sporothrix spp. infections were endemic in Brazil, with 2798 confirmed feline cases, primarily in Amazonas State, demonstrating significant public health relevance.
Bacterial pathogens: Tick-borne bacteria such as Anaplasma spp. and Borrelia burgdorferi were largely absent, while sporadic Brucella spp. exposure was observed (2.6–10%). Coxiella burnetii seroprevalence reached 12.3% in French Guiana dogs. Ehrlichia spp. ranged from 10–14.6% in Guyana and Brazil to 86% in Bolivia. Leptospira spp. were widespread, particularly in Ecuador (75%), with species-specific identification of L. borgpetersenii serovar Hardjo and L. interrogans. High exposure to Rickettsia spp. was observed across Brazil, Bolivia, and Peru, though molecular confirmation was limited.
Parasitic pathogens. Arthropod ectoparasites (Amblyomma spp., Rhipicephalus sanguineus) were common (5–22% infestation). Helminths (Ancylostoma, Toxocara, Trichuris vulpis) were highly prevalent; zoonotic cestodes (Echinococcus, Dipylidium) were detected at lower prevalence. Enteric protozoa (Cryptosporidium, Giardia) were widespread in Colombia. Vector-borne protozoa, including Babesia canis, Dirofilaria immitis, Trypanosoma cruzi, and T. evansi, showed variable but sometimes very high prevalence, indicating intense enzootic circulation. Leishmania spp. were among the most prevalent pathogens, with multiple species across Brazil, Colombia, Peru, Guyana, and French Guiana, showing seroprevalence and molecular detection rates often exceeding 40–70% in dogs; cats were confirmed hosts in several areas. Toxoplasma gondii exhibited consistently high seroprevalence (60–80%) in both dogs and cats, reflecting sustained environmental contamination and transmission.
4. Discussion
The absence of a formal risk of bias assessment represents a limitation of this review. However, the marked heterogeneity of study designs, data sources, host species, diagnostic approaches, and geographic contexts precluded the consistent application of a single standardized risk of bias tool. The literature included in this review encompasses a wide range of evidence types, including peer-reviewed articles, case reports, and theses, which differ substantially in methodological structure and reporting standards. Applying a uniform bias assessment framework under these conditions could have resulted in misleading or non-comparable evaluations. Nevertheless, this limitation has been taken into account in the interpretation of the results, and findings are discussed with appropriate caution, emphasizing patterns and knowledge gaps rather than quantitative synthesis or causal inference.
The georeferencing of studies on a regional map graphically shows how many of the studies evaluated multiple agents simultaneously, with a clear majority of parasites. Trypanosomiasis is one of the most frequently reported diseases in our literature review. Since its discovery in 1909, it has been widely documented from the southern United States to southern Argentina [88]. It constitutes an example of an enzootic disease that, originally limited to wild animals in uninhabited areas with little human impact, has become a zoonosis following the establishment of humans and domestic animals and the associated environmental transformations [89]. Almost two hundred species of mammalian hosts, both wild and domestic, play important roles in the epidemiological cycle as reservoirs [89], with particular emphasis on opossums (Didelphis marsupialis, D. albiventris, and others), considered key hosts in numerous studies [88,90,91]. Cats and especially dogs are identified as the most relevant domestic reservoirs [92], serving as a blood source for the vectors (hematophagous triatomine bugs—Rhodnius spp., Panstrongylus spp., and Triatoma spp.) [16]. The presence of dogs in the household makes the habitat more attractive to vectors, which translates into a higher infection risk for the owners [17].
Together with trypanosomiasis, leishmaniosis is the most frequently reported parasitic disease in the reviewed articles, with references to a wide variety of species affecting humans and animals and showing different degrees of pathogenicity (L. (chagasi) infantum, L. (V.) braziliensis, L. guyanensis, L. amazonensis, L. panamensis, L. pifanoi) [50,59,60,71,93]. Likewise, the associated epidemiological cycles, vectors, and reservoirs can also differ—for example, the relevance of the two-toed sloth Choloepus didactylus or the opossum Didelphis marsupialis in maintaining L. guyanensis, vectored by Lutzomyia umbratilis, whereas for L. amazonensis the main vector is Lutzomyia flaviscutellata and the reservoir is the rodent Proechimys cuvieri [94]. In deforested areas, however, it is the dog that emerges as the preferred food source for phlebotomine sandflies, becoming the main host for the parasite [95]. In addition to autochthonous transmission, imported cases are also reported, associated with the movement of domestic dogs, as in the case of L. infantum in French Guiana, introduced from endemic areas in Europe [74]. Although several authors suggest a progressive increase in the presence of domestic cats in the region, the studies testing this disease remain scarce [72]. In tropical areas, the great diversity of species and strains of the genus Leptospira creates an extensive network of mammals involved in its maintenance and transmission [96]. Besides the well-known role of rodents (ranging from purely wild species to synanthropic ones) [97,98], other mammals have become increasingly relevant due to their close contact with humans. This is evidenced by the prevalence found in swine, cattle or equine livestock [96,99,100]. Regarding pets, given their close contact with human households, they deserve special attention. Dogs showed variable infection rates with this bacterium, with prevalence reaching up to 75% [41]. As for cats, although the studies consulted reported absence of infection or low prevalence [37,42], their tendency to prey on rodents [101] means they should be closely monitored, even more so considering the growing presence of this species, increasingly common in human settlements in the region.
Toxoplasmosis is a disease obligatorily linked to the presence of felids, as only they can act as the final hosts of the protozoan Toxoplasma gondii [102]. The great diversity of wild species capable of fulfilling this role in our study area apparently relegates domestic cats to a secondary position compared to other geographical regions [103,104]. Mentions of domestic cat populations vary across the studies consulted, with cats generally considered scarce, and the primary sources of infection identified as the consumption of water contaminated with oocysts or tissue cysts in undercooked meat from intermediate hosts [18,104,105]. However, when domestic cats are abundant, which may occur locally, other infection routes have been reported, such as ingestion or even inhalation of oocysts present in the soil of inhabited areas [106]. The presence of highly virulent strains at the domestic–wildlife interface appears to be responsible for outbreaks affecting multiple people, including some fatal cases [18]. Apart from toxoplasmosis, other intestinal protozoa are scarcely reported in the studies reviewed. There is only a single mention of Cryptosporidium, Giardia, and Endolimax nana on the periphery of the region, already outside the study area [49].
Before our selected timeframe, some studies of canine dirofilariasis caused by Dirofilaria immitis were conducted in the Brazilian [107] and Colombian Amazon [108]. Dogs are considered the main source of human filariasis infection, of particular concern among native communities such as the Brazilian Yanomami [109]. Several studies have detected dirofilariosis in dogs [36,47,54,55,56], but only one in cats [42]. Regarding other related species, only one paper mentions Acanthocheilonema reconditum [47].
Ectoparasites are represented in this review by mites (genus Sarcoptes), fleas (genus Tunga), and ticks. Although dogs are the species most often reported as the source of zoonotic infection in humans by the mite Sarcoptes scabiei, the causative agent of sarcoptic mange [110], we found only one study referencing this disease [42], suggesting it is likely underreported. It is also a significant disease for wildlife, capable of causing episodes of high morbidity and mortality in susceptible and naïve populations [111,112,113], and has been diagnosed in wild canids from the region, such as the maned wolf (Chrysocyon brachyurus) [114,115,116] and crab-eating foxes (Cerdocyon thous) [117]. Tungiasis has been widely reported in countries of the region [118,119]; however, it is scarcely documented in our study area [86,87]. Regarding ticks, they are represented by Rhipicephalus sanguineus and, particularly, by species of the genus Amblyomma (A. ovale, A. scalpturatum, A. oblongoguttatum, A. latepunctatum, A. coelebs, A. naponense) [46]. These arachnids attract considerable interest among researchers, either due to their presence alone or because of the tick-borne diseases associated with them, as conditions such as ehrlichiosis [37,40], rickettsiosis [40,43,45], borreliosis [32], and babesiosis [31,40] are reported. Although one of the studies [36] investigates Anaplasma phagocytophilum as A. platys, the results are negative. No studies specifically addressing dipteran biting rates were identified. In contrast to other ectoparasites that remain on the host and can be directly collected and quantified, this methodological approach is not applicable to hematophagous flying insects. Given their recognized potential as vectors of multiple pathogens, targeted studies are required to quantify the relative concrete relevance of different dipteran species and their relevance to pathogen transmission dynamics.
Overall, fungi were the least frequently reported pathogens, followed by viruses and bacteria, which is a reasonable outcome in cases where dogs and cats are not common sources of human infection. This is the case of Q fever, caused by Coxiella burnetii, for which, although outbreaks originating from pets have been reported [120,121], they are much more frequently associated with contact with ungulates [122]. A similar situation occurs with brucellosis, in which Brucella canis, the species most associated with dogs (and which is the agent selected in the present review), is considered much less pathogenic than other members of the genus such as B. ovis, B. melitensis, or B. abortus [123]. However, diagnostic limitations may underestimate its true relevance [124,125].
Contrary to what occurs with trypanosomosis, it has been statistically demonstrated that dog ownership constitutes a protective factor against arboviroses such as Mayaro virus [29,126]. Studies in rural and urban communities of the Brazilian Amazon have found canine prevalence reaching 60.5% [29]. However, cat ownership does not reduce transmission to humans [29] and may even act as a higher transmission risk factor compared to other animals such as poultry or ungulates [126].
Lyssavirus (Rabies virus) is the most frequently reported viral agent in the reviewed studies. Its detection occurs both as part of preventive campaigns [33] and in response to human outbreak investigations, where suspected pets involved in aggressive incidents are often euthanized and analyzed [30]. Tracing the origin of outbreaks can be challenging, largely due to the presence of viral reservoirs such as hematophagous bats inhabiting the domestic–wildlife interface [30,31]. Rabies represents one of the zoonoses in which the greatest preventive efforts are invested, including pre-exposure prophylaxis (PEP) campaigns for at-risk personnel, widespread vaccination of the canine population, and surveillance of wild reservoirs.
The recent SARS-CoV-2 pandemic: despite the interest generated and the documented vulnerability of Indigenous populations to infection [127,128], only one article reports contagion in pets from isolated communities [34]. The COVID-19 pandemic exposed the fragility of health systems in Amazonian countries and the particular vulnerability of remote populations, especially Indigenous communities [129,130].
Sporotrichosis is considered an emerging disease throughout South America, with numerous studies reporting it [131]. However, we found only one investigation in Manaus city [35], which, with a large clinical sample size, highlights the need for further research given its ease of transmission to humans.
The heterogeneous yet widespread distribution of zoonotic pathogens in domestic animals across Amazonian territories highlights critical shortcomings in environmental governance that directly influence pathogen emergence, persistence, and spillover risk. Strengthening governance frameworks should therefore prioritize a One Health-oriented approach that explicitly integrates animal health surveillance within environmental management and public health policies [132,133]. Given the predominance and high prevalence of parasitic zoonoses, particularly vector-borne and environmentally mediated pathogens, governance strategies should emphasize sustained ecosystem monitoring, vector control, and the mitigation of key environmental drivers, including deforestation, land-use change, and unplanned urbanization, which have been repeatedly linked to altered transmission dynamics [134].
A sampling bias cannot be ruled out either. Peripheral areas and population centers have infrastructures and communication routes that facilitate both the spread of pathogens [135,136] and the work of research teams. However, conducting studies in remote or hard-to-reach areas is much more challenging, where logistical difficulties and cultural differences add further obstacles [137,138]. This may likely lead to undersampling in communities that, although isolated, also keep domestic animals [34]. Most of the studies coincide with areas affected by deforestation (especially in southern and eastern Brazil and northern Bolivia) (see Supplementary Materials Tables S1–S3). Habitat fragmentation and the land-use changes associated with it are considered a major environmental stressor linked to an increased risk of pathogen transmission at the domestic–wildlife interface [9,10,11,12]. Large urban centers represent another point of interest, especially those located in the Amazonian interior, such as Manaus or Iquitos. In these areas, the spread of pathogens is favored by high population density, poor waste management, and the proliferation of vectors [139]. Finally, a minority of results deviate from this trend, occurring in remote areas. Those human communities, although isolated, keep domestic animals that are closely connected to the environment and engage in activities that increase infection risk, such as hunting [43].
The pronounced geographic imbalance in available data further underscores the need to expand surveillance coverage to underrepresented and remote regions of the Amazon Basin. Environmental governance mechanisms should support decentralized and locally implemented monitoring programs, reducing reliance on sporadic, research-driven sampling and enabling continuous data generation. Strengthening institutional coordination among environmental, veterinary, and public health authorities at local, national, and transboundary levels is essential to ensure standardized data collection, interoperability of surveillance systems, and effective information sharing across Amazonian countries [140].
Given the frequent reliance on serological diagnostics and the high seroprevalence reported for multiple pathogens, governance frameworks should promote harmonization of diagnostic protocols and the routine integration of molecular tools to improve detection of active infections and transmission potential. Investment in regional laboratory capacity, workforce training, and long-term surveillance infrastructure is critical to support timely, evidence-based decision-making and outbreak preparedness [134].
Within an integrated strategy, the implementation of legal frameworks that ensure the sovereignty of local and Indigenous communities over their lands, along with the establishment and conservation of strictly protected areas, emerge as key mechanisms to curb deforestation and biodiversity loss [13,141,142], and consequently reduce the risk of emergence of zoonotic pathogens with the potential to cause future epidemic outbreaks. A redistribution of resources should also be promoted, aimed at monitoring neglected tropical pathogens both in areas under human pressure and in isolated communities located in remote regions. Finally, these findings point to an urgent need for inclusive, community-based governance models. The active engagement of Indigenous and rural communities—who often coexist closely with domestic animals and wildlife—is essential for effective environmental stewardship and early detection of zoonotic threats. Policies should support participatory surveillance, community-led reporting systems, and culturally appropriate education initiatives that align biodiversity conservation with health protection. Strengthening environmental governance through inclusive, multisectoral, and regionally coordinated One Health strategies is fundamental to mitigating zoonotic disease risk and enhancing resilience at the human–animal–environment interface in Amazonian ecosystems [132,133,134,143].
5. Conclusions
This synthesis of the available evidence indicates that zoonotic pathogens affecting domestic dogs and cats are widely distributed across Amazonian territories, with parasitic infections representing the most frequently reported and epidemiologically dominant group. In contrast, bacterial, viral, and fungal pathogens were reported less frequently and showed more heterogeneous and spatially restricted detection patterns.
The compiled literature reveals a pronounced geographic imbalance in research effort. Most studies were concentrated in Brazil and selected areas of Bolivia, Peru, and Colombia, while extensive portions of the Amazon Basin, particularly Guyana, Suriname, Venezuela, and remote transboundary regions, remain markedly understudied. Research was further clustered in peripheral Amazonian zones and urban centers, areas frequently exposed to anthropogenic stressors such as deforestation and land-use change. This uneven spatial coverage constrains the accurate characterization of pathogen circulation and may mask important zoonotic transmission hotspots.
Methodological limitations further restrict interpretation of the available data. The predominance of cross-sectional designs and heterogeneous diagnostic approaches, coupled with frequent reliance on serological evidence without molecular confirmation, limits the capacity to distinguish prior exposure from active infection and ongoing transmission. These gaps highlight the need for harmonized surveillance protocols integrating complementary diagnostic tools.
Collectively, these findings underscore the urgent need to strengthen inclusive, community-engaged One Health surveillance frameworks that prioritize zoonoses, expand coverage to understudied Amazonian regions, and reinforce monitoring of domestic animals at the human–animal–environment interface. Enhancing cross-sectoral coordination among environmental, veterinary, and public health institutions will be essential to improve early detection, reduce zoonotic risk, and support evidence-based disease prevention strategies across Amazonian ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens15010077/s1; Table S1: Zoonotic viral and fungal pathogens (expanded version); Table S2: Zoonotic bacterial pathogens (expanded version); Table S3: Zoonotic parasitic pathogens (expanded version).
Author Contributions
Conceptualization, C.J.V. and V.L.; methodology, V.L.; formal analysis and investigation, C.J.V., E.A.D., C.S. and V.L.; data curation, C.J.V., E.A.D., C.S. and V.L.; writing—original draft preparation, C.J.V. and V.L.; writing—review and editing, E.A.D. and C.S.; supervision, V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw files of the literature review are available upon request from the corresponding author.
Acknowledgments
During the preparation of this Review the authors acknowledge the use of ChatGPT (GPT-5, OpenAI, San Francisco, CA, USA) for checking the Excel files compiling scientific publications, in order to identify duplicates and resources published in languages other than those specified in Section 2. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Brazilian States | |
| (AC) | Acre State |
| (AM) | Amazonas State |
| (MA) | Maranhão State |
| (MS) | Mato Grosso do Sul State |
| (MT) | Mato Grosso State |
| (PA) | Pará State |
| (RO) | Rondônia State |
| (TO) | Tocantins State |
| Diagnostic test abbreviations | |
| AGID II | Agar Gel Immunodiffusion test (Type II) |
| BLASTn | Basic Local Alignment Search Tool–nucleotide |
| CF | Complement Fixation |
| ELISA | Enzyme-linked immunosorbent assay |
| ELISA-ICC | Enzyme-linked immunoassay with infected cultured cells as antigenic matrix |
| FITC | anti-rabies globulin: Fluorescein isothiocyanate-conjugated anti-rabies globulin |
| HRM | High-Resolution Melting Analysis |
| HWAT | Heartworm Antigen Test |
| ICT | Rapid immunochromatographic test |
| IFA | Immunofluorescence assay |
| IFAT | Indirect Fluorescent Antibody Test |
| IHA | Indirect Hemagglutination Test |
| mAT | Microscopic Agglutination Test |
| MAT | Modified Agglutination Test |
| MLMT | Microsatellite Marker Analysis |
| NNN Culture | Novy-Nicolle-MacNeal medium culture |
| nPCR | nested Polymerase Chain Reaction |
| PCR | Polymerase Chain Reaction |
| PCR-RFLP | Polymerase Chain Reaction–Restriction Fragment Length Polymorphism |
| qPCR | quantitative Polymerase Chain Reaction |
| RBT | Rose Bengal Test |
| RFFIT | Rapid Fluorescent Focus Inhibition Test |
| RFLP | Restriction Fragment Length Polymorphism |
| RICT | Rapid Immunochromatographic Test |
| RT-qPCR | Reverse Transcription quantitative Polymerase Chain Reaction |
| SAM | Serum Agglutination Microscopy |
| SAT | Slide agglutination test |
| TESA-blot | Trypomastigote Excreted-Secreted Antigen blot |
Appendix A
The complete search string with Boolean operators used in this Systematic review was: Zoonosis OR zoonotic OR emerging infectious disease OR EID OR neglected tropical disease OR NTD AND Amazon OR Amazonic OR Amazonas OR Amazonia OR Amazonian AND Brazil OR Peru OR Colombia OR Bolivia OR Venezuela OR Ecuador OR Guyana OR Suriname OR French Guiana AND dog OR canine OR cat OR feline.
References
- RAISG. Amazonía Bajo Presión; RAISG: Sao Paulo, Brazil, 2020. [Google Scholar]
- Gardner, T.A.; Barlow, J.; Sodhi, N.S.; Peres, C.A. A Multi-Region Assessment of Tropical Forest Biodiversity in a Human-Modified World. Biol. Conserv. 2010, 143, 2293–2300. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global Patterns of Terrestrial Vertebrate Diversity and Conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2602–E2610. [Google Scholar] [CrossRef]
- Reid, W.V.; Mooney, H.A.; Cropper, A.; Capistrano, D.; Carpenter, S.R.; Chopra, K.; Dasgupta, P.; Dietz, T.; Duraiappah, A.K.; Hassan, R.; et al. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; ISBN 1597260401. [Google Scholar]
- Chivian, E.; Bernstein, A. (Eds.) Sustaining Life How Human Health Depends on Biodiversity; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Suffredini, I.B.; Sader, H.S.; Gonçalves, A.G.; Reis, A.O.; Gales, A.C.; Varella, A.D.; Younes, R.N. Screening of antibacterial extracts from plants native to the Brazilian Amazon Rain Forest and Atlantic Forest. Braz. J. Med. Biol. Res. 2004, 37, 379–384. [Google Scholar] [CrossRef]
- Olival, K.J.; Hosseini, P.R.; Zambrana-Torrelio, C.; Ross, N.; Bogich, T.L.; Daszak, P. Host and Viral Traits Predict Zoonotic Spillover from Mammals. Nature 2017, 546, 646–650. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; Vittor, A.Y. Deforestation and Vector-Borne Disease: Forest Conversion Favors Important Mosquito Vectors of Human Pathogens. Basic Appl. Ecol. 2018, 26, 101–110. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Mordecai, E.A. Amazon Deforestation Drives Malaria Transmission, and Malaria Burden Reduces Forest Clearing. Proc. Natl. Acad. Sci. USA 2019, 116, 22212–22218. [Google Scholar] [CrossRef]
- Morand, S.; Lajaunie, C. Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated with Changes in Forest Cover and Oil Palm Expansion at Global Scale. Front. Vet. Sci. 2021, 8, 661063. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; Da Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond Diversity Loss and Climate Change: Impacts of Amazon Deforestation on Infectious Diseases and Public Health. An. Acad. Bras. Ciências 2020, 92, e20191375. [Google Scholar] [CrossRef]
- Garnett, S.T.; Burgess, N.D.; Fa, J.E.; Fernández-Llamazares, Á.; Molnár, Z.; Robinson, C.J.; Watson, J.E.M.; Zander, K.K.; Austin, B.; Brondizio, E.S.; et al. A Spatial Overview of the Global Importance of Indigenous Lands for Conservation. Nat. Sustain. 2018, 1, 369–374. [Google Scholar] [CrossRef]
- Garza, M.; Abascal Miguel, L. Health Disparities among Indigenous Populations in Latin America: A Scoping Review. Int. J. Equity Health 2025, 24, 119. [Google Scholar] [CrossRef]
- Haslam, P.A.; Ary Tanimoune, N. The Determinants of Social Conflict in the Latin American Mining Sector: New Evidence with Quantitative Data. World Dev. 2016, 78, 401–419. [Google Scholar] [CrossRef]
- Lascano, S.M. Molecular Epidemiology of Trypanosoma (Herpetosoma) Rangeli (Kinetoplastida: Trypanosomatidae) in Ecuador, South America, and Study of the Parasite Cell Invasion Mechanism In Vitro; Ohio University: Columbus, OH, USA, 2009. [Google Scholar]
- Cohen, J.E.; Gurtler, R.E. Modeling Household Transmission of American Trypanosomiasis. Science 2001, 293, 694–698. [Google Scholar] [CrossRef]
- Carme, B.; Demar-Pierre, M. Toxoplasmosis in French Guiana. Atypical (Neo-)Tropical Features of a Cosmopolitan Parasitosis. Med. Trop. 2006, 66, 495–503. [Google Scholar]
- Capdevila-Argüelles, L.; Iglesias-García, A.; Orueta, J.F.; Zilletti, B. Especies Exóticas Invasoras: Diagnóstico y Bases Para La Prevención y El Manejo; Organismo Autónomo Parques Nacionales: Madrid, Spain; Ministerio de Medio Ambiente: Madrid, Spain, 2006; ISBN 9788480146678. [Google Scholar]
- Stahl, P.W. Early Dogs and Endemic South American Canids of the Spanish Main. J. Anthropol. Res. 2013, 69, 515–533. [Google Scholar]
- Mendonça, T.O.; Perin, P.P.; Zanini, D.D.S.; de Souza, H.L.; Pires, P.H.K.; Muniz, I.M.; Tebaldi, J.H.; Mathias, L.A.; Bürger, K.P.; Lux-Hoppe, E.G. Parasitosis in Pet Dogs from Rondônia, Amazon Biome, and Human Perception of Zoonoses. Int. J. Environ. Res. Public Health 2024, 21, 138. [Google Scholar] [CrossRef]
- Mendoza Roldan, J.A.; Otranto, D. Zoonotic Parasites Associated with Predation by Dogs and Cats. Parasites Vectors 2023, 16, 55. [Google Scholar] [CrossRef]
- Salvarani, F.M.; Oliveira, H.G.D.S.; Correa, L.Y.S.; Soares, A.A.L.; Ferreira, B.C. The Importance of Studying Infectious and Parasitic Diseases of Wild Animals in the Amazon Biome with a Focus on One Health. Vet. Sci. 2025, 12, 100. [Google Scholar] [CrossRef]
- Lambin, E.F.; Gesit, H.J. Proximate Causes and Underlying Driving Forces of Tropical Deforestation: Tropical Forests Are Disappearing as the Result of Many Pressures, Both Local and Regional, Acting in Various Combinations in Different Geographical Locations. BioScience 2002, 52, 143–150. [Google Scholar]
- Une Seule Santé Par l’Organisation Mondiale de La Santé. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/one-health (accessed on 10 October 2025).
- Medina-Vogel, G. Ecología de Enfermedades Infecciosas Emergentes y Conservación de Especies Silvestres Ecology of Emerging Infectious Diseases and Wild Species Conservation. Arch. Med. Vet. 2010, 42, 11–24. [Google Scholar] [CrossRef]
- Malzoni Furtado, M.; Domingues de Ramos Filho, J.; Corrêa Scheffer, K.; Coelho, C.J.; Cruz, P.S.; Ikuta, C.Y.; de Almeida Jácomo, A.T.; de Oliveira Porfírio, G.E.; Silveira, L.; Sollmann, R.; et al. Serosurvey for Selected Viral Infections in Freeranging Jaguars (Panthera onca) and Domestic Carnivores in Brazilian Cerrado, Pantanal, and Amazon. J. Wildl. Dis. 2013, 49, 510–521. [Google Scholar] [CrossRef]
- Nava, A.F.D.; Cullen, L.; Sana, D.A.; Nardi, M.S.; Ramos Filho, J.D.; Lima, T.F.; Abreu, K.C.; Ferreira, F. First Evidence of Canine Distemper in Brazilian Free-Ranging Felids. Ecohealth 2008, 5, 513–518. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Grimmer, G.H.; de Paula, V.S.; Figueiredo, L.T.M.; Braga, W.S.M.; Luz, S.L.B. Mayaro Virus Infection in Amazonia: A Multimodel Inference Approach to Risk Factor Assessment. PLoS Negl. Trop. Dis. 2012, 6, e1846. [Google Scholar] [CrossRef]
- Meynard, J.-B.; Flamand, C.; Dupuy, C.; Mahamat, A.; Eltges, F.; Queuche, F.; Renner, J.; Fontanella, J.-M.; Hommel, D.; Dussart, P.; et al. First Human Rabies Case in French Guiana, 2008: Epidemiological Investigation and Control. PLoS Negl. Trop. Dis. 2012, 6, e1537. [Google Scholar] [CrossRef]
- Gaspari, M.M.F. Estudo Epidemiológico de Patógenos Circulantes Nas Populações de Onça-Pintada e Animais Domésticos Em Áreas Preservadas de Três Biomas Brasileiros: Cerrado, Pantanal e Amazônia; Universidade de São Paulo: São Paulo, Brazil, 2010. [Google Scholar]
- Bronson, E.; Emmons, L.H.; Murray, S.; Dubovi, E.J.; Deem, S.L. Serosurvey of Pathogens in Domestic Dogs on the Border of Noël Kempff Mercado National Park, Bolivia. J. Zoo Wildl. Med. 2008, 39, 28–36. [Google Scholar] [CrossRef]
- Widdowson, M.-A.; Morales, G.J.; Chaves, S.; McGrane, J. Epidemiology of Urban Canine Rabies, Santa Cruz, Bolivia, 1972–1997. Emerg. Infect. Dis. 2002, 8, 458–461. [Google Scholar] [CrossRef]
- Zambrano-Mila, M.S.; Freire-Paspuel, B.; Orlando, S.A.; Garcia-Bereguiain, M.A. SARS-CoV-2 Infection in Free Roaming Dogs from the Amazonian Jungle. One Health 2022, 14, 100387. [Google Scholar] [CrossRef]
- Mesquita, V.A.; Talhari, S.; Leturiondo, A.L.; de Souza, G.C.; de Brito, E.M.; de Andrade, S.L.; Fernandes, D.C.D.L.; Frota, M.Z.M.; Cruz, R.C.D.S.; Guimarães, J.D.A.R.; et al. Zoonotic Sporotrichosis Outbreak: Emerging Public Health Threat in the Amazon State, Brazil. PLoS Negl. Trop. Dis. 2024, 18, e0012328. [Google Scholar] [CrossRef]
- Milstein, M.S.; Shaffer, C.A.; Suse, P.; Marawanaru, A.; Heinrich, D.A.; Larsen, P.A.; Wolf, T.M. A Mixed-Methods Approach to Understanding Domestic Dog Health and Disease Transmission Risk in an Indigenous Reserve in Guyana, South America. PLoS Negl. Trop. Dis. 2022, 16, e0010469. [Google Scholar] [CrossRef]
- Furtado, M.M.; Gennari, S.M.; Ikuta, C.Y.; Jácomo, A.T.D.A.; de Morais, Z.M.; Pena, H.F.D.J.; Porfírio, G.E.D.O.; Silveira, L.; Sollmann, R.; de Souza, G.O.; et al. Serosurvey of Smooth Brucella, Leptospira Spp. and Toxoplasma Gondii in Free-Ranging Jaguars (Panthera onca) and Domestic Animals from Brazil. PLoS ONE 2015, 10, e0143816. [Google Scholar] [CrossRef]
- Rodriguez-Pazmiño, A.S.; Brito, C.M.; Salas-Rueda, M.; Orlando, S.A.; Garcia-Bereguiain, M.A. A First Insight into Seropositivity and Risk Factors for Brucella spp. and Coxiella burnetii in Free-Roaming Dogs in Ecuador. One Health 2024, 19, 100909. [Google Scholar] [CrossRef]
- Gardon, J.; Héraud, J.; Laventure, S.; Ladam, A.; Capot, P.; Fouquet, E.; Favre, J.; Weber, S.; Hommel, D.; Hulin, A.; et al. Suburban Transmission of Q Fever in French Guiana: Evidence of a Wild Reservoir. J. Infect. Dis. 2001, 184, 278–284. [Google Scholar] [CrossRef]
- Costa, A.P.D.; Costa, F.B.; Labruna, M.B.; Silveira, I.; Moraes-Filho, J.; Soares, J.F.; Spolidorio, M.G.; Guerra, R.d.M.S.N.d.C. A Serological and Molecular Survey of Babesia Vogeli, Ehrlichia Canis and Rickettsia Spp. among Dogs in the State of Maranhão, Northeastern Brazil. Rev. Bras. Parasitol. Veterinária 2015, 24, 28–35. [Google Scholar] [CrossRef]
- Guzmán, D.A.; Diaz, E.; Sáenz, C.; Álvarez, H.; Cueva, R.; Zapata-Ríos, G.; Prado-Vivar, B.; Falconí, M.; Pearson, T.; Barragan, V. Domestic Dogs in Indigenous Amazonian Communities: Key Players in Leptospira Cycling and Transmission? PLoS Negl. Trop. Dis. 2024, 18, e0011671. [Google Scholar] [CrossRef]
- Fiorello, C.V.; Deem, S.L.; Gompper, M.E.; Dubovi, E.J. Seroprevalence of Pathogens in Domestic Carnivores on the Border of Madidi National Park, Bolivia. Anim. Conserv. 2004, 7, 45–54. [Google Scholar] [CrossRef]
- Costa, F.B. Soroepidemiologia e Epidemiologia Molecular Das Infecções Por Rickettsia Spp Em Cães e Carrapatos de Ambientes Urbano e Rural Do Estado Do Maranhão; Universidade de São Paulo: São Paulo, Brazil, 2014. [Google Scholar]
- Tomassone, L.; Conte, V.; Parrilla, G.; De Meneghi, D. Rickettsia Infection in Dogs and Rickettsia Parkeri in Amblyomma Tigrinum Ticks, Cochabamba Department, Bolivia. Vector-Borne Zoonotic Dis. 2010, 10, 953–958. [Google Scholar] [CrossRef]
- Forshey, B.M.; Stewart, A.; Morrison, A.C.; Gálvez, H.; Rocha, C.; Astete, H.; Eza, D.; Chen, H.-W.; Chao, C.-C.; Montgomery, J.M.; et al. Epidemiology of Spotted Fever Group and Typhus Group Rickettsial Infection in the Amazon Basin of Peru. Am. Soc. Trop. Med. Hyg. 2010, 82, 683–690. [Google Scholar] [CrossRef]
- Costa, I.N.D.; Garcia, M.V.; Santos, V.P.D.; Costa, N.V.C.; Carioca, A.L.P.M.; Andreotti, R.; Medeiros, J.F.; Aguirre, A.D.A.R. Simultaneous Infestations in Dogs by Different Tick Species (Acari: Ixodidae) in Two Areas of the Western Brazilian Amazon. Rev. Bras. Parasitol. Veterinária 2025, 34, e020324. [Google Scholar] [CrossRef]
- de Argôlo, E.G.G.; Reis, T.; Fontes, D.A.T.; Gonçalves, E.C.; Giese, E.G.; Melo, F.T.D.V.; dos Santos, J.N.; Furtado, A.P. Canine Filariasis in the Amazon: Species Diversity and Epidemiology of These Emergent and Neglected Zoonoses. PLoS ONE 2018, 13, e0200419. [Google Scholar] [CrossRef]
- Dias-Correia, T.P.; Neves, L.B.D.; Bittencourt-Oliveira, F.; Giglio, G.C.B.; Pereira, T.C.; Almeida, F.B.D.; Rodrigues-Silva, R. Diversity of Helminths with Zoonotic Potential and Molecular Characterization of Toxocara Canis Infecting Domestic Dogs from Locations of Amazon and Atlantic Forest Brazilian Biomes. Rev. Bras. Parasitol. Veterinária 2023, 32, e012723. [Google Scholar] [CrossRef]
- Potes-Morales, C.; Crespo-Ortiz, M.D.P. Molecular Diagnosis of Intestinal Protozoa in Young Adults and Their Pets in Colombia, South America. PLoS ONE 2023, 18, e0283824. [Google Scholar] [CrossRef]
- Morais, A.N.; Sousa, M.G.; Meireles, L.R.; Kesper, N., Jr.; Umezawa, E.S. Canine Visceral Leishmaniasis and Chagas Disease among Dogs in Araguaína, Tocantins. Rev. Bras. Parasitol. Veterinária 2013, 22, 225–229. [Google Scholar] [CrossRef]
- Soares, H.S.; Camargo, L.M.A.; Gennari, S.M.; Labruna, M.B. Survey of Canine Tick-Borne Diseases in Lábrea, Brazilian Amazon: ‘Accidental’ Findings of Dirofilaria Immitis Infection. Rev. Bras. Parasitol. Veterinária 2014, 23, 473–480. [Google Scholar] [CrossRef]
- Barbosa, U.C.; Nava, A.F.D.; Ferreira Neto, J.V.; Dias, C.A.; Silva, V.C.D.; Mesquita, H.G.D.; Sampaio, R.T.D.; Barros, W.G.; Farias, E.D.S.; Silva, T.R.R.D.; et al. Dirofilaria Immitis Is Endemic in Rural Areas of the Brazilian Amazonas State Capital, Manaus. Rev. Bras. Parasitol. Veterinária 2023, 32, e000223. [Google Scholar] [CrossRef]
- Moreira, H.R.; Madeira, E.A.O.; Cunha, D.N.L.; Scofield, A.; Góes-Cavalcante, G.; Abel, I.; Guimarães, R.J.P.S.; Fernandes, J.I. Dirofilaria Immitis Infection in Dogs in Algodoal Island, Brazilian Amazon. Pesqui. Veterinária Bras. 2019, 39, 510–515. [Google Scholar] [CrossRef]
- Ogawa, G.M.; Cruz, E.N.D.; Cunha, P.N.A.; Camargo, L.M.A. Canine Heartworm Disease in Porto Velho: First Record, Distribution Map and Occurrence of Positive Mosquitoes. Rev. Bras. Parasitol. Veterinária 2013, 22, 559–564. [Google Scholar] [CrossRef]
- Gomes Zanfagnini, L.; Carvalho Bento, G.K.; Fernandes Nunes da Silva Malavazi, P.; Figueiredo Souza, S.; Duarte Pacheco, A. Primeira Descrição de Dirofilariose Canina Alóctone Em Rio Branco, XAcre: Relato de Caso. Rev. Med. Vet. 2024, 48, e1497. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Marié, J.-L.; Tahir, D.; Watier-Grillot, S.; Mediannikov, O.; Davoust, B. Detection of Canine Vector-Borne Filariasis and Their Wolbachia Endosymbionts in French Guiana. Microorganisms 2020, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- das Neves, L.B.; Teixeira, P.E.F.; Silva, S.; de Oliveira, F.B.; Garcia, D.D.; de Almeida, F.B.; Rodrigues-Silva, R.; Machado-Silva, J.R. First Molecular Identification of Echinococcus vogeli and Echinococcus granulosus (Sensu stricto) G1 Revealed in Feces of Domestic Dogs (Canis familiaris) from Acre, Brazil. Parasites Vectors 2017, 10, 28. [Google Scholar] [CrossRef]
- Carneiro, L.A.; Vasconcelos dos Santos, T.; Lima, L.V.D.R.; Ramos, P.K.S.; Campos, M.B.; Silveira, F.T. First Report on Feline Leishmaniasis Caused by Leishmania (Leishmania) Amazonensis in Amazonian Brazil. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100360. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.O. Investigação Do Perfil Sorológico e Detecção Do DNA de Leishmania Spp. Em Cães de Áreas Endêmicas Para Leishmaniose Tegumentar Americana No Estado Do Pará, Brasil; Universidade Federal do Pará: Belém, Brazil, 2012. [Google Scholar]
- Castillo-Castañeda, A.C.; Patiño, L.H.; Zuñiga, M.F.; Cantillo-Barraza, O.; Ayala, M.S.; Segura, M.; Bautista, J.; Urbano, P.; Jaimes-Dueñez, J.; Ramírez, J.D. An Overview of the Trypanosomatid (Kinetoplastida: Trypanosomatidae) Parasites Infecting Several Mammal Species in Colombia. Parasites Vectors 2022, 15, 471. [Google Scholar] [CrossRef]
- Santos, F.J.A.; Nascimento, L.C.S.; Silva, W.B.; Oliveira, L.P.; Santos, W.S.; Aguiar, D.C.F.; Garcez, L.M. First Report of Canine Infection by Leishmania (Viannia) Guyanensis in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2020, 17, 8488. [Google Scholar] [CrossRef]
- Cupolillo, E.; Cavalcanti, A.S.; Ferreira, G.E.M.; Boité, M.C.; Morgado, F.N.; Porrozzi, R. Occurrence of Multiple Genotype Infection Caused by Leishmania Infantum in Naturally Infected Dogs. PLoS Negl. Trop. Dis. 2020, 14, e0007986. [Google Scholar] [CrossRef]
- Basano, S.D.A.; Tarso, P.; Soares, H.S.; Costa, A.P.; Marcili, A.; Labruna, M.B.; Dias, R.A.; Camargo, L.M.A.; Gennari, S.M. Toxoplasma Gondii, Neospora Caninum and Leishmania Amazonensis Antibodies in Domestic Dogs in the Western Brazilian Amazon Region. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 1. [Google Scholar] [CrossRef]
- Nascimento de Campos, H.G.; Gennari, S.M.; da Silva, R.E.; Soares, H.S.; Costa, J.D.O.J.; de Azevedo, S.S.; Marcili, A. Molecular and Serological Detection of Leishmania Infantum (Trypanosomatida: Trypanosomatidae) in Domestic Dogs in Manaus City, Amazonas, Brazil. J. Med. Entomol. 2024, 61, 1519–1523. [Google Scholar] [CrossRef]
- Campolongo, C.; Silva, R.E.D.; Azevedo, R.C.D.F.E.; Pesenato, I.P.; Carioca, A.C.F.; Alves, B.F.; Castelli, G.S.N.; Onofrio, V.C.; Marcili, A. Prevalence of Leishmania Infantum in Dogs from Deforested Areas of the Amazon Biome. Vector-Borne Zoonotic Dis. 2022, 22, 108–113. [Google Scholar] [CrossRef]
- Lima, J.T.R.D.; Gennari, S.M.; Soares, H.S.; Minervino, A.H.H.; Malheiros, A.F.; Marques, F.S.; Laurenti, M.D.; Machado, R.Z.; Marcili, A.; Labruna, M.B.; et al. Serodiagnosis of Visceral and Cutaneous Leishmaniasis in Human and Canine Populations Living in Indigenous Reserves in the Brazilian Amazon Region. Rev. Soc. Bras. Med. Trop. 2017, 50, 61–66. [Google Scholar] [CrossRef]
- Rocha, A.V.V.O.; Moreno, B.F.S.; Cabral, A.D.; Louzeiro, N.M.; Miranda, L.M.; Santos, V.M.B.D.; Costa, F.B.; Nogueira, R.D.M.S.; Marcili, A.; Sperança, M.A.; et al. Diagnosis and Epidemiology of Leishmania Infantum in Domestic Cats in an Endemic Area of the Amazon Region, Brazil. Vet. Parasitol. 2019, 273, 80–85. [Google Scholar] [CrossRef]
- Valadas, S.; Minervino, A.H.H.; Lima, V.M.F.; Soares, R.M.; Ortolani, E.L.; Gennari, S.M. Occurrence of Antibodies Anti-Neospora Caninum, Anti-Toxoplasma Gondii, and Anti-Leishmania Chagasi in Serum of Dogs from Pará State, Amazon, Brazil. Parasitol. Res. 2010, 107, 453–457. [Google Scholar] [CrossRef]
- de Oliveira Porfirio, G.E.; Santos, F.M.; de Macedo, G.C.; Barreto, W.T.G.; Campos, J.B.V.; Meyers, A.C.; André, M.R.; Perles, L.; de Oliveira, C.E.; Xavier, S.C.D.C.; et al. Maintenance of Trypanosoma Cruzi, T. Evansi and Leishmania Spp. by Domestic Dogs and Wild Mammals in a Rural Settlement in Brazil-Bolivian Border. Int. J. Parasitol. Parasites Wildl. 2018, 7, 398–404. [Google Scholar] [CrossRef]
- Brilhante, A.F.; Lima, L.; Zampieri, R.A.; Nunes, V.L.B.; Dorval, M.E.C.; Malavazi, P.F.N.D.S.; Melchior, L.A.K.; Ishikawa, E.A.Y.; Cardoso, C.D.O.; Floeter-Winter, L.M.; et al. Leishmania (Viannia) Braziliensis Type 2 as Probable Etiological Agent of Canine Cutaneous Leishmaniasis in Brazilian Amazon. PLoS ONE 2019, 14, e0216291. [Google Scholar] [CrossRef] [PubMed]
- Fornazari, F.; Sevá, A.D.P.; Oliveira, K.M.M.; Assunçao, P.C.F.; Guimaraes, V.Y. Exposure of Dogs and Wild Carnivores to Canine Distemper Virus, Canine Parvovirus, Leishmania Infantum, and Toxoplasma Gondii in the Xingu River Basin, Brazilian Amazon: Prevalence, Spatial Distribution, and Association with Land Cover Types. Acta Amazon. 2023, 53, 325–335. [Google Scholar] [CrossRef]
- Moreno, B.F.S. Avaliação Sorológica Para Detecção de Anticorpos Anti-Leishmania Sp. Em Gatos de Uma Área Endêmica Para Leishmaniose Visceral; Universidad Estatal de Maranhão: São Luís, Brazil, 2017. [Google Scholar]
- Medkour, H.; Davoust, B.; Dulieu, F.; Maurizi, L.; Lamour, T.; Marié, J.-L.; Mediannikov, O. Potential Animal Reservoirs (Dogs and Bats) of Human Visceral Leishmaniasis Due to Leishmania Infantum in French Guiana. PLoS Negl. Trop. Dis. 2019, 13, e0007456. [Google Scholar] [CrossRef]
- Rotureau, B.; Ravel, C.; Aznar, C.; Carme, B.; Dedet, J.-P. First Report of Leishmania Infantum in French Guiana: Canine Visceral Leishmaniasis Imported from the Old World. J. Clin. Microbiol. 2006, 44, 1120–1122. [Google Scholar] [CrossRef]
- Reithinger, R. The Epidemiology and Control of Canine Leishmaniasis in Peru and Brazil; University of London: London, UK, 2004. [Google Scholar]
- Ziemniczak, H.M.; Maia, M.O.; Maia, M.O.; Ferreira, E.; Vieira, N.T.; Saturnino, K.C.; Bresciani, K.D.S.; Gomes, A.A.D.; Pacheco, R.D.C.; Santos-Doni, T.R. High Seroprevalence of Toxoplasma Gondii and Neospora Spp. in Stray Dogs from Rolim de Moura, Rondônia State, Western Brazilian Amazon. Semin. Cienc. Agrar. 2021, 42, 3535–3542. [Google Scholar] [CrossRef]
- Minervino, A.H.H.; Cassinelli, A.B.M.; de Lima, J.T.R.; Soares, H.S.; Malheiros, A.F.; Marcili, A.; Gennari, S.M. Prevalence of Anti-Neospora Caninum and Anti-Toxoplasma Gondii Antibodies in Dogs from Two Different Indigenous Communities in the Brazilian Amazon Region. J. Parasitol. 2012, 98, 1276–1278. [Google Scholar] [CrossRef]
- Campos, H.G.N.D.; Soares, H.S.; Azevedo, S.S.D.; Gennari, S.M. Occurrence of Toxoplasma Gondii and Neospora Caninum Antibodies and Risk Factors in Domiciliated Dogs of Manaus, Amazonas, Brazil. Rev. Bras. Parasitol. Veterinária 2022, 31, e020321. [Google Scholar] [CrossRef]
- Mercier, A.; Ajzenberg, D.; Devillard, S.; Demar, M.P.; de Thoisy, B.; Bonnabau, H.; Collinet, F.; Boukhari, R.; Blanchet, D.; Simon, S.; et al. Human Impact on Genetic Diversity of Toxoplasma Gondii: Example of the Anthropized Environment from French Guiana. Infect. Genet. Evol. 2011, 11, 1378–1387. [Google Scholar] [CrossRef]
- Tahir, D.; Davoust, B.; Heu, K.; Lamour, T.; Demar, M.; Marié, J.-L.; Blanchet, D. Molecular and Serological Investigation of Trypanosoma Cruzi Infection in Dogs in French Guiana. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 106–109. [Google Scholar] [CrossRef]
- Garces Quintero, E.Y. Caracterización Ecoepidemiológica de La Transmisión Peridoméstica de Trypanosoma Cruzi En El Municipio de Talaigua Nuevo; Departamento de Bolívar, Costa Caribe Colombiana, Universidad de Antioquía: Medellín, Colombia, 2012. [Google Scholar]
- Oliveira, M.J.D.; Anadão, L.D.S.; Souza, F.D.A.; Santos, R.S.D.; Fermino, B.R.; Rodrigues, C.M.F.; Carvalho, J.S.; Gasparotto, P.H.G.; Silva, H.S.D.; Santos, F.G.D.A. Infecção Natural Por Trypanosoma Evansi Em Cão No Estado de Rondônia, Brasil: Relato de Caso. Cad. Pedagógico 2024, 21, e4369. [Google Scholar] [CrossRef]
- Cantillo-Barraza, O.; Solis, C.; Zamora, A.; Herazo, R.; Osorio, M.I.; Garcés, E.; Xavier, S.; Mejía-Jaramillo, A.M.; Triana-Chávez, O. Enzootic Trypanosoma Cruzi Infection by Rhodnius Prolixus Shows Transmission to Humans and Dogs in Vichada, Colombia. Front. Cell. Infect. Microbiol. 2022, 12, 999082. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Xavier, S.C.C.; Gerhardt, M.; Silva, M.F.O.; Lima, V.S.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma Cruzi among Wild and Domestic Mammals in Different Areas of the Abaetetuba Municipality (Pará State, Brazil), an Endemic Chagas Disease Transmission Area. Vet. Parasitol. 2013, 193, 71–77. [Google Scholar] [CrossRef]
- Villena, F.E.; Puicón, V.H.; López, A.M.; Rivera, K.; Pannebaker, D.; Valdivia, H.O.; Arévalo, H. Parasitological and Molecular Detection of Trypanosoma Evansi in a Dog from Tocache, San Martin, Peru. Vet. Parasitol. Reg. Stud. Rep. 2023, 42, 100895. [Google Scholar] [CrossRef]
- Corrêa, R.D.S.; Araujo, J.A.S.D.; Leite, J.M.B.; Silva Filho, L.E.D.; da Silva, N.M. Tungiasis in Dogs Residing in the Community Nossa Senhora in the Livramento, Sustainable Development Reserve Tupé, Amazonas. Rev. Bras. Hig. E Sanidade Anim. 2014, 8, 79–87. [Google Scholar] [CrossRef]
- Chaguay Villamar, K.M.; Hernández Copello, V.M.; Toro-Valdivieso, C.; Rivera Gomez-Barris, B.M. First Report of Tunga Trimamillata Infection in a Dog. BMC Vet. Res. 2025, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.D.C.; Roque, A.L.R. Trypanosoma Cruzi Transmission in the Wild and Its Most Important Reservoir Hosts in Brazil. Parasites Vectors 2018, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L. Una Revisión Sobre Reservorios de Trypanosoma (Schizotrypanum) Cruzi (Chagas, 1909), Agente Etiológico de La Enfermedad de Chagas. Bol. Malariol. Salud Ambient. 2010, 50, 3–15. [Google Scholar]
- Herrera, L.; Urdaneta-Morales, S. Didelphis marsupialis: A Primary Reservoir of Trypanosoma Cruzi in Urban Areas of Caracas, Venezuela. Ann. Trop. Med. Parasitol. 1992, 86, 607–612. [Google Scholar] [CrossRef]
- Yeo, M.; Acosta, N.; Llewellyn, M.; Sánchez, H.; Adamson, S.; Miles, G.A.J.; López, E.; González, N.; Patterson, J.S.; Gaunt, M.W.; et al. Origins of Chagas Disease: Didelphis Species Are Natural Hosts of Trypanosoma Cruzi I and Armadillos Hosts of Trypanosoma Cruzi II, Including Hybrids. Int. J. Parasitol. 2005, 35, 225–233. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Cecere, M.C.; Lauricella, M.A.; Cardinal, M.V.; Kitron, U.; Cohen, J.E. Domestic Dogs and Cats as Sources of Trypanosoma Cruzi Infection in Rural Northwestern Argentina. Parasitology 2007, 134, 69–82. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Canine Leishmaniosis in South America. Parasites Vectors 2009, 2, S1. [Google Scholar] [CrossRef]
- Dedet, J.-P. Cutaneous Leishmaniasis in French Guiana: A Review. Am. J. Trop. Med. Hyg. 1990, 43, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Massey, A.L.; Ferreira da Silva, D.J.; Vieira, C.J.D.S.P.; Allen, J.M.; Canale, G.R.; Bernardo, C.S.S.; de Morais Bronzoni, R.V.; Peres, C.A.; Levi, T. Using IDNA to Determine Impacts of Amazonian Deforestation on Leishmania Hosts, Vectors, and Their Interactions. PLoS Negl. Trop. Dis. 2025, 19, e0012925. [Google Scholar] [CrossRef]
- Barragan, V.; Chiriboga, J.; Miller, E.; Olivas, S.; Birdsell, D.; Hepp, C.; Hornstra, H.; Schupp, J.M.; Morales, M.; Gonzalez, M.; et al. High Leptospira Diversity in Animals and Humans Complicates the Search for Common Reservoirs of Human Disease in Rural Ecuador. PLoS Negl. Trop. Dis. 2016, 10, e0004990. [Google Scholar] [CrossRef]
- Giraldo de León, G.; Orrego Uribe, A.; Betancurth, A.M. Los Roedores Como Reservorios de Leptospiras En Planteles Porcinos de La Zona Central Cafetera de Colombia. Arch. Med. Vet. 2002, 34, 69–78. [Google Scholar] [CrossRef]
- Ricardo, T.; Monje, L.D.; Landolt, N.; Chiani, Y.T.; Schmeling, M.F.; Beldoménico, P.M.; Vanasco, N.B.; Previtali, M.A. Primer Informe de Leptospira interrogans En El Roedor Sigmodontino Scapteromys aquaticus. Rev. Panam. Salud Pública 2018, 42, e83. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.A.; Arroyo, G.; Sáenz, C.; Mena, L.; Barragán, V. Leptospirosis in Horses: Sentinels for a Neglected Zoonosis? A Systematic Review. Vet. World 2023, 16, 2110–2119. [Google Scholar] [CrossRef]
- Orlando, S.A.; Perez, A.; Sanchez, E.; de la Cruz, C.; Rugel, O.; Garcia-Bereguiain, M.A. High Seroprevalence of Anti-Leptospira Spp. Antibodies in Domestic and Wild Mammals from a Mixed Use Rescue Center in Ecuador: Lessons for “One Health” Based Conservation Strategies. One Health 2020, 10, 100140. [Google Scholar] [CrossRef]
- Hartmann, K.; Egberink, H.; Pennisi, M.G.; Lloret, A.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hosie, M.J.; et al. Leptospira Species Infection in Cats. J. Feline Med. Surg. 2013, 15, 576–581. [Google Scholar] [CrossRef]
- Elmore, S.A.; Jones, J.L.; Conrad, P.A.; Patton, S.; Lindsay, D.S.; Dubey, J.P. Toxoplasma Gondii: Epidemiology, Feline Clinical Aspects, and Prevention. Trends Parasitol. 2010, 26, 190–196. [Google Scholar] [CrossRef]
- Cañon-Franco, W.A.; Araújo, F.A.P.D.; Gennari, S.M. Toxoplasma Gondii Em Pequenos Felinos Silvestres Neotropicais. Braz. J. Vet. Res. Anim. Sci. 2013, 50, 50. [Google Scholar] [CrossRef]
- Demar, M.; Ajzenberg, D.; Maubon, D.; Djossou, F.; Panchoe, D.; Punwasi, W.; Valery, N.; Peneau, C.; Daigre, J.-L.; Aznar, C.; et al. Fatal Outbreak of Human Toxoplasmosis along the Maroni River: Epidemiological, Clinical, and Parasitological Aspects. Clin. Infect. Dis. 2007, 45, e88–e95. [Google Scholar] [CrossRef]
- Gómez-Marín, J.E.; de-la-Torre, A.; Barrios, P.; Cardona, N.; Álvarez, C.; Herrera, C. Toxoplasmosis in Military Personnel Involved in Jungle Operations. Acta Trop. 2012, 122, 46–51. [Google Scholar] [CrossRef]
- do Carmo, E.L.; Póvoa, M.M.; Monteiro, N.S.; Marinho, R.R.; Nascimento, J.M.; Freitas, S.N.; Bichara, C.N.C. Surto de Toxoplasmose Humana No Distrito de Monte Dourado, Município de Almeirim, Pará, Brasil. Rev. Pan-Amaz. Saúde 2010, 1. [Google Scholar] [CrossRef]
- Souza, N.F.; Benigno, R.N.M.; Figueiredo, M.; Salim, S.K.; Silva, D.; Gonçalves, R.; Peixoto, P.C.; Serra-Freire, N.M. Prevalência de Dirofilaria Immitis Em Cães No Município de Belém/PA, Com Base Na Microfilaremia. Rev. Bras. Parasitol. Veterinária 1997, 6, 83–86. [Google Scholar]
- Vieira, C.; Vélez, I.D.; Montoya, M.N.; Agudelo, S.; Genchi, C.; Simón, F. Dirofilaria Immitis in Tikuna Indians and Their Dogs in the Colombian Amazon. Ann. Trop. Med. Parasitol. 1998, 92, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Portela, C.S.; Mendes de Araújo, C.P.; Moura Sousa, P.; Gomes Simão, C.L.; Silva de Oliveira, J.C.; Crainey, J.L. Filarial Disease in the Brazilian Amazon and Emerging Opportunities for Treatment and Control. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 5, 100168. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Bernigaud, C.; Guillot, J. Zoonotic Episodes of Scabies: A Global Overview. Pathogens 2022, 11, 213. [Google Scholar] [CrossRef]
- Rudd, J.L.; Clifford, D.L.; Cypher, B.L.; Hull, J.M.; Jane Riner, A.; Foley, J.E. Molecular Epidemiology of a Fatal Sarcoptic Mange Epidemic in Endangered San Joaquin Kit Foxes (Vulpes Macrotis Mutica). Parasites Vectors 2020, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Wallén, J.; Erlandsson, R.; Larm, M.; Meijer, T.; Norén, K.; Angerbjörn, A. Consequences of Repeated Sarcoptic Mange Outbreaks in an Endangered Mammal Population. Ecography 2024, 2024, e07291. [Google Scholar] [CrossRef]
- Unterköfler, M.S.; Schausberger, M.; Deutz, A.; Gressmann, G.; Kübber-Heiss, A.; Ferroglio, E.; Joachim, A. Sarcoptic Mange in Wild Ungulates in the European Alps—A Systematic Review. Int. J. Parasitol. Parasites Wildl. 2023, 22, 121–125. [Google Scholar] [CrossRef]
- Díaz Luque, J.A.; Müller, H.; González, L.; Berkunsky, I. Clinical Signs Suggestive of Mange Infestation in a Free-Ranging Maned Wolf (Chrysocyon Brachyurus) in the Moxos Savannahs of Beni, Bolivia. Mastozool. Neotrop. 2014, 21, 135–138. [Google Scholar]
- Fiori, F.; de Paula, R.C.; Boulhosa, R.L.P.; Dias, R.A. Clinical Evaluation of Sarcoptic Mange (Sarcoptes scabiei) in Maned Wolves (Chrysocyon brachyurus). Vet. Res. Commun. 2025, 49, 202. [Google Scholar] [CrossRef] [PubMed]
- Fiori, F.; de Paula, R.C.; Navas-Suárez, P.E.; Boulhosa, R.L.P.; Dias, R.A. The Sarcoptic Mange in Maned Wolf (Chrysocyon brachyurus): Mapping an Emerging Disease in the Largest South American Canid. Pathogens 2023, 12, 830. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, T.G.W.; Lima, P.A.; Stehling, P.C.; Oliveira Junior, I.M.; Varaschin, M.S.; Wouters, F.; Wouters, A.T.B. Sarcoptic Mange (Sarcoptes scabiei) in Wild Canids (Cerdocyon thous). Pesqui. Veterinária Bras. 2018, 38, 1444–1448. [Google Scholar] [CrossRef]
- Silva Paranhos, L.; Alves Soares Vaz de Castro, P.; Rotondo de Araújo, G.; Do Carmo Magalhães, F.; Trindade Bezerra, J.M. Prevalence of Tungiasis in Humans in Brazil and in Its Federative Units: A Systematic Review. Rev. Patol. Trop./J. Trop. Pathol. 2022, 51, 31–50. [Google Scholar] [CrossRef]
- Veraldi, S.; Valsecchi, M. Imported Tungiasis: A Report of 19 Cases and Review of the Literature. Int. J. Dermatol. 2007, 46, 1061–1066. [Google Scholar] [CrossRef]
- Buhariwalla, F.; Cann, B.; Marrie, T.J. A Dog-Related Outbreak of Q Fever. Clin. Infect. Dis. 1996, 23, 753–755. [Google Scholar] [CrossRef]
- Pinsky, R.L.; Fishbein, D.B.; Greene, C.R.; Gensheimer, K.F. An Outbreak of Cat-Associated Q Fever in the United States. J. Infect. Dis. 1991, 164, 202–204. [Google Scholar] [CrossRef]
- Celina, S.S.; Cerný, J. Coxiella burnetii in Ticks, Livestock, Pets and Wildlife: A Mini-Review. Front. Vet. Sci. 2022, 9, 1068129. [Google Scholar] [CrossRef]
- Chacón-Díaz, C.; Altamirano-Silva, P.; González-Espinoza, G.; Medina, M.-C.; Alfaro-Alarcón, A.; Bouza-Mora, L.; Jiménez-Rojas, C.; Wong, M.; Barquero-Calvo, E.; Rojas, N.; et al. Brucella Canis Is an Intracellular Pathogen That Induces a Lower Proinflammatory Response than Smooth Zoonotic Counterparts. Infect. Immun. 2015, 83, 4861–4870. [Google Scholar] [CrossRef]
- Djokic, V.; Freddi, L.; de Massis, F.; Lahti, E.; van den Esker, M.H.; Whatmore, A.; Haughey, A.; Ferreira, A.C.; Garofolo, G.; Melzer, F.; et al. The Emergence of Brucella Canis as a Public Health Threat in Europe: What We Know and What We Need to Learn. Emerg. Microbes Infect. 2023, 12, 2249126. [Google Scholar] [CrossRef]
- Lucero, N.E.; Escobar, G.I.; Ayala, S.M.; Jacob, N. Diagnosis of Human Brucellosis Caused by Brucella Canis. J. Med. Microbiol. 2005, 54, 457–461. [Google Scholar] [CrossRef]
- Davis, G.H.N.G. Estudos Epidemiológicos Sobre Arbovírus Em Populações Rurais e Urbanas Do Estado Do Amazonas; Universidade Federal do Amazonas: Manaus, Brazil, 2009. [Google Scholar]
- Ortiz-Prado, E.; Rivera-Olivero, I.A.; Freire-Paspuel, B.; Lowe, R.; Lozada, T.; Henriquez-Trujillo, A.R.; Garcia-Bereguiain, M.A. Testing for SARS-CoV-2 at the Core of Voluntary Collective Isolation: Lessons from the Indigenous Populations Living in the Amazon Region in Ecuador. Int. J. Infect. Dis. 2021, 105, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Sordillo, E.M.; Gotuzzo, E.; Zavaleta, C.; Caplivski, D.; Navarro, J.C.; Crainey, J.L.; Bessa Luz, S.L.; Noguera, L.A.D.; Schaub, R.; et al. SARS-CoV-2 in the Amazon Region: A Harbinger of Doom for Amerindians. PLoS Negl. Trop. Dis. 2020, 14, e0008686. [Google Scholar] [CrossRef]
- Morales-Jadán, D.; Vallejo-Janeta, A.P.; Freire-Paspuel, B.; Rodriguez-Pazmiño, Á.S.; Rivera-Olivero, I.; Henriquez-Trujillo, A.R.; Lozada, T.; Tapia, A.; Orlando, S.A.; Ortiz-Prado, E.; et al. COVID-19 Outbreaks in Endangered Indigenous Groups from the Amazonia during the First Wave of the COVID-19 Pandemic in Ecuador: A Retrospective Cross-Sectional Study. Rural. Remote Health 2024, 24, 1–9. [Google Scholar] [CrossRef]
- Muñoz-del-Carpio-Toia, A.; Bartolo-Marchena, M.; Benites-Zapata, V.A.; Herrera-Añazco, P. Mortality from COVID-19 in Amazonian and Andean Original Indigenous Populations of Peru. Travel. Med. Infect. Dis. 2023, 56, 102658. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.T.; Nascimento, L.F.D.J.; Barbosa, A.A.T.; Martins, M.P.; Tunon, G.I.L.; Santos, P.O.M.; Dantas-Torres, F.; Dolabella, S.S. The Rising Incidence of Feline and Cat-transmitted Sporotrichosis in Latin America. Zoonoses Public Health 2024, 71, 609–619. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Dhama, K.; Rodriguez-Morales, A.J. Revisiting the One Health Approach in the Context of COVID-19: A Look into the Ecology of This Emerging Disease. Adv. Anim. Vet. Sci. 2020, 8, 234–237. [Google Scholar] [CrossRef]
- Eisenberg, J.N.S.; Cevallos, W.; Ponce, K.; Levy, K.; Bates, S.J.; Scott, J.C.; Hubbard, A.; Vieira, N.; Endara, P.; Espinel, M.; et al. Environmental Change and Infectious Disease: How New Roads Affect the Transmission of Diarrheal Pathogens in Rural Ecuador. Proc. Natl. Acad. Sci. USA 2006, 103, 19460–19465. [Google Scholar] [CrossRef]
- Ferrante, L. A Road to the next Pandemic: The Consequences of Amazon Highway BR-319 for Planetary Health. Lancet Planet. Health 2024, 8, e524–e525. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.C.; Silva, E.A.R.T.; Gama, H.S.S.D.; Cordeiro, J.S.M.; Oliveira, A.P.S.; Araújo, J.A.; Dávila, R.N.; Amazonas Júnior, H.A.; Farias, A.S.; Sachett, J.A.G.; et al. Participatory Research towards the Control of Snakebite Envenoming and Other Illnesses in a Riverine Community of the Western Brazilian Amazon. PLoS Negl. Trop. Dis. 2025, 19, e0012840. [Google Scholar] [CrossRef]
- Garnelo, L.; Parente, R.C.P.; Puchiarelli, M.L.R.; Correia, P.C.; Torres, M.V.; Herkrath, F.J. Barriers to Access and Organization of Primary Health Care Services for Rural Riverside Populations in the Amazon. Int. J. Equity Health 2020, 19, 54. [Google Scholar] [CrossRef]
- Correa, L.L.; Pinheiro, A.D.S.F. Dynamics of Parasitic Diseases and the Environmental and Sanitation Context in Cities of the Brazilian Amazon. J. Parasit. Dis. Diagn. Ther. 2017, 2. [Google Scholar]
- Rodríguez-Prieto, V.; Vicente-Rubiano, M.; Sánchez-Matamoros, A.; Rubio-Guerri, C.; Melero, M.; Martínez-López, B.; Martínez-Avilés, M.; Hoinville, L.; VERGNE, T.; Comin, A.; et al. Systematic Review of Surveillance Systems and Methods for Early Detection of Exotic, New and Re-Emerging Diseases in Animal Populations. Epidemiol. Infect. 2015, 143, 2018–2042. [Google Scholar] [CrossRef] [PubMed]
- Sangat, S.S.; Rosero, M.; Olsson, E.; Nowakowski, A.J.; Drescher-Lehman, J.; Roehrdanz, P.R.; Noon, M.L.; McManus, N.; Perz, S.G.; Angel, M.; et al. Afro-Descendant Lands in South America Contribute to Biodiversity Conservation and Climate Change Mitigation. Commun. Earth Environ. 2025, 6, 458. [Google Scholar] [CrossRef]
- Nolte, C.; Agrawal, A.; Silvius, K.M.; Soares-Filho, B.S. Governance Regime and Location Influence Avoided Deforestation Success of Protected Areas in the Brazilian Amazon. Proc. Natl. Acad. Sci. USA 2013, 110, 4956–4961. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.D.; Irwin, P.J.; Lymbery, A.J.; Thompson, R.C.A. The Role of Companion Animals in the Emergence of Parasitic Zoonoses. Int. J. Parasitol. 2000, 30, 1369–1377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.